Several methods can be used to diagnose Helicobacter pylori infection. Most of them require upper gastrointestinal endoscopy for retrieval of a gastric biopsy specimen. For serology, no upper gastrointestinal endoscopy is required, but blood must be obtained to detect H. pylori antibodies. H. pylori serology is attractive in comparison with other diagnostic methods because it is simple, inexpensive, and less of a burden for the patient. Several kits for the detection of H. pylori by serology have become commercially available since the discovery of H. pylori by Warren (87) in 1983. Most of these H. pylori serology kits are based on various antibody preparations and different techniques.
The introduction of commercially available H. pylori kits has led to an increase in the number of studies that have evaluated kit characteristics. Recently, a systematic review comparing the accuracies of commonly used commercial serology kits for the detection of H. pylori infection has been conducted (48). To account for the different reference standards and designs used by various investigators, only studies that evaluated pairs of serology kits and that compared the kits only within those studies were included. A more appropriate method of comparing different diagnostic tests and the performance of different interpreters of one test is to calculate the area under the receiver operating characteristics curve (AURC) for each test (83). To correct for dependence between AURCs within the same study population, we used a random-effect model. By reviewing the literature, we also tried to determine whether H. pylori serology can accurately diagnose H. pylori infection. However, in contrast to the study by Loy et al. (48), we reviewed all the studies that evaluated commercially available H. pylori serology kits.
DATA COLLECTION AND EXTRACTION
Identification and eligibility of publications.
A computerized and manual literature search was performed in early 1998. Relevant publications were identified in MEDLINE (1983 to 1997) with the medical subject heading terms Helicobacter or pylori, Sero*, Sera*, Seru*, Sensitivity, and Human in checktags. Furthermore, additional publications were retrieved by reviewing references in publications found by MEDLINE. The criteria used to select publications were as follows: H. pylori infection was established before treatment; the H. pylori serology kit was commercially available; the number of patients, prevalence of infection, and the sensitivity and specificity of the H. pylori serology kit were described or could be calculated; and the studies were published in Dutch, English, French, or German.
Data analysis.
New diagnostic tests are mainly evaluated by determining the sensitivity and specificity of the test. For evaluative purposes, the sensitivity and specificity are less useful (83). On the basis of the receiver operating characteristic (ROC) curve, we calculated the AURC, which is a measure for the diagnostic performance of a test (42). It is independent of cutoff points and reasonably immune to selection bias. Depending on the serology kit result, one should use different methods to calculate the AURC. We used a method to estimate the AURC for kits with a quantitative test result by using one combination of a true-positive rate and a false-positive rate on the basis of the assumption that the data for the H. pylori-infected and noninfected persons were logistically distributed and had equal variances (84). On the other hand, for serology kits with a qualitative test result, we used the trapezium method to calculate the AURC (36). However, comparison of the H. pylori serology kits revealed that the diagnostic performance differed substantially depending on how the AURC was calculated. Therefore, we decided to estimate the AURCs by the trapezium method, irrespective of the distribution of the test result. Use of the trapezium method to estimate the AURC of a serology kit with a quantitative test result possibly underestimates its diagnostic performance (36).
The AURC was used to explore possible differences between clinical features of study populations and methodological aspects of the serology kits. The tests were stratified into the following: report type (abstract, letter, or article), publication year (1991 to 1997), whether the study population was a consecutive series or a selection of a relevant study population, whether or not the patients had dyspeptic symptoms, the nationality of the study population, the reference standard used, the serology kit used, kit scale (quantitative, qualitative), the type of immunoglobulin (immunoglobulin A [IgA], IgG, and IgM simultaneously, IgA alone, or IgG alone) used to detect serum antibodies, the analysis technique of the serology kits (agglutination, enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], fixation, or immunochemical analysis), and whether whole blood or serum was used. We could not examine whether the generation of the test influenced performance because few studies mentioned this.
Statistical methods.
We first tried to model the heterogeneity between the studies by means of an ordinary least-squares regression equation, in which all the clinical features and methodological aspects were simultanously included. Unfortunately, this was not possible because of convergence problems. A best subset analysis was also not possible for the same reasons. Therefore, we decided to perform a separate regression analysis for each clinical feature. It is very likely that the AURCs for different serology kits are correlated when they are used with the same study population. By introducing a random effect for study population, we could model dependency between kits within the same study population (24) (see the Appendix). Moreover, the imprecision of the AURCs varied per study. In order to correct for the heterogeneity in the precision of the AURCs caused by different study sizes, we also performed a weighted regression analysis with weights proportional to 1/SE2, where SE is the standard error (see the Appendix). Whenever AURC is equal to 1 or 0, SE will be 0. If this occurred, the study was excluded from the analysis.
For each regression model an overall F(NDF,DDF) test, where NDF is the degree of freedom in the numerator and DDF is the degree of freedom in the denominator of the F test, was used to examine whether the hypothesis β1 = 0, β2 = 0, . . ., βk = 0 (no fixed effect) should be rejected. Akaike’s information criterion (AIC) is given to choose between the ordinary least-squares model, the random-effects model, and the weighted-random-effects model for each feature. The higher the AIC, the better the model fit. We also performed a weighted-random-effect regression analysis, using the significant features from the previous model simultaneously, in order to correct for possible confounding. These analyses were performed with SAS software (70). For multiple comparisons the Bonferroni correction was used to keep the overall α level at 0.05.
DATA SYNTHESIS
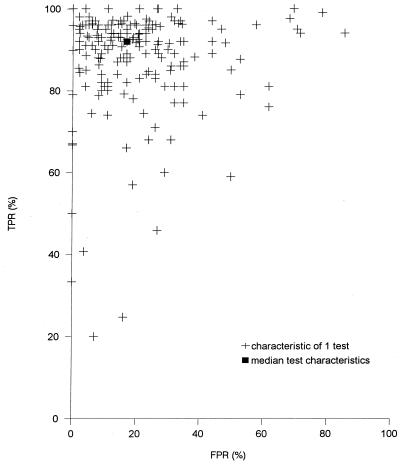
We found a total of 83 publications (1–23, 25–35, 37–41, 43–47, 49–69, 71–82, 85, 86, 88–91) with the MEDLINE search and by reviewing the references in the articles from the original search. Most publications had to be excluded because the H. pylori serology kit used was not commercially available. In those that could be included a total of 177 tests with 36 different commercially available H. pylori serology kits had been performed with 26,812 patients (Table 1). The medians (25 and 75% quantiles) of the sensitivity and specificity for H. pylori serology were 92% (85 and 96%) and 83% (73 and 92%), respectively. However, the sensitivities and specificities of the H. pylori serology kits ranged considerably between tests (Fig. 1).
TABLE 1.
H. pylori serology kits
| Kit | Manufacturer | Technique | No. of populations | Size of total population (no. of subjects) | Sensitivity (%)a | Specificity (%)a | AURCa |
|---|---|---|---|---|---|---|---|
| μPlate | Boehringer | ELISA | 1 | 102 | 93 | 92 | 0.90 |
| AccuMeter | ChemTrak | Immunochemical | 2 | 574 | 88 | 85 | 0.82 |
| Anti-Hp | Roche | ELISA | 18 | 3,558 | 94 (92–96) | 84 (74–92) | 0.85 (0.80–0.91) |
| Autoplate HF | Menarini | ELISA | 1 | 70 | 91 | 96 | 0.89 |
| Biolife | Biolife | ELISA | 1 | 67 | 57 | 81 | 0.56 |
| CFT H. pylori | Institute Virion | Fixation | 2 | 1,040 | 77 | 74 | 0.73 |
| Closer | Medical Instrument Corp. | EIA | 1 | 86 | 96 | 73 | 0.83 |
| Colorvue | Seradyn Clinical Diagnostics | EIA | 1 | 80 | 93 | 88 | 0.88 |
| Diesse | Senese | ELISA | 2 | 136 | 96 | 99 | 0.96 |
| Enzygnost | Behringwerke | ELISA | 2 | 180 | 85 | 90 | 0.86 |
| Flexpack | Abbott | Immunochemical | 1 | 205 | 70 | 100 | 0.85 |
| Flexsure | SmithKline | Immunochemical | 12 | 1,837 | 93 (92–96) | 75 (71–81) | 0.84 (0.78–0.85) |
| GAP | Bio-Rad | ELISA | 20 | 2,877 | 94 (88–97) | 79 (65–90) | 0.82 (0.68–0.88) |
| H. Pylori | Radim | ELISA | 5 | 537 | 90 (84–91) | 86 (83–90) | 0.84 (0.80–0.85) |
| Hel-p | Amrad | ELISA | 4 | 390 | 95 (93–98) | 88 (83–91) | 0.90 (0.86–0.92) |
| Helico-G | Porton | ELISA | 16 | 2,328 | 89 (82–96) | 75 (62–88) | 0.77 (0.67–0.84) |
| Helisal | Cortecs | EIA and ELISA | 5 | 754 | 82 (78–91) | 83 (81–91) | 0.79 (0.73–0.85) |
| Helori | Eurospital | ELISA | 3 | 2,742 | 89 (81–96) | 82 (68–96) | 0.80 (0.77–0.88) |
| HM-CAP | Enteric Products | ELISA | 8 | 1,216 | 94 (86–96) | 83 (75–93) | 0.86 (0.78–0.89) |
| Hp-G Screen | Genesis | ELISA | 1 | 84 | 93 | 91 | 0.89 |
| IIF Test | Bios | Immunochemical | 1 | 110 | 98 | 31 | 0.65 |
| ImmunoCard | Meridian Diagnostics | ELISA | 2 | 188 | 94 | 87 | 0.88 |
| Malakit | Biolab | ELISA | 8 | 925 | 89 (85–96) | 85 (74–96) | 0.84 (0.76–0.92) |
| Meddens | Biotech | ELISA | 1 | 102 | 98 | 95 | 0.96 |
| Microstar | Kenstar | ELISA | 1 | 84 | 97 | 76 | 0.86 |
| Premier | Biomedical | ELISA | 6 | 556 | 95 (89–100) | 88 (81–96) | 0.87 (0.85–0.90) |
| Pyloragen | Hycor Biomedical | ELISA | 1 | 306 | 95 | 83 | 0.87 |
| Pylori-Elisa | Bio-Whitaker | ELISA | 1 | 84 | 100 | 96 | 0.98 |
| Pylori-Stat | Whittaker | ELISA | 14 | 1,243 | 96 (88–96) | 88 (70–94) | 0.85 (0.78–0.91) |
| Pyloriset | Orion | ELISA | 15 | 1,807 | 90 (84–93) | 89 (79–98) | 0.84 (0.77–0.90) |
| Pyloriset latex | Orion | Agglutination | 12 | 1,418 | 86 (64–92) | 75 (66–91) | 0.73 (0.60–0.85) |
| Quickvue | Quidel | EIA | 3 | 456 | 92 | 88 | 0.78 (0.74–0.87) |
| Sia hp | Sigma | ELISA | 1 | 84 | 90 | 98 | 0.89 |
| SynElisa | Elias | ELISA | 2 | 196 | 86 | 81 | 0.79 |
| Trinity | Trinity Biotech | ELISA | 2 | 206 | 89 | 78 | 0.80 |
| Wampole | Carter-Wallace | ELISA | 1 | 57 | 91 | 73 | 0.80 |
Values are medians (25 and 75% quantiles).
FIG. 1.
Percent true-positive rates (TPR) and false-positive rates (FPR) for 177 tests.
Results for two tests were excluded from the regression analysis. Owing to their perfect diagnostic performance (the AURCs were 1) we could not calculate the SE of the AURC. According to the standard regression models, several clinical features and methodological aspects caused differences in diagnostic performance (Table 2). However, after correcting for the dependence between AURCs within the same study and the heterogeneity in the precision of the AURCs caused by different study sizes, only two aspects remained statistically significantly different. First, a major clinical feature of the study population that led to heterogeneity was the way in which the study population had been selected (Table 3). Second, because the investigated H. pylori serology kits were based on various antibody preparations, the diagnostic performances differed substantially. In the final weighted-random-effects regression model in which the features “consecutive yes/no” and “type of antibodies measured” were included, the estimated AURC for a nonconsecutive patient series was 0.053 (P = 0.01) higher than that for a consecutive patient series. The estimated AURC for kits that measured “IgA antibodies only” was 0.063 (P = 0.01) lower than that for kits that measured “IgG antibodies only,” while for kits that measured “IgA, IgG, and IgM simultaneously” it was 0.22 lower (P = <0.001). The P values for the overall F test in the multivariate weighted-random-effect regression analysis for the categories consecutive patients and antibodies were <0.001 and 0.013, respectively. The AURC for the serology kits that measured “IgA, IgG, and IgM antibodies” was 0.16 (P = 0.001) higher than that for the kits that measured “IgA antibodies alone.” After correcting for the way that the study population had been selected, an evaluation of only the serology kits that measured IgG antibodies with more than five test kits revealed that the diagnostic performance of the Helico-G serology kit was significantly lower (P = <0.001) than that of the Anti-Hp serology kit (Table 4). The overall F-test value for the serology kits with NDF equal to 8 and DDF equal to 47 was 4.07 (P = 0.001), and the overall F-test value for the consecutive category with NDF equal to 1 and DDF equal to 47 was 2.21 (P = 0.14).
TABLE 2.
Clinical features and methodological aspects of the selected studies associated with differences in test performance across studies
| Analysis | Standard regression
|
Random-effect regressiona
|
Weighted-random-effect regressiona
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| AICb | F(NDF,DDF)c | P value | AIC | F(NDF,DDF) | P value | AIC | F(NDF,DDF) | P value | |
| Manuscript | 121 | F(2,174) = 1.10 | 0.33 | 138 | F(2,96) = 0.44 | 0.64 | 144 | F(2,95) = 0.40 | 0.67 |
| Publication year | 118 | F(6,170) = 2.99 | <0.01 | 130 | F(6,95) = 0.77 | 0.59 | 135 | F(6,94) = 0.87 | 0.52 |
| Consecutive patients | 130 | F(1,175) = 15.9 | <0.01 | 143 | F(1,96) = 7.34 | <0.01 | 148 | F(1,95) = 7.36 | <0.01 |
| Patient population | 122 | F(2,174) = 2.17 | 0.12 | 138 | F(2,96) = 0.55 | 0.58 | 144 | F(2,95) = 0.55 | 0.58 |
| Nationality | 94 | F(25,151) = 1.02 | 0.44 | 111 | F(25,96) = 0.73 | 0.81 | 115 | F(25,95) = 0.70 | 0.84 |
| Reference standard | 106 | F(14,156) = 2.12 | 0.01 | 119 | F(14,93) = 1.23 | 0.27 | 124 | F(14,92) = 1.38 | 0.18 |
| Serology kit | 87 | F(35,141) = 1.24 | 0.19 | 97 | F(35,69) = 0.97 | 0.52 | 99 | F(35,68) = 0.96 | 0.55 |
| Kit scale | 123 | F(1,175) = 1.07 | 0.30 | 140 | F(1,95) = 1.01 | 0.31 | 146 | F(1,94) = 2.07 | 0.15 |
| Antibody | 135 | F(2,169) = 21.5 | <0.01 | 156 | F(2,90) = 30.4 | <0.01 | 161 | F(2,89) = 29.4 | <0.01 |
| Kit technique | 121 | F(4,172) = 2.30 | 0.06 | 136 | F(4,92) = 1.49 | 0.21 | 141 | F(4,91) = 1.65 | 0.17 |
| Blood or serum | 123 | F(1,175) = 0.23 | 0.63 | 140 | F(1,95) = 0.59 | 0.45 | 146 | F(1,94) = 1.55 | 0.22 |
Random effect for study population.
The higher the AIC, the better the model fit.
F(NDF,DDF), F test with degrees of freedom in the numerator (NDF) and denominator (DDF).
TABLE 3.
Multivariate weighted-random-effect regression analysis for the statistically significant sources of heterogeneitya
| Source of heterogeneity | Overall test
|
Test for each category
|
||||||
|---|---|---|---|---|---|---|---|---|
| F(NDF,DDF)b | P value | Category | No. of populations | Size of population (no. of subjects) | Regression coefficient | SE | P value | |
| Consecutive subjects | F(1,89) = 6.33 | 0.013 | Yes | 47 | 8,306 | Reference | ||
| No | 130 | 18,379 | 0.053 | 0.021 | 0.01 | |||
| Antibodiesc | F(2,89) = 28.71 | <0.001 | IgG | 151 | 22,497 | Reference | ||
| IgA | 7 | 1,272 | −0.220 | 0.030 | <0.01 | |||
| IgA, IgG, and IgM | 14 | 2,458 | −0.063 | 0.021 | 0.01 | |||
The AIC value was 161, and the intercept was 0.806 (AURC for tests with a consecutive patient series and IgG antibody testing [reference categories]).
See footnote c of Table 2.
Unknown for five tests.
TABLE 4.
Difference in estimated AURCs in at least six studies that used serology kits measuring IgG antibodies, using weighted-regression analysis with random effects for study population, corrected for the way that the patient population was selecteda
| Difference in AURCs (P value)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| HM-CAP | Malakit | Pylori-Stat | Premier | GAP | Pyloriset | Flexsure | Helico-G | |
| Anti-Hp | 0.011 (0.62) | 0.011 (0.61) | 0.012 (0.53) | 0.049 (0.08) | 0.057 (0.004) | 0.058 (0.01) | 0.063 (0.01) | 0.080 (<0.001)b |
| HM-CAP | <0.001 (0.98) | 0.001 (0.93) | 0.037 (0.16) | 0.046 (0.06) | 0.046 (0.05) | 0.052 (0.006) | 0.069 (0.005) | |
| Malakit | <0.001 (0.97) | 0.037 (0.24) | 0.046 (0.07) | 0.046 (0.07) | 0.051 (0.07) | 0.068 (0.008) | ||
| Pylori-Stat | 0.036 (0.16) | 0.044 (0.04) | 0.045 (0.03) | 0.051 (0.02) | 0.067 (0.002) | |||
| Premier | 0.007 (0.79) | 0.008 (0.78) | 0.014 (0.61) | 0.030 (0.28) | ||||
| GAP | <0.001 (0.99) | 0.006 (0.80) | 0.022 (0.26) | |||||
| Pyloriset | 0.006 (0.81) | 0.022 (0.31) | ||||||
| Flexsure | 0.016 (0.50) | |||||||
The AIC value was 110, and the overall F-test value for a serology kit with an NDF of 8 and a DDF of 47 was 4.07 (P = 0.001).
Statistically significant according to Bonferroni’s correction [α1 = (0.05/36) = 0.001).
IMPLEMENTATION OF DIAGNOSTIC TESTS
Before implementing new diagnostic tests in clinical practice, careful evaluations must be done. Three topics are of importance (83). First, the test must have been evaluated with the indicated study population, i.e., the population of patients suspected of having the disease in question. The relevant population in this case consisted of a consecutive series of patients with dyspeptic complaints referred for upper gastrointestinal endoscopy. However, most studies analyzed a highly selected sample of patients with dyspepsia referred for upper gastrointestinal endoscopy. For a highly selected sample, H. pylori serology has excellent diagnostic performance. If a population of consecutive patients is tested, the diagnostic performance decreases.
The second topic of importance is determination of the diagnostic performance. For diagnostic tests, sensitivity and specificity are the most commonly used measures of test performance. Sensitivity and specificity are important parameters for diagnostic purposes but not for evaluative purposes. First, the use of different cutoff points for test positivity leads to various sensitivities and specificities. Second, the distribution of the test results for H. pylori-positive and -negative patients can vary considerably among studies because of selection. To overcome these problems, the presentation of the entire range of sensitivities and specificities at various cutoff points by a ROC curve results in better comparability of diagnostic tests. However, ROC curves and/or test result distributions were very sparsely presented in the publications. Fortunately, it is possible to make a fairly accurate estimation of the underlying ROC curve if one sensitivity and one specificity are mentioned (42). We think that this method is more appropriate for evaluation of the diagnostic performance of tests than the summary ROC technique used by Loy et al. (48). First, by analyzing the AURCs, the different cutoff points used for the same kit with different study populations were of no importance. Second, to compare pairs of serology kits, Loy et al. (48) needed more studies that had used two kits. By our method we could compare kits without any restrictions. A statistical test could be used to evaluate whether one was better than another. If the hypothesis of “no kit effect” is rejected, a multiple-comparison method can be used to compare pairs of tests. By introducing a random effect for study population as an alternative for the paired t test used by Loy et al. (48), we allowed for dependence between kits in the same study. Third, the weighted-regression method took the various study population sizes into account and corrected for them. Small studies with low AURCs will have smaller weights than large studies with high AURCs. The summary ROC analysis used equal weights for the studies involved, while it ignored the different study sizes and AURCs. Fourth, it seems to us that our method is likely to be more efficient for the testing of kits and other covariables because it incorporated studies and all kits in one analysis.
One problem remains: we do not know whether the random-effect model assumptions are correct. It is not clear whether the correlation between two kits and the correlation between two other kits within the same study population are equal. Maybe different correlations between different pairs of kits are better descriptions of reality. Unfortunately, most kits were used in only a few studies, and the number of kits used within one study was too small to thoroughly test the model assumptions. We used the MIXED procedure from the SAS software for the analysis. This procedure did not converge when all clinical features were included in the models because of too few observations. An automatic subsets analysis is not part of this SAS procedure. Therefore, we had to perform a univariate analysis. We could only perform a multivariate weighted-regression analysis with correction for random effects, in which the statistically significant clinical features from the univariate analysis were simultaneously evaluated in order to correct for possible confounding. A necessary condition for confounding is that the confounding variable is related to the feature under study and to the outcome (AURC). If a clinical feature or methodological aspect is not significant in a univariate model, then it is very unlikely to be significant in a model in which more features are included. However, the differences found by our method were confirmed in the analysis of kits and studies that fulfilled the requirements of Loy et al. (48). In agreement with Loy et al. (48), the Anti-Hp serology kit performed better than the Helico-G serology kit. Furthermore, the Malakit serology kit also displayed a higher although not statistically significant AURC than the Helico-G serology kit.
Finally, the relation between a new test and current diagnostic tests needs to be established. Many methods for the diagnosis of H. pylori infection are available. Because there is no consensus about a reference standard, several methods were used to identify H. pylori infection. The definition of the reference standard used in the publications ranged from only one diagnostic method (histology, culture, or rapid urease testing) having to be positive to more methods having to be positive (culture, rapid urease testing, and the urea breath test). The selection of a test as a reference reflects the personal preference of the investigator, which might lead to bias. Furthermore, the sensitivity and specificity of biopsy specimen-based methods vary and are frequently about 90%. Therefore, it is inappropriate to use other imperfect diagnostic tests as reference methods to measure diagnostic performance. However, the diagnostic performance of H. pylori serology was not influenced by any of the 15 different reference standards used.
RECOMMENDATION
In contrast to the conclusion drawn by Loy et al. (48) the diagnostic performances of various serology kits differed substantially because commercially available serology kits were based on various antibody preparations and were used with different study populations. Our results showed that serology kits that measured IgA, IgG, and IgM simultaneously (Pyloriset latex, CFT H. pylori) or IgA alone (Pyloriset, GAP) for the detection of H. pylori antibodies in serum did not perform as well as those that measured only IgG antibodies. The overall performance of commercially available serology kits that measure IgG antibodies for the diagnosis of H. pylori infection showed that serology is an accurate means of diagnosing H. pylori infection in patients. Owing to the small differences in diagnostic performance between serology kits that measure IgG antibodies, other aspects, such as the price, ease of handling, or number of equivocal results, are becoming increasingly important when choosing a serology kit.
ACKNOWLEDGMENTS
We thank J. L. Severens and E. H. van de Lisdonk for comments that improved an earlier version of this paper.
Appendix
The regression equation used to model the heterogeneity between the studies by means of an ordinary least-squares regression analyses was as follows:
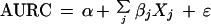 |
In this equation α is the intercept, βj, j = 1,...,k, is the regression coefficient, and Xj, j = 1,...,k, is the dummy variable indicating the category of the clinical feature. The term ɛ is the residual which is normally distributed with variance ςɛ2.
It is very likely that the AURCs for different serology kits are correlated when they are used within the same study population. By introducing a random effect for study population we could model dependency between kits within the same study population (24). The regression equation for the random effects model was as follows:
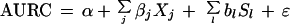 |
In this equation α, Xj, j = 1,...,k, and ɛ are as described above; βj, j = 1,...,k, is now called the fixed effect; and bl, l = 1,...,m, is the random effect which is independent and normally distributed with common variance ςb2. Sl, l = 1,...,m, is the dummy variable indicating the study population.
In order to correct for the heterogeneity in the precision of the AURCs caused by different study sizes, we also performed a weighted-regression analysis with weights proportional to 1/SE2. The SE was computed according to the expression given by Hanley and McNeil (36):
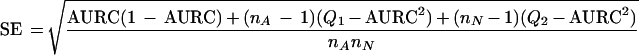 |
where nA is the number of abnormal individuals (H. pylori infected), and nN is the number of healthy individuals (H. pylori noninfected). The expression for Ql is given by Ql = AURC/(2 − AURC), and that for Q2 is given by Q2 = (2 × AURC2)/(1 + AURC). This formula was derived under the assumption that the ratings are on a scale that is sufficiently continuous not to produce “ties”. Although in our case we used dichotomous tests, we believed that the formula for SE would nevertheless be useful. We used the SE only for the weighting procedure and expected to obtain the same answer when the unknown “true” SE was proportional to the SE that we used.
REFERENCES
- 1.Alemohammad M M, Foley T J, Cohen H. Detection of immunoglobin G antibodies to Helicobacter pylori in urine by an enzyme immunoassay method. J Clin Microbiol. 1993;31:2174–2177. doi: 10.1128/jcm.31.8.2174-2177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allerberger F, Oberhuber G, Wrba F, Puspok A, Dejaco C, Dierich M P. Detection of Helicobacter pylori infection using single serum specimens: comparison of five commercial serological tests. Hepato-Gastroenterol. 1996;43:1656–1659. [PubMed] [Google Scholar]
- 3.Andersen L P, Wewer A V, Christiansen K M, Tvede M, Hansen J P, Henriksen F W, Krasnilikoff P A. The humoral immune response to Helicobacter pylori infection in children with recurrent abdominal pain. APMIS. 1994;102:457–464. [PubMed] [Google Scholar]
- 4.Anderson J C, Cheng E, Roeske M, Marchildon P, Peacock J, Shaw R S. Detection of serum antibodies to H. pylori by an immunochromatographic method. Am J Gastroenterol. 1997;92:1335–1339. [PubMed] [Google Scholar]
- 5.Antoine C, Lozniewski A, de Korwin J D, Conroy M C, Feldman L, Duprez A, Weber M. Etude comparative de quatre méthodes serologiques commercialisées pour le diagnostic de l’infection gastrique à Helicobacter pylori. Gastroenterol Clin Biol. 1995;19:182–188. [PubMed] [Google Scholar]
- 6.Best L M, Veldhuyzen van Zanten S J O, Bezanson G S, Haldane D J M, Malatjalian D A. Serological detection of Helicobacter pylori by a flow microsphere immunofluorescence assay. J Clin Microbiol. 1992;30:2311–2317. doi: 10.1128/jcm.30.9.2311-2317.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Best L M, Veldhuyzen van Zanten S J O, Sherman P M, Bezanson G S. Serological detection of Helicobacter pylori antibodies in children and their parents. J Clin Microbiol. 1994;32:1193–1196. doi: 10.1128/jcm.32.5.1193-1196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biemond I, Veenendaal R A, Lamers C B H W. United European Gastronenterology Week abstracts on disk. Amsterdam, The Netherlands: Excerpta Medica Medical Communications BV; 1997. Rapid diagnostic test for antibodies to Helicobacter pylori in comparison with the ELISA technique, abstr. 409. [Google Scholar]
- 9.Blecker U, Lanciers S, Hauser B, Vandenplas Y. Diagnosis of Helicobacter pylori in adults and children by using the Malakit Helicobacter pylori, a commercially available enzyme-linked immunosorbent assay. J Clin Microbiol. 1993;31:1770–1773. doi: 10.1128/jcm.31.7.1770-1773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodhidatta L, Hoge C W, Churnratanakul S, Nirdnoy W, Sampathanukiel P, Tungtaem C, Raktham S, Smith C D, Echeverrià P. Diagnosis of Helicobacter pylori infection in a developing country: comparison of two ELISAs and a seroprevalence study. J Infect Dis. 1993;168:1549–1553. doi: 10.1093/infdis/168.6.1549. [DOI] [PubMed] [Google Scholar]
- 11.Borody T J, Andrews P, Shortis N P. Evaluation of whole blood antibody kit to detect active Helicobacter pylori infection. Am J Gastroenterol. 1996;91:2509–2512. [PubMed] [Google Scholar]
- 12.Breslin P, Flynn S, Balbirnie E, O’Morain C. Digestive Disease Week abstracts on disk. Northfield, Minn: Marathon Multimedia; 1996. Validation of an “office” serological test for Helicobacter pylori. [Google Scholar]
- 13.Cammarota G, Fedeli G, Montalto M, et al. Proceedings of the EHPSG VIIth International Conference. 1994. The use of Helicobacter pylori serology in diagnosis and treatment monitoring. [Google Scholar]
- 14.Chen T S, Chang F Y, Lee S D. Serodiagnosis of Helicobacter pylori infection: comparison and correlation between enzyme-linked immunosorbent assay and rapid serological test results. J Clin Microbiol. 1997;35:184–186. doi: 10.1128/jcm.35.1.184-186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chey W D, Murthy U K, Linscheer W G, et al. Digestive Disease Week abstracts on disk. Northfield, Minn: Marathon Multimedia; 1996. A comparison between 4 different commercially available serology tests for Helicobacter pylori. [Google Scholar]
- 16.Chey W D, Murthy U, Barish C, et al. Digestive Disease Week abstracts on disk. Northfield, Minn: Marathon Multimedia; 1997. The Chemtrak Accumeter whole blood serology test for the detection of Helicobacter pylori infection. [Google Scholar]
- 17.Ching C K, Thompson S, Buxton C, Holgate C, Holmes G K T. Evaluation of a commercial enzyme-linked immunosorbent assay (ELISA) kit for serological diagnosis of Helicobacter pylori infection in a group of non-ulcer dyspepsia sufferers. Postgrad Med J. 1993;69:456–460. doi: 10.1136/pgmj.69.812.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cognein P, Costa A, Giacosa A. Serodiagnosis of Helicobacter pylori: evaluation of a rapid miniaturized immunochromatographic test. Eur J Cancer Prev. 1994;3:457–463. doi: 10.1097/00008469-199411000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree J E, Shallcross T M, Heatley R V, Wyatt J L. Evaluation of a commercial ELISA for serodiagnosis of Helicobacter pylori infection. J Clin Pathol. 1991;44:326–328. doi: 10.1136/jcp.44.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutler A F, Perry M. Digestive Disease Week abstracts on disk. Northfield, Minn: Marathon Multimedia; 1997. Comparison of commercial antibody tests to replace discontinued PyloriStat kit. [Google Scholar]
- 21.Debongnie J C, Delmee M, Pauwels S, Mainguet P. Campylobacter pylori gastritis: review of diagnostic methods. Acta Gastroenterol Belg. 1989;52:311–319. [PubMed] [Google Scholar]
- 22.de Boni M, De Bona M, Bellumat A, Barbazza R. United European Gastronenterology Week abstracts on disk. Amsterdam, The Netherlands: Excerpta Medica Medical Communications BV; 1995. Evaluation of a new rapid immunodiagnostic assay for H. pylori antibody detection: serological and histopathological correlations, abstr. 2112. [Google Scholar]
- 23.Denis P, de Koster E, Goossens H, et al. An evaluation of four kits for Helicobacter pylori serology. Gastroenterology. 1993;104:A70. [Google Scholar]
- 24.Diggle P J, Liang K Y, Zeger S L. Analysis of longitudinal data. Oxford, United Kingdom: Oxford Science Publications; 1996. [Google Scholar]
- 25.Edwards C N, Douglin C P, Prussia P R, Garriques S A, Levett P N. Epidemiology of Helicobacter pylori infection in Barbados. West Indian Med. 1997;46:3–7. [PubMed] [Google Scholar]
- 26.Enroth H, Rigo R, Hulten K, Engstrand L. Diagnostic accuracy of a rapid whole-blood test for detection of Helicobacter pylori. J Clin Microbiol. 1997;35:2695–2697. doi: 10.1128/jcm.35.10.2695-2697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabris P, Bozzola L, Benedetti P, Scagnelli M, Nicolin R, Manfrin V, Scarparo C, De Lalla F. H. pylori infection in HIV-positive patients. A serohistological study. Dig Dis Sci. 1997;42:289–292. doi: 10.1023/a:1018801532136. [DOI] [PubMed] [Google Scholar]
- 28.Fallone C A, Elizov M, Cleland P, et al. Detection of Helicobacter pylori infection by saliva IgG testing. Am J Gastroenterol. 1996;91:1145–1149. [PubMed] [Google Scholar]
- 29.Fedder A, Bartolone C, Harvey S, Mihalov M L, Anderson R, Goldstein J L. Digestive Disease Week abstracts on disk. Northfield, Minn: Marathon Multimedia; 1996. Flexsure Hp Fingerstick whole blood and serum qualitative testing for H. pylori antibodies are equally efficacious. [Google Scholar]
- 30.Figura N, Odera G, Verdiani S. Evaluation of a commercial ELISA kit for the serological diagnosis of Helicobacter pylori infection. Microbiologica. 1994;17:319–325. [PubMed] [Google Scholar]
- 31.Fraser A, Ali M R, Yeates N J, Haystead A. Diagnostic tests for Helicobacter pylori-can they help select patients for endoscopy. N Z Med J. 1996;109:95–98. [PubMed] [Google Scholar]
- 32.Goossens H, Glupczynski Y, Burette A, Van den Borre C, Butzler J. Evaluation of a commercially available second-generation immunoglobin G enzyme immunoassay for detection of Helicobacter pylori infection. J Clin Microbiol. 1992;30:176–180. doi: 10.1128/jcm.30.1.176-180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goossens H, Glupczynski Y, Burette A, Van den Borre C, DePrez C, Bodenmann J, Keller A, Butzler J P. Evaluation of commercially available complement fixation test for diagnosis of Helicobacter pylori infection and for follow-up after antimicrobial therapy. J Clin Microbiol. 1992;30:3230–3233. doi: 10.1128/jcm.30.12.3230-3233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gosciniak G, Matysiak-Budnik T, Przondo-Mordarska A. Evaluation of three serological tests for the detection of Helicobacter pylori-associated infection. Arch Immunol. 1993;41:315–319. [PubMed] [Google Scholar]
- 35.Granberg C, Mansikka A, Lehtonen O-P, Kujari H, Grönfors R, Nurmi H, Räihä J, Ståhlberg M J, Lein R. Diagnosis of Helicobacter pylori infection by using Pyloriset EIA-G and EIA-A for detection of serum immunoglobulin G (IgG) and IgA antibodies. J Clin Microbiol. 1993;31:1450–1453. doi: 10.1128/jcm.31.6.1450-1453.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanley J A, McNeil B J. The meaning and use of the AUC. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 37.Harries A D, Stewart M, Deegan K M, Mughogho G K, Wirima J J, Hommel M, Hart C A. H. pylori in Malawi, Central Africa. J Infect. 1992;24:269–276. doi: 10.1016/s0163-4453(05)80032-5. [DOI] [PubMed] [Google Scholar]
- 38.Hoek F J, Noach L A, Rauws E A J, Tytgat G N J. Evaluation of the performance of commercial test kits for detection of Helicobacter pylori antibodies in serum. J Clin Microbiol. 1992;30:1525–1528. doi: 10.1128/jcm.30.6.1525-1528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Italian Helicobacter pylori Study Group. Proceedings of the EHPSG VIIIth International Conference. 1995. 2579 Italian dyspeptic referred to 1st upper gastrointestinal endoscopy: a national multicenter study. [Google Scholar]
- 40.Jensen A K V, Andersen L P, Gaarslev K, Wachmann C H. Comparison of four second generation kits for detection of IgG antibodies against Helicobacter pylori in adults. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1993;280:221–226. doi: 10.1016/s0934-8840(11)80959-x. [DOI] [PubMed] [Google Scholar]
- 41.Jensen A K V, Andersen L P, Wachmann C H. Evaluation of eight commercial kits for Helicobacter pylori IgG antibody detection. APMIS. 1993;101:795–801. [PubMed] [Google Scholar]
- 42.Laheij R J F, Witteman E M, Bloemberg P, de Koning R W, Jansen J B M J, Verbeek A L M. Short-term follow-up by serology of patients given antibiotic treatment for H. pylori infection. J Clin Microbiol. 1998;36:1193–1196. doi: 10.1128/jcm.36.5.1193-1196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C, Ha T, Ferguson D A, Jr, Chi D S, Zhao R, Patel N R, Krishaswamy G, Thomas E. A newly developed PCR assay of H. pylori in gastric biopsy, salvia, and feces. Evidence of high prevalence of H. pylori in saliva supports oral transmission. Dig Dis Sci. 1996;41:2142–2149. doi: 10.1007/BF02071393. [DOI] [PubMed] [Google Scholar]
- 44.Lin S K, Lambert J R, Schembri M, et al. A comparison of diagnostic test to determine Helicobacter pylori infection. J Gastroenterol Hepatol. 1992;7:203–209. doi: 10.1111/j.1440-1746.1992.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 45.Loffeld R J L F, Vriese W T J, Stobberingh E E. Usefulness of several commercial enzyme-linked immunoassays for detection of Helicobacter pylori infection in clinical medicine. Eur J Gastroenterol Hepatol. 1993;5:333–337. [Google Scholar]
- 46.Lopez-Brea M, Martinez M J, Alarcon T, Sanz J C. Evaluation of a rapid dry latex aggutination test for serological diagnosis of Helicobacter pylori infection in children. Am J Gastroenterol. 1994;89:1341. . (Abstract.) [Google Scholar]
- 47.Lopez-Brea M, Martinez M J, Alarcon T, Sanz J C. Combined serological detection of specific IgG and IgA for the diagnosis of Helicobacter pylori infection in children. Am J Gastroenterol. 1994;89:1341. . (Absract.) [Google Scholar]
- 48.Loy C T, Irwig L M, Katelaris P H, Talley N J. Do commercial serology kits for Helicobacter pylori infection differ in accuracy? A meta-analysis. Am J Gastroenterol. 1996;91:1138–1144. [PubMed] [Google Scholar]
- 49.Lozniewski A, de Korwin J D, Conroy M C, Plenat F, Weber M. Evaluation of Pyloriset dry, a new rapid agglutination test for Helicobacter pylori antibody detection. J Clin Microbiol. 1996;34:1773–1775. doi: 10.1128/jcm.34.7.1773-1775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luzza F, Imeneo M, Maletta M, et al. United European Gastroenterology Week abstracts on disk. Amsterdam, The Netherlands: Excerpta Medica Medical Communications BV; 1997. Saliva IgG testing for diagnosis of Helicobacter pylori infection by means of a serological commercial kit, abstr. 414. [Google Scholar]
- 51.Madico G, Verastegui M. Serodiagnosis of Helicobacter pylori infection by enzyme-linked immunoelectrotransfer blot. J Diarrhoeal Dis Res. 1995;2:122–126. [PubMed] [Google Scholar]
- 52.Martin de Argila C, Boixeda D, Mir N, Valdezate S, Gisbert J P, de Rafael L, Canton R. United European Gastroenterology Week abstracts on disk. Amsterdam, The Netherlands: Excerpta Medica Medical Communications BV; 1996. Diagnostic value of a commercial IgA enzyme linked immunosorbent assay kit for H. pylori infection diagnosis. [Google Scholar]
- 53.Meijer B C, Thijs J C, Kleibeuker J H, van Zwet A A, Berrelkamp R J P. Evaluation of eight immunoassays for detection of immunoglobulin G against Helicobacter pylori. J Clin Microbiol. 1997;35:292–294. doi: 10.1128/jcm.35.1.292-294.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendall M A, Goggin P M, Marrero J M, et al. Role of Helicobacter pylori serology in screening prior to endoscopy. Eur J Gastroenterol Hepatol. 1992;4:713–717. [Google Scholar]
- 55.Meyer-Wyss B, Beglinger C, Baselgia L. Helicobacter pylori in healthy people. Gut. 1991;32:1429. doi: 10.1136/gut.32.11.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Midolo P D, Lambert J R, Russell E G, Lin S K. A practical single sample latex dry agglutination test for Helicobacter pylori antibody detection. J Clin Pathol. 1995;48:969–971. doi: 10.1136/jcp.48.10.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller S, Shama T K, Cutler A F. Digestive Disease Week abstracts on disk. Northfield, Minn: Marathon Multimedia; 1996. Prospective evaluation of test characteristics of a new method for detection of serum antibodies to Helicobacter pylori. [Google Scholar]
- 58.Moayyedi, P., A. M. Carter, R. M. Heppell, A. J. Catto, P. Grant, and A. T. R. Axon. 1995. Validation of a rapid whole blood test for the diagnosis of Helicobacter. Gut 36(Suppl. 1):A51.
- 59.Moayyedi P, Catto A, Carter A M, et al. United European Gastroenterology Week abstracts on disk. Amsterdam, The Netherlands: Excerpta Medica Medical Communications BV; 1996. Is Helicobacter pylori serology on plasma accurate. [Google Scholar]
- 60.Nair P, Watson B E, McNulty C A M, Uff J S, Valori R. United European Gastroenterology Week abstracts on disk. Amsterdam, The Netherlands: Excerpta Medica Medical Communications BV; 1995. A comparison of five commercial serological tests for Helicobacter pylori detection, abstr. 1370. [Google Scholar]
- 61.Nardone G, Coscione P, D’Armiento F P, Del Pezzo M, Pontillo M, Mossetti G, Lamberti C, Budillon G. Cirrhosis negatively affects the efficiency of serologic diagnosis of H. pylori infection. Ital J Gastroenterol. 1996;28:332–336. [PubMed] [Google Scholar]
- 62.Nilsson I, Ljungh A, Aleljung P, Wadstrom T. Immunoblot assay for serodiagnosis of Helicobacter pylori infections. J Clin Microbiol. 1997;35:427–432. doi: 10.1128/jcm.35.2.427-432.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peitz U, Labenz J, Tillenburg B, Bauman M, Borsch G. United European Gastroenterology Week abstracts on disk. Amsterdam, The Netherlands: Excerpta Medica Medical Communications BV; 1995. Validity of a new rapid whole blood test for Helicobacter pylori infection, abstr. 988. [Google Scholar]
- 64.Peitz U, Labenz J, Tillenburg B, Bauman M, Borsch G. Digestive Disease Week abstracts on disk. Northfield, Minn: Marathon Multimedia; 1996. Insufficient validity of a new rapid whole blood test for Helicobacter pylori infection. [Google Scholar]
- 65.Persico M, Suozzo R, De Seta M, Montella F, Torella R, Gentile S. Non-ulcer dyspepsia and H. pylori in type 2 diabetic patients: association with autonomic neuropathy. Diabetes Res Clin Pract. 1996;31:87–92. doi: 10.1016/0168-8227(96)01207-7. [DOI] [PubMed] [Google Scholar]
- 66.Plebani M, Basso D, Cassaro M, Brigato L, Scrigner M, Toma A, Di Mario F, Rugge M. Helicobacter pylori serology in patients with chronic gastritis. Am J Gastroenterol. 1996;91:954–958. [PubMed] [Google Scholar]
- 67.Pronovost A D, Rose S L, Pawlak J W, Robin H, Scheider R. Evaluation of a new immunodiagnostic assay for Helicobacter pylori antibody detection: correlation with histopathological and microbiological results. J Clin Microbiol. 1994;32:46–50. doi: 10.1128/jcm.32.1.46-50.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raymond J, Kalach N, Bergeret M, Barbet J P, Benhamou P H, Gendrel D, Dupont C. Evaluation of a serological test for diagnosis of Helicobacter pylori infection in children. Eur J Clin Microbiol Infect Dis. 1996;15:415–417. doi: 10.1007/BF01690102. [DOI] [PubMed] [Google Scholar]
- 69.Sadowski D, Cohen H, Laine L, et al. Digestive Disease Week abstracts on disk. Northfield, Minn: Marathon Multimedia; 1996. Evaluation of the flexsure Hp Fingerstick blood test for the detection of H. pylori infection. [Google Scholar]
- 70.SAS. SAS software, version 6.12. Cary, N.C: SAS Institute; 1996. [Google Scholar]
- 71.Schembri M A, Lin S K, Lambert J R. Comparison of commercial diagnostic tests for Helicobacter pylori antibodies. J Clin Microbiol. 1993;31:2621–2624. doi: 10.1128/jcm.31.10.2621-2624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma T K, Young E L, Miller S, Cutler A F. Evaluation of a rapid, new method for detecting serum IgG antibodies to H. pylori. Clin Chem. 1997;43:832–836. [PubMed] [Google Scholar]
- 73.Sim J G, Kim E C, Seo J K. The role of serology in the diagnosis of Helicobacter pylori infection in children. Clin Pediatr. 1995;34:458–462. doi: 10.1177/000992289503400901. [DOI] [PubMed] [Google Scholar]
- 74.Sternberg A, Coscas D, Wagner Y, Ausland L, Kaufstein M, Fireman Z. Comparison of various Helicobacter pylori detection methods: serology, histology and bacteriology. Isr J Med Sci. 1997;33:160–163. [PubMed] [Google Scholar]
- 75.Taha A S, Reid J, Boothmann P, Gemmell C G, Lee F D, Sturrock R D, Russell R I. Serological diagnosis of Helicobacter pylori—evaluation of four tests in the presence or absence of non-steroidal anti-inflammatory drugs. Gut. 1993;34:461–465. doi: 10.1136/gut.34.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Talley N J, Kost L, Haddad A, Zinsmeister A R. Comparison of commercial tests for detection of Helicobacter pylori antibodies. J Clin Microbiol. 1992;30:3146–3150. doi: 10.1128/jcm.30.12.3146-3150.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tham T C, McLaughlin N, Hughes D F, Ferguson M, Crosbie J J, Madden M, Namnyak S, O’Connor F A. Possible role of Helicobacter pylori serology in reducing endoscopy workload. Postgrad Med J. 1994;70:809–812. doi: 10.1136/pgmj.70.829.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trautmann M, Moldrzyk M, Vogt K, Korber J, Held T, Marre R. Use of a receiver operating characteristic in the evaluation of two commercial enzyme immunoassays for detection of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1994;13:812–819. doi: 10.1007/BF02111341. [DOI] [PubMed] [Google Scholar]
- 79.Tucci A, Donati M, Mazzoni C, Cevenini R, Sambri V, Varoli O, Bocus P, Ferrari A, Paparo G F, Caletti G. Value of serology (ELISA) for the diagnosis of Helicobacter pylori infection: evaluation in patients attending endoscopy and in those with fundic atrophic gastritis. Ital J Gastroenterol. 1996;28:371–376. [PubMed] [Google Scholar]
- 80.Uyub A M, Khairul A, Aiyar S. Reliability of two commercial serological kits for serodiagnosing Helicobacter pylori infection. South Asian J Trop Med. 1994;25:316–320. [PubMed] [Google Scholar]
- 81.van den Oever H L A, Loffeld R J L F, Stobberingh E E. Usefulness of a new serological test (Bio-Rad) to diagnose Helicobacter pylori-associated gastritis. J Clin Microbiol. 1991;29:283–286. doi: 10.1128/jcm.29.2.283-286.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vandenplas Y, Blecker U, Devreker T, Keppens E, Nijs J, Cadranel S, Pipeleers-Marichal M, Goosens A, Lauwers S. Contribution of the 13C-urea breath test to the detection of Helicobacter pylori gastritis in children. Pediatrics. 1992;90:608–611. [PubMed] [Google Scholar]
- 83.van der Schouw Y T, Verbeek A L M, Ruijs S H J. Guidelines for assessment of new diagnostic tests. Invest Radiol. 1995;30:334–340. doi: 10.1097/00004424-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 84.van der Schouw Y T, Straatman H, Verbeek A L M. ROC curves and the areas under them for dichotomized tests: emperical findings for logistically and normally distributed diagnostic test results. Med Decis Making. 1994;14:374–81. doi: 10.1177/0272989X9401400408. [DOI] [PubMed] [Google Scholar]
- 85.van de Wouw B A M, de Boer W A, Jansz A J, Roymans R T J M, Staals A P G. Comparison of three commercially available enzyme-linked immunosorbent assays and biopsy-dependent diagnosis for detecting Helicobacter pylori infection. J Clin Microbiol. 1996;34:94–97. doi: 10.1128/jcm.34.1.94-97.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van de Wouw B A M, de Boer W A, Jansz A R, Staals A P G, Roymans R T J M. Serodiagnosis of Helicobacter pylori infection: an evaluation of a commercially available ELISA-IgG. Neth J Med. 1995;47:272–277. doi: 10.1016/0300-2977(95)00104-2. [DOI] [PubMed] [Google Scholar]
- 87.Warren J R. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 88.Weiss J, Mecca J, da Silva E, Gassner D. Comparison of PCR and other diagnostic techniques for detection of Helicobacter pylori infection in dyspeptic patients. J Clin Microbiol. 1994;32:1663–1668. doi: 10.1128/jcm.32.7.1663-1668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Westblom T U, Madan E, Gudipati S, Midkiff B R, Czinn S. Diagnosis of Helicobacter pylori infection in adult and pediatric patients by using Pyloriset, a rapid latex agglutination test. J Clin Microbiol. 1992;30:96–98. doi: 10.1128/jcm.30.1.96-98.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Westblom T U, Lagging L M, Midkiff B R, Czinn S J. Evaluation of Quickvue, a rapid enzyme immunoassay test for the detection of serum antibodies to Helicobacter pylori. Diagn Microbiol Infect Dis. 1993;16:317–320. doi: 10.1016/0732-8893(93)90082-i. [DOI] [PubMed] [Google Scholar]
- 91.Wilcox M H, Dent T H S, Hunter J O, et al. Accuracy of serology for the diagnosis of Helicobacter pylori infection a comparison of eight kits. J Clin Pathol. 1996;49:373–376. doi: 10.1136/jcp.49.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]