Abstract
Background
Identifying individuals with a higher risk of developing severe coronavirus disease 2019 (COVID-19) outcomes will inform targeted and more intensive clinical monitoring and management. To date, there is mixed evidence regarding the impact of preexisting autoimmune disease (AID) diagnosis and/or immunosuppressant (IS) exposure on developing severe COVID-19 outcomes.
Methods
A retrospective cohort of adults diagnosed with COVID-19 was created in the National COVID Cohort Collaborative enclave. Two outcomes, life-threatening disease and hospitalization, were evaluated by using logistic regression models with and without adjustment for demographics and comorbidities.
Results
Of the 2 453 799 adults diagnosed with COVID-19, 191 520 (7.81%) had a preexisting AID diagnosis and 278 095 (11.33%) had a preexisting IS exposure. Logistic regression models adjusted for demographics and comorbidities demonstrated that individuals with a preexisting AID (odds ratio [OR], 1.13; 95% confidence interval [CI]: 1.09–1.17; P < .001), IS exposure (OR, 1.27; 95% CI: 1.24–1.30; P < .001), or both (OR, 1.35; 95% CI: 1.29–1.40; P < .001) were more likely to have a life-threatening disease. These results were consistent when hospitalization was evaluated. A sensitivity analysis evaluating specific IS revealed that tumor necrosis factor inhibitors were protective against life-threatening disease (OR, 0.80; 95% CI: .66–.96; P = .017) and hospitalization (OR, 0.80; 95% CI: .73–.89; P < .001).
Conclusions
Patients with preexisting AID, IS exposure, or both are more likely to have a life-threatening disease or hospitalization. These patients may thus require tailored monitoring and preventative measures to minimize negative consequences of COVID-19.
Keywords: N3C retrospective analysis, COVID-19 severity, immunosuppressants, TNF inhibitors, autoimmune disease
Patients with preexisting autoimmune disease and/or immunosuppressant exposure may require tailored monitoring because they are at risk of developing severe COVID-19, independent of vaccination and antiviral treatment. Tumor necrosis factor inhibitors are an exception and are protective against worse outcomes.
The coronavirus disease 2019 (COVID-19) pandemic has affected more than 664 million individuals and caused more than 6.7 million deaths worldwide as of 10 January 2023 [1]. The public health burden and magnitude of the pandemic underlie the importance of identifying patients at elevated risk of developing severe disease to inform targeted clinical monitoring and management. Centers for Disease Control and Prevention (CDC) guidelines provide a list of medical conditions, including, but not limited to, cancer, chronic kidney/liver/lung diseases, and diabetes, that increase the risk of worse outcomes from COVID-19 [2]. With the notable exception of type 1 diabetes, autoimmune diseases (AID) are excluded from this list. This is counterintuitive since these are common diseases (24 million people suffer from AID in the United States alone [3]), are typically life-long and incurable, and are often treated with an immunosuppressant (IS) that could theoretically modify immunological responses to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. Therefore, it is important to directly evaluate, on a population scale, the impact of AID and IS exposure on severity outcomes of COVID-19 to help inform healthcare guidelines and raise awareness for patients with AID so that they can appropriately protect themselves from severe outcomes of COVID-19.
To date, there is mixed evidence regarding the association between AID and the severity of COVID-19 outcomes. For example, SARS-CoV-2–infected patients with rheumatic and musculoskeletal diseases were reported to have a higher risk of developing COVID-19 and of having hospitalization and severe COVID-19, including requiring intensive care unit (ICU) admission and mechanical ventilation [4]. Another recent study observed a higher risk of respiratory failure among patients with rheumatic disease with COVID-19 [5–7]. In contrast, a retrospective study of patients with AID hospitalized with COVID-19 did not show increased risk of ICU admission, intubation, or death [8]. Another meta-analysis of observational and case-control studies, constrained to limited demographics (age, gender) and marked by considerable heterogeneity across studies, reported a high prevalence of COVID-19 in patients with AID yet similar hospitalization and mortality rates compared with patients without AID [9]. These studies are limited by relatively small sample size, limited number of AID evaluated, inadequate representative population sampling, and/or failure to adjust for key confounders and known risk factors. Thus, whether AID are significant risk factors for worse outcomes from COVID-19 in larger cohorts that include a broad demographic and across the gamut of AID remains unknown.
An additional important confounder is whether IS exposure also contributes to adverse outcomes from COVID-19. A recent study showed that solid-organ transplant patients exposed to chronic immunosuppression and later diagnosed with COVID-19 have overall more severe disease [10]. Further, patients treated with an IS for cancer and solid-organ transplantation may be at higher risk of severe COVID-19 outcomes, although patients with other AID may not be [11]. Another study concluded that individuals on a long-term IS have worse outcomes when hospitalized with COVID-19 compared with those not on these medications [12]. Immunosuppression may thus be as relevant to COVID-19 outcomes as the underlying diseases. A meta-analysis study demonstrated that exposure to glucocorticoids, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), or the combination of biologic or targeted synthetic DMARDs (b/tsDMARDs) and csDMARDs was associated with severe COVID-19, while b/tsDMARDs monotherapy (eg, anti–tumor necrosis factor [TNF] monotherapy) was associated with less severe COVID-19 [9]. These findings imply that some forms of IS for AID could be protective against COVID-19. Indeed, some clinical data suggest that prior treatment with TNF inhibitors may protect patients with psoriasis, at least compared with other forms of therapy [13]. Similarly, treatment with interleukin (IL)-1, IL-6, and Janus Kinase (JAK) inhibitors are beneficial in patients with more severe COVID-19, and emerging data from the Accelerating COVID-19 Therapeutic Interventions and Vaccines study [14] also suggest that anti-TNF therapy and abatacept may be beneficial in this context. Thus, a large-scale evaluation of IS in the context of AID to differentiate those that are protective from those that are harmful could help refine healthcare guidelines for patients who use these medications.
To definitively establish whether individuals with AID or those treated with an IS experience worse severity outcomes from COVID-19, we leveraged data from the National COVID Cohort Collaborative (N3C) enclave, which harmonizes and holds electronic health records from 75 health systems with 15 231 849 million individuals’ data throughout the United States, of which 5 858 748 have had COVID-19 [15, 16]. N3C represents the largest retrospective US cohort of SARS-CoV-2 patients. We hypothesized that patients with a prior diagnosis of an AID and/or exposure to an IS were more likely to have worse COVID-19 outcomes (manifesting life-threatening disease or hospitalization). To address this hypothesis, we leveraged logistic regression models and conducted sensitivity analyses to ensure results were robust to vaccination status and antiviral treatment, to different race and gender groups, and to identify whether TNF inhibitors were protective against worse disease outcomes.
METHODS
Cohort Definition
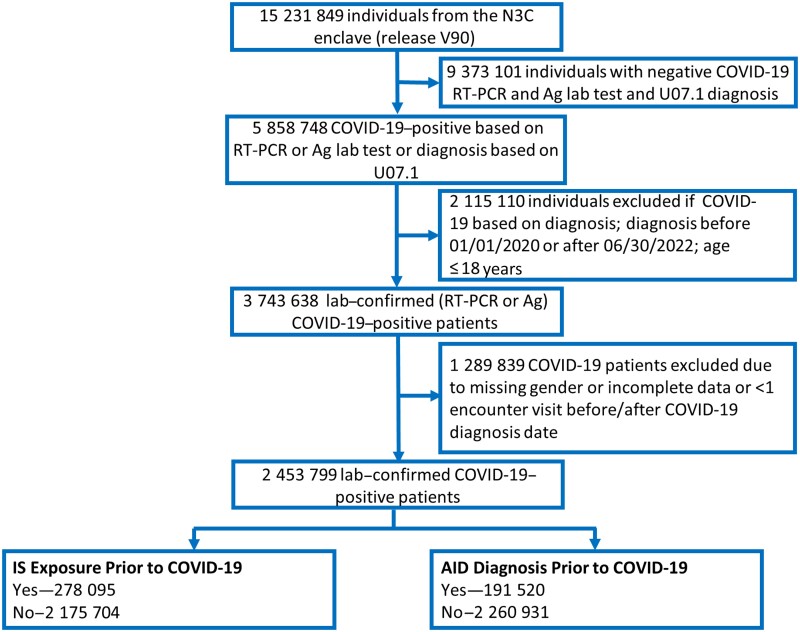
The N3C enclave [17] version V90 Limited DataSets data (N = 15 231 849 patients), including individuals entered on or before 25 August 2022, were used. We selected a subset of 2 453 799 patients who had a laboratory-confirmed positive COVID-19 diagnosis based on a positive SARS-CoV-2 polymerase chain reaction (PCR) or antigen (Ag) test between 1 January 2020 and 30 June 2022 inclusive (Figure 1). We excluded patients with age missing or aged ≤18 years, gender missing, with <1 encounter visit before or <1 encounter visit after COVID-19 diagnosis date, and patients from sites with data that did not meet quality check criteria.
Figure 1.
Workflow to define the AID cohort within N3C. A total of 15 231 849 patients were identified in the N3C enclave release version V90. Of these, 2 453 799 were COVID-19–positive between 1 January 2020 to 30 June 2022, as confirmed by an RT-PCR or Ag test, and had nonmissing values for age and gender. Patients were grouped based on whether they were diagnosed with an AID or exposed to an IS prior to COVID-19 diagnosis. See the Methods section for further details on inclusion/exclusion criteria. Abbreviations: Ag, antigen; AID, autoimmune disease; COVID-19, coronavirus disease 2019; IS, immunosuppressant; N3C, National COVID Cohort Collaborative; RT-PCR, reverse transcription-polymerase chain reaction; U07.1, 2023 ICD-10-CM Diagnosis Code for COVID-19.
Comorbidities, drug exposures, and other clinical information of patients diagnosed with COVID-19 are reported in N3C as far back as 1 January 2018 [12, 18]. Binarized comorbidities (Supplementary Table 1) were considered preexisting if their diagnosis date preceded that of COVID-19.
Severity Outcomes
COVID-19 severity outcomes in N3C are based on the clinical progression scale established by the World Health Organization (WHO) [15, 19]. Unaffected (WHO severity 0) patients were removed. Severity of the remaining patients was classified as mild (WHO severity 1–3), mild_ED (WHO severity 3), moderate (WHO severity 4–6, hospitalized patients without invasive ventilation), severe (WHO severity 7–9, hospitalized patients with invasive ventilation or extracorporeal membrane oxygenation), and mortality or hospice (WHO severity 10). We further categorized binary severity outcomes as follows: life-threatening disease (deceased/severe vs moderate/mild_ED/mild) and hospitalization (dead/severe/moderate vs mild/mild_ED).
Definition of Preexisting AID and IS Exposure
A curated list of 106 AID based on 2 previously published lists [20, 21] was used to identify COVID-19 patients with or without AID within N3C (Supplementary Table 2 for variable coding details, Supplementary Figure 1A). For defining IS exposure, 15 previously published drug classes representing 303 drugs [12] (Supplementary Figure 1B) were considered. Of note, the computable phenotypes presented here differ from what has been previously published from this database as it was developed by the authors rather than the Immunosuppressed/Compromised Clinical Domain Team.
A subanalysis of AID patients with and without prior exposure to TNF inhibitors included those exposed to etanercept, infliximab, afelimomab, adalimumab, certolizumab pegol, golimumab, or opinercept.
Definition of Vaccination Status and Antiviral Usage
We defined a subcohort of patients diagnosed with COVID-19 between 23 December 2021 and 30 June 2022 to enable adjustment of models for vaccination status and exposure to antivirals. This time frame was selected because oral antiviral therapy became available through the US Food and Drug Administration (FDA) emergency use authorization mechanism in late December 2021. Only sites with vaccination rates that reasonably matched CDC records for that site's geographic region were included. Patients were considered vaccinated if they had at least 1 vaccination administered prior to their COVID-19 diagnosis date. Patients exposed to antivirals were treated with at least 1 dose of any oral antiviral (Paxlovid [nirmatrelvir/ritonavir], LAGEVRIO [molnupiravir]) or 1 monoclonal antibody (bebtelovimab) for COVID-19 between the first COVID-19 diagnosis date and up to 10 days before/after.
Statistical Analyses
Statistical modeling (Supplementary Figure 2) was conducted within the N3C enclave using SQL, Python(3.6.7), statsmodels (version 0.12.2), Patsy (version 0.5.2), and scipy(1.6.2). Statistical significance was defined for P values < .05%; 95% confidence intervals (CIs) around the estimated odd ratios (ORs) are reported. The baseline characteristic table (Table 1) was created using the tableone python package [22].
Table 1.
Characteristics of Coronavirus Disease 2019–Positive Patients With and Without Autoimmune Diseases/Immunosuppressants Usage
| Characteristic | Overall N3C Sample (n = 2 453 799) |
Patients Without AID Prior to COVID-19 (n = 2 262 279) |
Patients With AID Prior to COVID-19 (n = 191 520) |
Patients Without IS Prior to COVID-19 (n = 2 175 704) |
Patients With IS Prior to COVID-19 (n = 278 095) |
|---|---|---|---|---|---|
| COVID-19 severity | |||||
| Life-threatening,a n (%) | 54 932 (2.2) | 46 335 (2.0) | 8597 (4.5) | 41 212 (1.9) | 13 720 (4.9) |
| Hospitalized,b n (%) | 220 353 (9.0) | 189 519 (8.4) | 30 834 (16.1) | 174 587 (8.0) | 45 766 (16.5) |
| Demographics | |||||
| Age, mean (SD), y | 47.4 (18.0) | 46.9 (18.0) | 53.8 (17.2) | 46.8 (18.0) | 52.3 (17.4) |
| Body mass index, mean (SD), kg/m2 | 32.3 (8.4) | 32.2 (8.3) | 32.9 (8.6) | 31.8 (8.2) | 33.9 (9.0) |
| Gender, n (%) | |||||
| Female | 1 454 673 (59.3) | 1 323 247 (58.5) | 131 426 (68.6) | 1 281 335 (58.9) | 173 338 (62.3) |
| Male | 999 126 (40.7) | 939 032 (41.5) | 60 094 (31.4) | 894 369 (41.1) | 104 757 (37.7) |
| Race, n (%) | |||||
| Black or African American | 342 511 (14.0) | 316 416 (14.0) | 26 095 (13.6) | 295 376 (13.6) | 47 135 (16.9) |
| White | 1 790 481 (73.0) | 1 644 325 (72.7) | 146 156 (76.3) | 1 590 559 (73.1) | 199 922 (71.9) |
| Asian | 61 597 (2.5) | 57 870 (2.6) | 3727 (1.9) | 56 531 (2.6) | 5066 (1.8) |
| Missing/Unknown/Other | 255 270 (10.4) | 239 977 (10.6) | 15 293 (8.0) | 229 739 (10.6) | 25 531 (9.2) |
| Ethnicity, n (%) | |||||
| Hispanic or Latino | 269 354 (11.0) | 252 829 (11.2) | 16 525 (8.6) | 244 580 (11.2) | 24 774 (8.9) |
| Not Hispanic or Latino | 1 966 475 (80.1) | 1 805 569 (79.8) | 160 906 (84.0) | 1 739 697 (80.0) | 226 778 (81.5) |
| Missing/Unknown | 217 970 (8.9) | 203 881 (9.0) | 14 089 (7.4) | 191 427 (8.8) | 26 543 (9.5) |
| Smoking status, n (%) | |||||
| Nonsmoker | 2 173 346 (88.6) | 2 014 857 (89.1) | 158 489 (82.8) | 1 959 001 (90.0) | 214 345 (77.1) |
| Current or former smoker | 280 453 (11.4) | 247 422 (10.9) | 33 031 (17.2) | 216 703 (10.0) | 63 750 (22.9) |
| Comorbidity | |||||
| Cardiovascular disease,c n (%) | 296 990 (12.1) | 237 822 (10.5) | 54 154 (28.3) | 216 891 (10.0) | 80 099 (28.8) |
| Dementia, n (%) | 32 023 (1.3) | 26 950 (1.2) | 5073 (2.6) | 25 810 (1.2) | 6213 (2.2) |
| Chronic pulmonary disease, n (%) | 333 222 (13.6) | 281 063 (12.4) | 52 159 (27.2) | 242 727 (11.2) | 90 495 (32.5) |
| Liver disease, mild, n (%) | 113 751 (4.6) | 91 069 (4.0) | 22 682 (11.8) | 79 800 (3.7) | 33 951 (12.2) |
| Liver disease, severe, n (%) | 13 463 (0.5) | 9817 (0.4) | 3646 (1.9) | 8378 (0.4) | 5085 (1.8) |
| Type 2 diabetes, n (%) | 308 690 (12.6) | 255 620 (11.3) | 53 070 (27.7) | 242 472 (11.1) | 66 218 (23.8) |
| Kidney disease, n (%) | 138 958 (5.7) | 108 921 (4.8) | 30 037 (15.7) | 97 804 (4.5) | 41 154 (14.8) |
| Cancer, n (%) | 137 706 (5.6) | 115 144 (5.1) | 22 562 (11.8) | 95 543 (4.4) | 42 163 (15.2) |
| Metastatic cancer, n (%) | 22 737 (0.9) | 19 051 (0.8) | 3686 (1.9) | 12 304 (0.6) | 10 433 (3.8) |
| Human immunodeficiency virus, n (%) | 10 959 (0.4) | 9882 (0.4) | 1077 (0.6) | 8625 (0.4) | 2334 (0.8) |
Distribution of all covariables differs between patients with/without AID and with/without IS exposure (all 2-tailed Student t test for continuous variables and χ2 test for categorical variables; P values <.001).
Abbreviations: AID, autoimmune disease; COVID-19, coronavirus disease 2019; IS, immunosuppressant; SD, standard deviation.
Life-threatening: death, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation vs moderate or mild_ED or mild.
Hospitalized: death, ECMO, or mechanical ventilation or moderate vs (mild_ED or mild).
Cardiovascular disease: myocardial infarction, congestive heart failure, peripheral vascular disease, stroke.
RESULTS
Cohort Description
We defined a large cohort within the N3C data enclave [17] to evaluate the impact of prior AID diagnosis and IS exposure on COVID-19 severity outcomes (Figure 1, Supplementary Figure 1). Of 15 231 849 individuals, 2 453 799 were diagnosed with COVID-19, as indicated by a positive reverse transcription polymerase chain reaction (RT-PCR) or antigen test between 1 January 2020 and 30 June 2022 inclusive. Among these 2 453 799 patients, 220 353 (9%) were hospitalized and 54 932 (2.2%) had life-threatening disease (Table 1). Patients were further categorized as those with a preexisting (prior to COVID-19 diagnosis) AID (n = 191 520), IS exposure (n = 278 095), or both (n = 56 813; Figure 1, Supplementary Figure 1A and 1B ).
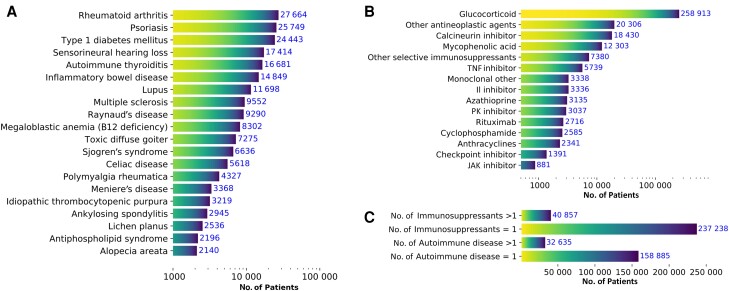
The top 3 most abundant AID were rheumatoid arthritis (n = 27 664), psoriasis (n = 25 749), and type 1 diabetes mellitus (n = 24 443; Figure 2A ). The top 3 most frequent IS drugs that patients were exposed to were glucocorticoids, other antineoplastic agents, and calcineurin inhibitors (Figure 2B ). Most patients with COVID-19 had a single preexisting AID diagnosis (n = 159 770) and exposure to a single IS (n = 237 238, representing 85.31% of patients with IS exposure; Figure 2C ). Last, we noted that the most frequent conditions and IS drugs showed a larger proportion of patients with life-threatening conditions or hospitalization (Supplementary Figure 3).
Figure 2.
Description of cohort exposures. Top 20 most abundant autoimmune diseases (AID) (A) and immunosuppressant exposures (B) prior to diagnosis of laboratory-confirmed coronavirus disease 2019. “Other selective immunosuppressants” include selective immunosuppressants that are not interleukin inhibitors, JK inhibitors, TNF alpha inhibitors, or monoclonal antibodies. “Other antineoplastic agents” includes monoclonal antibodies, Cancer Drugs L01 Other Cancer Therapies defined using World Health Organization Anatomical Therapeutic Chemistry Class L01 products that were not anthracyclines, checkpoint inhibitors, cyclophosphamide, or protein kinase inhibitors. "L01 other" includes other cancer therapies include therapies that are not anthracyclines, checkpoint inhibitors, cyclophosphamide, or protein kinase inhibitors. (C) Number of patients with single/multiple AID and patients with single/multiple immunosuppressant exposures. Abbreviations: JAK, Janus Kinase; PK, protein kinase; TNF, tumor necrosis factor.
Association Between Prior Exposure to AID, IS, or Both With COVID-19 Severity Outcomes
Two binary (presence/absence) clinically relevant COVID-19 severity outcomes were defined: life-threatening disease and hospitalization (see the Methods section). We tested whether a preexisting diagnosis of AID, IS exposure, or both were associated with these outcomes using univariate and multivariate models adjusted for demographics (Supplementary Table 3). In our final analyses (Table 2), the model was adjusted for demographics and preexisting comorbidities and showed that patients were almost 21% more likely to be hospitalized if they had a preexisting AID (OR, 1.21; 95% CI: 1.19–1.24; P < .001), 19% more likely if they had prior IS exposure (OR, 1.19; 95% CI: 1.17–1.21; P < .001), and 31% more likely if they had both (OR, 1.31; 95% CI: 1.28–1.34; P < .001). Similarly, when adjusting for demographics and comorbidities, patients were 13% more likely to develop life-threatening COVID-19 if they had a preexisting AID (OR, 1.13; 95% CI: 1.10–1.17; P < .001), 27% more likely if they had prior IS exposure (OR, 1.27; 95% CI: 1.24–1.30; P < .001), and 35% more likely if they had both (OR, 1.35; 95% CI: 1.29–1.40; P < .001; Table 2). Similar results were obtained when stratifying by race and gender for both COVID-19 outcomes (Supplementary Tables 4 and 5).
Table 2.
Multivariate Logistic Regression Model of Severity Outcomes Adjusted for Demographics and Comorbidities: 1 January 2020 to 30 June 2022 for n = 2 453 799
| Covariables | Life-Threatening Condition (Yes, 54 932; No, 2 398 867) OR (95% CI) |
P Value | Hospitalized Condition (Yes, 220 353; No, 2 233 446) OR (95% CI) |
P Value |
|---|---|---|---|---|
| AID/IS status | ||||
| AID only | 1.13 (1.09–1.17) | <.001 | 1.21 (1.19–1.24) | <.001 |
| IS only | 1.27 (1.24–1.30) | <.001 | 1.19 (1.17–1.21) | <.001 |
| AID + IS only | 1.35 (1.29−1.40) | <.001 | 1.31 (1.28–1.34) | <.001 |
| Demographics | ||||
| Age | 2.84 (2.81–2.88) | <.001 | 1.94 (1.93–1.95) | <.001 |
| Body mass index | 1.08 (1.07–1.09) | <.001 | 1.10 (1.09–1.11) | <.001 |
| Gender | ||||
| Female | 1 [Reference] | 1 [Reference] | … | |
| Male | 1.51 (1.48–1.53) | <.001 | 1.29 (1.28–1.30) | <.001 |
| Race | ||||
| White | 1 [Reference] | 1 [Reference] | … | |
| Other/Unknown | 1.20 (1.16–1.25) | <.001 | 1.18 (1.16–1.20) | <.001 |
| Black or African American | 1.37 (1.33–1.40) | <.001 | 1.77 (1.75–1.80) | <.001 |
| Asian | 1.32 (1.24–1.40) | <.001 | 1.35 (1.31–1.40) | <.001 |
| Ethnicity | ||||
| Not Hispanic or Latino | 1 [Reference] | 1 [Reference] | … | |
| Hispanic or Latino | 1.18 (1.13–1.22) | <.001 | 1.39 (1.36–1.41) | <.001 |
| Smoking status | ||||
| Nonsmoker | 1 [Reference] | 1 [Reference] | … | |
| Current or former | 1.37 (1.34–1.40) | <.001 | 1.61 (1.59–1.63) | <.001 |
| Comorbidity | ||||
| Cardiovascular diseasea | 1.69 (1.66–1.73) | <.001 | 1.67 (1.65–1.69) | <.001 |
| Dementia | 2.22 (2.16–2.30) | <.001 | 1.99 (1.94–2.04) | <.001 |
| Chronic pulmonary disease | 1.23 (1.21–1.26) | <.001 | 1.29 (1.27–1.30) | <.001 |
| Liver disease, mild | 1.34 (1.30–1.38) | <.001 | 1.25 (1.22–1.27) | <.001 |
| Liver disease, severe | 3.05 (2.89–3.23) | <.001 | 2.33 (2.23–2.42) | <.001 |
| Kidney disease | 1.77 (1.73–1.81) | <.001 | 1.88 (1.86–1.91) | <.001 |
| Cancer | 1.45 (1.41–1.49) | <.001 | 1.24 (1.22–1.26) | <.001 |
| Metastatic cancer | 3.31 (3.17–3.46) | <.001 | 2.19 (2.12–2.26) | <.001 |
| Type 2 diabetes mellitus | 1.32 (1.29–1.35) | <.001 | 1.46 (1.44–1.48) | <.001 |
| Human immunodeficiency virus | 1.24 (1.12–1.38) | <.001 | 1.26 (1.19–1.33) | <.001 |
Abbreviations: AID, autoimmune disease; CI, confidence interval; IS, immunosuppressant; OR, odds ratio.
Cardiovascular disease: myocardial infarction, congestive heart failure, peripheral vascular disease, stroke.
Association Between AID, IS, or Both With COVID-19 Severity Outcomes in a Cohort Subset Adjusting for COVID-19 Vaccination and Antiviral Exposure
The FDA has authorized antiviral medications and monoclonal antibodies to treat mild to moderate COVID-19 in outpatients diagnosed with COVID-19 who were prone to severe disease manifestations. We specifically considered 2 small-molecule antivirals (Paxlovid [nirmatrelvir/ritonavir], LAGEVRIO [molnupiravir]) and 1 monoclonal antibody (bebtelovimab) that were granted emergency use authorization by the FDA on or after December 2021 (the beginning of the Omicron epoch). Although bebtelovimab has since lost activity against the most recent dominant Omicron variants in the United States (BQ.1, BQ.1.1, and XBB), it was active against prior common US Omicron variants during the study period. Of the 248 743 patients diagnosed with COVID-19 between 23 December 2021 and 30 June 2022, 134 812 (54.2%) were vaccinated and 3974 (1.6%) were exposed to antivirals (see the Methods section, Supplementary Figure 1C). As expected, when adjusting for demographics and comorbidities, we found that usage of antivirals was protective (life-threatening disease: OR, 0.31; 95% CI: .21–.45; P < .001 and hospitalization: OR, 0.30; 95% CI: .25–.36; P < .001; Supplementary Table 6). Most importantly, independent of exposure to antivirals and vaccination status, patients with preexisting AID (OR, 1.34; 95% CI: 1.25–1.43; P < .001), IS exposure (OR, 1.61; 95% CI: 1.51–1.72; P < .001), or both (OR, 1.90; 95% CI: 1.73–2.10; P < .001) were more likely to be hospitalized. Similarly, patients with preexisting AID (OR, 1.18; 95% CI: 1.02–1.36; P < .001), prior IS exposure (OR, 1.60; 95% CI: 1.42–1.81; P < .001), or both (OR, 1.94; 95% CI: 1.63–2.30; P < .001) were more likely to have life-threatening disease, independent of exposure to antivirals and vaccination (Supplementary Table 6). We confirmed that associations of AID and/or IS exposure and worse COVID-19 outcomes were consistent in a subcohort comprising patients identified prior to vaccination rollout (Supplementary Tables 7 and 8).
Association Between TNF Inhibitors and Other ISs With COVID-19 Severity Outcomes in AID Patients
In view of prior data suggesting that TNF inhibitors may be protective against COVID-19 [13], we investigated the association of exposure to TNF inhibitors prior to COVID-19 diagnosis with COVID-19 severity outcomes in patients with a prior AID diagnosis. Of the 191 520 patients with a preexisting AID diagnosis, 4789 had been exposed to TNF inhibitors at least 14 days prior to their COVID-19 diagnosis. When adjusting for demographics and comorbidities, we found that exposure to TNF inhibitors protected against severe COVID-19 outcomes (life-threatening disease: OR, 0.80; 95% CI: .66–.96; P = .017 and hospitalization: OR, 0.80; 95% CI: .73–.89; P < .001; Table 3). While other IS were individually evaluated, only TNF inhibitors showed this protective effect.
Table 3.
Logistic Regression of Severity Outcomes in Autoimmune Disease Patients Only With Each Immunosuppressant Exposure Evaluated Individually
| Covariable | Life-Threatening Condition (Yes, 8597; No, 182 923) OR (95% CI) |
P Value | Hospitalized Condition (Yes, 30 834; No, 160 686) OR (95% CI) |
P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 2.09 (2.02–2.16) | <.001 | 1.55 (1.53–1.58) | <.001 |
| Body mass index | 1.04 (1.03–1.06) | <.001 | 1.06 (1.05–1.07) | <.001 |
| Gender | ||||
| Female | 1 [Reference] | 1 [Reference] | … | |
| Male | 1.36 (1.30–1.42) | <.001 | 1.29 (1.26–1.33) | <.001 |
| Race | ||||
| White | 1 [Reference] | 1 [Reference] | … | |
| Others/Unknown | 1.13 (1.02–1.25) | .02 | 1.15 (1.09–1.22) | <.001 |
| Black or African American | 1.25 (1.17–1.34) | <.001 | 1.63 (1.57–1.68) | <.001 |
| Asian | 1.26 (1.06–1.50) | .01 | 1.13 (1.03–1.25) | .01 |
| Ethnicity | ||||
| Not Hispanic or Latino | 1 [Reference] | 1 [Reference] | … | |
| Hispanic or Latino | 1.13 (1.03–1.25) | .01 | 1.28 (1.22–1.35) | <.001 |
| Smoking status | ||||
| Nonsmoker | 1 [Reference] | 1 [Reference] | … | |
| Current or former | 1.20 (1.13–1.27) | <.001 | 1.39 (1.35–1.44) | <.001 |
| Comorbidity | ||||
| Cardiovascular diseasea | 1.88 (1.78–1.98) | <.001 | 1.67 (1.62–1.72) | … |
| Dementia | 2.20 (2.04–2.38) | <.001 | 2.09 (1.97–2.23) | <.001 |
| Chronic pulmonary disease | 1.24 (1.18–1.31) | <.001 | 1.23 (1.20–1.27) | <.001 |
| Liver disease, mild | 1.19 (1.11–1.27) | <.001 | 1.13 (1.09–1.18) | <.001 |
| Liver disease, severe | 2.39(2.15–2.66) | <.001 | 1.93 (1.78–2.09) | <.001 |
| Kidney disease | 1.94 (1.85–2.05) | <.001 | 1.89 (1.83–1.95) | <.001 |
| Cancer | 1.32 (1.25–1.40) | <.001 | 1.16 (1.12–1.21) | <.001 |
| Metastatic cancer | 2.36 (2.12–2.62) | <.001 | 1.67 (1.54–1.81) | <.001 |
| Type 2 diabetes mellitus | 1.35 (1.29–1.42) | <.001 | 1.50 (1.46–1.55) | <.001 |
| Human immunodeficiency virus | 1.00 (.77–1.30) | .99 | 1.05 (.90–1.22) | .56 |
| Immunosuppressant | ||||
| Tumor necrosis factor inhibitor | .80 (.66–.96) | .02 | .80 (.73–.89) | <.001 |
| Calcineurin inhibitor | .95(.84–1.08) | .46 | .99 (.91–1.07) | .70 |
| Interleukin inhibitor | 1.03 (.84–1.27) | .76 | 1.08 (.96–1.21) | .221 |
| Other selective immunosuppressant | 1.04 (.91–1.20) | .55 | 1.15 (1.06–1.25) | <.001 |
| Cyclophosphamide | 1.05 (.77–1.44) | .74 | .90 (.72–1.12) | .35 |
| Glucocorticoid | 1.12 (1.06–1.18) | <.001 | 1.04 (1.01–1.08) | .01 |
| Azathioprine | 1.13 (.93–1.38) | .22 | 1.17 (1.04–1.31) | .01 |
| Other antineoplastic agents | 1.24 (1.12–1.37) | <.001 | 1.04 (.98–1.11) | .22 |
| JAK inhibitor | 1.28 (.91–1.79) | .16 | 1.37 (1.14–1.66) | .001 |
| Monoclonal other | 1.33 (1.07–1.65) | .01 | 1.09 (.92–1.29) | .30 |
| Mycophenolic acid | 1.44 (1.25–1.65) | <.001 | 1.60 (1.47–1.74) | <.001 |
| Anthracyclines | 1.54 (1.10–2.14) | .01 | 1.53 (1.19–1.98) | .001 |
| Rituximab | 1.62 (1.35–1.95) | <.001 | 1.73 (1.53–1.96) | <.001 |
| Protein kinase (PK) inhibitor | 1.63 (1.32–2.01) | <.001 | 1.76 (1.50–2.08) | <.001 |
| Checkpoint inhibitor | 1.70 (1.28–2.26) | <.001 | 1.29 (.99–1.67) | .06 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Cardiovascular disease: myocardial infarction, congestive heart failure, peripheral vascular disease, stroke.
DISCUSSION
Using N3C, we identified 2 453 799 patients diagnosed with PCR- or antigen testing–confirmed COVID-19, of whom 191 520 had a prior diagnosis of AID, 278 095 had a prior IS exposure, and 56 813 had both. Our cohort comprises data from the beginning of the pandemic to 30 June 2022, which includes epochs spanning the ancestral strain as well as 5 major variants (Alpha, Beta, Gamma, Delta, and Omicron). This cohort thus appropriately represents a broad population of patients diagnosed with COVID-19 and AID in the United States over time.
We found that COVID-19 patients with a prior diagnosis of AID, IS exposure, or both were more likely to have life-threatening disease or be hospitalized. These results were robust to adjustments for demographics and comorbidities. Further, a sensitivity analysis in a subset of our cohort confirmed that AID and/or IS exposure are risk factors for worse COVID-19, independent of exposure to antiviral treatments and/or having at least 1 COVID-19 vaccination dose. We note that our observed protective effect of vaccination against worse outcomes may be underestimated, given our inclusion criteria of a single vaccination dose. Nonetheless, our results help clarify the ambiguity in previous studies in answering the difficult question of whether prior AID diagnosis or IS exposure are risk factors for worse COVID-19 disease outcomes.
Race and gender are known to be associated with COVID-19 severity. For example, Asian American individuals have a higher risk of COVID-19 positivity and ICU admission than White individuals [23, 24], and socioeconomic disparity and clinical care quality are associated with COVID-19 mortality and incidence in racial and ethnic minority groups [24]. Further, severity and mortality of COVID-19 are higher in males than in females [25, 26]. Our results confirmed that the effects of AID, IS, or both on COVID-19 severity outcomes were significant across the different race and gender groups.
Finally, we clarified whether some specific IS showed contrary effects. Indeed, a recent study suggested TNF inhibitor monotherapy was associated with a lower risk of adverse COVID-19 outcomes compared with other commonly prescribed immunotherapy among patients with AID [27]. Here, we confirm that patients with a prior AID diagnosis and exposure to TNF inhibitors prior to infection are less likely to be hospitalized or have life-threatening COVID-19. We also confirm that this protective effect is unique to TNF inhibitors.
There are limitations to this study worth noting. First, the medical history of COVID-19 patients is limited to 1 January 2018 or later, with some patients having limited interaction with participating healthcare systems prior to their index diagnosis, making it difficult to fully assess preexisting conditions and comorbidities and to precisely determine the date of AID diagnosis. To mitigate these risks, patients with at least 1 encounter before diagnosis were included to increase the robustness of past medical history documentation. Second, N3C data are aggregated from many healthcare systems, covering 4 common data models that vary in granularity. Harmonization of these disparate data thus requires assumptions and inferences to be made that could incur systematic biases. Similarly, the ability to accurately determine race within N3C is diminished by variations in how race is reported in different healthcare systems [28]. Nonetheless, we highlight the meticulous efforts of the N3C collaborative in evaluating and improving the quality of phenotypes generated within N3C [29]. Third, missingness is a known issue with vaccination data, as patients may have received vaccine doses at pop-up clinics, drugstores, or at their place of employment, which may not be recorded in the patients’ records. To counteract this missingness, we only included sites whose rate of vaccination in N3C data was within range of the CDC vaccination rate for that site's geographic region [30]. Finally, we recognize limitations related to the retrospective design of this study; inability to handle all possible confounders, including biases in the standard of care across hospitals and physician behavior; and the possibility that follow-up data among patients could be incomplete (eg, patients seeking care in institutions not affiliated with N3C). Despite these limitations, this study is an important step toward increasing our understanding, at a population level, of whether prior exposure to AID, IS, or both pose an additional risk to patients in developing worse COVID-19 disease outcomes.
CONCLUSIONS
To the best of our knowledge, this study represents the largest, most comprehensive systematic analysis of the effects of AID, IS, or both on COVID-19 severity outcomes. Our study suggests that patients with a prior AID diagnosis, prior to IS exposure, or both have a higher risk of life-threatening COVID-19 disease or hospitalization. These associations were consistent in different race and gender subsets. Importantly, this study provides more definitive clarity of previous discrepant findings on whether patients with AID and/or IS exposure are at higher risk for worse COVID-19–related outcomes, providing clinicians with helpful data that may help guide their treatment and monitoring plans.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Arjun S Yadaw, National Center for Advancing Translational Sciences, National Institutes of Health, Rockville, Maryland, USA.
David K Sahner, National Center for Advancing Translational Sciences, National Institutes of Health, Rockville, Maryland, USA.
Hythem Sidky, National Center for Advancing Translational Sciences, National Institutes of Health, Rockville, Maryland, USA.
Behdad Afzali, Immunoregulation Section, Kidney Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Nathan Hotaling, National Center for Advancing Translational Sciences, National Institutes of Health, Rockville, Maryland, USA.
Emily R Pfaff, North Carolina Translational and Clinical Sciences Institute, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Ewy A Mathé, National Center for Advancing Translational Sciences, National Institutes of Health, Rockville, Maryland, USA.
Notes
National COVID Cohort Collaborative attribution. The analyses described here were conducted with data or tools accessed through the National Center for Advancing Translational Sciences (NCATS) National COVID Cohort Collaborative (N3C) Data Enclave (COVID.cd2h.org/enclave) and supported by the National Center for Data to Health (CD2H), the N3C IDeA Clinical Translational Research Collaboration (3U24TR002306–04S2 NCATS U24 TR002306). This research was possible because of the patients whose information is included within the data from participating organizations (COVID.cd2h.org/dtas) and the organizations and scientists (COVID.cd2h.org/duas) who have contributed to the ongoing development of this community resource (cite this https://doi.org/10.1093/jamia/ocaa196).
Institutional review board. The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol (IRB00249128) or individual site agreements with the National Institutes of Health (NIH). The N3C Data Enclave is managed under the authority of the NIH. Information can be found at https://ncats.nih.gov/n3c/resources.
Individual acknowledgments for core contributors. We gratefully acknowledge the following core contributors to N3C: Adam B. Wilcox, Adam M. Lee, Alexis Graves, Alfred (Jerrod) Anzalone, Amin Manna, Amit Saha, Amy Olex, Andrea Zhou, Andrew E. Williams, Andrew Southerland, Andrew T. Girvin, Anita Walden, Anjali A. Sharathkumar, Benjamin Amor, Benjamin Bates, Brian Hendricks, Brijesh Patel, Caleb Alexander, Carolyn Bramante, Cavin Ward-Caviness, Charisse Madlock-Brown, Christine Suver, Christopher Chute, Christopher Dillon, Chunlei Wu, Clare Schmitt, Cliff Takemoto, Dan Housman, Davera Gabriel, David A. Eichmann, Diego Mazzotti, Don Brown, Eilis Boudreau, Elaine Hill, Elizabeth Zampino, Emily Carlson Marti, Emily R. Pfaff, Evan French, Farrukh M. Koraishy, Federico Mariona, Fred Prior, George Sokos, Greg Martin, Harold Lehmann, Heidi Spratt, Hemalkumar Mehta, Hongfang Liu, Hythem Sidky, J. W. Awori Hayanga, Jami Pincavitch, Jaylyn Clark, Jeremy Richard Harper, Jessica Islam, Jin Ge, Joel Gagnier, Joel H. Saltz, Joel Saltz, Johanna Loomba, John Buse, Jomol Mathew, Joni L. Rutter, Julie A. McMurry, Justin Guinney, Justin Starren, Karen Crowley, Katie Rebecca Bradwell, Kellie M. Walters, Ken Wilkins, Kenneth R. Gersing, Kenrick Dwain Cato, Kimberly Murray, Kristin Kostka, Lavance Northington, Lee Allan Pyles, Leonie Misquitta, Lesley Cottrell, Lili Portilla, Mariam Deacy, Mark M. Bissell, Marshall Clark, Mary Emmett, Mary Morrison Saltz, Matvey B. Palchuk, Melissa A. Haendel, Meredith Adams, Meredith Temple-O'Connor, Michael G. Kurilla, Michele Morris, Nabeel Qureshi, Nasia Safdar, Nicole Garbarini, Noha Sharafeldin, Ofer Sadan, Patricia A. Francis, Penny Wung Burgoon, Peter Robinson, Philip R. O. Payne, Rafael Fuentes, Randeep Jawa, Rebecca Erwin-Cohen, Rena Patel, Richard A. Moffitt, Richard L. Zhu, Rishi Kamaleswaran, Robert Hurley, Robert T. Miller, Saiju Pyarajan, Sam G. Michael, Samuel Bozzette, Sandeep Mallipattu, Satyanarayana Vedula, Scott Chapman, Shawn T. O'Neil, Soko Setoguchi, Stephanie S. Hong, Steve Johnson, Tellen D. Bennett, Tiffany Callahan, Umit Topaloglu, Usman Sheikh, Valery Gordon, Vignesh Subbian, Warren A. Kibbe, Wenndy Hernandez, Will Beasley, Will Cooper, William Hillegass, and Xiaohan Tanner Zhang. Details of contributions are available at COVID.cd2h.org/core-contributors.
Data partners with released data. The following institutions have available or pending data. Available data: Advocate Health Care Network (UL1TR002389; Institute for Translational Medicine (ITM); Boston University Medical Campus (UL1TR001430; Boston University Clinical and Translational Science Institute); Brown University (U54GM115677; Advance Clinical Translational Research (Advance-CTR); Carilion Clinic (UL1TR003015; iTHRIV Integrated Translational Health Research Institute of Virginia); Charleston Area Medical Center (U54GM104942; West Virginia Clinical and Translational Science Institute (WVCTSI); Children's Hospital Colorado (UL1TR002535; Colorado Clinical and Translational Sciences Institute); Columbia University Irving Medical Center (UL1TR001873; Irving Institute for Clinical and Translational Research); Duke University (UL1TR002553; Duke Clinical and Translational Science Institute); George Washington Children's Research Institute (UL1TR001876; Clinical and Translational Science Institute at Children's National (CTSA-CN); George Washington University (UL1TR001876; CTSA-CN); Indiana University School of Medicine (UL1TR002529; Indiana Clinical and Translational Science Institute); Johns Hopkins University (UL1TR003098; Johns Hopkins Institute for Clinical and Translational Research); Loyola Medicine (Loyola University Medical Center); Loyola University Medical Center (UL1TR002389; ITM); Maine Medical Center (U54GM115516; Northern New England Clinical & Translational Research [NNE-CTR] Network); Massachusetts General Brigham (UL1TR002541; Harvard Catalyst); Mayo Clinic Rochester (UL1TR002377; Mayo Clinic Center for Clinical and Translational Science; Medical University of South Carolina (UL1TR001450; South Carolina Clinical & Translational Research Institute); Montefiore Medical Center (UL1TR002556; Institute for Clinical and Translational Research at Einstein and Montefiore); Nemours (U54GM104941; Delaware CTR ACCEL Program); NorthShore University Health System (UL1TR002389; ITM); Northwestern University at Chicago (UL1TR001422; Northwestern University Clinical and Translational Science Institute; OCHIN (INV-018455; Bill and Melinda Gates Foundation grant to Sage Bionetworks); Oregon Health & Science University (UL1TR002369; Oregon Clinical and Translational Research Institute); Penn State Health Milton S. Hershey Medical Center (UL1TR002014; Penn State Clinical and Translational Science Institute); Rush University Medical Center (UL1TR002389; ITM); Rutgers, State University of New Jersey (UL1TR003017; New Jersey Alliance for Clinical and Translational Science); Stony Brook University (U24TR002306); Ohio State University (UL1TR002733; Center for Clinical and Translational Science); State University of New York at Buffalo (UL1TR001412; Clinical and Translational Science Institute); University of Chicago (UL1TR002389; ITM); University of Iowa (UL1TR002537; Institute for Clinical and Translational Science); University of Miami Leonard M. Miller School of Medicine (UL1TR002736; University of Miami Clinical and Translational Science Institute); University of Michigan at Ann Arbor (UL1TR002240; Michigan Institute for Clinical and Health Research); University of Texas Health Science Center at Houston (UL1TR003167; Center for Clinical and Translational Sciences (CCTS); University of Texas Medical Branch at Galveston (UL1TR001439; Institute for Translational Sciences); University of Utah (UL1TR002538; Uhealth Center for Clinical and Translational Science); Tufts Medical Center (UL1TR002544; Tufts Clinical and Translational Science Institute); Tulane University (UL1TR003096; Center for Clinical and Translational Science); University Medical Center New Orleans (U54GM104940; Louisiana Clinical and Translational Science (LA CaTS) Center); University of Alabama at Birmingham (UL1TR003096; Center for Clinical and Translational Science); University of Arkansas for Medical Sciences (UL1TR003107; University of Arkansas for Medical Sciences Translational Research Institute); University of Cincinnati (UL1TR001425; Center for Clinical and Translational Science and Training); University of Colorado–Denver, Anschutz Medical Campus (UL1TR002535; Colorado Clinical and Translational Sciences Institute); University of Illinois at Chicago (UL1TR002003; University of Illinois at Chicago Center for Clinical and Translational Science); University of Kansas Medical Center (UL1TR002366; Frontiers: University of Kansas Clinical and Translational Science Institute); University of Kentucky (UL1TR001998; University of Kentucky Center for Clinical and Translational Science); University of Massachusetts Medical School–Worcester (UL1TR001453; UMass Center for Clinical and Translational Science); University of Minnesota (UL1TR002494; Clinical and Translational Science Institute); University of Mississippi Medical Center (U54GM115428; Mississippi Center for Clinical and Translational Research); University of Nebraska Medical Center (U54GM115458; Great Plains IDeA–Clinical & Translational Research); University of North Carolina at Chapel Hill (UL1TR002489; North Carolina Translational and Clinical Science Institute); University of Oklahoma Health Sciences Center (U54GM104938; Oklahoma Clinical and Translational Science Institute); University of Rochester (UL1TR002001; University of Rochester Clinical & Translational Science Institute); University of Southern California (UL1TR001855; Southern California Clinical and Translational Science Institute); University of Vermont (U54GM115516; NNE-CTR Network); University of Virginia (UL1TR003015; iTHRIV Integrated Translational Health Research Institute of Virginia); University of Washington (UL1TR002319; Institute of Translational Health Sciences); University of Wisconsin–Madison (UL1TR002373; University of Wisconsin Institute for Clinical and Translational Research); Vanderbilt University Medical Center (UL1TR002243; Vanderbilt Institute for Clinical and Translational Research); Virginia Commonwealth University (UL1TR002649; C. Kenneth and Dianne Wright Center for Clinical and Translational Research); Wake Forest University Health Sciences (UL1TR001420; Wake Forest Clinical and Translational Science Institute); Washington University in St. Louis (UL1TR002345; Institute of Clinical and Translational Sciences); Weill Medical College of Cornell University (UL1TR002384; Weill Cornell Medicine Clinical and Translational Science Center); West Virginia University (U54GM104942; WVCTSI. Submitted data: Icahn School of Medicine at Mount Sinai (UL1TR001433; ConduITS Institute for Translational Sciences); University of Texas Health Science Center at Tyler (UL1TR003167; CCTS); University of California–Davis (UL1TR001860; University of California–Davis Health Clinical and Translational Science Center); University of California–Irvine (UL1TR001414; University of California–Irvine Institute for Clinical and Translational Science); University of California–Los Angeles (UL1TR001881; University of California–Los Angeles Clinical Translational Science Institute); University of California–San Diego (UL1TR001442; Altman Clinical and Translational Research Institute); University of California–San Francisco (UL1TR001872; University of California–San Francisco Clinical and Translational Science Institute. Pending data: Arkansas Children's Hospital (UL1TR003107; UAMS Translational Research Institute); Baylor College of Medicine (none; voluntary); Children's Hospital of Philadelphia (UL1TR001878; Institute for Translational Medicine and Therapeutics); Cincinnati Children's Hospital Medical Center (UL1TR001425; Center for Clinical and Translational Science and Training); Emory University (UL1TR002378; Georgia Clinical and Translational Science Alliance); HonorHealth (none; voluntary); Loyola University–Chicago (UL1TR002389; ITM); Medical College of Wisconsin (UL1TR001436; Clinical and Translational Science Institute of Southeast Wisconsin); MedStar Health Research Institute (UL1TR001409; Georgetown–Howard Universities Center for Clinical and Translational Science); MetroHealth (none, voluntary); Montana State University (UL1TR001445; Langone Health's Clinical and Translational Science Institute); Ochsner Medical Center (U54GM104940; LA CaTS Center); Regenstrief Institute (UL1TR002529; Indiana Clinical and Translational Science Institute); Sanford Research (none; voluntary); Stanford University (UL1TR003142; Spectrum: Stanford Center for Clinical and Translational Research and Education); Rockefeller University (UL1TR001866; Center for Clinical and Translational Science); Scripps Research Institute (UL1TR002550; Scripps Research Translational Institute); University of Florida (UL1TR001427; University of Florida Clinical and Translational Science Institute); University of New Mexico Health Sciences Center (UL1TR001449; University of New Mexico Clinical and Translational Science Center); University of Texas Health Science Center at San Antonio (UL1TR002645; Institute for Integration of Medicine and Science); Yale New Haven Hospital (UL1TR001863; Yale Center for Clinical Investigation).
Author contributions. Concept and design: A. S. Y., B. A., D. S., E. A. M. Acquisition, analysis, or interpretation of the data: All authors. Drafting of the manuscript: A. S. Y. and E. A. M. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: A. S. Y., E. A. M., and H. S. Obtained funding: E. A. M. Administrative, technical, or material support: N. H. and H. S. Supervision: E. A. M.
Acknowledgments. We thank Sam Michael and Kenneth Gersing for their help and support in seamlessly obtaining access to the N3C data enclave.
Disclaimer. The content presented here is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the N3C program.
Financial support. This work was supported in part by the Intramural and Extramural Research Program of the National Center for Advancing Translational Sciences, NIH (ZICTR000410–03), and in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (project ZIA/DK075149). D. K. S. reports support for this work from NCATS (full-time contractor to NCATS through Axle). E. R. P. reports support for this work from NCATS (paid to institution). N. H. reports support for this work from NIH Intramural funds. H. S. reports support for this work from National Center for Advancing Translational Sciences.
See https://github.com/arjunyadaw/N3C-Autoimmune-Disease-model.git.
References
- 1. Johns Hopkins University & Medicine . Coronavirus Research Center. COVID-19 dashboard. Johns Hopkins University. 2021. Available at: https://coronavirus.jhu.edu/map.html. Accessed 3 January 2022.
- 2. Centers for Disease Control and Prevention. People with certain medical conditions. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed 12 August 2022.
- 3. National Institutes of Health. Autoimmune Diseases Coordinating Committee: autoimmune diseases research plan, March 2005. Available at: https://www.niaid.nih.gov/sites/default/files/adccfinal.pdf. Accessed March 2005.
- 4. Serling-Boyd N, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis 2021; 80:660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. D'Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US “hot spot.” Ann Rheum Dis 2020; 79:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ye C, Cai S, Shen G, et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann Rheum Dis 2020; 79:1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodríguez-Lago I, Ramírez de la Piscina P, Elorza A, Merino O, Ortiz de Zárate J, Cabriada JL. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque Country (Spain). Gastroenterology 2020; 159:781–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faye AS, Lee KE, Laszkowska M, et al. Risk of adverse outcomes in hospitalized patients with autoimmune disease and COVID-19: a matched cohort study from New York City. J Rheumatol 2021; 48:454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis 2021; 80:384–91. [DOI] [PubMed] [Google Scholar]
- 10. Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant 2020; 20:1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis 2021; 72:340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andersen KM, Bates BA, Rashidi ES, et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol 2022; 4:e33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kridin K, Schonmann Y, Damiani G, et al. Tumor necrosis factor inhibitors are associated with a decreased risk of COVID -19-associated hospitalization in patients with psoriasis—a population-based cohort study. Dermatol Ther 2021; 34:e15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health. Immune modulator drugs improved survival for people hospitalized with COVID 19. Available at: https://www.nih.gov/news-events/news-releases/immune-modulator-drugs-improved-survival-people-hospitalized-covid-19. Accesessed 2 June 2022.
- 15. Bennett TD, Moffitt RA, Hajagos JG, et al. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US National COVID Cohort Collaborative. JAMA Netw Open 2021; 4:e2116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haendel MA, Chute CG, Bennett TD, et al. The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc 2021; 28:427–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. GitHub . National COVID Cohort Collaborative, COVID-19 phenotype documentation, version 4.0 (last updated 3/11/2022). Available at: https://github.com/National-COVID-Cohort-Collaborative/Phenotype_Data_Acquisition/wiki/Latest-Phenotype. Accessed 30 September 2021.
- 18. Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med 2022; 182:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20:e192-e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Global Autoimmune Institute. Autoimmune disease list. Available at: https://www.autoimmuneinstitute.org/resources/autoimmune-disease-list/. Accessed 12 August 2022.
- 21. Autoimmune disease. Available at: http://www.ebi.ac.uk/efo/EFO_0005140. Accessed 12 August 2022.
- 22. Pollard TJ, Johnson AEW, Raffa JD, Mark RG. Tableone: an open source python package for producing summary statistics for research papers. JAMIA Open 2018; 1:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fu J, Reid SA, French B, et al. Racial disparities in COVID-19 outcomes among Black and White patients with cancer. JAMA Netw Open 2022; 5:e224304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic review and meta-analysis. JAMA Netw Open 2021; 4:e2134147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaturvedi R, Lui B, Aaronson JA, White RS, Samuels JD. COVID-19 complications in males and females: recent developments. J Comp Eff Res 2022; 11:689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pradhan A, Olsson PE. Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ 2020; 11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Izadi Z, Brenner EJ, Mahil SK, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open 2021; 4:e2129639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cook L, Espinoza J, Weiskopf NG, et al. Issues with variability in electronic health record data about race and ethnicity: descriptive analysis of the National COVID Collaborative data enclave. JMIR Med Inform 2022; 10:e39235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pfaff ER, Girvin AT, Gabriel DL, et al. Synergies between centralized and federated approaches to data quality: a report from the national COVID cohort collaborative. J Am Med Inform Assoc 2022; 29:609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brannock MD, Chew RF, Preiss AJ, et al. Long COVID risk and pre-COVID vaccination: an EHR-based cohort study from the RECOVER Program. Nat Commun 2023; 14:2914. 10.1038/s41467-023-38388-7.s [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.