Abstract
Background
Vaccine-induced phantosmia is a rare adverse effect of vaccination and has not been previously reported related to the Johnson & Johnson (J&J) COVID-19 vaccine.
Case Presentation
Three weeks after receiving the J&J COVID-19 vaccine, a 39-year-old veteran started smelling a burning odor in the absence of an identifiable source. At presentation to the clinic, his general and neurological examinations, brain magnetic resonance imaging, and electroencephalogram were all unremarkable. The episodes persisted for nearly 2 years (21 months postvaccination).
Conclusions
This is the only case of phantosmia reported after the use of the J&J COVID-19 vaccine and aligns with the literature that reports 1 case of phantosmia and 2 cases of hyposmia following the Pfizer-BioNTech COVID-19 mRNA vaccine. This information will help health care professionals understand the possible adverse effects of COVID-19 vaccination and be better equipped to counsel patients about the benign but potentially long-lasting adverse effects of the J&J COVID-19 vaccine.
The rapid development of multiple vaccines for COVID-19 significantly contributed to reducing the morbidity and mortality associated with COVID-19 infection.1 The vaccination campaign against COVID-19 started in December 2020 within the US Department of Veterans Affairs (VA) health care system with the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines followed by the Johnson & Johnson (J&J) vaccine in March 2021.2,3
Because of the importance of maintaining a safe vaccination campaign, surveillance reports documenting cases of malignant or benign adverse effects (AEs) are fundamental to generate awareness and accurate knowledge on these newly developed vaccines. Here we report the case of a veteran who developed olfactory hallucinations following the administration of the J&J COVID-19 vaccine.
CASE PRESENTATION
A 39-year-old veteran with a history of tension-type headaches presented to the neurology clinic with concern of a burning smell sensation in the absence of an identifiable source. He first noticed this symptom approximately 3 weeks after he received the J&J COVID-19 vaccine about 4 months prior. At the symptom’s first occurrence, he underwent a nasal swab antigen COVID-19 test, which was negative. Initially, symptoms would occur daily lasting about 1 hour. Thereafter, they started to decrease in duration, frequency, and intensity, and about 11 months postvaccination, milder episodes were occurring 1 to 2 times weekly. These episodes lasted nearly 2 years (21 months postvaccination). They happened randomly during the day and were not associated with any other symptoms. Specifically, there were no headaches, loss of consciousness, abnormal movements, nausea, vomiting, photophobia or phonophobia, or alteration of consciousness, such as confusion or drowsiness during or after the events. Additionally, there were no clear triggers the veteran could identify. The veteran did not sustain any head injuries or exposure to toxic odors before the onset of symptoms.
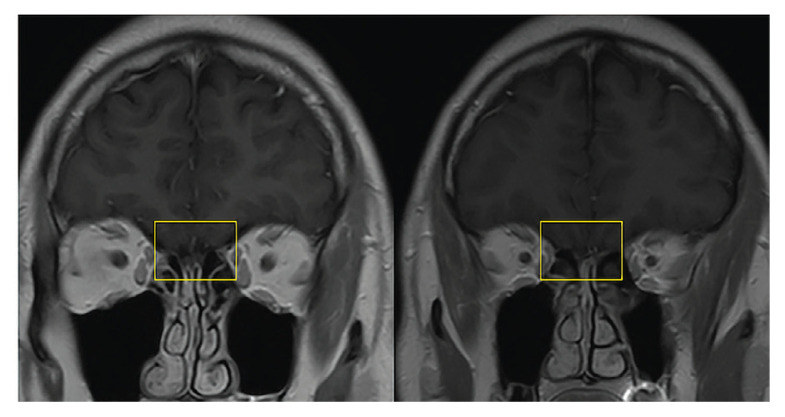
At the time of his presentation to the clinic, both his general and neurological examinations were unremarkable. Brain magnetic resonance imaging (MRI) showed chronic sinusitis but no other abnormalities (Figure 1), though the phantosmia persisted after the sinusitis had been treated with a course of doxycycline. An electroencephalogram (EEG) was obtained and was unremarkable as well (Figure 2). Notably, however, the EEG did not capture the episodes of phantosmia. Together the clinical and paraclinical evidence along with the timing of symptom onset in relation to vaccination point toward a new onset of phantosmia as an AE of the J&J COVID-19 vaccine.
FIGURE 1.
Brain Magnetic Resonance Imaging
T1-weighted coronal sequences showing no overt pathological changes of the olfactory bulb (yellow square).
FIGURE 2.
Sample segment of Electroencephalogram
Sample segment of electroencephalogram in a longitudinal bipolar montage showing normal awake background with a posterior predominant rhythm of 9 Hz. Bandpass filtered 1–70 Hz, Notch filter 60 Hz. Scale bar at the lower left depicts sensitivity and time scale.
DISCUSSION
It has been previously observed that infection with COVID-19 can lead to the loss of taste and smell, but only less commonly olfactory hallucination.4 The pathophysiology of olfactory hallucinations following COVID-19 infection is unknown, but several mechanisms have been proposed. These include obstruction of the olfactory cleft; infection of the sustentacular supporting cells, which express angiotensin-converting enzyme 2 (ACE-2); injury to olfactory sensory cells via neuropilin-1 receptors (NRP1); and injury to the olfactory bulb.5
The case we present represents the only report of phantosmia following a J&J COVID-19 vaccination. Phantosmia, featured by a burning or smoke odor, has been reported prior in a case of a 57-year-old woman following the administration of the Pfizer-BioNTech mRNA vaccine.6 Similar to our case, symptoms were not associated with a concurrent COVID-19 infection ruled out via a COVID-19 polymerase chain reaction test. For the Pfizer-BioNTech phantosmia case, a 3 Tesla (T) brain MRI showed left greater than right olfactory bulb and tract gadolinium enhancement on T1-weighted postcontrast images. On axial T2-weighted fluid-attenuated inversion recovery images, hyperintensity along the left olfactory bulb and bilateral olfactory tracts was noted and interpreted as edema. On sagittal thin sections of T2-weighted images, the olfactory nerve filia were thickened and clumped.6 On the contrary, in the case we present, a brain MRI obtained with a 1.5 T magnet showed no abnormalities. It is possible that a high-resolution scan targeting the olfactory bulb could have disclosed pathological changes. At the time when the veteran presented to the neurology clinic, symptoms were already improving, and repeat MRI was deferred as it would not have changed the clinical management.
Konstantinidis and colleagues reported hyposmia in 2 patients following Pfizer-BioNTech COVID-19 vaccination.5 Both patients, 42- and 39-year-old women, experienced hyposmia following their second dose of the vaccine with symptom onset 3 and 5 days after vaccination, respectively. The first patient reported improvement of symptoms after 1 week, while the second patient participated in olfactory training and experienced only partial recovery after 1 month. Multiple studies have reported cranial nerve involvement secondary to other COVID-19 vaccines, including olfactory dysfunction, optic neuritis, acute abducens nerve palsy, Bell palsy, tinnitus, and cochleopathy.7
There are no previous reports of phantosmia following the J&J COVID-19 vaccine. In our case, reported symptoms were mild, although they persisted for nearly 2 years following vaccination.
In the evaluation of this veteran, although the timing between symptom onset and vaccination was indicative of a possible link between the 2, other etiologies of phantosmia were ruled out. Isolated olfactory hallucination is most associated with temporal lobe epilepsy, which is the most common form of epilepsy to present in adulthood. However, given the absence of other symptoms suggestive of epilepsy and the duration of the episodes (approximately 1 hour), the clinical suspicion was low. This was reinforced by the EEG that showed no abnormalities in the temporal region. Notwithstanding these considerations, one must keep in mind that no episodes of phantosmia occurred during the EEG recording, the correlates of which are the gold standard to rule out a diagnosis of epilepsy.
A normal brain MRI argued against possible structural abnormalities leading to these symptoms. Thus, the origin of these symptoms remains unknown.
CONCLUSIONS
The emergency approval and use of vaccines against COVID-19 was a major victory for public health in 2021. However, given the rapid rollout of these vaccines, the medical community is responsible for reporting adverse effects as they are observed. The authors believe that the clinical events featuring the J&J COVID-19 vaccine in this veteran should not discourage the use of the COVID-19 vaccine. However, sharing the clinical outcome of this veteran is relevant to inform the community regarding this rare and benign possible adverse effect of the J&J COVID-19 vaccine.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Tennessee Valley Veteran Healthcare System (Nashville). The authors thank Dr. Martin Gallagher (Tennessee Valley Veteran Healthcare System) for providing clinical expertise with electroencephalogram interpretation.
Footnotes
Author disclosures
Francesca Bagnato, MD, PhD, has received consulting fees and other payments from Sanofi-Genzyme and consulting fees from Janssen Pharmaceuticals (Johnson & Johnson), Biogen, and Merck-Serono. Kelsey Barter reports no actual or potential conflicts of interest or outside sources of funding with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Ethics and consent
No informed consent was obtained from the patient; patient identifiers were removed to protect the patient’s identity.
References
- 1.Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020–July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520–1524. doi: 10.15585/mmwr.mm7043e2. Published 2021 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317–e324. doi: 10.1016/j.amepre.2021.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnato F, Wallin M. COVID-19 vaccine in veterans with multiple sclerosis: protect the vulnerable. Fed Pract. 2021;38(suppl 1):S28–S32. doi: 10.12788/fp.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Işlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine cases. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 2):2891–2893. doi: 10.1007/s12070-021-02505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399–1401. doi: 10.1002/alr.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133–137. doi: 10.1097/RMR.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 7.Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3–40. doi: 10.1007/s10072-021-05662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]