Abstract
A quantitative fit test is performed using a benchtop instrument (e.g., TSI PortaCount) to assess the fit factor provided by a respirator when assigned to a worker. There are no wearable instruments on the market to measure protection factors while the respirator is in use. The aim of this study is to evaluate two new, wearable, quantitative instruments—a dual-channel optical particle counter (DC OPC) and a dual-channel condensation particle counter (DC CPC)—that would enable in-situ, real-time measurement of respirator workplace protection factor. Respirator laboratory protection factors measured by the new instruments were compared to those measured with the TSI PortaCount on one test subject for three test aerosols (sodium chloride, incense, ambient) at target laboratory protection factors of 100, 300, and 1,000 for sodium chloride and ambient, and 75 and 500 for incense. Three replicates were performed for each test condition. Data were analyzed with a two-sided paired t-test at a significance level of 0.05. Laboratory protection factors measured with the DC CPC agree with those measured with the PortaCount whereas those from the DC OPC generally do not. Mean laboratory protection factors derived from the DC CPC are only statistically significantly different for mean values of a laboratory protection factor at ambient conditions for a target laboratory protection factor of 300 (p = 0.02) and for incense at a target laboratory protection factor of 75 (p = 0.03). Although statistically significant, the difference in laboratory protection factors derived from the DC CPC are not substantial in practice and may be explained by systematic uncertainty. In contrast, the DC OPC reports substantially larger mean laboratory protection factors, differing by about half an order of magnitude in extreme cases, and statistically significantly different mean laboratory protection factors for the sodium chloride aerosol for target laboratory protection factors of 100 and 300 (p = 0.01 and p = 0.01).
Keywords: Aerosol, laboratory protection factor, real-time measurement, respiratory protection
Introduction
Workers in the medical, construction, and general industries are exposed to airborne particulates that may cause harm when inhaled. Respirators are used to protect workers from these harmful inhalation exposures (NIOSH 2018). In the U.S., a worker required to wear a respirator must complete a fit test initially, after any changes in weight, after major dental work, and annually thereafter to identify if the respirator is able to protect the worker adequately (OSHA 2011). Despite these regulations and protocols, there is no guarantee that a worker is protected while performing workplace tasks in real time while using a respirator.
Passing a fit test depends on the ability for a specific make, model, and size respirator to completely seal with the face of the worker (OSHA 2004). There are two categories of fit tests currently approved by OSHA: qualitative or quantitative (OSHA 2011). A qualitative fit test is simply a pass/fail assessment of the employee’s response to either tasting or smelling a test agent during a simulated negative pressure of the respirator (OSHA 2004). A quantitative fit test provides a numerical evaluation of how well the respirator seals to the face. This numerical metric is referred to as the fit factor (FF) (OSHA 2011). The gold standard methodology to quantitatively determine respirator fit is the ambient aerosol condensation nuclei counter quantitative fit testing protocol using the TSI PortaCount (Coffey et al. 2002) (TSI Inc., Shoreview, MN). During the fit test the worker wearing the respirator conducts head and body movements designed to simulate workplace activities including normal breathing, deep breathing, turning the head side-to-side, grimacing, moving the head up and down, talking, and bending over at the waist (OSHA 2004). For each 60-sec exercise, a FF is calculated by taking an ambient sample before the exercise, purging the sample line, taking an inside mask sample, purging the sample line, and taking an additional ambient sample after the exercise. The ambient samples from before and after the exercise are averaged together and then divided by the mask sample. A harmonic mean of the exercise-specific FFs is computed to provide an overall FF (TSI Incorporated 2015).
The PortaCount, used during the ambient aerosol condensation nuclei counter quantitative fit test, is a bench top instrument (l 7×22×24 cm) that weighs 2.26 kg (Table 1). Two Tygon tubes conduct air from inside and outside the respirator mask to the PortaCount. The PortaCount has a single detector and uses a switching valve to change from monitoring conditions in either environment (TSI Incorporated 2015). The PortaCount measures particles larger than 0.015 μm and must be oriented in a stationary upright position to count particles accurately (Table 1).
Table 1.
Instruments used to count particles are listed and described below. The instruments vary in functional range, principle of operation, size, weight, and the flow rate per channel.
| Characteristic | PortaCount (model 8038) |
Instrument Dual-Channel Condensation Particle Counter (DC CPC) |
Dual-Channel Optical Particle Counter (DC OPC) |
|---|---|---|---|
| Counting method | Alcohol-based condensation | Water-based condensation | Optical |
| Size | 17 × 22 × 24 cm | 13 × 11 × 6.4 cm | 15 × 9.4 × 5.1 cm |
| Weight | 2.26 kg | 0.77 kg | 0.45 kg |
| Lower size cutoff | ~15 nm | ~7.5 nm | ~300 nm |
| Number of sensors | 1 | 2 | 2 |
| Flow rate per channel | 0.35 Lpm | 0.1 Lpm | 0.1 Lpm |
New wearable, dual-channel instruments have become available to measure respirator fit in realtime. These instruments report the laboratory protection factor (LPF) afforded by the respirator, which is defined as the ratio of the particle number concentration outside to that inside the facepiece. Similar to the PortaCount, the new instruments use two tubes to conduct air from within the respirator and outside the respirator. In contrast to the PortaCount, the tubes of the new instruments direct air to their own sensors for simultaneous measurement. Two sensors operating in tandem allow the novel instruments to calculate a respirator LPF second-by-second instead of as an average from multiple particle number concentrations. The dual-channel condensation particle counter CPC (DC CPC) being developed by TSI Incorporated uses two sensors to measure particles larger than 0.075 ~Lm. It is small (13×l l×6.4 cm) and lightweight (0.77 kg) (Table 1). TSI Incorporated is also developing a novel dual-channel optical particle counter (DC OPC). Similar to the DC CPC, the optical instrument is small (15 × 9.4 × 5.1 cm) and lightweight (0.45 kg) with two optical sensors for simultaneous measurement of conditions outside and inside the respirator (Table 1). The DC OPC detects particles larger than 0.3 μm. The size difference of minimum detectable particle size is due to the counting mechanism. Instead of growing the particle, the DC OPC detects when a particle passes through the sensor by the degree to which light is scattered. This technology is substantially less expensive than CPC technology.
Researchers have used two PortaCounts to measure respirator protection factor (PF) in real time during work tasks. In one study, two PortaCounts were strapped on military personnel during training to measure particle concentrations inside and outside a respirator simultaneously (Gijp and Steenweg 2004). The PortaCounts were operated without switching between channels in these studies. Each PortaCount simply measured the number concentration of an environment through one channel continuously. In similar studies, researchers mounted two PortaCounts on a cart and practicing registered nurses conducted simulated patient assessments, IV treatments, and wound care (Hauge et al. 2012). Additionally, first responders were assessed during simulated workplace activities (Sietsema et al. 2015). Gijp et al. and Hauge et al. were unable to make any conclusions about which exercises caused the respirator to fail. In the study conducted by Hauge et al., differences between tasks were not able to be evaluated with a change in PF because the PortaCount truncated all PFs above 200, resulting in homogeneous PF values. In contrast, Sietsema et al. reported how the PF achieved during a respirator fit test was related to PF during simulated exercises, and how PF changed among each exercise. These studies demonstrate that PFs can be measured in real time. However, the equipment is expensive, unreasonably bulky to mount on a person (Gijp and Steenweg 2004), and lacks ease of use by the operator, requiring a cart to hold instrumentation (Hauge et al. 2012).
The aim of this work is to evaluate and compare LPFs measured with new, wearable, real-time dual-channel instruments to those measured with the gold standard, a PortaCount. Laboratory protection factors from two instruments, a DC OPC and a DC CPC, were compared with the LPFs from the PortaCount.
Methods
Instruments
Respirator LPFs were monitored simultaneously using three instruments including: (1) the PortaCount Respirator Fit Tester model 8038 (TSI, Inc., Shoreview, MN) operated in PortaCount real-time mode; (2) a dual-channel condensation particle counter (in development by TSI, Inc., Shoreview, MN); and (3) a dual-channel optical particle counter (in development by TSI, Inc., Shoreview, MN). The terminology of LPF was chosen in accordance with the recommended terminology from Janssen and McKay (2017). Specialty software developed by TSI Incorporated was used to gather these data from the PortaCount. The data sampling rate of all three instruments was one sample per sec upstream, inside the respirator, and one sample per sec downstream, outside of the respirator. Before conducting experimental trials, the instruments were calibrated with a zero filter.
Experimental setup
Tests were conducted on a single member of the research team with an elastomeric half-mask respirator with Pl00 organic vapor cartridges (Model 65021HA1-C, 3M, St. Paul, MN). Small tubing and wire were inserted between the subject’s left cheek (opposite the testing port) and respirator to simulate leaks and achieve different target LPFs. To achieve a larger LPF, the size of the simulated leak would be decreased by inserting a smaller wire. To achieve a lower LPF a larger wire would be inserted. A single sampling probe was inserted on the right side of the facepiece approximately 25 mm below the top of the nose cup of the respirator. Two Y-connectors were added to allow all three instruments, using identical tubing for each instrument, to sample from the sampling probe simultaneously (Figure 1). The flow rate of the PortaCount was 0.35 Lpm and the flow rate of each dual-channel instrument was 0.1 Lpm (Table 1). Tubes used to measure ambient concentration were attached near the breathing zone to tubes measuring inside concentration.
Figure 1.
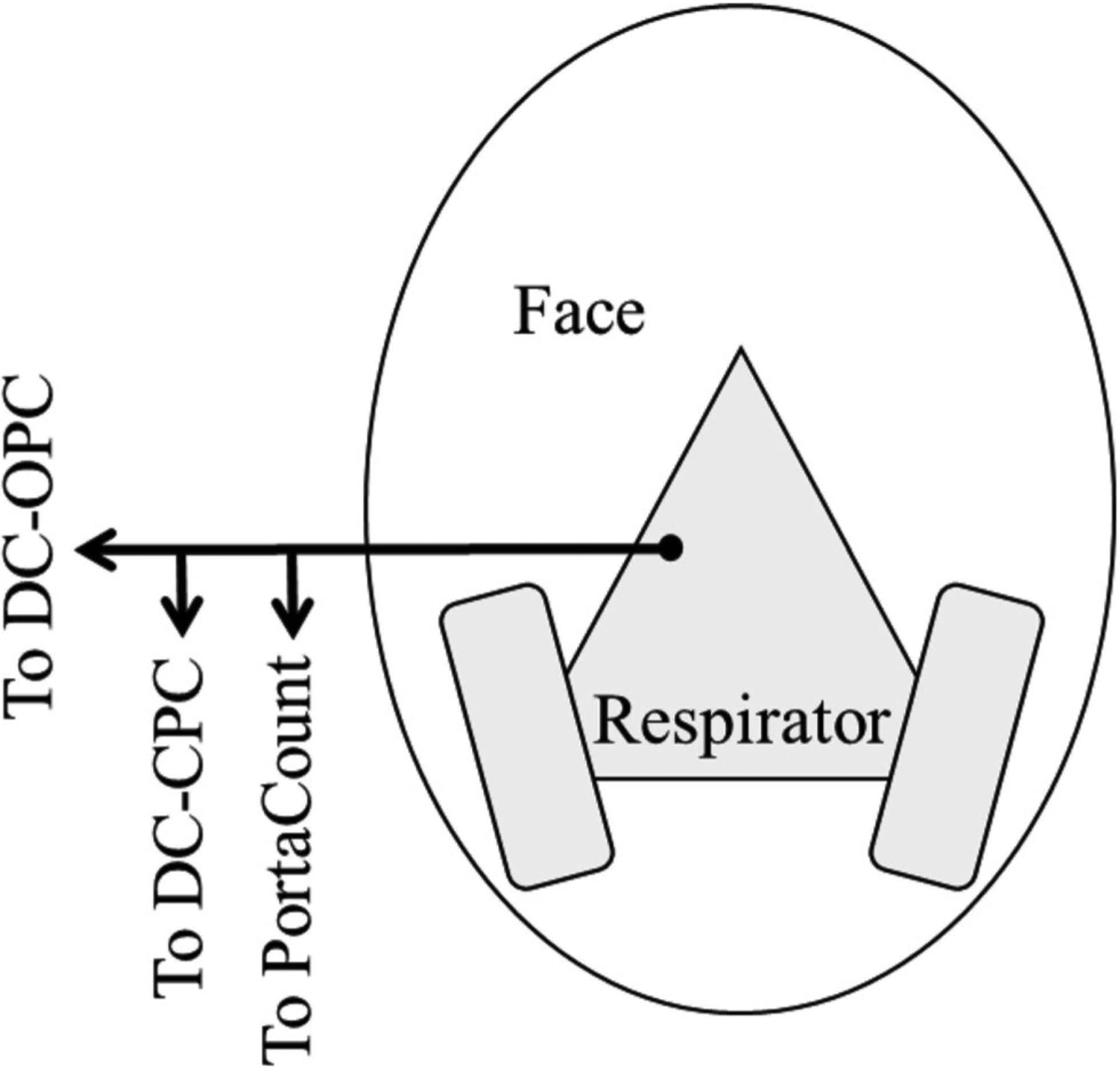
Schematic diagram representing the connections of the elastomeric half-mask respirator. A single artificial port on the subject’s right is connected to all three measuring instruments for consistent sampling from one location inside the respirator.
Three aerosols were used throughout the study: ambient aerosols present in the room, generated sodium chloride, and generated incense smoke. The trials for the generated sodium chloride and incense smoke aerosols were conducted in a small office (3.0 × 2.1 × 2.9 m) and the ambient aerosol tests were conducted in a dusty garage. Sodium chloride particles were generated using a TSI aerosol generator approximately 1 m from the test subject when standing to achieve target low and high concentrations for evaluating various LPFs (~7,000 particles/cm3 and ~40,000 particles/cm3) (TSI 8025, Shoreview, MN). Incense particles were generated by burning an incense stick inside the office (~20,000 particles/cm3). Incense particles were generated during testing approximately 50 cm in front of the test subject while standing. A small fan was used to facilitate mixing within the office during particle generation. Aerosol concentrations were monitored by using a TSI Condensation Particle Counter 3007 (TSI, Inc., Shoreview, MN).
Protocol - Fit testing
The LPF was investigated by conducting three repetitions, one right after another, at each of three target LPFs of 100, 300 and 1,000 for ambient and sodium chloride aerosols and target LPFs of 75 and 500 for the incense aerosol (3 LPFs × 3 reps × 2 aerosols + 2 LPFs × 3 reps × 1 aerosol = 24 tests). Due to the low number concentration of particles at ambient conditions that are detectable by the DC OPC, the DC OPC often did not detect any particles inside the respirator, especially for the highest target LPF value. Since it was unknown if the particle count was low inside the mask or if particle size was generally below the limit of detection of the DC OPC, the LPF was not analyzed for the DC OPC in ambient aerosol conditions. The target LPFs and environmental conditions were chosen to mimic desirable industry LPFs with industry related aerosols. Three of the OSHA recommended exercises were used to assess respirator LPF for each trial: normal breathing, deep breathing, and moving the head side-to-side. Although the three chosen exercises are not the most seal-breaking exercises, they are potentially the most commonly performed movements among the workforce. Each exercise was conducted for 60 sec, and the PortaCount ambient concentration was measured for 15 sec prior to the fit test exercises.
Data analysis
Laboratory protection factors were calculated for every second for each of the three instruments. For the PortaCount, an arithmetic mean concentration was calculated using the 15 sec of outside sample. LPF was then calculated for each second by dividing the mean outside concentration by the second-by-second inside-facepiece concentrations. LPFs for the DC CPC and the DC OPC were calculated using the simultaneous measurements of concentration outside and inside the facepiece, recorded by each of the two sensors:
All measurements of zero particles inside the respirator were changed to a concentration of 1 particle/cm3 in order to avoid error in the data from dividing by zero. This process was done only for the data from the dual-channel devices because the PortaCount did not report values of zero. The PortaCount did not report values of zero because when a zero value was detected, the PortaCount automatically substituted a value of one (1) (Sietsema and Brosseau 2018). Sietsema et al. performed similar methodology of replacing inside concentrations lower than the limit of detection with a concentration of 1 particle/cm3 for consistency with test software (Sietsema and Brosseau 2018). The average LPF for each environmental condition at each target LPF was calculated from a series of data reduction steps. First, values of zero (O) reported when concentrations were below the limit of detection, were replaced with a concentration of 1 particle/cm3, as the PortaCount does automatically. Then, 180 second-by-second LPFs were calculated for the duration of the trial. Next, the geometric mean of all second-by-second LPFs from each trial were calculated to deduce the trial LPF. Then, each of the three trial LPFs were averaged using an arithmetic mean to calculate the average LPF and standard deviation of each environmental condition and target LPF. To evaluate if the average arithmetic means of the LPFs were equivalent between the PortaCount and the novel instruments, separate two-sided paired t-tests (n = 3) were conducted for each dual-channel instrument and the PortaCount using an alpha value of 0.05. The null hypothesis stated that the mean of the LPF from the PortaCount was equivalent to the mean of the LPF of the DC OPC or the DC CPC. Data were determined to be substantially different in reference to a practical application if mean LPFs differed by half an order of magnitude. Data were manipulated in Microsoft Excel (Excel Windows 2019; Microsoft, Redmond, WA) and all statistical analyses were completed using R software (version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
Table 2 shows the mean LPF for each instrument averaged over all exercises, and Figure 2 shows a time series plot of LPFs with 1-sec resolution for one test. As indicated in bold in Table 2, statistically significantly different mean values for the DC CPC compared to the PortaCount occurred at ambient conditions for target LPFs of 300 (p = 0.02) and for incense smoke at a target LPF of 75 (p = 0.03). Figure 3(a) shows the DC CPC data vs. the PortaCount data, with error bars representing the standard deviations. Observe in this figure that the LPFs measured by both instruments differed by less than half an order of magnitude across almost all tests.
Table 2.
Arithmetic mean laboratory protection factors calculated over all exercises for each condition n = 3.
| Laboratory Protection Factor | ||||
|---|---|---|---|---|
| Aerosol | Target Laboratory Protection Factor |
PortaCount Mean ± St Dev |
DC CPC Mean ± St Dev, p-value* |
DC OPC Mean ± St Dev, p-value* |
| Ambient | 100 | 81 ± 11 | 79 ± 4.1, 0.72 | NA |
| 300 | 314 ± 27 | 218 ± 40, 0.02 | NA | |
| 1,000 | 1,920 ± 180 | 1,944 ± 274, 0.65 | NA | |
| Sodium chloride | 100 | 89 ± 7.7 | 77 ± 3.4, 0.05 | 156 ± 21, 0.01 |
| 300 | 370 ± 65 | 330 ± 52, 0.06 | 961 ± 54, 0.01 | |
| 1,000 | 1,550 ± 400 | 1,560 ± 270, 0.75 | 1,120 ± 100, 0.13 | |
| Incense | 75 | 71 ± 8.3 | 54 ± 3.5, 0.03 | 68 ± 6.6, 0.20 |
| 500 | 469 ± 297 | 351 ± 221, 0.05 | 1,180 ± 1,010, 0.14 | |
p-value associated with a two-sided paired t-test under the null hypothesis that the mean laboratory protection factor measured with the DC instrument is equal to that of the PortaCount. Balded values represent evidence to reject the null hypothesis that the mean of the PortaCount is equal to the mean of the dual-channel instrument.
Figure 2.
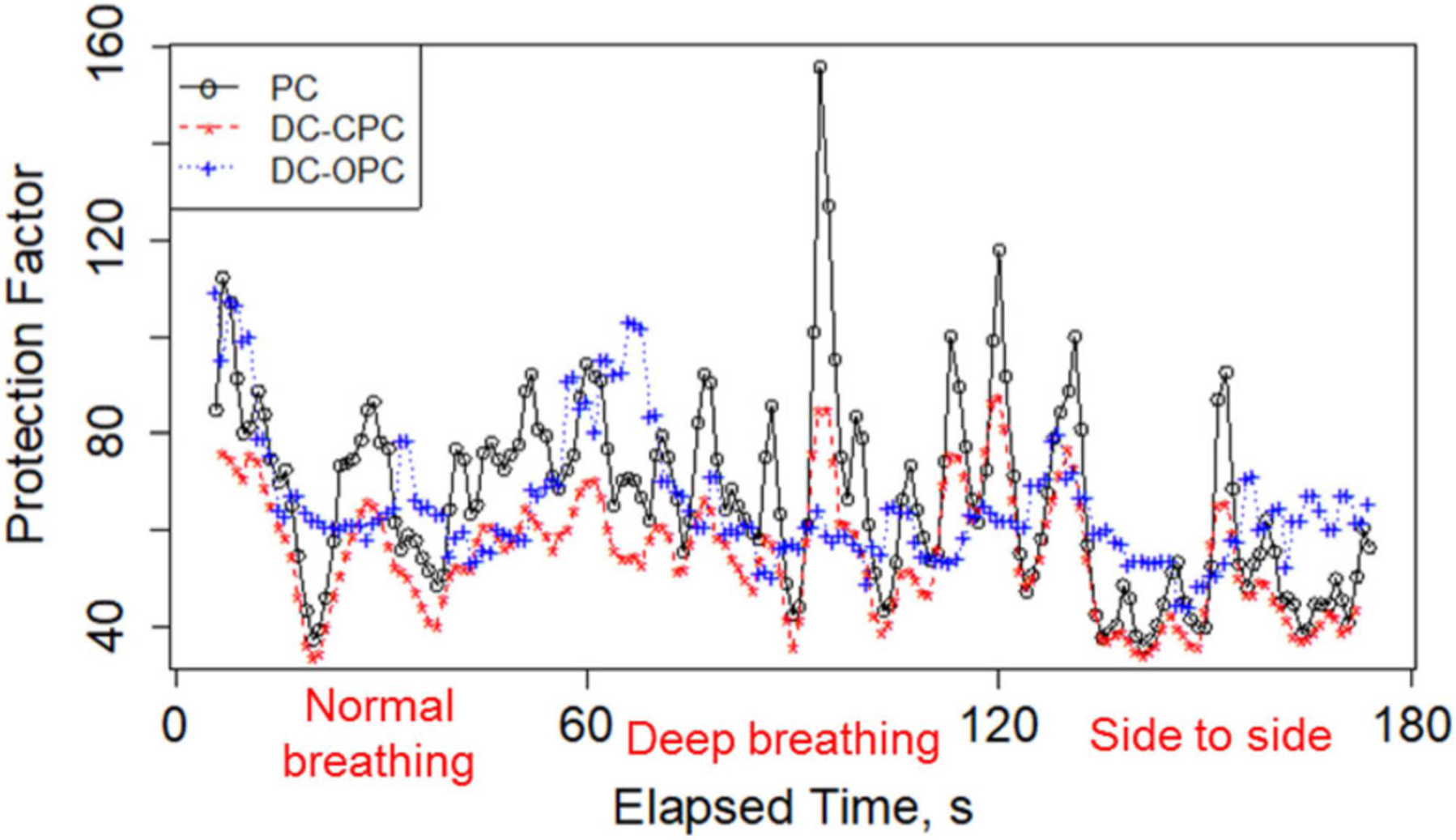
Laboratory protection factor by time (s) for a target laboratory protection factor of 75 and incense aerosol.
Figure 3.
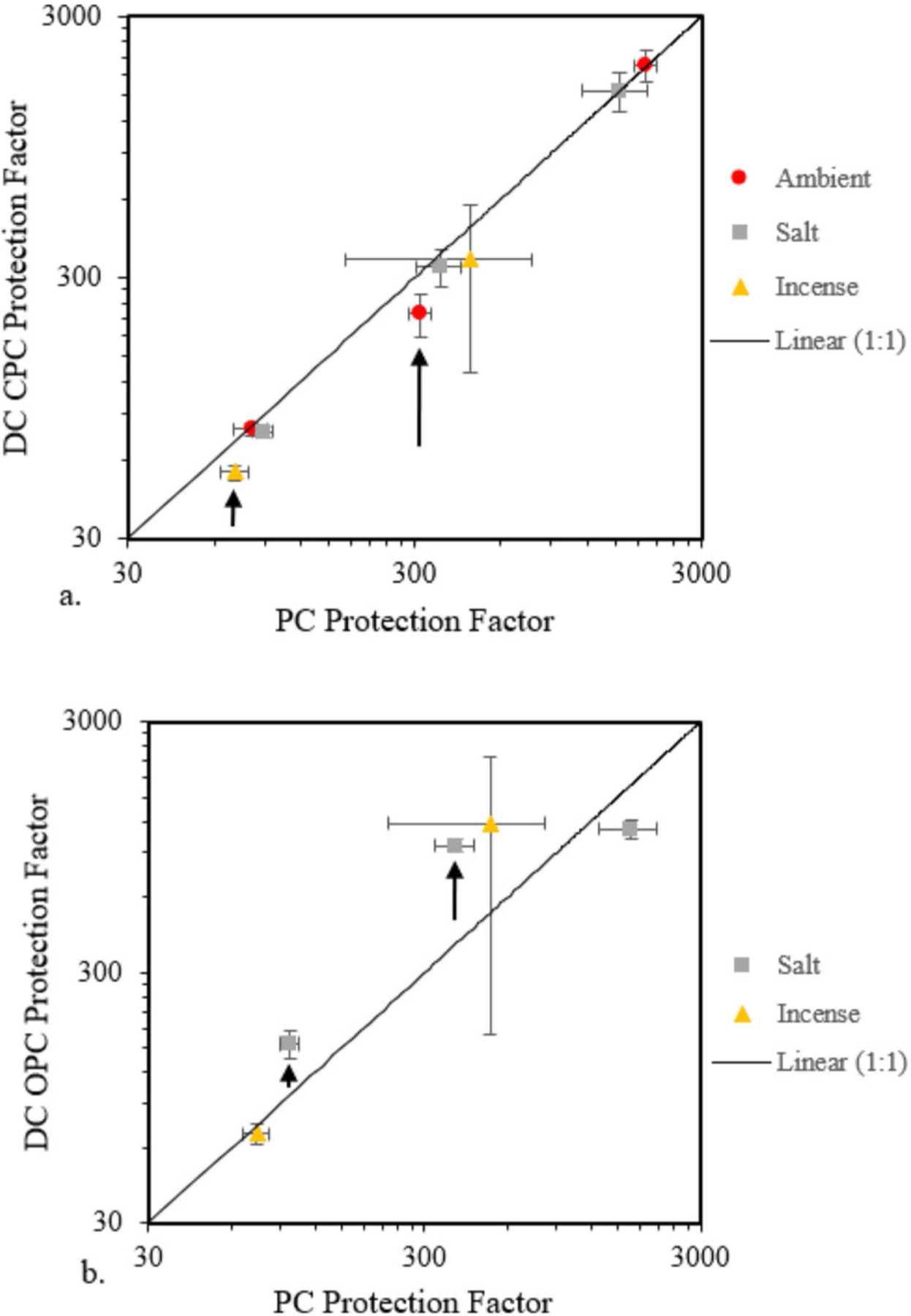
Arithmetic mean laboratory protection factor from the DC instruments compared to that from the PortaCount (indicated as PC) for (a) DC CPC and (b) DC OPC. The error bars represent the standard deviations for each of the three trials at each experimental level. The arrows represent the experimental levels where the null hypothesis is rejected. In these cases, the DC CPC underreported relative to the PC while the DC OPC generally over-reported relative to the PC.
As shown in Table 2, compared to the PortaCount the DC OPC reported statistically significantly different mean LPFs in the sodium chloride aerosol for the target LPFs of 100 and 300 (p = 0.01 and p = 0.01). The divergence between the DC OPC and the PortaCount is larger than half an order of magnitude in these two cases. Laboratory protection factors reported by the DC OPC that are substantially larger than the PortaCount should be dismissed when fine and ultrafine particles, not detectable by the DC OPC, are present. Figure 3(b) shows the DC OPC data vs. the PortaCount data, with error bars representing the standard deviations. The null hypothesis is rejected for the two data points indicated by the arrows. In Figure 3(b), the only practical case where the DC OPC and the PortaCount reported statistically similar values with low standard deviation was when measuring low LPFs (below ~75) in experimentally high concentration smoke aerosols (~ 20,000 particles/cm3). While there are many work environments with ultrafine particulate exposures near or exceeding 1 × 106 particles/cm3, average background levels have been reported to be approximately 1 × 104 particles/cm3 (Viitanen et al. 2017). In the present work, the high experimental concentration of 20,000 particles/cm3 is most representative of occupational setting background concentrations.
Figure 2 displays the LPFs with 1-sec resolution for a trial that had a target LPF of 75 using incense smoke aerosol. The PortaCount (black) reads LPFs larger than the dual-channel CPC at this LPF. However, there is a clear association between the measurements made using the PortaCount and those using the DC CPC because often, when the PortaCount measurement increases or decreases, so does the measurement of the DC CPC. This pattern is likely a consequence of the similarity of the DC CPC and PortaCount measurement methods, including the sampled ultrafine particle range. In contrast, the DC OPC reads LPFs similar in magnitude to the condensation-based instruments, but they are not well correlated with the results from the condensation-based instruments. It is interesting to note that in this particular test the measurements made using the DC OPC are close to those made using the CPCs despite the fact that the instruments are sampling two different particle size ranges. In this trial (LPF = 75) there is no obvious difference between LPFs among the three different exercises, as seen in Figure 2. The similar LPFs among the selected activities are not surprising as the three selected activities are not failure-prone physical motions.
Discussion
The DC CPC is a compact instrument that can assess the environment outside and inside of a respirator simultaneously to calculate real-time LPFs. In this work, the DC CPC performed similarly to the PortaCount and only reported statistically significantly different mean values in two test conditions. While the t-test for target LPFs of ambient aerosol at 300 and incense at a target LPF of 75 are statistically significant from the mean of the PortaCount, the origin of these differences is not clear. One explanation may be that the PortaCount data do not account for possible fluctuations in the ambient concentration which may contribute to data variability. If this is the case, then the DC CPC may provide a more representative LPF value. When the ambient concentration varies widely, such as in the present research and in many real work environments, the PortaCount is limited being a single-channel instrument and measuring the ambient concentration every 180 sec. There is no variability in the numerator for the calculation of LPFs because the PortaCount is using only a single value for concentration outside of the respirator. In contrast, the DC CPC and DC OPC measure both environments (inside and outside of the respirator) concurrently. The ability to measure through both channels may present a more accurate representation of the instantaneous LPF when the ambient particle concentration varies.
A possible explanation for the statistically different mean values for the target LPF in ambient aerosol may be the systematic error introduced by choosing the concentration of 1 particle/cm3 for the DC CPC data that were reported to be zero (0). Similar methodology of assigning 0.5 particles/cm3 was used by Hauge et al. in order to analyze data near the instrument’s limit of detection while Sietsema et al. used 1 particle/cm3• The effect of this assignment may be to calculate artificially low mean LPFs when there is a low concentration of particles in the respirator. In other words, at higher target LPFs, particularly for lower outside concentrations such as in the case of ambient aerosol, the calculated mean LPFs will be artificially low.
In addition, data were evaluated to investigate if the variability between the dual-channel instruments was within the 10% margin of error of the PortaCount (TSI Incorporated 2018). The dual-channel instruments were within the 10% margin of error for four conditions: the DC CPC at ambient conditions for a target LPF of 100 and 1,000, the DC CPC at a target LPF of 1,000 in generated sodium chloride aerosol, and the DC OPC at a target LPF of 75 in incense aerosol. Notably, the statistically significant conditions (DC CPC target LPF of 300 in ambient and 75 in incense and DC OPC at target LPFs of 100 and 300 in sodium chloride) were outside of the 10% margin of error of the PortaCount.
With the possible exception of measuring low LPFs (below ~ 75) in high concentration incense smoke aerosols (~ 20,000 particles/cm3), the DC OPC novel instrument does not reliably assess instantaneous measurements of environmental conditions to calculate real-time LPFs. The mean values of calculated LPFs from the DC OPC are substantially and significantly different or unreportable for five of the eight test conditions. Subject-generated particles or particles that are known to be most penetrating through mask filter materials (0.3 μm) could significantly add to the number of particles measured inside a respirator leading to artificially low LPFs for optical particle counters (OPCs) compared to the CPC method that detects much higher concentrations of ultrafine particles in the same environment. If the experimental setup limits the number of larger particles that can penetrate inside the facepiece compared to the more numerous and more easily penetrating ultrafines, then the OPC technique would report artificially high LPFs, especially at lower external concentrations. This systematic error may have been present for the salt aerosol, which may limit the ability to penetrate the small gap between the potentially moist skin and the elastomeric mask. The only practical case where the DC OPC and the PortaCount reported statistically similar values with low standard deviation was when measuring low LPFs in high concentration incense smoke aerosols. This result supports the possibility that there may be a difference in the ability of larger salt and smoke aerosols to penetrate the mask in the experimental setup which warrants further investigation.
Limitations of this work include only testing three aerosols, having one test subject, simulating the leak of the respirator, and limited resolution of the dual-channel instruments. There are various aerosols including metal fumes and volatile compounds that are prevalent in other respirator-requiring occupations that were not tested. By performing the methodology on just one test subject, there was only one face shape tested for the specific elastomeric respirator. The simulated leak in the cheek area of the respirator may not perform similarly to leaks near the chin or neck in an occupational setting where a leak may be more substantial. Additionally, the DC OPC only has 16 bits of resolution, reporting count values between O and 65,535 particles/cm3, limiting the count statistics at low concentrations and the maximum achievable second by second LPF (TSI Incorporated 2018). The DC CPC can measure from O to ~ 1.0 × 105 particles/cm3, similar to the PortaCount which reaches a maximum detectable concentration at 2.5 × 105 particles/cm3, (TSI Incorporated 2015) and the TSI Hand-Held CPC which ranges from O to 1.0 × 105 particles/cm3, allowing for a decrease in the margin of error when the number of sampled particles increases (TSI Incorporated 2012).
Future work with the dual-channel instruments includes testing within the occupational environment among a diverse workforce. A study sample should include a range of face shapes to increase generalizability to the workforce. Other work includes having workers wear the dual-channel instruments during typical work tasks to capture real-time data in the use environment, as well as to assess the ability of the tubing between the respirator and the instrument to maintain connectivity.
Conclusion
The DC CPC is a small, personal instrument that can assess conditions inside and outside a respirator to calculate real-time LPFs. In this work, with the possible exception of measuring low LPFs (below ~75) in high concentration smoke aerosols (~ 20,000 particles/cm3), the DC OPC in question does not reliably assess instantaneous measurements of environmental conditions to calculate real-time LPFs. Future work with the DC CPC includes evaluating the LPF in response to occupational specific tasks, testing with various occupational aerosols, and testing among a more diverse group of subjects.
References
- Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH). 2018. Respirator trusted-source information. https://www.cdc.gov/niosh/npptl/topics/respirators/lisp_part/respsourcelquestl.html.
- Coffey CC, Lawrence RB, Zhuang Z, Campbell DL, Jensen PA, Myers WR. 2002. Comparison of five methods for fit-testing N95 filtering-facepiece respirators. Appl Occup Environ Hyg. 17(10):723–730. doi: 10.1080/10473220290107002 [DOI] [PubMed] [Google Scholar]
- Gijp S, Steenweg L. 2004. Respirator performance during military field trials. ISRP. 2l(III&IV):135–141. [Google Scholar]
- Hauge J, Roe M, Brosseau L, Colton C. 2012. Real-time fit of a respirator during simulated health care tasks. J Occup Environ Hyg. 9(10):563–571. doi: 10.1080/15459624.2012.711699 [DOI] [PubMed] [Google Scholar]
- Janssen L, McKay R. 2017. Respirator performance terminology. J Occup Environ Hyg. 14(12):Dl81–Dl83. doi: 10.1080/15459624.2017.1359018 [DOI] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration (OSHA). 2004. Occupational Safety and Health Standards: Personal Protective Equipment. Code of Federal Regulations 1910.134. App A. Washington DC: U.S. Government Printing Office. Available March 24, 2020 from: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA. [Google Scholar]
- Occupational Safety and Health Administration (OSHA). 2011. Occupational Safety and Health Standards: respiratory protection. Code of Federal Regulations 1910.134. Washington DC: U.S. Government Printing Office. Available February 16, 2020 from: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134. [Google Scholar]
- Occupational Safety and Health Administration (OSHA). 2019. Occupational Safety and Health Standards: Additional Ambient Aerosol CNC Quantitative Fit Testing Protocols: Respiratory Protection Standard. Code of Federal Regulations 1910.134. Washington DC: U.S. Government Printing Office. Available February 16, 2020 from: https://www.osha.gov/laws-regs/federalregister/2019-09-26. [Google Scholar]
- Sietsema M, Bodurtha P, Dickson E, Brosseau L. 2015. Evaluating simulated workplaces for a first responder low-level protective ensemble. J Int Soc Respir Protect. 32(1):1–13. [Google Scholar]
- Sietsema M, Brosseau L. 2018. Are quantitative fit factors predictive of respirator fit during simulated healthcare activities? J Occup Environ Hyg. 15(12):803–809. doi: 10.1080/15459624.2018.1515490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSI Incorporated. 2012. Hand-held condensation particle counter model 3007 specifications sheet. Available February 16, 2020 from: https://www.tsi.com/getmedia/al852300-29bc-4a4b-acaa-4220bfd65334/3007_1930032-CPC-Spec-Sheet-US?ext=.pdf
- TSI Incorporated. 2015. PortaCount Pro 8030 and PortaCount Pro+ 8038 Respirator Fit Testers Operation and Service Manual. Available February 16, 2020 from: https://www.tsi.com/getmedia/76df3dbb-6d8d-4d78-aa24-5affl9e889e9/8030_8038_PortaCountPro_Manual_600l868?ext=.pdf.
- TSI Incorporated. 2018. Quantitative respirator fit testing: an analysis of utilizing Condensation Nuclei Counter (CNC) vs. Optical Particle Counter (OPC). Available April 18, 2020 from: https://www.tsi.com/getmedia/11,314,512-b32b-4ddf-9bl8-c5ed07daa58c/RFT-023_RevC_QRFT_CNC_vs_OPC_A4-web?ext=.pdf
- Viitanen A, Uuksulainen S, Koivisto A, Hameri K, Kauppinen T. 2017. Workplace measurements of ultrafine particles-a literature review. Ann Work Expo Health. 61(7):749–758. doi: 10.1093/annweh/wxx049 [DOI] [PubMed] [Google Scholar]


