ABSTRACT
Differential responses to viral infections are influenced by the genetic makeup of the host. Studies of resistance to retroviruses in human populations are complicated due to the inability to conduct proof-of-principle studies. Inbred mouse lines, which have a range of susceptible phenotypes to retroviruses, are an ideal tool to identify and characterize mechanisms of resistance and define their genetic underpinnings. YBR/Ei mice become infected with Mouse Mammary Tumor Virus, a mucosally transmitted murine retrovirus, but eliminate the virus from their pedigrees. Virus elimination correlates with a lack of virus-specific neonatal oral tolerance, which is a major mechanism for blocking the anti-virus response in susceptible mice. Virus control is unrelated to virus-neutralizing antibodies, cytotoxic CD8+ T cells, NK cells, and NK T cells, which are the best characterized mechanisms of resistance to retroviruses. We identified a single, dominant locus that controls the resistance mechanism, which we provisionally named attenuation of virus titers (Avt) and mapped to the distal region of chromosome 18.
IMPORTANCE
Elucidation of the mechanism that mediates resistance to retroviruses is of fundamental importance to human health, as it will ultimately lead to knowledge of the genetic differences among individuals in susceptibility to microbial infections.
KEYWORDS: retroviruses, genetic mechanism, immune response
INTRODUCTION
Genetic differences between distinct inbred mouse lines determine their ability to control retroviruses, rendering them either susceptible or resistant to infection and viral pathogenesis. Studies of retrovirus-resistant mouse strains lead to the identification of various mechanisms underlying resistance to retroviruses and the gene(s) that control them (1). Importantly, this animal model also allows for functional proof that one or more candidate genes mediate the mechanism(s) conferring resistance.
Mouse Mammary Tumor Virus (MMTV) is an oncogenic betaretrovirus that is used as a powerful tool to study host-retrovirus interactions. MMTV is transmitted either as an endogenous virus through the germline or as an exogenous virus via milk from infected mothers to newborn pups. In the neonatal intestine, the virus is transcytosed via microfold (M) cells in Peyers’ patches (2) and infects antigen-presenting cells (APCs), specifically dendritic cell (DCs) and B cells, as well as T cells (3, 4). Infected APCs present the virally encoded superantigen (SAg) on major histocompatibility class II (MHC class II) molecules to SAg-cognate CD4+ T cells bearing a particular T cell receptor (TCR) chain (5). In response to SAg presentation, SAg-cognate T cells proliferate and activate B cells to proliferate (6), generating a pool of activated, infection-competent cells (5). SAg-mediated stimulation of cognate CD4+ T cells is necessary for infection as mice that lack SAg-cognate T cells cannot be infected with MMTV (7, 8). In addition, MMTV lacking functional SAgs are not infectious (9, 10). MMTV amplification in lymphocytes enables transmission to mammary epithelial cells during puberty. Driven by lactogenic hormone-regulated expression in mammary epithelial cells, the virus is shed into the milk during lactation, therefore completing the replication cycle (11). Activated SAg-cognate T cells subsequently undergo deletion, which occurs in all infected mice. Deletion of SAg-cognate T cells is measured in peripheral blood and is used as an indicator of successful infection (12 - 15). Mammary tumorigenesis resulting from MMTV infection is not caused by an oncogene encoded by the virus, but rather is a result of provirus integration in the proximity of a cellular proto-oncogene leading to activation of its expression (11).
The neonatal immune system is relatively immature, which allows for the development of tolerance to the intestinal commensal microbial community and food antigens (16 - 18). MMTV has evolved to take advantage of neonatal oral tolerance by inducing a subversion pathway that involves the production of interleukin (IL)-10 driven by IL-6 produced in response to virally triggered Toll-like receptor (TLR) 4 (19, 20). This subversion pathway leads to immunological tolerance to MMTV, which is a hallmark of all neonatally infected mice from susceptible strains. Elimination of any component of the subversion pathway renders mice resistant to the virus (19, 20). Thus, MMTV-induced neonatal oral tolerance suppresses immune responses to viral antigens, supporting virus replication and spread.
The Andervont’s laboratory was the first to show that Y mice can be infected with the virus but resist MMTV-induced mammary tumors and that the resistant mechanism is dominantly inherited (21, 22). We have reproduced these data with YBR/Ei (YBR) mice [available from The Jackson Laboratory (TJL)], which are descendants of the Y strain (14). We have also shown that unlike susceptible mice, such as C3H/HeN, BALB/cJ, C57BL/6J, and 129S1/SvImJ (129) mice, YBR mice inhibit virus production, as evidenced by severely reduced viral titers in the mammary glands and in the milk (14, 19). This attenuation of virus titers leads to virus elimination through successive passages through YBR mice (14). The number of virus passages across generations required for viral clearance depends on the titer of the input virus, with higher input titers requiring more generations to clear the pathogen than lower input titers (14). Moreover, the virus resistance mechanism in YBR mice is unrelated to innate intrinsic restriction factors and is not due to the major histocompatibility locus or a lack of SAg-cognate CD4+ T cells (14).
MMTV-infected mice from the I/LnJ strain produce virus-neutralizing antibodies (Abs), which coat virions secreted by infected I/LnJ cells and prevent virus transmission to offspring (23). The gene conferring the ability to produce retrovirus-neutralizing Ab in I/LnJ mice was identified as H2-Ob, which together with H2-Oa forms H2-O, a negative regulator of MHC II peptide presentation (24). However, unlike MMTV-infected I/LnJ mice, infected YBR mice do not produce anti-virus Abs, and viral particles purified from their milk are not coated with Abs and remain infectious in susceptible mice (14). In addition, the H2-Ob allele of YBR is different from that of I/LnJ mice and is identical to the H2-Ob allele found in susceptible mice. Therefore, a virus-neutralizing Ab response does not underly the resistance mechanism inherited by YBR mice.
Here, we sought to define the mechanism that controls MMTV in YBR mice and to genetically map the locus conferring it.
RESULTS AND DISCUSSION
The mechanism of resistance inherited by YBR mice is not driven by MHC class I-mediated responses
Given that initial MMTV infection can be established in YBR mice, we reasoned that the mechanism controlling the virus must be adaptive. However, as previously shown, it is unrelated to Ab responses (14). To investigate the contribution of cytotoxic T cells (CTLs) and natural killer (NK) T cells to retrovirus restriction in YBR mice, we utilized YBR mice deficient in CD8, ß2m, and perforin. ß2m-deficient mice do not express MHC class I and CD1 (25) and thus lack functional MHC I-restricted CD8+ CTLs (26) (similar to CD8-deficient mice) and CD1-restricted NK T cells (25). ß2m-deficient mice also lack functional NK cells as they lose their ability to kill target cells in the absence of MHC I (27, 28). Perforin is a glycoprotein produced by cytotoxic CD8+T and NK cells responsible for pore formation in cell membranes of target cells (29).
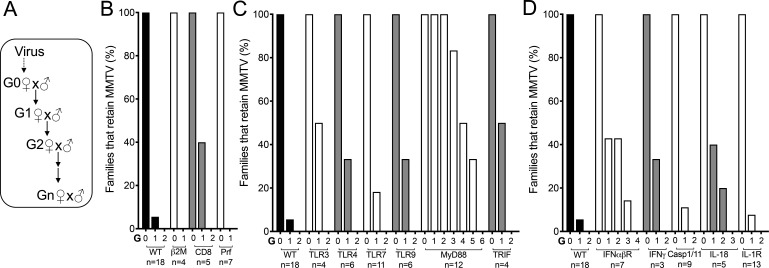
CD8-, ß2m-, and perforin-deficient YBR mice were MMTV-infected via fostering, and virus fate was followed in the infected mouse pedigrees (Fig. 1A). Like wild-type (WT) YBR mice, progenies of CD8-, perforin-, and ß2m-deficient infected YBR mice eliminated the virus (Fig. 1B). Thus, the retrovirus restriction mechanism in YBR mice is not due to CD8+ T, NK T, or NK cells.
Fig 1.
Most known innate sensors and effectors and MHC class I responses are not required to control MMTV in YBR mice. (A) Experimental design to follow the virus fate in infected mouse pedigrees. Female mice with the specific genotype were fostered by MMTV(C3H)-infected C3H/HeN viremic mothers (Generation 0, G0) and bred to produce subsequent generations (G1-N). (B–D) Infection status of mice at each generation was evaluated by monitoring deletion of SAg-cognate T cells at 10 weeks of age and by PCR specific for integrated proviruses in the spleen. Lines deficient in MHC class I-specific responses (B), innate immune sensors and adaptors (C), and specific cytokines and caspase 1/11 (D) were evaluated. n represents number of individual pedigrees per genotype followed. Three-eight mice per family per generation were analyzed.
The mechanism controlling MMTV in YBR mice was previously found to be dependent on Thy1-expressing cells (14), which include CD4+ T, CD8+ T, NK T, and NK cells. All cell subsets but CD4+ T cells were eliminated as effectors conferring retrovirus resistance in YBR (Fig. 1B). However, CD4-deficient mice cannot be infected with MMTV due to lack of SAg-cognate T cells required for infection (7, 8), making it is impossible to test for their direct involvement in the virus restriction.
Most known innate sensors and effectors are insufficient to control MMTV in YBR mice
Pathogen-specific immune responses require the recognition of pathogen-associated molecular patterns, highly conserved microbial structures that are not found in host cells, by pattern recognition receptors (PRRs) (30). Viral infections are recognized by cytosolic or endocytic PRRs that detect viral genomes or replication intermediates (e.g., nucleic acids) (31), by inflammasomes that detect the activities of virally encoded proteins (32), or by cytoplasmic factors that sense higher-order molecular structures such as the capsid lattice (33, 34). Innate immune sensors activate innate immune effectors, stimulate inflammatory responses, and direct the pathogen-specific adaptive responses. Different innate immune sensors drive distinct adaptive immune responses, each of which is tailored to a given pathogen. Thus, we reasoned that identification of the specific innate sensor directing the adaptive antiviral immune response in YBR mice would lead to a better understanding of how this response is orchestrated. To do this, we generated YBR mice with deficiencies in some PRRs known to be activated by viruses from various families, including retroviruses (35) and tested whether they could control MMTV. YBR mice deficient in TLR3 (which recognizes endosomal double-stranded RNA), four [which recognizes LPS bound to MMTV virions (19, 36)], seven (which recognizes endosomal single-stranded RNA), and nine (which recognizes endosomal DNA), as well as TRIF (the adaptor for TLR3 and TLR4 receptors) all eliminated the virus in successive generations (Fig. 1C). Although YBR mice deficient in the TLR adaptor MyD88 mice eliminated the virus it took more generations for virus clearance in these mouse lineages compared to other mouse lineages (Fig. 1C). TLR 4, 7, and 9 all signal through MyD88. Potentially, a loss of signaling through two or all three of these TLRs due to the MyD88-deficiency resulted in the elimination of a mechanism that controls the virus to some extent. However, this mechanism alone is insufficient to eliminate the virus in YBR mice.
Type I interferons are hallmark cytokines induced by various innate immune sensors, including some that we did not test, in response to various pathogens. All type I IFNs signal through the IFN-I receptor (IFNR). To test the role of type I IFNs in the anti-MMTV response in YBR mice, YBR mice deficient in IFNR were generated. YBR.Ifnr-deficient infected mouse pedigrees eliminated the virus similarly to WT mice (Fig. 1D), indicating that type I IFN are not involved in the retrovirus control in YBR mice.
IFN is a type II IFN that modulates both innate and adaptive immune responses and is induced by many viral infections including retroviral infections (37 - 43). We next tested whether it drives the anti-viral response in infected YBR mice. Accordingly, we followed the virus fate in YBR.Ifnγ-deficient mice. All mouse pedigrees deficient in IFNγ eliminated the virus (Fig. 1D), indicating that this cytokine is not involved in the retrovirus control in YBR mice.
A part of the host defense to fight viral infection includes programmed cell death (44). Pyroptosis is an inflammasome-dependent cell death executed following activation of caspase-1 or mouse caspase-11 (44, 45). To test whether caspase 1/11-dependent mechanism controls MMTV in YBR mice, we generated YBR lacking both caspases (YBR.Casp1/11-deficient mice) and followed the virus fate in the infected mouse pedigrees. All pedigrees of infected YBR.Casp1/11-deficient mice eliminated the virus (Fig. 1D).
IL-1 and -18, which are induced via inflammasomal activation, are known to contribute to adaptive anti-viral immune responses (46, 47). IL-1 acts either alone or in synergy with other cytokines to influence CD4+ and CD8+ T cell-specific responses (48), while IL-18 has the potential to induce plasticity of established Th1 cells (49). To test the contribution of these cytokines to retrovirus control, we fostered IL-1- and IL-18-deficient YBR mice on viremic mothers and followed virus fate. The virus was eliminated in both IL-1- and IL-18-deficient YBR-infected mouse pedigrees (Fig. 1D), indicating that IL-1- and IL-18-mediated immune responses are also not involved in the anti-retrovirus response in YBR mice.
’Clean’ STING-deficient YBR mice control the virus
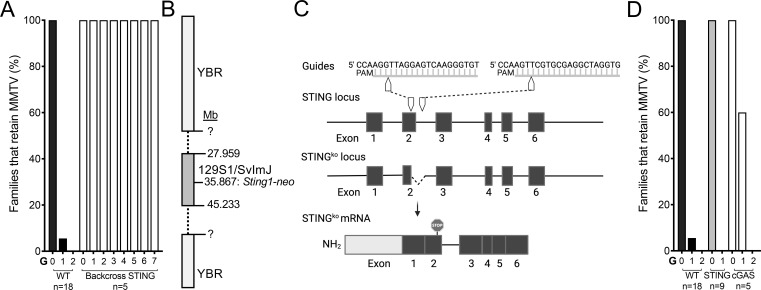
Cytoplasmic DNA is recognized by the cyclic GMP–AMP synthase (cGAS) and the cyclic GMP–AMP receptor stimulator of interferon genes (STING) thus triggering an innate immune reaction against microbial invaders. To test whether this pathway plays a critical role in the anti-MMTV response in YBR mice, we followed virus fate in STING-deficient YBR mice. None of STING-deficient YBR pedigree eliminated the virus (Fig. 2A), suggesting that STING may be critical factor in the anti-viral response. As all mice used in these studies, STING-deficient YBR mice were generated by repetitive backcrossing of C57BL/6J mice carrying the STING knockout (KO) allele produced using 129 mouse embryonic stem cells (50). Since 129 mice do not restrict MMTV (19), it was also possible that the retrovirus-restricting YBR gene which is mapped in the vicinity of Sting1 was replaced with the susceptible 129 gene during backcrossing. Therefore, we assessed the locus containing the Sting1 KO allele to determine the size of the non-recombinant 129 region, which was found to be greater than 20 Mb (Fig. 2B). Therefore, to rigorously evaluate whether the STING-dependent pathway orchestrates retrovirus resistance in YBR mice, we employed a CRISPR/Cas9 technology to generate a “clean” STING KO on the YBR background (Fig. 2C). When MMTV fate was followed in infected “clean” YBR STING-deficient lines, all families lost the virus (Fig. 4D). These data suggest that MMTV persistence in “backcrossed” YBR.Sting-deficient mice was not related to the lack of STING-mediated signaling but was due to the absence of the resistant YBR gene mapped in vicinity of STING. The fact that MMTV-infected cGAS-deficient YBR mice also lost the virus in infected mouse pedigrees (Fig. 2D) further confirmed that the STING-mediated pathway does not control the virus in YBR mice. These data also serve as a powerful reminder that findings obtained with “backcrossed” KO mice should be verified with additional genetic tools when possible.
Fig 2.
The cGAS-STING pathway is not involved in the anti-MMTV response in YBR mice. (A) YBR mice containing the disrupted Sting1-neo allele derived from 129 mice were fostered by viremic females and bred to follow the virus fate as in Fig. 1A. (B) Schematic of the Sting1-neo locus on chromosome 18 in “backcrossed” YBR.Sting−/− mice shown in (A). Mb, megabase. The beginning and end of the region from 129 mice were not defined before the mouse line was lost due to the COVID-19 pandemic. (C) CRISPR/Cas9 targeting strategy for the generation of “clean” YBR.Sting-deficient mice. Two guides were used to target exon 2 and intron 2–3, resulting in a 126-bp deletion and the introduction of a premature stop codon. (D) Virus fate in YBR mice with “clean” STING or cGAS deficiency. n represents number of individual pedigrees per genotype followed. Three-eight mice per family per generation were analyzed. KO, knockout.
Fig 4.
Neonatal oral tolerance to MMTV is not induced in YBR mice, despite an intact subversion pathway. (A and B) Nucleated splenic cells from indicated mice were incubated with LPS and IL-6 (A) and IL-10 (B) were measured in cell supernatants by enzyme-linked immunosorbent assay (ELISA). C3H/HeJ mice lack the functional TLR4 (51) and were thus, used as a negative control. Each dot shows individual mouse. (C) Mice of indicated strains were or were not immunized at 8 weeks of age with Triton X-100-treated MMTV(C3H) virions in complete Freund’s adjuvant. Reactivity of serum samples against MMTV antigens was measured by ELISA after the boost. MMTV(C3H) (+), mice were fostered by viremic females. Each dot shows individual mouse. Statistical significance was determined by two-tailed unpaired Welch’s t test (ns, P > 0.05; *, P ≤ 0.05; ***, P ≤ 0.001; ****, P ≤ 0.0001).
YBR resistance to MMTV is controlled by a single, dominant locus mapped to chromosome 18
Previously, we showed that the quantity of MMTV-specific transcripts and proviral load in infected mammary gland of YBR mice fostered by viremic foster mothers (G0 mice) is significantly lower compared to that of susceptible mice (14). To determine whether virus restriction also occurs at the early stages of infection, we compared proviral load in spleens of YBR and BALB/cJ G0 mice via MMTV-specific real-time PCR. Provirus load in the spleens in G0 resistant and susceptible mice were comparable (Fig. 3A). Thus, virus restriction does not occur at the initial stages of infection but develops at later stages either prior to or during infection of the mammary gland. Unlike in YBR G0 mice, reduction of proviral load was apparent in YBR G1 mice (Fig. 3C) and was due to severe attenuation of viral titers secreted by their G0 mothers (14).
Fig 3.
YBR resistance to MMTV is controlled by attenuation of virus titers (Avt), a single, dominant locus on chromosome 18. (A) MMTV(C3H) LTR-specific Taqman-based quantitative polymerase chain reaction (qPCR) was used to compare provirus load in splenic DNA of YBR and BALB/cJ G0 mice. Ct, Ct(MMTV) - Ct(beta-actin). Dotted line represents the limit of detection (average ΔCt from uninfected mice). Each dot shows individual mouse. G, generation. (B) Genetic crosses used to map Avt. (YBR × BALB/cJ)F1 females were fostered (F) by MMTV(C3H)-infected C3H/HeN mothers. Infected F1 females (G0)were bred to BALB/cJ males to generate N2 (G1) mice. A, the Avt dominant resistant YBR allele; a, the Avt recessive susceptible BALB/cJ allele. (C) MMTV(C3H) LTR-specific Taqman-based qPCR was used to compare provirus load in splenic DNA of N2 mice. Infected YBR and BALB/cJ G1 mice were used as controls. The ΔCt values for N2 mice ranged from 9.36 to 17.24 (mean ± SD = 11.89 ± 1.72). Arbitrary cut-offs were used to define N2 mice as resistant (ΔCt ≥11.89 + 1.72) and susceptible (ΔCt ≤11.89–1.72) based on the ΔCt mean and SD of total N2 mice. Res, resistant; Sus, susceptible. Each dot shows individual mouse. G, generation. (D) Linkage analysis across chromosome 18 in resistant and susceptible N2 mice. Proportion of mice heterozygous in each group at each single-nucleotide polymorphism (SNP) is plotted. Each dot shows individual mouse. Statistical significance was determined by two-tailed unpaired Welch’s t test (ns, P > 0.05; *, P ≤ 0.05; ****, P ≤ 0.0001). Mb, megabase.
The lack of virus restriction in the “backcrossed” YBR.Sting-deficient mice predicted that the gene(s) controlling the virus is located in the vicinity of sting on chromosome 18. We used an unbiased approach to verify this prediction. Since the resistance mechanism is dominant (14), we backcrossed MMTV-infected virus-restricting (YBR × BALB/cJ)F1 females (these mice are G0) to virus-susceptible BALB/cJ males and analyzed the infection status in their offspring, N2 mice (these mice are G1) (Fig. 3B) by comparing provirus load in splenic DNA via real-time PCR. We defined N2 mice as resistant (Res) or susceptible (Sus) if their Cts (Cts for MMTV integrated proviruses minus Cts for beta-actin) were similar to the Cts of YBR or BALB/cJ G1 mice, respectively. Animals with Cts which fell in between Cts of Res and Sus mice were excluded. Using these criteria, 23 mice were defined as Res and 24 as Sus (Fig. 3C). The 1:1 distribution of Res:Sus N2 mice suggests that a single dominant locus, provisionally named attenuation of the virus titers (Avt), drives retroviral resistance in YBR mice. Whereas YBR mice inherit the resistant allele, BALB/cJ mice inherit the susceptible allele of the gene mapped within the locus.
To map Avt, we performed a genome-wide scan that employed the Mini Mouse Universal Genotyping Array (MiniMUGA) platform (52) using DNA from 23 Res and 24 Sus N2 mice. As the YBR Avt allele is dominant and the BALB/cJ Avt allele is recessive, N2 Res animals were expected to be heterozygous (Aa), while N2 Sus animals were expected to be homozygous (aa) within the region encoding for the gene (Fig. 3B). Linkage was found on chromosome 18 from megabase (Mb) 50 and the end of the chromosome, as >65% of resistant mice and <30% of susceptible mice were heterozygous within this region (Fig. 3D). Thus, YBR resistance to MMTV is controlled by a single, dominant locus mapped to the distal part of chromosome 18, which coincides with the location of sting1. The region of interest on chromosome 18 (50 Mb end of chromosome) has 326 coding variants (SNPs and indels) unique to YBR mice compared to mice known to have the susceptible allele of Avt (BALB/c, C57BL/6J, C3H/HeN, and 129/SvImJ) (data not shown). Future studies will focus on fine mapping and the identification of Avt.
Neonatally MMTV-infected YBR mice do not develop oral tolerance to the virus
For successful transmission, retroviruses employ various mechanisms to evade host immune responses. Orally transmitted MMTV induces neonatal oral tolerance, so infected mice from susceptible strains are unresponsive to viral antigens (19). Induction of neonatal oral tolerance to the virus requires the production of immunosuppressive cytokine IL-10 in a TLR4/IL-6 dependent fashion (the subversion pathway) (19, 20). The virus stimulates the subversion pathway by incorporating mammalian lipopolysaccharide (LPS)-binding receptors into its membrane during viral egress (36). LPS binding receptors enable MMTV virions to bind LPS and activate the subversion pathway by triggering TLR4 (19, 36). When MMTV-susceptible mice were genetically manipulated to lack a component of the subversion pathway, they did not develop neonatal oral tolerance to viral antigens and eliminated the virus (19, 20, 53).
It was possible that YBR mice do not develop neonatal oral tolerance because they lack a component of the subversion pathway. To test whether the subversion pathway is intact in YBR mice, nucleated splenocytes from YBR and BALB/cJ mice were incubated with LPS and IL-6 and IL-10 production was analyzed. YBR splenocytes produced IL-6 (Fig. 4A) and IL-10 (Fig. 4B) to levels equal to BALB/cJ splenocytes, indicating that the subversion pathway is unaffected in YBR mice.
To test whether virus elimination in YBR mice coincided with the lack of neonatal virus tolerance, we immunized infected or uninfected YBR and BALB/cJ mice with detergent-treated MMTV virions mixed with complete Freund’s adjuvant and assayed their ability to produce anti-virus Abs. As previously shown, both BALB/cJ and YBR mice fostered on MMTV-laden milk failed to produce anti-virus Abs (Fig. 4C). However, in contrast to BALB/cJ mice, YBR mice produced anti-virus Abs upon immunization, indicating that they were not tolerant to viral antigens (Fig. 4C).
Taken together, data presented here suggest that MMTV is unable to induce neonatal oral tolerance in YBR mice, even in the presence of an intact subversion pathway. The lack of tolerance to viral antigens allows anti-virus response to be initiated and sustained in YBR mice, leading to virus elimination. This mechanism is adaptive and unrelated to the best currently characterized anti-retrovirus responses, namely, virus-neutralizing Abs, cytotoxic CD8+ T cells, NK cells, and NK T cells. We hypothesized that the resistant allele of the Avt gene mapped to chromosome 18 prevents the development of neonatal oral tolerance to the virus in YBR mice. The identity of Avt and the mechanism which leads to virus elimination in the absence of viral tolerance is the focus of future studies.
MATERIALS AND METHODS
Mice
Mice utilized in this study were bred and maintained at the animal facility of the University of Chicago. Males and females were used at a 50:50 ratio in all experiments. The studies described herein have been reviewed and approved by the Animal Care and Use Committee at the University of Chicago, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International).
YBR mice were purchased from TJL and were bred and maintained at the University of Chicago. C57BL/6J mice deficient in TLR3 (54), TLR4 (51), TLR9 (55), TRIF (56), Caspase 1/11 (57), IFN (58), IL-18 (59), IL-1R (60), Prf (61), 2M (62), CD8a (63), and cGAS (64) purchased from TJL and TLR7 (65), MyD88 (66), Ifnr1 (41), STING (50) obtained from Dr. Derry Roopenian (TJL), Dr. Ruslan Medzhitov (Yale University), Dr. Daniel Portnoy (University of California at Berkley) and Dr. Akiko Iwasaki (Yale University), respectively, were backcrossed to YBR mice for 10 generations to create mice with corresponding deficiencies on the YBR background. C3H/HeN mice were originally purchased from the National Cancer Institute Frederick Cancer Research Facility, Frederick, MD, USA, and maintained in a colony at the University of Chicago. C3H/HeJ mice were purchased from TJL and bred and maintained at the University of Chicago.
“Clean” YBR.Sting−/− mice were generated using CRISPR/Cas9 technology. Two guide RNAs targeting exon 2 (5′-ACACCCTTGACTCCTAACCT-3′) and intron 2–3 (5′-CACCTAGCCTCGCACGAACT-3′) were co-injected into single cell (YBR × C57BL/6J)F1 embryos along with Cas9. To identify mice with the targeted YBR Sting1 allele, F1 mice containing disrupted alleles were backcrossed to YBR using a speed-congenic approach. At each generation, mice carrying the disrupted Sting1 allele were genome-wide scanned using markers to identify backcross offspring with the highest levels of ancestry for the YBR genetic background. A founder line had a 126-bp deletion in the YBR Sting1 allele resulting in the canonical splice donor site of exon 2 being deleted and a premature stop codon generated due to a frameshift mutation (Fig. 2C). The deletion was confirmed at both DNA and RNA levels. Primers flanking the excised region (F: 5′-GCCAAACATCCAACTGAGGT-3′ and R: 5′-AGAGGTCACCGCTCCAAATA-3′) were used to genotype the founder mice and subsequent offspring.
Viral infection
The MMTV(C3H) viral variant was used for infection. The virus was propagated in C3H/HeN mice (67). Mice were infected by fostering on viremic females (the natural mechanism of infecting mice, as the virus is transmitted via the milk). Fostered mice are termed G0. To track virus fate, subsequent generations (G1-n) were generated. To produce N2 mice, (YBR × BALB/cJ)F1 females were fostered on viremic C3H/HeN females and bred to BALB/cJ males.
Immunization and Ab detection
Eight-week-old mice were immunized with 0.5% Triton X-100-treated MMTV(C3H) purified from milk in complete Freund’s adjuvant by subcutaneous injection in four locations in the back and one foot pad as before (19). Mice were challenged with the same dose of 0.5% Triton X-100-treated virus purified from milk in incomplete Freund’s adjuvant intraperitoneally 10 days after initial immunization and sera was collected 10 days post-boost immunization. Pre-immune sera were collected from infected or uninfected mice prior to initial immunization.
Ab production in response to MMTV was compared by ELISA as previously described (23). Briefly, MMTV virions obtained from milk from infected mice were isolated via sucrose cushion, freeze-thawed, and bound to plastic in borate-buffered saline overnight. About 10% fetal calf serum diluted in PBS was used as the blocking reagent. Mouse serum samples were diluted 1:200 and incubated at room temperature for 1 h, followed by incubation with donkey anti-mouse IgGs (Jackson ImmunoResearch) coupled to horseradish peroxidase. All samples were run in triplicate. Background optical density (OD450) values from incubation with secondary antibody alone were subtracted.
IL-6 and IL-10 production assays
Red blood cell-lysed splenocytes (3 × 106) were incubated with 10 ng/mL LPS from Escherichia coli, serotype O55:B5 (Enzo Life Sciences) in 300 L Click’s medium (Irvine Scientific) supplemented with 5% fetal calf serum (FCS) in 48-well plates for 14 h (IL-6) or 18 h (IL-10). IL-6 and IL-10 were measured in supernatants by ELISA MAX kits (BD Biosciences) as per the manufacturer’s protocols.
FACS analysis
The superantigen (SAg) of MMTV(C3H) has specificity for TCRs with a V14 chain (12). Deletion of CD4+V14+ SAg-cognate T cells was used to confirm MMTV infection. Mice were bled at 8 weeks of age and leukocytes were recovered from heparinized blood samples by centrifugation through a Ficoll-Hypaque cushion. Leukocytes were stained with FITC-coupled monoclonal antibody (mAb) against the V14 TCR chain, PE-coupled mAb against the V6 TCR chain, and APC-coupled mAb against CD4 (all from BioLegend). Peripheral blood lymphocytes were analyzed using a Fortessa (BD Biosciences) flow cytometer and FlowJo software (BD Biosciences).
Splenic genomic DNA isolation and MMTV(C3H) qPCR
To quantify integrated proviruses splenic, DNA was subjected to quantitative PCR using MMTV(C3H) LTR-specific TaqMan assay (ThermoFisher). Beta-actin-specific TaqMan probe was used for normalization. All samples were run in duplicate.
Chromosome 18 scan in backcrossed YBR.Sting-deficient mice
Genomic DNA from YBR mice was subjected to high-throughput whole-genome sequencing using the Illumina HiSeq platform with 20× coverage. To determine the genetic composition of chromosome 18 in backcrossed YBR.Sting-deficient mice, the chromosome was scanned with a series of PCR primers discriminating the length of polymorphic fragments produced from YBR and 129 mice. To design the PCR primers, insertions/deletions (indels) across the two strains of mice were identified. Genomic YBR reads were pairwise aligned to the reference genome (129, GRCm38/mm10) using Bowtie2. Then, reads aligned to chromosome 18 were extracted with Samtools and realigned to the reference genome using BWA-MEM to verify quality of alignment. Indels were called using the Haplotype Caller functions in GATK. Forward and reverse PCR primers were designed to encompass identified indels using SnapGene software.
Genome-wide scan of N2 mice and analysis of genome-wide scan data
Tail DNA samples collected from selected susceptible and resistant N2 mice were subjected to genome-wide SNP analysis. Genome-wide SNP analysis was carried out using the MiniMUGA platform offered by Transnetyx Inc. (Cordova, TN, USA). Percent heterozygosity at each SNP within was calculated using R Studio.
ACKNOWLEDGMENTS
The authors thank members of the Golovkina and Chervonsky labs for helpful discussions and Nik Bachmann for technical support. The authors also thank Dr Cameron MacDearmid for genotyping YBR.β2M-deficient mice during backcrossing. H.A.B. was supported by the Duchossois Family Institute at the University of Chicago.
This work was supported by P30 CA014599 and by the National Center for Advancing Translational Sciences of the National Institutes of Health through UL1 TR000430 to the University of Chicago. Whole-genome sequencing of mouse strains was performed by the University of Chicago Genomics Facility.
H.A.B., A.S., and T. G. performed most of the experiments reported in the paper. J.S. designed the CRISPR/Cas9 approach to target STING. F.B. identified single nucleotide polymorphisms between 129 and YBR genomes, designed primers, and performed the genetic scan of backcrossed mice for generation of YBR “clean” Sting KO mice. V.B. aided in computational analyses. N.N. aided in ELISA experiments. H.A.B. and T.G. wrote the manuscript. T.G. conceived the project.
Contributor Information
Tatyana Golovkina, Email: tgolovki@bsd.uchicago.edu.
Viviana Simon, Icahn School of Medicine at Mount Sinai, New York, New York, USA .
REFERENCES
- 1. Kane M, Golovkina TV. 2019. Mapping viral susceptibility loci in mice. Annu Rev Virol 6:525–546. doi: 10.1146/annurev-virology-092818-015544 [DOI] [PubMed] [Google Scholar]
- 2. Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. 1999. Organogenic role of B lymphocytes in mucosal immunity. Science 286:1965–1968. doi: 10.1126/science.286.5446.1965 [DOI] [PubMed] [Google Scholar]
- 3. Dzuris JL, Golovkina TV, Ross SR. 1997. Both T and B cells shed infectious mouse mammary tumor virus. J Virol 71:6044–6048. doi: 10.1128/JVI.71.8.6044-6048.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vacheron S, Luther SA, Acha-Orbea H. 2002. Preferential infection of immature dendritic cells and B cells by mouse mammary tumor virus. J Immunol 168:3470–3476. doi: 10.4049/jimmunol.168.7.3470 [DOI] [PubMed] [Google Scholar]
- 5. Acha-Orbea H, MacDonald HR. 1995. Superantigens of mouse mammary tumor virus. Annu Rev Immunol 13:459–486. doi: 10.1146/annurev.iy.13.040195.002331 [DOI] [PubMed] [Google Scholar]
- 6. Chervonsky AV, Xu J, Barlow AK, Khery M, Flavell RA, Janeway CA. 1995. Direct physical interaction involving CD40 ligand on T cells and CD40 on B cells is required to propagate MMTV. Immunity 3:139–146. doi: 10.1016/1074-7613(95)90166-3 [DOI] [PubMed] [Google Scholar]
- 7. Golovkina TV, Chervonsky A, Dudley JP, Ross SR. 1992. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell 69:637–645. doi: 10.1016/0092-8674(92)90227-4 [DOI] [PubMed] [Google Scholar]
- 8. Held W, Waanders GA, Shakhov AN, Scarpellino L, Acha-Orbea H, MacDonald HR. 1993. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell 74:529–540. doi: 10.1016/0092-8674(93)80054-i [DOI] [PubMed] [Google Scholar]
- 9. Golovkina TV, Dudley JP, Jaffe AB, Ross SR. 1995. Mouse mammary tumor viruses with functional superantigen genes are selected during in vivo infection. Proc Natl Acad Sci U S A 92:4828–4832. doi: 10.1073/pnas.92.11.4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Golovkina TV, Dudley JP, Ross SR. 1998. B and T cells are required for mouse mammary tumor virus spread within the Mammary gland. J Immunol 161:2375–2382. doi: 10.4049/jimmunol.161.5.2375 [DOI] [PubMed] [Google Scholar]
- 11. Dudley JP, Golovkina TV, Ross SR. 2016. Lessons learned from mouse mammary tumor virus in animal models. ILAR J 57:12–23. doi: 10.1093/ilar/ilv044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marrack P, Kushnir E, Kappler J. 1991. A maternally inherited superantigen encoded by a mammary tumour virus. Nature 349:524–526. doi: 10.1038/349524a0 [DOI] [PubMed] [Google Scholar]
- 13. Ignatowicz L, Kappler J, Marrack P. 1992. The effects of chronic infection with a superantigen-producing virus. J Exp Med 175:917–923. doi: 10.1084/jem.175.4.917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacDearmid CC, Case LK, Starling CL, Golovkina TV. 2006. Gradual elimination of retroviruses in YBR/Ei mice. J Virol 80:2206–2215. doi: 10.1128/JVI.80.5.2206-2215.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golovkina TV. 2000. A novel mechanism of resistance to mouse mammary tumor virus infection. J Virol 74:2752–2759. doi: 10.1128/jvi.74.6.2752-2759.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Billingham RE, Brent L, Medawar PB. 1953. Actively acquired tolerance of foreign cells. Nature 172:603–606. doi: 10.1038/172603a0 [DOI] [PubMed] [Google Scholar]
- 17. Adkins B, Leclerc C, Marshall-Clarke S. 2004. Neonatal adaptive immunity comes of age. Nat Rev Immunol 4:553–564. doi: 10.1038/nri1394 [DOI] [PubMed] [Google Scholar]
- 18. Garcia AM, Fadel SA, Cao S, Sarzotti M. 2000. T cell immunity in neonates. Immunol Res 22:177–190. doi: 10.1385/IR:22:2-3:177 [DOI] [PubMed] [Google Scholar]
- 19. Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. 2011. Successful transmission of a retrovirus depends on the commensal microbiota. Science 334:245–249. doi: 10.1126/science.1210718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jude BA, Pobezinskaya Y, Bishop J, Parke S, Medzhitov RM, Chervonsky AV, Golovkina TV. 2003. Subversion of the innate immune system by a retrovirus. Nat Immunol 4:573–578. doi: 10.1038/ni926 [DOI] [PubMed] [Google Scholar]
- 21. Andervont HB. 1940. Further studies on the susceptibility of hybrid mice to induced and spontaneous tumors. J Natl Cancer Inst 1:135–145. doi: 10.1093/jnci/1.2.135 [DOI] [Google Scholar]
- 22. Andervont HB. 1940. The influence of foster nursing upon the incidence of spontaneous mammary cancer in resistant and susceptible mice. J Natl Cancer Inst:147–153. doi: 10.1093/jnci/1.2.147 [Google Scholar]
- 23. Purdy A, Case L, Duvall M, Overstrom-Coleman M, Monnier N, Chervonsky A, Golovkina T. 2003. Unique resistance of I/LnJ mice to a retrovirus is due to sustained interferon gamma-dependent production of virus-neutralizing antibodies. J Exp Med 197:233–243. doi: 10.1084/jem.20021499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Denzin LK, Khan AA, Virdis F, Wilks J, Kane M, Beilinson HA, Dikiy S, Case LK, Roopenian D, Witkowski M, Chervonsky AV, Golovkina TV. 2017. Neutralizing antibody responses to viral infections are linked to the Non-classical MHC class II gene H2-Ob. Immunity 47:310–322. doi: 10.1016/j.immuni.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Melián A, Beckman EM, Porcelli SA, Brenner MB. 1996. Antigen presentation by CD1 and MHC-encoded class I-like molecules. Curr Opin Immunol 8:82–88. doi: 10.1016/s0952-7915(96)80109-9 [DOI] [PubMed] [Google Scholar]
- 26. Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. 1990. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature 344:742–746. doi: 10.1038/344742a0 [DOI] [PubMed] [Google Scholar]
- 27. Höglund P, Ohlén C, Carbone E, Franksson L, Ljunggren HG, Latour A, Koller B, Kärre K. 1991. Recognition of beta 2-microglobulin-negative (beta 2M-) T-cell blasts by natural killer cells from normal but not from beta 2M- mice: nonresponsiveness controlled by beta 2M- bone marrow in chimeric mice. Proc Natl Acad Sci U S A 88:10332–10336. doi: 10.1073/pnas.88.22.10332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. 1991. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science 253:199–202. doi: 10.1126/science.1853205 [DOI] [PubMed] [Google Scholar]
- 29. Osińska I, Popko K, Demkow U. 2014. Perforin: an important player in immune response. Cent Eur J Immunol 39:109–115. doi: 10.5114/ceji.2014.42135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Medzhitov R, Janeway CA. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295–298. doi: 10.1016/s0092-8674(00)80412-2 [DOI] [PubMed] [Google Scholar]
- 31. Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol 11:373–384. doi: 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- 32. Ichinohe T, Pang IK, Iwasaki A. 2010. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol 11:404–410. doi: 10.1038/ni.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pertel T, Hausmann S, Morger D, Züger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, Bisiaux A, Albert ML, Strambio-De-Castillia C, Mothes W, Pizzato M, Grütter MG, Luban J. 2011. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472:361–365. doi: 10.1038/nature09976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fletcher AJ, Vaysburd M, Maslen S, Zeng J, Skehel JM, Towers GJ, James LC. 2018. Trivalent RING assembly on retroviral Capsids activates TRIM5 ubiquitination and innate immune signaling. Cell Host Microbe 24:761–775. doi: 10.1016/j.chom.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xagorari A, Chlichlia K. 2008. Toll-like receptors and viruses: induction of innate antiviral immune responses. Open Microbiol J 2:49–59. doi: 10.2174/1874285800802010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilks J, Lien E, Jacobson AN, Fischbach MA, Qureshi N, Chervonsky AV, Golovkina TV. 2015. Mammalian lipopolysaccharide receptors incorporated into the retroviral envelope augment virus transmission. Cell Host Microbe 18:456–462. doi: 10.1016/j.chom.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chesler DA, Reiss CS. 2002. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev 13:441–454. doi: 10.1016/s1359-6101(02)00044-8 [DOI] [PubMed] [Google Scholar]
- 38. Cantin E, Tanamachi B, Openshaw H. 1999. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol 73:3418–3423. doi: 10.1128/JVI.73.4.3418-3423.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742–1745. doi: 10.1126/science.8456301 [DOI] [PubMed] [Google Scholar]
- 40. Lu B, Ebensperger C, Dembic Z, Wang Y, Kvatyuk M, Lu T, Coffman RL, Pestka S, Rothman PB. 1998. Targeted disruption of the interferon-gamma receptor 2 gene results in severe immune defects in mice. Proc Natl Acad Sci U S A 95:8233–8238. doi: 10.1073/pnas.95.14.8233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. 1994. Functional role of type I and type II Interferons in antiviral defense. Science 264:1918–1921. doi: 10.1126/science.8009221 [DOI] [PubMed] [Google Scholar]
- 42. Iwashiro M, Peterson K, Messer RJ, Stromnes IM, Hasenkrug KJ. 2001. CD4(+) T cells and gamma interferon in the long-term control of persistent friend retrovirus infection. J Virol 75:52–60. doi: 10.1128/JVI.75.1.52-60.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Case LK, Petell L, Yurkovetskiy L, Purdy A, Savage KJ, Golovkina TV. 2008. Replication of beta- and gammaretroviruses is restricted in I/Lnj mice via the same genetic mechanism. J Virol 82:1438–1447. doi: 10.1128/JVI.01991-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rex DAB, Keshava Prasad TS, Kandasamy RK. 2022. Revisiting regulated cell death responses in viral infections. Int J Mol Sci 23:7023. doi: 10.3390/ijms23137023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuriakose T, Kanneganti TD. 2019. Pyroptosis in antiviral immunity. Curr Top Microbiol Immunol. doi: 10.1007/82_2019_189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Orzalli MH, Smith A, Jurado KA, Iwasaki A, Garlick JA, Kagan JC. 2018. An antiviral branch of the IL-1 signaling pathway restricts immune-evasive virus replication. Mol Cell 71:825–840. doi: 10.1016/j.molcel.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zalinger ZB, Elliott R, Weiss SR. 2017. Role of the Inflammasome-related cytokines Il-1 and Il-18 during infection with murine coronavirus. J Neurovirol 23:845–854. doi: 10.1007/s13365-017-0574-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Van Den Eeckhout B, Tavernier J, Gerlo S. 2020. Interleukin-1 as innate mediator of T cell immunity. Front Immunol 11:621931. doi: 10.3389/fimmu.2020.621931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakanishi K. 2018. Unique action of interleukin-18 on T cells and other immune cells. Front Immunol 9:763. doi: 10.3389/fimmu.2018.00763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ishikawa H, Ma Z, Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788–792. doi: 10.1038/nature08476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCR mice: mutations in Tlr4 gene. Science 282:2085–2088. doi: 10.1126/science.282.5396.2085 [DOI] [PubMed] [Google Scholar]
- 52. Sigmon JS, Blanchard MW, Baric RS, Bell TA, Brennan J, Brockmann GA, Burks AW, Calabrese JM, Caron KM, Cheney RE, Ciavatta D, Conlon F, Darr DB, Faber J, Franklin C, Gershon TR, Gralinski L, Gu B, Gaines CH, Hagan RS, Heimsath EG, Heise MT, Hock P, Ideraabdullah F, Jennette JC, Kafri T, Kashfeen A, Kulis M, Kumar V, Linnertz C, Livraghi-Butrico A, Lloyd KCK, Lutz C, Lynch RM, Magnuson T, Matsushima GK, McMullan R, Miller DR, Mohlke KL, Moy SS, Murphy CEY, Najarian M, O’Brien L, Palmer AA, Philpot BD, Randell SH, Reinholdt L, Ren Y, Rockwood S, Rogala AR, Saraswatula A, Sassetti CM, Schisler JC, Schoenrock SA, Shaw GD, Shorter JR, Smith CM, St Pierre CL, Tarantino LM, Threadgill DW, Valdar W, Vilen BJ, Wardwell K, Whitmire JK, Williams L, Zylka MJ, Ferris MT, McMillan L, Manuel de Villena FP. 2020. Content and performance of the miniMUGA genotyping array: a new tool to improve rigor and reproducibility in mouse research. Genetics 216:905–930. doi: 10.1534/genetics.120.303596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilks J, Beilinson H, Golovkina TV. 2013. Dual role of commensal bacteria in viral infections. Immunol Rev 255:222–229. doi: 10.1111/imr.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. 2001. Recognition of double-stranded RNA and activation of nf-kappaB by toll-like receptor 3. Nature 413:732–738. doi: 10.1038/35099560 [DOI] [PubMed] [Google Scholar]
- 55. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. 2000. A toll-like receptor recognizes bacterial DNA. Nature 408:740–745. doi: 10.1038/35047123 [DOI] [PubMed] [Google Scholar]
- 56. Hou B, Reizis B, DeFranco AL. 2008. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity 29:272–282. doi: 10.1016/j.immuni.2008.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. 1995. Altered cytokine export and apoptosis in mice deficient in Interleukin-1 beta converting enzyme. Science 267:2000–2003. doi: 10.1126/science.7535475 [DOI] [PubMed] [Google Scholar]
- 58. Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739–1742. doi: 10.1126/science.8456300 [DOI] [PubMed] [Google Scholar]
- 59. Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383–390. doi: 10.1016/s1074-7613(00)80543-9 [DOI] [PubMed] [Google Scholar]
- 60. Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. 1997. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol 159:3364–3371. [PubMed] [Google Scholar]
- 61. Trapani JA, Smyth MJ. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol 2:735–747. doi: 10.1038/nri911 [DOI] [PubMed] [Google Scholar]
- 62. Koller BH, Marrack P, Kappler JW, Smithies O. 1990. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 248:1227–1230. doi: 10.1126/science.2112266 [DOI] [PubMed] [Google Scholar]
- 63. Fung-Leung WP, Schilham MW, Rahemtulla A, Kündig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW. 1991. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell 65:443–449. doi: 10.1016/0092-8674(91)90462-8 [DOI] [PubMed] [Google Scholar]
- 64. Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, Mar KB, Richardson RB, Ratushny AV, Litvak V, Dabelic R, Manicassamy B, Aitchison JD, Aderem A, Elliott RM, García-Sastre A, Racaniello V, Snijder EJ, Yokoyama WM, Diamond MS, Virgin HW, Rice CM. 2014. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505:691–695. doi: 10.1038/nature12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 3:196–200. doi: 10.1038/ni758 [DOI] [PubMed] [Google Scholar]
- 66. Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150. doi: 10.1016/s1074-7613(00)80596-8 [DOI] [PubMed] [Google Scholar]
- 67. Hook LM, Agafonova Y, Ross SR, Turner SJ, Golovkina TV. 2000. Genetics of mouse mammary tumor virus-induced mammary tumors: linkage of tumor induction to the gag gene. J Virol 74:8876–8883. doi: 10.1128/jvi.74.19.8876-8883.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]