Summary
Female receptivity in mating is crucial for successful copulation, but protocols for quantifying female behaviors reflecting receptivity are scarce compared to the analysis of male behaviors. Here, we present a protocol for assessing the sexual receptivity of female Drosophila that considers behaviors from both sexes. We describe steps for preparing and loading flies into a courtship chamber, video recording the behaviors of the pairs, and analyzing their behavioral displays. This protocol includes behavior recognition criteria suitable for typical laboratory settings.
For complete details on the use and execution of this protocol, please refer to Yang et al. (2023).1
Subject areas: Genetics, Model Organisms, Behavior
Graphical abstract
Highlights
-
•
A comprehensive protocol for quantifying Drosophila female receptivity
-
•
Assessment of key parameters indicative of female receptivity
-
•
Analyzing interplays between males and females during courtship
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Female receptivity in mating is crucial for successful copulation, but protocols for quantifying female behaviors reflecting receptivity are scarce compared to the analysis of male behaviors. Here, we present a protocol for assessing the sexual receptivity of female Drosophila that considers behaviors from both sexes. We describe steps for preparing and loading flies into a courtship chamber, video recording the behaviors of the pairs, and analyzing their behavioral displays. This protocol includes behavior recognition criteria suitable for typical laboratory settings.
Before you begin
The following protocol provides specific steps for testing and analyzing the sexual receptivity of both virgin and mated wild-type Drosophila females, achieved by pairing them with wild-type males. Importantly, this methodology is equally applicable to females of other genotypes.
Drosophila culture medium preparation
Timing: 1 day
-
1.Make 1 L of Drosophila food medium.
-
a.Prepare an anti-fungal solution by dissolving 1.5 g methyl p-hydroxybenzoate in 15 mL of EtOH. Set the solution aside.
-
b.Prepare food medium by adding 77.7 g cornmeal, 32.1 g yeast, 8 g agar, 0.726 g calcium chloride, 31.62 g sucrose, and 63.2 g glucose to 1000 mL water. Heat the mixture to boiling and continue stirring for 30 min.
-
c.Allow the food medium to cool to 70°C, and add the anti-fungal solution and 2 g potassium sorbate, serving as the additional preservative, to the medium.
-
a.
Note: To accelerate the cooling process, use a colder environment such as an ice bath or a fan.
-
2.Dispense food into culture containers.
-
a.UV sterilize empty vials (ø × H: 50 × 100 mm), tubes (2 mL Eppendorf tube), and sponge plugs for 1 h.
-
b.Dispense food into vials for rearing flies.
-
i.Pour 20 mL of food medium into each vial (Figure 1A).
-
ii.Cover the vials with gauze to prevent flies from entering. Wait for the food to solidify.
-
iii.UV sterilize the vials for half an hour.
-
iv.Cap the vials with sponge plugs.
-
v.Store the vials at 25°C or 4°C.
-
i.
-
c.Dispense food into tubes for isolation.
-
i.Transfer 0.5 mL of food into each 2 mL Eppendorf tube (Figure 1B).
-
ii.Cover the tubes with gauze to prevent flies from entering and wait for the food to solidify.
-
iii.Store tubes at 25°C or 4°C.
-
i.
-
a.
Note: The fly food can be stored at 25°C for a maximum of 2 weeks or at 4°C for up to 1 month. If the tubes are stored at 4°C, allow them to equilibrate at 25°C for half an hour before introducing flies.
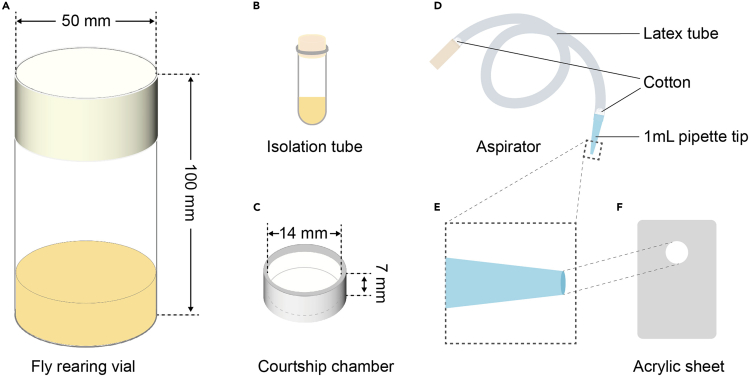
Figure 1.
Illustrations of the equipment and tools
(A) A vial with a sponge plug for rearing multiple flies. It contains 20 mL of food (yellow) and has a radius of 25 mm and a height of 100 mm.
(B) An isolation tube with a sponge plug, which is a 2 mL Eppendorf tube filled with 0.5 mL of food (yellow).
(C) The empty courtship chamber has an internal dimension with a radius of 7 mm and a height of 7 mm.
(D) An aspirator for transferring unanesthetized flies. It consists of a 1 mL pipette tip (blue) for holding flies, a hose (gray), and a clean connecter (brown) that is held in the mouth. The connections are plugged with cotton to prevent the inhalation of flies.
(E) An enlarged view of the trimmed pipette tip in the aspirator. Its opening is large enough for a female fly to pass through.
(F) A custom-made acrylic sheet with a hole that is the same size as the trimmed pipette tip for transferring unanesthetized flies from the pipette tip into a courtship chamber.
Culture and collection
Timing: 17 days
-
3.
Collect the parental flies (P0). For each cross, set up a vial containing 15 males and 40 females, all aged 5–10 days old.
-
4.Rear flies in the aforementioned food medium, with a temperature of 25°C, a relative humidity of 60% in a 12:12 h light:dark regimen (light on at 07:00 a.m.).
-
a.Discard P0 after 1–2 days.
-
b.If more offspring are needed, transfer P0 to a new vial for another 1–2 days.
-
a.
-
5.Collect F1 male flies within 24 h after eclosion, which occurs at approximately 10 days post-oviposition, under a stereo microscope using CO2 anesthesia. All the flies are age-synchronized.
-
a.Put 50–60 male flies into a vial, and plug the vial with a sponge.
-
a.
-
6.Similarly, Collect F1 virgin females within 4 h after eclosion. All the flies are age-synchronized.
-
a.Put one female fly in each Eppendorf tube for isolation, and plug the tube with a sponge.
-
b.Match the number of isolated virgin females and group-reared males.
-
a.
-
7.Rear F1 flies in an incubator under controlled conditions, including a temperature of 25°C, a relative humidity of 60%, and a 12:12 h light:dark cycle (with lights on at 07:00 a.m.).
-
a.On the 4th day after isolation, transfer the single-housed female to a new isolation tube to prevent mortality resulting from food desiccation.
-
b.On the 6th–8th day after collecting F1, conduct the behavior test.
-
a.
-
8.Obtain mated females.
-
a.Isolate F1 virgin females for 4–6 days and add one male fly to the isolation tube with a female to allow mating.
-
b.After the introduction of male flies, monitor the flies every 10 min for 1 h to select successful mating pairs. Under this circumstance, after 1 h, approximately 80%–95% of the fruit fly pairs had successfully mated.
-
c.Discard any flies that do not mate.
-
d.After 2 days, the mated females are used for the behavior test.
-
a.
Note: Anesthesia required during fly collection and setting up crosses can be performed with ice or CO2.
CRITICAL: The optimal number of P0 and the time for egg-laying may vary with vial sizes.
Courtship chamber preparation
Timing: 1 h
CRITICAL: Prepare fresh courtship chambers prior to the behavior test.
-
9.
Estimate the required number of courtship chambers (Figure 1C) for the behavior test.
-
10.
Prepare 0.8% agarose solution. Dissolve agarose powder in Milli-Q water by boiling it until fully dissolved. Use the agarose mixture before it solidifies.
-
11.
Arrange empty courtship chambers on a flat surface.
-
12.Add the agarose mixture into courtship chambers with a pipette. For different purposes, two types of chambers are prepared, which are different in the heights of their final internal space.
-
a.Type 1: 700 μL of agarose is used for each chamber.
-
i.Gently add agarose to the center, and allow the liquid to fill the periphery of the chamber employing surface tension.Note: The goal is to create a concave liquid surface, characterized by a gap of approximately 2.5 mm in height (from the final agarose surface to the top of the chamber) near the central region, while the gap gradually narrows towards the edges. This concave surface allows the fruit flies inside to assume a normal posture (dorsal orientation) near the center, while preventing them from walking on the wall of the chamber. Type 1 chamber is suitable for observing most courtship behaviors.
-
i.
-
b.Type 2: similar to Type 1, but with 530 μL of agarose used for each chamber.
-
i.Gently add agarose to the center, and allow the liquid to fill the periphery of the chamber.Note: The goal is to achieve a nearly uniform height of approximately 3 mm (from the final agarose surface to the top of the chamber) for the inside free space near both the center and the peripheral. This height allows flies to walk on the wall (lateral orientation) and is preferable for observing vaginal plate opening (VPO) and ovipositor extrusion (OE) of females.
-
i.
-
a.
-
13.
Allow the chambers to remain at 25°C for 30 min to ensure the agarose solidified.
Note: Avoid air bubbles while adding agarose.
CRITICAL: It is crucial to maintain consistent heights of agarose in each type of chamber.
Tools preparation
Timing: 1 h
-
14.Make an aspirator (Figures 1D and 1E) for transferring unanesthetized flies from vials or tubes to the courtship chamber.
-
a.Attach a cotton ball and 1 mL pipette tip to one end of the hose, and another cotton ball and a clean plastic connector to the other end.
-
a.
-
15.
Fashion an acrylic sheet featuring a hole (Figure 1F) to facilitate the transfer of unanesthetized flies into the courtship chamber. Ensure the hole is sufficiently large for the flies to pass through.
Note: Both aspirators and acrylic sheets are reusable.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Cornmeal | Beijing Zhongchang Huahui Supermarket Co., Ltd. | N/A |
| Yeast | AB Mauri | N/A |
| Agar powder | Beijing Zhongsheng Wanda Biotechnology Co., Ltd. | N/A |
| Sucrose | Vetec | CAS#V900116-500G |
| Calcium chloride | Beijing Yili Fine Chemicals Co., Ltd. | CAS#10043-52-4 |
| Glucose | Fufeng Group Ltd. | N/A |
| Potassium sorbate | Ningbo Wanglong Tech Co., Ltd. | URL: https://m.tb.cn/h.UIAR6B3?tk=a5nFdPJywLO |
| Methyl p-hydroxybenzoate | Beijing Zhongsheng Wanda Biotechnology Co., Ltd. | N/A |
| EtOH | Beijing Chemical Works | N/A |
| Agarose | Biowest | N/A |
| Experimental models: Organisms/strains | ||
| Drosophila melanogaster: wild-type Canton S | Li Liu | RRID: DGRC_ 105666 adult; both male and female flies; 6–8 days old |
| Software and algorithms | ||
| Prism 9 | GraphPad Software | URL: http://www.graphpad.com/; RRID: SCR_002798 |
| Adobe Illustrator | Adobe | URL: https://www.adobe.com/; RRID: SCR_010279 |
| Other | ||
| Stereo microscope | Olympus SZ61 | URL: https://www.olympus-lifescience.com/ |
| Camcorder | Panasonic HC-X1500 | URL: http://www.panasonic.com/ |
| Camcorder | Sony HDR-CX405 | URL: http://www.sony.com/ |
| Vial | Haimen Yichen Experimental Instrument Factory | Custom-made |
| Eppendorf tube | Haimen Dajin Experimental Equipment Business Department | URL: https://m.tb.cn/h.Ut15PZF?tk=aaLDdP7NlIg |
| Courtship chamber | Beijing Yiran Machinery Factory | Custom-made |
| Coverslip | Sail Brand | 18 × 18 mm |
| Acrylic sheet | Shanghai Xiyue Acrylic Processing Factory | URL: https://m.tb.cn/h.UIAk2fm?tk=BfdldPrZkij |
| Pipette tip | Haimen Med Laboratory Equipment Factory | CAT# 20180411 |
Materials and equipment
Fly food recipe
| Reagent | Final concentration | Amount |
|---|---|---|
| Cornmeal | 7.77% | 77.7 g |
| Yeast | 3.21% | 32.1 g |
| Agar | 0.80% | 8.00 g |
| Sucrose | 0.073% | 0.726 g |
| Calcium chloride | 3.16% | 31.6 g |
| Glucose | 6.32% | 63.2 g |
| Distilled H2O | 1000 mL |
Optional: 1.5 g methyl p-hydroxybenzoate dissolved in 15 mL EtOH and 2 g potassium sorbate are added for anti-fungal.
Note: The fly food can be stored at 25°C for up to 2 weeks, or at 4°C for up to 1 month. After that, it becomes too dry to be used.
Stereo Microscope: We generally use the Olympus SZ61 in our study, which offers a zoom magnification range of 5× to 10×, allowing for discriminating and sorting flies. Any commercial stereo microscopes with similar capacity are also suitable.
Camcorder: Any camcorder with a resolution of 1080p HD or higher is suitable for all behaviors except for OE and VPO. For recording OE and VPO, a camcorder with a resolution of 4K is required.
Backlight: A backlight with a transparent acrylic top is preferable to enhance the quality of video recordings for OE and VPO behaviors. The light source in our backlight is an array of white LEDs which brightness is adjustable. And normally the light intensity of 28 mW/cm2 is sufficient, measured by a spectrometer (CCS200/m, Thorlabs).
Vial: The internal measurements of the empty vial are 100 mm in height and 50 mm in width. These vials can be custom-made to specific dimensions using transparent and odorless materials like plastic or glass. The vials are manufactured by Haimen Yichen Experimental Instrument Factory, while any commercially available vials with the same dimensions are also suitable.
Eppendorf tube: The isolation tubes used in this protocol have a capacity of 2 mL and do not come with a lid. These tubes are manufactured by Haimen Dajin Experimental Equipment Business Department. However, any commercially available tubes with the same size (2 mL capacity, such as Eppendorf tube, Catalog No. 0030120094) are also suitable for use.
Courtship chamber: The empty chamber’s internal dimensions are 7 mm in height and 14 mm in diameter. Courtship chambers are made from acrylic or other transparent, odorless materials.
Coverslip: The dimensions of the coverslips are 18 × 18 mm, with a thickness between 0.13–0.17 mm.
Acrylic sheet: The sheet is made of acrylic material and has a height of 40 mm, a width of 25 mm, and a thickness of 1 mm. It also features a circular hole with a radius of 2.5 mm.
Pipette tip: The pipette tips used in this protocol have a capacity of 1 mL. Any commercially available pipette tips with the same size are also suitable for use.
Alternatives: Additional light sources are optional, but in many cases, help to improve video quality.
Step-by-step method details
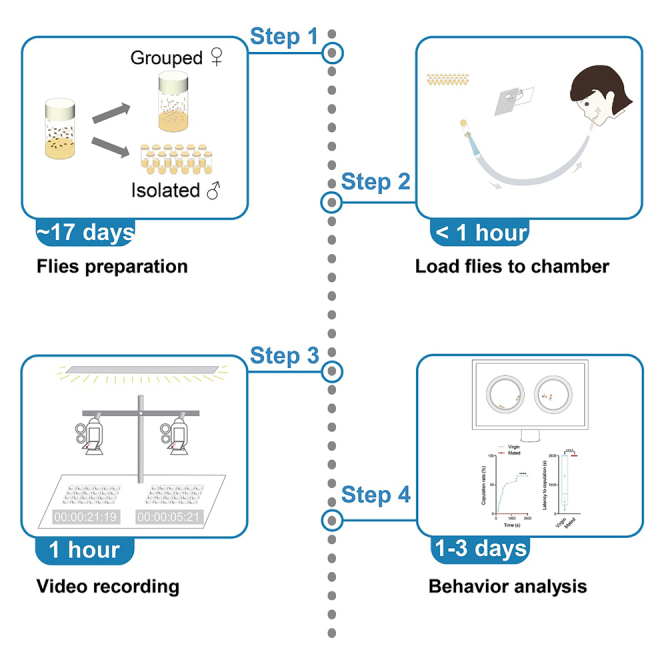
Behavior test and video recording
Timing: 3 h
This section aims to record the courtship behaviors of wild-type Canton S flies, aged 6–8 days old. The recorded videos will be utilized in the subsequent section for behavior analysis.
Note: Behaviors in fruit flies are regulated by circadian rhythms, and thus, the time of day for conducting the tests should be kept consistent to avoid any potential confounding effects.
-
1.
Set the room temperature to 25°C.
-
2.
Turn on the light source to optimize the brightness suitable for the recording device.
-
3.
Bring the flies and courtship chambers to the behavior testing room.
Note: Allow a minimum of 30 min for acclimation in the testing room.
-
4.
Turn on the camera to initiate recording, preferably at a frame rate of 50 frames per second (fps) or higher. Camcorders with a resolution of 1080p are suitable for recording all behaviors analyzed in the Behavior Analysis section, with the exception of VPO and OE. For capturing VPO and OE, camcorders with a resolution of 4K are recommended.
-
5.Pair a single female and a single male fly in the courtship chamber. The choice of using virgin or mated females depends on the purpose of experimental design. The selection of type 1 or type 2 chambers depends on the specific behaviors to be recorded.
-
a.Use the aspirator, with a brief inhale, to draw a single male fly from the group-reared vial, while ensuring that the fly does not escape.
-
b.Use the same aspirator to collect a single female fly from the isolation tube, while ensuring that the fly does not escape.
-
c.Cover a courtship chamber with an acrylic sheet, and connect the aspirator and courtship chamber through the hole in the acrylic sheet.
-
d.Hold the aspirator vertically, allowing the flies to climb upward into the courtship chamber.Note: A light source from above can be used to accelerate the climbing speed.
-
e.Replace the acrylic sheet with a transparent coverslip to enable video recording.Note: Block the hole in the acrylic sheet with a finger to prevent flies from escaping while replacing the acrylic sheet with the cover glass.
-
f.Place the covered courtship chamber under the recording camera.
-
a.
-
6.
Repeat step 5 according to the number of planned tests. A 1080p camcorder can capture up to 32 (4 × 8) chambers. A 4K camcorder for VPO and OE behaviors can record up to 6 (2 × 3) chambers.
-
7.
Continue recording for 1 h after the last chamber has been initiated.
-
8.
Stop recording, and transfer the video files to a computer for analysis.
Note: Refer to Methods video S1 for critical steps in video recording.
CRITICAL: Ensure that the testing room is quiet and free from any vibrations.
CRITICAL: Anesthesia should be avoided for the flies at least 48 h prior to the courtship behavior testing.
Pause point: Videos can be stored on disk and analyzed later according to the researcher's behavioral analysis schedule.
Behavior analysis
Timing: 1–3 days
In this section, the previously recorded videos will be analyzed to quantify female receptivity using multiple parameters. The neural mechanisms that control the sexual receptivity of female flies have recently been the focus of investigations, with different parameters used in various articles. In this study, we will use six parameters to quantify female receptivity in detail.
While the genotypes of female flies to be tested are different, we always pair them with wild-type males. With a short delay after pairing, males court females until copulation occurs. Copulation rate and latency to copulation are two parameters to quantify the courtship success, and reflect female receptivity in terms of the outcome of the courtship process.2
Successful copulation depends on dynamic interactions between male and female flies. When paired with a female, a male fly exhibits stereotyped courtship behaviors, including following, wing extension, proboscis extension, abdomen bending, and copulation attempts. Courtship index3 is the parameter used to measure male’s courtship attempts. When pursued by a male fly, a female fly pauses4 to wait for the male, and exhibit either VPO to accept courtship and initiate mating, or OE to reject courtship and prevent mating. The events of VPO and OE are quantified to reflect the willingness of females to accept courtship.5,6
-
9.Replay the videos and visually analyze related parameters as the following. The start time of each pair is when the chamber is placed under the video camera.
-
a.Analyze the outcome of courtship behaviors in 1 h.
-
i.Copulation rate is the percentage of pairs successfully mated within 1 h to the total number of pairs tested.
-
ii.Latency to copulation is the time interval from pairing to copulation.
-
i.
-
b.Analyze courtship attempts of male flies in the first 5 min.
-
i.Courtship index is the percentage of time of males’ displaying courtship behaviors, including following, wing extension, proboscis extension, abdomen bending, and copulation attempts.
-
i.
-
c.Analyze the behaviors of females in the first 10 min.
-
i.Frequency of pausing is the number of times that a female pauses when pursued by a male fly.
-
ii.Frequency of OE is the number of times that a female pauses and displays OE when pursued by a male fly.
-
iii.Frequency of VPO is the number of times that a female pauses and opens its vaginal plate when pursued by a male fly.
-
i.
-
a.
CRITICAL: A normal resolution (40–60 pixels in length, 15 pixels in width, or ∼500 pixels in area per fly) and type 1 chambers are sufficient for most parameters. To visualize VPO and OE, a higher video resolution (180–200 pixels in length, 55 pixels in width, or ∼9000 pixels in area per fly) and type 2 chambers are needed.
Note: Examples of the behaviors described above are shown in Methods video S2.
-
10.
Copy data to GraphPad Prism for data analysis and plotting.
Expected outcomes
In Drosophila, mating experience significantly influences female receptivity, and females mated within the past 48 h exhibit lower receptivity than virgin females.7 Our protocol utilizes multiple parameters to describe the interplay between males and females during courtship (Figure 2), and the results are consistent with reports in the literature.
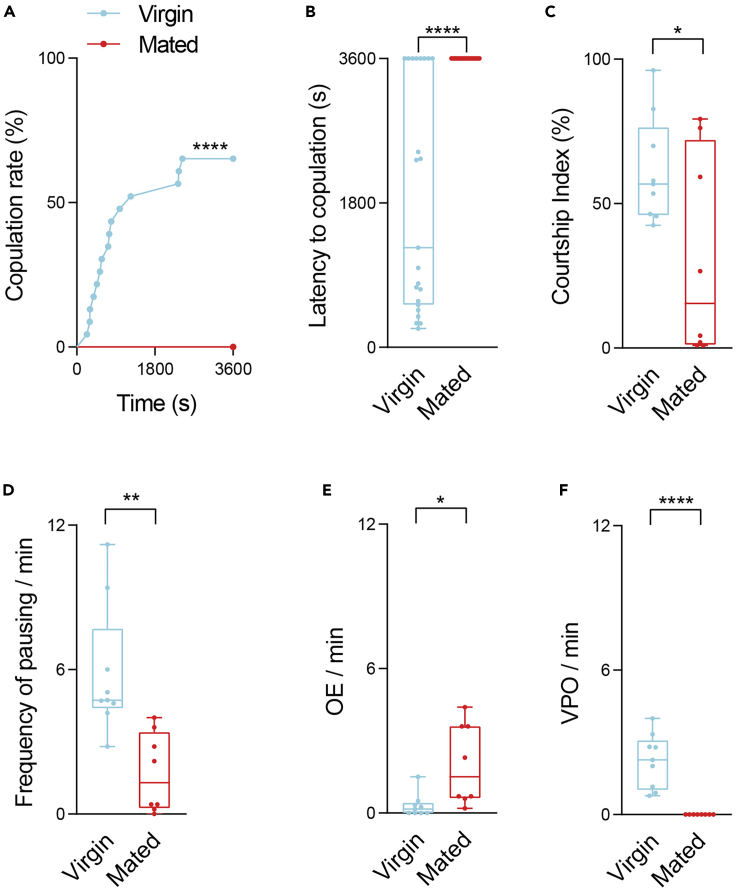
Figure 2.
Successful copulation decreases female receptivity
(A) Events of successful copulation over time when mated (red) and virgin (blue) females were paired with males (n = 23).
(B) Elapsed time before reaching successful copulation when mated (red) and virgin (blue) females were paired with males (n = 23).
(C) Courting attempts of males on mated (red) and virgin (blue) females (n = 8–9).
(D) The frequency of pausing per minute of mated (red) and virgin (blue) females toward the pursuing males (n = 8–9).
(E) The frequency of VPO bouts per minute of mated (red) and virgin (blue) females toward the pursuing males (n = 8–9).
(F) The frequency of OE bouts per minute of mated (red) and virgin (blue) females toward the pursuing males (n = 8–9). Wild type males and females were used in (A)-(F). All experimental conditions are indicated with the plots. In the box-and-whisker plot, the whiskers mark the minimum and maximum, the box includes the 25th to 75th percentiles, and the line within the box indicates the median of the data set. The Log-Rank test was applied to (A). The unpaired t-test was performed for (B)-(F). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001.
We expect to see a decrease in copulation rate (Figure 2A) and a prolonged latency to copulation (Figure 2B) when males are paired with mated females compared to virgins. The behaviors of males and females during the courtship ritual were further analyzed. Males exhibited lower courtship attempts (Figure 2C) toward mated females. Mated females paused less frequently (Figure 2D) and exhibited more rejection behaviors including OE (Figure 2E), compared to virgin females, who were more likely to show receptive behaviors including VPO (Figure 2F).
The changes in all six parameters between mated and virgin females indicate a general decline in courtship receptivity. Take precaution that manipulations such as activating a small group of neurons by optogenetics may not induce changes in the full range of parameters. This allows us to distinguish the role of specific neurons, based on the differential changes in these parameters, and to refine the neural circuit mechanisms that regulate female receptivity.
Limitations
This protocol has some limitations. Firstly, courtship is a highly dynamic and coordinated process involving both sexes; their behaviors are complex and diverse. This protocol incorporates several parameters to present features from these behaviors, but a versatile software for automatically extracting these features is not yet available. Secondly, the camera captures only top-view videos, and there are occasions that behaviors at certain angles can’t be readily discernible. Finally, additional but infrequent behaviors such as a female’s kick to reject a courting male are not included in this protocol.
Troubleshooting
Problem 1
During the transfer, flies are inclined to stay in the aspirator rather than climb into the courtship chamber (step-by-step method details step 5).
Potential solution
-
•
Move the aspirator closer to the light source which attracts the flies.
-
•
Flicking the pipette gently to stimulate the flies to initiate the climb.
Problem 2
Flies escape while being introduced into the courtship chamber (step-by-step method details step 5).
Potential solution
-
•
Practice makes perfect.
-
•
Block the hole in the acrylic sheet with your finger.
-
•
Keep an eye on the flies and be prepared to inhale when necessary.
Problem 3
It is difficult to discriminate OE and VPO (step-by-step method details step 9).
Potential solution
-
•
Check Methods video S2.
-
•
There is a visible extension of the tube-like ovipositor in OE, but not in VPO.
Problem 4
Water mist accumulated on the coverslip generates foggy video images, which may potentially cause missing fly behaviors (step-by-step method details step 7).
Potential solution
After the courtship chamber has been produced, allow them to cool to 25°C and wait at least half an hour before using them, while covering them with gauze to keep out other fruit flies in the testing room.
Problem 5
Insufficient number of mated females obtained within time window of 1 h to allow mating (before you begin step 8).
Potential solution
Take into consideration that the copulation rate is influenced by the genotypes of both sexes. Therefore, if not enough mated females are generated within the 1-h time window, utilize mated females obtained within a 2-h time frame. The performance of these females is similar to that of females from 1-h time window in the behavior test.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Yan Zhu (zhuyan@ibp.ac.cn).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We thank all members of the Zhu Lab for their beneficial discussions. We thank Ms. Meiyan Huang for technical assistance and Dr. Shan Gao for help in behavioral analyses. We thank Dr. Li Liu (CAS) for providing the fly strain.
This work was supported by NSFC grant (32071007, 9163210042), the Beijing Advanced Discipline Fund, Key Research Program of Frontier Sciences of CAS (QYZDY-SSW-SMC015), CAS Interdisciplinary Innovation Team, and Bill and Melinda Gates Foundation (OPP1119434) to Y.Z.
Author contributions
S.W.H. and Y.Z. designed the protocol, Y.T.Y. implemented the original protocol, Y.T.Y. analyzed the data, and Y.T.Y. and Y.Z. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102563.
Contributor Information
Yan Tong Yang, Email: yangyantong15@mails.ucas.edu.cn.
Yan Zhu, Email: zhuyan@ibp.ac.cn.
Data and code availability
-
•
This protocol did not generate original code.
-
•
Data is available from the lead contact upon request.
References
- 1.Yang Y.T., Hu S.W., Li X., Sun Y., He P., Kohlmeier K.A., Zhu Y. Sex peptide regulates female receptivity through serotoninergic neurons in Drosophila. iScience. 2023;26 doi: 10.1016/j.isci.2023.106123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishimoto H., Kamikouchi A. A Feedforward Circuit Regulates Action Selection of Pre-mating Courtship Behavior in Female Drosophila. Curr. Biol. 2020;30:396–407.e4. doi: 10.1016/j.cub.2019.11.065. [DOI] [PubMed] [Google Scholar]
- 3.Becnel J., Johnson O., Luo J., Nässel D.R., Nichols C.D. The Serotonin 5-HT7Dro Receptor Is Expressed in the Brain of Drosophila, and Is Essential for Normal Courtship and Mating. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bussell J.J., Yapici N., Zhang S.X., Dickson B.J., Vosshall L.B. Abdominal-B neurons control Drosophila virgin female receptivity. Curr. Biol. 2014;24:1584–1595. doi: 10.1016/j.cub.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F., Wang K., Forknall N., Parekh R., Dickson B.J. Circuit and Behavioral Mechanisms of Sexual Rejection by Drosophila Females. Curr. Biol. 2020;30:3749–3760.e3. doi: 10.1016/j.cub.2020.07.083. [DOI] [PubMed] [Google Scholar]
- 6.Wang K., Wang F., Forknall N., Yang T., Patrick C., Parekh R., Dickson B.J. Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature. 2021;589:577–581. doi: 10.1038/s41586-020-2972-7. [DOI] [PubMed] [Google Scholar]
- 7.Manning A. The control of sexual receptivity in female Drosophila. Anim. Behav. 1967;15:239–250. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This protocol did not generate original code.
-
•
Data is available from the lead contact upon request.