Abstract
Streptococcus gallolyticus subspecies pasteurianus, formerly classified as S. bovis biotype II/2 until 2003, is a rare cause of infant meningitis. Over the past 2 decades, only a few individual case reports and limited case series exist in the English-language literature. Moreover, the pathogenesis of S. gallolyticus subsp. pasteurianus meningitis in infants is unclear. Here we report a case of meningitis in a 6-week-old infant with hypothyroidism and preceding diarrhea. In this case, S. gallolyticus was cultured from cerebrospinal fluid, and then S. gallolyticus subspecies pasteurianus was identified by metagenomic next-generation Sequencing. The infant recovered uneventfully after a 4-week antibiotic course with ceftriaxone and vancomycin. Then combined with the literature of S. gallolyticus subsp. pasteurianus meningitis in infants, we discuss the possible etiology.
Keywords: Streptococcus gallolyticus subsp. pasteurianus, infant, meningitis, hypothyroidism, diarrhea
Introduction
The Streptococcus gallolyticus (S. gallolyticus), a Lancefield group D streptococcus, is one of the intestinal microflorae found in humans and animals.1 With new molecular analysis techniques leading to taxonomic changes, S. gallolyticus has been further subdivided into three subspecies: S. gallolyticus subsp. gallolyticus (S. gallolyticus), S. gallolyticus subsp. infantarius(S. infantarius), and S. gallolyticus subsp. pasteurianus(S. pasteurianus).1 It is important to have proper identification of the S. gallolyticus subspecies as it has its own clinical implications. While S. gallolyticus is linked to colonic carcinoma and endocarditis,1 S. infantarius is associated with non-colonic cancers,2 whereas S. pasteurianus is a pathogen causing meningitis and bacteremia in infants and adults.3,4 S. pasteurianus can be found as part of the normal human gastrointestinal microbiota. As a conditional pathogenic bacterium, it rarely causes meningitis in infants.1,5 The source and pathogenesis of S. pasteurianus meningitis in infants remains uncertain. Here we report a case of meningitis due to S. pasteurianus in a 6-week-old infant with hypothyroidism and preceding diarrhea. In addition, we review the literature of S. pasteurianus meningitis in infants to discuss possible etiology.
Case Report
A 6-week-old infant boy was born at term, weighing 3510g. His mother was diagnosed with maternal hypothyroidism during pregnancy, and then began to take levothyroxine tablets for treatment orally. On top of that, both the pregnancy and the delivery were uneventful. He was exclusively formula fed and developed abdominal distension and frequent vomit. At 5 weeks of life, the infant presented diarrhea, but this illness was not treated. Two days prior to the infant’s admission, he developed fever with a temperature of 39.6 Celsius and lethargy. Then, he was taken to Jiangsu Taizhou People’s Hospital. Physical examination at the hospital revealed his anterior fontanelle was full, but he did not present neurological deficits or other features of increased intracranial pressure. The abdomen was distended, but soft. Heart and lung examinations were unremarkable. A sepsis workup and lumbar puncture were performed, and the infant was empirically treated with meropenem (40 mg/kg of body weight every 8 h) and vancomycin (15 mg/kg of body weight every 8 h) for presumed sepsis and meningitis. Initial laboratory studies are presented in Table 1. According to these results and the symptoms, a diagnosis of bacterial meningitis was made. At the parents’ request, the baby was transported to our hospital for further investigation and treatment. Before the baby was transported, only two-dose meropenem and vancomycin were given.
Table 1.
Blood and Cerebrospinal Fluid Results (Jiangsu Taizhou People’s Hospital)
| Parameter (Reference Range) | Hospital Day (HOD) 1 |
|---|---|
| White blood cell count (8×109-12×109/L) | 5.85 |
| % Neutrophils | 58.1 |
| % Lymphocytes | 34.9 |
| C-reactive protein level (≤5mg/L) | 7.92 |
| CSF nucleated cell count (0–20×106/L) | 2723 |
| % Multinucleate cell | 71.2 |
| % Monocytes | 28.8 |
| CSF red blood cell count (0–10×106/L) | 0 |
| CSF glucose concn (2.8–4.5mmol/L) | 2.95 |
| CSF protein concn (150–450mg/L) | 1613 |
In our hospital, we continued the combination of meropenem (40 mg/kg of body weight every 8 h) and vancomycin (15 mg/kg of body weight every 8 h). Simultaneously, sepsis workup and lumbar puncture for cerebrospinal fluid (CSF) analysis were repeated. These results are presented in Table 2. CSF leukocytes, protein, and sugar all decreased significantly, and fever subsided on hospital day 2, suggesting meropenem and vancomycin were effective. On day 3 of admission, blood culture was negative, but CSF culture indicated Streptococcus gallolyticus (S. gallolyticus) sensitive to linezolid, penicillin, ceftriaxone, and vancomycin. S. gallolyticus subspecies pasteurianus was identified by metagenomic next-generation sequencing (mNGS) in CSF. According to the antimicrobial susceptibility test, meropenem was discontinued on day 4 of admission. However, the temperature went back up to 38.2° Celsius within 12 hours after meropenem was discontinued. Then treatment was changed to the combination of ceftriaxone (100 mg/kg of body weight every day) and vancomycin (15 mg/kg of body weight every 8 h) on day 5 of admission. The fever gradually disappeared, and clinical well-being improved significantly on day 6 of admission. A repeated CSF on day 7 of admission was negative for bacteria culture, but CSF leukocytes were still high (Table 2). A subsequent lumbar puncture was repeated on day 14 of admission, to assess the treatment response. The CSF analysis showed CSF leukocytes, protein, and sugar were all normal (Table 2). A magnetic resonance image of the brain with contrast showed meningeal enhancement of the left temporal pole on T2-Flair imaging, with no ventriculitis (Figure 1). Therefore, ceftriaxone and vancomycin were scheduled to continue for a total of 28 days. In addition, given that his mother had maternal hypothyroidism, and he had abdominal distension frequently, the serum thyroid function test was performed, and results showed free triiodothyronine (T3) of 2.60 pmol/L (reference range 2.85–7.78 pmol/L), free thyroxine (T4) of 7.95pmol/L (reference range 9.8–23.2 pmol/L), triiodothyronine of 0.84 nmol/L (reference range 0.9–2.8nmol/L), and thyroid-stimulating hormone (TSH) of 0.72μIU/mL(reference range 0.7–9.8μIU/mL). Endocrinologists consulted and suggested thyroxine replacement therapy. Thyroid function returned to normal after 2 weeks of treatment with levothyroxine. Moreover, cell-mediated immunity and humoral immunity were evaluated, but no impaired immune function was identified.
Table 2.
Blood and Cerebrospinal Fluid Results (Shanghai Children’s Hospital)
| Parameter (Reference Range) | HOD 1 | HOD 7 | HOD 14 |
|---|---|---|---|
| White blood cell count (8×109-12×109/L) | 7.51 | 5.9 | 7.4 |
| % Neutrophils | 59.4 | 12.8 | 32.1 |
| % Lymphocytes | 29.4 | 66.8 | 57.8 |
| % Monocytes | 10.9 | 15.3 | 8.2 |
| C-reactive protein level (≤5mg/L) | 11 | ≤5 | ≤5 |
| CSF WBC count (0–20×106/L) | 148 | 40 | 7 |
| % Neutrophils | 25 | 10 | / |
| % Lymphocytes | 10 | 58 | / |
| % Monocytes | 65 | 32 | / |
| CSF red blood cell count (0–10×106/L) | 10 | 25 | 180 |
| CSF glucose concn (2.8–4.5mmol/L) | 2.9 | 2.6 | 2.7 |
| CSF protein concn (150–450mg/L) | 980 | 550 | 590 |
Figure 1.
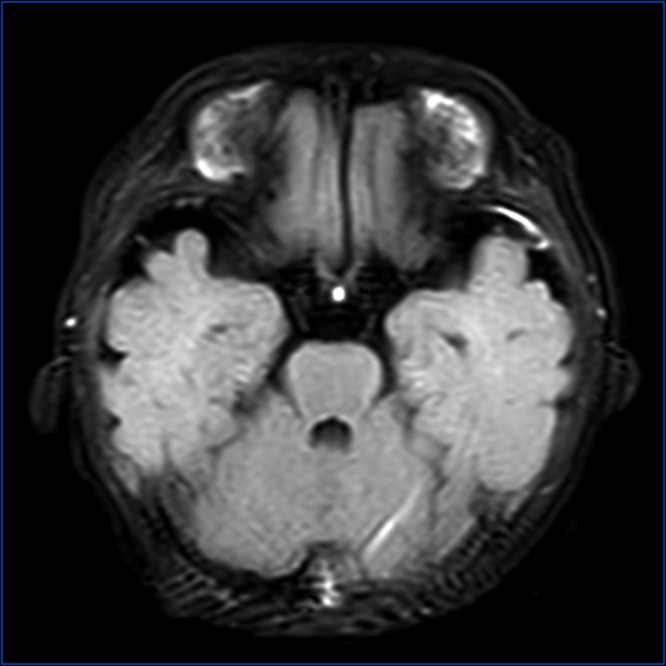
Magnetic resonance imaging of the brain, with contrast. Meningeal enhancement of the left temporal pole on T2-Flair imaging.
Discussion
In this case, S. gallolyticus was cultured from CSF, and then identified as S. gallolyticus subsp. pasteurianus on mNGS. To our knowledge, this is the first report of the use of mNGS to demonstrate that S. gallolyticus subsp. pasteurianus from CSF.
S. gallolyticus subsp. pasteurianus was formerly known as S. bovis biotype II.2 until 2003, when the taxonomy classification changed.6 S. gallolyticus subsp. pasteurianus is a normal bacterium found in the gut of humans and animals, especially ruminants.6,7 Noble7 demonstrated that S. bovis was isolated frequently from the stool samples of healthy neonates. Therefore, as a conditional pathogenic bacterium, S. gallolyticus subsp. pasteurianus has been infrequently reported as a cause of meningitis in infants. Much of the knowledge of S. gallolyticus subsp. pasteurianus meningitis in infants comes from individual case reports or limited case series.5,8–19 Over the past 2 decades, no one case has been reported in China Mainland and 28 cases have been reported in other countries or regions (Table 3). Almost all of the patients were neonates (≤30 days of life), and the oldest case among late onset was 6 weeks of life. Clinical presentation and examination findings of these cases are similar to those caused by group B streptococcal (GBS).20,21 In early onset sepsis, respiratory distress is the most common clinical manifestation. Hede et al and Chen et al reported five infants diagnosed with S. gallolyticus subsp. pasteurianus, and all of them had early onset sepsis and presented with respiratory distress.15,17 In late onset sepsis, meningitis is more common. Neurological symptoms such as irritability and seizures were more commonly reported in cases of late onset sepsis or meningitis.9,11,14,16 The target antimicrobial therapy often includes penicillin, ampicillin, or cefotaxime. After antibiotic therapy, all the reported cases survived with a relatively good prognosis. Neurological complications have rarely been reported. Park et al reported a case with delayed-onset subdural effusion and bilateral reduction of visual evoked potentials, and subsequent follow-up did not reveal any neurological sequelae in a 28-day-old male infant.14 Yoshiko et al reported the first case of ventriculitis diagnosed using follow-up magnetic resonance imaging (MRI). Antibiotic therapy was discontinued after the patient showed improvement, according to MRI findings, and the patient was discharged without sequelae.16 In addition, similar to patterns of GBS meningitis, most authors have proposed that this infection occurs via horizontal transmission or vertical contagion.12,15,20 The pathogenesis of invasive S. gallolyticus subsp. pasteurianus infection in infants remains unclear. Recent studies have suggested the gastrointestinal tract as a possible source. The late-onset case report from Takahashi et al demonstrated identical isolates of S. gallolyticus subsp. pasteurianus was isolated from blood, CSF, and stool, suggesting gastrointestinal tract as a possible source.13 The possible mechanism was translocation of previously commensal bacteria, with initiation of bacteremia and subsequent systemic dissemination and infiltration into CSF. In our case, 1 week prior to the infant’s admission, the infant developed diarrhea. However, S. gallolyticus subsp. pasteurianus was isolated from SCF but not from stool and blood.
Table 3.
Streptococcus Gallolyticus Subsp. Pasteurianus Meningitis in Infants
| Study (Case No.) [Country] | Age (day) | Sex | Gestational age | Clinical presentation | Site(s) of Isolation | Treatment | Complications | Outcome |
|---|---|---|---|---|---|---|---|---|
| Onoyama et al8
(n=1) [Japan] |
5 | F | Term | Fever | Blood, CSF | Cefotaxime | No | Lived |
| Punpanich et al9
(n=1) [Thailand] |
42 | M | Term | Fever, Seizure | Blood, CSF | Penicillin | No | Lived |
| Thatrimontrichai et al10 (n=1) [Thailand] |
2 | M | Term | Fever, Lethargic | CSF | Cefotaxime | No | Lived |
| Nagamatsu et al11
(n=1) [Japan] |
8 | M | Term | Fever, Seizure | CSF | Ampicillin | No | Lived |
| Klatte et al12
(n=4) [USA] |
2-5-5-13 | M-M-F-M | Term | Fever, Seizure | Blood, CSF | Cefotaxime, Ampicillin | No | Lived |
| Takahashi et al13
(n=1) [Japan] |
35 | M | Term | Fever, Diarrhea | Blood, CSF, Stool | Cefotaxime, Ampicillin | No | Lived |
| Park et al14 (n=1) [Korea] | 28 | M | Term | Fever, Lethargic | Blood, CSF | Cefotaxime, Ampicillin | Subdural effusion | Lived |
| Hede et al15
(n=2) [USA] |
21–21 | M-M | Preterm | RDS, Seizure | Blood, CSF | Ampicillin | No | Lived |
| Yamamura et al16
(n=1) [Japan] |
28 | M | Term | Fever, Lethargic | Blood, CSF | Ampicillin | Ventriculitis | Lived |
| Chen et al17
(n=3) [Chinese Taiwan] |
2-3-5 | F-F-M | Term- Preterm- Term | Fever, Tachypnea | Blood, CSF | Cefotaxime, Ampicillin | No | Lived |
| Beneteau et al18
(n=8) [France] |
NA | NA | NA | NA | NA | NA | NA | Lived |
| Orbea et al19
(n=4) [USA] |
NA | NA | NA | NA | NA | NA | NA | Lived |
Abbreviations: F, female; M, male; CSF, cerebrospinal fluid; N/A, not available; RDS, respiratory distress syndrome.
Hypothyroidism is a known risk factor for intestinal infections and sepsis, but its role in other neonatal infections remains unclear.22 The intestinal manifestations of hypothyroidism are varied, and their pathogenesis is not fully understood. In both hypothyroid animals and humans, the electrical and mechanical activities of the gastrointestinal tract has been documented to be decreased.23 Furthermore, hypothyroid subjects delayed neuronal transmission of the intestine due to myxedematous and round cell infiltration at the myoneural junction has been shown to cause peripheral neuropathy of the intestine, which can lead to reduced gut motility, ileus, abdominal distention, and compromised mesenteric blood flow.24 In the presence of formula feeding, such reduced intestinal motility may allow intestinal bacterial overgrowth with the resulting bacterial fermentation and gas production, which may lead to further distention of the intestine. In our case, the infant had hypothyroidism and abdominal distension, vomiting frequently. According to the frequent isolation of S. gallolyticus subsp. pasteurianus from stool samples of healthy neonates, we assume that hypothyroidism and gastrointestinal symptoms may cause translocation of previously commensal bacteria and subsequent bacteremia.
Impaired immune function is also a known risk factor for infection. The interplay between thyroid hormone action and the immune system has been established in physiological and pathological settings.25 Dendritic cells (DCs) are the main antigen-presenting cells at the interface between innate and adaptive immunity. Exposing immature DCs to physiological levels of T3 can induce the differentiation and maturation of antigen-presenting cells, leading to an enhancement in the proliferative capacity of T cells.25,26 Thyroid hormones can also affect the activity and function of macrophages. The inflammatory response exerted by macrophages was stimulated during hypothyroid condition and inhibited in the course of hyperthyroidism.27 On the other hand, abnormalities in immune function can also affect the synthesis and metabolism of thyroid hormones. For example, the “euthyroid sick syndrome” is distinctive of critically ill patients with severe infections or sepsis, being characterized by low serum T3 and in serious cases by also low serum T4 without the expected increase in TSH.25 In this case, the manifestation of thyroid dysfunction is similar to that seen in cases of infection-induced hypothyroidism. Therefore, their connection is complex and difficult to interpret, with an improved knowledge still necessary.
Our case has several limitations. Since S. gallolyticus subsp. pasteurianus is the cause of 8–29% cases of endocarditis and colon disease, it is supposed to identify whether a colonic disease or endocarditis is present by colonoscopy and echocardiography.28 As this is an observational case, colonoscopy and echocardiography were not performed.
Conclusion
In summary, we hypothesize that S. gallolyticus subsp. pasteurianus exists in the intestinal tract and then translocates into the blood because of hypothyroidism and invades the blood and CSF. These cases broaden the clinical diversity of infants with hypothyroidism, and also highlight the importance of a thorough evaluation of thyroid function of infant whose mother was diagnosed maternal hypothyroidism. We consider that this article provides important clues for the understanding of the pathogenesis of meningitis due to S. gallolyticus subsp. pasteurianus.
Abbreviations
CSF, cerebrospinal fluid; mNGS, metagenomic next-generation sequencing; T3, triiodothyronine; T4, free thyroxine; TSH, thyroid-stimulating hormone; GBS, group B streptococcal; HOD, Hospital Day; F, female; M, male; N/A, not available; RDS, respiratory distress syndrome.
Data Sharing Statement
Not shared as it contains confidential patient data.
Ethics Approval and Informed Consent
The parents of the patient provided informed consent for the case details to be published. Shanghai Children’s Hospital approval to publish the case details.
Consent for Publication
The parents of this patient provided the written permission to the publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- 1.Romero B, Morosini MI, Loza E, et al. Reidentification of Streptococcus bovis isolates causing bacteremia according to the new taxonomy criteria: still an issue? J Clin Microbiol. 2011;49(9):3228–3233. doi: 10.1128/JCM.00524-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corredoira J, Alonso MP, Coira A, Varela J. Association between Streptococcus infantarius (formerly S. bovis II/1) bacteremia and noncolonic cancer. J Clin Microbiol. 2008;46(4):1570. doi: 10.1128/JCM.00129-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturt AS, Yang L, Sandhu K, Pei Z, Cassai N, Blaser MJ. Streptococcus gallolyticus subspecies pasteurianus (biotype II/2), a newly reported cause of adult meningitis. J Clin Microbiol. 2010;48(6):2247–2249. doi: 10.1128/JCM.00081-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavin PJ, Thomson RB Jr, Horng SJ, Yogev R. Neonatal sepsis caused by Streptococcus bovis variant (biotype II/2): report of a case and review. J Clin Microbiol. 2003;41(7):3433–3435. doi: 10.1128/JCM.41.7.3433-3435.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen MT, Idriss S, Guzman E, De Oliveira ER. Neonatal meningitis, endocarditis, and pneumonitis due to Streptococcus gallolyticus subsp. pasteurianus: a case report. BMC Pediatr. 2019;19(1):265. doi: 10.1186/s12887-019-1645-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chlegel L, Grimont F, Ageron E, Grimont PAD, Bouvet A. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int J Syst Evol Microbiol. 2003;53(Pt 3):631–645. doi: 10.1099/ijs.0.02361-0 [DOI] [PubMed] [Google Scholar]
- 7.Noble CJ. Carriage of group D streptococci in the human bowel. J Clin Pathol. 1978;31:1182–1186. doi: 10.1136/jcp.31.12.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onoyama S, Ogata R, Wada A, Saito M, Okada K, Harada T. Neonatal bacterial meningitis caused by Streptococcus gallolyticus subsp. pasteurianus. J Med Microbiol. 2009;58(Pt 9):1252–1254. doi: 10.1099/jmm.0.006551-0 [DOI] [PubMed] [Google Scholar]
- 9.Punpanich W, Munsrichoom A, Dejsirilert S. Streptococcus gallolyticus subspecies pasteurianus meningitis in an infant: a case report and literature review. J Med Assoc Thai. 2012;95(12):1606–1612. [PubMed] [Google Scholar]
- 10.Thatrimontrichai A, Chanvitan P, Janjindamai W, Dissaneevate S, Maneenil G. Early onset neonatal bacterial meningitis caused by Streptococcus gallolyticus subsp. paste urianus. Southeast Asian J Trop Med Public Health. 2012;43(1):145–151. [PubMed] [Google Scholar]
- 11.Nagamatsu M, Takagi T, Ohyanagi T, et al. Neonatal meningitis caused by Streptococcus gallolyticus subsp. pasteurianus. J Infect Chemother. 2012;18(2):265–268. doi: 10.1007/s10156-011-0320-4 [DOI] [PubMed] [Google Scholar]
- 12.Klatte JM, Clarridge JE 3rd, Bratcher D, Selvarangan R. A longitudinal case series description of meningitis due to Streptococcus gallolyticus subsp. pasteurianus in infants. J Clin Microbiol. 2012;50(1):57–60. doi: 10.1128/JCM.05635-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi Y, Ishiwada N, Tanaka J, et al. Streptococcus gallolyticus subsp. pasteurianus meningitis in an infant. Pediatr Int. 2014;56(2):282–285. doi: 10.1111/ped.12254 [DOI] [PubMed] [Google Scholar]
- 14.Park JW, Eun SH, Kim EC, Seong MW, Kim YK. Neonatal invasive Streptococcus gallolyticus subsp. pasteurianus infection with delayed central nervous system complications. Korean J Pediatr. 2015;58(1):33–36. doi: 10.3345/kjp.2015.58.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hede SV, Olarte L, Chandramohan L, Kaplan SL, Hulten KG. Streptococcus gallolyticus subsp. pasteurianus infection in twin infants. J Clin Microbiol. 2015;53(4):1419–1422. doi: 10.1128/JCM.02725-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamura Y, Mihara Y, Nakatani K, Nishiguchi T, Ikebe T. Unexpected Ventriculitis Complication of Neonatal Meningitis Caused by Streptococcus gallolyticus Subsp. pasteurianus: a Case Report. Jpn J Infect Dis. 2018;71(1):68–71. doi: 10.7883/yoken.JJID.2017.053 [DOI] [PubMed] [Google Scholar]
- 17.Chen WC, Lee PI, Lin HC, et al. Clustering of Streptococcus gallolyticus subspecies pasteurianus bacteremia and meningitis in neonates. J Microbiol Immunol Infect. 2021;54(6):1078–1085. doi: 10.1016/j.jmii.2020.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Beneteau A, Levy C, Foucaud P, et al. Childhood meningitis caused by Streptococcus bovis group: clinical and biologic data during a 12-year period in France. Pediatr Infect Dis J. 2015;34(2):136–139. doi: 10.1097/INF.0000000000000513 [DOI] [PubMed] [Google Scholar]
- 19.Orbea M, Desai N, Foster C. Invasive Streptococcus Gallolyticus Infections in Infants At Texas Children’s Hospital: a 9-Year Retrospective Review. Pediatr Infect Dis J. 2022;41(11):e494–e497. doi: 10.1097/INF.0000000000003682 [DOI] [PubMed] [Google Scholar]
- 20.Doran KS, Benoit VM, Gertz RE, Beall B, Nizet V. Late-onset group B streptococcal infection in twins: insight to disease pathogenesis. J Perinatol. 2002;22:326–330. doi: 10.1038/sj.jp.7210675 [DOI] [PubMed] [Google Scholar]
- 21.Geetha O, Cherie C, Natalie TWH, Merchant K, Chien CM, Chandran S. Streptococcus gallolyticus subspecies pasteurianus causing early onset neonatal sepsis complicated by solitary liver abscess in a preterm infant. Access Microbiol. 2021;3(3):000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiran Z, Sheikh A, Humayun KN, Islam N. Neonatal outcomes and congenital anomalies in pregnancies affected by hypothyroidism. Ann Med. 2021;53(1):1560–1568. doi: 10.1080/07853890.2021.1970798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amer T, David R, Oberfield SE. Necrotizing enterocolitis and hypothyroidism in a newborn infant: treatment with intravenous L-thyroxine. Am J Perinatol. 1994;11(1):30–32. doi: 10.1055/s-2007-994530 [DOI] [PubMed] [Google Scholar]
- 24.Kliegman RM. Models of the pathogenesis of necrotizing enterocolitis. J Pediatr. 1990;117(1 Pt 2):S2–5. doi: 10.1016/S0022-3476(05)81123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montesinos MDM, Pellizas CG. Thyroid Hormone Action on Innate Immunity. Front Endocrinol (Lausanne). 2019;10:350. doi: 10.3389/fendo.2019.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florencia Soler M, Del Carmen Bravo-Miana R, María Negretti-Borga D, et al. Triiodothyronine-stimulated dendritic cell vaccination boosts antitumor immunity against murine colon cancer. Int Immunopharmacol. 2022;110:109016. doi: 10.1016/j.intimp.2022.109016 [DOI] [PubMed] [Google Scholar]
- 27.De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21(8):879–890. doi: 10.1089/thy.2010.0429 [DOI] [PubMed] [Google Scholar]
- 28.van Samkar A, Brouwer MC, Pannekoek Y, van der Ende A, van de Beek D. Streptococcus gallolyticus meningitis in adults: report of five cases and review of the literature. Clin Microbiol Infect. 2015;21(12):1077–1083. doi: 10.1016/j.cmi.2015.08.003 [DOI] [PubMed] [Google Scholar]


