Abstract
Purpose:
The underlying premise of prostate cancer active surveillance (AS) is that cancers likely to metastasize will be recognized and eliminated before cancer-related disease can ensue. Our study was designed to determine the prostate cancer upgrading rate when biopsy guided by magnetic resonance imaging (MRGBx) is used before entry and during AS.
Materials and Methods:
The cohort included 519 men with low- or intermediate-risk prostate cancer who enrolled in prospective studies (NCT00949819 and NCT00102544) between February 2008 and February 2020. Subjects were preliminarily diagnosed with Gleason Grade Group (GG) 1 cancer; AS began when subsequent MRGBx confirmed GG1 or GG2. Participants underwent confirmatory MRGBx (targeted and systematic) followed by surveillance MRGBx approximately every 12 to 24 months. The primary outcome was tumor upgrading to ≥GG3.
Results:
Upgrading to ≥GG3 was found in 92 men after a median followup of 4.8 years (IQR 3.1–6.5) after confirmatory MRGBx. Upgrade-free probability after 5 years was 0.85 (95% CI 0.81–0.88). Cancer detected in a magnetic resonance imaging lesion at confirmatory MRGBx increased risk of subsequent upgrading during AS (HR 2.8; 95% CI 1.3–6.0), as did presence of GG2 (HR 2.9; 95% CI 1.1e8.2) In men who upgraded ≥GG3 during AS, upgrading was detected by targeted cores only in 27%, systematic cores only in 25% and both in 47%. In 63 men undergoing prostatectomy, upgrading from MRGBx was found in only 5 (8%).
Conclusions:
When AS begins and follows with MRGBx (targeted and systematic), upgrading rate (≥GG3) is greater when tumor is initially present within a magnetic resonance imaging lesion or when pathology is GG2 than when these features are absent.
Keywords: image-guided biopsy, prostatic neoplasms, observation, magnetic resonance imaging
Study Need and Importance:
Active surveillance (AS) has become a preferred strategy for managing men with low-risk prostate cancer (PCa). Improved entry and followup criteria could help eliminate men up front who are destined to require active treatment and, during surveillance, identify those remaining men who progress. In most AS programs, men have entered based on an ultrasoundguided biopsy (USGBx) revealing Grade Group (GG) 1 PCa. However, in part because of the limitations of USGBx many men thus biopsied will ultimately exhibit PCa of increased risk and require active treatment. Biopsies employing guidance by magnetic resonance imaging (MRGBx) provide characterization of prostate pathology which is more accurate than USGBx. Thus, use of MRGBx is of increasing interest for men before entry and during AS.
What We Found:
We studied a cohort of 519 men on AS with low- or intermediate-risk PCa who enrolled in prospective studies between February 2008 and February 2020. All men underwent confirmatory MRGBx (targeted and systematic), followed by surveillance MRGBx approximately every 24 months. We found that cancer detected in a magnetic resonance imaging (MRI)-visible lesion at confirmatory MRGBx increased risk of subsequent upgrading during AS, as did presence of GG2 (see figure). In men undergoing prostatectomy after AS, upgrading from the most recent MRGBx was found in only 8%.
Limitations:
Limitations include lack of a comparator arm of men confirmed and followed with USGBx, and the results are from 2 centers with in-depth experience with MRGBx, potentially limiting generalizability.
Interpretation for Patient Care:
When AS begins and follows with MRGBx (targeted and systematic), upgrading rate (≥GG3) is greater when tumor is initially present within an MRI-visible lesion or when pathology is GG2 than when these features are absent.
Graphical Abstract
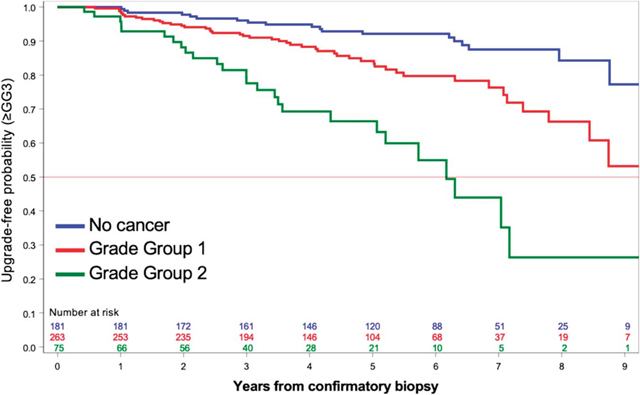
Figure. Probability of freedom from upgrading to ≥GG3 during AS of PCa after an MRI-guided confirmatory biopsy that showed no cancer (blue), GG1 (red) or GG2 (green).
Active surveillance (AS) has become the preferred strategy for managing men with low-risk prostate cancer (PCa).1,2 The proportion of men with low-risk PCa managed by AS increased from 15% in 2010 to 42% in 2015.3 When patients are selected and followed carefully, nearly all will avoid PCa-related disease in the near and intermediate term.4,5 In one study of 1,818 men with low-risk PCa who were followed in AS for many years, only 4 men died from PCa.6 However, the rate of definitive treatment in these men was 48% at 10 years.
Improved entry and followup criteria could help eliminate men up front who are destined to require active treatment and, during surveillance, identify those remaining men who progress. In most AS programs, men have entered on the basis of an ultrasound-guided biopsy (USGBx) revealing a cancer with Gleason Score of 6 (Gleason grade group [GG] 1), ie low risk, with periodic biopsies to confirm that low risk is maintained. However, because of the limitations of USGBx, many men thus biopsied will ultimately exhibit PCa of increased risk and require active treatment.6,7 Biopsies employing guidance by magnetic resonance imaging (MRGBx) provide characterization of prostate pathology which is more accurate than USGBx.8–10 Thus, use of MRGBx is of increasing interest for use in men before entry and during AS.11–15
The 2 centers reporting here initiated AS programs at approximately the same period when their work with MRGBx began.8,16 During that interval, prospectively acquired databases structured around MRGBx have been maintained at each site. In contrast to other studies, all patients in this large, combined series began AS with a confirmatory MRGBx; all patients had MRGBx at scheduled intervals per protocol during years of AS; at each biopsy session both targeted and systematic biopsies were obtained; and followup is among the longest yet reported using serial MRGBx to detect upgrading.
METHODS
Study Design
This cohort study used prospectively collected data from 2 institutions (University of California, Los Angeles [UCLA; NCT00949819] and National Cancer Institute [NCI; NCT00102544]) between February 2008 and February 2020. Both studies were approved by the respective institutional review boards to evaluate the use of image fusion devices for targeted biopsies (IRB No. 11–002281). The electromagnetic tracking device used at NCI is now commercially available as the UroNav® platform (Philips Healthcare, Eindhoven, Noord-Brabant, Netherlands) and the mechanical tracking image-fusion device used at UCLA is commercially available as the Artemis platform (Eigen, Grass Valley, California). Investigator experience with the MRI-ultrasound fusion systems has been described previously.17,18
Patients
All patients in this study had an initial diagnostic biopsy of GG1 PCa, obtained by various methods in community settings. Within 12 months of initial diagnostic biopsy subjects underwent confirmatory MRGBx. Patients were included in the analytical cohort if confirmatory biopsy revealed ≤GG2 cancer and at least 1 followup surveillance biopsy was obtained. Patients were excluded if they received immediate PCa treatment, were followed up elsewhere, withdrew consent or died from a cause unrelated to PCa. A flow diagram of participants is shown in figure 1.
Figure 1.
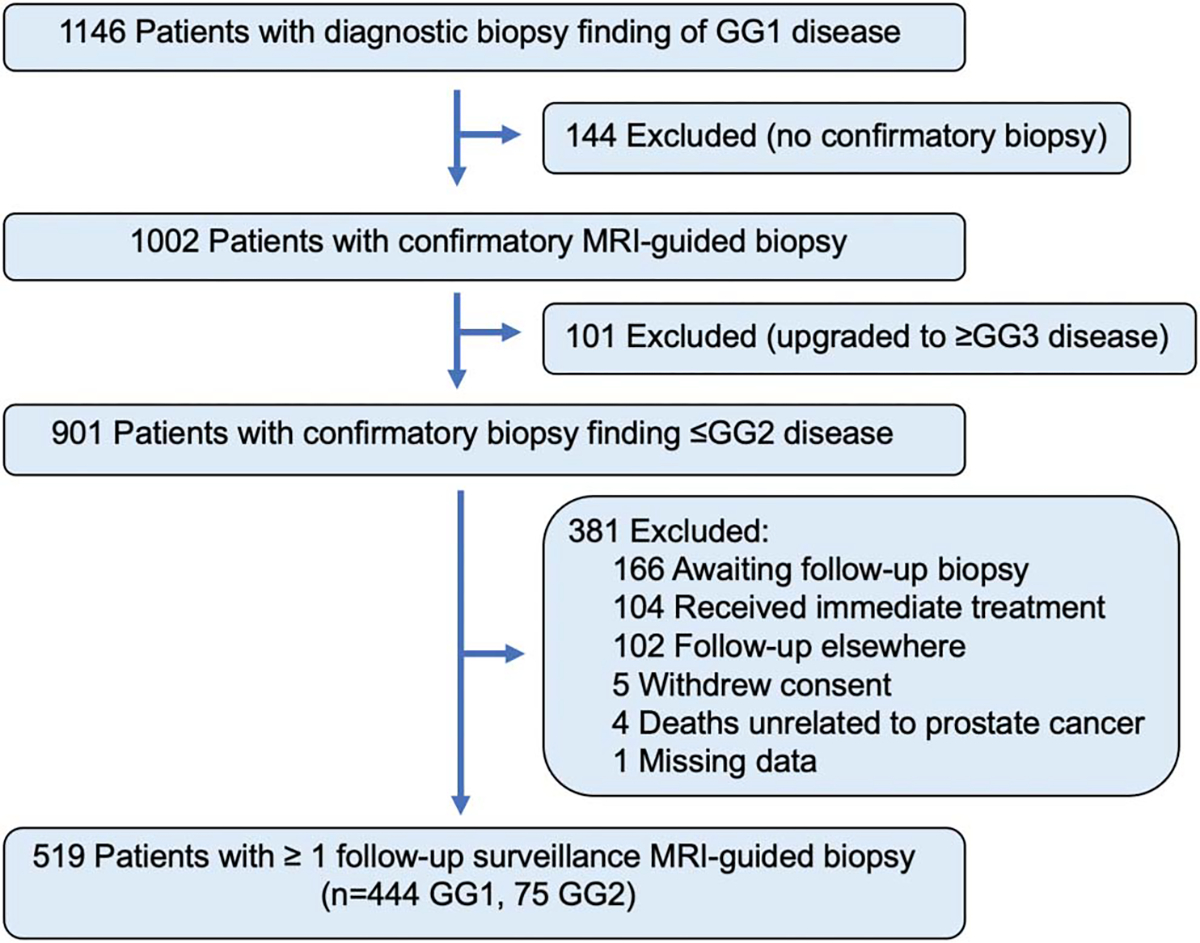
Flow diagram of participants.
Imaging and Biopsy Methods
All patients underwent 3.0 Tesla multiparametric magnetic resonance imaging (MRI) as previously described.8,15
All biopsies were performed using MRI-ultrasound fusion technology (Artemis at UCLA and UroNav or a pre-UroNav prototype at NCI). Each biopsy procedure combined conventional 12-core systematic sampling in addition to targeted biopsy directed to MRI visible lesions (at least 2 cores per Prostate Imaging–Reporting and Data System [PI-RADS®] 3–5 lesion).19 Targeted and systematic cores were obtained at each biopsy session per the protocol of each institution. MRI and pathology interpretations were performed by expert genitourinary radiologists and pathologists. Prior to the adoption of the PI-RADS scoring system, Likert scoring systems were used.20,21
Followup
After confirmatory biopsy, patients were monitored with digital rectal examination and prostate specific antigen (PSA) testing approximately every 12 to 24 months. Patients also underwent multiparametric MRI and MRI-guided surveillance biopsies approximately every 12 to 24 months.
Outcomes
The main outcome of interest was pathological upgrading of PCa to ≥GG3, a point at which AS is generally considered inappropriate. The secondary outcome was pathological upgrading to ≥GG2. Whole mount pathology was used to determine the pathological GG in men undergoing radical prostatectomy after AS.
Statistical Analyses
We conducted survival analyses for upgrading to ≥GG3 as well as ≥GG2 and present upgrade-free probabilities and Kaplan-Meier curves. Log-rank test was used to test strata. We calculated upgrade-free probabilities and 95% Wald confidence intervals in SAS® (SAS, Cary, North Carolina). Continuous variables that were not normally distributed were tested between groups using the Wilcoxon rank-sum or median test as appropriate. We conducted Cox proportional hazard regression to calculate the hazard ratios of upgrading, controlling for patient, biopsy and MRI characteristics from the confirmatory biopsy. We a priori chose the following variables to include in the regression: age, ethnicity (nonHispanic White vs African American vs Asian vs other/unknown), family history of PCa, PSA, PSA density (≥0.15 vs <0.15 ng/ml/cc), maximum cancer core length, number of positive systematic cores (0 vs 1 vs ≥2), number of positive targeted cores (0 vs 1 vs ≥2) and GG.
RESULTS
Characteristics of Patients
Patient characteristics at time of MRI-guided confirmatory biopsy are shown in table 1. From February 2008 to January 2019, a total of 519 patients (UCLA, 330; National Institutes of Health [NIH], 189) met inclusion criteria, signed consent forms and were enrolled in the study.
Table 1.
Baseline patient characteristics
| Total | UCLA | NIH | p Value | |
|---|---|---|---|---|
|
| ||||
| Total No. pts | 519 | 330 | 189 | |
| Mean yrs age (SD) | 62.2 (7.3) | 62.8 (7.6) | 61.2 (6.6) | 0.02 |
| No. ethnicity (%): | <0.01 | |||
| NonHispanic White | 414 (80) | 255 (77) | 159 (84) | |
| African American | 41 (8) | 21 (6) | 20 (11) | |
| Asian | 24 (5) | 20 (6) | 4 (2) | |
| Other/unknown | 40 (8) | 34 (10) | 6 (3) | |
| No. family history of PCa (%) | 145 (28) | 79 (24) | 66 (35) | <0.01 |
| No. abnormal rectal exam (%) | 12 (2) | 12 (4) | - | - |
| Median ng/ml PSA (IQR) | 5.2 (3.0–8.1) | 5.6 (2.7–8.3) | 5.0 (3.7–7.2) | 0.57 |
| Median cc MRI volume (IQR) | 48 (35–68) | 48 (35–71) | 48 (37–66) | 0.77 |
| No. ng/ml/cc PSA density (%): | 0.62 | |||
| <0.15 | 417 (80) | 263 (80) | 154 (81) | |
| ≥0.15 | 102 (20) | 67 (20) | 35 (19) | |
| No. MRI score (%): | 0.15 | |||
| Negative (1 or 2) | 156 (30) | 103 (31) | 53 (28) | |
| 3 | 226 (44) | 137 (42) | 89 (47) | |
| 4 | 105 (20) | 74 (22) | 31 (16) | |
| 5 | 31 (6) | 16 (5) | 15 (8) | |
| Median mm max Ca core length of highest tumor grade (IQR) | 2.5 (1.0–4.2) | 2.0 (1.0–4.0) | 3.0 (1.3–5.0) | 0.18 |
| Median No. systematic cores taken (IQR) | 12 (12–13) | 12 (11–13) | 12 (12–14) | < 0.01 |
| No. pos systematic cores (%): | 0.17 | |||
| 0 | 230 (45) | 146 (45) | 84 (44) | |
| 1 | 146 (29) | 99 (31) | 47 (25) | |
| >2 | 135 (26) | 24 (77) | 58 (31) | |
| Median No. targeted cores taken (IQR)* | 5 (4–6) | 5 (4–6) | 4 (4–6) | 0.77 |
| No. pos targeted cores (%):* | < 0.01 | |||
| 0 | 212 (59) | 145 (64) | 67 (49) | |
| 1 | 61 (17) | 39 (17) | 22 (16) | |
| ≥2 | 88 (24) | 41 (18) | 47 (35) | |
| No. GG (%): | 0.24 | |||
| No Ca | 181 (35) | 116 (35) | 67 (35) | |
| 1 | 263 (51) | 173 (52) | 89 (47) | |
| 2 | 75 (14) | 41 (12) | 33 (17) | |
Values in bold are statistically significant. Baseline Is considered at time of MRI-guided confirmatory biopsy.
From men with MRI score 3–5 only.
MRI-Guided Confirmatory Biopsy
Of the 519 men enrolled, confirmatory biopsy revealed PCa <GG2 in 444 and GG2 in 75 (fig. 1). A median of 5 (IQR 4–6) targeted cores were obtained from subjects with MRI-visible lesions (362) and a median of 12 (IQR 12–13) systematic cores were taken from all men (table 1). Targeted and systematic cores were both required to detect highest GG (supplementary table 2, https://www.jurology.com).
Followup During Surveillance
Followup data were available for outcomes through February 2020. The median followup period was 4.8 years (IQR 3.1–6.5) after MRI-guided confirmatory biopsy (table 2). Patients underwent a median of 1 (range 0–6) followup surveillance MRI study and a median of 2 (range 0–5) followup surveillance biopsy sessions within 5 years after confirmatory biopsy.
Table 2.
Summary of AS followup
| Total | UCLA | NIH | p Value | |
|---|---|---|---|---|
|
| ||||
| Total No. pts | 519 | 330 | 189 | |
| Median yrs length followup (IQR) | 4.8 (3.1–6.5) | 4.6 (3.0–6.4) | 5.0 (3.3–6.5) | 0.22 |
| Followup MRIs within 5 yrs: | <0.01 | |||
| Median total No. (range) | 1 (0–6) | 1 (0–5) | 3 (0–6) | |
| Median yrs between MRIs (IQR) | 1.1 (1.0–1.7) | 2.0 (1.2–2.1) | 1.1 (1.0–1.3) | |
| Followup biopsy sessions within 5 yrs: | <0.01 | |||
| Median total No. (range) | 2 (0–5) | 2 (1–5) | 1 (0–4) | |
| Median yrs between MRIs (IQR) | 1.2 (1.0–2.0) | 1.1 (1.0–2.0) | 1.7 (1.1 –2.0) | |
| No. upgrading at followup biopsy: | < 0.01 | |||
| Upgrading to ≥GG3 | 92 | 39 | 53 | |
| GG3 | 47 | 22 | 25 | |
| GG4 | 33 | 13 | 20 | |
| GG5 | 11 | 4 | 7 | |
| No. upgrading to ≥GG2 (among <GG2):* | 164 | 93 | 71 | < 0.01 |
| GG2 | 115 | 73 | 42 | |
| GG3 | 26 | 11 | 15 | |
| GG4 | 13 | 6 | 7 | |
| GG5 | 9 | 3 | 6 | |
| No. pts upgrading ≥GG3 by biopsy core type (%):† | < 0.01 | |||
| Targeted core only | 25 (2) | 17 (44) | 8 (15) | |
| Systematic core only | 23 (25) | 19 (49) | 4 (8) | |
| Both targeted and systematic cores | 43 (47) | 3 (8) | 40 (75) | |
| Data missing | 1 (1) | 0 (0) | 1 (2) | |
| No. pts upgrading ≥GG2 by biopsy core type (%):‡ | < 0.01 | |||
| Targeted core only | 50 (30) | 41 (44) | 9 (13) | |
| Systematic core only | 49 (30) | 40 (43) | 9 (13) | |
| Both targeted and systematic cores | 63 (38) | 12 (13) | 51 (72) | |
| Data missing | 2 (1) | 0 (0) | 2 (3) | |
| No. received treatment: | 116 | 73 | 43 | < 0.01 |
| Surgery | 67 | 36 | 31 | |
| Radiation therapy | 30 | 18 | 12 | |
| Focal therapy | 15 | 15 | 0 | |
| Other | 4 | 4 | 0 | |
Values in bold are statistically significant.
Of 444 cases.
Of 92 cases.
Of 164 cases.
Risk of Upgrading to ≥GG3
Upgrading to ≥GG3 occurred in 92 men (table 2). The median followup time for men who did not upgrade GG3 was 5.1 years (IQR 3.5–6.7). Upgrade-free probability at 2, 5 and 7 years after confirmatory biopsy were 0.95 (95% CI 0.93–0.96), 0.85 (95% CI 0.81–0.88) and 0.77 (95% CI 0.72–0.82), respectively (fig. 2, upper panel). The upgrade-free probability at 5 years for men who had cancer detected in targeted cores or combined biopsy was 0.75 (95% CI 0.59–0.86) and 0.69 (95% CI 0.56–0.79), respectively (fig. 2, middle panel). For men with cancer detected on systematic cores only, or when no cancer was detected, the upgrade-free probability at 5 years was 0.89 (95% CI 0.79–0.94) and 0.93 (95% CI 0.87–0.97), respectively.
Figure 2.
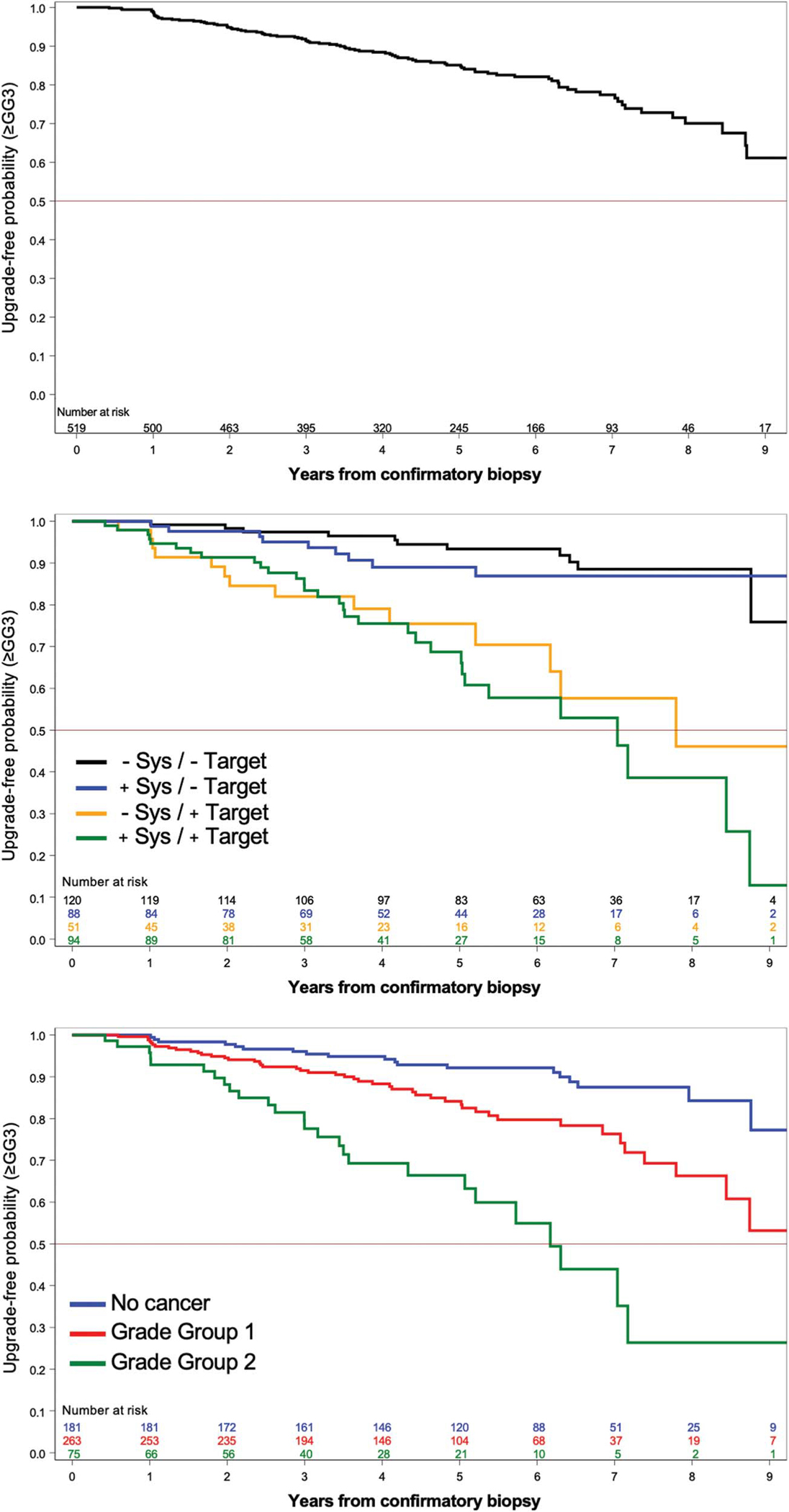
Probability of upgrading-free survival to ≥GG3 during AS of PCa after an MRI-guided confirmatory biopsy. Upper panel, overall probability of upgrading-free survival. Middle panel, probability of upgrading-free survival stratified by cancer-containing core type at confirmatory biopsy (black, negative systematic and negative targeted cores; blue, positive systematic and negative targeted cores; yellow, negative systematic and positive targeted cores; green, positive systematic and positive targeted cores). Lower panel, probability of upgrading-free survival stratified by GG at confirmatory biopsy (blue, no cancer; red, GG1; green, GG2). Sys, systematic biopsy.
At confirmatory MRGBx cancer found in targeted but not systematic cores was associated with tumor upgrading to ≥GG3 during AS (table 3). If tumor was identified in 1 targeted core (HR 2.75; 95% CI 1.25–6.03) or more than 1 targeted core (HR 3.38; 95% CI 1.65–6.91), men were more likely to upgrade during AS than if all targeted cores were cancer-free. Presence of cancer in systematic cores was not independently associated with tumor upgrading. Family history of PCa (HR 1.58; 95% CI 1.02–2.44) and GG2 identified at any confirmatory biopsy (HR 2.93; 95% CI 1.05–8.19) were independently associated with upgrading to ≥GG3. At followup surveillance biopsies upgrading to ≥GG3 was detected by only targeted cores in 27%, only systematic cores in 25% and both in 47% (table 2).
Table 3.
Risk factors of upgrading to ≥GG3 after MRI-guided confirmatory biopsy in 506 cases
| HR (95% CI) | |
|---|---|
|
| |
| Family history of PCa | 1.58 (1.02–2.44) |
| No. pos systematic cores (reference, 0): | |
| 1 | 0.95 (0.45–2.00) |
| ≥2 | 1.27 (0.65–2.50) |
| No. pos targeted cores (reference, 0): | |
| Neg MRI (no targeted cores) | 1.46 (0.79–2.70) |
| 1 | 2.75 (1.25–6.03) |
| ≥2 | 3.38 (1.65–6.91) |
| GG (reference, no cancer): | |
| 1 | 1.40 (0.57–3.44) |
| 2 | 2.93 (1.05–8.19) |
Values in bold are statistically significant.
Risk of Upgrading to ≥GG2
Upgrading to ≥GG2 occurred in 164 men. The median followup time for men who did not upgrade ≥GG2 was 4.7 years (IQR 3.3–6.3). Upgrade-free probability at 2, 5 and 7 years after confirmatory biopsy was 0.83 (95% CI 0.80–0.87), 0.62 (95% CI 0.56–0.67) and 0.55 (95% CI 0.49–0.61), respectively (see supplementary figure, https://www.jurology.com, upper panel).
At confirmatory MRGBx cancer found in targeted but not systematic cores was associated with tumor upgrading to ≥GG2 during AS (supplementary table 1, https://www.jurology.com). If tumor was identified in 1 targeted core (HR 2.07; 95% CI 1.17–3.65) or more than 1 targeted core (HR 1.90; 95% CI 1.10–3.29), men were more likely to upgrade during AS than if all targeted cores were cancer-free. Presence of cancer in systematic cores was not independently associated with tumor upgrading.
Radical Prostatectomy after AS
Of the 519 men entering AS after confirmatory MRGBx, 116 subsequently underwent active treatment. Sixty-seven men underwent radical prostatectomy a median interval of 30.5 months (IQR 17.9, 51.6) after confirmatory MRGBx. Study of excised prostates via whole mount pathology was available in 63 men (94%; table 4). In 58 men (92%), final GG was the same or lower than that found at the preceding MRGBx. Upgrading at surgery (≥GG3) was not associated with duration of AS (p=0.17). The time to surgery was not related to Grade Group (GG1 or GG2) at confirmatory MRGBx (p=0.11). At final pathology, 11 men (15%) had GG4–5 cancer and 2 (3%) had positive lymph nodes.
Table 4.
Radical prostatectomy outcomes after MRI-guided confirmatory biopsy
| Total | |
|---|---|
|
| |
| Total No. pts | 63 |
| No. final pathology after prostatectomy (%): | |
| No Ca | 1 (2) |
| GG1 | 3 (5) |
| GG2 | 41 (65) |
| GG3 | 7 (11) |
| GG4 | 8 (13) |
| GG5 | 3 (5) |
| No. change in final pathology from most recent MRI-guided biopsy (%): | |
| Upgrade | 5 (8) |
| Downgrade | 23 (37) |
| No change | 35 (56) |
| Median mos time to surgery after | |
| a confirmatory biopsy that diagnosed (IQR)*: | |
| Any GG | 30.5 (17.9, 51.6) |
| No Ca | 42.9 (22.6, 65.0) |
| GG1 | 33.0 (19.1, 54.6) |
| GG2 | 22.6 (16.0, 30.8) |
| Median mos time to surgery for final pathology (IQR)*: | |
| <GG3 | 28.2 (16.6, 49.4) |
| ≥GG3 | 35.4 (30.0, 54.8) |
| No. pathological pos lymph nodes at surgery (%)* | 2 (3) |
Of 61 patients.
DISCUSSION
The present work supports and expands that of other AS investigators.1,6,11–14 First, AS proved to be generally safe and effective throughout years of followup. The 5-year upgrading-free probability (≥GG3) was 85%. Second, the value of confirmatory MRGBx as a starting point for AS is established, since some 10% of men who were thought to be candidates for AS on the basis of conventional USGBx were found on MRGBx to have PCa ≥GG3. Third, tumor detected within an MRI lesion deserves special attention during AS, since the chances of tumor upgrading were increased threefold over others. Fourth, the role of both targeted and systematic biopsies during surveillance is reinforced, since 25% of ≥GG3 cancers seen during followup were diagnosed only by systematic biopsy. Fifth, with only an 8% upgrading rate at prostatectomy, MRGBx reliably characterizes the tumor. And sixth, we confirm the need for vigilance when GG2 lesions are surveilled because of a nearly threefold increase in upgrade rate compared to men entering with GG1.
The primary outcome was upgrading to ≥GG3. Men with GG1 or GG2 tumors are considered eligible for AS, but the presence of GG3 excludes AS from consideration.2,15 Men with ≥GG3 disease have significantly greater rates of biochemical recurrence and overall mortality compared to men with ≤GG2.22,23 Thus, MRGBx was used here to exclude men with ≥GG3 at the start of AS and to discontinue AS when ≥GG3 was found during surveillance. Since accurate characterization of prostate pathology is critical for men undergoing AS, and since MRGBx is more sensitive for cancer detection than USGBx,9 these data support the use of MRGBx for selection and followup of men undergoing AS.
The date of confirmatory biopsy was used as the start of AS, differentiating the present study from others which have used initial diagnostic biopsy (any method) as the starting point. When entry biopsies are performed via ultrasound guidance alone, later upgrading during AS may be as high as 30%.6 In the present study only 18% of men with GG1 pathology at confirmatory MRGBx experienced upgrading to ≥GG3 lesions. The results of the present study support the importance of MRGBx to confirm findings of conventional biopsy before entry into AS, and further suggest that such a confirmatory biopsy may be the appropriate starting point for AS.
A cancer-containing targeted core from an MRI lesion at confirmatory biopsy (GG1 or GG2) nearly tripled the risk of tumor upgrading compared to finding no tumor or tumor only in a systematic core. The overall risk of tumor upgrading to ≥GG3 after 7 years of AS was ~40% if cancer was found in a targeted core compared to only ~10% if cancer was found in a systematic core or no cancer was identified (fig. 2).
For men in AS, systematic biopsies provide important information beyond that obtained from targeted biopsy, as suggested by Klotz and colleagues and confirmed herein.24 In the present report, systematic cores detected upgrading missed by targeted biopsy in 25% of men. Tumor present outside MRI lesions may be the result of misregistration on fusion biopsy, segmentation error, operator or technical inaccuracies, MRI failing to identify cancer or any combination thereof.25 Further, combination of targeted and systematic cores most accurately predicted final tumor grade found at radical prostatectomy.8 In the present cohort, 63 men underwent radical prostatectomy (and had available whole mount slides for analysis) after elimination from AS; the upgrading rate in this group from prior MRGBx to final pathology was only 8% (vs 56% by systematic cores only, supplementary table 3, https://www.jurology.com) compared to historical rates of 20%–30% seen with USGBx.26
While an adverse MRI change during AS (increased PI-RADS or PRECISE scoring) has been associated with tumor upgrading,27 omission of surveillance biopsy based on a stable MRI may leave high-grade tumors undetected. In previous work, AS by serial MRI alone (without biopsy) failed to detect 17%–22% of tumor upgrades.11,13 These data are in keeping with the present study in which 14% of men with negative MRIs upgraded during AS. These findings may be due to the failure of MRI to visualize clinically significant cancers in as many as 15%–25% of cases.28–30 The possibility of a falsely negative MRI adds to the importance of combining systematic and targeted biopsy.
The present study, because it included men with GG2, adds information about upgrading for men entering AS with intermediate-risk pathology. Here the finding of GG2 at confirmatory biopsy was independently associated with increased risk of upgrading to ≥GG3. Thus is confirmed an earlier report showing that men with GG2 upgrade to ≥GG3 more often than men with GG1 by severalfold.15 Others have reported increased PCa progression and even metastasis during AS in men with GG2 initially diagnosed by USGBx.1,4 These data indicate that even with the accuracy of MRGBx, increased vigilance during surveillance of men with GG2 is warranted.
Since results were similar at the 2 sites despite use of different biopsy platforms, the findings appear to be unrelated to type of fusion device employed and may apply to other MRI-targeted methods, as well.
Limitations of this study include lack of a comparator arm of men confirmed and followed with USGBx. Relatively few men with GG2 were included (75); 80% of subjects were Caucasian. No attempt was made to stratify GG2 tumors, (eg percent pattern 4). Genomics were not studied. Men were able to choose definitive treatment at any time. And because the 2 centers have longstanding, in-depth experience with MRGBx, the results may not yet be generalizable. Despite the limitations, the prospective data herein provide evidence that for men in AS MRGBx can provide a degree of accuracy about prostate pathology that has not been available with USGBx.
CONCLUSIONS
When AS begins and follows with MRGBx (targeted and systematic), upgrading rate (≥GG3) is greater when tumor is initially present within an MRI lesion or when pathology is GG2 than when these features are absent.
Supplementary Material
Support:
Supported in part by R01CA218547, R01CA195505, the Intramural Research Program of the National Cancer Institute, the Center for Interventional Oncology, intramural NIH Grants Z1A CL040015, 1ZIDBC011242, UL1TR000124 from University of California, Los Angeles Clinical and Translational Science Institute, the University Hospital Foundation in Edmonton, Alberta, and generous donation from Mr. Leo Broks.
Abbreviations and Acronyms
- AS
active surveillance
- GG
Gleason grade group
- MRGBx
magnetic resonance imaging-guided biopsy
- MRI
magnetic resonance imaging
- NCI
National Cancer Institute
- NIH
National Institutes of Health
- PCa
prostate cancer
- PI-RADS®
Prostate Imaging eReporting and Data System
- PSA
prostate specific antigen
- UCLA
University of California, Los Angeles
- USGBx
ultrasound-guided biopsy
Footnotes
Conflict of Interest: Dr. Marks is co-founder of Avenda Health. Dr. Priester is data scientist at Avenda Health.
Disclosures: NIH and Philips have a Cooperative Research and Development Agreement.
Licensed Patents/Royalties: Philips: NIH receives royalties for licensed patents from Philips and in turn, BW, BT, PP, PC receive resulting royalties from NIH. NIH may own intellectual property in the field (multiple patents and patents pending-available upon request).
Intellectual Property: NIH & BW.
Non-Endorsement Language: The content of this manuscript does not necessarily reflect the views, policies, or opinions of the U.S. Department of Health and Human Services. The mention of commercial products, their source, or their use in connection with material reported herein is not to be construed as an actual or implied endorsement of such products by the United States government. Opinions expressed are those of the authors, not necessarily the NIH nor the NCI.
REFERENCES
- 1.Klotz L, Vesprini D, Sethukavalan P et al. : Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2014; 33: 272. [DOI] [PubMed] [Google Scholar]
- 2.Chen RC, Rumble RB, Loblaw DA et al. : Active surveillance for the management of localized prostate cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2016; 34: 2182. [DOI] [PubMed] [Google Scholar]
- 3.Mahal BA, Butler S, Franco I et al. : Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010–2015. JAMA 2019; 321: 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggi M, Cowan JE, Fasulo V et al. : The long-term risks of metastases in men on active surveillance for early stage prostate cancer. J Urol 2020; 204: 1222. [DOI] [PubMed] [Google Scholar]
- 5.Bokhorst LP, Valdagni R, Rannikko A et al. : A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol 2016; 70: 954. [DOI] [PubMed] [Google Scholar]
- 6.Tosoian JJ, Mamawala M, Epstein JI et al. : Active surveillance of grade group 1 prostate cancer: long-term outcomes from a large prospective cohort. Eur Urol 2020; 77: 675. [DOI] [PubMed] [Google Scholar]
- 7.Klotz L, Vesprini D, Sethukavalan P et al. : Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33: 272. [DOI] [PubMed] [Google Scholar]
- 8.Ahdoot M, Wilbur AR, Reese SE et al. : MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med 2020; 382: 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasivisvanathan V, Rannikko AS, Borghi M et al. : MRI-targeted or standard biopsy for prostatecancer diagnosis. N Engl J Med 2018; 378: 1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norris JM, Kinnaird A, Margolis DJ et al. : Developments in MRI-targeted prostate biopsy. Curr Opin Urol 2020; 30: 1. [DOI] [PubMed] [Google Scholar]
- 11.Chesnut GT, Vertosick EA, Benfante N et al. : Role of changes in magnetic resonance imaging or clinical stage in evaluation of disease progression for men with prostate cancer on active surveillance. Eur Urol 2020; 77: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klotz L, Pond G, Loblaw A et al. : Randomized study of systematic biopsy versus magnetic resonance imaging and targeted and systematic biopsy in men on active surveillance (ASIST): 2-year postbiopsy follow-up. Eur Urol 2019; 77: 311. [DOI] [PubMed] [Google Scholar]
- 13.Chu CE, Lonergan PE, Washington SL et al. : Multiparametric magnetic resonance imaging alone is insufficient to detect grade reclassification in active surveillance for prostate cancer. Eur Urol 2020; 78: 515. [DOI] [PubMed] [Google Scholar]
- 14.Stavrinides V, Giganti F, Trock B et al. : Five-year outcomes of magnetic resonance imaging-based active surveillance for prostate cancer: a large cohort study. Eur Urol 2020; 78: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayadevan R, Felker ER, Kwan L et al. : Magnetic resonance imaging–guided confirmatory biopsy for initiating active surveillance of prostate cancer. JAMA Netw Open 2019; 2: e1911019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filson CP, Natarajan S, Margolis DJA et al. : Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer 2016; 122: 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks L, Young S and Natarajan S: MRI—ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol 2013; 23: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natarajan S, Marks LS, Margolis DJA, et al. : Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol 2011; 29: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinreb JC, Barentsz JO, Choyke PL et al. : PI-RADS prostate imaging–reporting and data system: 2015, version 2. Eur Urol 2016; 69: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afshari Mirak S, Bajgiran AM, Hosseiny M et al. : PD64–06 Comparison of performance of PI-RADSV2 and a quantitiative PI-RADSV1 based protocol IN 3T multiparametric MRI for detection, grading and staging of prostate cancer using whole mount histopathology as reference standard IN 569 patients. J Urol, suppl., 2019; 201: e1183. [Google Scholar]
- 21.Gaur S, Harmon S, Mehralivand S et al. : Prospective comparison of PI-RADS version 2 and qualitative in-house categorization system in detection of prostate cancer. J Magn Reson Imaging 2018; 48: 1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson A, Sandeman K, Lahdensuo K et al. : New prostate cancer grade grouping system predicts survival after radical prostatectomy. Hum Pathol 2018; 75: 159. [DOI] [PubMed] [Google Scholar]
- 23.Epstein JI, Zelefsky MJ, Sjoberg DD et al. : A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol 2016; 69: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klotz L, Loblaw A, Sugar L et al. : Active surveillance magnetic resonance imaging study (ASIST): results of a randomized multicenter prospective trial. Eur Urol 2019; 75: 300. [DOI] [PubMed] [Google Scholar]
- 25.Kinnaird A, Sharma V, Chuang R et al. : Risk of prostate cancer after a negative magnetic resonance imaging guided biopsy. J Urol 2020; 204: 1180. [DOI] [PubMed] [Google Scholar]
- 26.Godtman RA, Schafferer M, Pihl CG et al. : Long-term outcomes after deferred radical prostatectomy in men initially treated with active surveillance. J Urol 2018; 200: 779. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor LP, Wang AZ, Yerram NK et al. : Changes in magnetic resonance imaging using the prostate cancer radiologic estimation of change in sequential evaluation criteria to detect prostate cancer progression for men on active surveillance. Eur Urol Oncol 2020; 4: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed HU, El-Shater Bosaily A, Brown LC et al. : Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815. [DOI] [PubMed] [Google Scholar]
- 29.Lovegrove CE, Miah S, El-Shater Bosaily A et al. : Comparison of transrectal ultrasound biopsy to transperineal template mapping biopsies stratified by multiparametric magnetic resonance imaging score in the PROMIS trial. J Urol 2019; 203: 100. [DOI] [PubMed] [Google Scholar]
- 30.Le JD, Tan N, Shkolyar E et al. : Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol 2015; 67: 569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


