TO THE EDITOR:
Hypoglycemia is a life-threatening complication of malignant insulinoma that can be refractory to maximal medical therapy that targets the tumor and insulin secretion and action simultaneously. We report the successful use of RZ358, a novel human anti–insulin receptor monoclonal antibody, in a patient with metastatic insulinoma in whom severe, refractory hypoglycemia developed after peptide receptor radionuclide therapy with lutetium Lu 177 dotatate.
A 55-year-old man presented with a 5-month history of abdominal pain and weight loss. Imaging showed a pancreatic mass (diameter, 1.8 cm) and numerous hepatic lesions (Fig. S1A in the Supplementary Appendix, available with the full text of this letter at NEJM.org). A liver biopsy revealed a pancreatic neuroendocrine tumor (Fig. S2) with a pathogenic MEN1 mutation. Despite octreotide therapy, the abdominal pain and the tumor mass increased, thereby prompting treatment with lutetium Lu 177 dotatate. Two days after the first dose, severe hypoglycemia with neuroglycopenia (with a minimum glucose level of 20 mg per deciliter [1.1 mmol per liter]) occurred in the patient in both fasting and postprandial states. Hormonal analysis, performed when the venous glucose level was 41 mg per deciliter (2.3 mmol per liter), revealed inappropriately high levels of insulin, at 45 μIU per milliliter (312 pmol per liter; reference value, <19.6 μIU per milliliter [141 pmol per liter]); C-peptide, at 6.5 ng per milliliter (reference range, 0.80 to 3.85); and proinsulin, at 453 pmol per liter (reference value, <18).
Intravenous 50% dextrose solution was infused through a central line, at a rate of up to 60 ml (30 g) per hour, which reflects very high glucose use. Despite enteral nutrition support and treatment with high-dose diazoxide, everolimus, dexamethasone, pasireotide, and glucagon, the patient remained hypoglycemic, with up to 58% of all sensor glucose values in each 24-hour period being less than 70 mg per deciliter [3.9 mmol per liter] and up to 19% being less than 54 mg per deciliter (3.0 mmol per liter) (Fig. 1). Additional liver-directed ablation or chemotherapy was not feasible owing to the extent of the hepatic metastases and severe hypoglycemia.1 Repeat imaging showed a substantial reduction in liver metastases (Fig. S1B) and a rising lactate dehydrogenase level (Fig. S3), findings that indicate a partial response and ongoing tumor lysis.
Figure 1. Clinical Course of Hypoglycemia in the Study Patient.
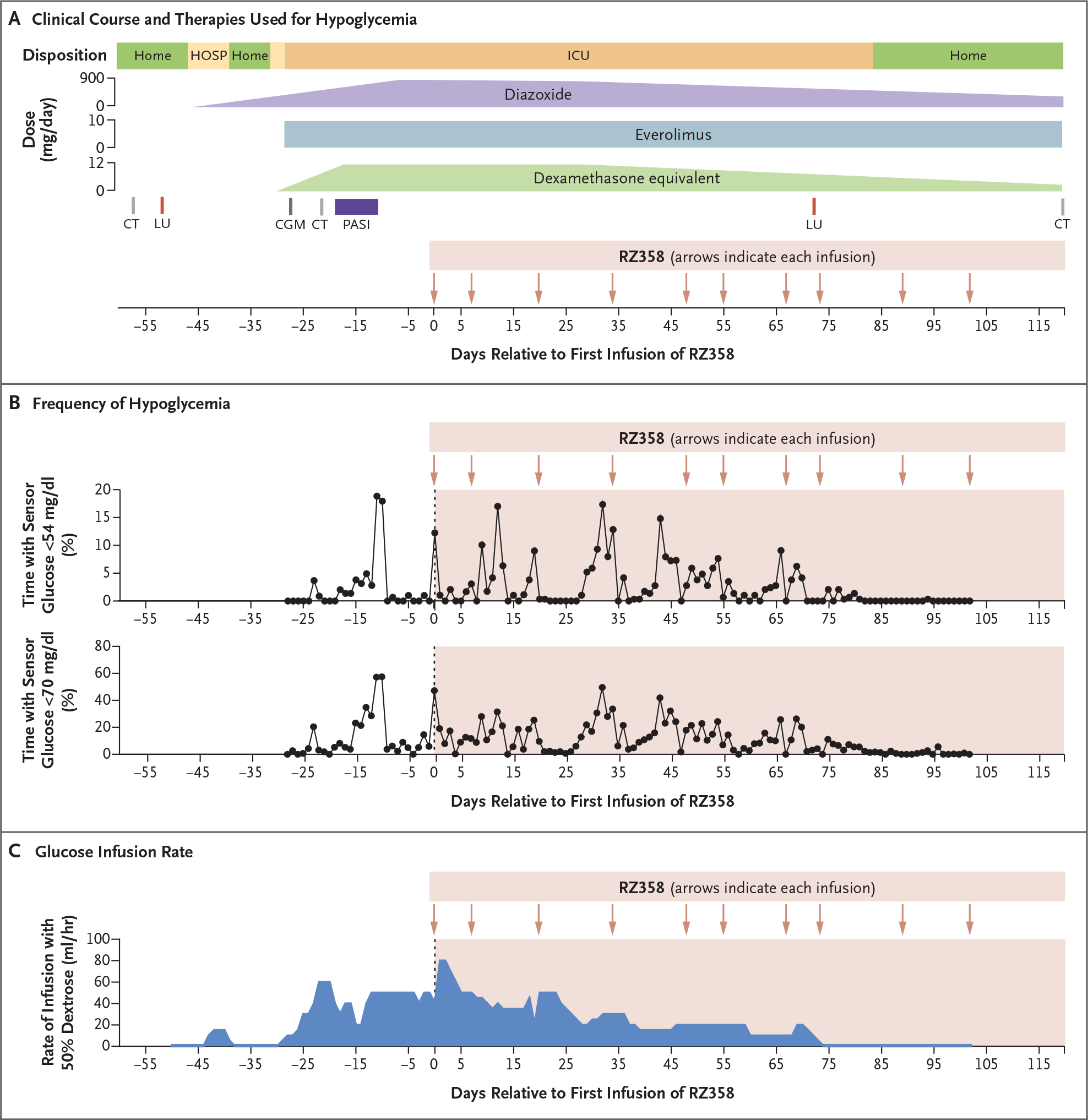
Panel A shows the clinical course and therapies used for hypoglycemia in a patient with metastatic insulinoma in whom severe, refractory hypoglycemia developed after peptide receptor radionuclide therapy with lutetium Lu 177 dotatate (LU). Panel B shows the frequency of hypoglycemia in view of the percentage of time with a sensor glucose measurement of less than 54 mg per deciliter (3.0 mmol per liter) (as monitored with the Dexcom G6 system, Dexcom) (upper graph) or with a measurement of less than 70 mg per deciliter (3.9 mmol per liter) (lower graph). To convert the values for glucose to millimoles per liter, multiply by 0.05551. Panel C shows the dextrose infusion rate (continuous infusion of 50% dextrose solution, not including rescue boluses). Hypoglycemia started after the first treatment with LU (day −50). The hypoglycemia was initially controlled with diazoxide but recurred with level 3 severity, leading to admission to an intensive care unit (ICU) despite sequential addition of multiple therapies directed at hypoglycemia. Initiation of the anti–insulin receptor monoclonal antibody RZ358 (day 0) was successful in reducing the frequency of hypoglycemia, and the patient was able to safely receive a second dose of LU (day 67), which allowed for discharge home on day 83 after initiation of RZ358 therapy. CGM denotes initiation of continuous glucose monitoring, CT computed tomography, HOSP hospitalization, and PASI pasireotide.
We then sought to administer additional therapies to restore euglycemia. RZ358 is a human monoclonal antibody that acts as a negative allosteric modulator of the insulin receptor by reducing insulin binding and downstream insulin signaling. RZ358 has been shown to induce whole-body insulin resistance and reverse insulin-stimulated hypoglycemia in both mice and healthy humans.2–5 We obtained emergency use authorization from the Food and Drug Administration, approval from the institutional review board of Beth Israel Deaconess Medical Center, and written informed consent from the patient.
After the second RZ358 infusion (at a dose of 6 mg per kilogram of body weight), the hypoglycemia attenuated, with less than 1% of all sensor glucose values in each 24-hour period being less than 54 mg per deciliter, a result that permitted a reduction in the rate of dextrose infusion. After four additional infusions of RZ358 (each at a dose of 9 mg per kilogram), metabolic stability allowed discontinuation of other therapies, despite persistent excessive insulin secretion, elevated lactate dehydrogenase level (Fig. S3), and high tumor burden (Fig. S1C). The patient has remained free of severe hypoglycemia after 9 months of RZ358 therapy, currently administered at monthly intervals, with no evidence of adverse effects.
Our case report shows that the anti–insulin receptor antibody RZ358 was used successfully in the treatment of severe hypoglycemic crisis in a patient with malignant insulinoma. Future studies are warranted to establish efficacy and the appropriate dose level and frequency of RZ358 in patients with malignant insulinoma. Applied more broadly, this approach of targeting the insulin receptor to modulate systemic metabolism in adults may be effective for the treatment of severe hypoglycemia and potentially expand the use of monoclonal antibody therapeutic agents for other metabolic disorders.
Supplementary Material
Acknowledgments
Supported by Diabetes Research Center Pilot and Feasibility program awards from the Joslin Diabetes Center (F32DK12643201 and P30 DK036836, to Dr. Vamvini) and grants from the National Institutes of Health (NIH R01 DK121995, to Dr. Patti, and P30 DK036836, to the Joslin Diabetes Center).
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Soravis Osataphan, Beth Israel Deaconess Medical Center Boston, MA
Maria Vamvini, Joslin Diabetes Center Boston, MA
Evan D. Rosen, Beth Israel Deaconess Medical Center Boston, MA
Lei Pei, Beth Israel Deaconess Medical Center Boston, MA
Natanie Erlikh, Beth Israel Deaconess Medical Center Boston, MA
Gurcharan Singh, Beth Israel Deaconess Medical Center Boston, MA
Poojaben Dhorajiya, Beth Israel Deaconess Medical Center Boston, MA
J. Anthony Parker, Beth Israel Deaconess Medical Center Boston, MA
Jonathan M. Dreyfuss, Joslin Diabetes Center Boston, MA
Ahmed Rattani, Beth Israel Deaconess Medical Center Boston, MA
Mary-Elizabeth Patti, Joslin Diabetes Center Boston, MA
References
- 1.Brown E, Watkin D, Evans J, Yip V, Cuthbertson DJ. Multidisciplinary management of refractory insulinomas. Clin Endocrinol (Oxf) 2018;88:615–24. [DOI] [PubMed] [Google Scholar]
- 2.Corbin JA, Bhaskar V, Goldfine ID, et al. Inhibition of insulin receptor function by a human, allosteric monoclonal antibody: a potential new approach for the treatment of hyperinsulinemic hypoglycemia. MAbs 2014;6:262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson KW, Neale A, Gordon A, et al. Attenuation of insulin action by an allosteric insulin receptor antibody in healthy volunteers. J Clin Endocrinol Metab 2017;102:3021–8. [DOI] [PubMed] [Google Scholar]
- 4.Patel P, Charles L, Corbin J, et al. A unique allosteric insulin receptor monoclonal antibody that prevents hypoglycemia in the SUR-1−/− mouse model of KATP hyperinsulinism. MAbs 2018;10:796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demirbilek H, Melikyan M, Galcheva S, et al. Results from a global, multi-center, phase 2b study (RIZE) in congenital hyperinsulinism: characterization of a high unmet treatment need and glycemic response to RZ358. ESPE Abstracts 2022;95:FC3.2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


