Abstract
People with osteoarthritis often experience pain and depression. These meta-analyses examined and compared nonpharmacological randomized controlled trials (RCTs) for pain and symptoms of depression in people living with osteoarthritis. RCTs published up until April 2022 were sourced by searching electronic databases EMBASE, PUBMED & MEDLINE, Web of Science, CINAHL and PEDro. Random-effects meta-analyses were performed to calculate pooled effect sizes (ES) and 95% confidence intervals (CI) for pain and depression. Subgroup analyses examined intervention subtypes. For pain, 29 interventions (n = 4382; 65 ± 6.9 years; 70% female), revealed a significant effect on reducing pain (ES = 0.43, 95% CI [0.25, 0.61], p < 0.001). Effect sizes were significant (p < 0.001) for movement meditation (ES = 0.52; 95% CI [0.35, 0.69]), multimodal approaches (ES = 0.37; 95% CI [0.22, 0.51]), and psychological therapy (ES = 0.21; 95% CI [0.11, 0.31]), and significant (p = 0.046) for resistance exercise (ES = 0.43, 95% CI [− 0.07, 0.94]. Aerobic exercise alone did not improve pain. For depression, 28 interventions (n = 3377; 63 ± 7.0 years; 69% female), revealed a significant effect on reducing depressive symptoms (ES = 0.29, 95% CI [0.08, 0.49], p < 0.001). Effect sizes were significant for movement meditation (ES = 0.30; 95% CI [0.06, 0.55], p = 0.008) and multimodal interventions (ES = 0.12; 95% CI [0.07, 0.18], p < 0.001). Resistance/aerobic exercise or therapy alone did not improve depressive symptoms. Mind–body approaches were more effective than aerobic/resistance exercise or therapy alone for reducing pain and depression in people with osteoarthritis.
Systematic review registration: PROSPERO CRD42022338051.
Subject terms: Osteoarthritis, Ageing, Depression
Introduction
Arthritis and psychological conditions (i.e. mental health conditions, for example, depression and anxiety) were in the top three most prevalent chronic conditions experienced in Australia during 2020–20211. Osteoarthritis (OA) is a degenerative joint condition where cartilage that cushions the ends of the bones deteriorates. It is the most common form of arthritis and most commonly affects hands, knees, hips and lower back and neck. Depression and anxiety are both common psychological co-morbidities that occur alongside OA2,3 in addition to increased pain and other mental health issues4. Chronic pain is experienced by one in five Australians aged 45 and over5.
OA has also been associated with increased dementia risk6,7, higher prevalence rates of dementia8, and is considered an early risk factor for cognitive decline7. Depression is another risk factor for dementia9 and is a common psychological symptom associated with dementia10. People living with dementia often experience other behavioural changes and psychological symptoms (e.g. agitation) that may be associated with underlying pain that is not always identified by care staff due to communication difficulties11. In Australia, dementia is the leading cause of death in women and second leading cause of death following heart disease in all Australians1,12. High rates of OA and dementia are also observed globally13.
There are no medications that can cure OA, though analgesics, non-steroidal anti-inflammatory drugs (NSAIDs) and topical therapies are often administered to relieve pain and inflammation. However, all medicines have side effects, for example, NSAIDS can increase risk of stomach ulcers and bleeding14, paracetamol can have adverse side effects when taken long-term15, and the use of opioids is controversial, with increasing evidence showing an increase in addiction, morbidity, and mortality16. Therefore, effective nonpharmacological interventions are needed to address pain and depression in OA, which are all well-established precursors to dementia. No previous systematic reviews have sought to determine the most effective approaches for reducing pain levels and symptoms of depression in OA. Reducing these co-morbidities will reduce dementia risk.
Physical and psychological health conditions rarely occur in isolation, and it is more likely people will go onto experience multiple comorbidities. For example, co-morbidities including psychological conditions are more likely in those with pain than those without5. The ABS reported that in 2020, 19% of Australians with long-term health conditions had two or more chronic conditions1. The interactions between physical and psychological conditions and associated symptoms are complex, and conditions may present independently or be highly intertwined. Common brain-related conditions (e.g. depression, anxiety, and dementia) can be exacerbated by physical conditions (e.g. OA, cardiovascular disease (CVD)) and vice-versa.
There are several models/theories that describe proposed mechanisms involved in these interactions. The biopsychosocial model which recognizes the contribution of all biological, psychological, social, and behavioural factors that dynamically interact with one another to generate the experience of pain17. Inflammation models describe how localized peripheral inflammation can trigger the development of neuroinflammation and subsequently the induction of dementia pathology18. The pain-sensitisation model recognises highly variable experiences of pain between individuals and the role of neuroplastic changes occurring in the peripheral and central nervous system, resulting in pain sensitisation, and impacting the person’s experience of pain19. The fear-avoidance model of musculoskeletal pain describes a psychological process where concern or fear about pain can lead to fear-avoidance behaviour which paradoxically can exacerbate pain and function20. The role of the gut microbiome has also been implicated with pain in OA21, depression22,23 and neurological conditions24.
There are clear associations between physical, psychological, and cognitive health outcomes (i.e. OA, anxiety, depression, and cognitive decline). However, it is not yet known what the most effective approaches are for improving psychological outcomes as well as physical outcomes (e.g. pain) in people living with OA. Physical activity interventions delivered by Accredited Exercise Physiologists (AEPs) have been shown to be highly effective for people living with 26 chronic conditions25, including OA26,27, anxiety and depression28,29, cognitive decline/dementia30,31, diabetes32 and cardiovascular disease33. Examples of effective exercise strategies include resistance training/strengthening exercises34, yoga35, and aquatic exercise36. Interventions that include lifestyle education and information on managing chronic conditions (e.g. information specific to OA, diabetes, depression, etc.) also provide additive beneficial effects37. Indeed, exercise and education are key components of clinical guidelines for OA. It is important that clients understand how to self-manage their pain and remain engaged with exercise to improve outcomes.
Research shows that therapeutic approaches such as cognitive behavioural therapy (CBT) are highly effective in treating mild-moderate depression and anxiety38. Furthermore, evidence suggests that the combination of exercise and behavioural therapies provides greater reduction in depressive symptomology than therapy alone39,40. Behavioural therapy approaches have been adapted or investigated in specific populations such as diabetes41, sleep disorders42, eating disorders43, and chronic pain44. Multimodal approaches that include a combination of exercise, therapeutic approaches and/or lifestyle education may be more effective than single mode approaches due to the focus on both psychological and physical health outcomes. However, there is limited evidence around the effect of multimodal treatment on multimorbidity.
Therefore, the aims of this systematic review and meta-analysis are to determine the most effective nonpharmacological approaches for reducing pain and symptoms of depression in people living with OA. We aimed to:
Identify effective approaches for improving pain and reducing symptoms of depression in people with OA.
Identify whether single or multi-modal approaches are more effective.
Present evidence-based recommendations for treatments for people with OA and associated depression.
In better understanding how to reduce OA-related symptoms and co-morbidities in populations who are at higher-risk of experiencing cognitive decline (i.e. people living with OA6), we may also reduce dementia prevalence rates, as well as improve psychological outcomes for people living with OA.
Methods
The review was preregistered with the international prospective register of systematic reviews (PROSPERO), in accordance with PRISMA guidelines (PROSPERO CRD42022338051)45.
Search strategy
Relevant studies published from electronic database inception until April 2022 were sourced by searching: EMBASE, PUBMED & MEDLINE, Web of Science, CINAHL and PEDro. Studies that included outcome measures relevant to physical outcomes (e.g. pain and physical function) and psychological outcomes (i.e. depression and anxiety symptomatology) were included in the initial search and this review. Search terms were Osteoarthritis OR OA; Depression OR anxiety OR mood OR affective OR psychological; Exercise OR physical activity OR education OR therapy OR CBT OR cognitive behavioural OR cognitive-behavioural OR lifestyle intervention OR mindfulness; RCT OR Randomised controlled trial OR Randomized controlled trial OR randomised controlled trial OR Randomized controlled trial (see Supplementary Material S1 for full list of search terms).
Eligibility criteria and study selection
Only randomized controlled trials (RCTs) were included in this review. Studies were included from all settings such as community clinics, hospitals, and in people’s homes via online platforms, if pain or symptoms of depression in people with OA were assessed. Studies were only included where two or more conditions were compared (control versus intervention). Exclusion criteria were studies having an active control group (e.g. aerobic activity versus resistance activity), studies where data was not presented separately for people with OA if they included people with other pain-related conditions (e.g. lower back pain), studies not available in English, and studies with insufficient information to conduct a meta-analysis. When means and standard deviations were not provided, authors were contacted and requested to share this data.
Only post-intervention scores were compared across conditions (i.e. post-intervention, control versus intervention group scores in pain and symptoms of depression). Cochrane methods and those developed by statisticians were used to convert data46–48. Pooled, weighted effect sizes were calculated for all approaches, followed by subgroups determined by intervention types (e.g. multimodal, aerobic activity only, resistance activity only, therapeutic only, movement meditation).
Interventions
Only nonpharmacological interventions were included that involved either exercise, lifestyle education, therapeutic and/or multimodal approaches. These were categorized into groups as they emerged and refined during the screening process. The final groups were ‘multimodal approaches’, ‘movement mediation’ (including yoga, tai chi, and chi gong), ‘resistance activity only’, ‘aerobic activity only’, and ‘therapeutic approaches only’. Studies were included if they had one or more active interventions and an inactive control group (e.g. treatment as usual). Only data from the combined intervention and control group were used for the meta-analyses.
Outcome measures
Studies were included that used several different measurement tools to determine pain and depression in people living with OA. For pain these included the Western Ontario and McMaster Universities Arthritis Index (WOMAC)49, Knee Injury and Osteoarthritis Outcome Score (KOOS)50, Hip Disability and Osteoarthritis Outcome Score (HOOS)51, 36-Item Short Form Survey (SF-36) (pain component)52, (PISF), Harris Hip Score (HHS) (pain component)53, and arthritis self-efficacy (ASE) (pain component)54. For depression these included the Centre for Epidemiological Studies Depression (CESD) scale55, Hospital Anxiety and Depression Scale (HADS)56, Geriatric Depression Scale (GDS)57, Beck Depression Inventory (BDI), (AIMS) (psychological component)34, Emotional Distress and Depression Short-Form (EDD-SF)58, Depression Anxiety Stress Scales (DASS21)59, and the Patient Health Questionnaire (PHQ-9)60. Studies were included with a range of intervention durations and some included follow-up periods (e.g. 6 months, 1-year) which were considered when rating of the quality of the research article was completed. Articles were only included if they were in English.
Quality rating
All studies were quality rated based on the study design, participant characteristics, outcome measures and statistical analyses using a Quality Rating Tool10 (see Supplementary Material S2) developed by adapting and combining several different previously used tools10,61,62. The tool ensured quality ratings reflected ability to determine potential statistical and clinical significance. Points were given based on study design and statistical analysis. Total scores on the quality rating tool ranged from 0 to 16 (i.e. higher scores indicated higher quality research).
Statistical analysis and clinical interpretation
The statistical analysis was conducted in Meta-Essentials developed by Suurmond and colleagues, Netherlands63. A random effects meta-analysis was completed with restricted maximum likelihood estimation (REML), assuming that the true effects differ across studies, as these studies vary in their study design and methodology (e.g. intervention type, duration, and outcome measure). Sensitivity analyses were performed to determine potential studies driving heterogeneity. Subgroup analyses were performed to investigate different types of interventions on clinical outcomes (pain and depression) and associated significance and effect sizes. The effect size of interventions was determined using Hedges’ g64 (page 110). Bias corrected standardized mean difference calculations (Hedges’ g) are recommended for studies with small sample sizes. According to Cohen, effect sizes less than 0.2 are trivial, 0.2 are small, 0.8 are medium, and 0.8 or higher are large65. Evidence for minimally clinically important differences (MCID) and moderate improvement estimates of change in OA-related pain had been published66. For the KOOS and HOOS short form pain measures, evidence has been published for MCID and moderate improvement estimates (2.2 and 15 respectively). Though reliability was much lower in knee compared to hip OA66.
A 95% confidence interval was used to determine the efficacy of combined interventions versus control on pain and symptoms of depression. Heterogeneity of the effects was assessed by calculating the I2 index and prediction intervals. The I2 index value reflects the proportion of true heterogeneity in the observed variance 69, 47], where 0% indicates no observed heterogeneity and increased heterogeneity is indicated by larger values where 25%, 50% and 75% reflect low, medium, and high heterogeneity respectively67. Prediction intervals are also presented throughout to ensure the predicted range of effects is unambiguous68. To understand the observed heterogeneity, we performed subgroup analyses to test intervention type. Publication bias was assessed graphically with contour-enhanced funnel plots and Egger’s regression test69. Funnel plots show if studies were missing only from areas of low statistical significance indicating that any asymmetry is likely to be caused by publication bias. The Egger regression gives “the degree of funnel plot asymmetry as measured by the intercept from regression of standard normal deviates against precision”69. If Egger’s test results in a p value less than 0.05, this implicates publication bias.
Results
Included studies
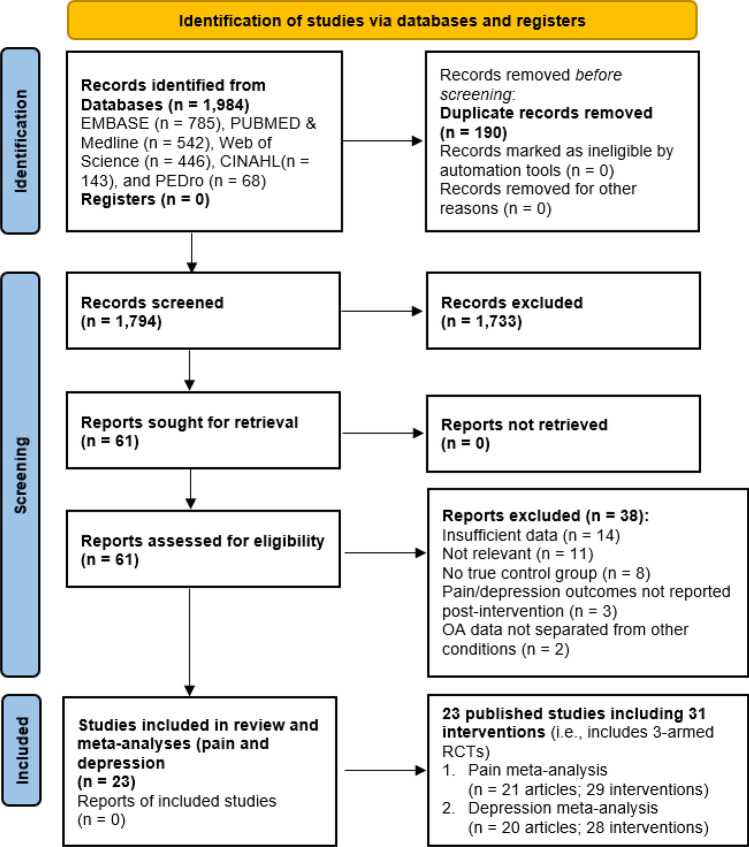
In total 1984 studies were found through the database search of studies published up until April 2022 (Fig. 1). Following removal of duplicates, article screening and removal of studies that did not meet eligibility criteria, 61 studies remained that included measures of pain and/or symptoms of depression in people with OA (Fig. 1). Thirty-eight studies were then excluded for not having sufficient data for meta-analysis, an active control condition, pain or depression outcomes not being reported post-intervention, and articles that did not meet methodology requirements (i.e. not RCTs, protocol papers). The mean quality rating of the 23 remaining studies (see Table 1) used in the final meta-analyses was high (mean: 13; SD = 1.6; total possible score: 16; range: 10–16). Participant characteristics are outlined in Table 2.
Figure 1.
PRISMA flow diagram.
Table 1.
Nonpharmacological approaches for pain levels and symptoms of depression in people with osteoarthritis (31 interventions from 23 studies).
| Author, year, country, setting | Interventions (description, frequency and duration) | Study design (randomisation method, number of participants, OA site/s, depression severity, blinding, follow-up) | Outcomes & measures (effect size; Hedge’s g) | Quality rating |
|---|---|---|---|---|
| Aerobic exercise only (4 interventions from 4 studies) | ||||
| *Cheung et al. 2017, USA70, Community and home |
A low-impact aerobic and strengthening exercise program that involved 15 min of mild aerobic exercise for a full-body warm-up, 30 min of strengthening exercises (isometric and isotonic exercises, i.e. without/with moving the joints). Participants were also asked to practice the aerobic portion 15–30 min/day and the strengthening portion 30 min/day on non-consecutive days at home. The program was progressive in nature 8 weeks: One weekly 45-min group session with 2–4 days per week home practice sessions |
Three-armed RCT (Aerobic strengthening, hatha yoga & control) Raters were blinded No post-intervention follow-up |
Pain levels: WOMAC d = 0.00 Depression: HADS d = − 0.12 |
13 |
| *French et al. 2013, Dublin71, Academic teaching hospitals |
Exercise therapy (ET) sessions were administered by a senior grade or clinical specialist physiotherapist. They included flexibility and strengthening exercises delivered using a semi-structured protocol. The protocol provided guidance on exercise prescription and progression but could be tailored to individual patient physical assessment findings. Strengthening focused on low-load exercise, commencing in non-weight-bearing positions, and progressing to functional positions. The key target muscles were the gluteal muscles. A daily home exercise program supplemented the clinic-based treatment. Participants were also encouraged to undertake aerobic exercise, such as walking, cycling, or swimming for at least 30 min, 5 days a week, and were given written and verbal information on the principles of aerobic conditioning, such as pacing, gradually progressing intensity and time of exercise, and incorporating exercise into daily life 8 weeks: One 30-min session per week (8 sessions in total) |
Three-armed RCT (exercise therapy, ET with adjunctive manual therapy, control) Raters were blinded No post-intervention follow-up |
Pain levels not reported Depression: HADS d = 0.16 |
15 |
| *Kuntz et al. 2018, Canada34, University setting |
The traditional exercise (TE) intervention reflected the current gold standard of strengthening exercise for knee OA. The program emphasized knee strengthening but also involved an aerobic warm-up, balance exercises, and stretching. TE was designed and supervised by kinesiologists and physical therapists and took place at a physical activity center. The sessions involved a ten-minute warm-up performed on a treadmill or cycle ergometer. Then, lower extremity strengthening was performed on pneumatically resisted exercise machines. Exercises included all major muscle groups of the lower extremity. The quadriceps were targeted at every session. Participants also completed balancing activities and static stretching. There was a progressive increase in the number of sets and resistance during strengthening exercises over the course of the intervention 12 weeks: 1-h, 3 times a week (36 sessions in total) |
Three-armed RCT (Traditional exercise, bio-mechanically-based yoga & control) Raters were blinded No post intervention follow-up |
Pain levels: KOOS d = 0.75 Depression: CESD d = − 0.09 |
13 |
| Lin et al. 2020, Taiwan72, Hospital clinic |
The active video games treatment involved using a computerised system called “Hot Plus” (Supreme Investment Co., Taipei). Two sessions were selected that required participants to move their trunk and lower limbs as fast as possible to tread on the step-sensing pad placed on the floor to complete on-screen tasks. The first session was ‘‘Whack-a-mole,’’ which required the player to stump moles into the holes when virtual moles appeared. It had 3 different levels. More and faster moles appeared simultaneously at higher difficulty levels. The second session was ‘‘Archery,’’ which required the player to step on the sensing pad to shoot an arrow when virtual dynamic targets appeared on the screen. This task also had 3 difficulty levels. Faster and smaller shooting targets appeared on the screen at higher difficulty levels 4 weeks: 20 min, 3 times a week (12 sessions in total) |
Two-armed RCT (active video games versus traditional therapeutic exercise Raters were blinded One- and three-months post intervention follow-up |
Pain levels: WOMAC d = 0.05 Depression: HADS d = − 0.03 |
12 |
| Movement meditation (7 interventions from 6 studies) | ||||
| *Cheung et al. 2017, USA70, Community and home |
The hatha yoga program was designed by a group of expert yoga teachers. Sessions included poses in the seated, supine, prone, and standing positions; breathing exercises; and relaxation/mindfulness training. Key yoga poses included “easy” seated pose, reclining bound angle, half locust variation, head to knee pose, warrior I and II, tree pose variation, bridge, reclining hamstring stretch with hip opener with strap, reclining twist and relaxation pose. A progressive series of poses with props such as yoga mats, blocks, straps, blankets, and chairs were used, and poses were modified when needed based on the participant’s physical abilities to increase confidence and the ability to remain in the pose and receive the benefits. Each yoga class consisted of 8–10 yoga poses with 2–3 new variable poses introduced at each session. A registered yoga instructor who was experienced in working with older adults with functional limitations taught the classes 8 weekly 45-min group classes with 2–4 days per week home practice sessions |
Three-armed RCT (Hatha yoga, aerobic strengthening & control) Raters were blinded No post-intervention follow-up |
Pain levels: WOMAC d = 0.51 Depression: HADS d = -0.32 |
13 |
| *Fransen et al. 2007, Australia27, Community |
Tai chi sessions were facilitated by four different Tai Chi instructors trained in a special program for arthritis designed by the study author Paul Lam. This program is a modification of 24 forms from the Sun style of Tai Chi and includes a preliminary 10-min warm-up session. Participants were able to purchase, if they desired, a Tai Chi video to assist with home practice 12 weeks: 1-h, 2 times a week (24 sessions in total) |
Three-armed RCT (Tai chi, hydrotherapy, waiting-list control) Raters were blinded 6-month post intervention follow-up |
Pain levels: WOMAC d = 0.52 Depression: DASS-21 d = 0.21 |
14 |
| *Kuntz et al. 2018, Canada34, University setting |
The biomechanically based yoga exercise program was led by a certified, trained yoga instructor and consisted of alignment-based postures that activate the lower limb musculature while maintaining a low knee abduction movement. The selected weight-bearing, static poses were performed barefoot and included squats and lunges with varying foot, trunk, and arm positioning. Careful attention was given to ideal alignment of the leg throughout the exercises. The classes began with a body-awareness exercise performed in supine followed by the strengthening postures and concluded with a closing deliberate relaxation exercise performed in supine. Exercise difficulty was progressively increased 12 weeks: 1-h, 3 times a week (36 sessions in total) |
Three-armed RCT (Traditional exercise, bio-mechanically-based yoga & control) Raters were blinded No post intervention follow-up |
Pain levels: KOOS d = 0.99 Depression: CESD d = 0.51 |
13 |
| Moonaz et al. 2015, Canada35, Hospital affiliated fitness centres |
The yoga program was designed by a registered yoga therapist (SM) with input from Johns Hopkins Arthritis Center faculty. Two yoga therapists with 10 + years of experience taught classes. Each class began with questions/comments (5 min), breathing exercises and chanting (5 min), warm-up and moving sequence (surya namaskara; 15 min), and isometric poses (asanas) (20 min) to increase strength, flexibility and balance. Classes ended with deep relaxation (sivasana; 10 min), a closing chant, and meditation (5 min). See supplemental file for sample class and modifications. Poses included gentle forward bends, backbends, twists, balances, standing, sitting, and lying poses, and were modified for individual at the discretion of the teacher and/or participant. Complexity of poses and intensity was standardized to allow gradual progression 8 weeks: 1-h, 2 times a week (16 sessions in total) |
Two-armed RCT (yoga versus wait-list control) Raters were blinded 9-month post intervention follow-up |
Pain levels: SF-36 (pain component) d = 0.62 Depression: CESD d = 0.84 |
13 |
| *Park et al. 2016, USA73, Community centres |
English linguistically tailored yoga program. Sit ‘N’ Fit Chair Yoga, designed for older adults with OA, is performed while sitting in a chair with arms, for easy access and standing. The chair is used for support for the standing poses. The intervention was developed by a research team of health care providers with a yoga teacher who has taught yoga for more than 15 years and is certified by the International Yoga Alliance. The yoga intervention consists of four components while using the support of a chair: breath of life (10 min), body proper (20 min), warrior in the body (5 min), and mind–body connection (10 min) 8 weeks: Two 45-min sessions per week (16 sessions in total) |
Four-armed RCT (chair yoga—English, chair yoga – Spanish, English/Spanish control: health education program) Raters were blinded One- and three-month post-intervention follow-up |
Pain levels: WOMAC d = 0.19 Depression: EDD-V1 d = 0.12 |
12 |
| *Park et al. 2016, USA73, Community centres |
Spanish linguistically tailored yoga program. Sit ‘N’ Fit Chair Yoga, designed for older adults with OA, is performed while sitting in a chair with arms, for easy access and standing. The chair is used for support for the standing poses. The intervention was developed by a research team of health care providers with a yoga teacher who has taught yoga for more than 15 years and is certified by the International Yoga Alliance. The yoga intervention consists of four components while using the support of a chair: breath of life (10 min), body proper (20 min), warrior in the body (5 min), and mind–body connection (10 min) 8 weeks: 45-min, 2 times a week (16 sessions in total) |
Four-armed RCT (chair yoga – Spanish, chair yoga – English, English/Spanish control: health education program) Raters were blinded One- and three-month post-intervention follow-up |
Pain levels: WOMAC d = 0.83 Depression: EDD-V1 d = 0.43 |
12 |
| Zhang et al. 2022, China74, Hospital |
Traditional Chinese Yijinjing Qigong exercise provided by professional instructors. Each session included a 5-min warm-up and cool-down period and a 30-min practice. Phase 1 (weeks 1–2) focused on fundamental principles, movement techniques, safety precautions. Phase 2 (weeks 3–4) focused on learning/practicing forms/associated movements. Phase 3 (weeks 5–12) involved completion of the family exercise plan, review video/homework materials 12 weeks: 40 min, 2 times a week (24 sessions in total) |
Two-armed RCT (qigong, stretching control) Raters were blinded (single-blinded) One- and three-month post intervention follow-up |
Pain levels: WOMAC d = 0.42 Depression: BDI d = 2.57 |
14 |
| Multimodal (10 interventions from 8 studies) | ||||
| *Ahn and Ham 2020, South Korea75, Community |
Experimental group 1 – Program outlined below with muscle strengthening plus stretching Health education and counselling combined with exercise classes based on the Interaction Model of Client Health Behaviour. The intervention involves 8 sessions and promotes cognitive-affective-behavioural skills including education and counselling on the disease characteristics and treatment options, encouraging self-responsibility, strengthening self-efficacy, self-monitoring, positive reinforcement, and emotional support regarding pain control, medication adherence, depression, and diet. Communication skills training was also provided to promote the client-professional interaction. Trained community health nurse practitioners led the intervention Duration not clear: 8 sessions (4 individual and 4 group), individual home visits were 30–60 min and group community sessions were 60–90 min |
Three-armed RCT (multimodal approach with muscle strengthening plus stretching, multimodal approach with muscle strengthening plus walking, control) Raters were blinded No post intervention follow-up |
Pain levels: WOMAC d = 0.66 Depression: CESD d = 0.07 |
13 |
| *Ahn and Ham 2020, South Korea75, Community |
Experimental group 2 – Program outlined above with muscle strengthening plus walking Duration not clear: 8 sessions (4 individual and 4 group), individual home visits were 30–60 min and group community sessions were 60–90 min |
Three-armed RCT (multimodal approach with muscle strengthening plus walking, multimodal approach with muscle strengthening plus stretching, control) Raters were blinded No post intervention follow-up |
Pain levels: WOMAC d = 0.78 Depression: CESD d = 0.11 |
13 |
| Barlow et al. 2000, UK76, Community (telephone-based) |
An RCT of the arthritis self-management programme (ASMP). ASMP is multicomponent and topics included: information about arthritis, an overview of self-management principles, exercise, cognitive symptom management (e.g. distraction, visualization, guided imagery), dealing with depression, nutrition, communication with family and health professionals, and contracting (setting of realistic goals during the forthcoming week). Participants report back to their group on their achievements at the weekly session. Participants are given a copy of The Arthritis Help Book (Lorig & Fries, 1995). Interactive course with group discussion, problem solving, role plays and mastery experience (trying out skills introduced) 4 months: 6 weekly 2-h sessions, delivered by pairs of lay leaders (most with arthritis) |
Two-armed RCT (self-management program versus waitlist-control) Unclear whether the raters were blinded 12-month post intervention follow-up |
Pain levels: ASE d = 0.31 Depression: HADS d = 0.12 |
11 |
| *French et al. 2013, Dublin71, Academic teaching hospitals |
Exercise therapy (ET) with adjunctive manual therapy (MT) The sessions included ET (as described above) and up to 15 min of MT in line with current clinical practice at participating sites. A choice of nonmanipulative MT techniques based on pain/stiffness relations and movement restrictions of the affected hip was available, with no more than 5 MT techniques allowed during an individual session 8 weeks: One 45-min session per week (8 sessions in total) |
Three-armed RCT (ET + MT, ET alone, control) Raters were blinded No post-intervention follow-up |
Pain levels not reported Depression: HADS d = 0.18 |
15 |
| Hurley et al. 2007, UK77, Primary care providers |
Rehabilitation program integrating exercise, self-management, and active coping strategies. The rehabilitation program combined discussion on specific topics regarding self-management and coping, etc., with an individualized, progressive exercise regimen. To ensure consistency in content and delivery, the same experienced physiotherapist devised, supervised, and progressed all sessions for all participants 6 weeks: Twice weekly (12 sessions in total) |
Two-armed RCT (rehabilitation program, usual care) Raters were blinded 6-month post intervention follow-up |
Pain levels: WOMAC d = 0.29 Depression: HADS d = 0.16 |
13 |
| *Somers et al. 2012, USA78, Community and clinic |
Lifestyle behavioural weight management (BWM) only. Each participant was given a copy of a manual, which focused on 5 elements related to weight loss: lifestyle, exercise, attitudes, relationships, and nutrition, and contains 16 weekly sessions. The content of most of the group sessions was based on the weekly topic(s). In addition, participants received Appetite Awareness Training for 2 sessions, which emphasized the importance of attending to internal hunger and fullness cues. The overall goal of the program was a weight loss of 1–2 pounds a week achieved by gradually decreasing calorie and fat intake through permanent lifestyle changes 24 weeks: One 60-min sessions per week for first 6 months, One 60-min session every other week for the second 6 months |
Four-armed RCT (PCST, BWM, PCST + BWM, standard care) Raters were blinded 4-weeks post intervention follow-up |
Pain levels: WOMAC d = 0.17 Depression: AIMS (psychological subscale) d = 0.00 |
14 |
| *Somers et al. 2012, USA78, Community and clinic |
Pain coping skills training (PCST) plus lifestyle behavioural weight management (BWM). Participants concurrently received the BWM protocol described above and the PCST protocol described under the therapeutic section of this table. During the first 12 weeks, participants attended 120 min of group sessions that first presented the BWM protocol and then the PCST protocol. During the first 12 weeks, participants also attended three 90-min supervised exercise sessions each week. During the last 12 weeks, participants attended 120 min of group sessions held every other week that first presented the BWM protocol and then the PCST protocol. All PCST + BWM groups were delivered by clinical psychologists referenced above 24 weeks: One 60-min sessions per week for first 6 months, One 60-min session every other week for the second 6 months |
Four-armed RCT (PCST, BWM, PCST + BMW, standard care) Raters were blinded 4-weeks post intervention follow-up |
Pain levels: WOMAC d = 0.72 Depression: AIMS (psychological subscale) d = 0.35 |
14 |
| Tak et al. 2005, Netherlands79, Community |
'Hop with the Hip’ exercise program with strength training and lifestyle advice versus self-initiated contact with their general practitioner (usual care). The program included strength training sessions using fitness equipment under supervision of a PT, OA education from PT, guidance for a home exercise program, personal ergonomic advice (given by an OT), education on dietary aspects (given by a dietician) and participants with BMI > 30 given personal consultation. Further information was also available via a special telephone line 8 weeks: one 1-h group session per week (8 sessions in total) |
Two-armed RCT (program, control) Raters were blinded 3-months post intervention follow-up |
Pain levels: Harris Hip Score (HHS) pain subscale d = 0.52 No depression measures |
10 |
| Walsh et al. 2020, UK80, Community (physio. clinic) |
The Facilitating Activity and Self-management in Arthritic Pain (FASA) program is a group exercise and self-management intervention facilitated by a physiotherapist. The intervention included approximately 20–25 min of group discussion and problem-solving session (with a supporting handbook) regarding issues of self-management. Topics included activity-rest cycling, use of ice and heat for pain relief, goal setting and action plans, exercise recommendations, healthy eating and managing changes in pain. After each discussion, participants undertook approximately 30–35 min of exercise, based on stations of strengthening, aerobic and co-ordination activities. Further to the exercises, each individual completed an action plan regarding exercise/activities they aimed to achieve over the following week 6 weeks: 60-min 2 times per week (12 sessions in total) |
Two-armed RCT (program, control) Raters were blinded 6-months post intervention follow-up |
Pain levels: DI-SMFA d = 0.13 Depression: HADS d = 0.07 |
16 |
| Yip et al. 2008, Hong Kong81, Hospital out-patient clinic and a Telehealth wellness centre |
Modified Arthritis Self-Management Programme (ASMP: Lorig et al. 1985)82 with an added exercise component. Small group classes (10–15 people) were led by registered nurses trained in leading small groups and self-management principles, standard ASMP plus goal-directed exercise component relevant to the group’s lifestyle habit: stretching exercises, walking, and Tai Chi types of movement – fluid, gentle, relaxed, slow – aimed at enhancing exercise for affected joints (Yip et al. 2007)83. The group practised the stretching exercise together twice each session, Tai Chi exercises were taught and reinforced for 30 min each session, intervention group were given pedometer for 3 days at baseline to reinforce walking. Routine conventional treatment prescribed by orthopaedic doctor or out-patient clinic was also provided 6 weeks: One 2-h session per week (6 sessions in total) |
Two-armed RCT (ASMP, usual care) Raters were blinded Results are reported following 1-year post-initiation of an ongoing program |
Pain levels: ASE (pain subscale; Lorig et al. 1985) d = 0.38 No depression measures |
12 |
| Resistance exercise only (3 interventions from 3 studies) | ||||
| Bossen et al. 2013, Netherlands84, Community (online in people’s homes) |
Web-based physical activity intervention 'Joint2Move'. The program incorporates a baseline test, goal setting, time-contingent PA objectives (i.e. on fixed time points), and text messages to promote PA. Positive reinforcement is provided of gradual PA, despite the presence of pain. The gradual increase in activities changes the perception that PA is related to pain and reinforces confidence to improve PA performance. The Join2move intervention is a fully automated web-based intervention that contains automatic functions (web-based text messaging and automatic emails) without human support 9 weeks: self-paced program in which a patient’s favourite recreational activity is gradually increased in a time-contingent way |
Two-arm RCT (web-based intervention, wait-list control) Raters were not blinded Three- and 12-month post-intervention follow-up |
Pain levels: KOOS/HOOS d = 0.19 Depression: HADS d = 0.13 |
13 |
| *Fransen et al. 2017, Australia27, Community |
Hydrotherapy sessions were facilitated by four different registered physiotherapists. The hydrotherapy program (Fransen et al.27: Appendix A) was designed by the senior rheumatology physiotherapist 12 weeks: 1-h, twice a week |
Three-armed RCT (Hydrotherapy, tai chi, waiting-list control) Raters were blinded 6-month post intervention follow-up |
Pain levels: WOMAC d = 0.71 Depression: DASS-21 d = 0.50 |
14 |
| Taglietti et al. 2018, Brazil36, Aquatic physiotherapy centre and primary health care unit |
Aquatic exercise protocol in a heated pool, one-to-one sessions provided by certified physiotherapists. The water temperature was maintained at approximately 32 °C, with a depth of 1.2 m. The exercise protocol consisted of specific exercises: 5 min of warm-up with walking, patellar mobilization; stretching the leg muscles (quadriceps, gluteus, adductors and abductors of hip, triceps surae, and hamstrings); 15 min of knee and hip isometric and dynamic exercises with elastic bands (gluteus, adductors and abductors, quadriceps, hamstrings, and triceps surae); 20 min of aerobic exercises (stationary running or deep water-running); 10 min of step training and proprioceptive exercises; and 10 min of cool down with massage and relaxation 8 weeks: Two 60-min sessions per week (16 sessions in total) |
Two-armed RCT (Hydrotherapy, education control) Raters were blinded Three-month post intervention follow-up |
Pain levels: WOMAC d = 3.33 Depression: GDS d = 2.17 |
13 |
| Therapeutic only (7 interventions from 7 studies) | ||||
| Allen et al. 2019, USA85, Community (telephone-based) |
Pain coping skills training for African Americans. Culturally tailored pain telephone-based coping skills training (CST) program Coping skills training counselors provided instruction in cognitive and behavioral pain coping skills and led participants in guided rehearsals of these skills. Content included progressive muscle relaxation, mini-relaxation practices, communication with significant others about pain and coping, managing unhelpful mood, activity pacing, pleasant activities, pleasant imagery and other distraction techniques, physical activity and OA, weight management, problem solving and maintenance. Participants were asked to engage in home-based practice of the skills to enhance their application in pain-related situations. During each phone call, the counselor reviewed participants’ home practice, including successes and barriers, encouraged problem solving, and worked to set goals for application of skills. Participants were given handouts to facilitate each session, along with an audio recording to guide progressive muscle relaxation 3 months: 30–45 min sessions (11 sessions in total) |
Two-armed RCT (intervention, wait-list control) Raters were blinded Three- and 9-months post intervention follow-up |
Pain levels: WOMAS d = 0.17 Depression: PHQ-8 d = 0.10 |
16 |
| Broderick et al. 2014, USA86 |
Pain coping skills training delivered by nurses. Training included cognitive and behavioural skills to manage pain and enhance self-perception of pain control. Four broad coping skills were taught across ten 30–45-min sessions: relaxation response, attention diversion techniques, altering activity and rest patterns as a way of increasing physical activity, reducing negative pain-related thoughts and emotions. The training included a treatment manual and home practice 10 weeks: One 30–45 min session per week (10 sessions in total) |
Two-armed RCT (intervention, usual care) Raters were blinded Six- and 12-months post intervention follow-up |
Pain levels: WOMAC d = 0.22 Depression: BDI d = 0.17 |
16 |
| Hausmann et al. 2017, USA87, Academic Veterans Affairs Medical Centre |
Positive psychology intervention program consisting of positive psychological skill-building activities drawn from the positive psychology literature and then refined based on qualitative input from patients. Included activities to build positive psychological skills focused on gratitude, kindness, optimism, mindfulness, self-affirmation, identifying and using personal strengths reflecting on good things, and forgiveness 6 weeks: 1 session per week (6 sessions in total) |
Two-armed RCT (therapy, control) Raters were not blinded Three- and 6-months post intervention follow-up |
Pain levels: WOMAC d = 1.08 Depression: PANAS d = 0.20 |
11 |
| Helminen et al. 2015, Finland88, Community (groups) |
Cognitive–behavioural group intervention (7 − 13 people) supervised by an experienced psychologist and a physiotherapist. Each session lasted for two hours with a 15 − 20 min break to enhance peer support and social bonding. The outline of the sessions included an introduction (15 min), lecture (knowledge and insight, max 15 min), problem solving (in pairs/teams, 15 − 20 min), skills training (15 − 20 min), homework assignments (15 min), and a résumé (feedback) of the session (15 min). A written example of a knee OA pain patient’s life was used throughout the intervention as a basis for discussion and practice in problem solving 6 weeks: One weekly 2-h session (6 sessions in total) |
Two-armed RCT (therapy, control) Raters were blinded Three- and 12-months post intervention follow-up |
Pain levels: WOMAC d = 0.18 Depression: BDI d = 0.02 |
15 |
| Lin et al. 2003, USA89, Primary care clinics |
Improving Mood-Promoting Access to Collaborative Treatment (IMPACT). Psychotherapeutic intervention using a collaborative care approach. Nurse/psychologist depression care management including psychosocial history, education, and behavioural activation, identify treatment preferences. Antidepressant medications and/or 6 to 8 sessions of psychotherapy (Problem-Solving Treatment in Primary Care). Usual care group received routinely available treatments including medication and referrals to speciality mental health services 1-year: 6–8 sessions |
Two-armed RCT (therapy, usual care) Unclear whether the raters were blinded Three-, 6- and 12-months post intervention follow-up |
Pain levels: RAND-36 (pain subscales) d = 0.18 Depression values not reported |
14 |
| O’Moore et al. 2018, Australia90, Community (online) |
Internet Cognitive-Behavioural Therapy (iCBT Sadness Program). iCBT Sadness Program consists of lessons representing best practice CBT, as well as regular homework assignments and access to supplementary resources. Each lesson comprises a cartoon narrative in which a character gains mastery over symptoms of depression by learning and implementing CBT skills. Participants could submit queries via email or phone 10 weeks: 6 online sessions |
Two-armed RCT (therapy, treatment as usual (TAU)) Raters were blinded Three-months post intervention follow-up |
Pain levels: WOMAC d = 0.28 Depression: PHQ-9 d = 1.01 |
13 |
| *Somers et al. 2012, USA78, Community and clinic |
Pain coping skills training (PCST) only. PCST was delivered by clinical psychologists with prior PCST experience (1 to 5 years) who were systematically trained by a senior clinical psychologist who is an expert in pain coping skills training. Training included role-playing, listening to the protocol delivered on audiotape, and observation of PCST being delivered in a group format. Psychologists delivering the treatment for this protocol met for supervision weekly with the senior psychologist; audiotapes of sessions were reviewed to evaluate the concurrence between the session delivery and the intervention protocol and role-playing for the next session was conducted. Four psychologists led the PCST groups during the study. The PCST intervention was designed to 1) decrease maladaptive pain catastrophizing; and 2) enhance participants’ ability to control and decrease pain by increasing use of adaptive coping strategies (e.g. distraction, relaxation, and changing activity patterns) 24 weeks: One 60-min sessions per week for first 6 months, One 60-min session every other week for the second 6 months |
Four-armed RCT (PCST, BWM, PCST + BWM, standard care) Raters were blinded 4-weeks post intervention follow-up |
Pain levels: WOMAC d = 0.23 Depression: AIMS (psychological subscale) d = -0.12 |
14 |
AIMS Arthritis Impact Measurement Scale, ASE Arthritis Self-Efficacy scale, BDI Beck’s Depression Inventory, CESD Center for Epidemiological Studies – Depression, DASS-21 Depression, Anxiety and Stress Scale – 21 items, DI-SMFA Dysfunction Index of the Short Musculoskeletal Functional Assessment, EDD-v1 Emotional Distress and Depression—version 1, GDS Geriatric Depression Scale, HADS Hospital Anxiety and Depression Scale, HHS Harris Hip Score, HOOS Hip injury Osteoarthritis Outcome Score, KOOS Knee injury Osteoarthritis Outcome Score, PHQ Patient Health Questionnaire, RAND-36 36-item health survey, PANAS Positive And Negative Affect Schedule, RCT Randomised Control Trial, SF-36 Short Form survey (36-item), WOMAC Western Ontario and McMaster Universities Osteoarthritis Index.
*studies where more than one intervention have been included, e.g. 3-armed RCTs.
Table 2.
Participant characteristics.
| Author, year | N | Sex N and (%) female | Age in years [mean ± SD or median (IQR)] | Osteoarthritis (type and severity if known) | Diagnosed depression | Other characteristics |
|---|---|---|---|---|---|---|
| Ahn and Ham 202075 | 90 | 80 (88) | 71.3 ± 7.1 | OA (not specified) | No | 49% had hypertension, majority of participants were farmers |
| Allen et al. 201985 | 248 | 122 (49) | 59.0 ± 10.3 | Hip/knee OA | No | None |
| Barlow et al. 200076 | 544 | 168 (31) |
I: 57.3 ± 13.2 C: 59.1 ± 12.3 |
OA (not specified) | No |
OA condition duration in years mean ± SD: I: 13.9 ± 10.6 C: 13.6 ± 9.1 Arthritis type: 104 OA, 70 RA, 25 other |
| Broderick et al. 201486 | 256 | Not stated |
I: 68.0 ± 8.7 C: 66.4 ± 10.3 |
Hip/knee OA Knee (vs. hip), 81%; 73% (of sample) Arthritis severity (K-L grading; I, C): 0 to 1; 17%, 27% > 1 to 2; 27%, 21% > 2 to 3; 26%, 23% > 3 to 4; 30%, 30% |
No |
OA condition duration in years mean ± SD: I: 10.7 ± 11.2 C: 11.3 ± 10.9 15–16% were receiving treatment for a psychiatric disorder and 10% had memory/thinking problems |
| Bossen et al. 201384 | 199 | 129 (65) |
I: 61 ± 5.9 C: 63 ± 5.4 |
Group: % of sample knee, hip, both OA I: 67%, 21%, 12% C: 60%, 20%, 19% |
No |
Number of comorbidities (I%, C%): None, (65, 60) One, (19, 16) Two or more, (16, 23) |
| Cheung et al. 201770 | 83 | 70 (84) |
I1: 68.9 ± 7.7 I2: 74.4 ± 7.5 C: 71.8 ± 8.0 |
Knee OA | No | None |
| Fransen et al. 200727 | 2152 | 112 (74) |
I1: 70.0 ± 6.3 I2: 70.8 ± 6.3 C: 69.6 ± 6.1 |
Hip/knee OA: % of sample for I1, I2, C Both knees: 74%, 61%, 80% Both hips: 20%, 29%, 29% Single knee: 93%, 73%, 88% Single hip joint: 7%, 27%, 12% |
No |
OA symptom duration: % of sample for I1, I2, C < 6 years: 31%, 46%, 22% 6–10 years: 35%, 27%, 46% > 10 years: 35%, 25%, 29% |
| French et al. 201371 | 131 | 84 (64) |
I1: 61.8 ± 9.7 I2: 64.8 ± 9.8 C: 60.8 ± 9.7 |
Knee OA | No | Mean number of comorbidities: I1: 2, I2: 2.6, C: 1.86 |
| Hausmann et al. 201787 | 42 | 7 (17) | 67.5 ± 10.3 |
% of sample hip/knee/both OA 45%, 55%, 45% |
33% of participants had anxiety and 48% depression | Comorbidities mean ± SD: 3.7 ± 2.8 |
| Helminem et al. 201588 | 111 | 77 (69) |
I: 64.5 ± 7.3 C: 62.8 ± 7.2 |
Knee OA | No | None |
| Hurley et al. 200777 | 418 | 294 (70) |
I: 68 (51–84) C: 67 (51–89) |
Knee OA | No |
OA symptom duration in years, median (IQR): I: 5 (3–11) C: 6 (3–5) |
| Kuntz et al. 201834 | 31 | 31 (100) |
I1: 65.5 ± 5.6 I2: 63.7 ± 9 C: 71.1 ± 9.3 |
Knee OA | No |
Comorbidities mean ± SD: I1: 2.1 ± 1.0 I2: 2.1 ± 1.7 C: 2.9 ± 1.4 |
| Lin et al. 200389 | 1001 | 684 (68) | 72 ± 7.4 | Knee OA | Yes (N, %): Major depression: 159, 16 Dysthymia: 288, 29 | None |
| Lin et al. 202072 | 80 | 41 (51) |
I: 55.9 ± 15.8 C: 58.1 ± 16.9 |
Knee OA | No |
Comorbidity (N) Yes (52) No (28) |
| Moonaz et al. 201535 | 75 | 72 (96) | 52 ± 12 | OA (not specified) | No |
OA condition duration in years mean ± SD I: 9.9 ± 8.7 C:8.6 ± 9.4 |
| O’Moore et al. 201890 | 69 | 55 (80) |
I: 63.2 ± 7.4 C: 59.7 ± 6.0 |
Knee OA | Yes. Major depressive disorder | None |
| Park et al. 201673 | 100 | 75 (75) | 75.3 ± 7.5 | Lower extremity OA | No | K-L score: 2.4 ± 1.1 |
| Somers et al. 201278 | 232 | 184 (79) | 58.0 ± 10.4 | Knee OA | No | None |
| Taglietti et al. 201836 | 60 | 41 (68) | 68.3 ± 4.8 | Knee OA | No | None |
| Tak et al. 200579 | 109 | 64 (68) |
I: 67.4 ± 7.6 C: 68.9 (SD not provided) |
Hip OA | No | None |
| Walsh et al. 202080 | 349 | 216 (62) |
I: 66.3 ± 8.1 C: 66.5 ± 8.4 |
% of sample Hip/knee only I: 57; C: 59 Lower back pain (LBP): I: 59; C: 56 Hip/knee and LBP: I: 65; C: 66 |
No | None |
| Yip et al. 200881 | 95 | 81 (85) |
I: 64.8 ± 10.1 C: 63.4 ± 10.7 |
Knee OA | No |
OA condition duration in years mean ± SD I: 8.0 ± 5.9; C: 6.7 ± 6.0 |
| Zhang et al. 202274 | 50 | 37 (74) |
I: 55.8 ± 8.4 C: 53.4 ± 10.7 |
Knee OA | No | None |
C control, I intervention, K-L Kellgren and Lawrence grading system, OA osteoarthritis, RA rheumatoid arthritis.
Meta-analyses
Pain
For pain, 29 interventions from 21 published articles were included in the meta-analysis (n = 4,382; 65 ± 6.9 years; 70% female; people with knee/hip OA at varying severity). There was a significant overall medium effect on reducing pain (ES = 0.43, 95% CI [0.25, 0.61], p < 0.001). Heterogeneity was significant and substantial (I2 = 71.5%, pq < 0.001) (Fig. 2a).
Figure 2.
Main meta-analysis (a) and sub-group meta-analysis (b) for pain. Subgroup meta-analysis results showing effect sizes (i.e. SMDs), CIs (black lines), PIs (red lines) and I2 values for interventions. Movement meditation included yoga, tai chi, and qigong. Resistance exercise included hydrotherapy. Multimodal approaches included a combination of exercise and therapeutic/education-based approaches. CI confidence interval, I2 percentage of variation across studies that is due to heterogeneity, PI prediction interval, SMD standardized mean difference, w weight.
Sensitivity analyses were performed to examine potential outliers. A study was excluded with a very large effect size that was in the intervention subtype defined as ‘resistance exercise only’36. This analysis included 28 interventions from 20 published articles (n = 4322). The overall effect was ES = 0.33, 95% CI [0.24, 0.42], p < 0.001, I2 = 32.33%, pq = 0.0052. According to Cohen, it is a small to medium effect. Heterogeneity was at an acceptable level.
Subgroup analysis was then performed using random effects models for type of intervention and were defined as aerobic exercise only, resistance exercise only, therapeutic only, multimodal approaches and movement meditation. Effect sizes for reducing pain were significant for movement meditation (7 interventions: ES = 0.52, 95% CI [0.35, 0.69], p < 0.001; I2 = 0.0%), multimodal approaches (9 interventions: ES = 0.37, 95% CI [0.22, 0.51], p < 0.001; I2 = 41.2%, pq = 0.0092), resistance exercise (2 interventions: ES = 0.43, 95% CI [− 0.07,0.94], p = 0.046; I2 = 74.4%, pq = 0.0048), and therapeutic approaches (7 interventions: ES = 0.21, 95% CI [0.11, 0.31], p < 0.001; I2 = 3.1%, pq = 0.402). Aerobic exercise alone (including 3 interventions) did not significantly improve pain (Fig. 2b). Analysis of variance revealed significant differences between subgroup effect sizes for pain between movement meditation and aerobic exercise (p < 0.05) and movement meditation and therapeutic approaches (p < 0.01). Heterogeneity was at an acceptable level for all subgroups except resistance exercise though there are too few studies in this group to do further subgroup analysis.
Depression
For depression, 28 interventions from 20 published articles were included in the meta-analysis (n = 3377; 63 ± 7.0 years; 69% female; people with knee/hip OA at varying severity). There was a significant overall small effect on reducing symptoms of depression (ES = 0.29, 95% CI [0.08, 0.49], p < 0.001). Heterogeneity was significant and substantial (I2 = 73.3%, pq < 0.001) (Fig. 3a).
Figure 3.
Main meta-analysis (a) and sub-group meta-analysis (b) for symptoms of depression. Subgroup meta-analysis results showing effect sizes (i.e. SMDs), CIs (black lines), PIs (red lines) and I2 values for interventions. Movement meditation included yoga, tai chi, and qigong. Resistance exercise included hydrotherapy. Multimodal approaches included a combination of exercise and therapeutic/education-based approaches. CI confidence interval, I2 percentage of variation across studies that is due to heterogeneity, PI prediction interval, SMD standardized mean difference, w weight.
For the sensitivity analysis two studies were excluded with very large effect sizes. They included a resistance intervention36 and a movement meditation intervention74. This analysis included 26 interventions from 18 published articles (n = 3274). The overall effect is small and significant ES = 0.16, 95% CI [0.08, 0.24], p < 0.001, I2 = 11.78%, pq = 0.293. Heterogeneity was at an acceptable level.
Subgroup analysis was then performed using random effects models for type of intervention and were defined as aerobic exercise only, resistance exercise only, therapeutic only, multimodal approaches and movement meditation. Effect sizes for reducing symptoms of depression were significant for movement meditation (6 interventions: ES = 0.30, 95% CI [0.06, 0.55], p = 0.008, I2 = 18.9%, pq = 0.291), and multimodal interventions (8 interventions: ES = 0.12, 95% CI [0.07, 0.18], p < 0.001, I2 = 0%, pq = 0.940). Resistance exercise, aerobic exercise, or therapeutic approaches alone (2, 4 and 6 interventions respectively) did not improve symptoms of depression (Fig. 3b). Analysis of variance revealed no significant differences between subgroup effect sizes for depression. Heterogeneity was at an acceptable level for movement meditation, multimodal approaches and aerobic exercise though was high for the remaining subgroups.
Publication bias
A funnel plot and the Egger test of asymmetry were used to examine publication bias (Fig. 4). The funnel plot was asymmetric. The Egger test was significant for pain (p < 0.001) suggesting potential publication bias and/or small-study effects91, though was not significant for depression (p = 0.216).
Figure 4.
Funnel plot of all included studies for (A) pain, and (B) symptoms of depression.
Discussion
Summary of main findings
Overall, meta-analyses from 23 high quality rated studies showed that nonpharmacological approaches significantly reduced pain (ES = 0.43, 95% CI [0.25, 0.61], 29 interventions) and symptoms of depression (ES = 0.29, 95% CI [0.08, 0.49], 28 interventions) in people with OA. Heterogeneity was lowered after removing outliers. For pain, subgroup analysis showed significance for movement meditation, multimodal approaches, therapeutic approaches, and resistance exercise only. However, heterogeneity was considerably high for resistance exercise. Movement meditation and multimodal approaches were also significant for symptoms of depression.
Based on these findings, approaches that are either multimodal (e.g. education/therapeutic approaches alongside exercise) or include focus on both the body and mind (e.g. focused breathing and mindfulness techniques used alongside movement, seen in yoga, qigong, and tai chi) are effective for reducing pain and symptoms of depression in people with knee/hip OA and warrant further investigation.
How these findings compare to others
Our findings support and expand on others regarding beneficial effects of exercise for reducing pain in people living with OA23,87. There are also differences between our findings and those of other studies. Fransen et al.’s26 Cochrane review based on 44 trials indicated that exercise significantly reduced pain and improved physical function26. Uthman et al.’s92 review based on 60 trials compared the effectiveness of different exercise interventions92.
Our analysis included only two resistance and three aerobic interventions. Both Cochrane reviews included more articles than our review. This was likely due to the main outcome of interest being pain and did not include depression, and because we only included studies with an inactive control group. Our findings align with other studies regarding reducing pain26,92, though extend our current understanding by investigating exercise type. In Fransen et al.’s review, no subgroup analysis was performed to examine potential different effects of exercise type, despite the heterogeneity and marked variability that was present regarding the exercise interventions assessed and other aspects of methodology26.
A more recent network meta-analysis investigated the relative efficacy of different types of exercise (aerobic, mind–body, strengthening, flexibility/skill or mixed) and found that aerobic had an equivalent beneficial effect (SMD = 1.11) to mind–body approaches in reducing pain93. Additionally, reporting that mixed exercise was the least effective. However, these approaches did not include education/therapeutic components as we did here, nor did they examine depression symptomatology. In another network analysis that examined different approaches on mental health measures in people with OA, the authors concluded that strengthening exercise was most beneficial for overall mental health, or mixed exercise for symptoms of depression94, though pain was not examined.
Strengths and limitations
Strengths of this study include that we were able to perform a meta-analysis including 23 studies where one or more intervention groups were compared with a control group. We also achieved high agreement levels between independent reviewers on ratings of study quality. We showed that similar approaches were effective for reducing both pain and symptoms of depression in OA, suggesting that these benefits will likely transfer to additional improvements in quality of life92,90. Further, identifying effective therapeutic options can support informed choice for people living with OA, and thus support shared decision-making processes in collaboration with healthcare professionals and family members to tailor treatments that best suit their individual needs and preferences95.
However, there are several limitations to consider when interpreting these results. Although the overall effect obtained for the meta-analyses for both pain and depression were significant, not all studies reported beneficial effects (e.g. pain72,85, symptoms of depression79,89). Possible explanations for observed non-significant effects in individual studies include low intervention dose, baseline depression scores and/or lack of formal depression diagnoses, and that participants differed between studies in the diagnostic site of OA, e.g. knee or hip. In our study, this information is included in Tables 1 and 2 where available.
Publication bias should also be considered, and some studies had very small sample sizes which may increase the likelihood of Type II errors. The funnel plots and Egger’s regression indicated publication bias was likely for pain level data though not for symptoms of depression, possibly due to pain more frequently being recorded as a primary outcome measure and depression a secondary outcome measure in these studies. Therefore, the overall effect size for pain may be an overestimation. This can be caused when small sample studies have not been published because significant effects were not obtained.
We included RCTs that measured pain and symptoms of depression in OA. Studies not measuring depression though were effective in reducing pain in OA may not have been captured in this review, for example therapeutic exercise and pain education96. Safety is a key consideration in intervention studies additional to effectiveness and adverse effects have been captured in other reviews26,97. However, we did not extract data for this outcome. Exercise is usually safe and well-tolerated for people with OA and depression, but this is based on limited data as studies seldom collect this as an outcome. Future trials would benefit from more focus and rigorous reporting on safety.
Clinical implications
In this systematic review we provide evidence for several approaches for reducing pain and symptoms of depression in OA, a condition with a substantial health burden in which co-morbidities are common. We highlight the importance of considering psychological health, i.e. symptoms of depression, in addition to physical health, i.e. pain, when providing quality healthcare to people living with OA. Interventions may be more effective when they are tailored to the individual and involve healthcare professionals working collaboratively across disciplines, e.g. nutrition, exercise, psychology, as well as with patients/clients. Further investigation is warranted on combining psychological and physical health focused interventions as well as multidisciplinary expertise to optimize patient outcomes.
Pain is experienced by at least half of people living with dementia and is most often attributed to OA98. Given the strong associations between OA and dementia6–8, 98, and that forty percent of dementia cases could be prevented or delayed by targeting risk factors including physical inactivity and associated chronic conditions31,99, targeting associated chronic conditions including OA will likely lead to reductions in dementia prevalence rates.
Conclusion
In this study, mind–body approaches were more effective than aerobic/resistance exercise or psychological therapy alone for reducing pain and depression in people with OA. Future research is required to determine the optimal approach for OA based on diagnostic site and symptom severity of comorbid conditions such as depression. Further, given the high prevalence rates of chronic conditions and people who experience multimorbidity, a better understanding of how the underlying mechanisms of co-occurring chronic conditions interact is also required. Incorporating qualitative studies such as focus groups and interviews to examine the experience, perspectives and preferences of people living with OA and depression will also add considerable value to this area.
Supplementary Information
Acknowledgements
This research was supported by philanthropic funding provided by Faye Williams.
Author contributions
C.B. designed the study, collected data, carried out quality ratings, analysed the data, and drafted the article. A.-N. Casey collected data, carried out quality ratings, analysed data and assisted with writing the article. B.P. supervised data collection and analysis and assisted with writing the article. M.J. and K.W. assisted with clinical interpretation of results and writing the article. All authors reviewed and authorized the final version of the article.
Data availability
The data will be made available on request to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-41709-x.
References
- 1.Australian Bureau of Statistics (ABS). Causes of Death, Australia, 2020. https://www.abs.gov.au/statistics/health/causes-death/causes-death-australia/latest-release#australia-s-leading-causes-of-death-2020 (Accessed 18 January 2023) (2022).
- 2.Sharma A, Kudesia P, Shi Q, Gandhi R. Anxiety and depression in patients with osteoarthritis: Impact and management challenges. Open Access Rheumatol. 2016;8:103–113. doi: 10.2147/OARRR.S93516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axford J, Butt A, Heron C, Hammond J, Morgan J, Alavi A, et al. Prevalence of anxiety and depression in osteoarthritis: use of the Hospital Anxiety and Depression Scale as a screening tool. Clin. Rheumatol. 2010;29(11):1277–1283. doi: 10.1007/s10067-010-1547-7. [DOI] [PubMed] [Google Scholar]
- 4.Australian Institute of Health and Welfare (AIHW) Osteoarthritis. https://www.aihw.gov.au/reports/chronic-musculoskeletal-conditions/osteoarthritis/contents/impact-of-osteoarthritis (Accessed 18 January 2023) (2020).
- 5.Australian Institute of Health and Welfare (AIHW). Chronic pain in Australia. https://www.aihw.gov.au/reports/chronic-disease/chronic-pain-in-australia/summary (Accessed 18 January 2023) (2022).
- 6.Guo R, Ou Y, Hu H. The Association Between Osteoarthritis with Risk of Dementia and Cognitive Impairment: A Meta-Analysis and Systematic Review. J. Alzheimer’s Dis. 2022;89(4):1159–1172. doi: 10.3233/JAD-220568. [DOI] [PubMed] [Google Scholar]
- 7.Chang K, Chung C, Weber A, Mak S, Berenbaum F, Sallam J. Association between osteoarthritis and increased risk of dementia: A systemic review and meta-analysis. Medicine. 2019;98(10):e14355. doi: 10.1097/MD.0000000000014355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S, Wang W, Chou L, et al. Osteoarthritis Increases the Risk of Dementia: A Nationwide Cohort Study in Taiwan. Scientific Reports. 2015;5:10145. doi: 10.1038/srep10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuring J, Mathias J, Ward L. Risk of dementia in persons who have previously experienced clinically-significant depression, anxiety, or PTSD: A systematic review and meta-analysis. J. Affect. Disord. 2020;274:247–261. doi: 10.1016/j.jad.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Burley CV, Burns K, Lam BC, Brodaty H. Nonpharmacological approaches reduce symptoms of depression in dementia: A systematic review and meta-analysis. Ageing Res. Rev. 2022;79:101669. doi: 10.1016/j.arr.2022.101669. [DOI] [PubMed] [Google Scholar]
- 11.May K, Scammell J. Nurses’ experiences of pain management in end-of-life dementia care: A literature review. Int. J. Palliat. Nurs. 2020;26(3):110–118. doi: 10.12968/ijpn.2020.26.3.110. [DOI] [PubMed] [Google Scholar]
- 12.Dementia Australia, 2022 Dementia statistics https://www.dementia.org.au/statistics (Accessed 18 January 2023).
- 13.WHO. Ageing and health. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (Accessed 18 January 2023) (2022).
- 14.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn’t the stomach digest itself? Physiol. Rev. 2008;88(4):1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 15.McCrae J, Morrison E, MacIntyre I, Dear J, Webb D. Long-term adverse effects of paracetamol–A review. Br. J. Clin. Pharmacol. 2018;84(10):2218–2230. doi: 10.1111/bcp.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yip K, Oettinger J. Why are we still using opioids for osteoarthritis? Int. J. Clin. Pract. 2020;74(1):e13416. doi: 10.1111/ijcp.13416. [DOI] [PubMed] [Google Scholar]
- 17.Bartley EJ, Palit S, Staud R. Predictors of osteoarthritis pain: The importance of resilience. Curr. Rheumatol. Rep. 2017;19(9):1–9. doi: 10.1007/s11926-017-0683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyrkanides S, Tallents RH, Miller JH, Olschowka ME, Johnson R, Yang M, et al. Osteoarthritis accelerates and exacerbates Alzheimer’s disease pathology in mice. J. Neuroinflamm. 2011;8(1):1–8. doi: 10.1186/1742-2094-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arendt-Nielsen L. Pain sensitisation in osteoarthritis. Clin. Exp. Rheumatol. 2017;107(5):68–74. [PubMed] [Google Scholar]
- 20.Bhatt NG, Sheth MS, Vyas NJ. Correlation of fear avoidance beliefs with pain and physical function in subjects with osteoarthritis of knee. Int. J. Ther. Rehabil. Res. 2015;4:117–121. [Google Scholar]
- 21.Sánchez Romero EA, Meléndez Oliva E, Alonso Pérez JL, Martín Pérez S, Turroni S, Marchese L, Villafañe JH. Relationship between the gut microbiome and osteoarthritis pain: Review of the literature. Nutrients. 2021;13(3):716. doi: 10.3390/nu13030716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017;7(4):987. doi: 10.4081/cp.2017.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine. 2023;90:104527. doi: 10.1016/j.ebiom.2023.104527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorboni SG, Moghaddam HS, Jafarzadeh-Esfehani R, Soleimanpour S. A Comprehensive review on the role of the gut microbiome in human neurological disorders. Clin. Microbiol. Rev. 2022;35(1):e0033820. doi: 10.1128/CMR.00338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen BK, Saltin B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports. 2015;25:1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 26.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: A Cochrane systematic review. Br. J. Sports Med. 2015;49(24):1554–1557. doi: 10.1136/bjsports-2015-095424. [DOI] [PubMed] [Google Scholar]
- 27.Fransen M, Nairn L, Winstanley J, Lam P, Edmonds J. Physical activity for osteoarthritis management: A randomized controlled clinical trial evaluating hydrotherapy or Tai Chi classes. Arthritis Care Res. 2007;57(3):407–414. doi: 10.1002/art.22621. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB. Physical activity interventions for people with mental illness: A systematic review and meta-analysis. J. Clin. Psychiatry. 2014;75(9):14465. doi: 10.4088/JCP.13r08765. [DOI] [PubMed] [Google Scholar]
- 29.McDowell CP, Dishman RK, Gordon BR, Herring MP. Physical activity and anxiety: A systematic review and meta-analysis of prospective cohort studies. Am. J. Prev. Med. 2019;57(4):545–556. doi: 10.1016/j.amepre.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Jia R, Liang J, Xu Y, Wang Y. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr. 2019;19(1):1–14. doi: 10.1186/s12877-019-1175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quirk H, Blake H, Tennyson R, Randell T, Glazebrook C. Physical activity interventions in children and young people with type 1 diabetes mellitus: A systematic review with meta-analysis. Diabet. Med. 2014;31(10):1163–1173. doi: 10.1111/dme.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polese JC, Ada L, Dean CM, Nascimento LR, Teixeira-Salmela LF. Treadmill training is effective for ambulatory adults with stroke: A systematic review. J. Physiother. 2013;59(2):73–80. doi: 10.1016/S1836-9553(13)70159-0. [DOI] [PubMed] [Google Scholar]
- 34.Kuntz AB, Chopp-Hurley JN, Brenneman EC, Karampatos S, Wiebenga EG, Adachi JD, et al. Efficacy of a biomechanically-based yoga exercise program in knee osteoarthritis: A randomized controlled trial. PLoS One. 2018;13(4):e0195653. doi: 10.1371/journal.pone.0195653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moonaz SH, Bingham CO, Wissow L, Bartlett SJ. Yoga in sedentary adults with arthritis: Effects of a randomized controlled pragmatic trial. J. Rheumatol. 2015;42(7):1194–1202. doi: 10.3899/jrheum.141129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taglietti M, Facci LM, Trelha CS, de Melo FC, da Silva DW, Sawczuk G, et al. Effectiveness of aquatic exercises compared to patient-education on health status in individuals with knee osteoarthritis: A randomized controlled trial. Clin. Rehabil. 2018;32(6):766–776. doi: 10.1177/0269215517754240. [DOI] [PubMed] [Google Scholar]
- 37.Gay C, Chabaud A, Guilley E, Coudeyre E. Educating patients about the benefits of physical activity and exercise for their hip and knee osteoarthritis. Systematic literature review. Ann. Phys. Rehabilit. Med. 2016;59(3):174–183. doi: 10.1016/j.rehab.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Brown G. Beck depression inventory–II. Psychol Assess (1996).
- 39.Gourgouvelis J, Yielder P, Clarke ST, Behbahani H, Murphy BA. Exercise leads to better clinical outcomes in those receiving medication plus cognitive behavioral therapy for major depressive disorder. Front. Psychiatry. 2018;9:37. doi: 10.3389/fpsyt.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourbeau K, Moriarty T, Ayanniyi A, Zuhl M. The combined effect of exercise and behavioral therapy for depression and anxiety: Systematic review and meta-analysis. Behav. Sci. 2020;10(7):116. doi: 10.3390/bs10070116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Xu D, Hu M, Tan Y, Zhang P, Li G, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for patients with diabetes and depression. J. Psychosom. Res. 2017;95:44–54. doi: 10.1016/j.jpsychores.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Rossman J. Cognitive-behavioral therapy for insomnia: An effective and underutilized treatment for insomnia. Am. J. Lifestyle Med. 2019;13(6):544–547. doi: 10.1177/1559827619867677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atwood ME, Friedman A. A systematic review of enhanced cognitive behavioral therapy (CBT-E) for eating disorders. Int. J. Eat. Disord. 2020;53(3):311–330. doi: 10.1002/eat.23206. [DOI] [PubMed] [Google Scholar]
- 44.Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80(1–2):1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 45.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021;10(1):1–11. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cochrane. Chapter 10: Analysing data and undertaking meta-analyses. https://training.cochrane.org/handbook/current/chapter-10 (Accessed 18 January 2023) (2022).
- 47.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14(1):1–13. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat. Med. 2000;19(22):3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 49.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 50.Bekkers J, de Windt TS, Raijmakers N, Dhert W, Saris D. Validation of the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the treatment of focal cartilage lesions. Osteoarthr. Cartil. 2009;17(11):1434–1439. doi: 10.1016/j.joca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 51.Nilsdotter AK, Lohmander LS, Klässbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS)–validity and responsiveness in total hip replacement. BMC Musculoskelet. Disord. 2003;4(1):1–8. doi: 10.1186/1471-2474-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McHorney CA, Ware Johne J, Anastasiae R. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Söderman P, Malchau H. Is the Harris hip score system useful to study the outcome of total hip replacement? Clin. Orthop. Relat. Res. 1976–2007. 2001;384:189–197. doi: 10.1097/00003086-200103000-00022. [DOI] [PubMed] [Google Scholar]
- 54.Barlow J, Williams B, Wright C. The reliability and validity of the arthritis self-efficacy scale in a UK context. Psychol. Health Med. 1997;2(1):3–17. [Google Scholar]
- 55.Devins GM, Orme CM, Costello CG, Binik YM, Frizzell B, Stam HJ, et al. Measuring depressive symptoms in illness populations: Psychometric properties of the center for epidemiologic studies depression (CES-D) scale. Psychol. Health. 1988;2(2):139–156. [Google Scholar]
- 56.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 57.Montorio I, Izal M. The geriatric depression scale: A review of its development and utility. Int. Psychogeriatr. 1996;8(1):103–112. doi: 10.1017/s1041610296002505. [DOI] [PubMed] [Google Scholar]
- 58.Liu H, Cella D, Gershon R, Shen J, Morales LS, Riley W, et al. Representativeness of the patient-reported outcomes measurement information system internet panel. J. Clin. Epidemiol. 2010;63(11):1169–1178. doi: 10.1016/j.jclinepi.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akin A, Çetin B. The depression anxiety and stress scale (DASS): The study of validity and reliability. Kuram ve Uygulamada Egitim Bilimleri. 2007;7(1):260. [Google Scholar]
- 60.Spitzer RL, Kroenke K, Williams JB, Patient Health Questionnaire Primary Care Study Group, Patient Health Questionnaire Primary Care Study Group Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 61.Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008;5(1):e20. doi: 10.1371/journal.pmed.0050020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verhagen AP, De Vet HC, De Bie RA, Boers M, Den Brandt Van, Piet A. The art of quality assessment of RCTs included in systematic reviews. J. Clin. Epidemiol. 2001;54(7):651–654. doi: 10.1016/s0895-4356(00)00360-7. [DOI] [PubMed] [Google Scholar]
- 63.Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of meta-essentials: A free and simple tool for meta-analysis. Res. Synth. Methods. 2017;8(4):537–553. doi: 10.1002/jrsm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981;6(2):107–128. [Google Scholar]
- 65.Cohen J. Statistical Power Analysis for the Behavioral Sciences, no. 1. Lwrence Earlbaum Associates Publishers; 1988. [Google Scholar]
- 66.Singh JA, Luo R, Landon GC, Suarez-Almazor M. Reliability and clinically important improvement thresholds for osteoarthritis pain and function scales: A multicenter study. J. Rheumatol. 2014;41(3):509–515. doi: 10.3899/jrheum.130609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-analysis. Wiley; 2021. [Google Scholar]
- 68.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res. Synth. Methods. 2017;8(1):5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 69.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheung C, Wyman JF, Bronas U, McCarthy T, Rudser K, Mathiason MA. Managing knee osteoarthritis with yoga or aerobic/strengthening exercise programs in older adults: A pilot randomized controlled trial. Rheumatol. Int. 2017;37(3):389–398. doi: 10.1007/s00296-016-3620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.French HP, Cusack T, Brennan A, Caffrey A, Conroy R, Cuddy V, et al. Exercise and manual physiotherapy arthritis research trial (EMPART) for osteoarthritis of the hip: A multicenter randomized controlled trial. Arch. Phys. Med. Rehabil. 2013;94(2):302–314. doi: 10.1016/j.apmr.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 72.Lin Y, Lee W, Hsieh R. Active video games for knee osteoarthritis improve mobility but not WOMAC score: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2020;63(6):458–465. doi: 10.1016/j.rehab.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 73.Park J, Newman D, McCaffrey R, Garrido JJ, Riccio ML, Liehr P. The effect of chair yoga on biopsychosocial changes in English-and Spanish-speaking community-dwelling older adults with lower-extremity osteoarthritis. J. Gerontol. Soc. Work. 2016;59(7–8):604–626. doi: 10.1080/01634372.2016.1239234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang S, Guo G, Li X, Yao F, Wu Z, Zhu Q, et al. The effectiveness of traditional Chinese Yijinjing Qigong exercise for the patients with knee osteoarthritis on the pain, dysfunction, and mood disorder: A pilot randomized controlled trial. Front. Med. 2022 doi: 10.3389/fmed.2021.792436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahn YH, Ham OK. Evaluation of the interaction model of client health Behavior-based multifaceted intervention on patient activation and osteoarthritis symptoms. Jpn. J. Nurs. Sci. 2020;17(2):e12306. doi: 10.1111/jjns.12306. [DOI] [PubMed] [Google Scholar]
- 76.Barlow JH, Turner AP, Wright CC. A randomized controlled study of the Arthritis Self-Management Programme in the UK. Health Educ. Res. 2000;15(6):665–680. doi: 10.1093/her/15.6.665. [DOI] [PubMed] [Google Scholar]
- 77.Hurley M, Walsh N, Mitchell H, Pimm TJ, Patel A, Williamson E, et al. Clinical effectiveness of a rehabilitation program integrating exercise, self-management, and active coping strategies for chronic knee pain: A cluster randomized trial. Arthritis Care Res. 2007;57(7):1211–1219. doi: 10.1002/art.22995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Somers TJ, Blumenthal JA, Guilak F, Kraus VB, Schmitt DO, Babyak MA, et al. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: A randomized controlled study. Pain. 2012;153(6):1199–1209. doi: 10.1016/j.pain.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tak E, Staats P, Van Hespen A, Hopman-Rock M. The effects of an exercise program for older adults with osteoarthritis of the hip. J. Rheumatol. 2005;32(6):1106–1113. [PubMed] [Google Scholar]
- 80.Walsh N, Jones L, Phillips S, Thomas R, Odondi L, Palmer S, et al. Facilitating activity and self-management for people with arthritic knee, hip or lower back pain (FASA): A cluster randomised controlled trial. Musculoskelet. Sci. Pract. 2020;50:102271. doi: 10.1016/j.msksp.2020.102271. [DOI] [PubMed] [Google Scholar]
- 81.Yip Y, Sit JW, Wong DY, Chong SY, Chung L. A 1-year follow-up of an experimental study of a self-management arthritis programme with an added exercise component of clients with osteoarthritis of the knee. Psychol. Health Med. 2008;13(4):402–414. doi: 10.1080/13548500701584030. [DOI] [PubMed] [Google Scholar]
- 82.Lorig, K. & Fries, J. The Arthritis Helpbook, 4th edn. Addison-Wesley, Reading, MA (1995).
- 83.Yip, Y.B. et al. Impact of a self-management arthritis programme with an added exercise component for osteoarthritic knee sufferers on improving pain, functional outcomes, and use of health care services: An experimental study. Patient Educ. Couns.65, 113–121 (2007). [DOI] [PubMed]
- 84.Bossen D, Veenhof C, Van Beek KE, Spreeuwenberg PM, Dekker J, De Bakker DH. Effectiveness of a web-based physical activity intervention in patients with knee and/or hip osteoarthritis: Randomized controlled trial. J. Med. Internet Res. 2013;15(11):e2662. doi: 10.2196/jmir.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Allen KD, Somers TJ, Campbell LC, Arbeeva L, Coffman CJ, Cene CW, et al. Pain coping skills training for African Americans with osteoarthritis: Results of a randomized controlled trial. Pain. 2019;160(6):1297–1307. doi: 10.1097/j.pain.0000000000001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Broderick JE, Keefe FJ, Bruckenthal P, Junghaenel DU, Schneider S, Schwartz JE, et al. Nurse practitioners can effectively deliver pain coping skills training to osteoarthritis patients with chronic pain: A randomized, controlled trial. Pain. 2014;155(9):1743–1754. doi: 10.1016/j.pain.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hausmann LR, Youk A, Kwoh CK, Ibrahim SA, Hannon MJ, Weiner DK, et al. Testing a positive psychological intervention for osteoarthritis. Pain Med. 2017;18(10):1908–1920. doi: 10.1093/pm/pnx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Helminen E, Sinikallio SH, Valjakka AL, Väisänen-Rouvali RH, Arokoski JP. Effectiveness of a cognitive–behavioural group intervention for knee osteoarthritis pain: A randomized controlled trial. Clin. Rehabil. 2015;29(9):868–881. doi: 10.1177/0269215514558567. [DOI] [PubMed] [Google Scholar]
- 89.Lin EH, Katon W, Von Korff M, Tang L, Williams JW, Jr, Kroenke K, et al. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: A randomized controlled trial. JAMA. 2003;290(18):2428–2429. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 90.O'Moore KA, Newby JM, Andrews G, Hunter DJ, Bennell K, Smith J, et al. Internet cognitive–behavioral therapy for depression in older adults with knee osteoarthritis: A randomized controlled trial. Arthritis Care Res. 2018;70(1):61–70. doi: 10.1002/acr.23257. [DOI] [PubMed] [Google Scholar]
- 91.Debray TP, Moons KG, Riley RD. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: A comparison of new and existing tests. Res. Synth. Methods. 2018;9(1):41–50. doi: 10.1002/jrsm.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uthman OA, van der Windt DA, Jordan JL, Dziedzic KS, Healey EL, Peat GM, et al. Exercise for lower limb osteoarthritis: Systematic review incorporating trial sequential analysis and network meta-analysis. BMJ. 2013;347:f5555. doi: 10.1136/bmj.f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goh S, Persson MS, Stocks J, Hou Y, Welton NJ, Lin J, et al. Relative efficacy of different exercises for pain, function, performance and quality of life in knee and hip osteoarthritis: Systematic review and network meta-analysis. Sports Med. 2019;49(5):743–761. doi: 10.1007/s40279-019-01082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hall M, Dodson F, Van Ginckel A, Nelligan RK, Collins NJ, Smith MD, Ross MH, Smits E, Bennell K. Comparative effectiveness of exercise programs for psychological well-being in knee osteoarthritis: A systematic review and network meta-analysis. Semin. Arthritis Rheum. 2021;51(5):1023–1032. doi: 10.1016/j.semarthrit.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 95.Johnson CB. A personalized shared decision-making tool for osteoarthritis management of the knee. Orthop. Nurs. 2021;40(2):64–70. doi: 10.1097/NOR.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Siddall B, Ram A, Jones MD, Booth J, Perriman D, Summers SJ. Short-term impact of combining pain neuroscience education with exercise for chronic musculoskeletal pain: A systematic review and meta-analysis. Pain. 2022;163(1):e20–e30. doi: 10.1097/j.pain.0000000000002308. [DOI] [PubMed] [Google Scholar]
- 97.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: An overview of cochrane reviews. Cochrane Database Syst. Rev. 2017;1(1):CD011279. doi: 10.1002/14651858.CD011279.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin P, Li C, Chou P, Chen Y, Lin L. Prevalence of pain-related diagnoses in patients with dementia: A nationwide study. J. Pain Res. 2018;11:1589–1598. doi: 10.2147/JPR.S172875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siette J, Taylor N, Deckers K, Köhler S, Braithwaite J, Valenzuela M, et al. Advancing Australian public health initiatives targeting dementia risk reduction. Australas. J. Ageing. 2022;41(2):e190–e195. doi: 10.1111/ajag.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be made available on request to the corresponding author.