Abstract
We report a case of mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) in a 31‐year‐old man. He had been diagnosed with mild COVID‐19 3 days earlier and presented to the emergency department with altered mental status. Brain magnetic resonance imaging (MRI) showed a high‐intensity area confined to the splenium of the corpus callosum on diffusion‐weighted imaging, which is consistent with MERS. MERS is characterized by a reversible change in the splenium of the corpus callosum. MERS secondary to COVID‐19 has been reported recently. It is important to consider MERS in COVID‐19 patients with impaired consciousness.
Keywords: COVID‐19, cytotoxic lesions of the corpus callosum, mild encephalitis with a reversible splenial lesion, SARS‐Cov‐2
We experienced a patient with mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) during COVID‐19. The brain MRI revealed a high‐intensity area confined to the splenium of the corpus callosum on diffusion‐weighted imaging, which is consistent with MERS. We should consider MERS in COVID‐19 patients with impaired consciousness.
1. INTRODUCTION
Since severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) first emerged in China in December 2019, it has rapidly spread worldwide, causing a global pandemic featuring numerous waves with changing predominant variants. 1 COVID‐19 is an infectious disease caused by SARS‐CoV‐2 and is often life‐threatening for elderly people and patients with comorbidities. Although respiratory symptoms were initially identified as the main clinical manifestations, it subsequently became clear that COVID‐19 can manifest in various organs, resulting in cardiac diseases, neurological abnormalities, and other disorders. 2 , 3 Here, we describe an adult patient with mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) secondary to COVID‐19 and review some of the relevant literature. Written informed consent was obtained from the patient for publication of this case report.
2. CASE PRESENTATION
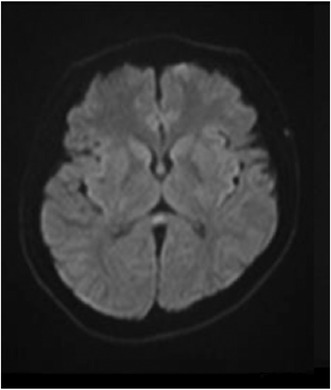
A 31‐year‐old man presented to the emergency department with altered mental status. He had no specific medical history, no allergic history, and no specific family history. He had not received the COVID‐19 vaccine. He had begun experiencing sore throat and fever and been diagnosed with mild COVID‐19 3 days earlier by a primary care doctor based on a SARS‐CoV‐2 antigen test. He rested at home after the diagnosis but became unable to speak and move on the day of admission. On arrival, he had a Japan Coma Scale score of II‐20, and his vital signs included body temperature of 36.4°C, blood pressure of 132/77 mmHg, pulse rate of 84 beats/min, respiratory rate of 16 breaths/min, and SpO2 of 96% (room air). Neurological tests could not be performed because of his drowsiness. Blood tests showed no signs of hypoglycemia or electrolyte abnormalities causing the altered mental status. Chest computed tomography (CT) revealed bilateral pneumonia (Figure 1), but no respiratory failure was observed. Considering the adverse effects of dehydration, 1500 mL of intravenous fluid was administered but his consciousness did not improve. Cerebrospinal fluid (CSF) examination was performed on suspicion of meningitis, but no increase in white blood cell count and protein level was observed. Brain magnetic resonance imaging (MRI) was performed to investigate the reason for his altered mental status and revealed a high‐intensity area confined to the splenium of the corpus callosum (SCC) on diffusion‐weighted imaging (DWI) and a slight decrease in the apparent diffusion coefficient (ADC) value at the site of the lesion on an ADC map (Figure 2). The patient was treated with remdesivir for 3 days, and his mental status fully recovered. On the sixth day of hospitalization, follow‐up MRI showed resolution of the high‐intensity area in the SCC (Figure 2), so the final diagnosis was MERS. This was the first time for him to have such symptoms.
FIGURE 1.
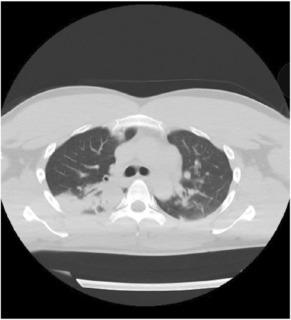
Chest CT on admission.
FIGURE 2.
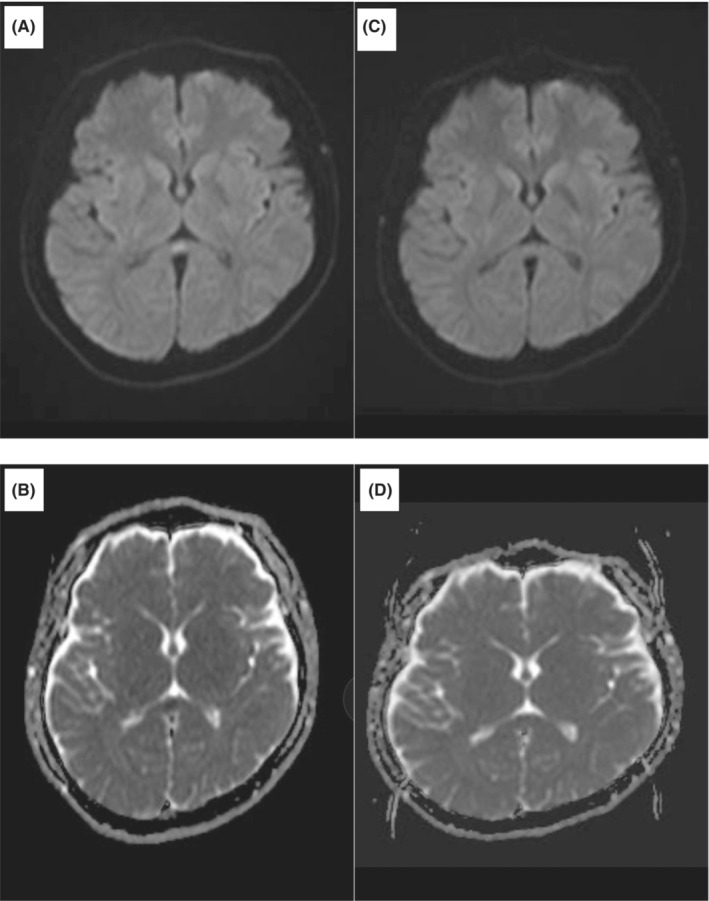
Brain MRI on admission (A: DWI; B: ADC map) and 6 days later (C: DWI; D: ADC map).
3. DISCUSSION
In 2004, Tada et al. 4 analyzed the clinical characteristics of 15 patients who had encephalitis/encephalopathy with a reversible lesion in the SCC and first referred to this as MERS. Subsequently, abnormalities similar to MERS on MRI have been defined as cytotoxic lesions of the corpus callosum (CLOCCs). 5 However, it is reported that CLOCCs are not always reversible. In 2012, Hoshino et al. 6 proposed diagnostic criteria for MERS, including the presence of neuropsychiatric symptoms, high signal intensity lesion in the SCC, and complete recovery without sequelae. Thus, CLOCCs and MERS partially overlap but are not the same. The patient in this case was an adult with MERS. Initially, MERS was thought to be more common in children, 4 , 7 but recently there have been many reported cases in adults. 8 Various causes of disease onset have been reported, including viral and bacterial infections. 6 Recently, it has been reported that MERS also occurs in patients with COVID‐19. In a review of 29 patients with COVID‐19‐associated MERS/CLOCCs, adult patients have been reported at various ages, the oldest being 75 years of age, and more common in men. The common symptoms were altered consciousness and ataxia, but various symptoms including altered mental status were reported. The median time from onset of COVID‐19 to onset of CLOCCs was 3 days (range: 0–13 days). Although the patients had various levels of COVID‐19 severity, most recovered from COVID‐19. 9 But a patient with mild COVID‐19 who have subarachnoid hemorrhage and CLOCCs progressed the encephalitis and dead because of progressing brain edema. This case suggests the CLOCCs accompanying with other MRI finding may have poor prognosis. 10 The present case met the criteria of MERS and was consistent with previously reported cases in that MERS developed within a short period after the onset of COVID‐19 and then resolved promptly. Laboratory tests did not show any notable findings that could explain MERS, suggesting that the cause was COVID‐19 in this patient. We performed SARS‐CoV‐2 PCR using CSF, and the result was negative, suggesting that the virus may not directly cause MERS. This suggests that MERS is different from meningitis or encephalitis caused by COVID‐19. 3 Many hypotheses are suspected for the pathogenesis of MERS, such as intramyelinic edema, axonal damage, hyponatremia, and oxidative stress. 7 , 8 The most possible hypothesis is that the activated immune response against COVID‐19 increases released inflammatory cytokines and may cause the MERS. The SCC is vulnerable to elevated inflammatory cytokines such as IL‐1 and IL‐6, which leads to focal edema, 5 but many aspects remain unknown and future study is warranted. The genetic factors are also suspected to be associated with MERS, 11 but our patient had no suspected family history of MERS. Most cases of MERS/CLOCCs have been reported during the initial phase of the pandemic. SARS‐CoV‐2 has made numerous waves with changing predominant variants. It has been revealed that the change in the predominant variants affected the major symptoms of COVID‐19. 12 We did not evaluate the variant of SARS‐CoV‐2 in this case, but the public survey for the SARS‐CoV‐2 variant in Japan showed that the predominant variant was the omicron sublineage BA.5 when our case was admitted to our hospital. It is true that the cases of MERS associated with other omicron sublineages have been reported, 9 , 13 but our case is the first report of MERS during the omicron sublineage BA.5 predominant era in Japan. Our case also shows that the alteration of the major variant does not affect the occurrence of MERS related to COVID‐19. We found two cases who developed MERS related to COVID‐19 despite being vaccinated for SARS‐CoV‐2, 9 , 13 suggesting we will continue to encounter MERS related to COVID‐19.
In this report, we described a case in which brain MRI was performed in a patient with mild COVID‐19 to investigate impaired consciousness, leading to a diagnosis of MERS. COVID‐19 is getting to be a common disease. Our case indicates that the generalist who treats COVID‐19 on a daily basis should be familiar with the various neurological symptoms related to MERS/CLOCCs and feel confident in taking action.
CONFLICT OF INTEREST STATEMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ETHICS APPROVAL STATEMENT
In our institution, case reports do not require institutional review board approval for publication.
PATIENT CONSENT STATEMENT
Written informed consent was obtained from the patient for publication of this case report.
CLINICAL TRIAL REGISTRATION
None.
ACKNOWLEDGMENTS
This study was supported by the AMED grant number JP22fk0108509 (to H. Araoka).
Nagae K, Haraguchi M, Sakoh T, Ishida K, Ogura S, Katoh‐Morishima M, et al. A case of mild encephalitis associated with COVID‐19. J Gen Fam Med. 2023;24:307–310. 10.1002/jgf2.646
REFERENCES
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 3. Islam MA, Cavestro C, Alam SS, Kundu S, Kamal MA, Reza F. Encephalitis in patients with COVID‐19: a systematic evidence‐based analysis. Cell. 2022;11(16):2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tada H, Takanashi J, Barkovich AJ, Oba H, Maeda M, Tsukahara H, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004;63(10):1854–1858. [DOI] [PubMed] [Google Scholar]
- 5. Starkey J, Kobayashi N, Numaguchi Y, Moritani T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. Radiographics. 2017;37(2):562–576. [DOI] [PubMed] [Google Scholar]
- 6. Hoshino A, Saitoh M, Oka A, Okumura A, Kubota M, Saito Y, et al. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev. 2012;34(5):337–343. [DOI] [PubMed] [Google Scholar]
- 7. Karampatsas K, Spyridou C, Morrison IR, Tong CY, Prendergast AJ. Rotavirus‐associated mild encephalopathy with a reversible splenial lesion (MERS)‐case report and review of the literature. BMC Infect Dis. 2015;15:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuan J, Yang S, Wang S, Qin W, Yang L, Hu W. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) in adults‐a case report and literature review. BMC Neurol. 2017;17(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kubo M, Kubo K, Kobayashi KI, Komiya N. Non‐severe COVID‐19 complicated by cytotoxic lesions of the corpus callosum (mild encephalitis/encephalopathy with a reversible splenial lesion): a case report and literature review. Int J Infect Dis. 2022;125:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J, Consortium C‐GU, et al. SARS‐CoV‐2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21(3):162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imamura T, Takanashi J, Yasugi J, Terada H, Nishimura A. Sisters with clinically mild encephalopathy with a reversible splenial lesion (MERS)‐like features; familial MERS? J Neurol Sci. 2010;290(1–2):153–156. [DOI] [PubMed] [Google Scholar]
- 12. Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS‐CoV‐2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet. 2022;399(10335):1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sassi C, Mehmed E, Alkhatib A, Forero‐Padilla MA, Goranov DS, Habermann S, et al. Persistent hiccups as main COVID‐19 manifestation with transient cytotoxic lesion of the corpus callosum splenium during the omicron wave in the post‐vaccination era. J Neurol. 2023;270(3):1211–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]


