Abstract
All World Health Organization (WHO) pre-qualified rabies vaccines for humans are inactivated tissue culture rabies virus formulations produced for intramuscular (IM) administration. Due to costs and vaccine shortage, dose-saving intradermal (ID) administration of rabies post-exposure prophylaxis (PEP) is encouraged by WHO. This study compared the immunogenicity of the ID 2-site, 3-visit Institut Pasteur Cambodge (IPC) PEP regimen to the IM 1-site, 4-visit 4-dose Essen regimen using Verorab vaccine (Sanofi). The development of neutralizing antibodies (nAbs) and T cell response was assessed in 210 patients with a category II or III animal exposure in a rabies-endemic country. At day 28, all participants developed nAbs (≥0.5 IU/mL), irrespective of PEP scheme, age, or administration of rabies immunoglobulin. T cell response and nAb titers were similar for both PEP schemes. This study demonstrated that the 1-week ID IPC regimen is as effective as the 2-week IM 4-dose Essen regimen in inducing an anti-rabies immune response under real-life PEP.
Keywords: rabies, vaccination, post-exposure prophylaxis, IPC regimen
A 1-week intradermal regimen of rabies post-exposure prophylaxis induces a similar antibody and T cell response as the standard 2-week intramuscular regimen. The shortened intradermal (ID) regimen saves vaccine doses, costs, and treatment time.
Graphical abstract
Rabies is a zoonotic encephalitis caused by rabies virus (RABV), which is transmitted through saliva of infected animals. Annually, RABV infections cause estimated 59 000 human deaths with half of cases occurring in children under 15 years old in Africa and Asia [1]. Cambodia is 1 of the countries with the highest rabies incidence with over 800 human rabies cases annually [2]. Although rabies is 100% lethal and there is no effective treatment [3, 4], the disease is preventable by post-exposure prophylaxis (PEP) when given before clinical symptoms appear. All World Health Organization (WHO) pre-qualified rabies vaccines for human use are inactivated tissue culture RABV formulations [5] that are produced for intramuscular (IM) administration. However, due to costs and vaccine shortages, dose-saving intradermal (ID) administration is encouraged [6] as it reduces the amount of total vaccine from several milliliters (2–4 mL depending on vaccination scheme) for IM administration to under 1 mL for ID administration. The ID use of the purified Vero cell rabies vaccine (PVRV, commercial name Verorab, Sanofi, Lyon, France) was intensively studied in the last decades (reviewed in [7]). However, these clinical trials either simulated a PEP treatment by enrolling healthy people rather than actual bite case victims or used extensive vaccination regimens with more than 2-site injections per visit and/or a PEP regimen that requires more than 3 visits.
Rabies neutralizing antibodies (nAb) are a proxy for protection and vaccination efficacy. According to WHO recommendations, an nAb concentration of ≥0.5 IU/mL measured at 14 and 28 or 30 days after vaccination start is considered as adequate immune response [8]. The role of T cells after RABV infection in humans remains unclear. CD4+ T cells are considered to play a protective role, as they enhance blood-brain barrier permeability in response to neurotrophic infection due to interferon (IFN)-γ expression [4, 9]. The primary response to rabies vaccination is mediated by CD4+ T cells, with peak at day 7 after the last vaccination dose scheme [10, 11]. These cells are functional with preserved ability to produce IFN-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-2 after peptide stimulation [12]. CD8+ T cells seem to have an insignificant role in the cellular immune response, as their frequencies tend to decrease after vaccination [13]. A previous study showed that the magnitude of type 1 and type 2 cytokine responses did not differ among the intramuscular and intradermal routes of PEP [14].
This study aimed to assess if the intradermal 2-site, 3-dose PEP scheme (Institut Pasteur Cambodge [IPC] regimen) has a similar immunogenicity compared to the intramuscular 1-site, 4-dose Essen regimen in actual bite victims by analyzing the development of antibodies and T cell response at D14 and D28 after initial PEP administration.
METHODS
Study Design
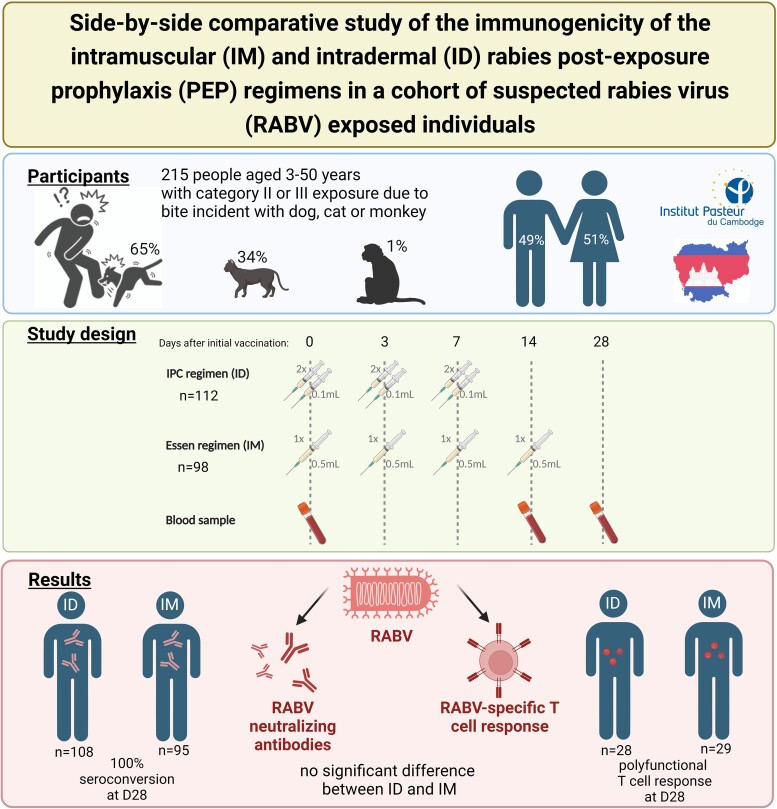
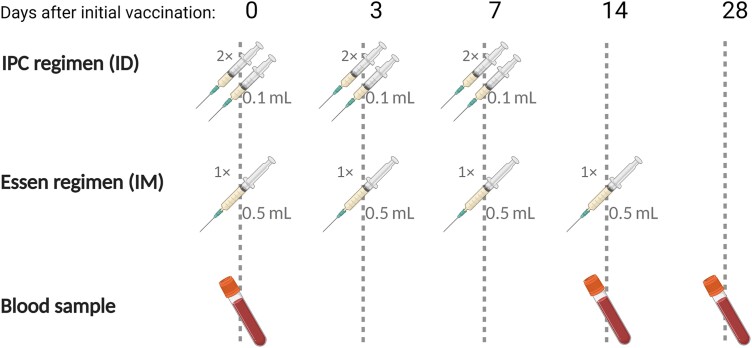
Study subjects were recruited from July 2020 to February 2021 among patients seeking rabies PEP at the Rabies Prevention Center of IPC after being bitten or scratched by an animal, and declared no previous vaccination against rabies. Written informed consent was obtained from patients (or legal representatives for patients aged under 18 years). Participants were randomly assigned to a PEP regimen group (ID or IM) and a stratification was used to balance the distribution of the two groups into each age category (≤15 years old and >15 years old). Administration of rabies immunoglobulin (RIG) was made according to necessity but was not part of the stratification. PEP consisted of Vero cell-based rabies vaccine (Verorab; Sanofi, Lyon, France). The day of the first PEP injection is referred to as day 0 (D0). Rabies vaccination protocol using ID administration included injections at two sites in the deltoid area per session with 0.1 mL of rabies vaccine on days 0, 3, and 7 (Figure 1). PEP using IM administration consisted of injection at one site in the deltoid area per session with 0.5 mL of rabies vaccine on days 0, 3, 7, and 14. Additional RIG (EQUIRAB, Bharat Serum and Vaccines Limited, India) was administered when the bite victim had a severe, deep wound or when the animal was rabies suspected (Supplementary Table 1). RIG was infiltrated into and around the wound as much as possible, the remaining RIG was administered by IM injection in the gluteal muscle. At D0 and at patient follow-ups contacts on days 3, 7, 14, 28, and at 6 months, the PEP protocol was provided, and a clinical and sociodemographic questionnaire (including the reporting of potential severe adverse events) was completed. This study was approved by Cambodia's National Ethics Committee (2020-103 NEHCR) and is registered at ClinicalTrials.gov (NCT05684185).
Figure 1.
Study design: Vaccination scheme for intradermal (ID) 2-site, 3-visit rabies vaccination (Institut Pasteur du Cambodge (IPC) regimen), and the intramuscular (IM) 1-site, 4-visit vaccination (4-dose Essen regimen), and the accompanying blood collection. Created with BioRender.com.
Sample Collection and Processing
Blood samples were collected before initial vaccination from participants at D0, on day 14 (D14) and on day 28 (D28) using dry tubes for serum collection and heparin tubes for acquisition of peripheral blood mononuclear cells (PBMCs, Figure 1). Serum was isolated by centrifugation and stored at −80°C until analysis. PBMCs were isolated by Ficoll-Paque (GE Healthcare) density gradient centrifugation and washed twice in RPMI-1640 medium (Gibco) and stored in liquid nitrogen in freezing medium (90% FBS (Gibco), 10% DMSO (Sigma-Aldrich)) until analysis.
Fluorescent Antibody Virus Neutralization Test (FAVNT)
The assay was performed according to standard procedure [15] to determine rabies nAb titers. The threshold for positivity is ≥0.5 IU/mL [16].
Rapid Fluorescent Focus Inhibition Test (RFFIT)
Rabies nAb were additionally determined with RFFIT, performed at the Sanofi Global Clinical Immunology laboratory (Swiftwater, Pennsylvania, USA) as described before [17]. The lower limit of quantification (LLOQ) for the RFFIT assay was 0.2 IU/mL; seropositivity was defined as nAb titers ≥0.2 IU/mL.
Activation-induced Markers (AIM) Assay
Frequencies of antigen-specific CD4+ and CD8+ T cells were evaluated by AIM assay in a subcohort of 57 patients (Supplementary Table 2) [18, 19]. Cells were thawed, washed, and assessed for cell viability. Samples with <0.5 × 106 PBMCs or viability of <75% were discarded. For each donor, 0.5–1 × 106 PBMCs per well were cultured for 24 hours at 37°C, 5% CO2 in a 96-well U-bottom plate in the presence of GlyRab peptide pool (103 15-mer peptides covering RABV glycoprotein, each peptide with overlapping by 10 residues, 1 µg/mL of each peptide, Mimotopes, Australia). Afterward, cells were stained for 20 minutes at 4°C with Zombie Aqua Fixable Viability kit (Biolegend). Following, a surface staining (Supplementary Table 3) was performed for 30 minutes at 4°C. Stained cells were resuspended in FACS buffer and analyzed using FACSAria Fusion (BD Biosciences). Negative controls without peptide stimulation were included for each donor. RABV-specific CD4+ T cells were identified as CD25+OX40+ CD4+ T cells, and RABV-specific CD8+ T cells as CD69+CD137+ CD8+ T cells (Supplementary Figure 1). RABV-specific CD4+ and CD8+ T cells were measured subtracting the unstimulated control from the peptide-stimulated sample.
Intracellular Staining (ICS) Assay
Functional RABV-specific CD4+ and CD8+ T cells were assessed by surface and ICS in a subset of individuals (Supplementary Table 2, Supplementary Figure 2) if sufficient amount of PBMCs were obtained. For each donor, 0.5–1 × 106 PBMCs per well were cultured for 6 hours at 37°C, 5% CO2 in a 96-well U-bottom plate in the presence of the GlyRAb peptide pool (see AIM, Monensin (Biolegend), 2 µM) and 100 µg/mL anti-human CD28/CD49d (BD Bioscience). Afterward, cells were stained for 20 minutes at 4°C with Zombie Aqua Fixable Viability kit (Biolegend), surface makers were stained for 30 minutes at 4°C, cells were fixed and permeabilized for 30 minutes with True-Nuclear™ Transcription Factor Buffer Set (Biolegend) and finally intracellular markers were stained for 30 minutes at 4°C (Supplementary Table 3). Stained cells were resuspended in FACS buffer and analyzed using a FACSAria Fusion. Antigen-specific CD4+ and CD8+ T cells were measured subtracting the unstimulated control from the peptide-stimulated sample (Supplementary Figure 2).
Statistical Analysis
Flow cytometry data were analyzed by FlowJo software version 10.7.1 (FlowJo LLC). Calculations, figures, and statistics were made using Prism 9 (GraphPad Software). Data were tested for statistical normality before applying appropriate statistical tests. Two groups were compared using Mann–Whitney test (non-paired data) or Wilcoxon matched-pairs signed rank test (paired data). Multiple groups were compared using Kruskal–Wallis test with Dunn's correction. Correlations between rabies virus neutralization antibody titers and T cells expression were evaluated using linear regression analysis. A statistical significance threshold of <0.05 was used in all computations and 95% CIs are shown for point estimates of effect size.
RESULTS
Study Cohort
Overall, 215 subjects were screened and initially enrolled (Table 1). Altogether, 210 subjects (97.7%) remained throughout the course of the study: 112 subjects (53.3%) received ID PEP and 98 (46.7%) received IM PEP. The mean age of the study participants was 21 years (3–50 years) with a female:male ratio of 1:1.02 (Table 2). The study participants were mainly victims from Phnom Penh (41.0%) and neighboring Kandal province (21.4%). At the date of initial vaccination, 29 study participants (13.8%) received additionally RIG treatment, with a similar RIG ratio for both ID and IM group (ID: 14, IM: 15, P = .56). None of the vaccinated individuals reported any side effects. Five subjects dropped out of the study unrelated to the vaccination due to absence at D3 or D28 (Table 1, eg, due to coronavirus disease 2019 [COVID-19] lockdowns or travel restrictions. The incidents were caused either by a dog (64.8%), cat (34.3%), or monkey (1.0%), and the majority of the bite cases (97.6%) were classified as category III exposure. As almost all animals appeared healthy at the time point of the bite incident (93.3%), a rabies diagnostic could not be performed, and therefore definite exposure of the study participants to RABV could not be proven. Animals that appeared ill (12 dogs, 1 cat, and 1 monkey) were not available for confirmatory diagnostic as they disappeared or were slaughtered before a sample could be taken. The study participants were contacted again 6 months after vaccination (M6, Table 1) to determine the survival. All except 1 individual with a category III incident (PEP without prescription of RIG) could be reached via phone call personally or their legal representatives for patients aged under 18 years.
Table 1.
Subject Inclusions
| Time Point | Action | ID Group n (%) |
IM Group n (%) |
Total n (%) |
|---|---|---|---|---|
| D0 | Blood sampling and vaccination | 115 (100.0) | 100 (100.0) | 215 (100.0) |
| RIG administered | 14 (12.2) | 15 (15.0) | 29 (13.5) | |
| D3 | Vaccination | 114 (99.1) | 99 (99.0) | 213 (99.1) |
| D7 | Blood sampling and vaccination | 113 (98.2) | 99 (99.0) | 212 (98.6) |
| D14 | Blood sampling | 112 (97.4) | 99 (99.0) | 211 (98.1) |
| Vaccination | NA | 99 (99.0) | NA | |
| D28 | Blood samplinga | 112 (97.4) | 98 (98.0) | 210 (97.7) |
| M6 | Survival follow-up | 112 (97.4) | 98 (98.0) | 210 (97.7) |
| Loss of follow-upb | 1 (0.9) | 0 (0.0) | 1 (0.5) | |
| Reachable and healthyb | 111 (99.1) | 98 (100.0) | 209 (99.5) |
Abbreviations: NA, not applicable; RIG, rabies immunoglobulin.
Drop outs until D28: 2 subjects refused later blood collection at D3, 1 at D7, 1 died in road accident before D14 blood collection, 1 subject could not attend D28 sample collection due to COVID-19 lock-down.
Including only individuals that where present through the follow-up until D28.
Table 2.
Cohort Characteristics (Patients Present Throughout D28)
| ID Group (n = 112) | IM Group (n = 98) | Total (n = 210) | |
|---|---|---|---|
| Age range (mean) years | 3–74 (22) | 3–74 (23) | 3–50 (21) |
| Sex n (%) Female Male |
61 (54.5) 51 (45.5) |
45 (45.9) 53 (54.1) |
106 (50.5) 104 (49.5) |
| Province of bite incident n (%) Banteay Meanchey Kampong Cham Kampong Chhnang Kampong Speu Kampong Thom Kampot Kandal Phnom Penh Prey Veng Svay Rieng Takeo |
0 (0.0) 7 (6.25) 4 (3.6) 8 (7.1) 1 (0.9) 2 (1.8) 25 (22.3) 44 (39.3) 11 (9.8) 2 (1.8) 8 (7.1) |
1 (1.0) 9 (9.2) 1 (1.0) 3 (3.1) 1 (1.0) 2 (2.0) 20 (20.4) 42 (42.9) 10 (10.2) 2 (2.0) 7 (7.1) |
1 (0.5) 16 (7.6) 5 (2.4) 11 (5.2) 2 (1.0) 4 (1.9) 45 (21.4) 86 (41.0) 21 (10.0) 4 (1.9) 15 (7.1) |
| WHO exposure n (%) Category II Category III |
3 (2.7) 109 (97.3) |
2 (2.0) 96 (98.0) |
5 (2.4) 205 (97.6) |
| Animal species n (%) Dog Cat Monkey |
77 (68.8) 35 (31.3) 0 (0.0) |
58 (59.2) 38 (38.8) 2 (2.0) |
135 (64.3) 73 (34.8) 2 (1.0) |
| Animal health status n (%) (at study enrollment) Healthy Ill |
104 (92.9) 8 (7.1) |
92 (93.9) 6 (6.1) |
196 (93.3) 14 (6.7) |
| RIG treatment n (%) | 14 (12.5) | 15 (15.3) | 29 (13.8) |
Abbreviations: ID, intradermal; IM, intramuscular; RIG, rabies immunoglobulin; WHO, World Health Organization.
Neutralizing Antibody Response
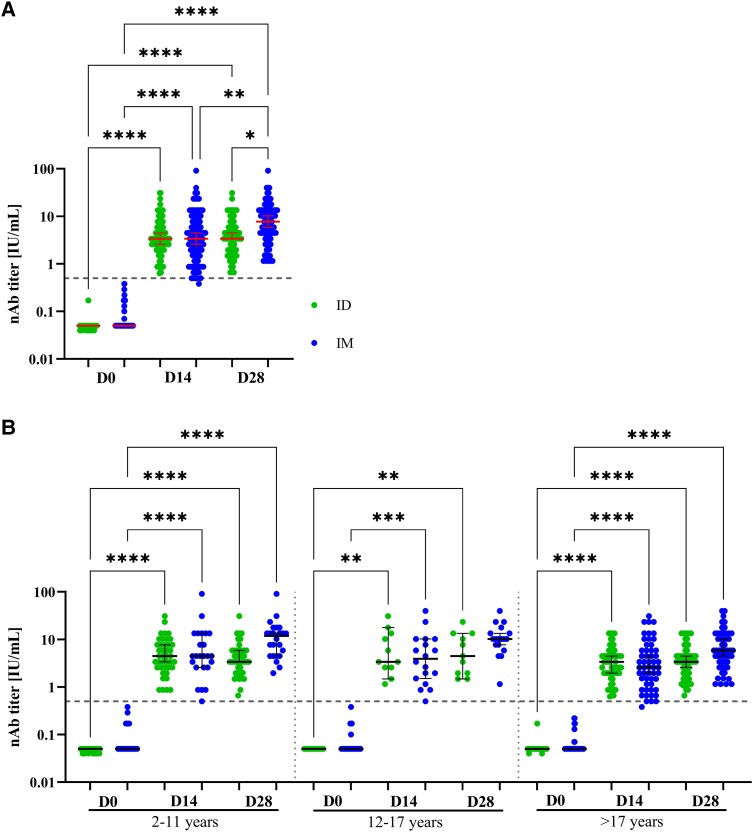
At D0 7 individuals (ID: 4, IM: 3) showed nAb titer ≥0.5 IU/mL (Supplementary Figure 3C, D and G) and were not included in ID-IM comparative analysis (Figure 2). At D28, all study participants seroconverted for rabies nAb tested by FAVNT (Figure 2A ) and RFFIT (100% concordance for D28 samples; Supplementary Table 4). All except 1 IM-vaccinated participant had detectable nAb at D14 and the median nAb titer was 3.38 for both administration groups. For ID group, the median nAb titer did not increase between D14 and D28 (Figure 2A, Supplementary Figure 3A), whereas for the IM group the median nAb titer rose from D14 to D28 (Figure 2A, Supplementary Figure 3B). The individual in the IM group with insufficient nAb titer at D14 (0.38 IU/mL) developed a positive nAb response (1.15 IU/mL) at D28, which indicates an inadequate immune response [8].
Figure 2.
Development nAb titers for intradermal (ID) and intramuscular (IM) vaccination: Titers of rabies neutralizing antibodies (nAb) measured by fluorescent antibody virus neutralization test (FAVNT) at baseline (D0), 14 d (D14) and 28 d (D28) after initial rabies vaccination either via ID (green) or IM administration (blue). A, Overall nAb development for full study cohort (n = 203, ID: 108, IM: 95). B, Results of nAb development stratified by age into children of 2–11 y (n = 65; ID: 41, IM: 24), teenagers of 12–17 y (n = 29; ID: 11, IM: 18), and adults of ≥17 y (n = 109; ID: 56, IM: 53). Each dot represents a single individual. Red lines represent median and interquartile range for each group. Dashed line indicates the threshold for seroprotection at 0.5 IU/mL. Statistics: Kruskal–Wallis with Dunn correction for multiple comparisons. *P < .05 **P ≤ .01, **P ≤ .001 and ****P ≤ .0001.
The classification into ≤15 years and >15 years old (Supplementary Figure 4) or in children (2–11 years), teenagers (12–17 years), and adults (≥18 years; Figure 2B ), revealed no age dependent nAb development. The additional RIG treatment (ID n = 14, IM n = 15) did not have a significant effect on the development of protective nAb titers nor on the seroconversion rate (Supplementary Table 4, Supplementary Figure 3E–G).
T Cell Response
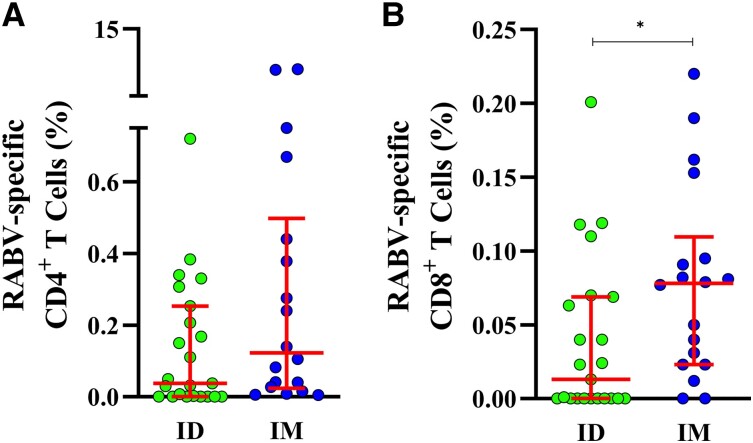
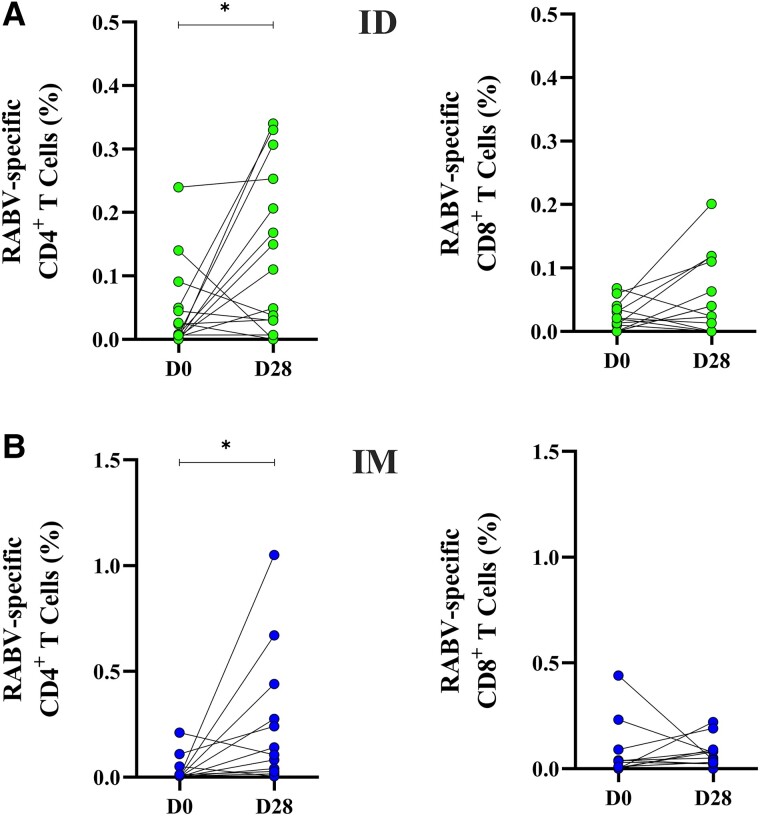
Overall, 57 patients were included for cellular immune response analyses (ID: 28, IM: 29 Supplementary Table 2). Percentages of RABV-specific CD4+ T and CD8+ T cells were significantly increased at D28 compared to D0 in IM-vaccinated individuals, but not in ID-vaccinated individuals (Supplementary Figure 5). The frequency of RABV-specific CD4+ T cells were not significantly different between ID and IM patients (Figure 3A ). However, the frequency of RABV-specific CD8+ T cells was higher among participants who received IM PEP compared to the ID scheme (P = .0199; Figure 3B ).
Figure 3.
Rabies virus (RABV)-specific CD4+ and CD8+ T cells 28 days after initial rabies vaccination: Frequencies of antigen-specific (A) CD4+ and (B) CD8+ T cells 28 days after vaccination with rabies vaccine administered Intradermal (ID, green, n = 23) and intramuscular (IM, blue, n = 18), after 24 h stimulation with GlyRab peptide pool. Induced expression of CD25 and OX40 was used to identify RABV-specific CD4+ T cells; and CD137 and CD69 to identify RABV-specific CD8+ T cells. Each dot represents a single individual. Lines represent median and interquartile range. Statistics: Mann–Whitney test. *P < .05.
The paired analysis of corresponding samples from D0 and D28 (Figure 4) revealed that CD4+ T cells responding to the GlyRab peptide pool increased significantly after ID (P = .0443) and IM PEP (P = .0210), whereas no significant effect of the vaccination was observed for CD8+ T cells. As CD4+ T cells are important to provide help to B cells for the production of Abs, we assessed if there was a correlation between RABV-specific CD4+ T cells and nAb titers. However, we found no statistically significant correlation between the frequency of RABV-specific CD4+ T cells and rabies nAb titers.
Figure 4.
Development of rabies virus (RABV)-specific CD4+ and CD8+ T cells 28 days after rabies vaccine: Frequency of antigen-specific CD4+ and CD8+ T cells, at baseline (D0) and 28 d after initial vaccination (D28) via intradermal (ID) scheme (green, A and C) or intramuscular (IM) scheme (blue, B and D) measured after 24 h stimulation with GlyRab peptide pool. Induced expression of CD25 and OX40 was used to identify RABV-specific CD4+ T cells; and CD137 and CD69 to identify RABV-specific CD8+ T cells. RABV-specific T cells were assessed for 17 ID-vaccinated individuals at D0 and D28, respectively, and for 12 IM-vaccinated study participants at D0 and D28, respectively. Each dot represents a single individual. Statistics: Wilcoxon matched-pairs signed rank test. *P < .05.
To assess the functionality of T cells by measuring cytokine production after stimulation with the rabies peptide pool (GlyRab) we measured the frequency of IFN-γ, IL-2, and TNF-α at D0 (ID n = 6, IM n = 10) and D28 (ID n = 6, IM n = 6). Percentages of CD4 + IFN-γ+ (P = .0649) were increased at D28 for ID (Supplementary Figure 6A) and IM group but not statistically significant (Supplementary Figure 6E). For both groups the RABV-specific CD4+ T cells were polyfunctional at D28, producing IFN-γ, IL-2, and TNF-α (significantly for ID vaccination, P = .0163; Supplementary Figure 6B). Similar results were obtained when assessing functionality of RABV-specific CD8+ T cells. Percentages of CD8+ IFN-γ+ were increased at D28 compared to D0 (significantly for ID vaccination, P = .0152; Supplementary Figure 6C) and the poly-functionality was also expanded at D28 compared to D0 (significantly for ID vaccination, P = .0362; Supplementary Figure 6D).
DISCUSSION
Cell-culture produced rabies vaccines were originally made for IM administration. However, due to recurrent vaccine shortages and the cost of the vaccines [20], dose-saving ID injections are recommended by WHO since 1992 and widely used, yet mostly off-label. This study is the first parallel, comparative trial among rabies PEP recipients with category II and III exposure investigating the humoral and cellular immune response of the 2-site 3-visit ID PEP (IPC regiment) with an IM-receiving control group (4-dose Essen regimen). Our study confirmed the immunogenicity of the ID administration as seroconversion and nAb titers were very similar for both PEP regimens, and in agreement to what was observed in other studies [7, 21]. Only a very small portion of studies performed parallel, comparative trials of ID and IM PEP vaccination schedules [22–25], but all these performed a simulated PEP on healthy unexposed people instead of actual bite victims. Most clinical trials either simulated a PEP treatment by enrolling healthy people rather than actual bite case victims or used extensive vaccination regimens with more than 2-site injections per visit and/or a regimen that requires more than 3 visits to complete the PEP. The only available study proving the efficacy of the 2-site, 3-visit ID regimen was done in Thailand in the 1980s vaccinating people with a low risk exposure (category I, touched or been licked by a rabid animal on unbroken skin [26]). Therefore, our study provides valuable data on the performance of the ID PEP in comparison to IM PEP in high risk exposure patients. The ID administration of rabies vaccine has immunological advantages over the IM administration, as the antigen is directly presented to skin-resident dendritic cells (DCs) via the ID route, which trigger the response of effector T cells. In contrast, skeletal muscle contains lower amounts of DCs than the skin and therefore the initial immune response at the vaccine delivery site is reduced.
Even though the role of T cell immunity in rabies infection and vaccination is not clearly understood, some studies have shown that cellular immune response may contribute to the clearance of rabies virus from the central nervous system [27–29]. Our study investigated the kinetics and functionality of both the CD4+ and CD8+ T cell compartments comparing the 2 PEP administration routes, IM and ID. We found no difference in the frequency of RABV-specific CD4+ T cells between ID and IM patients, whereas the frequency of RABV-specific CD8+ T cells was higher among participants who received the rabies vaccine via IM scheme compared to the ID scheme. Yet, how this relates to protection after PEP vaccination should be further investigated. Furthermore, the T cell analysis was restricted to a subset of the cohort as not all PBMC samples had viability sufficient to perform the relevant assays. Both CD4+ and CD8+ T cell compartments showed a polyfunctional response after peptide stimulation.
For this study, we followed the WHO recommendations measuring the RABV antibodies at D14 and D28 after PEP initialization as predictor for sufficient immunogenicity [8]. One caveat of this study design is that both schedules are not synchronized as the ID regimen is finished within 7 days, whereas the IM regimen takes 14 days. Furthermore, there was no further follow-up of the ID- and IM-vaccinated participants to monitor nAb and cellular immune response over a longer time. Studies with an extended follow-up have demonstrated that similar rates of pre-exposition prophylaxis (PrEP) recipients (2- and 3-visit regimens) remained seropositive [30] or showed similar mean nAb titers [31] up to one year after vaccination in healthy unexposed individuals. The RABV exposure in our study was also not definitely proven as none of the biting animals were available for diagnostic.
A shortened ID regimen not only saves vaccine doses but might increase the likelihood of patients completing the PEP because the ID PEP requires fewer visits Comparative studies in Vietnam and India have shown that the 4-visit ID regimen had higher completion rates that the 5-visit IM regimen [32, 33]. The completion rate at IPC's rabies prevention center in Phnom Penh is consistently over 90% [34] likely due to the lower out-of-pocket travel costs. In 2021, it was estimated that 4 return trips in Cambodia cost 64 USD per person, making the reduced number of visits required for ID PEP a significant financial relief for patients. This is especially important in Cambodia where more than half of bite victims are children under 17 who require adult accompaniment, effectively doubling the transportation costs and causing additional lack of income.
Overall, with this study we demonstrated under real-life PEP conditions in a direct comparative trial that 3-visit 1-week ID course is as sufficient at inducing an anti-rabies immune response as a 4-visit, 2-week IM regimen. Hence, this ID administration increases the availability of rabies vaccine and drastically reduces the vaccination costs itself but also the accompanying expenses for the PEP recipients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Heidi Auerswald, Virology Unit, Institut Pasteur du Cambodge, Pasteur Network, Phnom Penh, Cambodia.
Alvino Maestri, Immunology Unit, Institut Pasteur du Cambodge, Pasteur Network, Phnom Penh, Cambodia.
Sothy Touch, Rabies Prevention Center, Institut Pasteur du Cambodge, Pasteur Network, Phnom Penh, Cambodia.
Saraden In, Virology Unit, Institut Pasteur du Cambodge, Pasteur Network, Phnom Penh, Cambodia.
Nisa Ya, Immunology Unit, Institut Pasteur du Cambodge, Pasteur Network, Phnom Penh, Cambodia.
Borita Heng, Immunology Unit, Institut Pasteur du Cambodge, Pasteur Network, Phnom Penh, Cambodia.
Valérie Bosch-Castells, Global Medical, Sanofi, Lyon, France.
Christele Augard, Global Medical, Sanofi, Lyon, France.
Céline Petit, Global Medical, Sanofi, Lyon, France.
Philippe Dussart, Virology Unit, Institut Pasteur du Cambodge, Pasteur Network, Phnom Penh, Cambodia.
Yiksing Peng, Rabies Prevention Center, Institut Pasteur du Cambodge, Pasteur Network, Phnom Penh, Cambodia.
Tineke Cantaert, Immunology Unit, Institut Pasteur du Cambodge, Pasteur Network, Phnom Penh, Cambodia.
Sowath Ly, Epidemiology and Public Health Unit, Institut Pasteur du Cambodge, Pasteur Network, Phnom Penh, Cambodia.
Notes
Author contributions . Conceived and designed the study and the laboratory investigations: H. A., A. M., P. D., S. L., T. C., V. B. C. Study inclusion, sample collection and epidemiological analysis: S. L., S. T., Y. P. Performed the laboratory investigations: H. A., A. M., N. Y., B. H. Analyzed the data: H. A., A. M. Contributed field work/reagents/materials/analysis tools: C. P. Wrote the paper (original draft): H. A., A. M. Wrote the paper (comment and editing): T. C., S. L., Y. P., V. B. C., C. A.
Acknowledgments. The authors thank all study participants for their involvement in the study.
Financial support. This study (Code: RAB00056) is funded by Sanofi Pasteur SA (CISTM18). H. A. was supported by the German Centre for International Migration and Development (CIM) and HHMI-Wellcome. T. C. is an HHMI-Wellcome international research scholar (208710/Z/17/Z).
References
- 1. Hampson K, Coudeville L, Lembo T, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 2015; 9:e0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ly S, Buchy P, Heng NY, et al. Rabies situation in Cambodia. PLoS Negl Trop Dis 2009; 3:e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rupprecht CE. A tale of two worlds: public health management decisions in human rabies prevention. Clin Infect Dis 2004; 39:281–3. [DOI] [PubMed] [Google Scholar]
- 4. Katz ISS, Guedes F, Fernandes ER, dos Ramos Silva S. Immunological aspects of rabies: a literature review. Arch Virol 2017; 162:3251–68. [DOI] [PubMed] [Google Scholar]
- 5. Prequalified vaccines . 2019. Available at: https://extranet.who.int/pqweb/vaccines/prequalified-vaccines. Accessed 24 August 2022.
- 6. World Health Organization . Rabies vaccines: WHO position paper—April 2018. Wkly Epidemiol Rec 2018; 16:201–20. [Google Scholar]
- 7. Moulenat T, Petit C, Bosch Castells V, Houillon G. Purified Vero cell rabies vaccine (PVRV, Verorab®): a systematic review of intradermal use between 1985 and 2019. Trop Med Infect Dis 2020; 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . WHO Expert committee on biological standardization : fifty-sixth report.Geneva, Switzerland World Health Organization, 2007. Available at:.https://apps.who.int/iris/handle/10665/43594. Accessed 1 February 2023. [Google Scholar]
- 9. Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permeability changes during a central nervous system inflammatory response: TNF-alpha is neither necessary nor sufficient. J Immunol 2007; 178:7334–43. [DOI] [PubMed] [Google Scholar]
- 10. Moore SM, Wilkerson MJ, Davis RD, Wyatt CR, Briggs DJ. Detection of cellular immunity to rabies antigens in human vaccinees. J Clin Immunol 2006; 26:533–45. [DOI] [PubMed] [Google Scholar]
- 11. Herzog M, Fritzell C, Lafage M, Montaño Hirose JA, Scott-Algara D, Lafon M. T and B cell human responses to European bat lyssavirus after post-exposure rabies vaccination. Clin Exp Immunol 1991; 85:224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wijaya L, Tham CYL, Chan YFZ, et al. An accelerated rabies vaccine schedule based on Toll-like receptor 3 (TLR3) agonist PIKA adjuvant augments rabies virus specific antibody and T cell response in healthy adult volunteers. Vaccine 2017; 35:1175–83. [DOI] [PubMed] [Google Scholar]
- 13. Thraenhart O, Kreuzfelder E, Hillebrandt M, et al. Long-term humoral and cellular immunity after vaccination with cell culture rabies vaccines in man. Clin Immunol Immunopathol 1994; 71:287–92. [DOI] [PubMed] [Google Scholar]
- 14. Venkataswamy MM, Madhusudana SN, Sanyal SS, et al. Cellular immune response following pre-exposure and postexposure rabies vaccination by intradermal and intramuscular routes. Clin Exp Vaccine Res 2015; 4:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cliquet F, Aubert M, Sagné L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods 1998; 212:79–87. [DOI] [PubMed] [Google Scholar]
- 16. Moore SM, Hanlon CA. Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS Negl Trop Dis 2010; 4:e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Timiryasova TM, Luo P, Zheng L, et al. Rapid fluorescent focus inhibition test optimization and validation: improved detection of neutralizing antibodies to rabies virus. J Immunol Methods 2019; 474:112626. [DOI] [PubMed] [Google Scholar]
- 18. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021; 371:eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181:1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sreenivasan N, Li A, Shiferaw M, et al. Overview of rabies post-exposure prophylaxis access, procurement and distribution in selected countries in Asia and Africa, 2017–2018. Vaccine 2019; 37(Suppl 1):A6–A13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Denis M, Knezevic I, Wilde H, Hemachudha T, Briggs D, Knopf L. An overview of the immunogenicity and effectiveness of current human rabies vaccines administered by intradermal route. Vaccine 2019; 37(Suppl 1):A99–A106. [DOI] [PubMed] [Google Scholar]
- 22. Phanuphak P, Khawplod P, Sirivichayakul S, Siriprasomsub W, Ubol S, Thaweepathomwat M. Humoral and cell-mediated immune responses to various economical regimens of purified Vero cell rabies vaccine. Asian Pac J Allergy Immunol 1987; 5:33–7. [PubMed] [Google Scholar]
- 23. Sampath G, Madhusudana SN, Sudarshan MK, et al. Immunogenicity and safety study of Indirab: a Vero cell based chromatographically purified human rabies vaccine. Vaccine 2010; 28:4086–90. [DOI] [PubMed] [Google Scholar]
- 24. Khawplod P, Wilde H, Benjavongkulchai M, Sriaroon C, Chomchey P. Immunogenicity study of abbreviated rabies preexposure vaccination schedules. J Travel Med 2007; 14:173–6. [DOI] [PubMed] [Google Scholar]
- 25. Warrell MJ, Riddell A, Yu L-M, et al. A simplified 4-site economical intradermal post-exposure rabies vaccine regimen: a randomised controlled comparison with standard methods. PLoS Negl Trop Dis 2008; 2:e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phanuphak P, Khaoplod P, Benjavongkulchai M, Chutivongse S, Wilde H. What happens if intradermal injections of rabies vaccine are partially or entirely injected subcutaneously? Bull World Health Organ 1990; 68:83–5. [PMC free article] [PubMed] [Google Scholar]
- 27. Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol 1998; 72:3711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hooper DC, Roy A, Barkhouse DA, Li J, Kean RB. Rabies virus clearance from the central nervous system. Adv Virus Res 2011; 79:55–71. [DOI] [PubMed] [Google Scholar]
- 29. Flamand A, Raux H, Gaudin Y, Ruigrok RWH. Mechanisms of rabies virus neutralization. Virology 1993; 194:302–13. [DOI] [PubMed] [Google Scholar]
- 30. Quiambao BP, Lim JG, Bosch Castells V, et al. One-week intramuscular or intradermal pre-exposure prophylaxis with human diploid cell vaccine or Vero cell rabies vaccine, followed by simulated post-exposure prophylaxis at one year: a phase III, open-label, randomized, controlled trial to assess immunogenicity and safety. Vaccine 2022; 40:5347–55. S0264-410X(22)00923–9. [DOI] [PubMed] [Google Scholar]
- 31. Endy TP, Keiser PB, Wang D, et al. Serologic response of 2 versus 3 doses and intradermal versus intramuscular administration of a licensed rabies vaccine for preexposure prophylaxis. J Infect Dis 2020; 221:1494–8. [DOI] [PubMed] [Google Scholar]
- 32. Tran CH, Afriyie DO, Pham TN, et al. Rabies post-exposure prophylaxis initiation and adherence among patients in Vietnam, 2014–2016. Vaccine 2019; 37(Suppl 1):A54–63. [DOI] [PubMed] [Google Scholar]
- 33. Aggarwal S, Chaugule R, Haralkar S, Aswar N, Khandare K, Kumavat A. A cross-sectional study—intradermal versus intramuscular anti rabies prophylaxis. J Res Med Den Sci 2015; 3:31. [Google Scholar]
- 34. Tarantola A, Blanchi S, Cappelle J, et al. Rabies postexposure prophylaxis noncompletion after dog bites: estimating the unseen to meet the needs of the underserved. Am J Epidemiol 2018; 187:306–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.