Abstract
Vagus nerve stimulation (VNS) is used to deliver electric current to stimulate the vagus nerve. The aim of this study is to carry out a systematic review and meta-analysis to determine its effects on motor function in patients with stroke. PubMED, Embase, Web of Science (WoS), and Scopus were searched. Data on time since stroke, and mean scores and standard deviation on outcomes such as level of impairment and motor function were extracted. The results showed that invasive (MD 2.66, 95% CI 1.19–4.13, P = 0.0004) and non-invasive (MD 24.16, 95% CI 23.56–24.75, P = 0.00001) VNS are superior at improving level of motor impairment than the control post intervention and at follow-up respectively. Similarly, VNS improved motor function post intervention (MD 0.28, 95% CI 0.15–0.41, P < 0.0001); and there was no significant difference in adverse events between invasive VNS and control (OR 2.15, 95% CI 0.97–4.74, P = 0.06), and between non-invasive VNS and control (OR 4.54, 95% CI 0.48–42.97, P = 0.19). VNS can be used to improve motor function in patients with stroke.
Subject terms: Neuroscience, Neurology
Introduction
Stroke is a neurological disease caused by impairment in the supply of blood to the brain due to critical stenosis or occlusion and/ or rupture of the blood vessels supplying the brain1,2. Consequently, the survival of the cells is put in danger, and as such they may get damaged or die, and subsequently injure or lead to the death of the neighboring healthy neuronal cells3. Damage or death of these cells will lead to the impairment in functions of the brain, and subsequently disability in carrying out activities of daily living (ADL)4–6.
Currently, there are about 101 million people with stroke globally7. By 2050, the expected yearly incidence of stroke is 200 million8. Out of this number, many will survive the stroke and will eventually live with long-term disabilities especially in carrying out ADL9–11. In addition, although, growing evidence supports the importance of rehabilitation intervention after stroke, strategies to reduce the risk of long-term post-stroke disability beyond a year remain unclear12. This may be partly because of the severity of their impairment, as there are not many rehabilitation techniques that are used for severe impairment in motor function following stroke13. Therefore, strategies that will help enhance cortical reorganization by directly targeting the neuromodulatory systems such as the vagus nerve stimulation (VNS) are needed14.
Vagus nerve stimulation (VNS) is a technique used to deliver electric current to stimulate the vagus nerve15,16. The vagus nerve extends from the brainstem down to the colon, and in doing so, it traverses many structures that are vital to human body functions17,18. In addition, the nerve serves both motor and sensory functions in both the afferent and efferent regards17,19. The afferent function is sub-served by the afferent fibers arising from the nodose ganglion and projecting largely to the nucleus of the solitary tract (NTS)20,21. Projection of these afferent fibers to the NTS particularly helps to rapidly activate the cholinergic and the noradrenergic systems, which regulate various aspects of brain function, including sensory, motor and cognitive functions, and learning and memory22–27.
Vagus nerve stimulation (VNS) was initially started as an invasive technique where an implantable device was used to stimulate the vagus nerve; but later advances partly due to concerns about the safety of the invasive method, and the transcutaneous accessibility of the superficial branch of the vagus nerve, led to the use of a non-invasive method, where electrical current is delivered transcutaneously14,16,28,29. Stimulation of the vagus nerve either invasively or non-invasively, is presumed to help induce a brain environment that might increase the potential for experience-dependent plasticity15. However, for any rehabilitation technique to widely be accepted, what it is, for whom it is suitable, how and why it is used, and its safety need to be clearly delineated. The aim of this study is to carry out a systematic review and meta-analysis to determine the effects and adverse events of VNS; and the relationships in the reported effects and adverse events between groups in the included studies. This will help clinicians to make the most appropriate clinical decision as per as VNS is concerned. Secondly, to the best of our knowledge, this is the first study as well as a systemic review and meta-analysis to statistically determine the relationships in the reported effects and adverse events between groups in the included studies following the use of VNS in patients with stroke.
Materials and methods
This study was registered in PROSPERO (registration number, CRD42022380312), and it was carried out using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline.
Eligibility criteria
Studies eligible for inclusion in this study were randomized controlled trials (RCT) that compared the use of VNS (invasive or non-invasive) with a sham VNS and/or a control intervention for the rehabilitation of upper limb function in patients with stroke. Moreover, the participants included in the studies must be 18 years old or above.
Searching the literature
Four databases, PubMED, Embase, Web of Science (WoS), and Scopus were searched from their inceptions to December, 2022 using the key words, stroke AND vagus nerve stimulation OR auricular vagus nerve stimulation. However, the search strategies used were adapted based on the specific requirements of each database. Appendix I provides the details of the search strategy used in most of the databases. Moreover, additional search was carried out manually in the reference lists of the included studies and previous reviews on the subject matter.
One of the researchers, (AA) carried out the search independently. However, the search was verified by the two other researchers (TWLW and SSMN).
Study selection and data extraction
Rayyan software was used to select eligible studies for inclusion30. The selection was performed independently by two of the researchers (AA and TWLW). These two researchers excluded some ineligible studies based on the information in their titles and abstracts. For the remaining studies, the ineligible ones were only excluded after their full texts were read by the researchers. However, for some of the studies that the two researchers could not agree on their eligibility for inclusion, the other researcher (SSMN) was consulted for discussions on how to arrive at a consensus.
In addition, data on characteristics of the study participants such as the mean age, time since stroke, type of stroke, side affected, the protocols of the VNS and the control groups including the intensity of the interventions, the outcomes assessed such as motor function, level of motor impairment and, ADL, quality of life and their mean scores post intervention and at follow-up, and the sample sizes in the studies were extracted by one of the researchers (AA). However, to ensure the data extraction was of sufficient quality, two of the other researchers (TWLW & SSMN) verified the data extracted.
The characteristics of the studies included in the review are presented in a table.
Risks of bias and methodological quality assessment
Cochrane Risk of Bias Assessment tool and PEDro scale were used to assess the risk of bias and methodological quality of the included studies respectively. The Cochrane Risk of Bias Assessment tool is used to assess selection bias (random sequences generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and any other bias not covered in the previous items of the scale31. The results of this assessment are presented in risks of bias graph and summary table.
The PEDro scale is an 11-items scale32. The first item is used to assess external validity of studies. The other ten items are used to assess internal validity of studies, and they are rated on a two-point scale, 0 and 1 which mean no and yes to a question in an item respectively. The total score from the scale is considered low or moderate or high methodological quality when it is zero to three or four to five or six to ten respectively33–35. The results of this assessment are presented in a table.
Both assessments were carried out independently by two of the researchers (AA and TWLW). However, where there was disagreement, the other researcher (SSMN) was consulted to help arrive at a consensus.
Analysis of the extracted data
Narrative and quantitative syntheses were used to analyze the extracted data. The narrative synthesis involved summarizing the characteristics, risks of bias and methodological quality of the included studies. The quantitative synthesis of the effects of VNS involved the use of fixed or random effect models (where applicable) meta-analysis of the mean scores and standard deviation of the outcomes of interest, and the number of participants in the studies (for the VNS and control groups) post intervention and at follow-up.
The quantitative synthesis of the adverse events in the studies involved the use of fixed or random effect models (where applicable) meta-analysis of the number of events and sample sizes in both the VNS and the control groups post intervention and at follow-up. Similarly, the quantitative synthesis of the relationship between studies in the effects of VNS and adverse events involved meta-analysis of the correlation coefficients, r (converted from the odd ratios and mean difference of the studies) in the outcomes of interest between groups post intervention, and the sample sizes of the studies. The following formulae were used to convert odd ratios and effect size to correlation coefficient (r): and 36. Where π = 3.14, d = effect size, r = correlation coefficient and (where n1 and n2 = number of participants in group 1 and 2 respectively). In addition, percentage of variation across the studies due to heterogeneity (I2) was deemed significant when it is between 50 and 90% at P < 0.05.
Furthermore, the meta-analyses for the effects and adverse events were carried out using RevMan software; while the meta-analyses for the correlation were carried out using MedCalc® software.
Making sense of the evidence
To make sense of the evidence, body of evidence matrix of the Australian National Health and Medical Research Council's (NHMRC) evidence hierarchy was adapted37.
Result
Narrative synthesis
Selection of eligible studies
Electronic search of the databases provided a total of 733 studies. Out of this number, only seven studies were eligible for inclusion in the study38–44. However, in one of the studies, two papers were published43,45. See Fig. 1 for the details of the literature search process and the selection of the studies.
Figure 1.
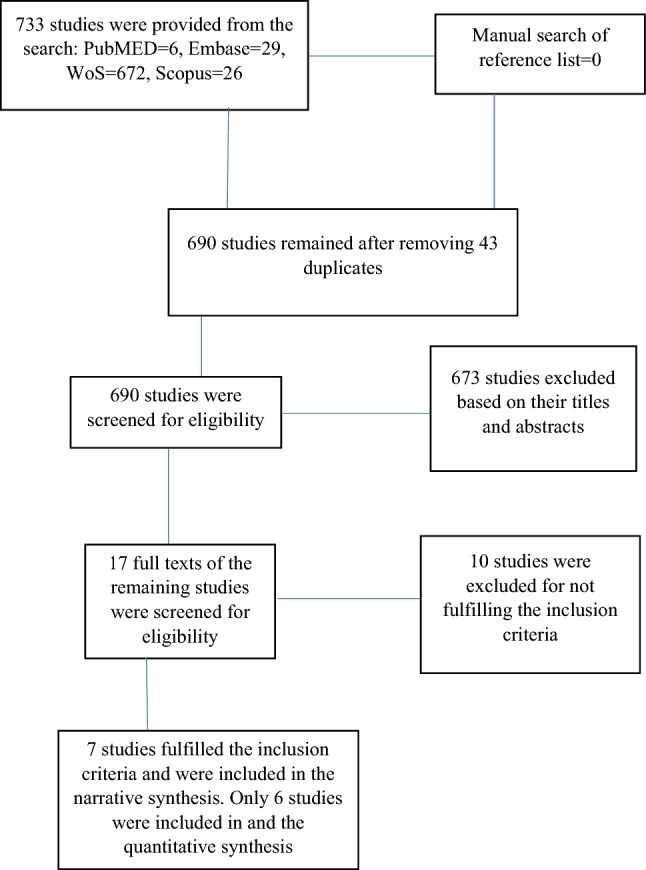
The study flowchart.
Characteristics of the included studies
The included studies have a total sample size of 274 patients with stroke (range 12–108), mean age range, 53.71 ± 5.88 to 69.2 ± 12.3 years and mean time since stroke range, 10.4 ± 6.9 days to about 93.71 ± 38.81 months. Out of this number, 102 were female, and the type of stroke the patients had include both ischaemic and haemorrhagic stroke. Consequently, the studies included 255 and 19 patients with ischaemic and haemorrhagic stroke respectively. Out of this, patients with ischaemic stroke were exclusively included in all the studies that used invasive VNS39,40,43; and one study that used non-invasive VNS41. However, the remaining studies that used non-invasive VNS included participants with both ischaemic and haemorrhagic stroke.
Similarly, only five studies reported the sides affected, which were 107 and 119 right and left sided hemiplegia respectively38–44. In addition, five studies used 125 participants with dominant hand stroke38–40,42–44; while, two participants in two studies were ambidextrous40,43.
For the period of enrolment of participants in the studies, one study each enrolled participants during the acute stage44; during the acute and subacute stages41; and during the subacute and chronic stages40. All the remaining studies enrolled participants during the chronic stage38,39,42,43.
One study included participants with moderate impairment in motor function38. Five studies included participants with moderate to severe impairment in motor function, a score of 15–50 on action research arm test (ARAT)39; and a score of 20–50 on Fugl Meyer motor assessment upper extremity (FMA-UE)40,42–44. However, one study did not specify the degree of impairment in motor function41. Similarly, the included studies used different types of VNS. Three studies used invasive VNS39,40,43; whereas, the remaining four studies used non-invasive VNS38,41,42,44.
In three studies, the stimulation parameters used were 0.8 mA, constant current, charge balanced pulses (100-μs pulse width, 30-Hz frequency39,40,43; in three studies, the stimulation parameters used were 600 pulses (intratrain pulse frequency = 20 Hz; pulse duration = 0:3 ms) 38,41,44; and in one study, the stimulation parameters used were single 500 ms bursts with a frequency of 30 Hz and a pulse width of 0.3 ms42.
In all the studies, participants in both the experimental and the control groups received upper limb rehabilitation training. In three studies, the participants received goal oriented upper limb training39,40,43; in two studies, the participants received conventional upper limb rehabilitation41,43; and in two studies, the participants received robotic rehabilitation of the upper limb38,42. In four studies, the stimulation was delivered simultaneously with the upper limb training39,40,42,43; whereas, it was delivered immediately after the training in three studies38,41,44.
The outcomes assessed in the studies include level of motor impairment, motor function, quantity and quality of use of the arm in the real world, hand function and/or manual dexterity, hand grip strength, muscle strength, activities of daily living (ADL), stage of recovery, spasticity, muscle electrical activity, depression, anxiety, quality of life, infarct volume and adverse events. The level of motor impairment was assessed using FMA-UE38–44. The motor function was assessed using Wolf motor function test (WMFT)40–44; and ARAT39. The quantity and quality of use of the arm in the real world was assessed using Motor Activity Log [MAL]40,43. The hand function and/or manual dexterity was assessed using Box and Block test, and 9-hole peg test39,40. The hand grip strength was assessed using hand-held dynamometer39. The muscle strength was assessed using Medical Research Council scale42. The activities of daily living (ADL) was assessed using Functional Independence Measure41. The stage of recovery was assessed using Bruunstrom recovery stage41. The spasticity was assessed using Modified Tardieu scale42. The muscle electrical activity was assessed using surface EMG42. The depression and anxiety were assessed using Beck Depression Index and Hospital Depression and Anxiety scale 43,44. The quality of life was assessed using stroke impact scale39,40; and stroke specific quality of life questionnaire43. The infarct volume was assessed using Magnetic Resonance Imaging39.
Both groups demonstrated improvement in most of the outcomes post intervention and at follow-up. In addition, the VNS group demonstrated a greater reduction in infarct volume post intervention. See Table 1 for the details of the characteristics of the included studies.
Table 1.
Characteristics of the included studies.
| References | N | Stroke duration | Mean age (years) | Intervention | Outcomes | Findings | Adverse events |
|---|---|---|---|---|---|---|---|
| Dawson et al.39 | N = 20; VNS (n = 9, females = 2); control (n = 11, females = 2) | VNS = 1.8 ± 1.0 months; control = 1.7 ± 1.3 months | VNS = 57.9 ± 17.2; control = 60.7 ± 10.7 |
Participants in both groups received a 6-week course of 2-h therapy sessions, 3 times a week. During each session, participants performed at least 300–400 movements In addition, the VNS group received a 500-ms burst of VNS via an implanted electrode attached to the left vagus nerve in the left carotid sheath during each movement. Each simulation consisted of fifteen 0.8-mA, constant current, charge balanced pulses (100-μs pulse width, 30-Hz frequency) |
Level of motor impairment (FMA-UE), motor function (ARAT), grip and pinch strength (hand-held dynamometer), quality of life (Stroke Impact Scale), manual dexterity (Box and Block test, and 9-hole peg test), safety (adverse events), feasibility (compliance with VNS) and infarct volume, CST overlap volume, fractional anisotropy ratio, and mean diffusivity Ratio (MRI) |
Feasibility: All participants completed all their treatment session, and only one required removal of the implant Safety: Eight and three participants in VNS and control respectively reported adverse events; however, they were majorly not serious adverse event Efficacy: Only level of motor function attained a meaningful clinical improvement post intervention in the VNS group. However, level of motor impairment, motor function and quality of life significantly improved in VNS groups at one-year follow-up There was also greater reduction in infarct volume in VNS group post intervention |
Vocal cord palsy, dysphagia, taste disturbance after the surgery (metallic taste), atrial fibrillation, reduced oxygen saturation, and chest pain |
| Capone et al.38 | N = 12; VNS (n = 7, females = 3); control (n = 5, females = 2) | VNS = 93.71 ± 38.81 months; control = 46.00 ± 21.85 months | VNS = 53.71 ± 5.88; control = 55.60 ± 7.12 |
tVNS was delivered as trains lasting 30 s and composed by 600 pulses (intratrain pulse frequency = 20 Hz; pulse duration = 0.3 ms) repeated every 5 min for 60 min for 10 consecutive days VNS group: Electrodes were placed in the left external acoustic meatus at the inner side of the tragus Sham group: Electrodes were attached to the left ear lobe, an anatomical area that is outside the innervation of the auricular branch of the vagus nerve In addition, both groups received robotic therapy of the upper limb immediately after the stimulation |
Level of motor impairment (FMA-UE), safety (blood pressure and heart rate) and tolerability (questions on unpleasant sensation and/ or discomfort) | VNS was safe. In addition, VNS significantly improved level of motor impairment better than the control | No any adverse event |
| Kimberly et al.40 | N = 17; VNS (n = 8, females = 4); control (n = 9, females = 4) | VNS = 18 (1143) months, mean (range); control = 18 (6.3–53) months, mean (range) | VNS = 59.5 ± 7.4; control = 60.0 ± 13.5 |
VNS = In-clinic rehabilitation paired with active VNS (0.8 mA), 3 × a week for 6 weeks Control = In-clinic rehabilitation paired with sham VNS (0.0 mA), 3 × a week for 6 weeks |
Level of motor impairment (FMA-UE), motor function (WMFT), quality and quantity of use of the limb in daily life (MAL), quality of life (SIS), manual dexterity (box and block test, and 9PHT), and safety (adverse events) | VNS was significantly superior only at improving motor function at 90 days follow-up compared to the control | Skin rediness in one patient in VNS group |
| Wu et al.41 | N = 21; VNS (n = 10, females = 5); control (n = 11, females = 3) | VNS = 36:30 ± 9:23 days; control = 35:55 ± 6:47 days | VNS = 64:50 ± 9:97; control = 61:82 ± 10:63 |
VNS: The left auricular branch vagus nerve was stimulated by the modified dot-like electrodes that were fitted to the cymba conchae. The parameters were selected as follows: 600 pulses (intratrain pulse frequency = 20 Hz; pulse duration = 0:3 ms), lasting 30 s each time, stimulating once every 5 min. Stimulation was performed for 30 min per day for 15 consecutive days Control: Electrodes were fixed to the cymba conchae of the left ear without electrical stimulation After the stimulation, participants in both groups performed conventional rehabilitation training involving postural control, proprioception exercises, neuromuscular facilitation, gait training, and always at the upper limit of their capacity for 30 min each day |
Level of motor impairment (FMA-UE), motor function (WMFT), activities of daily living (FIM), and recovery stage (upper limb Brunnstrom stage) | Level of motor impairment, motor function, and activities of daily living significantly improved better in VNS than the control post intervention and at follow-up | Skin rediness in one patient in VNS group |
| Chang et al.42 | N = 36; VNS (n = 18, females = 5); control (n = 18, females = 3) | 2.16 ± 0.39 years | 59.02 ± 1.98 (27.9–81.1) | 9 sessions of shoulder/elbow robotic therapy (3x/week for 3 weeks) paired with active taVNS or sham taVNS delivered in a single 500 ms bursts with a frequency of 30 Hz and a pulse width of 0.3 ms to the left cymba conchae during the onset of a visual cue for extension movements | Electrical activity of upper limb muscles (sEMG), level of motor impairment (FMA-UE), muscle power (MRC muscle power scale), motor function (WMFT), and spasticity (modified Tardieu Scale) | All outcomes significantly in both groups except in spasticity where VNS group improved significantly better post intervention. However, there was no significant difference in any of the outcomes at discharge or follow-up | No serious adverse events were reported |
| Dawson et al.43 | N = 108; VNS (n = 53, females = 19); control (n = 55, females = 19) | VNS = 3.1 ± 2.3; control = 3.3 ± 2.6 | VNS = 59.1 ± 10.2; control = 61.1 ± 9.2 |
Participants in both groups performed 30–50 repetitions, task-based, functional, individualised, and progressive upper limb exercises such as reach and grasp, gross movement, object flipping, simulated eating tasks, inserting objects, and opening/closing containers daily for 6 weeks In addition, VNS group received 0.8 mA (or 0.7 and 0.6 mA in two participants as described above), 100 μs, 30 Hz stimulation pulses, lasting 0.5 s, during each movement repetition. The control group received 0 mA pulses Both groups all performed 30 min therapist prescribed home exercises during the period. The VNS group was asked to put on their VNS during the home exercise |
Level of motor impairment (FMA-UE), motor function (WMFT), quality and quantity of use of the limb in daily life (MAL), quality of life (SIS, SS-QOL, EQ-5D), and depression (BDI) Safety: adverse event (MedDRA, version 22) |
All outcome improved higher in the VNS group post intervention and at follow-p compared to the control group | About 334 adverse events (163 VNS, 171 Control) were reported. However, majority were mild |
| Li et al.44 | N = 60; VNS (n = 30, females = 15); control (n = 30, females = 16) | VNS = 10.8 ± 7.7 days; control = 10.4 ± 6.9 days | VNS = 69.2 ± 12.3; control = 68.3 ± 12.1 |
Participants in the VNS and control received active or sham VNS respectively delivered by an auricular transcutaneous electrical nerve stimulation apparatus using a 0.3-ms square pulses at 20 Hz for 30 s and repeated every 5 min, 20 min a day for 20 working days (5 days a week for 4 weeks) All participants received a 4-week course of 30-min rehabilitation therapy sessions, five times per week in the hospital |
Motor function (WMFT), level of motor impairment (FMA-UE and FMA-LE), quality of life (SIS), anxiety and depression (HADS) and safety (adverse events) | All outcomes improved in both groups post intervention and at follow-up. However, the improvements were significantly higher in the VNS group | Two participants in VNS group reported skin redness |
VNS vagus nerve stimulation, FMA-UE Fugl Meyer motor assessment- upper extremity, ARAT Action research arm test, MRI magnetic resonance imaging, tVNS transcutaneous vagus nerve stimulation, WMFT Wolf motor function test, MAL motor activity log, SIS stroke impact scale, 9HPT Nine Hole Peg test, FIM Functional independence measure, taVNS transcutaneous auricular vagus nerve stimulation, sEMG surface electromyography, MRC Medical research council, SS-QOL stroke specific quality of life questionnaire, EQ-5D EURO-QOL five-dimension, BDI Beck depression index, MedDRA Medical Dictionary for Regulatory Activities, FMA-LE Fugl Meyer motor assessment-lower extremity, HADS Hospital anxiety and depression scale.
Furthermore, only five studies reported adverse events39,40,43,44. Although the adverse events are majorly mild or not serious; in one of the studies, they (Vocal cord palsy, dysphagia, taste disturbance after the surgery (metallic taste), atrial fibrillation, reduced oxygen saturation, and chest pain) seem to be serious39.
Methodological quality and risks of bias
Four of the included studies have good methodological quality38,41–43; two have excellent methodological quality40,44; and one study has fair methodological quality38. Scores of < 4, 4–5, 6–8 and 9–10 are considered as poor, fair, good and excellent methodological quality respectively46,47. See Table 2 for the details of the methodological quality of the included studies.
Table 2.
Methodological quality of the included studies.
| Study | Eligibility criteria specified | Random allocation | Concealed allocation | Comparable subjects | Blind subjects | Blind therapists | Blind assessors | Adequate follow-up | Intention to treat analysis | Between group comparison | Point estimation and variability | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dawson et al.39 | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 5/10 |
| Capone et al.38 | Yes | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7/10 |
| Kimberly et al.40 | Yes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10/10 |
| Wu et al.41 | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 7/10 |
| Chang et al.42 | Yes | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8/10 |
| Dawson et al.43 | Yes | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8/10 |
| Li et al.44 | Yes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9/10 |
In addition, generally, the studies have low risks of bias except in selection bias41,42,45; attrition bias38,39,43,44; and performance bias41,42. See Fig. 2a,b for the risks of bias graph and summary respectively.
Figure 2.
(a) Risks of bias graph. (b) Risks of bias summary.
Quantitative synthesis
Effect of VNS compared with control on level of motor impairment (measured using FMA-UE)
The result showed that, invasive VNS is significantly better than control at improving level of motor impairment (MD 2.66, 95% CI 1.19–4.13, P = 0.0004) post intervention, with no significant heterogeneity between studies, (I2 = 44%, P = 0.17) (see Fig. 3a for the details of the result). However, there was a significant correlation between improvements in both groups post intervention (r = 0.77, 95% CI 0.708–0.822, P < 0.001), possibly suggesting that, upper limb rehabilitation training used in both groups contributed to the improvement immensely (see Fig. 3b for the details of the result). In addition, the relatively large confidence interval in the result of the effect, suggests uncertainty concerning the effect of invasive VNS.
Figure 3.
(a) Effect of VNS compared with control on level of motor impairment post intervention. (b) Relationship between VNS and control group in improving level of motor impairment post intervention. (c) Effect of non-invasive VNS compared with control on level of motor impairment at follow-up. (d) Effect of invasive VNS compared with control on motor function post intervention. (e) Difference in proportions of adverse events between VNS and control. (f) Relationship between VNS and control in adverse events.
For the non-invasive VNS, the result showed that, there was no significant difference between groups post intervention (MD 0.28, 95% CI − 0.07 to 0.62, P = 0.12), with significant heterogeneity between studies, I2 = 67%, P = 0.05) (see Fig. 3a for the details of the result). However, at follow-up, the result, showed that, non-invasive VNS is significantly better than control at improving level of motor impairment (MD 24.16, 95% CI 23.56–24.75, P = 0.00001), with significant heterogeneity between studies, (I2 = 99%, P < 0.00001) (see Fig. 3c for the details of the result).
Effect of VNS compared with control on motor function
The result showed that, invasive VNS is significantly better than control at improving motor function (MD 0.28, 95% CI 0.15–0.41, P < 0.0001) post intervention, with significant heterogeneity between studies, (I2 = 65%, P = 0.09) (see Fig. 3d for the details of the result).
Presence of adverse events
The results showed that, there was no significant difference in the presence of adverse events between invasive VNS and control (OR 2.15, 95% CI 0.97–4.74, P = 0.06), with significant heterogeneity between studies (I2 = 55%, P = 0.11); and no-invasive VNS and control (OR 4.54, 95% CI 0.48–42.97, P = 0.19) with no significant heterogeneity between studies (I2 = 0%, P = 0.87) (see Fig. 3e for more details). However, there was no significant correlation between groups in adverse events (r = 0.0942, 95% CI − 0.0405 to 0.225, P = 0.170), suggesting that, VNS may produce adverse events higher than the control (see Fig. 3f for more details).
Interpretation of the evidence
The evidence seems to be excellent, satisfactorily consistent and applicable, excellently generalizable and has good clinical impact. Therefore, the body of evidence can be trusted to guide practice in most cases. See Table 3 for the body of the evidence matrix.
Table 3.
Body of evidence matrix.
| Component | Grade | Comments |
|---|---|---|
| 1. Evidence |
A-Excellent Several Level II studies |
Quantity: a total of 7 studies Participants: 274 patients with stroke Level II studies: 7 |
| 2. Consistency | C-Satisfactory | There is significant heterogeneity between studies for one of the outcomes, I2 > 50% |
| 3. Clinical impact | B-Good | Several studies reported that the effect attained clinically meaningful39,40,42,43 |
| 4. Generalizability | A-Excellent | The studied population is the same as the target population (patients with stroke) |
| 5. Applicability | C-Satisfactory | The evidence may be applicable globally since the studies were carried out in 4 different countries (China, Italy, UK and USA) in three different continents |
| Recommendation | B = Body of evidence can be trusted to guide practice in most cases |
Discussion
The aim of this study was to carry out a systematic review and meta-analysis to determine the effects, adverse events of VNS and the relationships in the reported effects and adverse events between groups in the included studies. The results showed that VNS improves outcomes such as level of motor impairment and motor function. In addition, there was no significant difference between groups in terms of adverse events. These findings are important since search for effective rehabilitation techniques for the rehabilitation of upper limb function in patients with stroke remain an important goal for clinicians, patients and their families14. Therefore, VNS can serve as a means to reach centrally located neurological structures to help patients with stroke recover upper limb function17. This is because the vagus nerve serves both motor and sensory functions, which are essential for recovery of upper limb functional activities17,19.
The afferent function of the vagus nerve is promoted by the afferent fibers that arise from the nodose ganglion and project majorly to the NTS20,21. This helps to activate the cholinergic and noradrenergic systems that are required for various normal functions of the brain22–24,28. This is made possible because most cholinergic and adrenergic neurons are located in subcortical regions and have axons that innervate many brain regions, including cortices and the hippocampus48,49. Consequently, the engagement of these neuromodulatory systems by VNS led to the prediction that brief bursts of VNS paired with sensory or motor experience could enhance cortical plasticity that was specific to the paired experience14.
However, from the results of the present study, there was significant correlation in improvements in level of motor impairment between the VNS and control groups, suggesting that upper limb rehabilitation training that was used in both groups may have a crucial effect on the improvement. This is because, the types of interventions used for the upper limb trainings in the included studies are known to also improve upper limb function50,51. Thus, VNS may be used as an adjunct therapy to other rehabilitation techniques, which re-echoes previous claim that neuromodulation techniques should be used in combination with other rehabilitation techniques13. Similarly, hybrid therapy, where two or more techniques are combined has been advocated during stroke rehabilitation for optimal gain52. In addition, there was no significant correlation between groups in presence of adverse events, suggesting that, one of the interventions may produce higher or more serious adverse events.
Another issue concerning the results of the present study that needs discussing, is the characteristics of patients with stroke who are most suitable for VNS. This is because, from the results of the included studies, the participants used were those with mild to moderate impairment in motor and cognitive functions. However, VNS is a passive technique and it does not require active performance by the patients. Similarly, it has also been reported to help improve cognitive function53,54. Therefore, VNS can be used for patients with stroke who have severe impairment in motor and cognitive function. This is a scientific breakthrough as there is as yet not many rehabilitation techniques used for patients with severe impairment in motor function13.
Another issue is that, considering the cost and potential risks of adverse events with the use of invasive VNS, especially the risk of introducing infection and having wound due to surgery, the non-invasive technique should be given a special attention especially for research. However, the non-invasive technique is still not widely used as there is no quality evidence of its superiority over control interventions55. Similarly, future studies should try and compare the use of invasive VNS versus non-invasive VNS for the rehabilitation upper limb function following stroke.
Conclusion
The evidence for the use of VNS for the rehabilitation of upper limb function in patients with stroke seems to be excellent, satisfactorily consistent and applicable, excellently generalizable and has good clinical impact. Therefore, the body of evidence can be trusted to guide practice in most cases. However, further studies are needed specially to compare the effects of invasive VNS with non-invasive VNS, and the presence of adverse events following them.
Supplementary Information
Acknowledgements
We would like to thank the Research Centre for Chinese Medicine Innovation of The Hong Kong Polytechnic University (Grant no. P0041139) and PolyU Distinguished Postdoctoral Fellowship Scheme (Grant no. P0035217) for their support to carry out this research.
Author contributions
All authors contributed in the design, analysis and writing of the study.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-42077-2.
References
- 1.Kuriakose D, Xiao Z. Pathophysiology and treatment of stroke: Present status and future perspectives. Int. J. Mol. Sci. 2020;21(20):7609. doi: 10.3390/ijms21207609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ. Res. 2017;120(3):472–495. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC. Neuronal cell death. Physiol. Rev. 2018;98:813–880. doi: 10.1152/physrev.00011.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zorowitz RD, Gillard PJ, Brainin M. Poststroke spasticity: Sequelae and burden on stroke survivors and caregivers. Neurol. 2013;80:S45–S52. doi: 10.1212/WNL.0b013e3182764c86. [DOI] [PubMed] [Google Scholar]
- 5.Cullen B, O’Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J. Neurol. Neurosurg. Psychiatry. 2007;78(8):790–799. doi: 10.1136/jnnp.2006.095414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wurzinger EH, Abzhandadze T, Rafsten L, Sunnerhagen KS. Dependency in activities of daily living during the first year after stroke. Front. Neurol. 2021;12:736684. doi: 10.3389/fneur.2021.736684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brainin M, Feigin VL, Norrving B, Martins SCO, Hankey GJ, Hachinski V. Global prevention of stroke and dementia: The WSO Declaration. Lancet Neurol. 2020;19:487–488. doi: 10.1016/S1474-4422(20)30141-1. [DOI] [PubMed] [Google Scholar]
- 9.Hobeanu C, Lavallée PC, Charles H, Labreuche J, Albers GW, Caplan LR, Donnan GA, Ferro JM, Hennerici MG, Molina CA, Rothwell PM, Steg PG, Touboul PJ, Uchiyama S, Vicaut E, Wong KSL, Amarenco P. Risk of subsequent disabling or fatal stroke in patients with transient ischaemic attack or minor ischaemic stroke: An international, prospective cohort study. Lancet Neurol. 2022;21(10):889–898. doi: 10.1016/S1474-4422(22)00302-7. [DOI] [PubMed] [Google Scholar]
- 10.Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, Elkind MS. Long-term functional recovery after first ischemic stroke: The Northern Manhattan Study. Stroke. 2009;40(8):2805–2811. doi: 10.1161/strokeaha.109.549576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: Impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34(9):2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Shi YZ, Zhang N, Wang S, Ungvari GS, Ng CH, Wang YL, Zhao XQ, Wang YJ, Wang CX, Xiang YT. The disability rate of 5-year post-stroke and its correlation factors: A national survey in China. PLoS ONE. 2016;11(11):e0165341. doi: 10.1371/journal.pone.0165341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullahi A, Wong TWL, Ng SSM. Rehabilitation of severe impairment in motor function after stroke: Suggestions for harnessing the potentials of mirror neurons and the mentalizing systems to stimulate recovery. Brain Sci. 2022;12(10):1311. doi: 10.3390/brainsci12101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, Hays SA. Targeted vagus nerve stimulation for rehabilitation after stroke. Front. Neurosci. 2019;13:280. doi: 10.3389/fnins.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neren D, Johnson MD, Legon W, Bachour SP, Ling G, Divani AA. Vagus nerve stimulation and other neuromodulation methods for treatment of traumatic brain injury. Neurocrit. Care. 2016;24(2):308–319. doi: 10.1007/s12028-015-0203-0. [DOI] [PubMed] [Google Scholar]
- 16.Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8(3):624–636. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny, B. J., Bordoni, B. Neuroanatomy, Cranial Nerve 10 (Vagus Nerve). In StatPearls. (StatPearls Publishing, 2022). [PubMed]
- 18.Trepel, M. Neuroanatomy—Structure and Function (Urban & Fischer, 2017).
- 19.Terra VC, Amorim R, Silvado C, de Oliveira AJ, Jorge CL, Faveret E, Ragazzo P, De Paola L. Vagus nerve stimulator in patients with epilepsy: Indications and recommendations for use. Arq. Neuropsiquiatr. 2013;71(11):902–906. doi: 10.1590/0004-282X20130116. [DOI] [PubMed] [Google Scholar]
- 20.Boon P, Vonck K, De Reuck J, Caemaert J. Vagus nerve stimulation for refractory epilepsy. Seizure. 2001;10:448–455. doi: 10.1053/seiz.2001.0626. [DOI] [PubMed] [Google Scholar]
- 21.Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998;39:677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 22.Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp. Neurol. 2017;289:21–30. doi: 10.1016/j.expneurol.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagena H, Hansen N, Manahan-Vaughan D. β-adrenergic control of hippocampal function: subserving the choreography of synaptic information storage and memory. Cereb. Cortex. 2016;26:1349–1364. doi: 10.1093/cercor/bhv330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen N, Manahan-Vaughan D. Hippocampal long-term potentiation that is elicited by perforant path stimulation or that occurs in conjunction with spatial learning is tightly controlled by beta-adrenoreceptors and the locus coeruleus. Hippocampus. 2015;25:1285–1298. doi: 10.1002/hipo.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, Rennaker RL, 2nd, Kilgard MP. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb. Cortex. 2012;22:2365–2374. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 26.Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011;189:207–214. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006;1119:124–132. doi: 10.1016/j.brainres.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mertens A, Raedt R, Gadeyne S, Carrette E, Boon P, Vonck K. Recent advances in devices for vagus nerve stimulation. Expert Rev. Med. Devices. 2018;15:527–539. doi: 10.1080/17434440.2018.1507732. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Zhan G, Cai Z, Jiao B, Zhao Y, Li S. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci. Biobehav. Rev. 2021;127:37–53. doi: 10.1016/j.neubiorev.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—A web and mobile application for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group. Cochrane Statistical Methods Group The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003;83:713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 33.Herbert R, Moseley A, Sherrington C. PEDro: A database of randomized controlled trials in physiotherapy. Health Inf. Manag. 1998;28:186–188. doi: 10.1177/183335839902800410. [DOI] [PubMed] [Google Scholar]
- 34.Moseley AM, Herbert RD, Maher CG, Sherrington C, Elkins MR. Reported quality of randomized controlled trials of physiotherapy interventions has improved over time. J. Clin. Epidemiol. 2011;64:594–601. doi: 10.1016/j.jclinepi.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 35.da Costa BR, Hilfiker R, Egger M. PEDro’s bias: Summary quality scores should not be used in meta-analysis. J. Clin. Epidemiol. 2013;66:75–77. doi: 10.1016/j.jclinepi.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, editors. Introduction to Meta-analysis. Wiley; 2009. [Google Scholar]
- 37.Hillier S, Grimmer-Somers K, Merlin T, Middleton P, Salisbury J, Tooher R. FORM: An Australian method for formulating and grading recommendations in evidence-based clinical guidelines. BMC Med. Res. Methodol. 2011;11:23. doi: 10.1186/1471-2288-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capone F, Miccinilli S, Pellegrino G, Zollo L, Simonetti D, Bressi F, Florio L, Ranieri F, Falato E, Di Santo A, Pepe A, Guglielmelli E, Sterzi S, Di Lazzaro V. Transcutaneous vagus nerve stimulation combined with robotic rehabilitation improves upper limb function after stroke. Neural Plast. 2017 doi: 10.1155/2017/7876507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, Hilmi O, McLean J, Forbes K, Kilgard MP, Rennaker RL, Cramer SC, Walters M, Engineer N. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke. 2016;47(1):143–150. doi: 10.1161/STROKEAHA.115.010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimberley TJ, Pierce D, Prudente CN, Francisco GE, Yozbatiran N, Smith P, Tarver B, Engineer ND, Alexander Dickie D, Kline DK, Wigginton JG, Cramer SC, Dawson J. Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke. Stroke. 2018;49(11):2789–2792. doi: 10.1161/STROKEAHA.118.022279. [DOI] [PubMed] [Google Scholar]
- 41.Wu D, Ma J, Zhang L, Wang S, Tan B, Jia G. Effect and safety of transcutaneous auricular vagus nerve stimulation on recovery of upper limb motor function in subacute ischemic stroke patients: A randomized pilot study. Neural Plast. 2020;2020:8841752. doi: 10.1155/2020/8841752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang JL, Coggins AN, Saul M, Paget-Blanc A, Straka M, Wright J, Datta-Chaudhuri T, Zanos S, Volpe BT. Transcutaneous auricular vagus nerve stimulation (tAVNS) delivered during upper limb interactive robotic training demonstrates novel antagonist control for reaching movements following stroke. Front. Neurosci. 2021;15:767302. doi: 10.3389/fnins.2021.767302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawson J, Liu CY, Francisco GE, Cramer SC, Wolf SL, Dixit A, Alexander J, Ali R, Brown BL, Feng W, DeMark L, Hochberg LR, Kautz SA, Majid A, O'Dell MW, Pierce D, Prudente CN, Redgrave J, Turner DL, Engineer ND, Kimberley TJ. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): A randomised, blinded, pivotal, device trial. Lancet. 2021;397(10284):1545–1553. doi: 10.1016/S0140-6736(21)00475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li JN, Xie CC, Li CQ, Zhang GF, Tang H, Jin CN, Ma JX, Wen L, Zhang KM, Niu LC. Efficacy and safety of transcutaneous auricular vagus nerve stimulation combined with conventional rehabilitation training in acute stroke patients: A randomized controlled trial conducted for 1 year involving 60 patients. Neural Regener. Res. 2022;17(8):1809–1813. doi: 10.4103/1673-5374.332155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson J, Engineer ND, Prudente CN, Pierce D, Francisco G, Yozbatiran N, Tarver WB, Casavant R, Kline DK, Cramer SC, Van de Winckel A, Kimberley TJ. Vagus nerve stimulation paired with upper-limb rehabilitation after stroke: One-year follow-up. Neurorehabil. Neural Repair. 2020;34(7):609–615. doi: 10.1177/1545968320924361. [DOI] [PubMed] [Google Scholar]
- 46.Foley NC, Teasell RW, Bhogal SK, Speechley MR. Stroke rehabilitation evidence-based review: Methodology. Top Stroke Rehabil. 2003;10(1):1–7. doi: 10.1310/Y6TG-1KQ9-LEDQ-64L8. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez GZ, Moseley AM, Maher CG, Nascimento DP, Costa LDCM, Costa LO. Methodologic quality and statistical reporting of physical therapy randomized controlled trials relevant to musculoskeletal conditions. Arch. Phys. Med. Rehabil. 2018;99(1):129–136. doi: 10.1016/j.apmr.2017.08.485. [DOI] [PubMed] [Google Scholar]
- 48.Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. Immunocytochemical localization of choline acetyltransferase in rat cerebral cortex: A study of cholinergic neurons and synapses. J. Comp. Neurol. 1985;234:17–34. doi: 10.1002/cne.902340103. [DOI] [PubMed] [Google Scholar]
- 49.Bouarab C, Thompson B, Polter AM. VTA GABA neurons at the interface of stress and reward. Front. Neural Circuits. 2019;13:78. doi: 10.3389/fncir.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, van Wijck F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014;2014(11):CD010820. doi: 10.1002/14651858.CD010820.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, Dispa D, Bleyenheuft Y. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 2016;10:442. doi: 10.3389/fnhum.2016.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hung CS, Hsieh YW, Wu CY, Chen YJ, Lin KC, Chen CL, Yao KG, Liu CT, Horng YS. Hybrid rehabilitation therapies on upper-limb function and goal attainment in chronic stroke. OTJR (Thorofare N J). 2019;39(2):116–123. doi: 10.1177/1539449218825438. [DOI] [PubMed] [Google Scholar]
- 53.Choi S, Jang DC, Chung G, Kim SK. Transcutaneous auricular vagus nerve stimulation enhances cerebrospinal fluid circulation and restores cognitive function in the rodent model of vascular cognitive impairment. Cells. 2022;11(19):3019. doi: 10.3390/cells11193019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Guo Z, Qu Y, Zhao Y, Yang Y, Du J, Yang C. Cognitive function and brain activation before and after transcutaneous cervical vagus nerve stimulation in healthy adults: A concurrent tcVNS-fMRI study. Front. Psychol. 2022;13:1003411. doi: 10.3389/fpsyg.2022.1003411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keser Z, Feng W. Vagus nerve stimulation for stroke motor recovery-what is next? Transl. Stroke Res. 2023;14(4):438–442. doi: 10.1007/s12975-022-01041-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.




