Graphical abstract
Keywords: Ferulic acid, Intracerebral hemorrhage, COVID-19, Key targets, Pharmacological mechanism
Highlights
-
•
All eleven core targets in ferulic acid and COVID-19/ICH were identified.
-
•
The anti-COVID-19 and ICH benefits were characterized.
-
•
Ferulic acid might bind to EGFR protein with potent binding energy.
-
•
Ferulic acid exerted potential activities against COVID-19 and ICH.
Abstract
Intracerebral hemorrhage (ICH) refers to severe stroke subtype that may be life-threatening or even cause death. It is clinically observed that coronavirus disease 2019 (COVID-19) may be associated with the high mortality in ICH patients. Ferulic acid, one of the functional bioactive ingredients from medicinal herbs, has been preclinically proven with beneficial activities, including neuroprotection and anti-inflammation actions. Based on current findings, we assumed that ferulic acid may play the potentials against COVID-19 when ICH. In this study, preclinical approach including network pharmacology and molecular docking was applied to detect and identify the core targets and pharmacological mechanisms involved in ferulic acid on COVID-19 and ICH. The network pharmacology analysis identified total eleven core targets in ferulic acid and COVID-19/ICH. The molecular mechanisms of ferulic acid against COVID-19 and ICH were mostly involved in induction of antiviral activity, modulation of inflammatory reaction. Molecular docking model revealed that ferulic acid might effectively bind to epidermal growth factor receptor (EGFR) protein based on strong binding capability. Current findings reflected the preclinical pharmacological activities of ferulic acid that might use for management of COVID-19 and ICH. Although there are the limitations that are absence of experimental validation, these bioinformatic results underline that ferulic acid may exert simultaneous potentials against COVID-19 and ICH through modulating integrative mechanisms and key biotargets.
1. Introduction
Intracerebral hemorrhage (ICH) is a parenchyma lesion in the brain, characterized with neurological dysfunction and complications (Manno, 2012). ICH can induce increased risk of developing stroke and result in up to 50% mortality when there is no timely treatment (Xue and Yong, 2020). ICH is the clinical manifestation that can lead to secondary brain lesion and neurological disorder (Watson et al, 2022). It is clinically reported that the recurrence of ICH is high as severe comorbidity may cause mid- or long-term death rate (Poon et al, 2014). Thus, the medical guidelines of ICH aim to reduction of bleeding, hematoma size, blood pressure and neuroinflammation in the brain. In clinical treatment, drug therapy such as tranexamic acid or hemostatic drugs have certain therapeutic effectiveness and then other adverse effects may occur inevitably (Powers et al, 2018). In the past few years, COVID-19 has impacted world human health thoroughly due to virus variation, imperfect vaccination, and absence of specific medicine (Li et al, 2022a). In clinical observation, COVID patients may detect with ICH because intracerebral angiotensin-converting enzyme 2 receptor (ACE2) is disrupting by SARS-CoV-2 infection, and thus hypertension, coagulopathy, disseminated intravascular coagulation may occur (Osman et al, 2023). We significantly reasoned that newly explored effective agents against ICH and COVID-19 are imperative before further clinical trial, such as natural compounds from medicinal herbs or Traditional Chinese medicine (TCM). Some clinical findings demonstrate that TCM may facilitate hematoma dissolution and then meliorate ICH-caused symptoms (DU et al, 2021). TCM has exerted an exceptional effectiveness to relieve COVID-19 in China when mass epidemic period (Li et al, 2022b). It can be speculated that TCM-isolated effective functional ingredients may be potential for the treatment of ICH and COVID-19. Recent studies have provided compelling evidence of neuroprotective properties in functional ingredients. Syringic acid may be a therapeutic compound for the treatment of Parkinson's disease in vivo through exerting anti-oxidant and anti-inflammatory actions for neuroprotection (Güzelad al, 2021). Besides, syringic acid may improve neurological and behavioral functions for the potential treatment of neurological disorder (Ogut et al, 2022). Ferulic acid, a bioactive compound rich in Chinese herbs, has been associated with the promising health benefits, such as preventing cerebral ischemia/reperfusion injury (Liu et al, 2023), and anti-neurological disease (Zhou and Dong, 2023). Preclinical study shows that ferulic acid exerts neuroprotective effect against neuroinflammation via inactivating the expression of NACHT, LRP and PYD domains-containing protein 3 (NLRP3) inflammasome. Additionally, ferulic acid may be potential for reducing depressive-like behaviour through inhibiting neuroinflammation and enhancing neuroprotection (Mallik et al, 2023). Interestingly, our previous study using bioinformatics findings uncover the potential therapeutic targets and molecular mechanisms of ferulic acid against traumatic brain injury (Dong et al, 2022). Ferulic acid suppresses the replication of porcine parvovirus possibly via regulating apoptosis-dependent pathway (Ma et al, 2020). Other preclinical reports indicate that ferulic acid may play potential treatment for COVID-19 and osteosarcoma characterized with autophagy-associated targets and signalling mechanisms (Pang et al, 2022). Taken together, we conclude that ferulic acid may have the pharmacological activity for the treatment of ICH and COVID-19. Notably, it is increasingly popular to reveal the therapeutic targets and mechanism of bioactive compounds isolated TCM and other agents against clinical disorders through application of network pharmacology approach, such as extract of Tinospora cordifolia Miers against encephalitis (Tiwari et al, 2023), abscisic acid against Japanese encephalitis virus (Bhimaneni et al, 2022), auranofin against bacterial infection (Sharma et al, 2021). Thus, in this study, we aimed to identify the pharmacological actions and targets, as well molecular mechanisms of ferulic acid against ICH and COVID-19 via bioinformatics determination including network pharmacology and molecular docking model before virtual validation and clinical experiment.
2. Materials and methods
2.1. Identification of ferulic acid, COVID-19, ICH-related targets
The Drugbank (https://www.drugbank.ca), Swiss Target Predicition (https://www.swisstargetprediction.ch) databases were used to obtain the potential targets of ferulic acid. The Drugbank (https://go.drugbank.com/), Online Mendelian Inheritance in Man (OMIM, https://omim.org) and Therapeutic Target Database (https://db.idrblab.net/ttd/) by using the “intracerebral hemorrhage”, “COVID-19”, “Coronavirus disease 2019” followed by screening with the term of “Homo sapiens” were evaluated, and the repetitive targets were excluded. Subsequently, all identified targets associated with ferulic acid, COVID-19, ICH were further mapped by using Venn platform (Jia et al, 2021). The intersection genes were selected and identified as candidate targets among ferulic acid, COVID-19 and ICH.
2.2. Protein–protein interaction (PPI) network construction
Cytoscape software (https://cytoscape.org) was employed to establish a target network of PPI, and the minimum interaction value was designed as “medium confidence > 0.09”. The “Network Analyzer” plugin was applied to determine the degree value of identified genes, in which the node and color settings were expressed with larger and deeper degree values, respectively. Furthermore, the core targets were screened via the larger degree values, in which the identifying standard was greater than two times the median of the degree value for screening (Majeed and Mukhtar, 2023).
2.3. Detection of gene ontology (GO) function and kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment
The “GOplot” package in R-software was subjected to investigate all screened core genes for respective assays of GO function and KEGG pathway. By use of the P less than 0.05 as calculative criterion, the GO functional annotations and KEGG enrichment pathways involved in core genes were validated computationally. In addition, the R-software was applied to visually present the enrichment findings for different figures (Wu et al, 2021).
2.4. Integrated visualization of network relationship
The Cytoscape software (https://cytoscape.org) was used to construct and characterize the interaction and function of all core targets in GO and KEGG network findings for comprehensively revealing the pharmacological mechanism of ferulic acid in the treatment of ICH and COVID-19 (Chen et al, 2021).
2.5. Molecular docking study
The chemical structure of ferulic acid was downloaded from PubChem dataset (https://pubchem.ncbi.nlm.nih.gov/). The Protein Data Bank (PDB, https://www.pdbus.org) was used to present the crystal structure of the core protein of EGFR. Crystal EGFR was modified through the AutoDock Vina (Maya, 2023) program to exclude water molecules, add hydrogen atoms before being saved the data as pdbqt files. Then Pymol software (Al-Ahmary et al, 2014) was used to score out the unrelated small molecule. The ferulic acid molecule was energy minimized via ChemBio3D (Abdelrehim et al, 2020) and was used as target ligand or receptor, characterized with the grid box’s center location, length, width, and height. Finally, Pymol software was applied to characterize the energetically favored binding conformation of ligand in the active site of the core protein of EGFR.
3. Results
3.1. Candidate, interaction targets of ferulic acid, COVID-19 and ICH
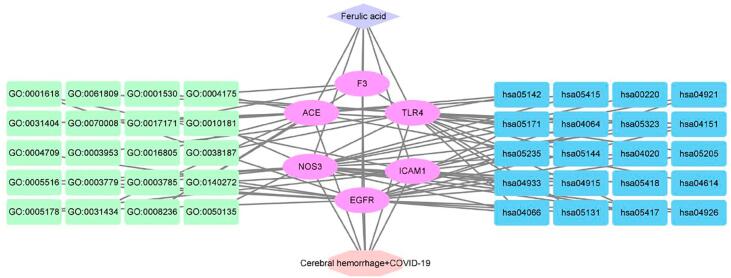
After being screened by bioinformatics analysis, a total of 184 targets were identified to interact with ferulic acid, and another 2624 targets were identified to interact with COVID-19, as well as other 403 targets were identified to interact with ICH. The interaction targets of ferulic acid, COVID-19 and ICH were acquired through Venn diagram analysis. And then total 11 interaction targets of ferulic acid for the treatment of COVID-19 and ICH were ascertained, and these interaction targets were characterized with relevancy. The Venn analysis and analytical diagram is presented in Fig. 1.
Fig. 1.
Venn diagram analysis visualized the candidate genes including respective, correlative targets in ferulic acid, COVID-19, ICH. And the correlative target network was characterized.
3.2. Target network construction and core target identification
To characterize network topology findings, a PPI network involved in 11 interaction targets of ferulic acid, COVID-19 and ICH was established. In this diagram, the target clusters in the correlative network resulted in 6 core genes in ferulic acid against COVID-19 and ICH, including EGFR, intercellular adhesion molecule 1 (ICAM1), angiotensin converting enzyme 2 (ACE), F3, Toll-like receptor 4 (TLR4), nitric oxide synthase 3 (NOS3). These core targets were shown in Fig. 2.
Fig. 2.
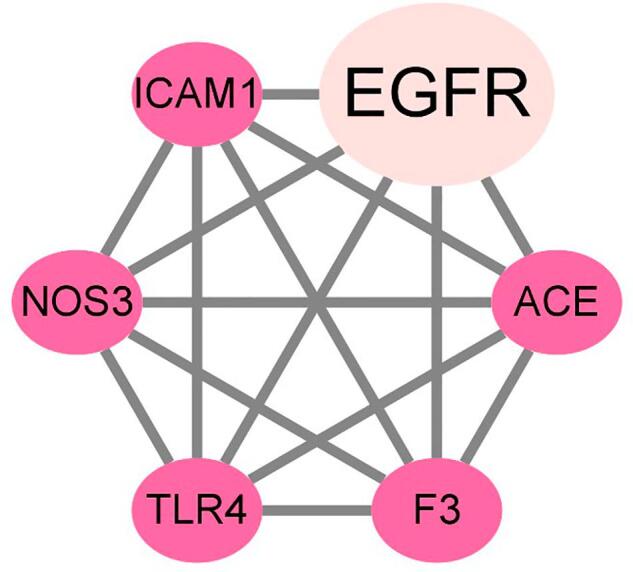
Identification of core targets in ferulic acid in the potential treatment of COVID-19 and ICH, including EGFR, ICAM1, ACE, F3, TLR4, NOS3.
3.3. Findings of GO and KEGG enrichment analysis
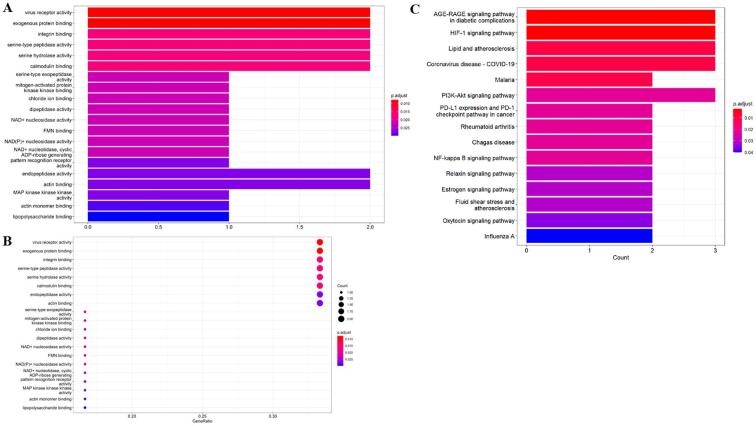
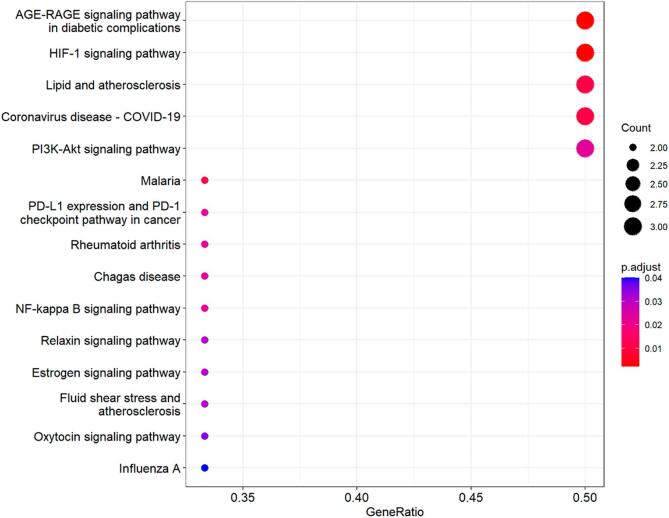
As revealed in Fig. 3, GO-based enrichment findings were assigned as three main sections, including biological process (BP), molecular function (MF), and cellular component (CC). Dissimilar categories of BP, MF, and CC were characterized and colored accordingly in bar chart (Fig. 3A and C) and bubble chart (Fig. 3B). The enrichment data indicated that top 20 biological functions involved in GO annotations were characterized and illustrated the potential use of ferulic acid in the treatment of COVID-19 and ICH. To further demonstrate the molecular mechanism of ferulic acid against COVID-19 and ICH, we performed KEGG pathway enrichment assay with core targets to identify the KEGG signaling pathways (P < 0.05). The classification bubble diagram of KEGG findings was indicated in Fig. 4. The top 20 signaling pathways included advanced glycation end product (AGE)- receptor of AGE (RAGE) signaling pathway in diabetic complications, hypoxia-inducible factor-1 (HIF-1) signaling pathway, Lipid and atherosclerosis, Coronavirus disease-COVID-19, Malaria, phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway, Programmed death ligand 1 (PD-L1) expression and Programmed death 1 (PD-1) checkpoint pathway in cancer, Rheumatoid arthritis, Chagas disease, Nuclear factor-kappa B (NF-kappa B) signaling pathway, Relaxin signaling pathway, Estrogen signaling pathway, Fluid shear stress and atherosclerosis, Oxytocin signaling pathway, Influenza A. In a word, integrated bioinformatics findings in current report were characterized and visualized in Fig. 5.
Fig. 3.
The GO functional enrichment annotations of core targets in bar diagram and bubble chart, including biological process (A), molecular function (B), and cellular component (C).
Fig. 4.
The classification bubble chart of KEGG signaling pathways was characterized.
Fig. 5.
Integrated overview map in bioinformatics findings from current report was visualized.
3.4. Findings of molecular docking
Firstly, core target proteins were evaluated before being used for molecular docking validation, and the EGFR protein (PDB ID:4LQM) was screened out for further analysis. By using active cavity box model, ferulic acid was determined to bind the active pocket of EGFR protein. As a result, ferulic acid molecule formed hydrogen bonds with 4LQM residue on EGFR, including MET-793 (2.3 Å), ASP-855 (2.3 Å), THR-854 (2.4 Å). And the binding energy within ferulic acid and EGFR was −5.9 kJ/mol, implying the effective binding ability between them. The molecular docking results were displayed in Fig. 6.
Fig. 6.
Molecular docking model of ferulic acid binding to the core target protein, characterized by ferulic acid docked to the surface of EGFR with MET-793, ASP-855, THR-854.
4. Discussion
During the epidemic of COVID-19, vaccine treatment has achieved great benefits against SARS-CoV-2. However, certain potential side-effects have been reported, such as allergic and anaphylactic reactions, thrombocytopenia, autoimmunity disorder (Lamprinou et al, 2023). In addition to the use of vaccine, the chemotherapy using antiviral drug, kinase inhibitors, and neutralizing antibody is prescribed clinically (Yuan et al, 2023). In pathological features, the SARS-CoV-2 infection may cause multiple organ injuries due to pneumonia, systemic inflammation, including cerebrovascular complications (Hingorani KS, Bhadola, 2023). It is clinically reported that COVID-19 patient may develop the SARS-CoV-2-caused neurological complications including encephalitis or meningitis (Huo et al, 2021), indicating that effective and timely therapy is urgently needed. Thus, it is of great significance to explore bioactive agent, especially natural ingredient, against neurological symptoms in COVID-19. After severe SARS-CoV-2 infection, neurological disorders induced by neurovirulence may occur, such as ICH, ageusia, anosmia (Aghagoli et al, 2021). A narrative review suggests that incidence of primary and secondary ICH is detected in COVID-19 patients, and it may conclude that SARS-CoV2 infection can lead to ICH (Margos et al, 2021). Compared to non-SARS-CoV2 infection, COVID-19 and ICH patients may present greater mortality owing to higher mortal comorbidities and adverse events (Qureshi et al, 2022). The underlying pathological mechanism may be involvement with SARS-CoV-2 binding to ACE II receptors in cerebral vasculature resulting in overactive immune response (Fayed et al, 2020). Therefore, we aim to further screen and develop candidate bioactive ingredient in the potential application against COVID-19 and ICH. Ferulic acid, a neuroprotective phytochemical, has exerted anti-inflammatory activity for protective action against nerve injury (Jiang et al, 2021). In pharmacokinetic analysis in vivo using ultra-high performance liquid chromatography approach, the data show that the peak concentration of ferulic acid is 27.50 min under 0.199 mg/L. The elimination half-life and area under the concentration–time curve of ferulic acid are 14.835 μg min/mL in 131.27 min. The absorption constant and volume of distribution of ferulic acid are (0.524 ± 0.157) min−1 and (11713 ± 7618.68) L/kg. The results of pharmacokinetic profiles of ferulic acid suggest that ferulic acid is rapidly absorbed and distributed (Qiu et al, 2011). Notably, ferulic acid has garnered attention due to its pronounced neuroprotective attributes. Emerging research suggests ferulic acid’s capacity to mitigate cellular damage and bolster the resilience of the nervous system against detrimental factors (Thapliyal et al, 2021). Preliminary investigations have suggested a putative role for ferulic acid in mitigating the impact of the viral infection, although comprehensive studies are still warranted to establish its therapeutic efficacy definitively. A comprehensive analysis using molecular docking, molecular dynamics indicates inhibitory potential of ferulic acid derivatives against the main protease (Mpro) of SARS-CoV-2 (Antonopoulou et al, 2022). Some of ferulic acid derivatives have been found with promising anti-viral activity against SARS-CoV2, characterized with the inhibition of viral replication of SARS-CoV2 by 82% (Pasquereau et al, 2022). In the current study, results from network pharmacology analysis ascertained total eleven intersection targets among ferulic acid, COVID-19 and ICH. To further integrate functional relevancy within intersection genes, the PPI network characterized protein–protein interactions before further identified all core targets based on degree value assessment, and the core targets included EGFR, ICAM1, ACE, F3, TLR4, NOS3. These core genes might act as the pharmacological targets in ferulic acid against COVID-19 and ICH before functional verification of the biological features in these core genes. Further drug combination using ferulic acid and medicine targeted core proteins may be optimized for clinical treatment of COVID-19 and ICH. Additionally, GO and KEGG functional assays were conducted to reveal the molecular mechanisms of ferulic acid against COVID-19 and ICH. We integrated signaling pathways from core targets-based annotations. Among current pathways including Coronavirus disease-COVID-19, and Influenza A were chiefly implicated in viral infectious disorders, implying that ferulic acid might possess a potential antiviral mechanism against COVID-19. Other molecular pathways, including phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway and NF-kappa B signaling pathway should be characterized accordingly in current enrichment analysis. It is reported that the nuclear factor kappaB (NF-κB) pathway relates to release of proinflammatory cytokines induced by infection (Yu et al, 2022). The PI3K/AKT signaling pathway is found with positive involvement in inflammation development (He et al, 2022). Collectively, it is surmised that cytokine storm is one of the leading causes of COVID-19 patients with ICH. Thus, anti-viral and anti-inflammatory functions may be involved in the preliminary beneficial mechanism in ferulic acid in the treatment of COVID-19 and ICH. As highlighted in enriched signaling pathways, we speculated that anti-COVID-19 and ICH mechanisms of ferulic acid might exerted via regulating multiple KEGG molecular pathways. Among identified core targets, molecular docking analysis was applied to determine the binding capability of core targets with ferulic acid. As shown in EGFR protein, the report indicated that the binding free energy of ferulic acid with EGFR was −5.9 kJ/mol, revealing the potent binding force between ferulic acid and EGFR. EGFR is a crucial kinase receptor that can be responsible for cell proliferation, differentiation, division, and cell survival (Sabbah et al, 2020). Preclinical evidence shows that EGFR and the signaling pathway may be involved in brain injury caused by ICH in vivo (Qian et al, 2018). A bioinformatics study indicates that EGFR may be a potential anti-COVID-19 target for candidate natural compound (Du et al, 2021). In brief, it is speculated that regulation of EGFR activity may be one of pharmacological activities in ferulic acid against COVID-19 and ICH. Further drug combination using ferulic acid and EGFR inhibitor may be prescribed for clinical management of COVID-19 and ICH. However, in this study, there are still some limitations, including absence of further experimental validations, preclinical studies, drug absorption of ferulic acid and clinical formulation with ferulic acid in pharmaceutical considerations before future clinical test.
5. Conclusion
In current study, we characterized the molecular mechanisms and pharmacological targets of ferulic acid against COVID-19 and ICH through application of a network pharmacology and molecular docking approach. Ferulic acid may inhibit inflammatory reaction, enhance neurological function, and exert antiviral action via synthetically achieved by multi-targets and multi-pathways against COVID-19 and ICH. On the other hand, ferulic acid may be used as an adjuvant treatment with future chemotherapy against COVID-19 patients with ICH.
Finding.
This research was supported by the Construction of Key Projects with Traditional Chinese Medical Science Characteristics and Advantages (Critical Treatment Capacity, No. 2023-CZXM-280).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- Abdelrehim E.M., El-Sayed D.S. A new synthesis of poly heterocyclic compounds containing [1,2,4]triazolo and [1,2,3,4]tetrazolo moieties and their dft study as expected anti-cancer reagents. Curr. Org. Synth. 2020;17(3):211–223. doi: 10.2174/1570179417666200226092516. [DOI] [PubMed] [Google Scholar]
- Aghagoli G., Gallo Marin B., Katchur N.J., Chaves-Sell F., Asaad W.F., Murphy S.A. Neurological involvement in covid-19 and potential mechanisms: a review. Neurocrit. Care. 2021;34(3):1062–1071. doi: 10.1007/s12028-020-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ahmary K.M., Al-Solmy E.A., Habeeb M.M. Spectroscopic characterization of hydrogen-bonded proton transfer complex between 4-aminopyridine with 2,6-dichloro-4-nitrophenol in different solvents and solid state. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;126:260–269. doi: 10.1016/j.saa.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Antonopoulou I., Sapountzaki E., Rova U., Christakopoulos P. The inhibitory potential of ferulic acid derivatives against the sars-cov-2 main protease: molecular docking, molecular dynamics, and admet evaluation. Biomedicines. 2022;10:1787. doi: 10.3390/biomedicines10081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhimaneni S., Kumar A. Abscisic acid and aloe-emodin against NS2B-NS3A protease of Japanese encephalitis virus. Environ. Sci. Pollut. Res. Int. 2022;29(6):8759–8766. doi: 10.1007/s11356-021-16229-8. [DOI] [PubMed] [Google Scholar]
- Chen L., Zhang Y.-H., Wang ShaoPeng, Zhang YunHua, Huang T., Cai Y.-D., Liu B. Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS One. 2017;12(9):e0184129. doi: 10.1371/journal.pone.0184129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q., Yang S., Liao H., He Q., Xiao J. Preclinical findings reveal the pharmacological targets of ferulic acid in the treatment of traumatic brain injury. Food Sci. Nutr. 2022;10(12):4403–4410. doi: 10.1002/fsn3.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H.X., Zhu J.Q., Chen J., Zhou H.F., Yang J.H., Wan H.T. Revealing the therapeutic targets and molecular mechanisms of emodin-treated coronavirus disease 2019 via a systematic study of network pharmacology. Aging (Albany NY) 2021;13(11):14571–14589. doi: 10.18632/aging.203098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayed I., Pivazyan G., Conte A.G., Chang J., Mai J.C. Intracranial hemorrhage in critically ill patients hospitalized for COVID-19. J. Clin. Neurosci. 2020;81:192–195. doi: 10.1016/j.jocn.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güzelad Ö., Özkan A., Parlak H., Sinen O., Afşar E., Öğüt E., Yıldırım F.B., Bülbül M., Ağar A., Aslan M. Protective mechanism of Syringic acid in an experimental model of Parkinson’s disease. Metab. Brain Dis. 2021;36(5):1003–1014. doi: 10.1007/s11011-021-00704-9. [DOI] [PubMed] [Google Scholar]
- He X., Li Y., Deng B., Lin A., Zhang G., Ma M., Wang Y., Yang Y., Kang X. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: mechanisms and therapeutic opportunities. Cell Prolif. 2022;55:e13275. doi: 10.1111/cpr.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani K.S., Bhadola S., Cervantes-Arslanian A.M. COVID-19 and the brain. Trends Cardiovasc. Med. 2022;32(6):323–330. doi: 10.1016/j.tcm.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L., Xu K.L., Wang H. Clinical features of SARS-CoV-2-associated encephalitis and meningitis amid COVID-19 pandemic. World J. Clin. Cases. 2021;9(5):1058–1078. doi: 10.12998/wjcc.v9.i5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia A., Xu L., Wang Y. Venn diagrams in bioinformatics. Brief. Bioinform. 2021;22(5):bbab108 doi: 10.1093/bib/bbab108. [DOI] [PubMed] [Google Scholar]
- Jiang X., Yu X., Chen J., Jing C., Xu L., Chen Z., Liu F., Chen L. Ferulic acid improves motor function induced by spinal cord injury in rats via inhibiting neuroinflammation and apoptosis. Acta Cir. Bras. 2021;36(7):e360705. doi: 10.1590/ACB360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprinou M., Sachinidis A., Stamoula E., Vavilis T., Papazisis G. COVID-19 vaccines adverse events: potential molecular mechanisms. Immunol. Res. 2023;71(3):356–372. doi: 10.1007/s12026-023-09357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Lou F., Fan H. SARS-CoV-2 variant Omicron: currently the most complete “escapee” from neutralization by antibodies and vaccines. Signal Transduct. Target. Ther. 2022;7(1):28. doi: 10.1038/s41392-022-00880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhu H., Liu Y., Lu Y., Sun M., Zhang Y., Shi J., Shi N., Li L., Yang K., Sun X., Liu J., Ge L., Huang L. Role of traditional chinese medicine in treating severe or critical covid-19: a systematic review of randomized controlled trials and observational studies. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.926189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Mu J., Gong W., Zhang K., Yuan M., Song Y., Li B., Jin N., Zhang W., Zhang D. In vitro diagnosis and visualization of cerebral ischemia/reperfusion injury in rats and protective effects of ferulic acid by raman biospectroscopy and machine learning. ACS Chem. Nerosci. 2023;14(1):159–169. doi: 10.1021/acschemneuro.2c00612. [DOI] [PubMed] [Google Scholar]
- Ma X., Guo Z., Zhang Z., Li X., Wang X., Liu Y., Wang X. Ferulic acid isolated from propolis inhibits porcine parvovirus replication potentially through Bid-mediate apoptosis. Int. Immunopharmacol. 2020;83:106379. doi: 10.1016/j.intimp.2020.106379. [DOI] [PubMed] [Google Scholar]
- Majeed A., Mukhtar S. Protein-protein interaction network exploration using cytoscape. Methods Mol. Biol. 2023;2690:419–427. doi: 10.1007/978-1-0716-3327-4_32. [DOI] [PubMed] [Google Scholar]
- Mallik S.B., Mudgal J., Kinra M., Hall S., Grant G.D., Anoopkumar-Dukie S., Nampoothiri M., Zhang Y., Arora D. Involvement of indoleamine 2, 3-dioxygenase (IDO) and brain-derived neurotrophic factor (BDNF) in the neuroprotective mechanisms of ferulic acid against depressive-like behaviour. Metab. Brain Dis. 2023 doi: 10.1007/s11011-023-01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno E.M. Update on intracerebral hemorrhage. Continuum (Minneap Minn). 2012;18(3):598–610. doi: 10.1212/01.CON.0000415430.99394.3e. [DOI] [PubMed] [Google Scholar]
- Margos N.P., Meintanopoulos A.S., Filioglou D., Ellul J. Intracerebral hemorrhage in COVID-19: a narrative review. J. Clin. Neurosci. 2021;89:271–278. doi: 10.1016/j.jocn.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya C. Using PyMOL to Understand Why COVID-19 Vaccines Save Lives. J. Chem. Educ. 2023;100(3):1351–1356. doi: 10.1021/acs.jchemed.2c00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogut E., Armagan K., Gül Z. The role of syringic acid as a neuroprotective agent for neurodegenerative disorders and future expectations. Metab. Brain Dis. 2022;37(4):859–880. doi: 10.1007/s11011-022-00960-3. [DOI] [PubMed] [Google Scholar]
- Osman W., Awad El Gharieb H., Ibrahim H., Alanazi A., Meshref M. Intracerebral Hemorrhage associated COVID-19 patient with normal coagulation profile after ECMO treatment: a case report“. Brain Hemorrhages. 2023;4(2):65–68. doi: 10.1016/j.hest.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang G., Yi T., Luo H., Jiang L. Preclinical findings: The pharmacological targets and molecular mechanisms of ferulic acid treatment for COVID-19 and osteosarcoma via targeting autophagy. Front Endocrinol (Lausanne). 2022;13 doi: 10.3389/fendo.2022.971687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau S., Galais M., Bellefroid M., Pachón Angona I., Morot-Bizot S., Ismaili L., Van Lint C., Herbein G. Ferulic acid derivatives block coronaviruses HCoV-229E and SARS-CoV-2 replication in vitro. Sci. Rep. 2022;12(1):20309. doi: 10.1038/s41598-022-24682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon M.T., Fonville A.F., Al-Shahi S.R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2014;85(6):660–667. doi: 10.1136/jnnp-2013-306476. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49(3):46-110.
- Qian H., Hu K., Xie M., Wu H., Li W., Wu B., Man R., Nie M. Intracerebroventricular injection of miR-7 inhibits secondary brain injury induced by intracerebral hemorrhage via EGFR/STAT3 pathway in rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018;34:141–147. [PubMed] [Google Scholar]
- Qiu X.J., Huang X., Chen Z.Q., Ren P., Huang W., Qin F., Hu S.H., Huang J., He J., Liu Z.Q., Zhou H.H. Pharmacokinetic study of the prokinetic compounds meranzin hydrate and ferulic acid following oral administration of Chaihu-Shugan-San to patients with functional dyspepsia. J. Ethnopharmacol. 2011;137(1):205–213. doi: 10.1016/j.jep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Qureshi A.I., Baskett W.I., Huang W., Myers D., Lobanova I., Ishfaq M.F., Naqvi S.H., French B.R., Chandrasekaran P.N., Siddiq F., Gomez C.R., Shyu C.-R. Intracerebral hemorrhage and coronavirus disease 2019 in a cohort of 282,718 hospitalized patients. Neurocrit. Care. 2022;36(1):259–265. doi: 10.1007/s12028-021-01297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah D.A., Hajjo R., Sweidan K. Review on epidermal growth factor receptor (egfr) structure, signaling pathways, interactions, and recent updates of egfr inhibitors. Curr. Top. Med. Chem. 2020;20(10):815–834. doi: 10.2174/1568026620666200303123102. [DOI] [PubMed] [Google Scholar]
- Sharma N., Singh A., Sharma R., Kumar A. Repurposing of auranofin against bacterial infections: an in silico and in vitro study. Curr. Comput. Aided Drug Des. 2021;17(5):687–701. doi: 10.2174/1386207323666200717155640. [DOI] [PubMed] [Google Scholar]
- Thapliyal S., Singh T., Handu S., Bisht M., Kumari P., Arya P., Srivastava P., Gandham R. A review on potential footprints of ferulic acid for treatment of neurological disorders. Neurochem. Res. 2021;46(5):1043–1057. doi: 10.1007/s11064-021-03257-6. [DOI] [PubMed] [Google Scholar]
- Tiwari P., Ali S.A., Puri B., Kumar A., Datusalia A.K. Tinospora cordifolia Miers enhances the immune response in mice immunized with JEV-vaccine: a network pharmacology and experimental approach. Phytomedicine. 2023;119:154976. doi: 10.1016/j.phymed.2023.154976. [DOI] [PubMed] [Google Scholar]
- Watson N., Bonsack F., Sukumari-Ramesh S. Intracerebral hemorrhage: the effects of aging on brain injury. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.859067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Hu E., Xu S., Chen M., Guo P., Dai Z., Feng T., Zhou L., Tang W., Zhan L., Fu X., Liu S., Bo X., Yu G. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb) 2021;2(3) doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Yong V.W. Neuroinflammation in intracerebral haemorrhage: immunotherapies with potential for translation. Lancet Neurol. 2020;19(12):1023–1032. doi: 10.1016/S1474-4422(20)30364-1. [DOI] [PubMed] [Google Scholar]
- Yu G., Yu H., Yang Q., Wang J., Fan H., Liu G., Wang L., Bello B.K., Zhao P., Zhang H., Dong J. Vibrio harveyi infections induce production of proinflammatory cytokines in murine peritoneal macrophages via activation of p38 MAPK and NF-κB pathways, but reversed by PI3K/AKT pathways. Dev. Comp. Immunol. 2022;127:104292. doi: 10.1016/j.dci.2021.104292. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Jiao B., Qu L., Yang D., Liu R. The development of COVID-19 treatment. Front. Immunol. 2023;14:1125246. doi: 10.3389/fimmu.2023.1125246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Dong X. Neuroprotective properties of ferulic acid in preclinical models of alzheimer's disease: a systematic literature review. Curr. Med. Chem. 2023;30(24):2796–2811. doi: 10.2174/0929867329666220906110506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.