Abstract
BACKGROUND:
Pulmonary function test (PFT) impairments are common after allogeneic hematopoietic stem cell transplantation. The prognostic significance of these declines on outcomes is not well understood.
The objectives were to determine the frequency of declines in pulmonary function (FVC, FEV1, and diffusing capacity for carbon monoxide [DLCO]) in the early post-transplantation period; and to determine the prognostic significance of these declines on mortality or development of bronchiolitis obliterans syndrome.
METHODS:
This was a retrospective cohort study conducted at Mayo Clinic, Rochester, Minnesota. PFTs were obtained at baseline and at day +100. Competing risk survival models were developed, which accounted for pre-transplantation pulmonary function and relapse status.
RESULTS:
Between January 1, 2005, and December 31, 2020, 1,145 subjects underwent allogeneic hematopoietic stem cell transplantation and had a pre-transplantation PFT performed. Of these, 900 (78.6%) survived to day 100 and had post-transplantation PFTs performed (median [interquartile range] 97 [94–103] d). A decline of ≥10% in FEV1, FVC, or DLCO was seen in 401 of 900 subjects (44.5%). Declines of ≥20% in FEV1 (hazard ratio 1.65, 95% CI 1.07–2.56; P = .02), FVC (hazard ratio 1.72, 95% CI [1.11–2.67]; P = .02), and DLCO (hazard ratio 1.46, 95% CI 1.04–2.07; P = .028) were all associated with reduced survival when compared with those with < 10% decline in PFT measures. These findings were independent of pre-transplantation pulmonary function or relapse status. Bronchiolitis obliterans syndrome was diagnosed in 118 subjects (10.3%), and there was no relationship between early PFT decline and a subsequent diagnosis of bronchiolitis obliterans syndrome. The subjects who received myeloablative conditioning with cyclophosphamide plus total body irradiation or cyclophosphamide plus fludarabine plus total body irradiation were more likely to have lower spirometry values after hematopoietic stem cell transplantation. The subjects who received reduced intensity conditioning or nonmyeloablative conditioning with fludarabine plus total body irradiation were more likely to have higher post–hematopoietic stem cell transplantation FEV1, FVC, and DLCO.
CONCLUSIONS:
An absolute decline of ≥20% in FEV1, FVC, or DLCO were associated with reduced survival independent of pre-transplantation pulmonary function or relapse status. In contrast to previous work, early declines in PFT measures were not associated with future development of bronchiolitis obliterans syndrome.
Keywords: Bone marrow transplantation, pulmonary function, spirometry, diffusing capacity, bronchiolitis obliterans
Introduction
Pulmonary complications are common after hematopoietic stem cell transplantation.1–5 Pulmonary function testing (PFT) is part of the routine pre-transplantation evaluation.6 These PFTs provide baseline values when diagnosing pulmonary toxicities from chemotherapy and radiation therapy,7 and in diagnosing specific post–hematopoietic stem cell transplantation syndromes, for example, bronchiolitis obliterans syndrome. PFTs also have a prognostic component: poorer baseline PFT is associated with an increased risk of post-transplantation pulmonary complications and reduced overall survival.3,8–11 Given the prognostic importance of pre-transplantation pulmonary function, the hematopoietic stem cell transplantation comorbidity index incorporates pre-transplantation FEV1 and diffusing capacity for carbon monoxide (DLCO) in its composite risk score.12–15
Previous studies that looked at the significance of early post-transplantation spirometry predominantly focused on to what extent early declines in expiratory flow, especially FEV1, are predictive of subsequent bronchiolitis obliterans syndrome.16,17 The largest such study found that the absolute values of FEV1 and forced expiratory flow in the mid-part of the FVC maneuver at the day +80 PFT were associated with subsequent bronchiolitis obliterans syndrome development.16 Studies that looked at impact of early post–hematopoietic stem cell transplantation lung function on overall survival are more limited. One study that assessed this determined that lower absolute values of FVC, FEV1, and DLCO collected at day +80 after hematopoietic stem cell transplantation were associated with reduced survival.18 However, this study did not incorporate pre-transplantation reference values, so it is unclear if the worse outcomes seen in those with greater impairments in pulmonary function after hematopoietic stem cell transplantation were merely a reflection of preexisting pulmonary dysfunction. The impact of the changes in pulmonary function between the time of the pre-transplantation PFT and the early post–hematopoietic stem cell transplantation period is not well studied. Our objective with this study was to determine the frequency of early decline in pulmonary function and whether such declines have prognostic implications beyond that of preexisting pulmonary function parameters.
QUICK LOOK.
Current Knowledge
Pulmonary complications are a major threat to the survivorship of the recipient of an allogeneic hematopoietic stem cell transplantation. Pre-transplantation lung function is a known predictor of non-relapse mortality in recipients of an allogeneic hematopoietic stem cell transplantation. After allogeneic hematopoietic stem cell transplantation, declines in lung function are commonly seen, but it is not known precisely how frequently these occur or what threshold of decline should prompt concern.
What This Paper Contributes to Our Knowledge
In this retrospective cohort study, 20% absolute declines in FEV1, FVC, and DLCO were associated with an increased risk of non-relapse mortality, independent of pre-transplantation lung function. We found that early declines in lung function from pre-transplantation baseline is a predictor of mortality and determined a 20% absolute decline should be particularly concerning when recipients are seen at their day +100 milestone visit.
Methods
This was a retrospective cohort study conducted at Mayo Clinic, Rochester. The Mayo Clinic Institutional Review Board (Rochester, Minnesota) approved the study protocol (13 - 002869) before study initiation. The requirement for written informed consent was waived by the institutional review board. The study population included adult subjects (ages ≥ 18 years) who underwent first-time hematopoietic stem cell transplantation at Mayo Clinic, Rochester, Minnesota, between January 1, 2005, and December 31, 2020. For those subjects who had >1 hematopoietic stem cell transplantation, the first hematopoietic stem cell transplantation was used as the index event. Follow-up occurred until August 31, 2022. The subjects were included if (a) they provided consent for the use of the medical records for research purposes, (b) they had a pre-transplantation PFT performed, and (c) they had at least one PFT performed after hematopoietic stem cell transplantation.
A bronchiolitis obliterans syndrome diagnosis was performed via manual chart review by 2 authors (HY, MH) in line with 2014 National Institutes of Health Consensus Statement19 for diagnosis of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation and before published work by our group.20 Specifically, this included (a) FEV1 < 75% of predicted and an irreversible ≥ 10% decline in < 2 years; (b) the FEV1–to–vital capacity less than either 0.7 or the lower limit normal; (c) absence of infection; and (d) one of the following: (i) a preexisting diagnosis of chronic graft-versus-host disease, (ii) air trapping by expiratory computed tomography scan of the chest, or (iii) air trapping on PFTs as measured by residual volume. All 4 criteria were met in 90 of 118 subjects, whereas 28 of 118 subjects only met 3 of the 4 criteria. Specifically, these subjects met criteria a, c, and d but had a FEV1/FVC above the lower limit of normal. These 28 of 118 subjects were diagnosed and treated as having bronchiolitis obliterans syndrome by their primary clinician.
All spirometry and diffusing capacity measurements were performed by a trained pulmonary function technician in the Pulmonary Function Laboratory at Mayo Clinic, Rochester. The American Thoracic Society and European Respiratory Society technical standards were followed in the performance and interpretation of all testing.21 The PFT laboratory follows American Thoracic Society and European Respiratory Society guidelines for quality assurance with daily calibrations and regular biologic quality control checks.22–24 DLCO whenever possible. To account for the effects of age-related decline in lung function (∼20–30 mL/year for FEV1 and FVC),25 all comparison measures were performed with the percent of predicted values, which accounted for these age-related declines. When we reported differences in percentages, this reflects an absolute difference in the percent of predicted unless otherwise stated. For example, a subject whose FEV1 percent of predicted went from 80% to 60% would be said to have a 20% decline in FEV1. Global Lung Initiative reference equations were used for normative value calculations.26,27 For the pre-transplantation reference value, the spirometry and diffusing capacity measurement immediately before transplantation was used. For post–hematopoietic stem cell transplantation spirometry and diffusing capacity measurements, the first PFT performed was used. If the subjects had pre- and post-bronchodilator measurements taken at the same visit, the best value was used.
Subject demographics and clinical characteristics were described by using proportions and percentages for dichotomous and categorical variables. Categorical variables were summarized as counts and percentages, and were compared across case status by using the chi-square and Fisher exact tests. Continuous variables were summarized as mean ± SD and compared between subjects with a paired t test and across case status by using an unpaired t test. Survival analyses were analyzed in a competing risks model, with relapse and death considered as competing events.28–30 Results from 2 competing risk models were reported: cause-specific hazard models and subdistribution hazard models.31–34 A 2-sided P < .05 was considered statistically significant. JMP Pro software (SAS Institute, Cary, North Carolina) was used for data collection and handling. Data analysis was performed in R 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) by using the R Studio 2022.02 integrated development environment (PBC, Boston, Massachusetts).15
Results
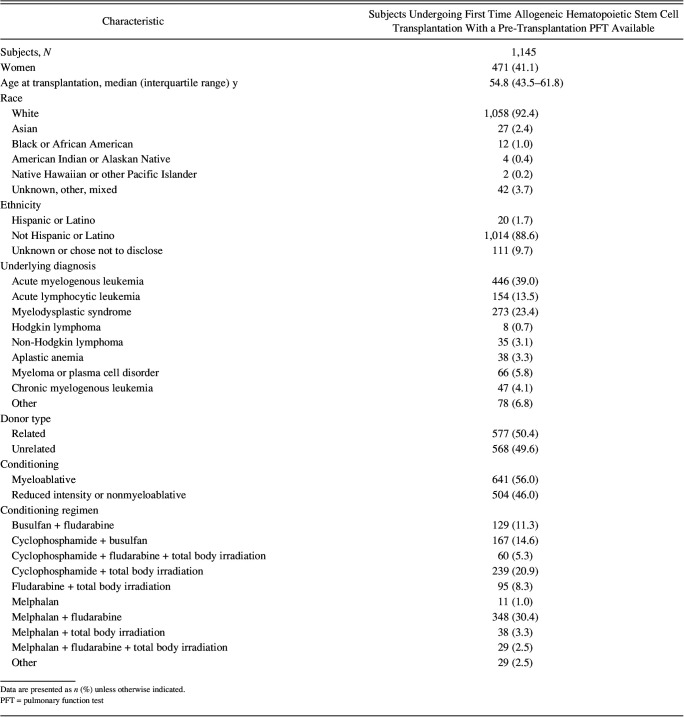
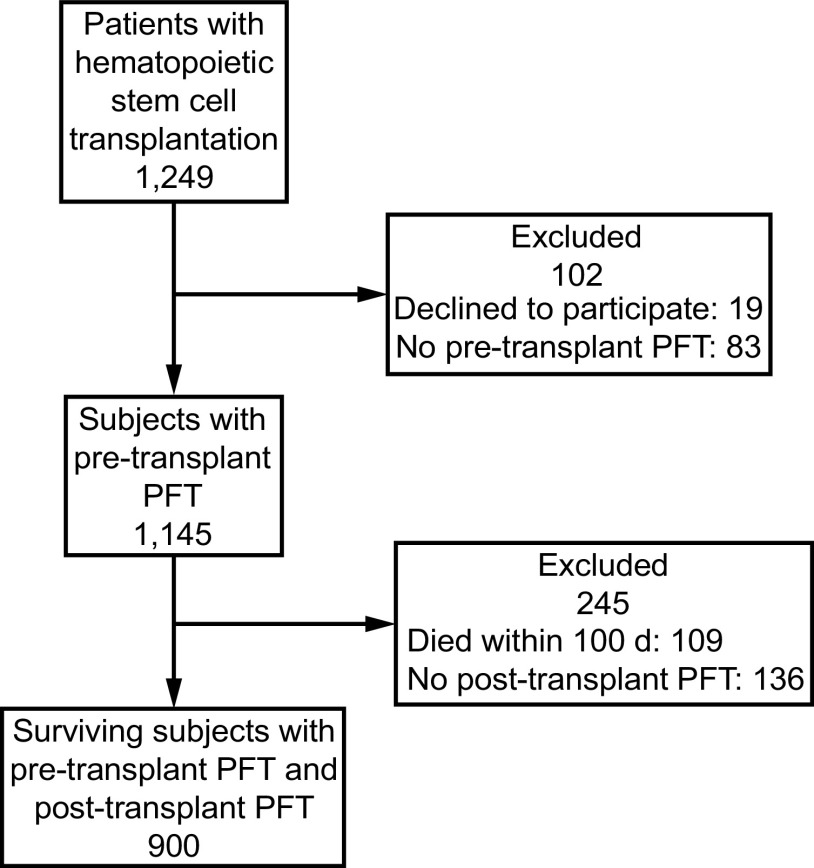
Between January 1, 2005, and December 31, 2020, 1,249 patients underwent first-time allogeneic hematopoietic stem cell transplantation. Of these, 19 declined the use of their medical records for research purposes and 83 did not have a pre-transplantation PFT performed. Therefore, 1,145 subjects were eligible for inclusion in the study. Of these 1,145, 1,036 (90.5%) were alive at day 100 and 838 (73.2%) were alive at 1-year post–hematopoietic stem cell transplantation. Baseline characteristics are outlined in Table 1. A study flow diagram is outlined in Figure 1.
Table 1.
Baseline Characteristics of the Study Population
Fig. 1.
Flow chart. PFT = pulmonary function testing.
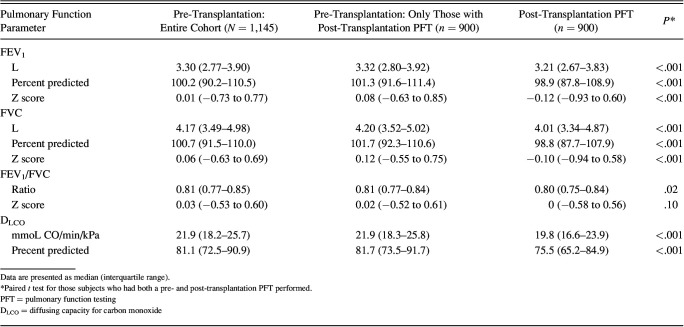
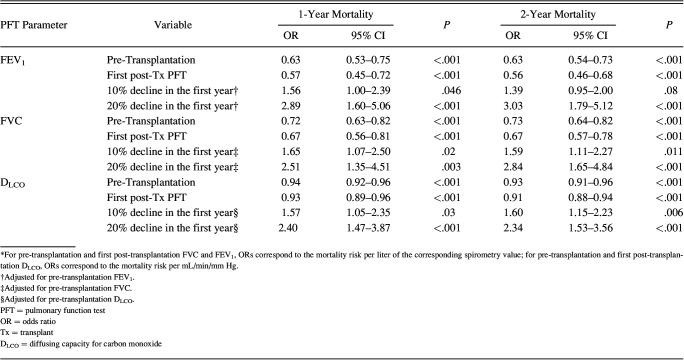
Of the 1,145 subjects, 900 (78.6%) had a PFT done within 6 months of hematopoietic stem cell transplantation at a median (interquartile range) of 97 (94–103) d, typically coordinating with the day +100 milestone visit (Table 2). All the subjects were able to complete spirometry assessment. Twenty-two subjects of 900 were unable to perform the maneuvers required for diffusing capacity assessment. Declines in PFT measures were common: 180 (20.0%) had a >10% decline in FEV1, 188 (20.8%) had a >10% decline in FVC, and 326 (36.2%) had a >10% decline in DLCO compared with their pre-transplantation referent value. In total, 401 of 900 (44.5%) had a decline in at least one of the 3 measures. Lower absolute values of either the pre-transplantation or the first post-transplantation FEV1, FVC, and DLCO were associated with reduced overall survival at 1- and 2-years (Table 3). For the 1-year survival, the odds ratios (OR) for mortality were 0.63 (95% CI 0.53–0.75; P = .003) and 0.57 (95% CI 0.45–0.72; P < .001) per liter increase in FEV1 for the pre- and post-transplantation measures, respectively. Similarly, the ORs for mortality were 0.72 (95% CI 0.63–0.82; P < .001) and 0.67 (95% CI 0.56–0.81; P < .001) per liter increase in FVC for the pre- and post-transplantation measures, respectively. Also, the ORs for mortality were 0.94 (95% CI 0.92–0.96; P < .001) and 0.93 (95% CI 0.89–0.96; P < .001) per mL/min/mm Hg DLCO for the pre- and post-transplantation measures, respectively.
Table 2.
Pulmonary Function Measurements Before and After Hematopoietic Stem Cell Transplantation
Table 3.
The Association Between First Post-Transplantation Spirometry and Diffusing Capacity Measures and Overall Survival At 1 And 2 Years After Hematopoietic Stem Cell Transplantation
After adjusting for pre-transplantation reference values, 10% declines in FEV1 (OR 1.56, 95% CI 1.00–2.39; P = .046), FVC (OR 1.65, 95% CI 1.07–2.50; P = .02), and DLCO (OR 1.57, 95% CI 1.05–2.35; P = .026) were associated with reduced overall survival. After adjusting for pre-transplantation reference values, >20% declines in all 3 parameters were associated with reduced overall survival: FEV1 (OR 2.89, 95% CI 1.60–5.06; P < .001), FVC (OR 2.51, 95% CI 1.35–4.51; P = .003), and DLCO (OR 2.40, 95% CI 1.47–3.87; P < .001). Broadly, similar findings were seen for the 2-year survival (Table 3).
Across all measures of PFT decline, effect estimates were greater in those with a ≥ 20% decline in PFT measures compared with those with a 10–20% decline in PFT measures. One hundred and thirty-four subjects (14.9%) had a ≥ 10% decline in DLCO, alongside a ≥ 10% decline in either FEV1 or FVC. The OR for mortality at 2 years for those with a ≥ 10% decline in DLCO plus spirometry measure was 1.80, 95% CI 1.20-2.67 (P = .004). Thirty-nine subjects (4.3%) had a ≥ 20% decline in DLCO as well as a ≥ 20% decline in either FEV1 or FVC. The OR for mortality at 2 years for those with a ≥ 20% decline in DLCO plus spirometry measure was 4.15, 95% CI 2.14–8.15 (P < .001). We, in addition, analyzed non-relapse mortality with similar results seen (Supplementary Table 2, see the supplementary materials at http://www.rcjournal.com). Specifically, a decline of > 20% resulted in an OR for mortality at 1 year of 3.02 (95% CI 1.54–5.64; P < .001) for FEV1, 2.75 (1.35–5.28) for FVC, and 3.16 (1.86–5.31) for DLCO. A decline of > 20% resulted in an OR for mortality at 2-years of 3.07 (1.68–5.52) for FEV1, 3.21 (1.74–5.82) for FVC, and 2.91 (1.82–4.62) for DLCO.
Of 1,145 subjects, 118 were diagnosed with bronchiolitis obliterans syndrome at a median of 14 months after hematopoietic stem cell transplantation. Absolute values of PFT parameters at the early post–hematopoietic stem cell transplantation PFT were not associated with subsequent bronchiolitis obliterans syndrome development. Specifically, there was no difference between the subjects with bronchiolitis obliterans syndrome versus the subjects without bronchiolitis obliterans syndrome for FEV1 (95.1% vs 98.1% of predicted; P = .08), FVC (97.4% vs 98.0% of predicted; P = .72), or DLCO (73.6% vs 75.4% of predicted; P = .21). Declines of 10% or 20% in PFT measures from the pre-transplantation reference value were also not associated with subsequent bronchiolitis obliterans syndrome development. Specifically, 25 of 118 subjects (21.1%) who went on to develop bronchiolitis obliterans syndrome had a decline in FEV1 of 10% at the time of the first PFT compared with 155 of 614 subjects (20.1%) who did not go on to develop bronchiolitis obliterans syndrome (P = .79). Eight of 118 subjects (6.8%) who went on to develop bronchiolitis obliterans syndrome had a decline of ≥20% in FEV1 compared with 57 of 712 (7.4%) of those who did not go on to develop bronchiolitis obliterans syndrome (P = .80).
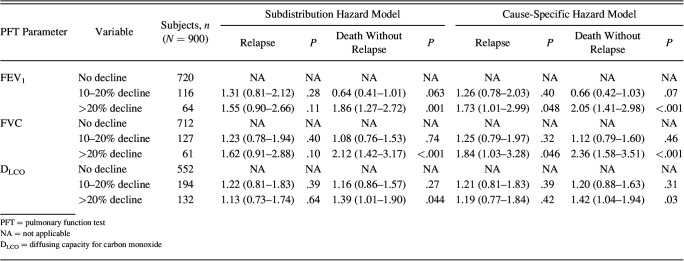
Declines in FEV1, FVC, and DLCO were modeled in an overall survival analysis (Figure 2). Hazard ratios for 5-year survival were then calculated with relapse and death considered as competing risks (Figure 3, Table 4). Results were consistent with the 2 competing risk models used. In the subdistribution hazard model for those without relapse, declines of ≥ 20% in FEV1 (hazard ratio 1.65, 95% CI 1.07–2.56; P = .02), FVC (hazard ratio 1.72 [1.11–2.67]; P = .02), and DLCO (hazard ratio 1.46, 95% CI 1.04–2.07; P = .03) were all associated with reduced survival when compared with those with a <10% decline in PFT measures. There was no significant difference in mortality in those with a 10–20% decline in PFT measures compared with those who had a <10% decline in PFT. As a sensitivity analysis, we repeated this survival analysis, including only those subjects who did not go on to develop bronchiolitis obliterans syndrome. Findings were similar to the parent analysis (Supplementary Table 1, see the supplementary materials at http://www.rcjournal.com). A decline in pulmonary function parameters stratified by year of transplantation is outlined in Supplementary Figure 1 (see the supplementary materials at http://www.rcjournal.com). Declines in DLCO and FEV1 remained stable over time. A decline in FVC at the first post-transplantation PFT was significantly lower in the subjects who underwent hematopoietic stem cell transplantation toward the end of the cohort compared with those at the start of the cohort (P = .02 for trend).
Fig. 2.
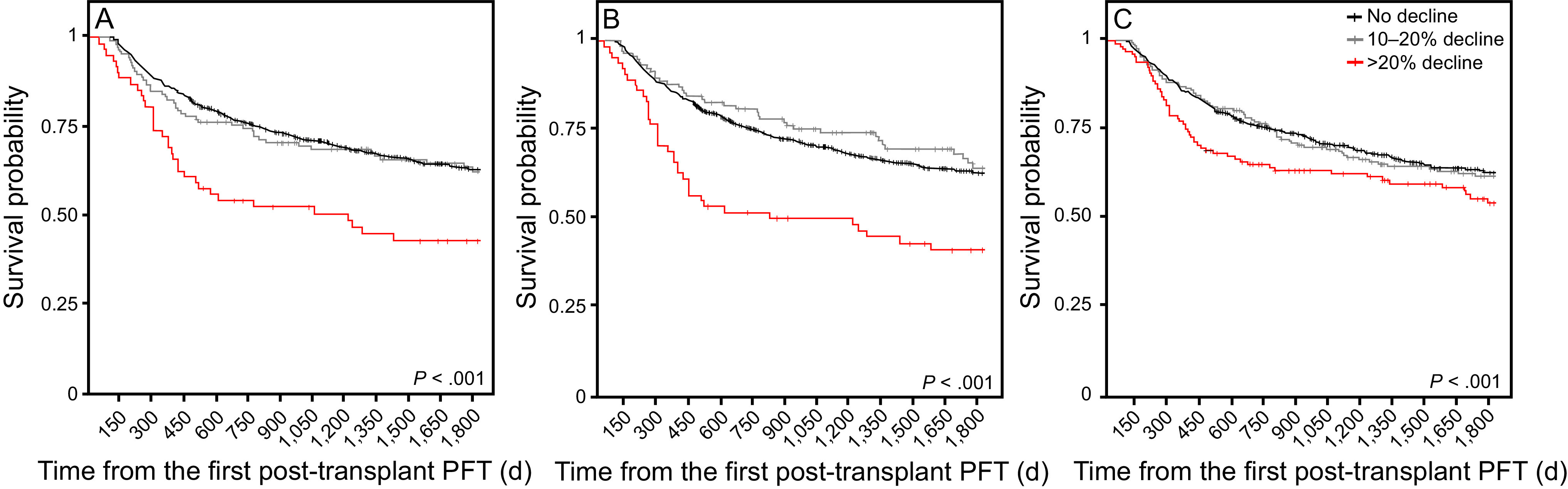
Overall survival stratified by degree of decline at the time of the first post-transplantation pulmonary function test (PFT) performed within 6 mo, typically around the day +100 visit. A: Forced vital capacity, B: FEV1, and C: DLCO. There were no differences between the survival curves for a 10–20% decline in PFT parameters and < 10% decline; there were significant differences in survival probability for those subjects who had > 20% declines in FEV1 and FVC (but not diffusing capacity for carbon monoxide [DLCO]).
Fig. 3.
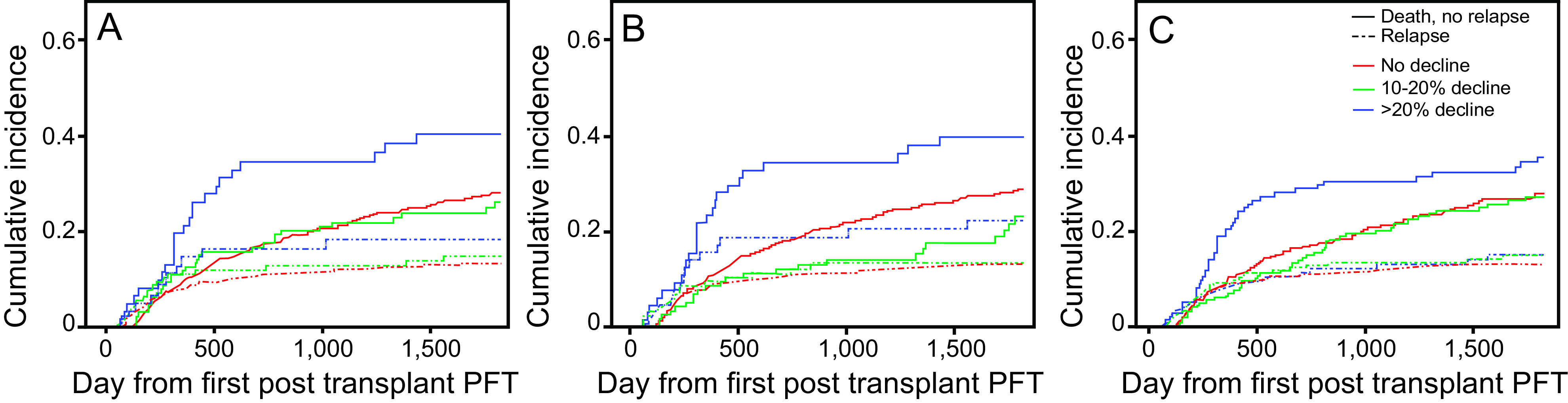
Cumulative incidence of relapse and death without relapse stratified by degree of decline at the time of the first post-transplantation pulmonary function test. A: Forced vital capacity, B: FEV1, and C: DLCO. Hazard ratios for mortality are presented in Table 5.
Table 4.
Hazard Ratios for Either Relapse or Death Based on the First Post-Transplantation PFT By Using Competing Risk Survival Modeling: 2 Different Competing Risk Models are Reported (subdistribution hazard modeling and cause-specific hazard modeling) with Consistent Results Across Both Models
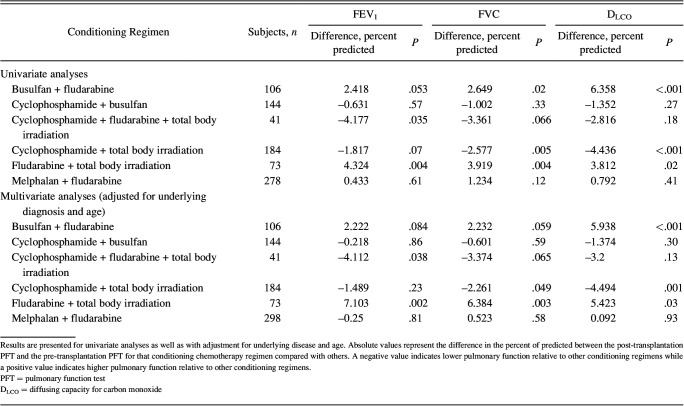
As an exploratory analysis, we evaluated the relationship between the 6 most common conditioning chemotherapy regimens and the change in pulmonary function parameters after hematopoietic stem cell transplantation (Table 5). After adjusting for age and underlying disease, cyclophosphamide plus fludarabine plus total body irradiation was associated with lower post-transplantation FEV1 percent of predicted (4.1% lower, P = .038), whereas cyclophosphamide plus total body irradiation was associated with lower post–hematopoietic stem cell transplantation FVC percent of predicted (2.3% lower, P = .049). Certain conditioning regimens (given in reduced intensity conditioning or nonmyeloablative conditioning) were associated with higher post-transplantation pulmonary function. Specifically, the subjects who received fludarabine plus total body irradiation had higher FEV1 (7.1% higher, P = .002), FVC (6.4% higher, P = .004), and DLCO (5.4% higher, P = .03) percent of predicted. Busulfan plus fludarabine was also associated with better post-transplantation DLCO percent of predicted (5.9% higher, P < .001).
Table 5.
Relationship Between the 6 Most Common Conditioning Chemotherapy Regimens and PFT Changes
Discussion
In this large study of subjects undergoing first-time allogeneic hematopoietic stem cell transplantation, we demonstrated that declines in early post-transplantation pulmonary function was associated with mortality. The key findings of our study are that (a) almost half of patients who undergo allogeneic hematopoietic stem cell transplantation will have a decline of ≥10% or one PFT measure; (b) declines of ≥20% in any of FEV1, FVC, and DLCO were associated with reduced survival and this risk was independent of both pre-transplantation pulmonary function and relapse status; and (c) in contrast to previous reports, early post-transplantation spirometry changes do not seem to be associated with subsequent bronchiolitis obliterans syndrome development. Clinicians who are looking after recipients of allogeneic hematopoietic stem cell transplantation are often unclear about the significance of changes in pulmonary function after allogeneic hematopoietic stem cell transplantation. Our findings are not only novel but also provide much needed context for clinicians interpreting PFTs after allogeneic hematopoietic stem cell transplantation.
A key finding of our study was that pulmonary function declined in almost half of the recipients of allogenic hematopoietic stem cell transplantation at the day +100 visit, irrespective of whether the subjects went on to develop bronchiolitis obliterans syndrome. Previous studies indicate that post-transplantation spirometric and DLCO values were associated with increased mortality.18 However, these analyses looked at absolute indices rather than accounting for pre-transplantation lung function. In that context, it is unclear to what extent the mortality risk is due to the baseline pulmonary dysfunction and to what extent it is due to the decline in PFT measures after transplantation. Our study found that absolute values of FEV1, FVC, and DLCO were significantly associated with mortality risk when using either pre- or post-transplantation pulmonary function measures. Interestingly, the effect estimates for these 2 time points are similar, which suggests that the mortality difference seen in previous studies may have reflected pre-transplantation pulmonary function. Although smaller declines (10–20%) in pulmonary function measures do not seem to be associated with increased mortality risk, declines of ≥20% were associated with mortality risk. These findings remained robust after adjustment for pre-transplantation pulmonary function and relapse status as well as in sensitivity analyses that excluded those subjects who went on to develop bronchiolitis obliterans syndrome. The data with regard to the additive risk of mortality attributable to a decline in pulmonary function had not been previously reported.
Further work is needed to also determine why these declines in pulmonary function are associated with reduced survival. Some pulmonary conditions with high mortality, such as ARDS and idiopathic pneumonia syndrome (IPS), are associated with impairments in pre-transplantation pulmonary function.3,8 Because PFTs are effort dependent, they may be a marker of overall health, debility, and functional decline. Such patients may have had more post-hematopoietic stem cell transplantation complications and, as such, may have a greater risk of subsequent illness. Irrespective, our study highlights that patients who experience ≥ 20% declines in percent of predicted of any pulmonary function parameter should have closer clinical monitoring.
In contrast to previous studies, we did not find that pre- or post-transplantation pulmonary function parameters were associated with subsequent bronchiolitis obliterans syndrome development. There are several possible reasons for this discrepancy. First, our study was smaller than the largest previous study on this topic, which had a 3-fold greater number of subjects.16 Given the P value of the difference in FEV1 between the subjects with bronchiolitis obliterans syndrome and the subjects without bronchiolitis obliterans syndrome was 0.08, it is possible that this smaller sample size led to a type-2 error. Second, previous studies used a protocolized definition of bronchiolitis obliterans syndrome in line with National Institutes of Health criteria. These criteria are specific but may lack adequate sensitivity in capturing the real-world spectrum of bronchiolitis obliterans syndrome seen after allogenic hematopoietic stem cell transplantation.35
In chronic lung allograft dysfunction after lung transplantation, ∼20–30% of patients have a mixed and/or restrictive phenotype.36 Unlike chronic lung allograft dysfunction, hematopoietic stem cell transplantation–bronchiolitis obliterans syndrome phenotypes are less well defined. Bergeron et al17 did identify a subset of subjects with predominantly restrictive features (FEV1 < 80%, FVC < 80%, normal FEV1/FVC). These patients may have a restrictive form of lung graft-versus-host disease or have restrictive PFTs due to chest wall limitation from graft-versus-host of other organs (eg, pleura). Many such patients, who do not qualify for inclusion if a strict definition of the 2014 National Institutes of Health bronchiolitis obliterans syndrome criteria is used, are nonetheless managed as having bronchiolitis obliterans syndrome by their hematologist. As such, we included this broader definition of hematopoietic stem cell transplantation–bronchiolitis obliterans syndrome in our cohort because we felt that was more reflective of contemporary clinical practice. This difference in inclusion criteria may be another reason why our findings differed from previous reports on the significance of early post–hematopoietic stem cell transplantation FEV1 on subsequent bronchiolitis obliterans syndrome development.
Another notable finding of our study was the association between 2 myeloablative regimens (cyclophosphamide plus fludarabine plus total body irradiation and cyclophosphamide plus total body irradiation) and reduced spirometry measures after hematopoietic stem cell transplantation (Table 5). Although the association between cyclophosphamide-based conditioning regimens and post–hematopoietic stem cell transplantation pneumonitis has been well described,37 this was the first evidence that specific conditioning chemotherapy regimens are associated with reduced pulmonary function after hematopoietic stem cell transplantation. Similarly certain reduced intensity conditioning or nonmyeloablative conditioning regimens were associated with better post–hematopoietic stem cell transplantation pulmonary function (fludarabine plus total body irradiation and busulfan plus fludarabine). Notably, not all reduced intensity conditioning or nonmyeloablative conditioning regimens had this finding. Melphalan plus fludarabine is a common reduced intensity conditioning or nonmyeloablative conditioning regimen and was not associated with any differences in pulmonary function compared with other conditioning regimens. These exploratory findings will be informative when considering future studies that evaluate the impact of chemotherapy and conditioning regimens on long-term pulmonary function.
Our study had several strengths worth noting. This was a large, contemporary cohort of recipients with allogeneic hematopoietic stem cell transplantation and with high rates of follow-up and protocolized post-transplantation PFT monitoring at regular intervals after hematopoietic stem cell transplantation. The subjects had standardized measurement of spirometry indices and diffusing capacity in a large pulmonary function laboratory with rigorous quality assurance and control procedures in place. PFT is highly dependent on subject effort, and performance of testing in a laboratory with rigorous quality assurance and control ensures accurate assessment of pulmonary function. Our statistical analyses were also robust. When assessing overall survival (Table 4), we adjusted for pre-transplantation pulmonary function when assessing post-transplantation PFT changes. Effect estimates were consistent across PFT measures and between 1- and 2-year survival, and seemed to be linear: increasing impairments of pulmonary function were associated with greater mortality risk. When assessing relapse-free survival, we had consistent results with 2 different competing risk models, again adding to the confidence of our findings.
Our study also had several limitations. The single-center nature of the study limits generalizability. The retrospective nature of the study meant that we were reliant on clinician ordering of PFTs. Although our institution protocolizes performance of PFTs at routine milestone visits, this is not always carried out. A prospective study that evaluates pulmonary function after allogeneic hematopoietic stem cell transplantation would address these issues but clearly represents a substantial resource burden. The protocolized nature of our PFT also means that we do not routinely perform lung volume measurement. Rather, lung volume (ie, total lung capacity) measurements are performed if FVC, FEV1, or DLCO are below the lower limit of normal. Because that is uncommon before hematopoietic stem cell transplantation, few subjects in our cohort had baseline lung volume measurements (total lung capacity or residual volume).
However, our study highlights the importance of PFT monitoring after hematopoietic stem cell transplantation and supports such studies being performed in the future. The demographics of our study population were predominantly white, which limits generalizability to more diverse populations. The subjects were classified as having or not having bronchiolitis obliterans syndrome based on retrospective chart review. This likely adds imprecision with regard to the true number of patients who develop bronchiolitis obliterans syndrome. Moreover, we did not have the data to characterize the frequency of specific post–hematopoietic stem cell transplantation pulmonary complications, an important analysis for future work. Such pulmonary complications could lead to reduced PFT measures and increased mortality. The subjects with early relapse or mortality may not undergo PFTs either because their goals of care change or they are never well enough to undergo a more comprehensive out-patient evaluation. Also, it is possible that those with severe pulmonary complications were hospitalized before PFTs could be performed and our study was not able to capture their decline in pulmonary function parameters.
Conclusions
Pulmonary function declines are common after hematopoietic stem cell transplantation. Absolute declines of ≥20% of predicted in FEV1, FVC, or DLCO were associated with increased mortality, independent of relapse status or pre-transplantation lung function or whether the patient goes on to develop bronchiolitis obliterans syndrome. Further research is needed to better understand why these impairments lead to increased mortality and whether further physiologic monitoring might be warranted in patients particularly at high risk.
Footnotes
The authors have disclosed no conflicts of interest.
Support was provided by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) grant K23HL151671 (recipient: Dr Yadav) and NIH/NHLBI grant R01HL062150 (recipient: Dr Limper). This manuscript's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Supplementary material related to this paper is available at http://www.rcjournal.com.
REFERENCES
- 1. Afessa B, Abdulai RM, Kremers WK, Hogan WJ, Litzow MR, Peters SG. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest 2012;141(2):442–450. [DOI] [PubMed] [Google Scholar]
- 2. Yadav H, Nolan ME, Bohman JK, Cartin-Ceba R, Peters SG, Hogan WJ, et al. Epidemiology of acute respiratory distress syndrome following hematopoietic stem cell transplantation. Crit Care Med 2016;44(6):1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herasevich S, Frank RD, Bo H, Alkhateeb H, Hogan WJ, Gajic O, Yadav H. Pretransplant risk factors can predict development of acute respiratory distress syndrome after hematopoietic stem cell transplantation. Ann Am Thorac Soc 2021;18(6):1004–1012. [DOI] [PubMed] [Google Scholar]
- 4. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. ; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315(8):788–800. [DOI] [PubMed] [Google Scholar]
- 5. Rios F, Iscar T, Cardinal-Fernandez P. What every intensivist should know about acute respiratory distress syndrome and diffuse alveolar damage. Rev Bras Ter Intensiva 2017;29(3):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Efrati O, Toren A, Duskin H, Modan-Moses D, Bielorai B, Goldstein G, et al. Pulmonary function studies in children treated by chemoradiotherapy and stem cell transplantation. Pediatr Blood Cancer 2008;51(5):684–688. [DOI] [PubMed] [Google Scholar]
- 7. Leger P, Limper AH, Maldonado F. Pulmonary toxicities from conventional chemotherapy. Clin Chest Med 2017;38(2):209–222. [DOI] [PubMed] [Google Scholar]
- 8. Wenger DS, Triplette M, Crothers K, Cheng G-S, Hill JA, Milano F, et al. Incidence, risk factors, and outcomes of idiopathic pneumonia syndrome after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2020;26(2):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest 1992;101(5):1257–1264. [DOI] [PubMed] [Google Scholar]
- 10. Ghalie R, Szidon JP, Thompson L, Nawas YN, Dolce A, Kaizer H. Evaluation of pulmonary complications after bone marrow transplantation: the role of pretransplant pulmonary function tests. Bone Marrow Transplant 1992;10(4):359–365. [PubMed] [Google Scholar]
- 11. Ho VT, Weller E, Lee SJ, Alyea EP, Antin JH, Soiffer RJ. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001;7(4):223–229. [DOI] [PubMed] [Google Scholar]
- 12. Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veeraputhiran M, Yang L, Sundaram V, Arai S, Lowsky R, Miklos D, et al. Validation of the hematopoietic cell transplantation-specific comorbidity index in nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2017;23(10):1744–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raimondi R, Tosetto A, Oneto R, Cavazzina R, Rodeghiero F, Bacigalupo A, et al. Validation of the hematopoietic cell transplantation-specific comorbidity index: a prospective, multicenter GITMO study. Blood 2012;120(6):1327–1333. [DOI] [PubMed] [Google Scholar]
- 15. Sorror ML, Sandmaier BM, Storer BE, Franke GN, Laport GG, Chauncey TR, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA 2011;306(17):1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jamani K, He Q, Liu Y, Davis C, Hubbard J, Schoch G, et al. Early post-transplantation spirometry is associated with the development of bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2020;26(5):943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergeron A, Chevret S, Peffault de Latour R, Chagnon K, de Margerie-Mellon C, Riviere F, et al. Noninfectious lung complications after allogeneic haematopoietic stem cell transplantation. Eur Respir J 2018;51(5):1702617. [DOI] [PubMed] [Google Scholar]
- 18. Walter EC, Orozco-Levi M, Ramirez-Sarmiento A, Vigorito A, Campregher PV, Martin PJ, et al. Lung function and long-term complications after allogeneic hematopoietic cell transplant. Biol Blood Marrow Transplant 2010;16(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21(3):389–401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hefazi M, Langer KJ, Khera N, Adamski J, Roy V, Winters JL, et al. Extracorporeal photopheresis improves survival in hematopoietic cell transplant patients with bronchiolitis obliterans syndrome without significantly impacting measured pulmonary functions. Biol Blood Marrow Transplant 2018;24(9):1906–1913. [DOI] [PubMed] [Google Scholar]
- 21. Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of Spirometry 2019 Update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019;200(8):e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CPM, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26(4):720–735. [DOI] [PubMed] [Google Scholar]
- 23. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 24. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26(3):511–522. [DOI] [PubMed] [Google Scholar]
- 25. Thomas ET, Guppy M, Straus SE, Bell KJL, Glasziou P. Rate of normal lung function decline in ageing adults: a systematic review of prospective cohort studies. BMJ Open 2019;9(6):e028150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ; ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stanojevic S, Graham BL, Cooper BG, Thompson BR, Carter KW, Francis RW, et al. ; Global Lung Function Initiative TLCO working group; Global Lung Function Initiative (GLI) TLCO. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017;50(3):1700010. [DOI] [PubMed] [Google Scholar]
- 28. Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant 2007;40(4):381–387. [DOI] [PubMed] [Google Scholar]
- 29. Iacobelli S; EBMT Statistical Committee. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2013;48(Suppl 1):S1–S37. [DOI] [PubMed] [Google Scholar]
- 30. Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res 2007;13(2 Pt 1):559–565. [DOI] [PubMed] [Google Scholar]
- 31. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133(6):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang X, Zhang M-J, Fine J. A proportional hazards regression model for the subdistribution with right-censored and left-truncated competing risks data. Stat Med 2011;30(16):1933–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170(2):244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017;36(27):4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glanville AR, Benden C, Bergeron A, Cheng G-S, Gottlieb J, Lease ED, et al. Bronchiolitis obliterans syndrome after lung or haematopoietic stem cell transplantation: current management and future directions. ERJ Open Res 2022;8(3):00185-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levy L, Huszti E, Renaud-Picard B, Berra G, Kawashima M, Takahagi A, et al. Risk assessment of chronic lung allograft dysfunction phenotypes: validation and proposed refinement of the 2019 International Society for Heart and Lung Transplantation classification system. J Heart Lung Transplant 2020;39(8):761–770. [DOI] [PubMed] [Google Scholar]
- 37. Sampath S, Schultheiss TE, Wong J. Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int J Radiat Oncol Biol Phys 2005;63(3):876–884. [DOI] [PubMed] [Google Scholar]