Abstract
Lyme disease, a tick-borne multisystem disease, is caused by spirochete Borrelia burgdorferi (sensu lato). It is a common illness in temperate countries, especially the United States, but the incidence is increasing across continents due to increasing reforestation, travel and adventure tourism, increased intrusion in the vector habitat, and changing habitat of the vector. Transmission primarily occurs via bite of an infected tick (Ixodes spp.). The appearance of an erythema migrans rash following a tick bite is diagnostic of early Lyme disease even without laboratory evidence. Borrelia lymphocytoma and acrodermatitis chronica atrophicans along with multisystem involvement occur in late disseminated and chronic stages. A two-step serologic testing protocol using an enzyme-linked immunosorbent assay (ELISA) followed by confirmation of positive and equivocal results by Western immunoblot is recommended for the diagnosis. Transplacental transmission to infant occurs in the first trimester with possible congenital Lyme disease making treatment imperative during antenatal period. The treatment is most effective in the early stages of the disease, whereas rheumatological, neurological, or other late manifestations remain difficult to treat with antibiotics alone. Treatment with oral doxycycline is preferred for its additional activity against other tick-borne illnesses which may occur concurrently in 10%–15% of cases. New-generation cephalosporins and azithromycin are alternative options in patients with doxycycline contraindications. No vaccine is available and one episode of the disease will not confer life-long immunity; thus, preventive measures remain a priority. The concept of post–Lyme disease syndrome versus chronic Lyme disease remains contested for want of robust evidence favoring benefits of prolonged antibiotic therapy.
Keywords: Acrodermatitis chronica atrophicans, Borrelia, borrelia lymphocytoma, erythema migrans, Ixodes, Lyme borreliosis
Introduction
Lyme disease (syn: Lyme borreliosis), a vector-borne anthropozoonotic multisystem disease, occurs worldwide across continents. Although several genospecies of causative spirochete Borrelia burgdorferi (sensu lato) have been identified to be associated with human disease across and within the continents, Lyme disease is primarily caused by three genospecies comprising B.burgdorferi (sensu stricto) in North America, and B.afzelii and B.garinii in Asia and Europe.[1] The human transmission is primarily via an infected tick (Ixodes spp.) bite.[2] Table 1 depicts important historical milestones in Lyme disease.[3,4]
Table 1.
Important events in the history of Lyme disease
| Year | Historical events |
|---|---|
| 1883 | Alfred Buchwald, a German physician, described what is now termed as acrodermatitis chronica atrophicans. |
| 1912 | Arvid Afzelius, a Swedish dermatologist, described erythema chronicum migrans, now known as erythema migrans. |
| 1920s | Garin and Bujadoux described erythema migrans, meningoencephalitis, and painful sensory radiculitis in a patient following a tick bite and attributed to a spirochetal infection. |
| 1930s | Ixodes (deer tick) bite-associated neurological manifestations (meningoencephalitis) were recognized. |
| 1940s | Several cases of chronic lymphocytic meningitis and polyradiculoneuritis with or without erythematous skin lesions were described in United States by Bannwarth. B. burgdorferi was found in tick specimens collected on the eastern Long Island, New York (USA). |
| 1950s | Cutaneous manifestations of erythema migrans were described in the European literature. Wilhelm Burgdorfer confirmed Borrelia as the causative agent after isolating a borrelial organism in Ixodes ticks and patients with clinical Lyme disease. Development of antibody tests for the disease led to identification of different Borrelia strains. |
| 1970s | Cluster of juvenile arthritis cases occurred in the region around Lyme, Connecticut (USA). Epidemiological investigations by Allen Steere and others from Yale led to the discovery of the causative agent and its ecology, expanding geographic range and clinical manifestations. |
| 1983 | For the first time, seven cases of erythema migrans from a single center was reported in India. A few more isolated cases have been reported since then.* |
*No epidemiological surveys have been carried out in India and the disease largely remains underreported for lack of clinical suspicion perhaps
Epidemiology
Lyme disease is mainly endemic in temperate countries; more than 30,000 cases of Lyme disease are reported to the US Center for Disease Control and Prevention (CDC) each year.[5] Depending on the habitat of Ixodes ticks, it is also endemic in parts of North America, Europe, the Middle East, Australia, South East Asia, and the former Soviet Union.[6] However, it is being reported increasingly across continents, with relatively high Borrelia positive seroprevalence in central Europe and western and eastern Asia.[7] This increasing trend has been attributed to reforestation, increased deer and tick populations, and high tick exposure because of people moving into forests/deer habitats. The hikers, workers in animal parks, thickets, forests, woodland, groves or meadows, and travelers to endemic regions are at increased risk.[8] While large/white-tailed deer, small rodents, domestic cattle and few birds are natural hosts for B.burgdorferi, the disease is mostly incidental in humans. The principal vector in northeast and central USA and Canada is Ixodes scapularis, I. ricinus in Europe, I. pacificus on the Pacific coast, and I. persulcatus in Asia.[2] In India, cases of Lyme disease including those with Lyme disease–associated monoarthritis and neuroretinitis have been reported from Himachal Pradesh, Haryana, Bihar, Uttarakhand, Uttar Pradesh, Maharashtra, and parts of south India.[4,9,10,11,12,13,14,15,16,17] Praharaj et al.[18] also reported Borrelia positive seroprevalence in 13% of the population in Indian northeastern states. The presence of Ixodes ticks too has been recorded in the Himalayan region.[19]
Pathogenesis
B. burgdorferi colonizes and infects Ixodes ticks that transmit infection to hosts including humans. The life cycle of ticks usually spans two years. The ova laid by adult ticks during spring hatch in summer and the emerged larvae molt as nymphs, which, after molting as adults by autumn, feed on the (infected) host animal. Although adult ticks carry the infection two times more than the nymph, 90% of the human disease is transmitted by nymphs as they are far more abundant and there is generally more human outdoor activity during summers when feeding season of nymphs/adult female ticks is at its peak. Small size makes detection and removal of nymphs less likely giving them time to attach fully for long enough; 36–48 hours for nymphs and 48–72 hours for adults are needed to transmit the infection.[20]
Generally, disease pathogenesis depends on the adaptation of B. burgdorferi to the environmental and host conditions by changing the composition of spirochete membrane proteins or different gene expression and the host immune response. The spirochetes in the mid-gut of an infected tick are transferred to the salivary glands and introduced into the skin following a bite. Thereafter, the spirochetes may be eliminated by the host’s immunity or remain viable and localized to the skin, causing characteristic erythema migrans (EM) lesion(s). The spirochetes will disseminate hematogenously or via lymphatics within one to two weeks (average 3–30 days), remain latent, or progress to disseminated disease in about 50% of untreated cases.[21] The spirochetes show an apparent affinity for skin, joints, nervous system, heart, and eyes, but have also been demonstrated in the lymph nodes, bone marrow, liver, spleen, testes, and placenta during early hematogenous spread. In about 10% of patients with EM without systemic symptoms, B. burgdorferi or its DNA can be detected in the bloodstream or cerebrospinal fluid (CSF) indicating spirochetemia and early CNS penetration.[21] However, only a few genotypes of B. burgdorferi are responsible for a majority of the cases with disseminated/chronic disease.[22] Clinically, B. afzelii is mostly associated with acrodermatitis chronica atrophicans (ACA) and B. garinii with neurological symptoms, whereas the arthritis and neuroborreliosis are mainly linked to B. burgdorferi sensu stricto.[23] Furthermore, antibodies against spirochetal protein membrane epitopes possibly cross-react with neural and connective tissues leading to an autoimmune inflammatory reaction and clinical symptoms ascribed to molecular mimicry.
Clinical Manifestations
As occurrence of Lyme disease depends on the presence of the vector, the infective organism, and environment conducing for its transmission, no age, gender, or race is spared. However, boys are affected more often than girls and women are affected more than men among adults aged above 30 years.[24,25] The age distribution is usually bimodal and the first peak occurs at 5–14 years and second peak happens at 45–54 years of age owing to increased outdoor activity and environmental exposure. After infecting, B. burgdorferi produces three distinct clinical stages of Lyme disease (vide infra), which have overlapping manifestations [Table 2].
Table 2.
Lyme disease: classical clinical features and treatment options
| Clinical Stage | Incubation Period | Clinical Types | Cutaneous Features and Histology | Systemic Features | Treatment in Adults (for Children) |
|---|---|---|---|---|---|
| Stage 1 | 1-30 days (average 1-3 weeks) | Early localized or primary Lyme disease | • Erythema migrans (EM) • Histology is characterized by perivascular infiltrate of lymphohistiocytes with sparse interspersed plasma cells in the dermis. Neutrophils, macrophages, dermal edema, and panniculitis are infrequent. |
Minor constitutional symptoms of fever, chills, fatigue, malaise, transient oligoarthralgia, myalgia, headache, neck stiffness. | Doxycycline, orally 100mg twice daily (4mg/kg/d divided in two doses in children aged ≥8 years, maximum dose - as in adults) or Cefuroxime axetil, orally 500mg twice daily (30mg/kg/d in two divided doses, maximum 500mg/dose) or Azithromycin, orally 500mg once daily (10mg/kg/d, maximum 500mg/day) or Amoxicillin, orally 500mg three times daily (50mg/kg/d in three divided doses) |
| Stage 2 | Weeks to months (average 3-20 weeks) | Early disseminated Lyme disease | • Multiple EM, secondary EM • Borrelia lymphocytoma (BL) Histology of BL shows dense lymphocytic infiltrate with nodules and germinal centers in the dermis and subcutis |
Multisystem involvement occurs as myalgia, migratory polyarticular enthesitis, arthralgia, lymphocytic meningitis (waxing and waning mild-to-severe headache, neck stiffness, photophobia), facial nerve palsy, encephalopathy (mild confusional state, disturbances in sleep, mood, memory and concentration, depression, irritability, and personality disorder), cardiac symptoms (chest pain, palpitations, dizziness, syncope, dyspnea, atrioventricular block) | Ceftriaxone, intravenous 2g/day (50-75mg/kg/day, maximum 2g/day) or Cefotaxime, intravenous 2g every 8 hourly (150-200mg/kg/day, maximum 6g/day, in three to four divided doses or Doxycycline, orally 200-400mg in two divided doses daily (4-8mg/kg/day, maximum 100-200mg/day, in two divided doses in children aged ≥8 years) |
| Stage 3 | Months to years | Chronic or late Lyme disease | • Acrodermatitis chronica atrophicans. • Histologically, a dense patchy periappendageal and perivascular inflammatory infiltrate of lymphohistiocytes and plasma cells seen in the early inflammatory stage, while epidermal atrophy with normal subepidermal zone, lymphocytic and plasma cell-rich infiltrate in the sclerotic upper dermis with dilated blood vessels will feature in the late stage. |
Joints symptoms: Mono or oligoarthritis without neurological involvement. Knee arthritis is the hallmark of chronic Lyme disease. | Oral antibiotics as used for erythema migrans. Symptomatic treatment. |
| Neuropsychiatric symptoms: Encephalomyelitis, subacute encephalopathy, myelitis (in 50% of cases), Bannwarth syndrome, progressive spastic paraparesis or quadriparesis, fibromyalgia, peripheral neuropathy, cognitive and affective disorders Ocular symptoms: Follicular conjunctivitis, photophobia, macular edema, uveitis/iridocyclitis, retinal vasculitis, choroiditis, retinal vein occlusion, white dot syndrome, stromal keratitis, episcleritis Cardiac symptoms: Conduction defects, arrhythmias, myocarditis, pericarditis, postural orthostatic tachycardia syndrome | Intravenous antibiotics as used for early disseminated disease. Symptomatic treatment. |
Remarks: (1) Treatment is recommended for 2-3 weeks. (2) For adults, prophylactic doxycycline in a single 200mg dose (4mg/kg of body weight or maximum up to 200mg in children aged ≥8 years) is recommended orally within 72 hours of tick removal when it had remained attached for 36 hours or longer, or when risk of infection is high as in endemic areas. (3) Doxycycline remains contraindicated in patients with doxycycline hypersensitivity, children aged <8 years, and pregnant and lactating women. (4) Bannwarth syndrome is characterized by late axonal neuropathy and radiculopathy with pleocytosis
Primary or early localized disease (stage 1) has pronounced cutaneous symptoms (dermatoborreliosis) manifesting as characteristic EM rash at the site of tick bite over lower extremities or upper trunk in 80% of cases.[26] It generally happens within 30 days of the tick bite and 7–14 days after tick removal. Classically, EM is centrifugally expanding, uniformly erythematous, or showing various hues of erythema, oval to circular patch of 5–70 cm (median 16 cm) in diameter and is occasionally associated with pruritus, burning, or pain. A prodrome of mild nonspecific constitutional symptoms within days to one week of infection is common in 50% of cases. EM, usually a targetoid rash [Figure 1] with a tick-bite mark or occasionally with a tick still attached in the center in about 19% of cases, may attain a vesiculobullous, erythematous papular, erysipeloid, purpuric or lymphangitic form.[4,20,27,28] Smaller and multiple lesions may occur in up to 10%–20% of cases.[29,30] This morphological heterogeneity is attributed to variation in genospecies and host response to skin-bound spirochete as nearly all such patients are seropositive irrespective of the disease duration.[31] The lesion resolves spontaneously within weeks to months but may recur in 20% of cases. Approximately 75% of patients develop further symptoms of early disseminated or chronic disseminated disease when untreated.[3]
Figure 1.
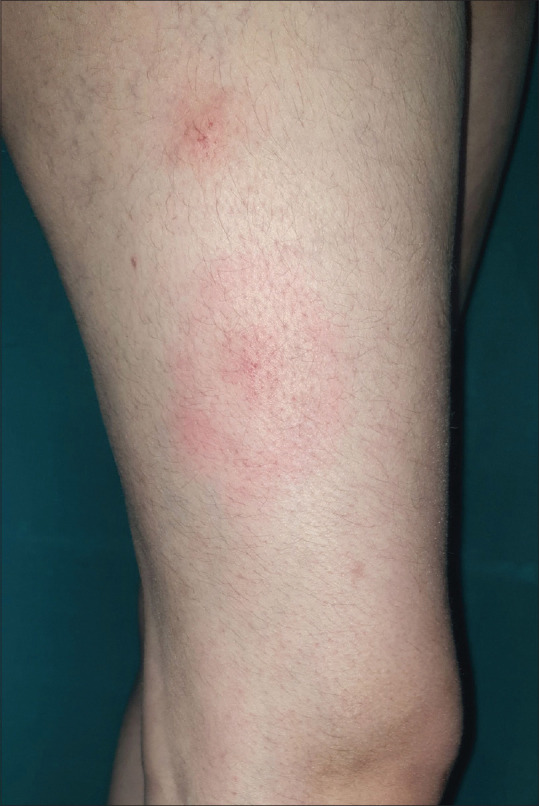
Erythema migrans over the thigh in a 36-year-old woman
The early disseminated disease (stage 2) usually develops 3–10 weeks after the tick bite wherein musculoskeletal and neurological symptoms predominate more compared to dermatological or cardiac symptoms.[24,32] Secondary EM lesions distant from tick bite over face and extremities occur in 17%–57% of patients indicating hematogenous/lymphatic dissemination.[27] Secondary EM lesions are light-colored, usually of the same size/shape, multiple, come in one to two crops, and have less edema, central clearing, and local symptoms compared to primary EM. Presence of secondary EM without obvious primary EM reflects poor host response but these patients are usually seropositive.[27,33] Low grade fever and regional lymphadenopathy are usual but few patients may develop isolated EM only.[21]
Borrelia lymphocytoma typically occurs 30–45 days after the tick bite, and can occur earlier than or as late as six months during its clinical course, but is uncommon in early disseminated disease. It presents over the ear lobe of children as a solitary bluish-red nodule/plaque sized one to few centimeters [Figure 2], at the areola of nipple (may be slightly painful) in adults, and rarely involves the scrotum, nose, and extremities.[27]
Figure 2.
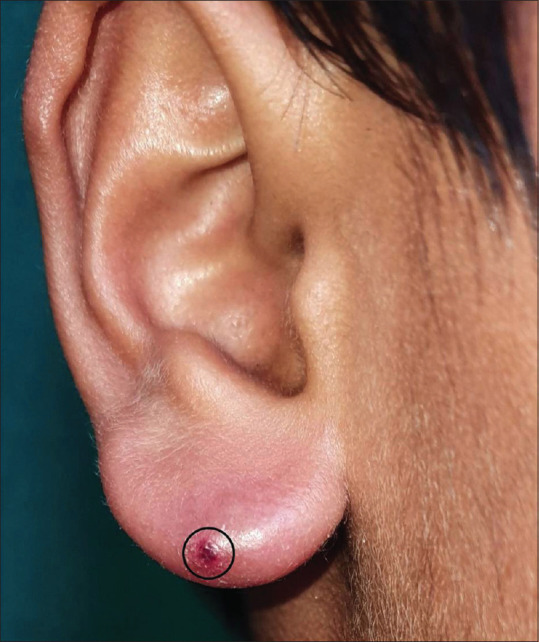
Borrelia lymphocytoma cutis involving the ear lobe in a 12-year-old boy (encircled area is post biopsy)
Migratory polyarticular enthesitis evolves in one to two days into a monoarticular disease involving the knee, ankle, or wrist joints lasting for about a week with propensity to recur every two to three months when untreated but ultimately resolving over a period of 10 years. Neurological involvement occurs in 5%–20% of patients with early Lyme disease and cranial neuropathy, facial nerve palsy in particular (in about 3% of patients and especially in children with meningeal symptoms), is the most common manifestation.[3,34] The symptoms of meningitis usually occur 2–10 weeks after the infection. Ocular symptoms such as diplopia, when present, are usually due to cranial neuropathy, while keratitis and iritis will cause blurred vision and eye pain. Borrelial encephalopathy commonly manifests after months to years of infection.
Chronic or late Lyme disease (stage 3) manifests several months or years after initial infection or a period of latency. It is characterized by persistence of nonspecific symptoms and signs with or without clinical/laboratory evidence [Table 3].[20,35,36] ACA, rheumatological, and neurological manifestations are the hallmark of this stage.
Table 3.
Categories of chronic Lyme disease
| Category | Description |
|---|---|
| 1 | Patients have nonspecific signs and symptoms suggestive of Lyme disease but no objective clinical or laboratory evidence of infection. |
| 2 | Patients may have a history of tick exposure with symptoms suggestive of Lyme disease but have an alternative identifiable illness to explain said symptoms. |
| 3 | Patients have serological evidence suggestive of Lyme disease but do not have clinical symptoms consistent with Lyme disease. |
| 4 | Also referred as post-Lyme disease syndrome, such patients have clinical and laboratory evidence of past Lyme disease but have persistent symptoms of fatigue, pain, and neurocognitive impairment for longer than six months despite appropriate antibiotic therapy. |
ACA, the characteristic cutaneous feature of this stage, occurs months to years after the tick bite. Affecting mostly the elderly, it reflects persistent infection and is imputed to T cell–mediated immune reaction with restricted cytokine expression and down-regulation of MHC class II molecules on Langerhans cells.[27] Unlike EM, it tends to involve bony prominences and cool distal extremities, especially elbows, forearms, dorsal surfaces of hands, knees, legs, and feet [Figures 3 and 4]. Minimally symptomatic, ill-defined bluish-red erythematous patches of initial inflammatory phase tend to spread peripherally [Figure 5a] and evolve into well-defined plaques, rarely with papules, nodules or edema associated with inflammation and discoloration; severe atrophy ensues later.[33] Fibrotic nodules adjoining joints, mainly around the knees and elbows, develop concurrently or prior to ACA[Figure 5b]. Lymphadenopathy and peripheral neuropathy can occur. Spontaneous healing is not a feature of ACA, and atrophic phase over the years evolves into dry, translucent, alopecic, and papery thin patches coalescing into large areas of atrophic hyperesthetic skin with visible superficial veins. The atrophy may extend to the underlying structures and minor trauma will cause slow-to-heal ulcerations, while development of squamous cell carcinoma or sarcoma in lesions is reported rarely.[37]
Figure 3.
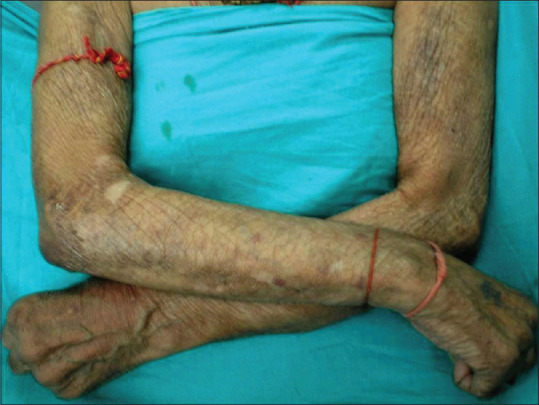
Acrodermatitis chronica atrophicans in a 60-year-old man involving cool dorsal surfaces of the upper extremities
Figure 4.
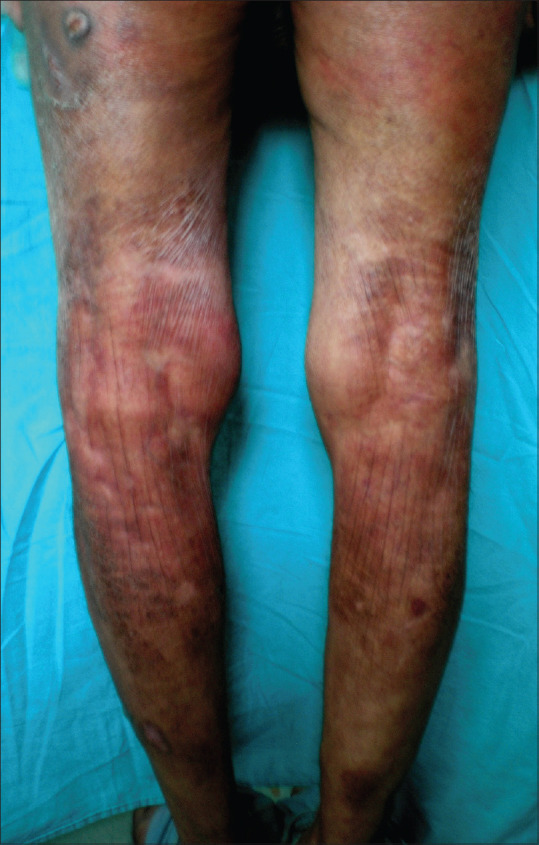
Characteristic papery thin patches coalescing into large areas of finely wrinkled atrophic skin bereft of hair in acrodermatitis chronica atrophicans involving the lower limbs
Figure 5.
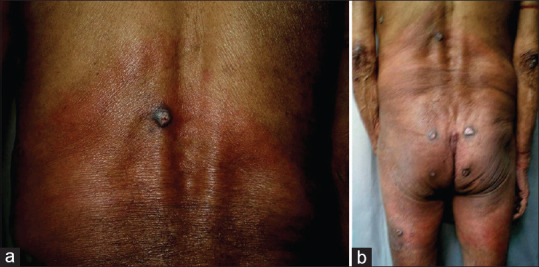
(a) Large, ill-defined, bluish-red erythematous patch with tendency to spread peripherally over the lower trunk and (b) back of upper thighs. Note concurrently occurring fibrotic papulonodules over the patch and near the joints, mainly around the elbows and sacroiliac joints
Chronic arthritis involving large joints, primarily the knee (in 90% patients) and hip joints, develops about six months after the infection in about 60% of untreated patients. Massive inflammatory synovial proliferation in the knee is a feature of post-infectious Lyme arthritis while autoimmune arthritis will manifest as rheumatoid arthritis, psoriatic arthritis, or peripheral spondyloarthropathy.[32,38]
Neurological involvement (Lyme neuroborreliosis) of both the central and the peripheral nervous systems can occur in up to 15% of patients of chronic stage.[20] Borrelial encephalomyelitis is an uncommon severe syndrome wherein relapsing and remitting symptoms of hemiparesis, ataxia, seizures, bladder dysfunction, hearing loss, and cognitive impairment remain gradually progressive with partial improvement after the episode. Most of these patients do not remember of EM but other manifestations such as aseptic meningitis, unilateral or rarely bilateral cranial neuropathy (particularly facial nerve palsy), mononeuritis multiplex, ataxia, peripheral neuropathy (paresthesias, dysesthesias), or neuropsychiatric dysfunction may coexist or might have occurred in the past.[20] The patients with abnormal host response may develop autoimmune chronic idiopathic demyelinating polyneuropathy and post–Lyme disease syndrome [category 4 in Table 3].[38] It was proposed that prolonged antibiotic therapy for months to years may be required for these patents to eradicate latent intracellular infection associated with antibiotic-refractory arthritis.[36] However, the concept of post–Lyme disease syndrome itself remains contested, as prolonged antibiotic therapy showed no benefit in randomized clinical trials.[39,40,41] Instead, these continued symptoms have been ascribed to an autoimmune response between Lyme disease and certain HLA haplotypes, and prolonged antibiotic therapy is not recommended.[35,36,42,43,44,45,46]
Lyme Disease in Pregnant Women and Children
Transplacental transmission of Borrelia to the fetus starts in the first month unlike Treponema pallidum where transplacental transmission happens in the second trimester.[2,47] Microbiologically confirmed case of a congenital B. garinii infection with multiple EM at birth and rapid recovery after antibiotic therapy in a child born to a mother with EM during pregnancy has been reported.[48] Borrelia was also isolated from blood in seven antenatal women with EM in another study, and early treatment with intravenous ceftriaxone led to an uneventful gestational outcome reflecting that treatment of at-risk pregnant women will be prophylactic for the newborns.[49]
Children having non-specific fever, malaise, or fatigue without characteristic features of early, disseminated, or late disease probably do not have Lyme disease but follow-up is imperative. However, the diagnosis of Lyme disease must be suspected in children living in endemic regions or who are potentially exposed to tick bites, are seropositive, or have EM involving the face frequently with conjunctivitis and/or photophobia, and/or compatible symptomatology including migratory myo-arthralgia (especially involving temporomandibular joint), arthritis, or carditis. Though extremely rare, ACA has been reported in children too.[50]
Diagnosis of Lyme Disease
The diagnosis of Lyme disease can be missed, especially in non-endemic regions, as pathognomic skin lesions can mimic several other dermatoses [Table 4].[27,35,51,52,53] For not-so-pathognomic histology [Table 2], a skin biopsy is rarely indicated for diagnosis but will exclude other differentials. Since laboratory culture of B. burgdorferi is difficult, confirming its presence in an affected organ remains uncertain. Thus, the diagnosis of Lyme disease is usually from history of tick bite or visit to endemic area, compatible manifestations, and positive serology. Since only 25%–30% of patients of early Lyme disease can recall the tick bite, an epidemiological context remains crucial for diagnosis. Erythrocyte sedimentation rate (ESR) is usually elevated, whereas leukocyte counts and complement (C3, C4) levels are normal or slightly elevated. The serum transaminases may be elevated while rheumatoid factor and ANA tests are negative. Electrocardiogram, echocardiogram, CSF analysis, and imaging studies are prerequisites when indicated. When pathognomic skin lesions are subtle or absent, presence of cranial neuritis, lymphocytic meningitis, radicular pain with dermatomal sensory-motor neuropathy, carditis, or joint swelling will be indicative and needs positive serology/immunoblot test for confirmation.[54] The diagnosis of Lyme neuroborreliosis is definite in the presence of compatible neurological symptoms, lymphocytic pleocytosis, and intrathecal antibodies against B. burgdorferi.[55]
Table 4.
Common differentials of cutaneous lesions of Lyme disease
| Cutaneous lesions | Common differentials | Remarks |
|---|---|---|
| Erythema migrans (EM) | Tinea corporis, | EM lesions most often occur over sites (back, abdomen, axilla, popliteal fossa) that are usually not involved by these mimickers. |
| Erythema multiforme, | EM lesion has rapid and prolonged expansion. | |
| Mycosis fungoides, | Spirochetes can be demonstrated with Warthin- | |
| Erythema gyratum repens, | Starry stain histology sections in 50%-65% of primary EM biopsies. | |
| Erythema annulare centrifugum, | ||
| Granuloma annulare, | ||
| Erysipelas, | ||
| Urticaria (sometimes), | ||
| Nummular eczema, | ||
| Fixed drug eruptions. | ||
| Borrelia lymphocytoma (BL) | Sarcoidosis, | BL lesions usually resolve after about three weeks of treatment with antibiotics unlike other mimickers. |
| Granuloma faciale, | BL lesions show characteristic histological features and organisms in Warthin-Starry-stained sections. | |
| Polymorphous light eruptions, | Cutaneous marginal zone lymphoma has CD20, CD22, CD79a and | |
| Chilblain lupus, | BCL-2 positivity and high κ/λ light chain ratio. | |
| Leukemic infiltrate, | ||
| Cutaneous marginal zone lymphoma. | ||
| Acrodermatitis chronica atrophicans (ACA) | Aging skin, | The diagnosis is mainly from compatible histopathology. |
| Venous or arterial insufficiency of legs resemble ACA over legs, | B .burgdorferi can be cultured from lesions even up to 10 years after its onset. | |
| Parry-Romberg syndrome when ACA involves face, | ||
| Localized scleroderma, | ||
| Lichen sclerosis et atrophicus, | ||
| Eczema, | ||
| Xerosis, | ||
| Skin atrophy secondary to other inflammatory dermatoses healing with atrophy (e.g., lepromatous leprosy) |
Remarks: The ever-expanding spectrum of Lyme disease manifestations involving the skin, joints, heart, eye, and nervous system vary from region to region; however, cutaneous lesions remain consistent and the most classic
Microscopy and Laboratory Culture
Direct visualization of the spirochete may be possible in Warthin–Starry-stained tissue sections or under focus floating microscopy and electron microscopy but low Borrelia load remains a limitation. Although the culture of B. burgdorferi from clinical samples of skin biopsy, blood, or CSF on modified Kelly–Pettenkofer, Barbour–Stoenner–Kelly II (BSK II) or BSK-H medium is the gold standard to diagnose active infection, it is not practiced routinely for being time consuming, technically demanding, and less sensitive.[56,57,58,59,60]
Serodiagnosis and Molecular Diagnostic Techniques
Laboratory evidence by serology for specific antibodies is from a two-test procedure comprising screening by enzyme-linked immunosorbent assay (ELISA), enzyme immunoassay (EIA), enzyme-linked fluorescence assay (ELFA), chemiluminescence immunoassay (CLIA), or fluoro-immunoassay (FIA) and confirmation of positive or equivocal results by Western immunoblot test.[2] The IgM antibody to B. burgdorferi can be detected two to four weeks after appearance of skin lesion(s) while IgG will rise in four to six weeks after the infection.[27] The levels peak up to six months and remain elevated for months to years. The diagnostic sensitivity of serological tests varies greatly depending on clinical manifestations, and an average sensitivity of 50% for EM, 77% for neuroborreliosis, and 97% for ACA has been reported.[52] False-negative results are common in early stage while false positive results can be from mononucleosis, syphilis, or autoimmune disorders. Measurement of IgG to a peptide from the sixth invariant region (C6) of the variable major protein-like sequence-expressed (VlsE) B. burgdorferi lipoprotein is more sensitive in patients with EM; it also effectively differentiates southern tick-associated illness from patients getting infected in Europe.[56] However, its utility remains limited in routine office practice.
In late Lyme arthritis or neuroborreliosis, PCR testing is highly sensitive in synovial fluid or CSF, respectively, whereas testing the CSF samples for intrathecal antibody production is preferred for selected cases.[35,61,62,63] However, the sensitivity of PCR to detect B. burgdorferi nucleic acids is highly variable and depends on factors such as the test material (skin biopsy, blood, CSF, synovial fluid), DNA extraction protocols, PCR targets, and the methodology used (nested PCR, real-time PCR, PCR followed by hybridization or digital PCR).[2] Low spirochete concentration and lack of standardization also remain as its major limitations.[64,65 66] Urine antigen test is generally not recommended because of high false-positivity.[67,68]
In view of limitations of diagnostic techniques, a case of Lyme disease is deemed confirmed for standardized surveillance when there is (a) presence of EM with known tick exposure or laboratory evidence of infection and; (b) late manifestations of the disease with laboratory evidence with or without history of tick exposure.[27] However, patients living in endemic regions, having possible EM, and recent tick exposure should be treated even without laboratory evidence to prevent long-term complications.[69]
Treatment
The Infectious Diseases Society of America, American College of Rheumatology, and American Academy of Neurology have issued joint consensus guidelines for the prevention, diagnosis, and treatment of Lyme disease [Table 2].[70] The removal of ticks with fine-tipped forceps close to the skin without compressing its body within 24 hours of attachment will prevent disease progression. Treatment is most effective in early stages, whereas rheumatological, neurological, or other late manifestations, being unresponsive to antibiotics alone, remain difficult to treat. The treatment is mainly directed towards clinical manifestations and oral regimens are recommended for early localized disease. Patients with refractory arthritis, symptomatic neurological or cardiac disease need treatment with intravenous regimens. Oral doxycycline is preferred for prophylaxis and treatment for its additional efficacy against other tick-borne diseases (babesiosis, human granulocytic anaplasmosis) seen concurrently in 10%–15% of patients.[35,71] Cephalosporins and azithromycin/erythromycin are other alternatives when doxycycline is contraindicated. However, amoxicillin showed no significant effect on development of EM or seroconversion in a randomized controlled trial and is not recommended for post-exposure prophylaxis or doxycycline contraindications.[35,72] As Jarisch Herxheimer reaction following treatment remains possible, the patient needs to be kept under observation.
Prevention
Routine chemoprophylaxis after a tick bite is not recommended and prevention remains an essential first-step management, especially for at-risk individuals. Educating patients about preventive measures, minimizing tick bite risk, early consultation after tick exposure/clinical manifestations, and significance of early treatment and treatment adherence will benefit in the long term. Patients should be counseled about protracted clinical course in the absence of adequate treatment, about one episode of EM not conferring life-long immunity, and about avoiding places with high deer/tick burden with priority.[68,72,73,74] Avoiding tick habitats, wearing light-colored protective clothes, use of tick repellants (diethyltoluamide), inspecting clothing/pets for ticks within 36 hours, and bathing within 2 hours after outdoor activities can reduce the risk of acquiring disease in endemic areas.[74] Fencing to prevent deer/animal intrusion, cleaning barns for leaf/wood litter, and spraying acaricides (carbaryl, deltamethrin) will reduce the population of ticks.
A vaccine (LYMErix) composed of antibodies directed against recombinant outer-surface lipoprotein A (OspA) of B. burgdorferi with reported efficacy of 70%–80% after three injections was approved by the US Food and Drug Administration (USFDA) for use in individuals aged 16 years and above, but it is no longer manufactured/available.[72,75]
Lyme Disease and Morphea, Scleroderma and Other Dermatoses
B. burgdorferi, with its ability to adhere and penetrate fibroblasts, has been implicated in the etiopathogenesis of morphea or localized scleroderma and sclerotic lesions observed in about 10% of patients with ACA. Coexistence or clinicopathological similarities between ACA and sclerotic lesions, detection of B. burgdorferi, its DNA or antibodies to it in few patients with morphea or early lichen sclerosus et atrophicus (LSA), and response to antimicrobials in such cases favored its pathogenic role.[76] However, absence of B. burgdorferi in 7 of 10 patients with morphea and in 37% of 60 cases of LSA in controlled studies remains irreconcilable.[76,77] Similarly, the pathogenic relationship between B. burgdorferi and atrophoderma of Pasini and Pierini, Parry–Romberg syndrome, cutaneous B-cell lymphoma, and mycosis fungoides needs more robust evidence,[78,79,80] whereas its association with eosinophilic fasciitis, benign lymphocytic infiltration (Jessner-Kanof), granuloma annulare, erythema multiforme, urticaria, urticarial vasculitis, Gianotti–Crosti syndrome, and panniculitis perhaps remains fortuitous at best.
Comments
Lyme disease is being recognized increasingly beyond its endemic regions. When treated early with appropriate antibiotic regimens, the prognosis for these patients is generally good but recurrent infection remains possible. However, an early diagnosis remains challenging in the absence of an epidemiological context and classic clinical features or affordable investigations of high diagnostic sensitivity. The new diagnostic techniques such as TickPlex assay, T cell response tests (lymphocyte transformation test), enzyme-linked immunospot (ELISpot) assay, Luminex-based techniques for the simultaneous detection of plasmid contents of different B. burgdorferi strains, and immuno-PCR (iPCR) assay have limited application in clinical practice as they are technically demanding and not cost-effective.[2,81,82,83,84,85] Metabolic profiling and the measurement of interferon (IFN)-g after incubating blood with Borrelia antigens even after antibiotic treatment are other potentially useful diagnostic approaches.[86,87] However, development of affordable easy-to-use cutting edge diagnostic tools with shorter turnaround time remains highly desirable. There also remains a scope for development of an affordable, safe, and effective vaccine against Borrelia antigen(s) and therapeutic modalities that can cure the disease in a shorter period, treat disseminated forms effectively, and prevent long-term complications. Increased public awareness and public health surveillance for the disease are some urgent needs, and making it a notifiable disease in India will perhaps be a step in the right direction.
Statement of ethics
No ethical approval was required as it was not an experimental study involving human subjects or animals. Informed consent was obtained from patients/guardians for publication of clinical images with the understanding that names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Feder HM., Jr Lyme disease in children. Infect Dis Clin North Am. 2008;22:315–26. doi: 10.1016/j.idc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Trevisan G, Bonin S, Ruscio M. A practical approach to the diagnosis of Lyme Borreliosis: From clinical heterogeneity to laboratory methods. Front Med. 2020;7:265. doi: 10.3389/fmed.2020.00265. doi: 10.3389/fmed. 2020.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyerhoff JO, Steele RW, Zaidman GW, Diamond HS. Lyme disease. [[Last accessed on 2022 Mar 27]]. Available from: https://emedicine.medscape.com/article/330178 .
- 4.Sharma RC, Sharma NL. Erythema chronic migrans in Simla, Himachal Pradesh. Indian J Dermatol Venerel Leprol. 1983;49:18–83. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Lyme disease: Data and Surveillance. CDC. [[Last accessed on 2022 Mar 27]]. Available from: http://www.cdc.gov/lyme/stats/index.html?s_cid=cs_281 .
- 6.Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone star tick-vectored Lyme-like illness. Infect Dis Clin North Am. 2008;22:361–76. doi: 10.1016/j.idc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y, Zhou G, Cao W, Xy X, Zhang Y, Ji Z, et al. Global seroprevalence and sociodemographic characteristics of Borrelia burgdorferi sensu lato in human populations: A systematic review and meta analysis. BMJ Global Health. 2022;7:e007744. doi: 10.1136/bmjgh-2021-007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: A diagnostic dilemma. Indian J Dermatol. 2014;59:595–7. doi: 10.4103/0019-5154.143530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Guleria S, Sharma R, Sharma A. Lyme disease: A case report with typical and atypical lesions. Indian Dermatol Online J. 2017;8:124–7. doi: 10.4103/2229-5178.202271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patial RK, Kashyap S, Bansal SK, Sood A. Lyme disease in a Shimla boy. J Assoc Physicians India. 1990;38:503–4. [PubMed] [Google Scholar]
- 11.Jairath V, Sehrawat M, Jindal N, Jain VK, Aggarwal P. Lyme disease in Haryana, India. Indian J Dermatol Venereol Leprol. 2014;80:320–3. doi: 10.4103/0378-6323.136894. [DOI] [PubMed] [Google Scholar]
- 12.Gupta N, Chaudhry R, Valappil VE, Soneja M, Ray A, Kumar U, et al. Lyme arthritis: A prospective study from India. J Family Med Prim Care. 2019;8:4046–7. doi: 10.4103/jfmpc.jfmpc_859_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachan S, Chaudhry R, Pathania S, Suvirya S, Verma P, Reddy HD, et al. Coexistence of primary erythema migrans and erythema multiforme in early Lyme disease. Indian J Dermatol Venereol Leprol. 2022;88:396–8. doi: 10.25259/IJDVL_53_2021. [DOI] [PubMed] [Google Scholar]
- 14.Sinha P, Oberoi B, Sirohi YS, Sood A, Bhattacharjee A. A case report of early disseminated Lyme disease. Neurol India. 2020;68:16–8. doi: 10.4103/0028-3886.293476. [DOI] [PubMed] [Google Scholar]
- 15.Handa R, Wali JP, Singh S, Aggarwal P. A prospective study of Lyme arthritis in north India. Indian J Med Res. 1999;110:107–9. [PubMed] [Google Scholar]
- 16.Babu K, Murthy PR. Neuroretinitis as a manifestation of Lyme disease in South India: A case report. Ocul Immunol Inflamm. 2010;18:97–8. doi: 10.3109/09273940903359733. [DOI] [PubMed] [Google Scholar]
- 17.Rajeev KR. Lyme disease outbreak in Wayanad. The Times of India. 2013. [[Last accessed on 2022 Mar 27]]. Available from: https://timesofindia.indiatimes.com/city/kozhikode/lyme-disease-outbreak-in-wayanad/articleshow/18758675.cms .
- 18.Praharaj AK, Jetley S, Kalghatgi AT. Seroprevalence of Borrelia burgdorferi in north eastern India. Med J Armed Forces India. 2008;64:26–8. doi: 10.1016/S0377-1237(08)80140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geevarghese G, Fernandes S, Kulkarni SM. A check list of Indian ticks (Acari: Ixodoidea) Indian J Animal Sci. 1997;67:17–25. [Google Scholar]
- 20.Wright WF, Riedel DJ, Talwani R, Gilliam BL. Diagnosis and management of Lyme disease. A Fam Physician. 2012;85:1086–93. [PubMed] [Google Scholar]
- 21.Wormser GP, McKenna D, Carlin J, Nadelman RB, Cavaliere LF, Holmgren D, et al. Brief communication: Hematogenous dissemination in early Lyme disease. Ann Intern Med. 2005;142:751–5. doi: 10.7326/0003-4819-142-9-200505030-00011. [DOI] [PubMed] [Google Scholar]
- 22.Wormser GP, Brisson D, Liveris D, Hanincová K, Sandigursky S, Nowakowski J, et al. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis. 2008;198:1358–64. doi: 10.1086/592279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strnad M, Honig V, Ruzek D, Grubhoffer L, Rego ROM. Europe wide meta-analysis of Borrelia burgdorferisensu lato prevalence in questing Ixodes ricinus ticks. Appl Environ Microbiol. 2017;83:e00609–17. doi: 10.1128/AEM.00609-17. doi: 10.1128/AEM.00609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease--United States, 1992-2006. MMWR Surveill Summ. 2008;57:1–9. [PubMed] [Google Scholar]
- 25.Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2015;29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 26.DePietropaolo DL, Powers JH, Gill JM, Foy AJ. Diagnosis of Lyme disease. Am Fam Physician. 2005;72:297–304. (Corrections in Am Fam Physician 2006; 73: 776) [PubMed] [Google Scholar]
- 27.Vasudevan B, Chattrjee M. Lyme borreliosis and skin. Indian J Dermatol. 2013;58:167–74. doi: 10.4103/0019-5154.110822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doughty H, O’Hern K, Barton DT, Carter JB. Vesiculbullous Lyme disease: A case series. JAAD Case Rep. 2022;24:56–8. doi: 10.1016/j.jdcr.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;297:2617–27. doi: 10.1001/jama.297.23.2617. [DOI] [PubMed] [Google Scholar]
- 30.Aucott J, Morrison C, Munoz B, Rowe PC, Schwarzwalder A, West SK. Diagnostic challenges of early Lyme disease: Lessons from a community case series. BMC Infect Dis. 2009;9:79. doi: 10.1186/1471-2334-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wormser GP, Nowakowski J, Nadelman RB, Visintainer P, Levin A, Aguero-Rosenfeld ME. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519–22. doi: 10.1128/CVI.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams D, Fullerton K, Jajosky R, Sharp P, Onweh D, Schley A, et al. Summary of notifiable infectious diseases and conditions - United States, MMWR Morb Mortal Wkly Rep. 2013;2015;62:1–122. doi: 10.15585/mmwr.mm6253a1. doi: 10.15585/mmwr.mm6253a1. [DOI] [PubMed] [Google Scholar]
- 33.Stanek G, Strle F. Lyme disease: European perspective. Infect Dis Clin North Am. 2008;22:327–39. doi: 10.1016/j.idc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Nigrovic LE, Thompson AD, Fine AM, Kimia A. Clinical predictors of Lyme disease among children with a peripheral facial palsy at an emergency department in a Lyme disease-endemic area. Pediatrics. 2008;122:e1080–5. doi: 10.1542/peds.2008-1273. [DOI] [PubMed] [Google Scholar]
- 35.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. (Corrections in Clin Infect Dis 2007; 45: 941) [DOI] [PubMed] [Google Scholar]
- 36.Marques A. Chronic Lyme disease: A review. Infect Dis Clin North Am. 2008;22:341–60. doi: 10.1016/j.idc.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leverkus M, Finner AM, Pokrywka A, Franke I, Gollnick H. Metastatic squamous cell carcinoma of the ankle in long-standing untreated acrodermatitis chronica atrophicans. Dermatology. 2008;217:215–8. doi: 10.1159/000142946. [DOI] [PubMed] [Google Scholar]
- 38.Steere AC. Post treatment Lyme disease syndromes: Distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 2020;130:2148–51. doi: 10.1172/JCI138062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
- 40.Krupp LB, Hyman LG, Grimson R, Coyle PK, Melville P, Ahnn S, et al. Study and treatment of post Lyme disease (STOP-LD): A randomized double masked clinical trial. Neurology. 2003;60:1923–30. doi: 10.1212/01.wnl.0000071227.23769.9e. [DOI] [PubMed] [Google Scholar]
- 41.Fallon BA, Keilp JG, Corbera KM, Petkova E, Britton CB, Dwyer E, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70:992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d. [DOI] [PubMed] [Google Scholar]
- 42.Marques AR. Lyme disease: A review. Curr Allergy Asthma Rep. 2010;10:13–20. doi: 10.1007/s11882-009-0077-3. [DOI] [PubMed] [Google Scholar]
- 43.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 44.American Academy of Pediatrics. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove, Ill: American Academy of Pediatrics; 2009. [[Last accessed on 2022 Mar 27]]. Available from: https://portaldeboaspraticas.iff.fiocruz.br/wp-content/uploads/2019/04/RB2009.pdf . [Google Scholar]
- 45.Halperin JJ, Shapiro ED, Logigian EL, Belman AL, Dotevall L, Wormser GP, et al. Practice parameter: Treatment of nervous system Lyme disease (an evidence-based review): Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2007;69:91–102. doi: 10.1212/01.wnl.0000265517.66976.28. (Correction in Neurology 2008: 70; 1223) [DOI] [PubMed] [Google Scholar]
- 46.Appropriateness of parenteral antibiotic treatment for patients with presumed Lyme disease. A joint statement of the American College of Rheumatology and the Council of the Infectious Diseases Society of America. Ann Intern Med. 1993;119:518. [PubMed] [Google Scholar]
- 47.Waddell LA, Greig J, Lindsay LR, Hinckley AF, Ogden NH. A systematic review on the impact of gestational Lyme disease in humans on the fetus and newborn. PLoS One. 2018;13:e0207067. doi: 10.1371/journal.pone.0207067. doi: 10.1371/journal.pone. 0207067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trevisan G, Stinco G, Cinco M. Neonatal skin lesions due to a spirochetal infection: A case of congenital Lyme borreliosis? Int J Dermatol. 1997;36:677–80. doi: 10.1046/j.1365-4362.1997.00217.x. [DOI] [PubMed] [Google Scholar]
- 49.Maraspin V, Ruzic-Sabljic E, Pleterski-Rigler D, Strle F. Pregnant women with erythema migrans and isolation of borreliae from blood: Course and outcome after treatment with ceftriaxone. Diagn Microbiol Infect Dis. 2011;71:446–8. doi: 10.1016/j.diagmicrobio.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Menni S, Pistritto G, Piccinno R, Trevisan G. Acrodermatitis chronica atrophicans in an Italian child. Acta Derm Venereol. 1996;76:243. doi: 10.2340/0001555576243243. doi: 10.2340/0001555576243243. [DOI] [PubMed] [Google Scholar]
- 51.James AM, Liveris D, Wormser GP, Schwartz I, Montecalvo MA, Johnson BJ. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J Infect Dis. 2001;183:1810–4. doi: 10.1086/320721. [DOI] [PubMed] [Google Scholar]
- 52.Leeflang MM, Ang CW, Berkhout J, Bijlmer HA, Van Bortel W, Brandenburg AH, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: A systematic review and meta-analysis. BMC Infect Dis. 2016;16:140. doi: 10.1186/s12879-016-1468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selva R, Violetti SA, Delfino C, Grandi V, Cicchelli S, Tomasini C, et al. A literature revision in primary cutaneous B-cell lymphoma. Indian J Dermatol. 2017;62:146–57. doi: 10.4103/ijd.IJD_74_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. National notifiable diseases surveillance system. In: Lyme Disease (Borrelia Burgdorferi) 2011 Case Definition. Atlanta, GA: Centers for Disease Control and Prevention; 2017. Available from: https://wwwn.cdc.gov/nndss/conditions/lyme-disease/case-definition/2017/ [Google Scholar]
- 55.Mygland A, Ljostad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I. European federation of neurological societies: EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol. 2010;17:8–16. doi: 10.1111/j.1468-1331.2009.02862.x. [DOI] [PubMed] [Google Scholar]
- 56.Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for lyme disease. Clin Infect Dis. 2008;472:188–95. doi: 10.1086/589242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cerar T, Ruzic-Sabljic E, Glinsek U, Zore A, Strle F. Comparison of PCR methods and culture for the detection of Borrelia spp. in patients with erythema migrans. Clin Microbiol Infect. 2008;14:653–8. doi: 10.1111/j.1469-0691.2008.02013.x. [DOI] [PubMed] [Google Scholar]
- 59.Veinovic G, Cerar T, Strle F, Ruzic-Sabljic E. Influence of MKP medium stored for prolonged periods on growth and morphology of Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. APMIS. 2014;122:230–5. doi: 10.1111/apm.12129. [DOI] [PubMed] [Google Scholar]
- 60.Wilske B, Fingerle V, Schulte-Spechtel U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol Med Microbiol. 2007;49:13–21. doi: 10.1111/j.1574-695X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 61.Coulter P, Lema C, Flayhart D, Linhardt AS, Aucott JN, Auwaerter PG, et al. Two-year evaluation of Borrelia burgdorferi culture and supplemental tests for definitive diagnosis of Lyme disease. J Clin Microbiol. 2005;43:5080–4. doi: 10.1128/JCM.43.10.5080-5084.2005. (Correction in: J Clin Microbiol 2007;45:277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dumler JS. Molecular diagnosis of Lyme disease: Review and meta analysis. Mol Diagn. 2001;6:1–11. doi: 10.1054/modi.2001.21898. [DOI] [PubMed] [Google Scholar]
- 63.Babady NE, Sloan LM, Vetter EA, Patel R, Binnicker MJ. Percent positive rate of Lyme real-time polymerase chain reaction in blood, cerebrospinal fluid, synovial fluid, and tissue. Diagn Microbiol Infect Dis. 2008;62:464–6. doi: 10.1016/j.diagmicrobio.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Ružić-Sabljić E, Cerar T. Progress in the molecular diagnosis of Lyme disease. Expert Rev Mol Diagn. 2017;17:19–30. doi: 10.1080/14737159.2016.1246959. [DOI] [PubMed] [Google Scholar]
- 65.Bonin S. Diagnostic tools for Borrelia assessment in humans. Open Dermatol J. 2016;10(Suppl 1):62–9. [Google Scholar]
- 66.van Dam AP. Molecular diagnosis of Borrelia bacteria for the diagnosis of Lyme disease. Expert Opin Med Diagn. 2011;5:135–49. doi: 10.1517/17530059.2011.555396. [DOI] [PubMed] [Google Scholar]
- 67.Klempner MS, Schmid CH, Hu L, Steere AC, Johnson G, McCloud B, et al. Intra laboratory reliability of serologic and urine testing for Lyme disease. Am J Med. 2001;110:217–9. doi: 10.1016/s0002-9343(00)00701-4. [DOI] [PubMed] [Google Scholar]
- 68.Bratton RL, Whiteside JW, Hovan MJ, Engle RL, Edwards FD. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566–71. doi: 10.4065/83.5.566. [DOI] [PubMed] [Google Scholar]
- 69.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 70.Lantos PM, Rumbaugh J, Bockenstedt LK, Falck-Ytter YT, Maria E Aguero-Rosenfeld ME, Auwaerter PG, et al. Clinical practice guidelines by the Infectious Diseases Society of America, American Academy of Neurology, and American College of Rheumatology: 2020 Guidelines for the prevention, diagnosis, and treatment of Lyme disease. Neurology. 2021;96:262–73. doi: 10.1212/WNL.0000000000011151. [DOI] [PubMed] [Google Scholar]
- 71.Centers for Disease Control and Prevention. Lyme disease: Tick removal. [[Last accessed on 2022 Mar 27]]. Available from: http://www.cdc.gov/lyme/removal/index.html .
- 72.Clark RP, Hu LT. Prevention of Lyme disease and other tick-borne infections. Infect Dis Clin North Am. 2008;22:381–96. doi: 10.1016/j.idc.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murray TS, Shapiro ED. Lyme disease. Clin Lab Med. 2010;30:311–28. doi: 10.1016/j.cll.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Connally NP, Durante AJ, Yousey-Hindes KM, Meek JI, Nelson RS, Heimer R. Peridomestic Lyme disease prevention: Results of a population-based case-control study. Am J Prev Med. 2009;37:201–6. doi: 10.1016/j.amepre.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention. Lyme disease vaccine. CDC. December 21. 2018. [[Last accessed on 2022 Mar 27]]. Available from: https://www.cdc.gov/lyme/prev/vaccine.html .
- 76.Ozkan S, Atabey N, Fetil E, Erkizan V, Günes AT. Evidence for Borrelia burgdorferi in morphea and lichen sclerosus. Int J Dermatol. 2000;39:278–83. doi: 10.1046/j.1365-4362.2000.00912.x. [DOI] [PubMed] [Google Scholar]
- 77.Eisendle K, Grabner T, Kutzner H, Zelger B. Possible role of Borrelia burgdorferi sensu lato infection in lichen sclerosus. Arch Dermatol. 2008;144:591–8. doi: 10.1001/archderm.144.5.591. [DOI] [PubMed] [Google Scholar]
- 78.Goodlad JR, Davidson MM, Hollowood K, Batstone P, Ho-Yen DO. Borrelia burgdorferi-associated cutaneous marginal zone lymphoma: A clinicopathological study of two cases illustrating the temporal progression of B. burgdorferi-associated B-cell proliferation in the skin. Histopathology. 2000;37:501–8. doi: 10.1046/j.1365-2559.2000.01003.x. [DOI] [PubMed] [Google Scholar]
- 79.Tothova SM, Bonin S, Trevisan G, Stanta G. Mycosis fungoides: Is it a Borrelia burgdorferi-associated disease? Br J Cancer. 2006;94:879–83. doi: 10.1038/sj.bjc.6602997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stinco G, Trevisan G, Martina Patriarca M, Ruscio M, Di Meo N, Patrone P. Acrodermatitis chronica atrophicans of the face: A case report and a brief review of the literature. Acta Dermatovenerol Croat. 2014;22:205–8. [PubMed] [Google Scholar]
- 81.Norris SJ, Howell JK, Odeh EA, Lin T, Gao L, Edmondson DG. High-throughput plasmid content analysis of Borrelia burgdorferi B31 by using Luminex multiplex technology. Appl Environ Microbiol. 2011;77:1483–92. doi: 10.1128/AEM.01877-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jahfari S, Sarksyan DS, Kolyasnikova NM, Hovius JW, Sprong H, Platonov AE. Evaluation of a serological test for the diagnosis of Borrelia miyamotoi disease in Europe. J Microbiol Methods. 2017;136:11–6. doi: 10.1016/j.mimet.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 83.Wang HY, Wu SQ, Jiang L, Xiao RH, Li T, Mei L, et al. Establishment and optimization of a liquid bead array for the simultaneous detection of ten insect-borne pathogens. Parasit Vectors. 2018;11:442. doi: 10.1186/s13071-018-2996-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Theel ES. The past, present, and (possible) future of serologic testing for Lyme disease. J Clin Microbiol. 2016;54:1191–6. doi: 10.1128/JCM.03394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Halpern MD, Jain S, Jewett MW. Enhanced detection of host response antibodies to Borrelia burgdorferi using immuno-PCR. Clin Vaccine Immunol. 2013;20:350–7. doi: 10.1128/CVI.00630-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molins CR, Ashton LV, Wormser GP, Hess AM, Delorey MJ, Mahapatra S, et al. Development of a metabolic bio signature for detection of early Lyme disease. Clin Infect Dis. 2015;60:1767–75. doi: 10.1093/cid/civ185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Callister SM, Jobe DA, Stuparic-Stancic A, Miyamasu M, Boyle J, Dattwyler RJ, et al. Detection of IFN-gamma secretion by T cells collected before and after successful treatment of early Lyme disease. Clin Infect Dis. 2016;62:1235–41. doi: 10.1093/cid/ciw112. [DOI] [PMC free article] [PubMed] [Google Scholar]


