Abstract
BACKGROUND
KRAS p.G12C mutation occurs in approximately 1 to 2% of pancreatic cancers. The safety and efficacy of sotorasib, a KRAS G12C inhibitor, in previously treated patients with KRAS p.G12C–mutated pancreatic cancer are unknown.
METHODS
We conducted a single-group, phase 1–2 trial to assess the safety and efficacy of sotorasib treatment in patients with KRAS p.G12C–mutated pancreatic cancer who had received at least one previous systemic therapy. The primary objective of phase 1 was to assess safety and to identify the recommended dose for phase 2. In phase 2, patients received sotorasib at a dose of 960 mg orally once daily. The primary end point for phase 2 was a centrally confirmed objective response (defined as a complete or partial response). Efficacy end points were assessed in the pooled population from both phases and included objective response, duration of response, time to objective response, disease control (defined as an objective response or stable disease), progression-free survival, and overall survival. Safety was also assessed.
RESULTS
The pooled population from phases 1 and 2 consisted of 38 patients, all of whom had metastatic disease at enrollment and had previously received chemotherapy. At baseline, patients had received a median of 2 lines (range, 1 to 8) of therapy previously. All 38 patients received sotorasib in the trial. A total of 8 patients had a centrally confirmed objective response (21%; 95% confidence interval [CI], 10 to 37). The median progression-free survival was 4.0 months (95% CI, 2.8 to 5.6), and the median overall survival was 6.9 months (95% CI, 5.0 to 9.1). Treatment-related adverse events of any grade were reported in 16 patients (42%); 6 patients (16%) had grade 3 adverse events. No treatment-related adverse events were fatal or led to treatment discontinuation.
CONCLUSIONS
Sotorasib showed anticancer activity and had an acceptable safety profile in patients with KRAS p.G12C–mutated advanced pancreatic cancer who had received previous treatment. (Funded by Amgen and others; CodeBreaK 100 ClinicalTrials.gov number, NCT03600883.)
In 2020, pancreatic cancer accounted for 495,773 new cases of cancer and 466,003 cancer-related deaths worldwide.1 Aside from surgery, the treatment of pancreatic cancer includes chemotherapy with FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin), albumin-conjugated paclitaxel plus gemcitabine, and nanoliposomal irinotecan plus leucovorin and fluorouracil.2–5 Although these chemotherapy regimens offer modest survival and quality-of-life benefits, they cause toxic effects and are unsuitable for many patients because of age, performance status, or disease-related frailty. Several other therapies have been approved for the treatment of pancreatic cancer. Olaparib, a poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitor, has been approved as maintenance treatment for pretreated metastatic pancreatic ductal adenocarcinoma harboring a germline BRCA mutation, which occurs in 4 to 7% of patients. Pembrolizumab has been approved to treat unresectable or metastatic, microsatellite-instability–high or defective DNA mismatch-repair–deficient solid tumors (in 1 to 2% of pancreatic ductal adenocarcinomas). Larotrectinib and entrectinib are tropomyosin receptor kinase inhibitors approved to treat solid tumors with an NTRK gene fusion.6–11 Additional therapies targeting various genetic mutations in pancreatic cancer are currently being explored.12
Mutations in the Kirsten rat sarcoma viral oncogene homologue (KRAS) gene are found in approximately 90% of pancreatic ductal adenocarcinomas, which is the most prevalent histologic type of pancreatic cancer,13–15 with KRAS p.G12C (glycine-to-cysteine substitution at codon 12) mutation occurring in approximately 1 to 2% of patients.16 Sotorasib is a small molecule that specifically and irreversibly inhibits KRAS G12C. The Food and Drug Administration recently granted accelerated approval to sotorasib for the treatment of patients with KRAS p.G12C–mutated non–small-cell lung cancer who had received at least one previous systemic therapy.17 Here, we report the results in heavily pretreated patients with KRAS p.G12C–mutated pancreatic cancer from the phase 1 and phase 2 portions of the CodeBreaK 100 trial.
METHODS
TRIAL DESIGN AND END POINTS
We conducted an international, multicenter, open-label, phase 1–2 trial to evaluate the efficacy and safety of sotorasib as monotherapy in patients with KRAS p.G12C–mutated pancreatic cancer. The primary objectives of phase 1 were to evaluate the safety and side-effect profile of sotorasib monotherapy and to identify the recommended dose for phase 2. In phase 2, we assessed the efficacy of sotorasib at the dose recommended from phase 1 (960 mg daily). The primary end point was a centrally confirmed objective response (defined as a complete or partial response). In both phases, patients received sotorasib treatment until the occurrence of disease progression, development of unacceptable side effects, or withdrawal of consent.
Here, we report the results of the safety and efficacy analyses of sotorasib therapy in the combined populations from phase 1 and phase 2. Efficacy end points included objective response, duration of response, time to objective response, disease control (defined as an objective response or stable disease), progression-free survival, and overall survival. Tumor response was assessed in both phases by blinded independent central review according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1).
PATIENTS
Key inclusion criteria were an age of 18 years or older; pathologically documented, locally advanced or metastatic pancreatic cancer with KRAS p.G12C mutation identified by means of locally performed molecular testing; treatment with at least one previous systemic therapy (unless the patient was ineligible for available therapies known to provide clinical benefit or these therapies would have caused unacceptable adverse events); and an Eastern Cooperative Oncology Group (ECOG) performance-status score of 2 or less (with scores ranging from 0 to 5 and higher scores indicating greater disability) for patients enrolled in phase 1 or a score of 0 or 1 for patients enrolled in phase 2. Complete eligibility criteria are provided in the protocol, available with the full text of this article at NEJM.org.
ASSESSMENTS
Imaging and tumor assessments were performed with the use of magnetic resonance imaging or contrast-enhanced computed tomography or both at screening and every 6 weeks (within a window of ±1 week) for the first eight assessments and every 12 weeks (within a window of ±1 week) until the occurrence of disease progression or until sotorasib treatment was stopped, whichever occurred later. Response outcomes were determined on the basis of blinded independent central review according to RECIST, version 1.1. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. Plasma specimens that were obtained at baseline were analyzed for genomic alterations in cell-free DNA with the use of the Guardant360 assay (Guardant Health), with mutations reported according to the specifications of the test manufacturer. Additional details regarding adverse events and analysis of genomic alterations are provided in the Supplementary Appendix, available at NEJM.org.
TRIAL OVERSIGHT
The trial was conducted in accordance with the Good Clinical Practice guidelines of the International Council for Harmonisation and the principles of the Declaration of Helsinki. The protocol and amendments were approved by regulatory authorities of participating countries and the institutional review board at each participating site. All the patients provided written informed consent. The trial was designed by employees of the sponsor (Amgen) in collaboration with the investigators. The data were collected by the investigators and analyzed by statisticians employed by the sponsor. A medical writer employed by the sponsor assisted the authors with the first draft of the manuscript and provided editorial assistance with subsequent drafts. All the authors contributed to the interpretation of the data, reviewed the first draft of the manuscript, and provided input for revisions. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol.
STATISTICAL ANALYSIS
In phase 1, we planned to enroll approximately 20 patients with advanced solid tumors of varying types for dose exploration and 60 patients for dose expansion. In phase 2, a sample of 60 patients with tumor types that were not lung or colorectal cancer was selected on the basis of enrollment feasibility. Patients with pancreatic cancer were included in both phases. We did not establish a prespecified benchmark for response, and we did not plan hypothesis testing in the cohort of patients with tumor types other than lung or colorectal cancer. The protocol specified that the analysis would be performed when sufficient follow-up time had accrued among the patients with tumor types other than lung or colorectal cancer (approximately 8.5 months after the last patient was enrolled).
The percentage of patients with an objective response was summarized with exact 95% confidence intervals, calculated with the use of the Clopper–Pearson method. Time-to-event end points were summarized with the use of Kaplan–Meier estimates and 95% confidence intervals with log–log transformation. The time to objective response was summarized as a continuous variable with the use of mean, standard deviation, minimum, and maximum values. The confidence intervals for the efficacy end points were not adjusted for multiplicity. Censoring rules for time-to-event end points are outlined in the statistical analysis plan (available with the protocol). Additional details are provided in the Supplementary Appendix.
RESULTS
TRIAL POPULATION
From July 3, 2019, to January 25, 2021, investigators at 25 centers in seven countries enrolled a total of 38 patients with KRAS p.G12C–mutated pancreatic cancer; 12 patients were enrolled in phase 1, and 26 patients were enrolled in phase 2 (Fig. S1 in the Supplementary Appendix). All 38 patients received oral sotorasib at a dose of 960 mg daily and were included in the analysis. The median duration of treatment was 18 weeks (range, 1 to 48); 25 patients (66%) received treatment for 3 months or longer, and 8 (21%) received treatment for 6 months or longer. Two patients (5%) continued to receive treatment after disease progression according to the investigator’s assessment that there would be a continued clinical benefit. As of the data-cutoff date (November 1, 2021), 36 patients (95%) had discontinued treatment, with disease progression as the most common reason (in 32 patients [84%]). Of the 36 patients who discontinued treatment, 26 (72%) died or withdrew from the trial and did not receive subsequent anticancer therapy. A majority of the remaining 10 patients were treated with chemotherapy (Table S1).
Overall, the baseline characteristics of the patients enrolled in phase 1 and phase 2 were similar (Table 1). A majority of the patients were men (29 patients [76%]), and the median age was 65.5 years (range, 45 to 81). A total of 21 patients (55%) had stage IV disease at initial diagnosis, and all the patients had metastatic disease at enrollment. Liver metastasis was observed in 31 patients (82%). A total of 14 patients (37%) had a history of pancreatic resection, including 1 patient who had had stage IV disease at initial diagnosis. The patients had received a median of 2 lines (range, 1 to 8) of therapy previously, with 30 patients (79%) having received 2 or more previous lines. Additional details regarding previous regimens are provided in the Supplementary Results section in the Supplementary Appendix. Analysis of the plasma next-generation sequencing data from 28 patients revealed multiple common co-mutations at baseline such as TP53 (in 75% of patients), CDKN2A (in 18%), BRCA2 (in 18%), and SMAD4 (in 11%) (Fig. S2). Because of the small sample size, interpretation of the data was difficult and precluded our ability to perform subgroup analyses.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Phase 1 (N = 12) | Phase 2 (N = 26) | Combined Phase 1–2 (N = 38) |
|---|---|---|---|
| Sex — no. (%) | |||
| Male | 9 (75) | 20 (77) | 29 (76) |
| Female | 3 (25) | 6 (23) | 9 (24) |
| Median age (range) — yr | 62.5 (45–75) | 67 (53–81) | 65.5 (45–81) |
| ECOG performance-status score — no. (%)† | |||
| 0 | 1 (8) | 11 (42) | 12 (32) |
| 1 | 7 (58) | 15 (58) | 22 (58) |
| 2 | 4 (33) | 0 | 4 (11) |
| Disease stage at initial diagnosis — no. (%) | |||
| I | 2 (17) | 1 (4) | 3 (8) |
| II | 4 (33) | 7 (27) | 11 (29) |
| III | 1 (8) | 2 (8) | 3 (8) |
| IV | 5 (42) | 16 (62) | 21 (55) |
| Stage IV disease at screening — no. (%) | 12 (100) | 26 (100) | 38 (100) |
| Histopathological subtype — no. (%) | |||
| Adenocarcinoma | 11 (92) | 26 (100) | 37 (97) |
| Other or unknown | 1 (8) | 0 | 1 (3) |
| No. of sites of metastatic disease — no. (%) | |||
| 1 | 4 (33) | 13 (50) | 17 (45) |
| 2 | 5 (42) | 10 (38) | 15 (39) |
| ≥3 | 3 (25) | 3 (12) | 6 (16) |
| Site of metastases — no. (%) | |||
| Liver | 9 (75) | 22 (85) | 31 (82) |
| Lung | 5 (42) | 11 (42) | 16 (42) |
| Brain | 0 | 1 (4) | 1 (3) |
| Bone | 3 (25) | 1 (4) | 4 (11) |
| Previous lines of anticancer therapy — no. (%) | |||
| 1 | 2 (17) | 6 (23) | 8 (21) |
| 2 | 3 (25) | 10 (38) | 13 (34) |
| 3 | 2 (17) | 7 (27) | 9 (24) |
| ≥4 | 5 (42) | 3 (12) | 8 (21) |
| Median lines of previous anticancer therapy (range) — no. | 3 (1–5) | 2 (1–8) | 2 (1–8) |
| Type of previous anticancer therapy — no. (%) | |||
| Chemotherapy | 12 (100) | 26 (100) | 38 (100) |
| Fluoropyrimidine | 11 (92) | 23 (88) | 34 (89) |
| Irinotecan | 11 (92) | 22 (85) | 33 (87) |
| Oxaliplatin or cisplatin | 11 (92) | 21 (81) | 32 (84) |
| Gemcitabine | 7 (58) | 21 (81) | 28 (74) |
| Nab-paclitaxel | 7 (58) | 18 (69) | 25 (66) |
| Liposomal irinotecan | 2 (17) | 3 (12) | 5 (13) |
| Erlotinib | 1 (8) | 0 | 1 (3) |
| Select regimens | |||
| FOLFIRINOX | 11 (92) | 18 (69) | 29 (76) |
| Gemcitabine + nab-paclitaxel | 7 (58) | 18 (69) | 25 (66) |
| Fluorouracil + liposomal irinotecan | 2 (17) | 3 (12) | 5 (13) |
Percentages may not total 100 because of rounding. FOLFIRINOX denotes fluorouracil, leucovorin, irinotecan, and oxaliplatin, and nab-paclitaxel nanoparticle albumin–bound paclitaxel.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability.
EFFICACY
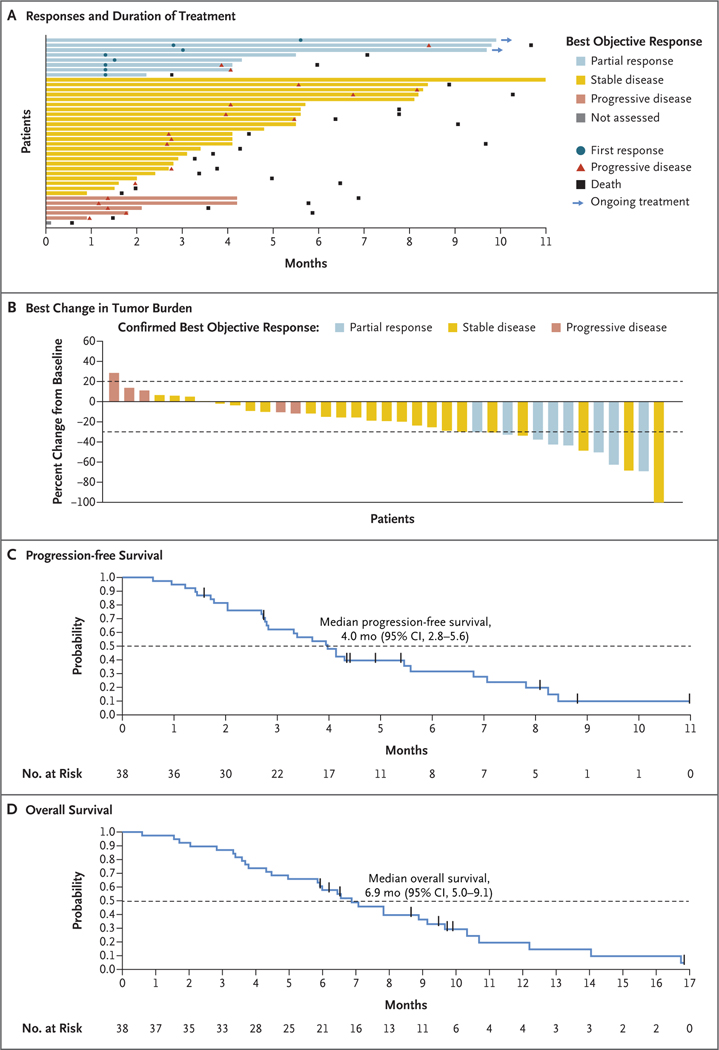
Among the 38 patients in the two phases, 8 patients (21%; 95% confidence interval [CI], 10 to 37) had a confirmed partial response as assessed by blinded independent central review (Table 2). No patients had a complete response. Responses according to investigator assessment are provided in Table S2. Among these 8 patients, the median time to a response was 1.5 months (range, 1.3 to 5.6), and the duration of response was 5.7 months (95% CI, 1.6 to could not be evaluated) (Table 2). In 5 of these patients (62%), a response was observed at the time of the first tumor assessment at approximately week 6. As of the data-cutoff date, 2 of the 8 patients (25%) were continuing to receive treatment, with an ongoing treatment duration of approximately 10 months (Fig. 1A).
Table 2.
Efficacy of Sotorasib Therapy.*
| Variable | Phase 1 (N = 12) | Phase 2 (N = 26) | Combined Phase 1–2 (N = 38) |
|---|---|---|---|
| Best overall response — no. (%)† | |||
| Confirmed complete response | 0 | 0 | 0 |
| Confirmed partial response | 3 (25) | 5 (19) | 8 (21) |
| Stable disease | 6 (50) | 18 (69) | 24 (63) |
| Progressive disease | 2 (17) | 3 (12) | 5 (13) |
| Could not be evaluated | 0 | 0 | 0 |
| Not assessed | 1 (8) | 0 | 1 (3) |
| Percentage of patients with objective response (95% CI) — % | 25 (6–57) | 19 (7–39) | 21 (10–37) |
| Percentage of patients with disease control (95% CI) — %‡ | 75 (43–95) | 89 (70–98) | 84 (69–94) |
| Median time to objective response (range) — mo§ | 1.4 (1.3–1.5) | 2.8 (1.3–5.6) | 1.5 (1.3–5.6) |
| Median duration of response (95% CI) — mo§¶ | — | — | 5.7 (1.6–NE) |
An objective response was defined as a complete or partial response. NE denotes could not be evaluated.
The best overall response was determined by blinded independent central review.
Disease control was defined as an objective response or stable disease.
The median time to objective response and the median duration of response were calculated for the patients who had a confirmed objective response.
The median duration of response (Kaplan–Meier estimates) is not provided for individual phases because of the small number of patients.
Figure 1. Efficacy Analyses of Sotorasib Therapy.
Panel A shows the responses and the duration of treatment in all patients as assessed by blinded independent central review (which could be different from investigator-assessed timing of progressive disease). Five patients with a best objective response of progressive disease as assessed by blinded independent central review ended treatment within one cycle after the occurrence of progressive disease according to the decision of the investigator. Panel B shows the best percentage change from baseline in tumor burden (defined as the sum of the diameters of all target lesions). The upper dashed line indicates a 20% increase in tumor burden (progressive disease), and the lower dashed line indicates a 30% decrease in tumor burden (partial response). Progressive disease with less than a 20% increase (from nadir) in the sum diameter of target lesions was due to unequivocal progressive disease in the nontarget lesion or the presence of a new lesion. Panel C shows the Kaplan–Meier curve of progression-free survival; the dashed line indicates 50% progression-free survival probability. Panel D shows the Kaplan–Meier curve of overall survival; the dashed line indicates 50% overall survival probability. The vertical bars in Panels C and D indicate censored data.
Tumor shrinkage of target lesions of any magnitude was observed in 30 patients (79%) (Fig. 1B). Tumor shrinkage of target lesions according to investigator assessment is provided in Figure S3.
The median progression-free survival was 4.0 months (95% CI, 2.8 to 5.6) (Fig. 1C). The median progression-free survival as assessed by blinded independent central review and according to investigator assessment is provided in Table S3. The Kaplan–Meier estimate of progression-free survival was 31.6% (95% CI, 16.7 to 47.7) at 6 months and 9.9% (95% CI, 2.0 to 25.6) at 9 months. At a median follow-up of 16.8 months (95% CI, 9.5 to could not be evaluated), the median overall survival was 6.9 months (95% CI, 5.0 to 9.1) (Fig. 1D). The Kaplan–Meier estimate of overall survival at 12 months was 19.6% (95% CI, 7.2 to 36.3).
SAFETY
Overall, the safety profile of sotorasib in the combined population of patients in the two phases (38 patients) was similar to that observed in the cohorts in phase 1 (12 patients) and phase 2 (26 patients). Adverse events of any grade that occurred during treatment, regardless of attribution, were observed in all the patients (100%); the most common adverse events were abdominal pain (in 14 patients [37%]), diarrhea and nausea (in 9 patients [24%] each), and vomiting and pyrexia (in 8 patients [21%] each). Overall, 24 patients (63%) had adverse events of grade 3 or higher.
Adverse events of any grade that were considered by the investigators to be related to treatment were reported in 16 patients (42%); 6 of these events (16%) were grade 3 in severity (Table 3). The most common grade 3 treatment-related adverse events were diarrhea and fatigue (in 2 patients [5%] each). No treatment-related adverse events of grade 4 or 5 were reported. Treatment-related serious adverse events were reported in 3 patients (8%). A total of 14 patients (37%) had fatal events; none were considered by the investigators to be related to treatment. Treatment-related adverse events leading to dose reduction or interruption of sotorasib therapy occurred in 5 patients (13%). Among these events, grade 3 diarrhea that resolved in 4 days and grade 2 or 3 diarrhea that resolved in 16 days were observed in 2 patients (5%). No treatment-related adverse events resulted in the discontinuation of sotorasib therapy. The full list of adverse events that occurred during each phase and that occurred in the combined phase 1 and 2 population is provided in Table S4.
Table 3.
Adverse Events.*
| Adverse Event | Adverse Events Reported during Treatment | Treatment-Related Adverse Events | ||||
|---|---|---|---|---|---|---|
| Phase 1 (N = 12) | Phase 2 (N = 26) | Combined Phase 1–2 (N = 38) | Phase 1 (N = 12) | Phase 2 (N = 26) | Combined Phase 1–2 (N = 38) | |
| number of patients (percent) | ||||||
| Any adverse event | 12 (100) | 26 (100) | 38 (100) | 5 (42) | 11 (42) | 16 (42) |
| Grade ≥2 | 12 (100) | 23 (89) | 35 (92) | 3 (25) | 9 (35) | 12 (32) |
| Grade ≥3 | 9 (75) | 15 (58) | 24 (63) | 0 | 6 (23) | 6 (16) |
| Grade ≥4 | 7 (58) | 7 (27) | 14 (37) | 0 | 0 | 0 |
| Serious adverse event | 9 (75) | 15 (58) | 24 (63) | 0 | 3 (12) | 3 (8) |
| Adverse event leading to dose reduction or interruption of therapy | 5 (42) | 9 (35) | 14 (37) | 0 | 5 (19) | 5 (13) |
| Adverse event leading to discontinuation of therapy | 1 (8) | 1 (4) | 2 (5) | 0 | 0 | 0 |
| Fatal adverse event | 7 (58) | 7 (27) | 14 (37) | 0 | 0 | 0 |
Shown are adverse events that occurred during treatment and those that occurred within 30 days after the last dose of sotorasib or the end of the trial, whichever occurred earlier. Relatedness to treatment was determined by the investigators. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
DISCUSSION
In the RAS family of genes, KRAS is the most commonly mutated isoform in pancreatic cancer, with oncogenic driver mutations occurring most frequently in codons 12, 13, and 61.2,18 The mutated KRAS protein was considered to be “undruggable” for many years, owing mainly to its complex biochemical characteristics, high affinity for guanosine triphosphate, and limited number of binding sites.19,20 Analysis of the crystal structure of the KRAS G12C bound to guanosine diphosphate (GDP) revealed a previously unknown pocket beneath the switch II region that allowed for direct targeting of the mutated protein.21 Sotorasib irreversibly and selectively binds to this pocket, which is present in the mutant KRAS G12C, and locks it in the inactive GDP-bound state, thereby inhibiting oncogenic signaling. On the basis of the potential of sotorasib to treat pancreatic cancer harboring KRAS p.G12C mutation, we evaluated the safety and efficacy of sotorasib in this phase 1–2 clinical trial. In this trial, sotorasib therapy had clinically meaningful efficacy and an acceptable safety profile in patients with KRAS p.G12C–mutated metastatic pancreatic cancer. A total of 21% of the patients had a centrally confirmed response, and the median time to response was 1.5 months; disease control was observed in 84% of the patients. The median progression-free survival was 4.0 months, and the overall survival was 6.9 months.
The results of this trial are promising in the context of the outcomes observed with approved regimens of second-line treatment of advanced pancreatic cancer.5,22–24 In the final analysis of the phase 3 NAPOLI-1 trial, second-line treatment with a combination of liposomal irinotecan, fluorouracil, and leucovorin in patients with metastatic pancreatic ductal adenocarcinoma who had received previous gemcitabine-based treatment resulted in a median overall survival of 6.2 months and a progression-free survival of 3.1 months.5 In an open-label, randomized, phase 3 trial involving patients with gemcitabine-resistant advanced pancreatic cancer (the Charité Onkologie 003 [CONKO-003] trial), second-line treatment with oxaliplatin, leucovorin, and fluorouracil in patients with gemcitabine-refractory, advanced pancreatic cancer resulted in a median overall survival of 5.9 months and a progression-free survival of 2.9 months.22 Although the results for the median progression-free survival and overall survival in the current trial are encouraging, additional data from larger trials are needed to confirm these findings.
On the basis of the results of the Pancreas Cancer Olaparib Ongoing (POLO) trial, olaparib was approved as maintenance therapy in patients with germline BRCA-mutated metastatic pancreatic cancer.6 Although the percentage of patients with a response reported in the POLO trial (23%) was similar to that reported in this trial, it must be acknowledged that the POLO trial was a randomized, placebo-controlled, phase 3 trial, whereas the current study is a nonrandomized, single-group, phase 1–2 trial. In the POLO trial, patient eligibility was limited to those who did not have disease progression after 4 months of first-line platinum-based therapies, which enriched the trial with patients who would most likely benefit from PARP inhibitors. In contrast, approximately 79% of the patients enrolled in the current trial were heavily pretreated with two or more lines of anticancer therapies. Furthermore, clinical studies evaluating various chemotherapeutic regimens as second-line therapy for patients with advanced pancreatic cancer have reported a response in less than 10% of patients.24–27
The response that was observed with sotorasib therapy in this analysis (21%) was numerically lower than that among patients with KRAS p.G12C–mutated non–small-cell lung cancer (37.1%; 95% CI, 28.6 to 46.2) and greater than that among patients with KRAS p.G12C–mutated colorectal cancer (9.7%; 95% CI, 3.6 to 19.9).28–30 At baseline, a majority of patients in those lung and colon cancer cohorts were heavily pretreated with a median of two or three previous lines of anticancer therapy, which predominantly included chemotherapy, checkpoint inhibitors, or bevacizumab. In addition, the patients with KRAS p.G12C–mutated lung and colorectal cancer who were enrolled in phase 2 of this trial had an ECOG performance-status score of 0 or 1. (The results in these patients are not reported here.) In the combined phase 1–2 patient population in the current trial, 11% of the patients with an ECOG performance-status score of 2 were enrolled. (The other 89% of patients had an ECOG performance-status score of 0 or 1.) Although the response observed in this trial is promising, the mechanisms in various tumor types with a sensitivity to KRAS pathway inhibition are unknown, and further cancer-cell biology studies are warranted.
An analysis of the data from patients with colorectal cancer who were treated with KRAS G12C inhibitors suggests that higher receptor tyrosine kinase signaling in these tumors and reactivation of receptors (particularly epidermal growth factor receptor [EGFR]) with RAS inhibition attenuates response to KRAS G12C inhibitors, and combination treatment with KRAS G12C and EGFR inhibitors improves efficacy.31 It is unclear whether a similar receptor reactivation limits sotorasib monotherapy activity in pancreatic cancer. Studies examining new sotorasib combinations, such as CodeBreaK 101 (ClinicalTrials.gov number, NCT04185883), are actively under way. Preliminary results from studies evaluating the combinations of KRAS G12C inhibitors with EGFR inhibitors for the treatment of patients with KRAS p.G12C–mutated colorectal cancer have shown a response in 30 to 43% of patients.32–34 Although these data are encouraging, additional analyses in patients with KRAS p.G12C–mutated pancreatic cancer are needed to assess the efficacy of these combinations.
Overall, sotorasib was associated with mainly low-grade toxic effects in the heavily pretreated population in this trial, and the safety findings were consistent with those previously reported in other CodeBreaK 100 trials.28–30 Diarrhea and fatigue were the most frequently occurring treatment-related adverse events (in 5% of patients). None of these events were fatal, and none resulted in the discontinuation of sotorasib therapy. This safety profile compares favorably with current standard regimens used for the treatment of pancreatic cancer.3–5 However, a higher number of patients were enrolled in the pivotal trials evaluating the safety and efficacy of these regimens than were enrolled in the current trial. Therefore, studies with larger cohorts are needed to clarify the prognostic effect of KRAS p.G12C mutation in patients with pancreatic cancer.
Pancreatic cancer is one of the most difficult cancers to diagnose and treat. In this phase 1–2 trial, sotorasib monotherapy showed promising anticancer activity in patients with heavily pretreated KRAS p.G12C–mutated advanced pancreatic cancer. The clinical activity of sotorasib shown in this trial provides evidence that targeting KRAS is a viable strategy for the treatment of advanced pancreatic cancer. In addition, the clinical activity of sotorasib against the KRAS p.G12C mutation should invigorate efforts aimed at the design and development of inhibitors relevant to the forms of KRAS mutations that are more common in pancreatic ductal adenocarcinomas. Studies assessing the safety and efficacy of sotorasib in combination with other anticancer therapies are ongoing.
Supplementary Material
Acknowledgments
Supported by Amgen, a Cancer Center Core Grant (P30 CA 008748 [to Memorial Sloan Kettering Cancer Center]), an M.D. Anderson Cancer Center Support Grant (P30 CA016672), a Clinical Translational Science Award (1UL1 TR003167), and a grant (RP150535) from the Cancer Prevention Research Institute of Texas Precision Oncology Decision Support Core. The Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy provided molecular and other services.
We thank the patients and their families for participating in the trial; Maya Shehayeb, Pharm.D., Timothy Harrison, Pharm.D., and Jennifer Martucci, B.F.A. (all employed by Amgen), for operational planning assistance; Robert Dawson, B.A., for graphics assistance; and Advait Joshi, Ph.D., of Cactus Life Sciences (part of Cactus Communications) for medical writing support with an earlier version of the manuscript.
Footnotes
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–49. [DOI] [PubMed] [Google Scholar]
- 2.Waters AM, Der CJ. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb Perspect Med 2018; 8(9): a031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;3 64: 1817–25. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369:1 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang-Gillam A, Hubner RA, Siveke JT, et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: final overall survival analysis and characteristics of long-term survivors. Eur J Cancer 2019; 108: 78–87. [DOI] [PubMed] [Google Scholar]
- 6.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019; 381: 317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora S, Balasubramaniam S, Zhang H, et al. FDA approval summary: olaparib monotherapy or in combination with bevacizumab for the maintenance treatment of patients with advanced ovarian cancer. Oncologist 2021; 26(1):e 164–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luchini C, Brosens LAA, Wood LD, et al. Comprehensive characterisation of pancreatic ductal adenocarcinoma with microsatellite instability: histology, molecular pathology and clinical implications. Gut 2021; 70: 148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad V, Kaestner V, Mailankody S. Cancer drugs approved based on biomarkers and not tumor type-FDA approval of pembrolizumab for mismatch repair-deficient solid cancers. JAMA Oncol 2018; 4: 157–8. [DOI] [PubMed] [Google Scholar]
- 10.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018; 378: 731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol 2020;2 1:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leroux C, Konstantinidou G. Targeted therapies for pancreatic cancer: overview of current treatments and new opportunities for personalized oncology. Cancers (Basel) 2021; 13:7 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016; 531: 47–52. [DOI] [PubMed] [Google Scholar]
- 14.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012; 491: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun 2015; 6: 6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo J. KRAS mutation in pancreatic cancer. Semin Oncol 2021; 48: 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima EC, Drezner N, Li X, et al. FDA approval summary: sotorasib for KRAS G12C-mutated metastatic NSCLC. Clin Cancer Res 2022; 28: 1482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prior IA, Hood FE, Hartley JL. The Frequency of Ras mutations in cancer. Cancer Res 2020; 80: 2969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gysin S, Salt M, Young A, McCormick F. Therapeutic strategies for targeting ras proteins. Genes Cancer 2011; 2:3 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spoerner M, Herrmann C, Vetter IR, Kalbitzer HR, Wittinghofer A. Dynamic properties of the Ras switch I region and its importance for binding to effectors. Proc Natl Acad Sci U S A 2001; 98: 4944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 2013;5 03: 54851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014;3 2: 2423–9. [DOI] [PubMed] [Google Scholar]
- 23.Ko AH, Tempero MA, Shan Y-S, et al. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. Br J Cancer 2013; 109: 920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo C, Hwang JY, Kim JE, et al. A ran-domised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 2009; 101: 1658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong HQ, Varadhachary GR, Blais JC, Hess KR, Abbruzzese JL, Wolff RA. Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer 2008; 113:2 046–52. [DOI] [PubMed] [Google Scholar]
- 26.Pelzer U, Stieler J, Roll L, et al. Second-line therapy in refractory pancreatic cancer: results of a phase II study. Onkologie 2009; 32:9 9–102. [DOI] [PubMed] [Google Scholar]
- 27.Zaniboni A, Aitini E, Barni S, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol 2012; 69: 1641–5. [DOI] [PubMed] [Google Scholar]
- 28.Hong DS, Fakih MG, Strickler JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med 2020; 383:1 207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med 2021; 384: 2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fakih MG, Kopetz S, Kuboki Y, et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (Code-BreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol 2022; 23:1 15–24. [DOI] [PubMed] [Google Scholar]
- 31.Amodio V, Yaeger R, Arcella P, et al. EGFR blockade reverts resistance to KRASG12C inhibition in colorectal cancer. Cancer Discov 2020; 10:1 129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuboki Y, Yaeger R, Fakih MG, et al. Sotorasib in combination with panitumumab in refractory KRAS G12C-mutated colorectal cancer: safety and efficacy for phase Ib full expansion cohort. Proceedings and Abstracts of the 2022 Congress of the European Society for Medical Oncology, September 9–13, 2022. Paris: European Society for Clinical Oncology, 2022. [Google Scholar]
- 33.Weiss J, Yaeger RD, Johnson ML, et al. KRYSTAL-1: adagrasib (MRTX849) as monotherapy or combined with cetuximab (Cetux) in patients (Pts) with colorectal cancer (CRC) harboring a KRASG12C mutation. Ann Oncol 2021; 32:Suppl 5: S1294. abstract. [Google Scholar]
- 34.Weiss L. ESMO 2021 — highlights in colorectal cancer. Memo 2022; 15: 114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.