Abstract
Background
Neurocognitive impairment (NCI) in people with HIV (PWH) on antiretroviral therapy (ART) is common and may result from persistent HIV replication in the central nervous system.
Methods
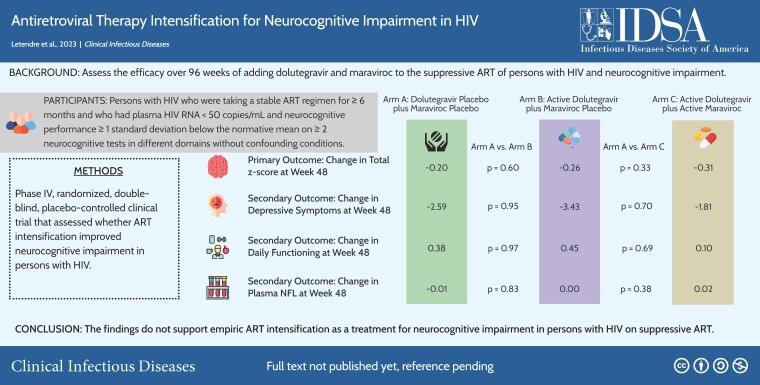
A5324 was a randomized, double-blind, placebo-controlled, 96-week trial of ART intensification with dolutegravir (DTG) + MVC, DTG + Placebo, or Dual - Placebo in PWH with plasma HIV RNA <50 copies/mL on ART and NCI. The primary outcome was the change on the normalized total z score (ie, the mean of individual NC test z scores) at week 48.
Results
Of 357 screened, 191 enrolled: 71% male, 51% Black race, 22% Hispanic ethnicity; mean age 52 years; mean CD4+ T-cells 681 cells/µL. Most (65%) had symptomatic HIV-associated NC disorder. Study drug was discontinued due to an adverse event in 15 (8%) and did not differ between arms (P = .17). Total z score, depressive symptoms, and daily functioning improved over time in all arms with no significant differences between them at week 48 or later. Adjusting for age, sex, race, study site, efavirenz use, or baseline z score did not alter the results. Body mass index modestly increased over 96 weeks (mean increase 0.32 kg/m2, P = .006) and did not differ between arms (P > .10).
Conclusions
This is the largest, randomized, placebo-controlled trial of ART intensification for NCI in PWH. The findings do not support empiric ART intensification as a treatment for NCI in PWH on suppressive ART. They also do not support that DTG adversely affects cognition, mood, or weight.
Keywords: HIV, cognition, brain, antiretroviral therapy
A5324 assessed whether ART intensification with dolutegravir and maraviroc compared with placebo would benefit persons with human immunodeficiency virus and neurocognitive impairment. Neurocognitive performance, depressive symptoms, and daily functioning improved over 96 weeks but did not differ by treatment arm.
Clinical Trials Registration . NCT02519777.
Graphical abstract
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/challenges-in-the-long-term-management-of-patients-with-coccidioidal-meningitis-a-retrospective-analysis-of-treatment-and-outcomes
Despite effective suppression of plasma human immunodeficiency virus (HIV) RNA below the lower limit of quantification (LLQ) with antiretroviral therapy (ART), persons with HIV (PWH) often have neurocognitive (NC) impairment (NCI), which can range from mild and asymptomatic to severe. Large, observational studies have found that NCI occurred in up to 69.5% of PWH, including those taking suppressive ART [1–7]. A meta-analysis of more than 35 000 PWH from 123 published reports found that the prevalence of NCI was 42.6% (95% confidence interval [CI], 39.7%–45.5%) [8].
NCI may be accompanied by depressive symptoms [9–11], and each of these can adversely affect daily functioning [12–16]. NCI in treated PWH may be due to 1 or more factors, including irreversible injury that occurred prior to ART, persistent HIV replication in the central nervous system (CNS) [17], persistent CNS inflammation [11, 18–20], or ART toxicity [21–23]. Controversy remains as to whether ART that better distributes into the CNS is important in treating NCI in PWH since findings from observational studies are mixed [24–26], with some finding that better “CNS-penetrating” ART is associated with better NC performance [27–35], while others have reported no association or even worse performance [36–41].
This trial assessed the efficacy over 96 weeks of adding 2 well-tolerated drugs, dolutegravir (DTG) and maraviroc (MVC), to the suppressive ART regimen of PWH who had NCI. MVC was chosen because its cerebrospinal fluid (CSF) concentrations are in the therapeutic range [42, 43]; the primary target cells in the CNS express predominantly CC chemokine receptor 5; and inhibition of CC chemokine receptor 5 may decrease migration of lymphocytes into the CNS [44]. DTG was chosen because its CSF concentrations are also in the therapeutic range [45] and it has reliable antiviral activity in integrase inhibitor–naive individuals.
METHODS
Overall Design
A5324 was a phase 4, randomized, double-blind, placebo-controlled trial that assessed whether ART intensification improved NC performance in PWH with NCI on suppressive ART. Eligible participants were PWH who were on a stable ART regimen for at least 6 months that did not contain an integrase inhibitor or MVC and who had plasma HIV RNA <50 copies/mL and NC performance ≥1 standard deviation below the normative mean on ≥2 NC tests in different domains (consistent with an HIV-associated NC disorder [HAND]) [46]. Exclusion criteria included severe neuropsychiatric conditions that affect functioning and are summarized in Supplementary Table 1. As summarized in the CONSORT (Consolidated Standards of Reporting Trials) diagram (Supplementary Figure 1), 357 participants provided informed consent at 24 sites. Of these, 191 were eligible to participate and were enrolled between April 2016 and November 2018 in the United States (n = 156), Brazil (n = 5), South Africa (n = 15), and Thailand (n = 15). Participants were randomized 1:1:1 to add dual-placebo, DTG and placebo for MVC (DTG + placebo), or DTG and MVC (DTG + MVC). Randomization was stratified by CD4+ T-cell nadir (≤100 vs >100 cells/µL) and HAND severity (asymptomatic: asymptomatic neurocognitive impairment [ANI] vs symptomatic: mild neurocognitive disorder [MND] and HIV-associated dementia [HAD]). Minor ART regimen changes were allowed, for example, changing tenofovir disoproxil fumarate to tenofovir alafenamide or changing ritonavir to cobicistat. NC testing was performed at screening within 90 days of entry and then repeated at weeks 24, 48, 72, and 96. The primary outcome was NC performance change at 48 weeks with a secondary end point at 96 weeks. The institutional review board at each site approved all study procedures.
Neuropsychiatric Assessment
The NC test battery was designed to adhere to the recommendations of the Frascati consensus and is summarized in Supplementary Table 2. The tests in the battery have been implemented successfully in international, multisite clinical trials, and extensive training materials within the AIDS Clinical Trials Group (ACTG) exist. Personnel who administered the tests completed initial training and certification as well as annual recertification. Country and site-specific normative data were used for comparison, and individual z scores were calculated using a demographically appropriate norming process [5–7]. Individual scores were separated into 6 component domains (see Supplementary Table 2), and the z score for each domain was calculated as the average of standardized individual z scores listed under the domain. The total z score was the average of the z scores of each domain. The primary outcome measure of change over time was calculated by subtracting the total z score at week 48 from the total z score at baseline. Alternative versions of the tests were administered at subsequent visits to reduce practice effects. The dual-placebo arm provided an estimate of practice effects and natural history in our analyses. Self-report assessments of depressive symptoms (the Beck Depression Inventory-II [BDI-II] in the United States and the Patient Health Questionnaire-9 outside of the United States) were administered, as was the revised Lawton and Brody instrumental activities of daily living scale (IADLs).
Safety, Clinical, and Biomarker Assessments
Clinical safety evaluation and monitoring were performed at weeks 2, 4, 12, and 24 and then every 24 weeks. Blood was collected for clinical hematology, chemistry panels, and CD4+ and CD8+ T cells at local Clinical Laboratory Improvement Amendments (CLIA)-approved, College of American Pathologists (CAP)-certified, virology/immunology quality assured, ACTG-affiliated laboratories. Specimens were stored at −80°C prior to plasma HIV RNA quantification (Abbott m2000sp/rt; assay LLQ 40 RNA copies/mL) at a central laboratory. CSF was collected by lumbar puncture in consenting participants. Biomarkers were measured in stored plasma (at baseline, 12 weeks, and 48 weeks; n = 162) and CSF (at baseline and 48 weeks; n = 34). Plasma levels of soluble tumor necrosis factor receptor II, soluble vascular cell adhesion molecule-1, soluble CD14, macrophage inflammatory protein (MIP)-1β (R&D Systems), and neurofilament light chain (NfL; Uman Diagnostics) were measured using enzyme-linked immunosorbent assay (ELISA) per manufacturers’ instructions. Duplicates of 15% of the samples were included in each plate, and results were analyzed using the BioTek EL×800 reader using KCjunior software (version 1.6). In CSF, MIP-1β, interferon inducible protein (IP)-10 (R&D Systems), NfL, and neopterin (Genway) were measured in duplicate using ELISA.
Statistical Analyses
A sample size of 62 participants per group, with a total of 186 participants, was selected to achieve 90% power to detect a difference of at least 0.5 higher for the DTG + MVC arm compared with the dual-placebo arm. The sample size accounted for multiple comparisons of the efficacy outcome between arms using Bonferroni correction, 1 interim analysis of efficacy, and a 15% loss to follow-up. The primary analysis and secondary analyses used the modified intent-to-treat principle, which did not include 1 participant who did not start the study drug. The 2-sample t test was used for 3 pairwise comparisons of total z score between any 2 arms. Treatment comparisons were also evaluated using linear regression models adjusting for covariates such as sex, race, and location (United States vs others). Proportion of adverse events and discontinuation of the study drug were evaluated using Fisher exact tests. Time to discontinuation was compared between arms using the Kaplan–Meier method and log-rank test. Comparisons over time between arms for IADLs, BDI-II, CD4+ T cells, and CD8+ T cells were evaluated using mixed repeated measures models. Changes over time within arms were evaluated using the Wilcoxon signed rank test. Spearman correlations were used to assess associations between biomarkers.
RESULTS
Participant Characteristics at Baseline
As summarized in Table 1, participants were mostly assigned male sex at birth (71%), had a mean age of 52 years, and had self-reported racial identity as Black (51%) and ethnic identity as non-Hispanic (78%). Gender identity was not collected. All participants had plasma HIV RNA <50 copies/mL at screening, although 7 values were between 50 and 200 copies/mL at baseline (after randomization). Mean CD4+ T cells were 703 cells/µL, and 30% had nadir CD4+ T cells ≤100/µL. The most frequently used ART drugs at baseline were emtricitabine (83%), tenofovir disoproxil fumarate (63%), efavirenz (32%), tenofovir alafenamide (27%), and darunavir (24%). The most common ART regimens at baseline were coformulated efavirenz-emtricitabine-tenofovir disoproxil fumarate (50, 26.3%), coformulated rilpivirine-emtricitabine-tenofovir alafenamide (23, 12.1%), and darunavir-ritonavir-emtricitabine-tenofovir disoproxil fumarate (16, 8.4%). All participants had NCI at baseline with nearly two-thirds meeting criteria for symptomatic HAND. Participants averaged more than 4 comorbid conditions at baseline, most commonly hypertension (74, 39%), hyperlipidemia (29, 15%), asthma (23, 12%), osteoarthritis (23, 12%), and type 2 diabetes mellitus (18, 9%). None differed between arms, nor did the number of concomitant medications.
Table 1.
Participant Characteristics
| Characteristic | Total (N = 191) | Dual Placebo (n = 63) | DTG + Placebo (n = 67) | DTG + MVC (n = 61) |
|---|---|---|---|---|
| Age, ya | 52 (8) | 52 (7) | 52 (9) | 52 (8) |
| Sex at birth, femaleb | 56 (29%) | 15 (24%) | 23 (34%) | 18 (30%) |
| Race, Black or African Americanb | 97 (51%) | 30 (48%) | 30 (45%) | 37 (61%) |
| Ethnicity, Hispanicb | 42 (22%) | 15 (24%) | 17 (25%) | 10 (16%) |
| Primary language, Englishb | 127 (66%) | 40 (63%) | 44 (66%) | 43 (70%) |
| History of injection Drug useb | 14 (7%) | 2 (3%) | 8 (12%) | 4 (7%) |
| HIV RNA, <50 copies/mLb | 184 (96%) | 62 (98%) | 64 (96%) | 58 (95%) |
| CD4+ T-cell count, per µLa | 703 (300) | 681 (294) | 703 (278) | 726 (331) |
| Nadir CD4+ T-cell count ≤100/µL2 | 57 (30%) | 19 (30%) | 20 (30%) | 18 (30%) |
| CD4+/CD8+ T-cell ratioa | 1.00 (0.51) | 0.95 (0.46) | 1.00 (0.48) | 1.05 (0.59) |
| Body mass index, kg/m2a | 29.0 (6.7) | 29.5 (6.6) | 29.0 (6.8) | 28.6 (6.8) |
| Efavirenz useb | 60 (32%) | 24 (38%) | 18 (27%) | 18 (30%) |
| Number of comorbid conditionsa | 4.49 (4.68) | 4.32 (3.81) | 4.35 (5.03) | 4.84 (5.15) |
| Polypharmacy, >5 concomitant drugsb | 73 (38%) | 21 (33%) | 26 (39%) | 26 (43%) |
| Average z scorea | −1.00 (0.73) | −0.96 (0.79) | −0.97 (0.70) | −1.09 (0.70) |
| Symptomatic HIV-associated neurocognitive disorder diagnosis (mild neurocognitive disorder or HIV-associated dementia)b | 124 (65%) | 42 (67%) | 44 (66%) | 38 (62%) |
Abbreviations: DTG, dolutegravir; HIV, human immunodeficiency virus; MVC, maraviroc.
Mean (standard deviation).
Number (percent).
Safety of the Intervention
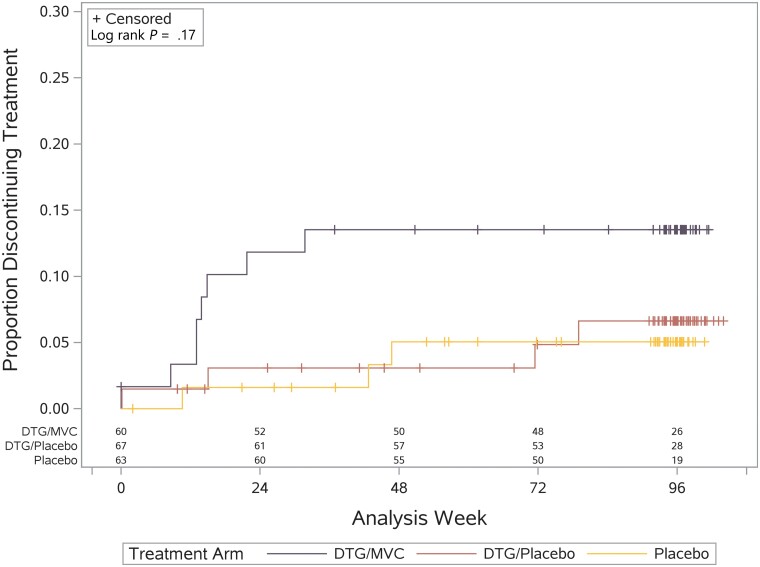
Fifteen adverse events were attributed to the study drug, had grades between 1 and 3, and did not differ between arms (all P values >.10; Supplementary Table 3). The most common were decreased creatinine clearance (6 participants, 3.1%) [47] and gastrointestinal disorders (5 participants, 2.6%), with 5 other adverse events occurring in 1 participant each (nervous system disorders, psychiatric disorders, respiratory disorders, urinary tract infection, increased serum total bilirubin). Five participants experienced virological failure, which was defined as plasma HIV RNA >200 copies/mL with repeat confirmation. Four of these occurred in the dual-placebo arm (at weeks 16, 17, 24, and 28) and 1 in the DTG + MVC arm (at week 34). The study drug was discontinued in 15 (7.8%) participants. Most discontinuations occurred prior to week 48 and totaled 3 (5%) in the dual-placebo arm, 4 (6%) in the DTG + placebo arm, and 8 (13%) in the DTG + MVC arm (P = .19). Time to discontinuation did not differ between arms (P = .17; Figure 1).
Figure 1.
Time to discontinuation of study drug. Abbreviations: DTG, dolutegravir; MVC, maraviroc.
Effects of the Intervention on NC Test Performance, Depressive Symptoms, and IADLs
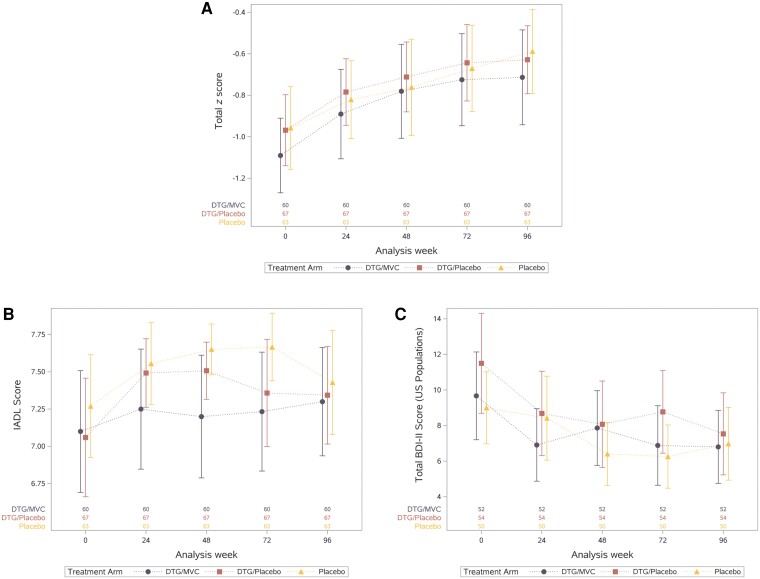
Global NC test performance, BDI-II values, and IADL values over time by treatment arm are shown in Figure 2. In all arms, the total z score improved between baseline and the primary outcome time point, week 48 (mean, 0.25; 95% CI, .16–.34), consistent with practice effects [48]. This change did not differ between arms (DTG + placebo vs dual-placebo, P = .60; DTG + MVC vs dual-placebo, P = .33). Total z score also did not differ between arms over 96 weeks (DTG + placebo vs dual-placebo, P = .79; DTG + MVC vs dual-placebo, P = .95). Individual domain z scores also improved over time in the entire group and did not differ between arms (all P values >.05; Table 2), except for verbal memory (weeks 48 and 72) and verbal learning (week 48) domains, which improved more in the DTG + MVC arm than in the dual-placebo arm. BDI-II and IADL values also improved over time and did not differ between arms (P > .10).
Figure 2.
Summary of neuropsychological performance (A), IADLs (B), and BDI-II (C) over time by study arm. Abbreviations: BDI-II, Beck Depression Inventory-II; DTG, dolutegravir; IADL, instrumental activities of daily living; MVC, maraviroc.
Table 2.
Mean and 95% Confidence Interval of Change in Cognitive Domain z Scores at Week 48
| Cognitive Domain | Total (N = 190) | Arm A (Dual-Placebo) (n = 63) |
Arm B (DTG + Placebo) (n = 67) |
Arm C (DTG + MVC) (n = 60) |
|---|---|---|---|---|
| Attention/working memory | 0.32 (0.21 to 0.43) | 0.24 (0.04 to 0.43) | 0.44 (0.28 to 0.60) | 0.28 (0.07 to 0.50) |
| Executive function | 0.25 (0.15 to 0.35) | 0.26 (0.05 to 0.46) | 0.24 (0.07 to 0.41) | 0.25 (0.09 to 0.42) |
| Fine motor skills | 0.25 (0.16 to 0.35) | 0.38 (0.22 to 0.54) | 0.19 (0.03 to 0.35) | 0.18 (0.00 to 0.37) |
| Speed of information processing | 0.23 (0.09 to 0.37) | 0.17 (−0.09 to 0.44) | 0.36 (0.13 to 0.60) | 0.14 (−0.12 to 0.40) |
| Verbal learninga | 0.23 (0.07 to 0.39) | 0.01 (−0.28 to 0.30) | 0.17 (−0.09 to 0.43) | 0.53 (0.26 to 0.79) |
| Verbal memorya | 0.13 (−0.14 to 0.40) | −0.15 (−0.62 to 0.32) | −0.08 (−0.57 to 0.42) | 0.64 (0.24 to 1.04) |
Abbreviations: DTG, dolutegravir; MVC, maraviroc.
Verbal learning and verbal memory improved more in the DTG + MVC arm than in the dual-placebo arm (both P values = .01) or the DTG + placebo arm (verbal learning P = .06, verbal memory P = .03).
Planned analyses stratified total z scores by HAND diagnosis (ANI vs MND + HAD) and nadir CD4+ T cells (≤100 vs >100/µL). Performance did not differ between treatment arms for participants with either asymptomatic or symptomatic HAND (P > .10). Similarly, performance did not differ between treatment arms by nadir CD4+ T-cell stratum (P > .10). Results were unchanged in post hoc analyses that adjusted for the influence of age, sex, education, study site, baseline z score, or efavirenz use (all P values >.10).
Sensitivity analyses that assessed the impact of missing NC test data using imputation by 3 methods (minimum, median, and maximum total z score by arm) also found no significant differences between arms. Per-protocol analyses included 163 (86%) participants and found no significant differences between arms.
Effects of the Intervention on Cellular and Soluble Biomarkers
The DTG + MVC arm had greater increases in CD4+ (P = .019) and CD8+ (P = .018) T cells over time than the dual-placebo arm, but these arms did not differ in the CD4/CD8 T-cell ratio over time. The DTG + placebo arm did have higher CD4/CD8 T-cell ratios over time than the dual-placebo arm (P = .037). Supplementary Figure 2A is a correlation matrix of the soluble biomarkers. The strongest correlations were between CSF IP-10 and either CSF neopterin (r = 0.58, P < .001) or CSF NfL (r = 0.54, P < .001). Trend-level correlations were present between worse total z score and higher plasma NfL (P = .068), CSF IP-10 (P = .074), and CSF NfL (P = .089) at week 48 but not at other time points. Change in soluble biomarkers over time did not correlate with change in total z score over time. Change in biomarkers did not differ by treatment arm (eg, plasma NfL; Supplementary Figure 2B), with the exception that the DTG + MVC arm had greater increases over time in plasma MIP-1β than the other 2 arms (Supplementary Figure 2C).
Effect of the Intervention on Body Mass Index
Because integrase inhibitors may increase weight [49], a post hoc analysis was performed and found that body mass index increased over 96 weeks in the entire cohort (P = .006; Supplementary Figure 3). The increase was modest (mean, 0.32 kg/m2; 95% CI, 0.11–0.74 kg/m2); was numerically greatest in the dual-placebo arm; and did not differ between arms at week 96 (P > .10).
DISCUSSION
A5324 was the largest, randomized, placebo-controlled trial of ART intensification for NCI in PWH. The premise for the trial was that NCI in PWH who are taking ART is due to persistent HIV replication [50–54], but the findings do not support this conclusion: NC performance improved over 96 weeks and did not differ by arm. Stratification and sensitivity analyses did not identify subgroups that benefitted from active study drug, nor did adjustments for demographic or other characteristics. Results of NC testing were further supported by a lack of differences between arms in depressive symptoms, IADLs, or concentrations of NfL in plasma or CSF [55, 56]. These findings provide strong evidence that ART intensification with currently available ART drugs is not an effective strategy for treating existing NCI in PWH who are already taking suppressive therapy. Instead, the negative findings support alternative explanations, such as prior CNS injury, cellular shedding of viral products [54, 57–59], persistent inflammation, or comorbid disease [60].
Many studies have assessed the influence of ART on cognition [27–34, 36–40], but few have assessed the influence of ART intensification. Two small, open-label, single-center trials of MVC intensification found evidence of NC improvement [61, 62]. A third open-label trial of ART intensification found evidence of NC improvement after 96 weeks [63]. As a randomized, placebo-controlled, multisite, international clinical trial, A5324 substantially improved on the design of these prior trials and found that ART intensification did not improve NC performance over 96 weeks compared with placebo. The trial did confirm that the addition of MVC (in combination with DTG) increased CD4+ and CD8+ T cells (but not CD4/CD8 ratio) [64] and MIP-1β in blood; however, these changes were not associated with NC performance, depressive symptoms, or IADLs.
Multiple reasons for these negative findings are possible. Foremost is that ART intensification would not reduce shedding of neurotoxic viral products in transcriptionally active cells in the CNS more than an existing suppressive ART regimen. Second, ART drug concentrations may be substantially higher in the brain than in CSF [65–67], which could also eliminate the benefit of intensification. While pharmacology and measurement of viral products are not reported here, future analyses will measure ART drug concentrations, cell-associated HIV DNA, and extracellular single-copy HIV RNA using stored biospecimens from the trial. Another possibility is that NCI in PWH is due to comorbid conditions that occur more frequently in PWH than in the general population. For instance, multiple reports show that conditions such as vascular disease [60] and metabolic syndrome [68, 69] are more strongly associated with NCI in virally suppressed PWH than indicators of HIV disease severity. Another possibility is that NCI was due to static brain injury that occurred prior to ART rather than to reversible processes that might respond to an intervention. The heterogeneous nature of NCI in PWH may also reduce the power of an intervention that targets just 1 step in pathogenesis. Even if NCI is associated with HIV disease indicators (eg, high levels of cell-associated HIV DNA [54], CSF viral escape [70], or HIV compartmentalization [71–74]), only a subgroup of PWH typically has these conditions. Finally, the drugs chosen for this trial may also not have been ideal. For example, since the trial began, data have emerged on possible DTG neurotoxicity [75–78]. While larger analyses did not find evidence of DTG neurotoxicity in adults [79, 80], if DTG does cause neurotoxicity in a subgroup of PWH, then this may have also influenced our results. Of note, however, participants in the DTG arms did not have worse NC performance than those in the dual-placebo arm.
The trial has important limitations. Chief among them may be insufficient power. The trial found no evidence of NC benefit, but the intervention may have only been beneficial in a subgroup for the reasons summarized above. Since the trial did not target PWH who had, for example, higher cell-associated HIV DNA or CSF viral escape, it may not have selected the group most likely to benefit. The multisite design that includes participants from low- and middle-income countries strengthens generalizability and also necessitated the use of different NC test protocols (Supplementary Table 2) to optimize cultural relevance (eg, different languages), which introduced variability [81]. The use of ecologically relevant normative data from each region should have mitigated this, and accounting for the influence of study site did not alter the findings. Domain-specific and global cognitive performance improved similarly in each treatment arm. However, the magnitude of change in test performance was within the range of measurement error for the test protocol. Other studies report that cognitive change with repeat testing of PWH is within measurement error. Practice effects may also have contributed to the improvement observed in each treatment arm. The influence of practice may have been reduced by our use of alternate forms for verbal learning and memory, but similar options were not available for other cognitive domains. Multiple baseline testing is another strategy that might have reduced the influence of practice, but this adds burden for study participants and staff. Our assessment is that measurement error and practice effects did not substantively influence the outcomes in this trial.
In summary, we report the primary results of the largest, randomized, placebo-controlled trial of ART intensification for NCI in PWH to date. The negative findings do not support the use of ART intensification to treat this condition in PWH who are already taking stable, suppressive ART. As noted, future analyses will quantify ART drug concentrations in blood and CSF as well as cell-associated HIV DNA and single-copy HIV RNA in plasma to assess whether these characteristics influenced the findings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Scott L Letendre, University of California–San Diego, San Diego, California, USA.
Huichao Chen, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Ashley McKhann, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Jhoanna Roa, DLH Corporation, Silver Spring, Maryland, USA.
Alyssa Vecchio, University of North Carolina, Chapel Hill, North Carolina, USA.
Eric S Daar, Lundquist Institute at Harbor–University of California–Los Angeles Medical Center, Torrance, California, USA.
Baiba Berzins, Northwestern University, Chicago, Illinois, USA.
Peter W Hunt, University of California–San Francisco, San Francisco, California, USA.
Christina M Marra, University of Washington School of Medicine, Seattle, Washington, USA.
Thomas B Campbell, University of Colorado School of Medicine, Denver, Colorado, USA.
Robert W Coombs, University of Washington School of Medicine, Seattle, Washington, USA.
Qing Ma, University at Buffalo, Buffalo, New York, USA.
Shobha Swaminathan, Rutgers New Jersey Medical School, Newark, New Jersey, USA.
Bernard J C Macatangay, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Gene D Morse, University at Buffalo, Buffalo, New York, USA.
Thomas Miller, DLH Corporation, Silver Spring, Maryland, USA.
David Rusin, DLH Corporation, Silver Spring, Maryland, USA.
Alexander L Greninger, University of Washington School of Medicine, Seattle, Washington, USA.
Belinda Ha, ViiV Healthcare Ltd, Research Triangle Park, North Carolina, USA.
Beverly Alston-Smith, Division of AIDS, National Institutes of Health, Rockville, Maryland, USA.
Kevin Robertson, University of North Carolina, Chapel Hill, North Carolina, USA.
Robert Paul, University of Missouri, St. Louis, Missouri, USA.
Serena Spudich, Yale School of Medicine, New Haven, Connecticut, USA.
Notes
Acknowledgments. Support was provided by the National Institute of Mental Health, the Statistical and Data Management Center, ACTG Specialty Laboratories, and ViiV Healthcare. Participating clinical research sites included the following: University of Pittsburgh, University of Witwatersrand, Durban International, Instituto de Pesquisa Clinica Evandro Chagas, University of Washington AIDS, Johns Hopkins University, Washington University, Ohio State University, Cincinnati, Case, Northwestern University, Miriam Hospital, Trinity Health and Wellness, Houston AIDS Research Team, Chiang Mai University, New Jersey Medical School, University of Rochester, Whitman-Walker Institute, Thai Red Cross AIDS Centre, Chapel Hill, Greensboro, Vanderbilt Therapeutics, Puerto Rico ACTU, University of California–Los Angeles CARE Center, Harbor University of California–Los Angeles Center, Penn Therapeutics, University of California–San Diego Antiviral Research Center, Weill Cornell Uptown, Weill Cornell Chelsea, and the Family Clinical Research Unit. As part of this project, biospecimens were stored at the ACTG Repository (http://www.specimenrepository.org/RepositorySite/home.html). B. H. reports that ViiV Healthcare provided study drugs (dolutegravir and maraviroc) for the duration of the study. S. Sp. reports that ViiV Healthcare, Inc provided study medications for the A5324 clinical trial.
Additional information: Coauthor Kevin Robertson, PhD, died 16 June 2019.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the NIH under awards UM1 AI068634, UM1 AI068636, and UM1 AI106701. S. L. L. reports support for this work from the AIDS Clinical Trial Group. R. W. C. reports the following support for this work: UM1-AI-068618 and P30-AI-027757. Q. M. reports the following support for this work: NIH UM1AI069511, NIH R01AG063659, and Gilead Sciences (CO-US-380-5529), all paid to institution. T. M. reports support for this work from Frontier Science and Technology–Data Management. R. P. reports support to institution from NIH-AIDS Clinical Trials Group.
References
- 1. Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007; 21:1915–21. [DOI] [PubMed] [Google Scholar]
- 2. Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010; 24:1243–50. [DOI] [PubMed] [Google Scholar]
- 4. Robertson K, Jiang H, Kumwenda J, et al. Improved neuropsychological and neurological functioning across three antiretroviral regimens in diverse resource-limited settings: AIDS Clinical Trials Group study a5199, the International Neurological Study. Clin Infect Dis 2012; 55:868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robertson K, Kumwenda J, Supparatpinyo K, et al. A multinational study of neurological performance in antiretroviral therapy-naive HIV-1-infected persons in diverse resource-constrained settings. J Neurovirol 2011; 17:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robertson K, Liner J, Heaton R. Neuropsychological assessment of HIV-infected populations in international settings. Neuropsychol Rev 2009; 19:232–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robertson K, Jiang H, Evans SR, et al. International neurocognitive normative study: neurocognitive comparison data in diverse resource-limited settings: AIDS clinical trials group A5271. J Neurovirol 2016; 22:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Liu M, Lu Q, et al. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology 2020; 95:e2610–21. [DOI] [PubMed] [Google Scholar]
- 9. Rubin LH, Maki PM. HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Curr HIV/AIDS Rep 2019; 16:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan P, Kerr SJ, Kroon E, et al. Cognitive trajectories after treatment in acute HIV infection. AIDS 2021; 35:883–8. [DOI] [PubMed] [Google Scholar]
- 11. Anderson AM, Ma Q, Letendre SL, Iudicello J. Soluble biomarkers of cognition and depression in adults with HIV infection in the combination therapy era. Curr HIV/AIDS Rep 2021; 18:558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sacktor N. Changing clinical phenotypes of HIV-associated neurocognitive disorders. J Neurovirol 2018; 24:141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marquine MJ, Flores I, Kamat R, et al. A composite of multisystem injury and neurocognitive impairment in HIV infection: association with everyday functioning. J Neurovirol 2018; 24:549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paul R, Tsuei T, Cho K, et al. Ensemble machine learning classification of daily living abilities among older people with HIV. EClinicalMedicine 2021; 35:100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morgan EE, Iudicello JE, Weber E, et al. Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr 2012; 61:341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamat R, Morgan E, Marcotte TD, et al. Implications of apathy and depression for everyday functioning in HIV/AIDS in Brazil. J Affect Disord 2013; 150:1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol 2002; 59:923–8. [DOI] [PubMed] [Google Scholar]
- 18. Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 2010; 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eden A, Marcotte TD, Heaton RK, et al. Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS One 2016; 11:e0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edén A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslén M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis 2007; 196:1779–83. [DOI] [PubMed] [Google Scholar]
- 21. Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol 2012; 18:388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 2009; 23:1359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslén M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr 2008; 47:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arentoft A, Troxell K, Alvarez K, et al. HIV antiretroviral medication neuropenetrance and neurocognitive outcomes in HIV+ adults: a review of the literature examining the central nervous system penetration effectiveness score. Viruses 2022; 14:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michael HU, Youbi E, Ohadoma SC, Ramlall S, Oosthuizen F, Polyakova M. A meta-analytic review of the effect of antiretroviral therapy on neurocognitive outcomes in adults living with HIV-1 in low-and middle-income countries. Neuropsychol Rev 2022; 32:828–54. [DOI] [PubMed] [Google Scholar]
- 26. Webb AJ, Borrelli EP, Vyas A, Taylor LE, Buchanan AL. The effect of antiretroviral therapy with high central nervous system penetration on HIV-related cognitive impairment: a systematic review and meta-analysis [manuscript published online ahead of print 18 July 2022]. AIDS Care 2022. 10.1080/09540121.2022.2098231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008; 65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ciccarelli N, Fabbiani M, Colafigli M, et al. Revised central nervous system neuropenetration-effectiveness score is associated with cognitive disorders in HIV-infected patients with controlled plasma viraemia. Antivir Ther 2013; 18:153–60. [DOI] [PubMed] [Google Scholar]
- 29. Fabbiani M, Grima P, Milanini B, et al. Antiretroviral neuropenetration scores better correlate with cognitive performance of HIV-infected patients after accounting for drug susceptibility. Antivir Ther 2015; 20:441–7. [DOI] [PubMed] [Google Scholar]
- 30. Casado JL, Marín A, Moreno A, et al. Central nervous system antiretroviral penetration and cognitive functioning in largely pretreated HIV-infected patients. J Neurovirol 2014; 20:54–61. [DOI] [PubMed] [Google Scholar]
- 31. Vassallo M, Durant J, Biscay V, et al. Can high central nervous system penetrating antiretroviral regimens protect against the onset of HIV-associated neurocognitive disorders? AIDS 2014; 28:493–501. [DOI] [PubMed] [Google Scholar]
- 32. Ghate M, Mehendale S, Meyer R, et al. The effects of antiretroviral treatment initiation on cognition in HIV-infected individuals with advanced disease in Pune, India. J Neurovirol 2015; 21:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carvalhal A, Gill MJ, Letendre SL, et al. Central nervous system penetration effectiveness of antiretroviral drugs and neuropsychological impairment in the Ontario HIV Treatment Network Cohort Study. J Neurovirol 2016; 22:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS 2011; 25:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vassallo M, Fabre R, Durant J, et al. A decreasing CD4/CD8 ratio over time and lower CSF-penetrating antiretroviral regimens are associated with a higher risk of neurocognitive deterioration, independently of viral replication. J Neurovirol 2017; 23:216–25. [DOI] [PubMed] [Google Scholar]
- 36. Cross HM, Combrinck MI, Joska JA. HIV-associated neurocognitive disorders: antiretroviral regimen, central nervous system penetration effectiveness, and cognitive outcomes. S Afr Med J 2013; 103:758–62. [DOI] [PubMed] [Google Scholar]
- 37. Ellis RJ, Letendre S, Vaida F, et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis 2014; 58:1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson MJ, Martin-Engel L, Vassileva J, Gonzalez R, Martin EM. An investigation of the effects of antiretroviral central nervous system penetration effectiveness on procedural learning in HIV+ drug users. J Clin Exp Neuropsychol 2013; 35:915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kahouadji Y, Dumurgier J, Sellier P, et al. Cognitive function after several years of antiretroviral therapy with stable central nervous system penetration score. HIV Med 2013; 14:311–5. [DOI] [PubMed] [Google Scholar]
- 40. Caniglia EC, Cain LE, Justice A, et al. Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology 2014; 83:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Santos GMA, Locatelli I, Metral M, et al. Cross-sectional and cumulative longitudinal central nervous system penetration effectiveness scores are not associated with neurocognitive impairment in a well treated aging human immunodeficiency virus-positive population in Switzerland. Open Forum Infect Dis 2019; 6:ofz277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tiraboschi JM, Niubo J, Curto J, Podzamczer D. Maraviroc concentrations in cerebrospinal fluid in HIV-infected patients. J Acquir Immune Defic Syndr 2010; 55:606–9. [DOI] [PubMed] [Google Scholar]
- 43. Melica G, Canestri A, Peytavin G, et al. Maraviroc-containing regimen suppresses HIV replication in the cerebrospinal fluid of patients with neurological symptoms. AIDS 2010; 24:2130–3. [DOI] [PubMed] [Google Scholar]
- 44. Rossi R, Lichtner M, De Rosa A, et al. In vitro effect of anti-human immunodeficiency virus CCR5 antagonist maraviroc on chemotactic activity of monocytes, macrophages and dendritic cells. Clin Exp Immunol 2011; 166:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Letendre SL, Mills AM, Tashima KT, et al. ING116070: a study of the pharmacokinetics and antiviral activity of dolutegravir in cerebrospinal fluid in HIV-1-infected, antiretroviral therapy-naive subjects. Clin Infect Dis 2014; 59:1032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cottrell ML, Hadzic T, Kashuba AD. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet 2013; 52:981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cysique LA, Franklin D Jr, Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol 2011; 33:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Avdoshina V, Fields JA, Castellano P, et al. The HIV protein gp120 alters mitochondrial dynamics in neurons. Neurotox Res 2016; 29:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hategan A, Bianchet MA, Steiner J, et al. HIV Tat protein and amyloid-beta peptide form multifibrillar structures that cause neurotoxicity. Nat Struct Mol Biol 2017; 24:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rahimian P, He JJ. Exosome-associated release, uptake, and neurotoxicity of HIV-1 Tat protein. J Neurovirol 2016; 22:774–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 2016; 12:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spudich S, Robertson KR, Bosch RJ, et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J Clin Invest 2019; 129:3339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 2016; 3:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gisslén M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis 2007; 195:1774–8. [DOI] [PubMed] [Google Scholar]
- 57. DeMarino C, Cowen M, Pleet ML, et al. Differences in transcriptional dynamics between T-cells and macrophages as determined by a three-state mathematical model. Sci Rep 2020; 10:2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Valcour VG, Shiramizu BT, Shikuma CM. HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol 2010; 87:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Anderson AM, Tang B, Vaida F, et al. Low-level HIV RNA in cerebrospinal fluid and neurocognitive performance: a longitudinal cohort study. J Acquir Immune Defic Syndr 2021; 87:1196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 2009; 73:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS 2016; 30:591–600. [DOI] [PubMed] [Google Scholar]
- 62. Ndhlovu LC, Umaki T, Chew GM, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neurovirol 2014; 20:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Force G, Ghout I, Ropers J, et al. Improvement of HIV-associated neurocognitive disorders after antiretroviral therapy intensification: the Neuro+3 study. J Antimicrob Chemother 2021; 76:743–52. [DOI] [PubMed] [Google Scholar]
- 64. Chan ES, Landay AL, Brown TT, et al. Differential CD4+ cell count increase and CD4+ : CD8+ ratio normalization with maraviroc compared with tenofovir. AIDS 2016; 30:2091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Curley P, Rajoli RK, Moss DM, et al. Efavirenz is predicted to accumulate in brain tissue: an in silico, in vitro, and in vivo investigation. Antimicrob Agents Chemother 2017; 61:e01841-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferrara M, Bumpus NN, Ma Q, et al. Antiretroviral drug concentrations in brain tissue of adult decedents. AIDS 2020; 34:1907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Srinivas N, Rosen EP, Gilliland WM Jr, et al. Antiretroviral concentrations and surrogate measures of efficacy in the brain tissue and CSF of preclinical species. Xenobiotica 2019; 49:1192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pasipanodya EC, Montoya JL, Campbell LM, et al. Metabolic risk factors as differential predictors of profiles of neurocognitive impairment among older HIV+ and HIV- adults: an observational study. Arch Clin Neuropsychol 2021; 36:151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pope CN, Montoya JL, Vasquez E, et al. Association of HIV serostatus and metabolic syndrome with neurobehavioral disturbances. J Neurovirol 2020; 26:888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 2010; 50:773–8. [DOI] [PubMed] [Google Scholar]
- 71. Eggers C, Müller O, Thordsen I, Schreiber M, Methner A. Genetic shift of env V3 loop viral sequences in patients with HIV-associated neurocognitive disorder during antiretroviral therapy. J Neurovirol 2013; 19:523–30. [DOI] [PubMed] [Google Scholar]
- 72. Evering TH, Kamau E, St Bernard L, Farmer CB, Kong XP, Markowitz M. Single genome analysis reveals genetic characteristics of neuroadaptation across HIV-1 envelope. Retrovirology 2014; 11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Oliveira MF, Chaillon A, Nakazawa M, et al. Early antiretroviral therapy is associated with lower HIV DNA molecular diversity and lower inflammation in cerebrospinal fluid but does not prevent the establishment of compartmentalized HIV DNA populations. PLoS Pathog 2017; 13:e1006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog 2009; 5:e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. de Boer MG, van den Berk GE, van Holten N, et al. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS 2016; 30:2831–4. [DOI] [PubMed] [Google Scholar]
- 76. Hoffmann C, Welz T, Sabranski M, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med 2017; 18:56–63. [DOI] [PubMed] [Google Scholar]
- 77. Zash R, Makhema J, Shapiro RL. Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med 2018; 379:979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med 2019; 381:827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Elzi L, Erb S, Furrer H, et al. Adverse events of raltegravir and dolutegravir. AIDS 2017; 31:1853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fettiplace A, Stainsby C, Winston A, et al. Psychiatric symptoms in patients receiving dolutegravir. J Acquir Immune Defic Syndr 2017; 74:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chan P, Goh O, Kroon E, et al. Neuropsychiatric outcomes before and after switching to dolutegravir-based therapy in an acute HIV cohort. AIDS Res Ther 2020; 17:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.