ABSTRACT
Checkpoint blockade immunotherapy transforms many types of cancer; however, in the field of metastatic colorectal cancer, checkpoint blockers are only effective in microsatellite-unstable tumors, which represent only a minority of patients. Microsatellite-stable tumors are thought to be immunoresistant. A recent publication demonstrates that, contrary to the standard view point, the combination of chemo-immunotherapy could trigger a tumor-specific immune response, leading to clinical benefit.
KEYWORDS: Checkpoint blockade, clinical trial, colorectal cancer, immunogenic cell death
Introduction
Colorectal cancer (CRC) is a heterogeneous disease classified by its genetic characteristics. Treatment is based upon such molecular status. Most metastatic patients are treated in palliative care with chemotherapies regimen and target therapies, because when surgical removal of metastases is not feasible, there is no curative treatment.1 A particular genetic subset of CRC is tumors with microsatellite instability (MSI). Such tumors present large immune infiltrates, and because of abnormalities in DNA repair enzymes, they also have a high tumor mutational burden (TMB), inducing significant recruitment of the immune system to the tumor. In 2015, it was demonstrated that immunotherapy using a monoclonal antibody targeting PD-1 is highly effective for MSI tumors and is rapidly becoming the first line of treatment for this population. By contrast, in MSS CRC, which represents 95% of CRC patients, immunotherapy is ineffective as monotherapy.2 Resistance to immunotherapy in MSS tumors is thought to be due both to low rates of tumor neoantigens, which impede tumor cell recognition, and to immunoexclusion due to activation of the beta catenin pathway.3
However, many studies showed that a high T-cell infiltrate in the tumor is associated with a better prognosis in localized or metastatic CRC,4 suggesting that MSS CRC could be recognized by the immune system. Immunogenic cell death inducers could be proposed to fight against immunoresistant tumors. Thus, our preclinical data showed in the CT26 model that combining a PD-1 inhibitor with a classical immunogenic cell death inducer, such as oxaliplatin, and 5-fluorouracil, a drug capable of eliminating myeloid-derived immunosuppressive cells, could enhance the efficacy of immunotherapy5 (Figure 1). Indeed, oxaliplatin induces apoptotic death in cancer cells, leading to the expression of immunogenic death stigmas such as membrane exposure of calreticulin, release of ATP and HGMB1, and secretion of IFNα and CXCL10 (Figure 1).6 Based on these data, we generated the phase 1b/2 MEDITREME trial (NCT03202758).
Figure 1.
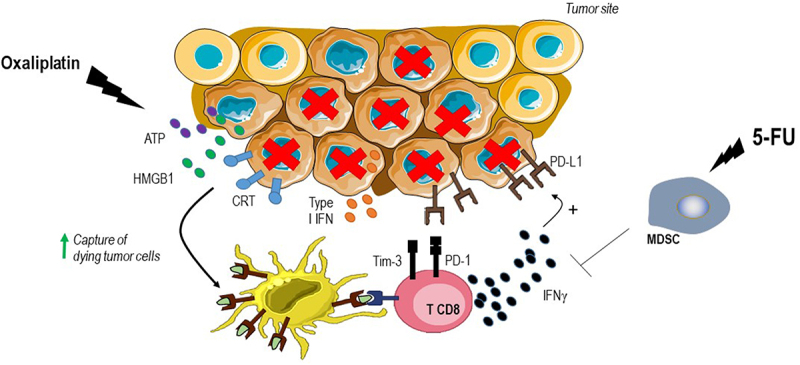
Strategy to counteract immune escape in MSS CRC.
Clinical and translational results
In this study, recently published in Nature Medicine,7 patients were treated for 3 months with a modified FOLFOX6 regimen (six cycles) in combination with durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA-4) as induction therapy, followed by maintenance therapy with durvalumab until progression. Fifty‐seven patients with RAS‐mutant unresectable metastatic CRC were treated. Phase I demonstrated the safety of the combination. The phase II primary objective of efficacy in terms of 3-, 6- and 12-month PFS was met, with 3-month PFS of 90.7% [95% CI: 79.2%–96%], 6-month PFS of 60.4% [95% CI: 45.2%–72.6%] and 12-month PFS of 26.9% [95% CI: 15.3%–39.9%]. The response rate achieved was 64%, which is one of the best response rates in the first-line metastatic CRC in a clinical trial testing a doublet of chemotherapy plus targeted therapies. Importantly, 15% of patients remain free from relapse at 3 years despite having received only 3 months of chemotherapy.
In translational fashion, we have observed that patients with high tumor mutational burden and low chromosomal instability gain benefits from therapy. Baseline immune infiltrates with high CD8 and PD-L1 expression are associated with better treatment efficacy. These findings mirror those of the AtezoTRIBE trial, which also observed better efficacy of atezolizumab plus FOLFOXIRI in patients with a high immunoscore IC, which also consists of CD8 and PD-L1 analysis, and a high TMB.8 On the contrary, the fibroblastic reaction and the stigmata of immunoexclusion are associated with a lack of treatment efficacy. Importantly, high CTLA-4 expression in the tumor is associated with a better response to treatment, suggesting the importance of CTLA-4 therapy in MSS CRC. Previous reports testing the combination of an anti-CTLA-4 and an anti-PD-1/PD-L1 in third-line CRC (botensilimab plus balstilimab or durvalumab plus tremelimumab)9,10 have also showed some levels of efficacy of these combinations, reinforcing our findings and providing arguments for the use of an anti-CTLA-4 and an anti-PD-1/PD-L1 combination in MSS CRC.
FOLFOX is a regimen well-known as an inducer of immunogenic cell death, however the demonstration of the in vivo relevance of immunogenic cell death in patients still remains to be determined. In a subset of patients, a biopsy performed after induction of FOLFOX treatment showed increased T-cell infiltration and M×1induction, raising the argument to validate that the anticancer efficacy of the regimen is linked to the activation of immunogenic cell death.
When looking at the antitumor immune response of T cells, we observed the induction of a CD4 T-cell immune response against both the tumor antigens NY‐ESO1 and telomerase in the blood after chemo-immunotherapy, which is associated with a better outcome. Moreover, we were able to demonstrate that treatment induced an antitumor CD8 immune response against neoantigens in the blood of 7 of the 10 patients tested. At the tumor site, using single-cell RNA sequencing and TCR sequencing, we could unravel the accumulation of a clonal T cell response with polyfunctional T cells with high cytotoxic and cytokine producing properties. These intra-tumoral T cells have been shown to recognize CD8 dependent tumor neoantigens. These data clearly demonstrate the ability of our treatment combination to elicit a specific anti-tumor immune response and contrast with the dogma suggesting that MSS CRC is immuno-ignored.
Conclusions
In this study, we report the favorable clinical efficacy of a first-line of chemo-immunotherapy for unresectable metastatic MSS CRC using FOLFOX immunogenic cell death inducing-regimen and the combination of an anti-PD-L1 and an anti-CTLA-4. This is the first positive trial of immunotherapy in metastatic MSS CRC and highlights the rationale to continue developing immunotherapy combinations in these patients that are able to generate a specific antitumor T-cell immune response. We believe that the selection of patients with a high immune infiltrate and the expression of PD-L1 and CTLA-4 could be key biomarkers for increasing patient response rate. This study is the first step in introducing chemo-immunotherapy using an immunogenic cell death inducer with an anti-CTLA-4 and anti-PD-1/PD-L1 to transform the treatment and the outcome of this frequent cancer.
Acknowledgment
F. Ghiringhelli is granted by Ligue National contre le cancer and INCA.
Funding Statement
The study was supported by Astra-Zeneca.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB.. Colorectal cancer. Lancet. 2019;394(10207):1467–3. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 2.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghiringhelli F, Fumet J-D. Is there a place for immunotherapy for metastatic microsatellite stable colorectal cancer? Front Immunol. 2019;10:1816. doi: 10.3389/fimmu.2019.01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 5.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell–dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487–500. doi: 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
- 7.Thibaudin M, Fumet J-D, Chibaudel B, Bennouna J, Borg C, Martin-Babau J, Cohen R, Fonck M, Taieb J, Limagne E, et al. First-line durvalumab and tremelimumab with chemotherapy in RAS-mutated metastatic colorectal cancer: a phase 1b/2 trial. Nat Med. 2023;29(8):2087–2098. doi: 10.1038/s41591-023-02497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M, Zaniboni A, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 9.Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, Ahmad CE, Goffin JR, Kavan P, Harb M, et al. Effect of combined immune Checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: the Canadian cancer trials group CO.26 study. JAMA Oncol. 2020;6(6):831–838. doi: 10.1001/jamaoncol.2020.0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullock A, Grossman J, Fakih M, Lenz H, Gordon M, Margolin K, Wilky B, Mahadevan D, Trent J, Bockorny B, et al. LBA O-9 Botensilimab, a novel innate/adaptive immune activator, plus balstilimab (anti-PD-1) for metastatic heavily pretreated microsatellite stable colorectal cancer. Ann Oncol. 2022;33:S376. doi: 10.1016/j.annonc.2022.04.453. [DOI] [Google Scholar]


