Abstract
Background
Despite data suggesting elevated morbidity and mortality among people who have survived tuberculosis disease, the impact of respiratory tuberculosis on healthcare utilization in the years following diagnosis and treatment remains unclear.
Methods
Using linked health administrative data from British Columbia, Canada, we identified foreign-born individuals treated for respiratory tuberculosis between 1990 and 2019. We matched each person with up to four people without a tuberculosis diagnosis from the same source cohort using propensity score matching. Then, using a controlled interrupted time series analysis, we measured outpatient physician encounters and inpatient hospital admissions in the 5 years following respiratory tuberculosis diagnosis and treatment.
Results
We matched 1216 individuals treated for respiratory tuberculosis to 4864 non-tuberculosis controls. Immediately following the tuberculosis diagnostic and treatment period, the monthly rate of outpatient encounters in the tuberculosis group was 34.0% (95% confidence interval [CI]: 30.7%, 37.2%) higher than expected, and this trend was sustained for the duration of the post-tuberculosis period. The excess utilization represented an additional 12.2 (95% CI: 10.6, 14.9) outpatient encounters per person over the post-tuberculosis period, with respiratory morbidity a large contributor to the excess healthcare utilization. Results were similar for hospital admissions, with an additional 0.4 (95% CI: .3, .5) hospital admissions per person over the post-tuberculosis period.
Conclusions
Respiratory tuberculosis appears to have long-term impacts on healthcare utilization beyond treatment. These findings underscore the need for screening, assessment, and treatment of post-tuberculosis sequelae, as it may provide an opportunity to improve health and reduce resource use.
Keywords: post-tuberculosis, healthcare utilization, epidemiology
Using an interrupted time series analysis, we found that people treated for respiratory tuberculosis in a high-resource setting experience elevated healthcare utilization in the years following the completion of their tuberculosis treatment.
Tuberculosis is frequently portrayed as a curable infectious disease with limited long-term effects after successful treatment. However, recent studies highlight chronic lung disease, disability, and elevated mortality among tuberculosis survivors [1–3]. Although long- COVID (coronavirus disease) has raised similar questions on the long-term impacts of severe respiratory infections, the post-tuberculosis research agenda has received much less attention, with almost four times as many papers published about long-COVID than post-tuberculosis [4].
One question that remains unclear is the long-term impact of tuberculosis on healthcare utilization. Information on post-tuberculosis healthcare utilization is necessary to estimate the burden of post-tuberculosis morbidity on the healthcare system, predict resource needs, and identify potential intervention points to reduce morbidity and mortality. These data are also necessary to accurately evaluate the clinical and programmatic value of tuberculosis preventive treatment. The current paradigm of viewing tuberculosis as an acute illness impairs our ability to accurately value the benefits of prevention and develop indicators for chronic illness as part of measurements of tuberculosis burden.
Evidence suggests healthcare use increases in the months leading up to tuberculosis diagnosis, primarily due to misdiagnosis and prolonged tuberculosis diagnostic workup [5]. However, there are also reasons to believe healthcare utilization in the years following treatment may be higher than expected due to the long-term consequences of tuberculosis. For example, people who survive respiratory tuberculosis have high rates of airway disease and infectious pulmonary complications [6]. They may also experience higher rates of cardiovascular disease and mood disorders and remain at high risk for recurrence, particularly within the first 2 years after treatment completion [1, 7, 8]. Lastly, although tuberculosis treatment has saved millions, drug toxicity may also contribute to increased healthcare utilization in the post-tuberculosis period due to harmful effects on the liver, kidneys, and hearing, especially in those treated for drug-resistant tuberculosis [9, 10].
In this study, we sought to examine the relationship between treated respiratory tuberculosis and post-tuberculosis healthcare utilization. Our primary objective was to measure outpatient physician encounters and inpatient hospital admissions among individuals with treated respiratory disease in the years following diagnosis and treatment. Our secondary objective was to explore the reasons for outpatient encounters in the years following diagnosis and treatment.
METHODS
Study Setting
British Columbia (BC) is a Canadian province with an annual tuberculosis incidence of 6.0 per 100 000 residents [11]. In 2020, approximately 86% of people diagnosed with tuberculosis in British Columbia were born outside of Canada, despite representing only 22% of the population [11].
The BC Medical Services Plan (MSP) is the universal health insurance program administered by the provincial government of British Columbia. Enrolment is mandatory for all eligible residents, including those with Canadian citizenship and permanent residents who meet certain conditions [12]. For people with provincial health insurance, there are no out-of-pocket expenses for physician and hospital care [12].
The BC Center for Disease Control runs a centralized provincial tuberculosis program and maintains a provincial tuberculosis registry that includes diagnosis and treatment data. Mandatory reporting by public health agencies, routine reporting from the centralized provincial mycobacteriology laboratory, and access to publicly funded tuberculosis medications make this registry virtually complete for tuberculosis disease diagnosis and treatment information in the province [11].
Data Source
The source cohort construction and data linkage for this analysis have been described elsewhere [13]. Briefly, the source cohort contains linked administrative health data accessed through Population Data BC, covering all foreign-born permanent residents to Canada who immigrated between 1 January 1985 and 31 December 2019 and established residency in British Columbia. Data elements include demographics, immigration information, MSP registration and physician billings, hospital discharge, and provincial disease registries, including the Provincial Tuberculosis Registry [14–19].
Study Design and Population
We conducted a controlled interrupted time series analysis by measuring healthcare utilization at multiple time points before and after the tuberculosis diagnosis date. Our study consisted of 3 specific periods, which are illustrated in Supplementary Figure 1:
The pre-tuberculosis period: up to the 4 years preceding the tuberculosis period.
The tuberculosis period: beginning 2 years before the tuberculosis diagnosis date to account for an increase in healthcare utilization associated with tuberculosis diagnosis [5] and ending 1 year after the tuberculosis diagnosis date to account for tuberculosis disease and treatment (typically 6–9 months) [20]. This period was not included in our regression models.
The post–tuberculosis period: up to the 5 years after the tuberculosis period.
Our study population included foreign-born individuals who established residency in British Columbia, Canada, between 1 January 1990, and 31 December 2019, and completed treatment for incident respiratory tuberculosis between those dates, as coded in the Provincial Tuberculosis Registry.
People treated for tuberculosis were matched to people without a tuberculosis diagnosis from the same source cohort. For the non-tuberculosis controls, we imputed a proxy tuberculosis diagnosis date based on the distribution of time-to-diagnosis in the tuberculosis group. Imputation covariates included age of arrival to Canada, sex, tuberculosis incidence in country of origin, World Health Organization region, immigration class, and year of arrival to Canada.
Propensity score matching was then used to account for differences in covariates that might influence healthcare utilization [21]. The propensity score was the predicted probability of being diagnosed with tuberculosis based on covariates chosen through literature review and our knowledge of tuberculosis and healthcare utilization [22]. All covariates were defined using a covariate assessment window, which began on the date their residency in British Columbia was established and ended 1 year later [13]. Individuals with a tuberculosis diagnosis date (or proxy diagnosis date) within the comorbidity assessment window or without covariate information were excluded from the study population to ensure adequate time to assess demographic characteristics and pre-existing comorbidities among our study population. Age and sex at immigration date, year of immigration, immigration class, and tuberculosis incidence in country of birth were obtained from the Immigration, Refugees, and Citizenship Canada database [18]. MSP registration data were used to determine years since establishing residency in BC and health service delivery area [17]. Neighborhood income decile from Census data as a proxy measure for socioeconomic status and comorbidities were measured using the Walraven Elixhauser Comorbidity Index [23].
We matched each person with treated tuberculosis with up to 4 non-tuberculosis controls, using nearest neighbor matching without replacement and a caliper of 0.1 standard deviations of the logit of the propensity score [21, 24]. Group balance was assessed using standardized mean differences, with a standardized difference of <0.1 indicating good balance [25].
Outcomes
Our primary outcome was healthcare utilization, captured by 2 key variables:
Inpatient hospital admissions: information on hospital admissions were obtained from the Discharge Abstracts Database [14]. We included all hospital admissions in BC acute care hospitals, including elective and urgent admissions and admissions via the emergency department.
Outpatient physician encounters: information on outpatient physician encounters was obtained from the MSP database [17]. Each physician encounter counted was based on a unique combination of patient ID, provider ID, and service date, regardless of the number of fee items billed during that encounter. We included all physician encounters, regardless of International Classification of Diseases-9 (ICD-9) codes, provided in British Columbia by fee-for-service practitioners.
For each measure of healthcare use, we summed the total number of physician encounters or hospital admissions for each month in both populations. We then divided the sum by the population total for that monthly interval, based on the number of people in each population alive and enrolled in MSP during that month.
For our secondary outcomes, we examined the impact treated respiratory tuberculosis had on out-patient physician encounters captured under specific ICD-9 codes for (1) diseases of the respiratory system, (2) diseases of the circulatory system, (3) mental disorders, (4) diseases of the genitourinary system, (5) infectious and parasitic disease, (6) injury and poisoning, (7) endocrine, nutritional, and metabolic diseases, and immunity disorders, and (8) physician encounters related to ill-defined conditions (Supplementary Table 1). These categories were chosen based on the post-tuberculosis morbidity literature [1, 2, 26–28]. Laboratory and additional diagnostic procedures are not captured within these site-specific categories, and physician encounters related to tuberculosis-associated codes are captured within the infectious and parasitic diseases category [29].
Statistical Analysis
For our primary objective, we ran 2 separate segmented linear regression models to estimate the change in trend and level of healthcare utilization following the tuberculosis period; 1 for the monthly rate of outpatient physician encounters and the other for the monthly rate of inpatient hospital admissions [30]. We examined the data for linearity and autocorrelation using scatterplots, plots of residuals, and partial autocorrelation functions. Preliminary analyses indicated that a linear trend did not provide a reasonable fit, so we included a quadratic term to fit the expected line for the post-tuberculosis period, which improved model fit based on the likelihood ratio test. We also included a 1-month autoregressive term to adjust for detected autocorrelation [30]. We then used the segmented regression models to estimate the absolute and relative difference, with 95% confidence intervals, in observed versus predicted monthly physician encounters and monthly hospital admissions during the first and the fifth year of the post-tuberculosis period. For each category of healthcare utilization, we also calculated the excess healthcare utilization per person over the 5-year post-tuberculosis period by subtracting the total number of physician encounters or hospital admissions observed during the post-tuberculosis period in the tuberculosis population from the expected number of physician encounters or hospital admissions, respectively.
We used the same methodology for outpatient physician encounters detailed in our primary analysis for our secondary objective but ran separate segmented regression models for each physician encounter category. We also undertook 2 sensitivity analyses. First, given that estimates from propensity score approaches are sensitive to model specifications, we used coarsened exact matching, rather than propensity score matching, as this method does not rely on the correct specifications of a propensity score model [31]. We used the same variables as we did for the propensity score model and group balance was assessed using standardized mean differences. Next, because our primary analysis encompasses 30 years, where patterns of healthcare utilization may have changed remarkably, we restricted our analysis to individuals treated for respiratory tuberculosis between 2000 and 2019. All analyses were conducted in R (V.4.0.5) [32]. Propensity score matching and coarsened exact matching were conducted using the MatchIt Package (V.4.5.2) [33].
Ethical Consideration and Reporting
Ethical approval was provided by the University of British Columbia Clinical Research Ethics Board (Certificate number H16-00265). We reported this study following the guidelines for the Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) statement (Supplementary Appendix) [34].
RESULTS
Of the 1589 people who completed treatment for incident respiratory disease during the study period, 1216 were eligible for inclusion (Supplementary Figure 2). In total, we matched all 1216 people to 4864 non-tuberculosis controls (Table 1). The monthly population denominators and median follow-up times are summarized in Supplementary Tables 2 and Table 3, respectively.
Table 1.
Baseline Characteristics of the Propensity Score Matched Tuberculosis and Non-tuberculosis Control Populations
| Characteristics | Non-tuberculosis Control Population N (%) |
Tuberculosis Population N (%) |
Standardized Mean Difference |
|---|---|---|---|
| N | 4864 | 1216 | … |
| Medical Service Plan start year | … | … | 0.023 |
| 1990–1995 | 1885 (38.8) | 465 (38.2) | … |
| 1996–2000 | 1152 (23.7) | 296 (24.3) | … |
| 2001–2005 | 705 (14.5) | 181 (14.9) | … |
| 2006–2010 | 651 (13.4) | 161 (13.2) | … |
| 2011–2015 | 382 (7.9) | 92 (7.6) | … |
| 2016–2019 | 89 (1.8) | 21 (1.7) | … |
| Age at arrival to Canada, median (IOR) | 43.0 (30.0, 59.0) | 43.0 (29.0, 59.0) | 0.003 |
| Sex | … | … | 0.014 |
| Male | 2781 (57.2) | 687 (56.5) | … |
| Female | 2083 (42.8) | 529 (43.5) | … |
| WHO tuberculosis incidence in country of origin | … | … | 0.032 |
| < 30 per 100 000 | 120 (2.5) | 26 (2.1) | … |
| 30 to 100 per 100 000 | 1579 (32.5) | 407 (33.5) | … |
| 101 to 200 per 100 000 | 1621 (33.3) | 407 (33.5) | … |
| > 200 per 100 000 | 1544 (31.7) | 376 (30.9) | … |
| WHO region of origin | … | … | 0.031 |
| Western Pacific Region | 3000 (61.7) | 761 (62.6) | … |
| South-East Asia Region | 1333 (27.4) | 332 (27.3) | … |
| Eastern Mediterranean Region | 189 (3.9) | 44 (3.6) | … |
| African Region | 141 (2.9) | 33 (2.7) | … |
| European Region | 115 (2.4) | 28 (2.3) | … |
| Region of the Americans | 86 (1.8) | 18 (1.5) | … |
| Immigration class | … | … | 0.020 |
| Family | 2565 (52.7) | 647 (53.2) | … |
| Economic | 1638 (33.7) | 412 (33.9) | … |
| Refugee | 438 (9.0) | 104 (8.6) | … |
| Other | 223 (4.6) | 53 (4.4) | … |
| Elixhauser comorbidity score, median (IQR) | 0.01 (0.1) | 0.01 (0.1) | 0.055 |
| Neighborhood income | … | … | 0.027 |
| Lowest 20% | 1799 (37.0) | 442 (36.3) | … |
| Low-middle 20% | 1250 (25.7) | 325 (26.7) | … |
| Middle 20% | 869 (17.9) | 214 (17.6) | … |
| Middle-high 20% | 514 (10.6) | 131 (10.8) | … |
| Highest 20% | 432 (8.9) | 104 (8.6) | … |
| Health service delivery area | … | … | 0.023 |
| Metro | 4141 (85.1) | 1037 (85.3) | … |
| Urban—Rural | 611 (12.6) | 155 (12.7) | … |
| Rural—Remote | 112 (2.3) | 24 (2.0) | … |
| Years since arrival, median (IQR) | 8.0 (4.0. 14.0) | 8.0 (4.0, 14.0) | 0.020 |
| Tuberculosis diagnosis year | … | … | 0.089 |
| 1990–1995 | 187 (3.8) | 55 (4.5) | … |
| 1996–2000 | 518 (10.6) | 131 (10.8) | … |
| 2001–2005 | 821 (16.9) | 200 (16.4) | … |
| 2006–2010 | 1157 (23.8) | 250 (20.6) | … |
| 2011–2015 | 1321 (27.2) | 295 (24.3) | … |
| 2016–2019 | 860 (17.7) | 285 (23.4) | … |
Abbreviations: IQR, interquartile range; WHO, World Health Organization.
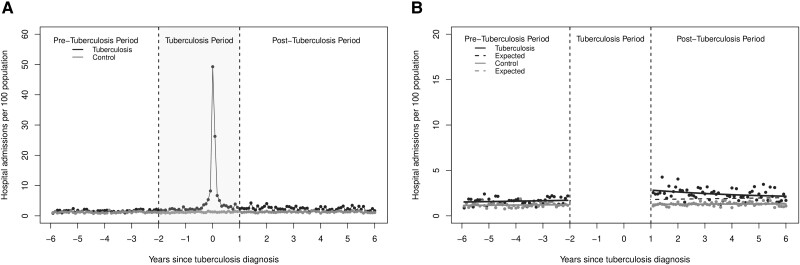
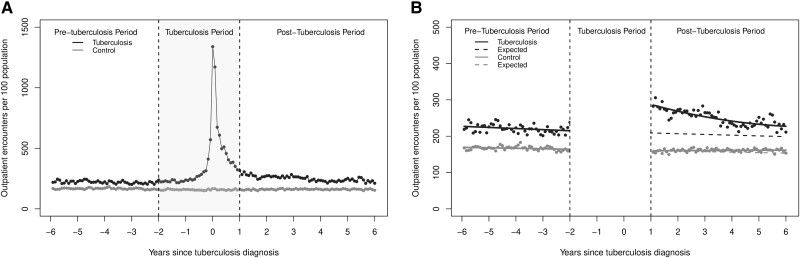
The monthly rates of inpatient hospital admissions and outpatient physician encounters are presented in Figure 1A and Figure 2A , respectively. As expected, those with tuberculosis had a steep increase in inpatient hospital admissions and outpatient encounters in the months leading up to the tuberculosis diagnosis date.
Figure 1.
(A) The monthly rate of in-patient hospital admissions per 100 population for all people who completed treatment for incident respiratory tuberculosis and (B) results of the segmented regression analysis.
Figure 2.
(A) The monthly rate of out-patient physician encounters per 100 population for all people who completed treatment for incident respiratory tuberculosis and (B) results of the segmented regression analysis.
Inpatient Hospital Admissions
At the start of the post-tuberculosis period, the monthly rate of inpatient hospital admissions in the tuberculosis group was 1.1 (95% confidence interval [CI]: .8, 1.4) hospital admission per 100 population higher than would have been expected in the absence of tuberculosis, a relative increase of 60.4% (95% CI: 43.2%, 77.6%). However, this trend was not sustained and decreased to the expected rate by year 5 of the post-tuberculosis period (Figure 1B , Table 2). The excess hospital admissions represent an additional 0.4 (95% CI: 0.3, 0.5) hospital admissions per person over the 5-year post-tuberculosis study period. In contrast, there was no significant change in the level or trend for hospital admissions in the non-tuberculosis group.
Table 2.
Changes in Monthly Inpatient Hospital Admissions, Outpatient Physician Encounters, and Respiratory-related Physician Encounters per 100 Population at 1 and 5 Years Post-tuberculosis for People Diagnosed With Tuberculosis
| One year Post-tuberculosis | Five years Post-tuberculosis | |||||||
|---|---|---|---|---|---|---|---|---|
| Expected Monthly Ratea | Observed Monthly Ratea (95% CI) |
Absolute Difference (95% CI) |
Relative Change (95% CI) |
Expected Monthly Ratea | Observed Monthly Ratea (95% CI) |
Absolute Difference (95% CI) |
Relative Change (95% CI) |
|
| Inpatient hospital admissions | 1.7 | 2.8 (2.5, 3.1) |
1.1 (0.8, 1.4) |
60.4% (43.2, 77.6) |
1.9 | 2.2 (2.1, 2.4) | 0.3 (0.2, 0.5) | 17.8% (12.4, 28.5) |
| Outpatient physician encountersb | 213.6 | 286.2 (279.3, 293.0) |
72.6 (65.7, 79.4) |
34.0% (30.7, 37.2) |
207.9 | 233.3 (229.9, 236.6) |
25.5 (22.1, 28.8) |
12.3% (10.6, 13.8) |
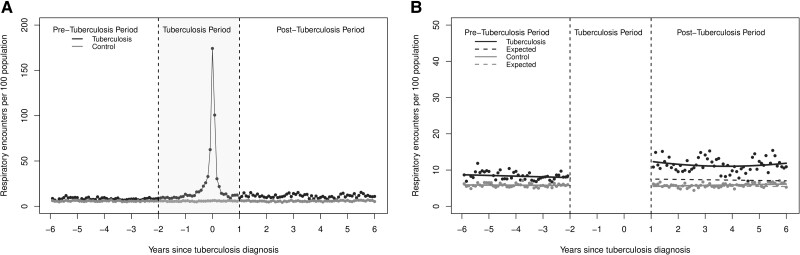
| Respiratory related outpatient physician encountersc | 7.7 | 12.4 (11.5, 13.3) |
4.6 (3.7, 5.5) |
59.5% (47.9, 71.0%) |
7.8 | 11.3 (10.9, 11.8) |
3.5 (3.1, 4.0) |
44.9% (39.8, 51.3) |
Abbreviation: CI, confidence interval.
Per 100 population.
Captures all unique physician encounters, regardless of ICD-9 billing codes, including laboratory and additional diagnostic procedures.
Only captures unique physician encounters coded for respiratory diseases.
Outpatient Physician Encounters
At the start of the post-tuberculosis, the monthly rate of outpatient physician encounters in the tuberculosis group was 72.6 (95% CI: 65.7, 79.4) encounters per 100 population higher than expected, a relative increase of 34.0% (95% CI: 30.7, 37.2) The monthly rate of outpatient encounters remained elevated above the expected rate for the duration of the post-tuberculosis period (Figure 2B , Table 2). The excess outpatient encounters represent an additional 12.2 (95% CI: 10.6, 14.9) physician encounters per person over the post-tuberculosis period. Similar to hospital admissions, there was no significant change in the level or trend for outpatient encounters in the non-tuberculosis control group.
Reasons for Outpatient Physician Encounters
At the start of the post-tuberculosis, there was an increase in all outpatient encounter categories except for diseases of the circulatory system. Although above the non-tuberculosis controls, circulatory-related physician encounters were at the expected rate for the post-tuberculosis period. Infectious and parasitic disease, injury, and poisoning-related encounters returned to expected rates within 4–5 years of the post-tuberculosis period (Supplementary Figure 3, Supplementary Table 4).
In contrast, respiratory-related physician encounters remained elevated higher than would be expected for the duration of the post-tuberculosis period (Figure 3, Table 2). There were also sustained increases in physician encounters related to endocrine disorders, ill-defined conditions, and diseases of the genitourinary system (Supplementary Figure 3, Supplementary Table 4). Lastly, although there was an immediate increase in mental-disorder-related physician encounters, the monthly rate remained below the expected for the post-tuberculosis period (Supplementary Figure 3, Supplementary Table 4).
Figure 3.
(A) The monthly rate of out-patient respiratory encounters per 100 population for all people who completed treatment for incident respiratory tuberculosis and (B) results of the segmented regression analysis.
Sensitivity Analysis
For the coarsened exact matching analysis, we matched 1108 people treated for incident pulmonary tuberculosis to 1108 controls, and our results were consistent with findings from our primary analysis (Supplementary Table 5, Supplementary Figure 4). Similarly, restricting our analysis to individuals treated for respiratory tuberculosis between 2000 and 2019 also resulted in consistent findings (Supplementary Figure 5).
DISCUSSION
Our results demonstrate that foreign-born people treated for respiratory tuberculosis in a low-incidence setting experience elevated healthcare utilization in the years following the completion of tuberculosis therapy. Moreover, our results indicate that respiratory morbidity is a large contributor to the elevated healthcare utilization seen. These findings, combined with emerging literature showing respiratory tuberculosis, can cause irreversible lung damage, including obstructive lung disease, bronchiectasis, restrictive disease and infectious complications, underscores the need for comprehensive programs addressing post-tuberculosis lung disease [35–37].
Notably, we also saw sustained increases in physician encounters related to endocrine disorders and diseases of the genitourinary system after the tuberculosis period. Although diabetes and chronic kidney disease are risk factors for tuberculosis, these conditions may also be exacerbated by tuberculosis disease [38, 39]. Studies have shown tuberculosis can lead to impaired glucose tolerance and new-onset diabetes [28]. Similarly, respiratory infections can lead to faster disease progression in people with chronic kidney disease and expedited dialysis dependency, which in turn may increase post-tuberculosis healthcare utilization [40].
Some of the most effective ways to avert the short and long-term consequences of tuberculosis are to avoid diagnostic delays and to provide preventive treatment to people with tuberculosis infection [41]. Yet even in well-resourced tuberculosis programs, only a small proportion of people with tuberculosis infection receive preventive treatment, largely due to its perceived limited benefits [41, 42]. Currently, the clinical and programmatic value of tuberculosis preventive treatment is based on the potential to avert morbidity and mortality associated with the acute illness and treatment period, typically assuming tuberculosis survivors experience “cure” with limited long-term morbidity. Our findings, along with recent literature on post-tuberculosis disease, dispute this notion and suggest researchers reconsider risk-benefit calculations of preventive treatment.
A major strength of this study is its design and the use of a control group. An interrupted time series methodology is one of the strongest quasi-experimental research designs and has the distinct advantage of being methodologically rigorous but easily interpretable by non-technical audiences [30]. Additionally, our use of provincial tuberculosis data and near-complete capture of hospital admissions and physician encounters added to the strength of this study.
However, this study also has limitations. First, as a retrospective study using linked administrative data, we encountered analytical and sampling challenges inherent to administrative data. In our analysis, even in the pre-tuberculosis period, those who went on to develop tuberculosis had higher healthcare utilization rates than matched controls, suggesting there may be residual or unmeasured confounding related to measures of socioeconomic status, smoking history, or severity of comorbidities [43–45]. For our propensity score match, we used neighborhood income decile as a proxy measure for socioeconomic status because we did not have the necessary variables to calculate a deprivation index [46]. While income is a powerful indicator of health, it does not adequately capture all domains of socioeconomic status [46]. Thus, our use of a proxy measure may have resulted in a higher proportion of people from lower socioeconomic groups in the tuberculosis population. In addition to being at an increased risk of tuberculosis and chronic conditions, people from lower socioeconomic groups report higher rates of smoking and often live or work in poorly ventilated conditions [47, 48]. They also have limited access to high-quality healthcare and have less power to act on existing knowledge of healthy behaviors, all factors associated with increased healthcare use [47–51].
Next, in Canada, approximately 30% of TB diagnoses among people who are foreign-born are temporary visa holders, which includes tourists, visitors, students, or temporary workers [52]. Unfortunately, our data only includes information on individuals who have received permanent landing status, we were unable to capture the healthcare utilization patterns of this group. Third, we used data from a high-resource, low-tuberculosis-incidence region with universal healthcare to generate these findings. Thus, interpretations may not be generalizable in settings with different characteristics. Lastly, we did not include the costs associated with the increase in post-tuberculosis healthcare utilization. For future research, we recommend that a complete costing analysis be undertaken to capture the costs of diagnosis, treatment, and post-tuberculosis care incurred by patients and the healthcare system.
A growing number of studies highlight chronic lung disease, long-term disability, and elevated mortality among individuals who have survived tuberculosis. Our study adds to this body of literature and is the first to capture healthcare use post-tuberculosis treatment comprehensively. It shows that healthcare utilization in a high-resource setting remains elevated among individuals who have completed treatment for respiratory disease. Adjusting burden and cost estimates to consider post-tuberculosis healthcare utilization may substantially influence the perceived benefits of tuberculosis prevention, while incorporating post-tuberculosis care as part of routine care may provide an opportunity to reduce resource use.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Kamila Romanowski, Provincial Tuberculosis Services, British Columbia Centre for Disease Control, Vancouver, British Columbia, Canada; Department of Medicine, The University of British Columbia, Vancouver, British Columbia, Canada.
Michael R Law, Centre for Health Services and Policy Research, The University of British Columbia, Vancouver, British Columbia, Canada; School of Population and Public Health, Faculty of Medicine, The University of British Columbia, Vancouver, British Columbia, Canada.
Mohammad Ehsanul Karim, School of Population and Public Health, Faculty of Medicine, The University of British Columbia, Vancouver, British Columbia, Canada; Centre for Health Evaluation and Outcome Sciences, St. Paul’s Hospital, Vancouver, British Columbia, Canada.
Jonathon R Campbell, Respiratory Epidemiology and Clinical Research Unit, Centre for Outcomes Research & Evaluation, Research Institute of the McGill University Health Centre, Montreal, Canada; McGill International TB Centre, McGill University, Montreal, Quebec, Canada; Departments of Medicine & Global and Public Health, Faculty of Medicine and Health Sciences, McGill University, Montreal, Canada.
Md Belal Hossain, School of Population and Public Health, Faculty of Medicine, The University of British Columbia, Vancouver, British Columbia, Canada.
Mark Gilbert, School of Population and Public Health, Faculty of Medicine, The University of British Columbia, Vancouver, British Columbia, Canada; Clinical Prevention Services, British Columbia Centre for Disease Control, Vancouver, British Columbia, Canada.
Victoria J Cook, Provincial Tuberculosis Services, British Columbia Centre for Disease Control, Vancouver, British Columbia, Canada; Department of Medicine, The University of British Columbia, Vancouver, British Columbia, Canada.
James C Johnston, Provincial Tuberculosis Services, British Columbia Centre for Disease Control, Vancouver, British Columbia, Canada; Department of Medicine, The University of British Columbia, Vancouver, British Columbia, Canada; McGill International TB Centre, McGill University, Montreal, Quebec, Canada.
Notes
Data availability statement . The data from this study are held in a secure research environment managed by Population Data BC (https://www.popdata.bc.ca/data). Access to the data provided by the Data Steward(s) is subject to approval but can be requested for research projects through the Data Steward(s) or their designated service providers (dataaccess@popdata.bc.ca). All inferences, opinions, and conclusions drawn in this manuscript are those of the authors and do not reflect the views and policies of the Data Steward(s).
Author contributions. Conceptualization: K. R., M. E. K., M. G., V. J. C., J. C. J.
Funding acquisition: K. R., J. C. J.
Data curation: K. R.
Formal analysis: K. R.
Methodology: K. R., M. R. L., M. E. K., J. C. J.
Resources: K. R., M. R. L., M. E. K., M. B. H., J. R. C.
Supervision: J. C. J.
Writing—original draft: K. R.
Writing—review and editing: M. R. L., M. E. K., M. B. H., J. R. C., M. G., V. J. C., and J. C. J.
Financial support. K. R. is supported by the Canadian Institutes for Health Research (Doctoral Fellowship Award, 2020-2023). M. R. L. received salary support from Canada Research Chairs. M. E. K. is supported by the Michael Smith Health Research BC (ID 17661). J. R. C. receives salary support from the McGill University Health Centre (Foundation) and McGill University (Department of Medicine). M. B. H. is supported by the University of British Columbia (4 Year Doctoral Fellowship 2020-2024, and the Harry and Florence Dennison Fellowship in Medical Research 2022-2023). M. G. receives salary support from the Canadian Institutes of Health Research (Applied Public Health Chair Program). J. C. J. is supported by Michael Smith Health Research BC and the Canadian Institutes for Health Research (grant # PJT-153213). The researchers were independent of their sources of support, which had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Basham CA, Smith SJ, Romanowski K, Johnston JC. Cardiovascular morbidity and mortality among persons diagnosed with tuberculosis: a systematic review and meta-analysis. PLoS One 2020; 15:e0235821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis 2015; 32:138–46. [DOI] [PubMed] [Google Scholar]
- 3. Romanowski K, Baumann B, Basham CA, Khan FA, Fox GJ, Johnston JC. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2019; 19:1129–37. [DOI] [PubMed] [Google Scholar]
- 4. Allwood B. The forgotten form of TB that can carry on forever. Bhekisisa. 2023. Available at: https://bhekisisa.org/article/2023-03-24-the-forgotten-form-of-tb-that-can-carry-on-forever/. Accessed 10 April 2023.
- 5. Sreeramareddy CT, Panduru KV, Menten J, Van den Ende J. Time delays in diagnosis of pulmonary tuberculosis: a systematic review of literature. BMC Infect Dis 2009; 9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 2018; 27:170077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basham CA, Karim ME, Cook VJ, Patrick DM, Johnston JC. Tuberculosis-associated depression: a population-based cohort study of people immigrating to British Columbia, Canada, 1985–2015. Ann Epidemiol 2021; 63:7–14. [DOI] [PubMed] [Google Scholar]
- 8. Cox H, Kebede Y, Allamuratova S, et al. Tuberculosis recurrence and mortality after successful treatment: impact of drug resistance. PLoS Med 2006; 3:e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Global tuberculosis report 2022. Available at: https://www.who.int/publications-detail-redirect/9789240037021. Accessed 1 November 2022.
- 10. Wu S, Zhang Y, Sun F, et al. Adverse events associated with the treatment of multidrug-resistant tuberculosis: a systematic review and meta-analysis. Am J Ther 2016; 23:e521–30. [DOI] [PubMed] [Google Scholar]
- 11. BC Centre for Disease Control . CPS Monthly Surveillance Report.2022. Available at: http://www.bccdc.ca/resource-gallery/Documents/StatisticsandResearch/StatisticsandReports/STI/CPSMonthlySurveillanceReport-September2022_FINAL.pdf. Accessed 21 November 2022.
- 12. Ministry of Health. Eligibility for MSP - Province of British Columbia [Internet] . Province of British Columbia. 2022. Available from: https://www2.gov.bc.ca/gov/content/health/health-drug-coverage/msp/bc-residents/eligibility-and-enrolment/are-you-eligible. Accessed 4 November 2022.
- 13. Ronald LA, Campbell JR, Balshaw RF, et al. Predicting tuberculosis risk in the foreign-born population of British Columbia, Canada: study protocol for a retrospective population-based cohort study. BMJ Open 2016; 6:e013488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute for Health Information (2019): Discharge Abstract Database (Hospital Separations). Population Data BC [publisher]. Data Extract. MOH (2021).
- 15.BC Centre for Disease Control (2019): BC Provincial TB Registry. Population Data BC. Data Extract. BCCDC (2021).
- 16.BC Vital Statistics Agency (2021): BC Vital Statistics. Population Data BC. Data Extract. BC Vital Statics Agency (2021).
- 17. British Columbia Ministry of Health (2015): Consolidation File (MSP Registration & Premium Billing). Population Data BC. Data Extract. MOH (2014).
- 18. Immigration, Refugees, and Citizenship Canada . Permanent Resident Database. V2. Population Data BC. Data Extract. IRCC (2021).2021.
- 19. Statistics Canada . Statistics Canada Income Band Data. V2. Population Data BC. Data Extract. Population Data BC (2021).2021.
- 20. Johnston JC, Cooper R, Menzies D. Chapter 5: treatment of tuberculosis disease. Can J Respir Crit Care Sleep Med 2022; 6:66–76. [Google Scholar]
- 21. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Setia MS, Quesnel-Vallee A, Abrahamowicz M, Tousignant P, Lynch J. Access to health-care in Canadian immigrants: a longitudinal study of the national population health survey. Health Soc Care Community 2011; 19:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009; 47:626–33. [DOI] [PubMed] [Google Scholar]
- 24. Rosenbaum PR. Modern algorithms for matching in observational studies. Annu Rev Stat Its Appl 2020; 7:143–76. [Google Scholar]
- 25. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duko B, Bedaso A, Ayano G. The prevalence of depression among patients with tuberculosis: a systematic review and meta-analysis. Ann Gen Psychiatry 2020; 19:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romanowski K, Balshaw RF, Benedetti A, et al. Predicting tuberculosis relapse in patients treated with the standard 6-month regimen: an individual patient data meta-analysis. Thorax 2019; 74:291–7. [DOI] [PubMed] [Google Scholar]
- 28. Yorke E, Atiase Y, Akpalu J, Sarfo-Kantanka O, Boima V, Dey ID. The bidirectional relationship between tuberculosis and diabetes. Tuberc Res Treat 2017; 2017:e1702578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ministry of Health. Diagnostic Code Descriptions (ICD-9) - Province of British Columbia [Internet]. Province of British Columbia. 2017. Available from: https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/msp/physicians/diagnostic-code-descriptions-icd-9. Accessed 14 December 2022.
- 30. Lopez Bernal J, Cummins S, Gasparrini A. The use of controls in interrupted time series studies of public health interventions. Int J Epidemiol 2018; 47:2082–93. [DOI] [PubMed] [Google Scholar]
- 31. Iacus SM, King G, Porro G. Causal inference without balance checking: coarsened exact matching. Polit Anal 2012; 20:1–24. [Google Scholar]
- 32. R Core Team . R: A language and environment for statistical computing. R Found Stat Comput. 2021.
- 33. Ho D, Imai K, King G, Stuart EA. Matchit: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011; 42:1–28. [Google Scholar]
- 34. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med 2015; 12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Migliori GB, Marx FM, Ambrosino N, et al. Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int J Tuberc Lung Dis 2021; 25:797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allwood BW, Byrne A, Meghji J, Rachow A, van der Zalm MM, Schoch OD. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration 2021; 100:751–63. [DOI] [PubMed] [Google Scholar]
- 37. Basham CA, Karim ME, Cook VJ, Patrick DM, Johnston JC. Post-tuberculosis airway disease: a population-based cohort study of people immigrating to British Columbia, Canada, 1985–2015. EClinicalMedicine 2021; 33:100752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campbell JR, Winters N, Menzies D. Absolute risk of tuberculosis among untreated populations with a positive tuberculin skin test or interferon-gamma release assay result: systematic review and meta-analysis. BMJ 2020; 368:m549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bollenbecker S, Czaya B, Gutierrez OM, Krick S. Lung-kidney interactions and their role in chronic kidney disease-associated pulmonary diseases. Am J Physiol-Lung Cell Mol Physiol 2022; 322:L625–40. [DOI] [PubMed] [Google Scholar]
- 41. WHO consolidated guidelines on tuberculosis: module 1: prevention: tuberculosis preventive treatment. Available at: https://www.who.int/publications-detail-redirect/9789240001503. Accessed 20 November 2022.
- 42. Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16:1269–78. [DOI] [PubMed] [Google Scholar]
- 43. Natarajan S, Nietert PJ. Hypertension, diabetes, hypercholesterolemia, and their combinations increased health care utilization and decreased health status. J Clin Epidemiol 2004; 57:954–61. [DOI] [PubMed] [Google Scholar]
- 44. Marais BJ, Lönnroth K, Lawn SD, et al. Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infect Dis 2013; 13:436–48. [DOI] [PubMed] [Google Scholar]
- 45. van Zyl Smit RN, Pai M, Yew WW, et al. Global lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. Eur Respir J 2010; 35:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pampalon R, Hamel D, Gamache P, Raymond G. A deprivation index for health planning in Canada. Chronic Dis Can 2009; 29:178–91. [PubMed] [Google Scholar]
- 47. Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med 2009; 68:2240–6. [DOI] [PubMed] [Google Scholar]
- 48. Gilson L, Doherty J, Loewenson R, Francis V. Knowledge network on health systems—WHO Commission on Social Determinants of Health. Final report.2007.
- 49. Azagba S, Sharaf MF, Xiao Liu C. Disparities in health care utilization by smoking status in Canada. Int J Public Health 2013; 58:913–25. [DOI] [PubMed] [Google Scholar]
- 50. Fitzpatrick T, Rosella LC, Calzavara A, et al. Looking beyond income and education: socioeconomic status gradients among future high-cost users of health care. Am J Prev Med 2015; 49:161–71. [DOI] [PubMed] [Google Scholar]
- 51. Roos NP, Mustard CA. Variation in health and health care use by socioeconomic status in Winnipeg, Canada: does the system work well? Yes and no. Milbank Q 1997; 75:89–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Public Health Agency of Canada. Tuberculosis in Canada. 2008—2018 Data. 2020. Available at: https://www.canada.ca/en/public-health/services/diseases/tuberculosis/surveillance.html. Accessed 4 April 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.