Abstract
Background
The leaves of Serjania erecta Radlk (Sapindaceae) are renowned in ethnobotany for their medicinal properties and are significant as a medicinal resource for traditional Brazilian communities. As necrotic spots are common on these leaves, indicating interaction with phytopathogenic fungi, it was hypothesized that biotrophic fungal species colonize the leaf tissues of S. erecta.
Methods
To test this hypothesis, we employed standard techniques in plant anatomy, which enabled us to investigate the interaction of fungal structures with plant tissues and describe the morphoanatomical and histochemical characteristics of the epidermis and limbus of S. erecta.
Results
The anatomical analysis showed the existence of leaf teeth on the leaf tips. Additionally, hyphae, conidiospores, and spores of Bipolaris/Curvularia species were detected on the adaxial epidermis. Moreover, melanized microsclerotia were found in glandular areas of the leaf teeth and the phloem, providing evidence of biotrophic behavior. The hypothesis that biotrophic phytopathogenic fungi interact with S. erecta leaf tissues was confirmed, despite the presence of many bioactive compounds (such as flavonoids, alkaloids, and essential oils), as evidenced by histochemical analyses. The presence of tector, glandular, and scabiform trichomes on the leaf teeth and epidermis was also revealed. This study presents, for the first time, the synthesis of essential oils and alkaloids in the leaves of S. erecta. Additionally, it investigates previously unexplained aspects of the anatomy and histochemistry of the species, as well as its interaction with resident microorganisms. Therefore, it is recommended that future research focus on extracting and characterizing the oils and alkaloids of S. erecta, as well as exploring other aspects related to its microbiome and its relationship.
Keywords: Five-leaf liana, Bioactive compounds, Leaf teeth, Biotrophic fungi, Trichomes
Introduction
Sapindaceae is the largest and most important family of the order Sapindales (APG IV, Angiosperm Phylogeny Group IV), with about 140 genera and 1,900 species mainly distributed in tropical regions (Acevedo-Rodríguez et al., 2011). In Brazil, the family is represented by 32 genera and 436 species, of which 191 are endemic (Flora do Brasil, 2020). This family comprises many species of economic and medicinal importance (Heywood et al., 2007). Among them Serjania erecta Radlk, commonly known as Cipó de cinco folhas. S. erecta is characterized by erect subshrubs with flexuous growth. Its leaves are compound, with leaflets up to 15 cm long and up to 10 cm wide. Additionally, it has inflorescences in solitary thyrsus with white flowers. The fruits are schizocarpic, slightly corded, and up to 3.5 cm long, with subglobose and slightly arilate seeds (Guarim Neto & Macedo, 2009). It is a native plant that grows throughout the Brazilian cerrado and has been reported for its various ethnobotanical applications, including its use in teas and extracts to treat ulcers and inflammatory infections (Rodríguez & Pinto, 2014; Guarim Neto, Santana & Silva, 2000). The populations living along the riverside of the Paraná and Cuiabá River basins use tea made from plant leaves to alleviate flu symptoms (Reis & Bellini, 2007). Additionally, there are reports that indigenous communities in Mato Grosso State use the roots of the plant to manage hypertension (Guarim Neto & Macedo, 2009).
The biological effects attributed to S. erecta in traditional and indigenous medicine may be related to the bioactive compounds found in this species. Qualitative analyses have already identified the presence of saponins, flavonoids, triterpenoids, steroids, tannins, and catechins in the hydroalcoholic extract of the leaves (Gomig et al., 2008; Ventura et al., 2021). Similarly, analyses of the crude extract using nuclear magnetic resonance showed the presence of flavonoids such as camferol, epicatechins, apigenins, vitexin, isovitexin, and quercetin (Guimarães et al., 2015; Cardoso et al., 2013).
Research demonstrates that the interaction between S. erecta leaf extract, animals, and microbial cells produces various effects. The hydroalcoholic extract showed topical anti-inflammatory activity in mice (Gomig et al., 2008). Additionally, the chloroform extract exhibited gastroprotective action and reduced the ulcerative process (Arruda et al., 2009). Memory disorders appear to be mitigated by the use of the crude extract of the plant, which has a synergistic effect with pharmacological drugs (Broggini et al., 2010).
Other trials have shown nematicidal effects against Pratylenchus zeae and P. jaehni (Slomp et al., 2009), as well as antimicrobial effects against key human pathogens, including Mycobacterium tuberculosis, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella setubal, Candida albicans, Saccharomyces cerevisiae, and Escherichia coli (Cardoso et al., 2013). Metabolites present in S. erecta can also affect insects, as it directly interferes with the growth, metabolism, and development of caterpillars (De Freitas et al., 2022).
Despite the considerable phytochemical potential of S. erecta, extensive studies on this plant are scarce. This species has not yet been evaluated for its anatomy and histochemistry, although studies describe its biotechnological potential and promote its conservation. According to Guarim Neto & Macedo (2009), the conservation of this species deserves more attention, considering the serious and irreversible losses caused to the Cerrado domain.
Furthermore, there needs to be more information regarding the morphoanatomical and histochemical aspects of the plant and the microbiota residing in the leaves of S. erecta. Given that it is a medicinal plant, any epiphytes or endophytes present must exhibit traits of resistance or insensitivity to the secondary metabolites found in its tissues.
During field observations, it was discovered that S. erecta leaves display necrotic spots even on healthy plants, indicating interaction with phytopathogenic fungal species that release toxins and cause local tissue damage (Salvatore & Andolfi, 2021; Doehlemann et al., 2017; Horbach et al., 2011). However, many phytopathogenic species can establish themselves as endophytes and promote plant growth (Abdel-Motaal et al., 2020; Toghueo & Boyom, 2020; Javed et al., 2019). Research has shown that certain necrotrophic pathogens can transition to hemibiotrophy or biotrophy, where they feed on living tissue without causing the death of the host plant.
Biotrophic phytopathogenic fungi were hypothesized to interact with the leaf tissues of S. erecta. To test this hypothesis, we employed standard analytical techniques in plant anatomy, which enabled us to investigate not only the interaction of fungal structures with plant tissues but also uncover aspects of the plant’s anatomy and histochemistry not previously elucidated. This study aims to enhance our understanding of the biodiversity of the Cerrado by reducing the information gap regarding the S. erecta species. Specifically, we investigated the leaf tissue organization and its interaction with microorganisms that form part of its microbiome.
Materials & Methods
Plant material collection
Five S. erecta individuals were sampled at Fazenda Fontes do Saber, Rio Verde/GO (−17.783262°S, −50.967928°W), which is characterized by a transitional physiognomy between “cerrado sensu stricto” and “cerradão” vegetation. Field collections were allowed
by the Post-Graduate Direction of the Instituto Federal Goiano Campus Rio Verde (project number: CMRV DPPG 011/2021). The specimens were intended for morphoanatomical characterization analyses, fungal colonization of leaves, histochemical tests using light microscopy, and electron microscopy analyses. Adult specimens with a shrubby habit were collected. An exsiccate was deposited at the herbarium of the Instituto Federal Goiano, Rio Verde campus, for identification confirmation, and it was assigned the catalog number 545.
Morphoanatomical characterization and evaluation of leaf colonization by fungi
Leaf samples measuring 3 mm2 were collected from the central region of the adult leaves of all five individuals of the species. Initially, the samples were fixed in FAA70 solution (formalin, acetic acid, and ethyl alcohol) for 24 h. Following this period, the plant material was stored in 70% alcohol and dehydrated using an increasing series of ethyl alcohol. It was then pre-infiltrated and infiltrated with historesin, following the manufacturer’s instructions (Leica, Wetzlar, Germany).
Afterward, the S. erecta leaf samples were cut into 5 µm-thick cross-sections using a rotating microtome. The sections were then stained with toluidine blue-polychrome stain (0.05% 0.1 M phosphate buffer, pH 6.8), following the method detailed by O’Brien, Feder & McCully (1964). Images were captured using a microscope with the bright field option. Subsequently, morphoanatomical observations were made on the epidermis of both the adaxial and abaxial faces, as well as the mesophyll. Observations were also made using ultraviolet (UV) light to observe autofluorescent structures.
The leaf diaphanization technique utilized in this study adhered to the protocol outlined by Shobe & Lersten (1967) and Fonsêca, Proença & Gonçalves (2007). Briefly, the fully developed leaves were divided into four sections: apical, middle, margin, and basal thirds. Impressions were taken from the epidermis of both the adaxial and abaxial faces using fresh material and instant adhesive (Segatto et al., 2004); these impressions were then used to prepare semi-permanent slides.
The analyses were conducted at the Plant Anatomy Laboratory of the Instituto Federal Goiano, Campus Rio Verde. The material was photographed using an Olympus microscope (BX61; Olympus, Tokyo, Japan) with a DP-72 camera. Next, the images were recorded using the low gamma option and the highest image contrast. These images were utilized to characterize the morphology and anatomy of S. erecta leaf tissues and to evaluate the presence of hyphae and fungal structures colonizing the tissues.
Scanning electron microscopy
Leaf fragments of approximately 3 mm2 were fixed in formalin, acetic acid, and 70% ethyl alcohol and then subjected to a critical point drying process. After compositional analysis, they were coated with gold as a conductive element for image acquisition.
The images were captured using a Jeol JSM7100F field emission scanning electron microscope (SEM-FEG) with a 5 keV electron acceleration voltage in the secondary electron detection (SED) mode. The data was utilized to assess the interaction between hyphae, fungal structures, and S. erecta leaf tissues.
Histochemical tests
To conduct the histochemical tests, serial sections of S. erecta leaf samples were first fixed in FAA70 solution and then embedded in historesin. These sections were subjected to the following treatments: potassium dichromate (original method: Gabe, 1968) and toluidine blue staining (original method: O’Brien, Feder & McCully, 1964) for identification of phenolic compounds; NADI for essential oils and oleoresins (original method: (David & Carde, 1964); and Schiff’s reagent/periodic acid (PAS) for polysaccharide identification (original method: O’Brien & McCully, 1981). The following tests were performed using fresh material: hydrochloric vanillin for tannin identification (original method: Mace & Howell, 1974); aluminum chloride and fluorescence under UV light for flavonoid identification; and Wagner’s reagent for alkaloid reaction (original method: Furr & Mahlberg, 1981).
Results
Leaf morphological characterization
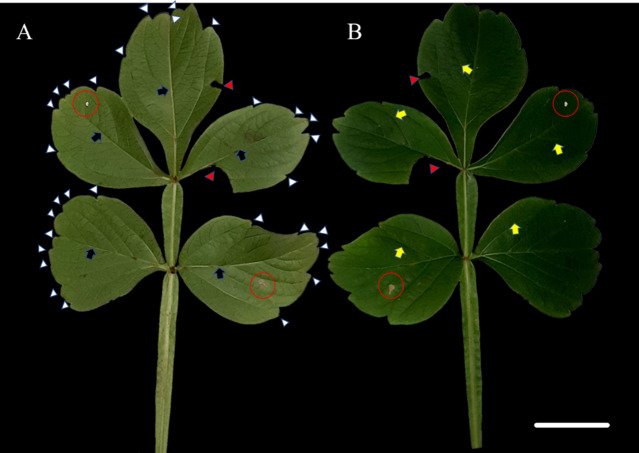
The species S. erecta has a compound leaf consisting of five leaflets. Light-colored leaf teeth were present in the apical region of the leaflets (Fig. 1A). Spots of leaf necrosis are a common indication of the interaction between phytopathogenic fungal species and leaf tissues (Figs. 1A–1B). However, the leaf may still retain visible signs of good health.
Figure 1. Leaf morphology of Serjania erecta Radlk (Sapindaceae).
(A) The abaxial surface and (B) the adaxial surface. Red arrows indicate herbivore attacks, while red circles indicate necrotic spots. Blue arrows point to the primary veins; yellow arrows indicate the secondary veins; and white arrows point to the leaf teeth. 5 cm scale bar.
Interaction with fungi
Epidermal impressions revealed the presence of septate, branched, melanized hyphae on the adaxial side of the S. erecta leaf samples (Figs. 2A–2F). These hyphae were identified as belonging to the Bipolaris-Cochliobolus-Curvularia complex because they have septate conidiophores, which are typically observed in this complex. Bipolaris and Curvularia are anamorphs of Cochliobolus in this complex. Colonization by the anamorphs was confirmed by the presence of typical spores, specifically conidia, that were subcylindrical to narrowly clavate and straight to slightly curved. Phragmospores and didymospores were also documented (Figs. 3A–3F).
Figure 2. Presence of septate and melanized hyphae in the epidermis of the adaxial side of the S. erecta Radlk (Sapindaceae) leaf.

(A–F) Arrows indicate the presence of conidiophores. 8 µm scale bar.
Figure 3. The presence of spores typical of Bipolaris/Curvularia anamorphs was observed in the epidermis of the S. erecta Radlk (Sapindaceae) adaxial leaf surface.
(A–F) Conidia; (C and D) phragmospores; and (F) didymospores. 5.5 µm scale bar.
Electron microscopy images confirmed the presence of fungi that directly interacted with the adaxial epidermis of S. erecta. Septate hyphae were observed spreading across the tissue surface (Fig. 4A). On the contrary, hyphae and fungal spores were frequently observed colonizing the inner leaf tissues (Fig. 4B). Microsclerotia were observed in the phloem region (Fig. 4C).
Figure 4. An electron micrograph of S. erecta Radlk (Sapindaceae).
(A) Septate hyphae on the surface of the leaf epidermis; (B) hyphae and fungal spores within the inner tissues of the leaf lamina; and (C) microsclerotia in the phloem of the central leaf vein. (A) 100 µm scale bar; (B) 40 µm scale bar; and (C) 30 µm scale bar.
Leaf anatomical characterization
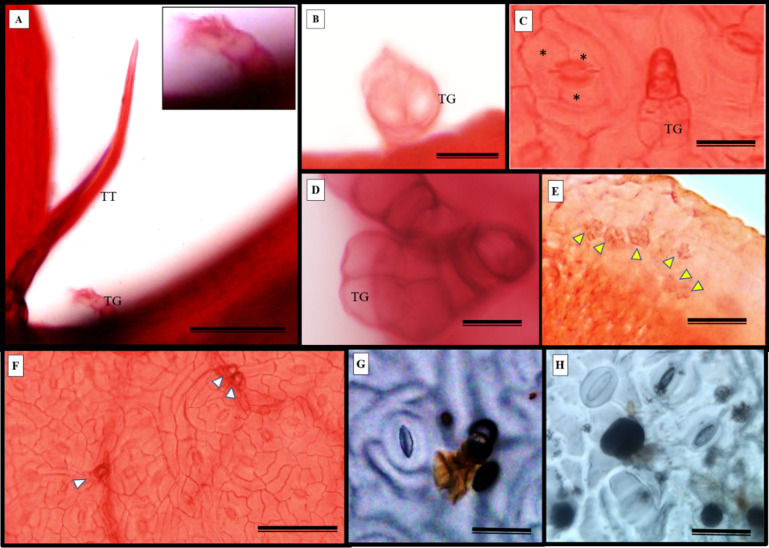
S. erecta leaves have both tectors and glandular trichomes, as shown in Figs. 5A–5B. The glandular trichomes are composed of a multicellular peduncle that is attached to a unicellular base. The multicellular head comprises two visible elliptical layers overlapping each other (Figs. 5B–5D). The observed stomata were classified as reniform and anisocytic (Figs. 5C, 5F–5H). Accumulations of melanized microsclerotia were observed in the leaf tooth region (Fig. 5E).
Figure 5. Fuchsin-stained diaphanization (A–F) and epidermal impression (G and (H) of S. erecta Radlk (Sapindaceae) under light microscopy.
Apex of toothed leaf with tector trichome and glandular structures: (A) glandular trichomes; (B–D) stomata; (C, F–H) S. erecta toothed leaf with fungal microsclerotia; (E) tector trichome (TT), glandular trichome (TG); and an asterisk (*) indicates subsidiary cells. White arrows indicate glandular trichomes, and yellow arrows indicate microsclerotia. (A, D, and F) 100 µm scale bar; (B and H) 25 µm scale bar; (C and G) 50 µm scale bar; (E) 125 µm scale bar.
Glandular (Fig. 6A) and tectonic trichomes (Fig. 6B) were observed, as well as secretions resulting from rupturing due to mechanical pressure (Figs. 6B–6D). Electron microscopy images confirmed the presence of trichomes on the leaf teeth (Figs. 7A–7C) as well as scaly trichomes on the limbus (Fig. 7D).
Figure 6. Impression of the S. erecta Radlk (Sapindaceae) epidermis in light microscopy.
Adaxial side of S. erecta. (A) Glandular trichome; (B) tector and glandular trichome; (C) secretion from disruption of glandular trichome; (D) stomata and glandular trichome. Yellow arrows indicate glandular trichomes; blue arrows indicate secretion; and white arrows indicate stomata. TT, Tector Trichome. (A) 200 µm scale bar; (B) 50 µm scale bar; and (C–D) 100 µm scale bar.
Figure 7. Electron micrographs of S. erecta Radlk (Sapindaceae) leaf teeth (A, B, and C) and leaf blade (D).
(A) Glandular trichome; (B and C) tector trichomes; (D) scamiform trichome. (A and D) 10 µm scale bar; (B and C) 50 µm scale bar.
In addition to the teeth on the leaves, endophytic fungi were also observed colonizing the area surrounding the veins. S. erecta has a biconvex vein (Fig. 8A), and fungi can inhabit the secretory canal. Glandular trichomes were also observed on the adaxial epidermis, and exudates were present (Fig. 8B). Secretory channels were also observed in the vicinity of the toothed leaf (Fig. 8C). However, Fig. 8D indicates that the leaf margin does not possess any secretory structures. The species has mesophyll composed of a palisade and spongy parenchyma layer comprising four to eight cells (Fig. 8E).
Figure 8. Transverse anatomical section of Serjania erecta Radlk (Sapindaceae) fresh leaves.
(A) Biconvex primary vein; (B) secretory canal, fungus, and glandular trichome; (C) toothed leaf; (D) margin; (E) leaf limb. EB, Abaxial epidermis; PP, Palliçadic parenchyma; PS, Spongy parenchyma; CS, Secretory canal; ED, Adaxial epidermis; EP, Toothed leaf epidermis; VF, Vascular bundle; TG, Glandular trichome; an asterisk (*) indicates fungal microsclerotia. (A) 250 µm scale bar; B, 25 µm scale bar; (C–D) 100 µm scale bar; (E) 66 µm scale bar.
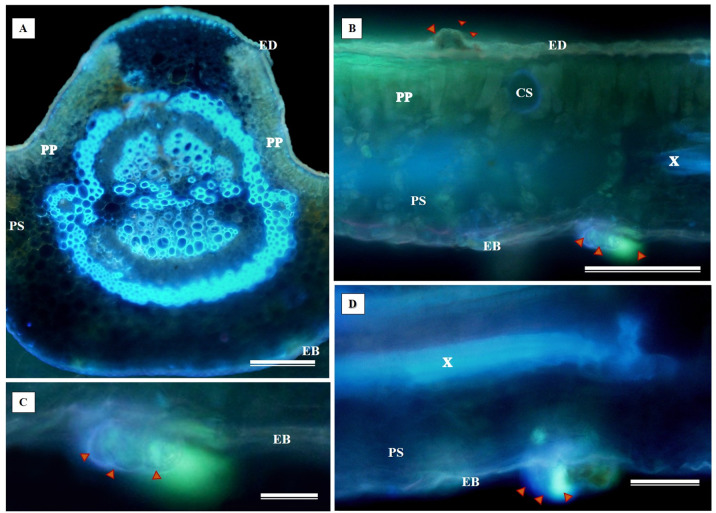
Glandular trichomes are also present in the abaxial epidermis of S. erecta (Fig. 9). The secretory channel, located near the tooth and epidermal cells, emits blue fluorescence at approximately 460 nm (Fig. 9C). Fluorescence was also detected from the vascular bundles (Fig. 9D), vessel elements, and secretory channels in the epidermal cells (Fig. 9E).
Figure 9. Transverse anatomical sections of fresh S. erecta Radlk (Sapindaceae) leaves were observed under ultraviolet light.
(A) Biconvex primary vein; (B) glandular trichome; (C) toothed leaf; (D) margin; (E) leaf lamina. AE, Abaxial epidermis; PP, Palisade parenchyma; SD, Secretory duct; AE, Adaxial epidermis; Ep, Epidermis; VB, Vascular bundle; GT, Glandular trichome. (A) 250 µm scale bar; (B) 25 µm scale bar; (C–E) 100 µm scale bar.
Histochemical tests
The histochemical tests, namely hydrochloric vanillin, potassium dichromate, and toluidine blue staining, confirmed the presence of phenolic compounds in various tissues in S. erecta (Fig. 10), including the palisade parenchyma (Fig. 10A), the secretory canal amidst the fungal microsclerotia (Fig. 10B), the primary vein amidst the fibers and xylem walls (Fig. 10C), and the glandular structures in the epidermis (Fig. 10D). The potassium dichromate test detected phenolic compounds mainly in the palisade parenchyma and fibers found in the primary vein (Fig. 10E). Using the toluidine blue test, we were able to identify phenolic compounds in the cells of the palisade parenchyma and leaf teeth (Fig. 10F).
Figure 10. Histochemical tests were conducted to detect phenolic compounds in S. erecta Radlk (Sapindaceae) leaf specimens under light microscopy.
(A–D) Hydrochloric vanillin; (E) potassium dichromate; (F) toluidine blue. ED, Adaxial epidermis; EB, Abaxial epidermis; PP, Palisade parenchyma; PS, Spongy parenchyma; CS, Secretory duct; TT, Tector trichome; an asterisk (*) indicates the presence of fungus. Arrows indicate the presence of phenolic compounds. (A and B) 25 µm scale bar; (C and F) 250 µm scale bar; (E) 100 µm scale bar.
The histochemical test for flavonoids was performed using aluminum chloride, which stained the abaxial and adaxial epidermis as well as the cell walls of the vessel elements in the primary vein (Fig. 11A). The section xylem, abaxial epidermis (Fig. 11B), and glandular secretory structures (Figs. 11C–11D) were identified on the leaf limb.
Figure 11. Histochemical testing for flavonoids using aluminum chloride was conducted on S. erecta Radlk (Sapindaceae) leaves.
ED, Adaxial epidermis; EB, Abaxial epidermis; PP, Palisade parenchyma; SP, Spongy parenchyma; SD, Secretory duct; X, Xylem. Arrows indicate glandular secretory structures.
NADI tests revealed that the adaxial epidermis tested positive for essential oils and oleoresins (Figs. 12A–12C).
Figure 12. Histochemical test for essential oils in Serjania erecta Radlk (Sapindaceae) leaves under light microscopy.
(A–C) NADI reagent. ED, Adaxial Epidermis; EB, Abaxial Epidermis; PP, Palisade Parenchyma; SP, Spongy Parenchyma. Arrows indicate the presence of essential oils. A and C represent 100 µm scale bars, while B represents a 200 µm scale bar.
The histochemical test for total polysaccharides using the periodic acid Schiff’s reaction (PAS) correctly identified the cell walls of S. erecta leaves (Fig. 13A), which comprise cellulose. Polysaccharides were marked within the secretory channel, which could indicate the presence of fungi (Fig. 13B). The presence of idioblasts, which contain polysaccharides, was observed in the leaf margins (Fig. 13C) as well as in the cells of the spongy parenchyma (Fig. 13D).
Figure 13. Histochemical testing for total polysaccharides (PAS) was conducted on S. erecta leaves under light microscopy.
(A–D) Toothed leaf. ED, Adaxial Epidermis; EB, Abaxial Epidermis; PP, Palisade Parenchyma; PS, Spongy Parenchyma; CS, Secretory Duct. 25 µm scale bar.
The histochemical test further confirmed the presence of alkaloids in the leaves, particularly in the leaf apices where idioblasts were observed (Figs. 14A and 14B).
Figure 14. The histochemical test for alkaloids (using Wagner’s reagent) was conducted on S. erecta Radlk (Sapindaceae) leaves under light microscopy.
Black indicates the presence of starch, while orange indicates the presence of alkaloid compounds. The bars represent 50 µm.
Discussion
Bipolaris and Curvularia species colonize S. erecta leaf tissues
Many species of Cochliobolus have asexual states, which are also known as synonyms for both Bipolaris and Curvularia. Therefore, these fungi are classified within a species complex (Elkhateeb, Kolaibe & Daba, 2021). The presence of Bipolaris/Curvularia in the epidermis of S. erecta was evidenced by necrotic leaf spots on the limbus. These spots occur as a plant response to phytotoxin production by pathogens, such as ophiobolins synthesized by Bipolaris or radicinin produced by Curvularia (Srivastava et al., 2021; Xiao et al., 1991). Data suggests that phytotoxins synthesized by Curvularia luneta can cause general disruptions in the plasma membrane, leading to a decrease in active transport, which is reflected by the inhibition of protein precursors and loss of ions (Vianello, Macri & Passera, 1976). These toxins damage membranes, which trigger systemic responses in plants that ultimately result in cell death. Bipolaris/Curvularia fungi are phytopathogens of various plants, particularly grasses (Dos Santos et al., 2022; Gilardi et al., 2022; Ferdinandez et al., 2021). They are known to cause devastating epidemics globally in economically important food crops (such as wheat, rice, and corn) (Berbee, Pirseyedi & Hubbard, 1999; Scheffer, 1997). However, research shows that Bipolaris/Curvularia can establish themselves as endophytes in plants sampled from the Cerrado biome. Rosa et al. (2010) found that Bipolaris/Cochliobolus acted as endophytes in healthy Piptadenia adiantoides tissues, and Teles et al. (2005) isolated Curvularia sp. from Ocotea corymbosa, a plant native to this biome. This is the first report on the interaction between endophytic fungi and S. erecta tissues.
Evidence of biotrophism has been observed in the interaction between Bipolaris/Curvularia and S. erecta leaf tissues
The first study to characterize leaf teeth, conducted by Hickey & Wolfe (1975), identified the teeth of the Sapindales order as Cunonioides (with a small glandular apex and the main vein running towards the apex). Within this order, the Sapindaceae family exhibits a wide range of indumentum variability, with non-glandular trichomes being more frequent. Multicellular glandular, papilliform, fasciculate, stellate, or scamiform trichomes occur less frequently, and stomata are classified as reniform and anisocytic (Tanaka, Pinto & Mourão, 2014; Acevedo-Rodríguez et al., 2011). The morphological data obtained for S. erecta support the observations made for Sapindaceae and indicate the presence of three types of trichomes (non-glandular, glandular, and scamiform) on the leaves of this species.
Trichomes are widely recognized as structural defense traits of plants (Da Silva & Batalha, 2011), which also serve as a barrier against water loss through evapotranspiration (Agrawal & Fishbein, 2006). Trichome density negatively affects the oviposition, feeding preference, survival, and growth rate of herbivorous insects (Agrawal & Fishbein, 2006). As a potential defense mechanism, S. erecta produces secretory structures near the toothed leaf. In this region, we observed the presence of tector, scabiform, and glandular trichomes, as well as secretions from the latter. In addition to the aforementioned protective strategies, S. erecta allows colonization of its epidermal surface by Bipolaris/Curvularia. This is evident from observing hyphae and conidia associated with epidermal cells. Furthermore, the plant provides colonization of its leaf tissues, as evidenced by melanized microsclerotia in intra-foliar structures such as secretory ducts and sieve tube elements. Research has demonstrated that phytopathogenic species can produce microsclerotia by establishing endophytic symbiotic relationships (Liu et al., 2022; Luo et al., 2019). Patil, Kulkarni & More (1966) showed that in Curvularia hyphae, aggregates of thickened or chlamydospore-like cells result in microsclerotia.
On the epidermal surface, fungi can act as a line of defense against pathogens (Wang, Wen & Asiegbu, 2022; Wen, Terhonen & Asiegbu, 2022) by exerting direct antibiosis. Benzopyrans from Curvularia exhibited antifungal activity against Cladosporium sphaerospermum and Cladosporium cladosporioides (Dos Reis, Do Vale & Lorenzi, 2022). Radicinin and its novel metabolite, O-demethylated-zeaenol, are effective in controlling the phytopathogens Magnaporthe grisea and Valsa mali (Yin et al., 2018; Zhang et al., 2011). Moreover, antifungal peptides have been isolated from pathogenic cultures of Bipolaris (Khiralla et al., 2019). A by-product mutualism interaction (Ruotsalainen et al., 2021) has been suggested between Bipolaris/Curvularia and S. erecta. This means that the investment made by S. erecta benefits Bipolaris/Curvularia as a by-product. In by-product mutualism, an organism benefits from the self-serving characteristics of another organism (Connor, 1995; Leimar & Hammerstein, 2010; Bronstein, 2015), as seen in microorganisms that utilize the metabolic waste products of their hosts (Harcombe et al., 2018). Evidence was found of endophytic fungi surviving in glandular regions and the phloem. In these regions, the primary and secondary metabolites of S. erecta are directed towards nourishing the hyphae and microsclerotia, which benefits the fungus.
Research has already demonstrated the saprophytic survival of Bipolaris and Curvularia, which depend on substrate degradation for nutrition (Stohr & Dighton, 2004; Duveiller et al., 2002; Reis et al., 1998). However, because of their saprotrophic ability to break down organic matter in soil and colonize plant tissues, fungi such as dark septate endophytes (DSE) could be classified as by-product mutualists. These fungi enhance the performance and vitality of their host plants by providing benefits without requiring significant investment from the host. Moreover, the literature has provided evidence of the transition from saprotrophic to hemibiotrophic and biotrophic states during evolution (Zhang et al., 2009). Bipolaris/Curvularia can be considered biotrophic when they derive their nutrition from the metabolism of S. erecta. Biotrophic pathogens can only survive by parasitizing living plant tissue. Thus, they rarely kill their hosts since they rely on them for nourishment. Evidence for a hemi-biotrophic lifestyle has already been proposed for Bipolaris/Curvularia (Lu et al., 2021; Poudel et al., 2019; Xue et al., 2008; Chen et al., 2003). Additionally, there are reports of a growth-promoting endophytic strain that, under the influence of a hormonal imbalance of indole acetic acid and brassinosteroids, can transform into a biotrophic pathogen (Yousaf et al., 2021).
Bipolaris/Curvulariaare insensitive to or feed on metabolites present in the toothed leaf of S. erecta
The removal of metabolites from the toothed leaf may be linked to the function of secretory channels and glandular trichomes. Some studies have linked these components to temperature gradients to understand their ecological and evolutionary importance (Peppe et al., 2011; Nicotra et al., 2011). Royer & Wilf (2006) hypothesized that toothed leaves increase sap flow, providing nutrients and other solutes to emerging and young leaves. In habitats with low temperatures, toothed leaves may be crucial in maximizing carbon gain potential and early-season growth. According to Hickey & Wolfe (1975), the presence of toothed leaves alongside glands is crucial to comprehending their function. The exudates observed from S. erecta trichomes may have a varied composition (Fahn, 1979) and are likely utilized in protection strategies against pathogen attacks or metabolized by biotrophic species.
Histochemistry of S. erecta
Histochemical tests conducted on S. pernambucensis, another species of the genus Serjania, revealed that its stem contains a complex secretion consisting of various substances in three secretory structures: idioblasts, glandular trichomes, and laticifers (Da Cunha Neto et al., 2017). These data demonstrate the abundance of compounds detected in the leaves of S. erecta, including phenolic compounds, alkaloids, lipids, polysaccharides, proteins, essential oils, and other substances. In general, the presence of phenolic compounds is associated with responses to environmental stresses and/or plant protection (Jachula, Konarska & Denisow, 2018). Phytochemical analyses of plant extracts obtained from S. erecta have indicated the presence of phenolic compounds, including tannins and flavonoids (Gomig et al., 2008; Slomp et al., 2009; Broggini et al., 2010; Fernandes et al., 2011; Cardoso et al., 2013; Guimarães et al., 2015). These compounds protect the plant against herbivores and pathogens, attracting pollinators and dispersers. Furthermore, they can perform other functions, such as protecting against UV radiation or exhibiting allelopathic effects (Taiz & Zeiger, 2013). Studies have shown that these secondary metabolites can be produced directly by the plant or the endophytic fungi it hosts (Rocha et al., 2020; Amirzakariya & Shakeri, 2022).
Moreover, tannins isolated from S. erecta have already demonstrated inhibitory effects on Bothrops jararacussu venom (Fernandes et al., 2011). This suggests that the plant has anticoagulant properties and potential pharmaceutical applications. Guimarães et al. (2015) showed that S. erecta has three main flavonoids: quercetin, vitexin, and isovitexin. Vitexin protects PC12 cells (a cell line derived from an adrenal medulla pheochromocytoma) against toxicity. Additionally, it inhibits the induced generation of nitric oxide in these cells.
The detection of polysaccharides within the secretory cavity provides further evidence for the presence of fungi in these structures because fungi have cell walls composed of polysaccharides such as chitin and glycan (Curto et al., 2021; Brown, Esher & Alspaugh, 2020).
The presence of essential oils and oleoresins in S. erecta leaves was also observed. Resins of this type are composed of a combination of substances, including terpenoids, flavonoids, and lipids (Gupta et al., 2012). This study is the first to report the presence of essential oils and alkaloids in this species. Therefore, we recommend conducting additional research to clarify the composition of these oils and alkaloids and uncover their biotechnological potential. This study highlights the significance of conserving the biodiversity of the Cerrado region, not only for understanding how plants interact with microorganisms but also because these plants could potentially serve as sources for bioactive compounds in the future. This study aligns with initiatives to attribute value to the biodiversity of the Cerrado region and stimulate conservation initiatives and policies.
Conclusions
Bipolaris/Curvularia species colonize S. erecta leaf tissues. Hyphae, conidiophores, and spores are observed in the adaxial epidermis, along with melanized microsclerotia in glandular regions and the phloem, which provide evidence of biotrophic behavior. The hypothesis that biotrophic phytopathogenic fungi interact with S. erecta leaf tissues was confirmed, despite large amounts of bioactive compounds as evidenced by histochemical analyses. On both the toothed leaf and epidermis of S. erecta tector, glandular, and scamiform trichomes have been demonstrated. Furthermore, we are reporting, for the first time, the synthesis of essential oils and alkaloids in the leaves of this species. Therefore, it is recommended that future studies focus on extracting and characterizing these compounds as well as exploring other aspects related to the interaction between S. erecta and the microorganisms in its microbiome.
Acknowledgments
The authors are grateful to the Foundation de Amparo à Pesquisa do Estado de Goiás (Goiás State Research Foundation, FAPEG) and the Rio Verde Campus of the Instituto Federal Goiano (Federal Institute Goiano) for providing the infrastructure and the students involved in this study.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare that there are no competing interests.
Author Contributions
Samylla Tássia Ferreira de Freitas performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Giselle Faria performed the experiments, prepared figures and/or tables, and approved the final draft.
Fabiano Guimarães Silva conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Marco Aurélio Batista performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Damiana Souza Santos Augusto performed the experiments, prepared figures and/or tables, and approved the final draft.
Fábio Henrique Dyszy analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Luciana Cristina Vitorino conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field collections were allowed by the Post-Graduate Direction of the Instituto Federal Goiano Campus Rio Verde (project number: CMRV DPPG 011/2021).
Data Availability
References
- Abdel-Motaal et al. (2020).Abdel-Motaal F, Kamel N, El-Zayat S, Abou-Ellail M. Early blight suppression and plant growth promotion potential of the endophyte Aspergillus flavus in tomato plant. Annals of Agricultural Sciences. 2020;65(2):117–123. doi: 10.1016/j.aoas.2020.07.001. [DOI] [Google Scholar]
- Acevedo-Rodríguez et al. (2011).Acevedo-Rodríguez P, Van Welzen PC, Adema F, Van Der Ham RWJM. Sapindaceae, flowering plants. Eudicots: Sapindales, Cucurbitales, Myrtaceae. 2011;35:7–407. [Google Scholar]
- Agrawal & Fishbein (2006).Agrawal AA, Fishbein M. Plant defense syndromes. Ecology. 2006;87:S132–S149. doi: 10.1890/0012-9658(2006)87[132:PDS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Amirzakariya & Shakeri (2022).Amirzakariya BZ, Shakeri A. Bioactive terpenoids derived from plant endophytic fungi: an updated review (2011–2020) Phytochemistry. 2022;197:113130. doi: 10.1016/j.phytochem.2022.113130. [DOI] [PubMed] [Google Scholar]
- APG IV (Angiosperm Phylogeny Group IV).APG IV (Angiosperm Phylogeny Group IV) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society. 2016;181:1–20. doi: 10.1111/boj.12385. [DOI] [Google Scholar]
- Arruda et al. (2009).Arruda BN, Castelo APC, Coelho RG, Honda NK, Ferrazoli C, Pott A, Hiruma-Lima CA. Gastroprotective effect of Serjania erecta Radlk (Sapindaceae): involvement of sensory neurons, endogenous nonprotein sulfhydryls, and nitric oxide. Journal of Medicinal Food. 2009;141:1–1415. doi: 10.1089/jmf.2008.0269. [DOI] [PubMed] [Google Scholar]
- Berbee, Pirseyedi & Hubbard (1999).Berbee ML, Pirseyedi M, Hubbard S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 1999;91:964–977. doi: 10.1080/00275514.1999.12061106. [DOI] [Google Scholar]
- Broggini et al. (2010).Broggini LS, Fernandes RS, Nogueira T, Suzano FR, Caetano AL, Buck HS, Couto LB, França SC, Pereira PS. Behavioral and enzymatic bioassays with Serjania erecta Radlk., Sapindaceae, correlated with cognitive dysfunctions. Revista Brasileira de Farmacognosia. 2010;20:519–528. doi: 10.1590/S0102-695X2010000400010. [DOI] [Google Scholar]
- Bronstein (2015).Bronstein JL, editor. Mutualism. Oxford, United Kingdom: Oxford University Press; 2015. [Google Scholar]
- Brown, Esher & Alspaugh (2020).Brown HE, Esher SK, Alspaugh JA. Chitin: a hidden figure in the fungal cell wall. Current Topics in Microbiology and Immunology. 2020;425:83–111. doi: 10.1007/82_2019_184. [DOI] [PubMed] [Google Scholar]
- Cardoso et al. (2013).Cardoso CAL, Coelho RG, Honda NK, Pott A, Pavan FR, Leite CQF. Phenolic compounds and antioxidant, antimicrobial and antimycobacterial activities of Serjania erecta Radlk. (Sapindaceae) Brazilian Journal of Pharmaceutical Sciences. 2013;49:775–782. doi: 10.1590/S1984-82502013000400017. [DOI] [Google Scholar]
- Chen et al. (2003).Chen J, Yan H, Gao Z, Xue C, Zhuang J. Identification techniques for physiological differentiation of Curvularia lunata in maize. Acta Phytopathologica Sinica. 2003;33:121–125. [Google Scholar]
- Connor (1995).Connor RC. The benefits of mutualism: a conceptual framework. Biological Reviews. 1995;70:427–457. doi: 10.1111/j.1469-185X.1995.tb01196.x. [DOI] [Google Scholar]
- Curto et al. (2021).Curto MÁ, Butassi E, Ribas JC, Svetaz LA, Cortes JC. Natural products targeting the synthesis of β (1, 3)-D-glucan and chitin of the fungal cell wall. Existing drugs and recent findings. Phytomedicine. 2021;88:153556. doi: 10.1016/j.phymed.2021.153556. [DOI] [PubMed] [Google Scholar]
- Da Cunha Neto et al. (2017).Da Cunha Neto IL, Martins FM, Somner GV, Tamaio N. Secretory structures in stems of five lianas of Paullinieae (Sapindaceae): morphology and histochemistry. Flora. 2017;235:29–40. doi: 10.1016/j.flora.2017.09.001. [DOI] [Google Scholar]
- Da Silva & Batalha (2011).Da Silva DM, Batalha MA. Defense syndromes against herbivory in a cerrado plant community. Plant Ecology. 2011;212(2):181–193. doi: 10.1007/s11258-010-9813-y. [DOI] [Google Scholar]
- David & Carde (1964).David R, Carde JP. Coloration différentielle dês inclusions lipidique et terpeniques dês pseudophylles du Pin maritime au moyen du reactif Nadi. Comptes Rendus Hebdomadaires Dês Séances de L’ Academie Dês Sciences Paris. Série D. 1964;258:1338–1340. [Google Scholar]
- De Freitas et al. (2022).De Freitas STF, Rodrigues ARDS, Ataídes ACC, De Oliveira Menino GC, De Faria GS, Vitorino LC, Silva FG, Dyszy FH. Inhibitory effects of Serjania erecta on the development of Chrysodeixis includens. Scientific Reports. 2022;12(1):1–8. doi: 10.1038/s41598-021-99269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlemann et al. (2017).Doehlemann G, Ökmen B, Zhu W, Sharon A. Plant pathogenic fungi. Microbiology Spectrum. 2017;5(1):5–1. doi: 10.1128/microbiolspec.funk-0023-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Reis, Do Vale & Lorenzi (2022).Dos Reis JBA, Do Vale HMM, Lorenzi AS. Insights into taxonomic diversity and bioprospecting potential of Cerrado endophytic fungi: a review exploring an unique Brazilian biome and methodological limitations. World Journal of Microbiology and Biotechnology. 2022;38(11):202. doi: 10.1007/s11274-022-03386-2. [DOI] [PubMed] [Google Scholar]
- Dos Santos et al. (2022).Dos Santos PRR, De Souza Carlos Mourão D, Dalcin MS, Osorio PRA, De Oliveira Lima FS, dos Santos GR. Pathogenicity of fungi associated with Andropogon grass seeds. Journal of Plant Pathology. 2022;104(2):565–573. doi: 10.1007/s42161-022-01038-w. [DOI] [Google Scholar]
- Duveiller et al. (2002).Duveiller E, Chand R, Singh HV, Joshi AK. Physiological and morphological aspects of Bipolaris sorokiniana conidia surviving on wheat straw. The Plant Pathology Journal. 2002;18(6):328–332. doi: 10.5423/PPJ.2002.18.6.328. [DOI] [Google Scholar]
- Elkhateeb, Kolaibe & Daba (2021).Elkhateeb WA, Kolaibe AG, Daba GM. Cochliobolus, Drechslera, Bipolaris, Curvularia different nomenclature for one potent fungus. Journal of Pharmaceutics and Pharmacology Research. 2021;4(1):1–6. doi: 10.31579/2693-7247/031. [DOI] [Google Scholar]
- Fahn (1979).Fahn A. Secretory tissues in plants. Academic Press; London: 1979. [Google Scholar]
- Ferdinandez et al. (2021).Ferdinandez HS, Manamgoda DS, Udayanga D, Deshappriya N, Munasinghe MS, Castlebury LA. Molecular phylogeny and morphology reveal three novel species of Curvularia (Pleosporales, Pleosporaceae) associated with cereal crops and weedy grass hosts. Mycological Progress. 2021;20:431–451. doi: 10.1007/s11557-021-01681-0. [DOI] [Google Scholar]
- Fernandes et al. (2011).Fernandes RS, Costa TR, Marcussi S, Bernardes CP, Menaldo DL, Rodriguéz Gonzaléz II, Pereira PS, Soares AM. Neutralization of pharmacological and toxic activities of Bothrops jararacussu snake venom and isolated myotoxins by Serjania erecta methanolic extract and its fractions. Journal of Venomous Animals and Toxins Including Tropical Diseases. 2011;17(1):85–93. doi: 10.1590/S1678-91992011000100011. [DOI] [Google Scholar]
- Flora do Brasil (2020).Flora do Brasil Jardim Botânico do Rio De Janeiro. 2020. http://floradobrasil.jbrj.gov.br/ [07 January 2023]. http://floradobrasil.jbrj.gov.br/
- Fonsêca, Proença & Gonçalves (2007).Fonsêca LCM, Proença CEB, Gonçalves EG. Descrição do padrão de venação foliar em Spathicarpa Hook. (Araceae) Acta Botanica Brasilica. 2007;21:213–221. doi: 10.1590/S0102-33062007000100020. [DOI] [Google Scholar]
- Furr & Mahlberg (1981).Furr M, Mahlberg PG. Histochemical analyses of laticifers and glandular trichomes in Cannabis sativa. Journal of Natural Products. 1981;44:153–159. doi: 10.1021/np50014a002. [DOI] [Google Scholar]
- Gabe (1968).Gabe M. Techniques histologiques. Masson et Cie; Paris: 1968. [Google Scholar]
- Gilardi et al. (2022).Gilardi G, Mocioni M, Gullino ML, Guarnaccia V. Curvularia americana and Curvularia tropicalis cause leaf and crown necrosis on Bermuda grass in Italy. Phytopathologia Mediterranea. 2022;61:431–437. [Google Scholar]
- Gomig et al. (2008).Gomig F, Pietrovski EF, Guedes A, Dalmarco EM, Calderari MT, Guimarães CL, Pinheiro RM, Cabrini DA, Otuki MF. Topical anti-inflammatory activity of Serjania erecta Radlk (Sapindaceae) extracts. Journal of Ethnopharmacology. 2008;118(2):220–224. doi: 10.1016/j.jep.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Guarim Neto & Macedo (2009).Guarim Neto G, Macedo M. Utilização de vegetais na medicina tradicional. I. Serjania erecta Radlk.(Cinco-folhas) FLOVET-Boletim Do Grupo de Pesquisa Da Flora, Vegetação e Etnobotânica. 2009;1(1):14–20. [Google Scholar]
- Guarim Neto, Santana & Silva (2000).Guarim Neto G, Santana SR, Silva JVBD. Notas etnobotânicas de espécies de Sapindaceae Jussieu. Acta Botanica Brasilica. 2000;14:327–334. doi: 10.1590/S0102-33062000000300009. [DOI] [Google Scholar]
- Guimarães et al. (2015).Guimarães CC, Oliveira DD, Valdevite M, Saltoratto ALF, Pereira SIV, De Castro França S, Pereira AMS, Pereira PS. The glycosylated flavonoids vitexin, isovitexin, and quercetrin isolated from Serjania erecta Radlk (Sapindaceae) leaves protect PC12 cells against amyloid- β25-35 peptide-induced toxicity. Food and Chemical Toxicology. 2015;86:88–94. doi: 10.1016/j.fct.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Gupta et al. (2012).Gupta P, Sharma U, Schulz TC, McLean AB, Robins AJ, West LM. Bicyclic C21 terpenoids from the marine sponge Clathria compressa. Journal of Natural Products. 2012;75:1223–1227. doi: 10.1021/np300265p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcombe et al. (2018).Harcombe WR, Chacón JM, Adamowicz EM, Chubiz LM, Marx CJ. Evolution of bidirectional costly mutualism from byproduct consumption. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(47):12000–12004. doi: 10.1073/pnas.1810949115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood et al. (2007).Heywood VH, Brummitt RK, Culham A, Seberg O. Flowering plant families of the world (Vol. 88) Firefly books; Ontario: 2007. [Google Scholar]
- Hickey & Wolfe (1975).Hickey LJ, Wolfe JA. The bases of angiosperm phylogeny: vegetative morphology. Annals of the Missouri Botanical Garden. 1975;62(3):538–589. doi: 10.2307/2395267. [DOI] [Google Scholar]
- Horbach et al. (2011).Horbach R, Navarro-Quesada AR, Knogge W, Deising HB. When and how to kill a plant cell: infection strategies of plant pathogenic fungi. Journal of Plant Physiology. 2011;168(1):51–62. doi: 10.1016/j.jplph.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Jachula, Konarska & Denisow (2018).Jachula J, Konarska A, Denisow B. Micromorphological and histochemical attributes of flowers and floral reward in Linaria vulgaris (Plantaginaceae) Protoplasma. 2018;255:1763–1776. doi: 10.1007/s00709-018-1269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed et al. (2019).Javed A, Shah AH, Hussain A, Khan SA, Khan SA, Hamayun M, Hassan I, Jan SA. Identification and characterization of Penicillium chrysogenum T8 as potent plant growth promoting endophytic fungi. FEB-Fresenius Environmental Bulletin. 2019;4896 [Google Scholar]
- Khiralla et al. (2019).Khiralla A, Spina R, Saliba S, Laurain-Mattar D. Diversity of natural products of the genera Curvularia and Bipolaris. Fungal Biology Reviews. 2019;33(2):101–122. doi: 10.1016/j.fbr.2018.09.002. [DOI] [Google Scholar]
- Leimar & Hammerstein (2010).Leimar O, Hammerstein P. Cooperation for direct f itness benefits. Philosophical Transactions of the Royal Society B. 2010;365:2619–2626. doi: 10.1098/rstb.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2022).Liu HH, Huang CC, Lin YH, Tseng MN, Chang HX. Superoxide initiates the hyphal differentiation to microsclerotia formation of Macrophomina phaseolina. Microbiology Spectrum. 2022;10(1):e02084–21. doi: 10.1128/spectrum.02084-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2021).Lu Y, Sun J, Gao Y, Liu K, Yuan M, Gao W, Wang F, Fu D, Chen N, Xiao S, Xue C. The key iron assimilation genes ClFTR1, ClNPS6 were crucial for virulence of Curvularia lunata via initiating its appressorium formation and virulence factors. Environmental Microbiology. 2021;23(2):613–627. doi: 10.1111/1462-2920.15101. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2019).Luo X, Xie C, Dong J, Yang X. Comparative transcriptome analysis reveals regulatory networks and key genes of microsclerotia formation in the cotton vascular wilt pathogen. Fungal Genetics and Biology. 2019;126:25–36. doi: 10.1016/j.fgb.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Mace & Howell (1974).Mace ME, Howell CR. Histochemistry and identification of condensed tannin precursor in roots of cotton seedlings. Canadian Journal of Botany. 1974;52:2423–2426. doi: 10.1139/b74-314. [DOI] [Google Scholar]
- Nicotra et al. (2011).Nicotra AB, Leigh A, Boyce CK, Jones CS, Niklas KJ, Royer DL, Tsukaya H. The evolution and functional significance of leaf shape in the angiosperms. Functional Plant Biology. 2011;38(7):535–552. doi: 10.1071/FP11057. [DOI] [PubMed] [Google Scholar]
- O’Brien, Feder & McCully (1964).O’Brien TP, Feder N, McCully ME. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma. 1964;59:367–373. [Google Scholar]
- O’Brien & McCully (1981).O’Brien TP, McCully ME. The study of plant structure. Principles and selected methods. Melbourne: Termacarphi Pty Ltd; 1981. [Google Scholar]
- Patil, Kulkarni & More (1966).Patil PL, Kulkarni NB, More BB. Curvularia leaf blight of bajra (Pennisetum typhoides Stapf.) in India. Mycopathologia Et Mycologia Applicata. 1966;28(4):348–352. doi: 10.1007/BF02145111. [DOI] [PubMed] [Google Scholar]
- Peppe et al. (2011).Peppe DJ, Royer DL, Cariglino B, Oliver SY, Newman S, Leight E, Enikolopov G, Fernandez-Burgos M, Herrera F, Adams JM, Correa E, Currano ED, Mark Erickson J, Hinojosa LF, Hoganson JW, Iglesias A, Jaramillo CA, Johnson KR, Jordan GJ, Kraft NJB, Lovelock EC, Lusk CH, Niinemets Ü, Peñuelas J, Rapson G, Wing SL, Wright IJ. Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytologist. 2011;190(3):724–739. doi: 10.1111/j.1469-8137.2010.03615.x. [DOI] [PubMed] [Google Scholar]
- Poudel et al. (2019).Poudel A, Navathe S, Chand R, Mishra VK, Singh PK, Joshi AK. Hydrogen peroxide prompted lignification affects pathogenicity of hemi-biotrophic pathogen Bipolaris sorokiniana to wheat. The Plant Pathology Journal. 2019;35(4):287–300. doi: 10.5423/PPJ.OA.09.2018.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis et al. (1998).Reis EM, Silva CEL, Casa RT, Medeiros CA. Decomposition of wheat crop residues and saprophytic survival of Bipolaris sorokiniana. Fitopatologia Brasileira. 1998;23(1):62–64. [Google Scholar]
- Reis & Bellini (2007).Reis SLA, Bellini LM. Conhecimento e uso da flora para fins medicinais em comunidades ribeirinhas dos rios Paraná, PR e Cuiabá, MT. 2007. http://www.educadores.diaadia.pr.gov.br/arquivos/File/2010/artigos_teses/2010/Biologia/artigos/flora_ribeirinha.pdf http://www.educadores.diaadia.pr.gov.br/arquivos/File/2010/artigos_teses/2010/Biologia/artigos/flora_ribeirinha.pdf
- Rocha et al. (2020).Rocha PDSD, Paula VMB, Olinto SCF, Dos Santos EL, De Picoli Souza K, Estevinho LM. Diversity, chemical constituents and biological activities of endophytic fungi isolated from Schinus terebinthifolius Raddi. Microorganisms. 2020;8(6):859. doi: 10.3390/microorganisms8060859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez & Pinto (2014).Rodríguez RH, Pinto AC. Constituintes Químicos e Propriedades Biológicas de Espécies do Gênero Serjania. Revista Virtual de Química. 2014;6:1583–1606. [Google Scholar]
- Rosa et al. (2010).Rosa LH, Gonçalves VN, Caligiorne RB, Alves T, Rabello A, Sales PA, Romanha AJ, Sobral MEG, Rosa CA, Zani CL. Leishmanicidal, trypanocidal, and cytotoxic activities of endophytic fungi associated with bioactive plants in Brazil. Brazilian Journal of Microbiology. 2010;41:420–430. doi: 10.1590/S1517-83822010000200024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer & Wilf (2006).Royer DL, Wilf P. Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy. International Journal of Plant Sciences. 2006;167(1):11–18. doi: 10.1086/497995. [DOI] [Google Scholar]
- Ruotsalainen et al. (2021).Ruotsalainen AL, Kauppinen M, Wäli PR, Saikkonen K, Helander M, Tuomi J. Dark septate endophytes: mutualism from by-products? Trends in Plant Science. 2021;27:247–254. doi: 10.1016/j.tplants.2021.10.001. [DOI] [PubMed] [Google Scholar]
- Salvatore & Andolfi (2021).Salvatore MM, Andolfi A. Phytopathogenic fungi and toxicity. Toxins. 2021;13(10):689. doi: 10.3390/toxins13100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer (1997).Scheffer RP. The nature of disease in plants. Cambridge University Press; New York: 1997. [Google Scholar]
- Segatto et al. (2004).Segatto FB, Bisognin DA, Benedetti M, Costa LC, Rampelotto MV, Nicolodo FT. Técnica para o estudo da anatomia da epiderme foliar de batata. Ciência Rural. 2004;34(5):1597–1601. doi: 10.1590/S0103-84782004000500042. [DOI] [Google Scholar]
- Shobe & Lersten (1967).Shobe WR, Lersten NR. Uma técnica para limpar e tingir folhas de gimnosperma. Botanical Gazette. 1967;128(2):150–152. doi: 10.1086/336391. [DOI] [Google Scholar]
- Slomp et al. (2009).Slomp L, Pereira PS, Franca SDC, Zingaretti S, Beleboni RO. In vitro nematocidal effects of medicinal plants from Sao Paulo state, Brazil. Pharmaceutical Biology. 2009;47(3):230–235. doi: 10.1080/13880200802434658. [DOI] [Google Scholar]
- Srivastava et al. (2021).Srivastava AK, Kapkoti DS, Gupta M, Rout PK, Bhakuni RS, Samad A. Enhanced production of phytotoxic polyketides isolated from Curvularia lunata by applying chemical stresses. Industrial Crops and Products. 2021;160:113156. doi: 10.1016/j.indcrop.2020.113156. [DOI] [Google Scholar]
- Stohr & Dighton (2004).Stohr SN, Dighton J. Effects of species diversity on establishment and coexistence: a phylloplane fungal community model system. Microbial Ecology. 2004;48:431–438. doi: 10.1007/s00248-003-1064-1. [DOI] [PubMed] [Google Scholar]
- Taiz & Zeiger (2013).Taiz L, Zeiger E. Fisiologia vegetal. 5th edition Editora Artmed; Porto Alegre: 2013. [Google Scholar]
- Tanaka, Pinto & Mourão (2014).Tanaka BMM, Pinto DD, Mourão KSM. Ontogeny of the pericarp of Serjania communis Camb, and Urvillea ulmacea Kunth (Sapindaceae) with emphasis on the dispersion apparatus. Acta Scientiarum. Biological Sciences. 2014;36(4):457–465. doi: 10.4025/actascibiolsci.v36i4.20666. [DOI] [Google Scholar]
- Teles et al. (2005).Teles HL, Silva GH, Castro-Gamboa I, Da Silva Bolzani V, Pereira JO, Costa-Neto CM, Haddad R, Eberlin MN, Young MCM, Araújo ÂR. Benzopyrans from Curvularia sp., an endophytic fungus associated with Ocotea corymbosa (Lauraceae) Phytochemistry. 2005;66(19):2363–2367. doi: 10.1016/j.phytochem.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Toghueo & Boyom (2020).Toghueo RMK, Boyom FF. Endophytic Penicillium species and their agricultural, biotechnological, and pharmaceutical applications. 3 Biotech. 2020;10(3):107. doi: 10.1007/s13205-020-2081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura et al. (2021).Ventura AS, Correa Filho RA, Spica LN, Silva ACF, Araújo AM, Cardoso CA, Jeronimo GT, Povh JA. Toxicological, biochemical and morphophysiological effects of Serjania erecta leaf aqueous extract on Piaractus mesopotamicus. Anais Da Academia Brasileira de Ciências. 2021;93:e20190479. doi: 10.1590/0001-3765202120190479. [DOI] [PubMed] [Google Scholar]
- Vianello, Macri & Passera (1976).Vianello A, Macri F, Passera C. Effect of Curvularia lunata phytotoxin on membrane permeability of corn roots. Canadian Journal of Botany. 1976;54(24):2918–2923. doi: 10.1139/b76-314. [DOI] [Google Scholar]
- Wang, Wen & Asiegbu (2022).Wang K, Wen Z, Asiegbu FO. The dark septate endophyte Phialocephala sphaeroides suppresses conifer pathogen transcripts and promotes root growth of Norway spruce. Tree Physiology. 2022;42(12):2627–2639. doi: 10.1093/treephys/tpac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, Terhonen & Asiegbu (2022).Wen Z, Terhonen E, Asiegbu FO. The dark septate endophyte Phialocephala sphaeroides confers growth fitness benefits and mitigates pathogenic effects of Heterobasidion on Norway spruce. Tree Physiology. 2022;42(4):891–906. doi: 10.1093/treephys/tpab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao et al. (1991).Xiao JZ, Tsuda M, Doke N, Nishimura S. Phytotoxins produced by germinating spores of Bipolaris oryzae. Phytopathology. 1991;81:58–64. doi: 10.1094/Phyto-81-58. [DOI] [Google Scholar]
- Xue et al. (2008).Xue C, Xiao S, Zhai Y, Gao Y, Gao Z, Chen J. Pathogenicity differentiation of Curvularia lunata. Acta Phytopathologica Sinica. 2008;38:6–12. [Google Scholar]
- Yin et al. (2018).Yin C, Jin L, Sun F, Xu X, Shao M, Zhang Y. Phytotoxic and antifungal metabolites from Curvularia crepinii QTYC-1 isolated from the gut of Pantala flavescens. Molecules. 2018;23(4):951. doi: 10.3390/molecules23040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf et al. (2021).Yousaf MJ, Hussain A, Hamayun M, Iqbal A, Irshad M, Kim HY, Lee IJ. Transformation of Endophytic Bipolaris spp., into biotrophic pathogen under auxin cross-talk with brassinosteroids and abscisic acid. Frontiers in Bioengineering and Biotechnology. 2021;9:657635. doi: 10.3389/fbioe.2021.657635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2011).Zhang YL, Kong LC, Jiang DH, Yin CP, Cai QM, Chen Q, Zheng JY. Phytotoxic and antifungal metabolites from Curvularia sp. FH01 isolated from the gut of Atractomorpha sinensis. Bioresource Technology. 2011;102(3):3575–3577. doi: 10.1016/j.biortech.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2009).Zhang Y, Schoch CL, Fournier J, Crous PW, De Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD. Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology. 2009;64(1):85–102. doi: 10.3114/sim.2009.64.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
All data used in the preparation of the article are available in Figs. 1–14.