Abstract
Ivermectin has emerged as a therapeutic option for various parasitic diseases, including strongyloidiasis, scabies, lice infestations, gnathostomiasis, and myiasis. This study comprehensively reviews the evidence-based indications for ivermectin in treating parasitic diseases, considering the unique context and challenges in Peru. Fourteen studies were selected from a systematic search of scientific evidence on ivermectin in PubMed, from 2010 to July 2022. The optimal dosage of ivermectin for treating onchocerciasis, strongyloidiasis, and enterobiasis ranges from 150 to 200 μg/kg, while lymphatic filariasis requires a higher dose of 400 μg/kg (Brown et al., 2000). However, increased dosages have been associated with a higher incidence of ocular adverse events. Scientific evidence shows that ivermectin can be safely and effectively administered to children weighing less than 15 kg. Systematic reviews and meta-analyses provide strong support for the efficacy and safety of ivermectin in combating parasitic infections. Ivermectin has proven to be an effective treatment for various parasitic diseases, including intestinal parasites, ectoparasites, filariasis, and onchocerciasis. Dosages ranging from 200 μg/kg to 400 μg/kg are generally safe, with adjustments made according to the specific pathology, patient age, and weight/height. Given Peru's prevailing social and environmental conditions, the high burden of intestinal parasites and ectoparasites in the country underscores the importance of ivermectin in addressing these health challenges.
Keywords: Ivermectin, Antiparasitic agents, Scientific evidence, Helminthiasis, Peru
1. Introduction
In pursuit of novel antiparasitic agents, a new compound called avermectin was discovered. From this compound, ivermectin, a semisynthetic molecule, was derived and initially introduced as a commercial product for animal health purposes. (González Canga et al., 2008). In humans, the first specialty containing ivermectin was Mectizan, which has a long history of use in the medical field (González Canga et al., 2008). Over the years, numerous studies have been conducted and published investigating the efficacy and safety of Mectizan in treating various parasitic diseases. These studies have helped establish Mectizan as a cornerstone in the management of parasitic infections, highlighting its role in improving public health outcomes. The extensive body of research on Mectizan has not only informed clinical practice but also contributed to our understanding of ivermectin's potential applications, paving the way for its expanded use in the treatment of other parasitic diseases.
Ivermectin is a secondary metabolite produced by the bacterium Streptomyces avermitilis, which belongs to the soil-dwelling actinomycetes group. In this context, a secondary metabolite is a compound that is not essential for the basic growth, development, or reproduction of the organism but serves other important functions, such as defending against predators or inhibiting the growth of competing organisms. Streptomyces avermitilis is a type of soil-dwelling actinomycete, which are filamentous bacteria known for their ability to produce a wide variety of biologically active compounds, including antibiotics and other pharmaceuticals.
This compound exhibits lipophilic properties, meaning it has an affinity for lipid-rich environments. As a result, individuals with a higher body mass index (BMI) may experience lower plasma concentrations of the compound due to its increased distribution within fatty tissues. This can lead to a longer half-life (Schulz et al., 2019), which is the time it takes for the concentration of the substance in the body to decrease by half. Consequently, it is essential to prescribe the dose of this compound based on the patient's body weight, using a specific unit such as micrograms per kilogram (μg/kg) (Duthaler et al., 2019), in order to ensure its therapeutic efficacy and minimize potential side effects.
Ivermectin has demonstrated its effectiveness in combating a broad spectrum of parasitic infections. As an antiparasitic agent, it targets a diverse range of parasites, including those responsible for diseases such as onchocerciasis (river blindness), strongyloidiasis, lymphatic filariasis, and scabies. The drug works by disrupting the normal functioning of the parasite's nervous system, ultimately leading to paralysis and death. This allows the host's immune system to effectively eliminate the invaders. Ivermectin's wide-ranging efficacy against various parasites makes it a valuable tool in the treatment and control of multiple parasitic diseases, contributing to significant improvements in global public health.
In helminths (parasitic worms), ivermectin works by targeting the chloride channels that are sensitive to the neurotransmitter glutamate (Fox, 2006). By opening these channels, the drug disrupts the normal functioning of the parasite's central nervous system, leading to paralysis and eventual death. A notable example of ivermectin's effectiveness is its action against human onchocerciasis, a disease caused by the parasitic worm Onchocerca volvulus. Onchocerciasis, also known as river blindness, is a vector-borne disease transmitted through the bites of infected Simulium species, commonly known as black flies (Aziz et al., 1982). By incapacitating the adult worms and preventing the release of microfilariae (immature larvae), ivermectin aids in controlling the spread and impact of this debilitating disease.
Ivermectin is also effective against certain protozoa, which, along with helminths, are prevalent in children and pose a significant public health concern, particularly in developing countries (Vasquez-Elera et al., 2022). Protozoa are single-celled microorganisms that can cause various diseases in humans. Both protozoan and helminthic infections can lead to serious health issues, especially in children, who may suffer from impaired growth, cognitive development, and immune function as a result. By targeting these parasites, ivermectin plays a crucial role in addressing these health challenges and improving the overall well-being of affected populations, especially in resource-limited settings where access to healthcare may be limited.
In Latin America, it is estimated that more than 40 million children are exposed to an intestinal parasite type, which is one of the main public health problems that affect more than 30% of the world population. The prevalence and intensity of the parasite diseases are associated with a higher risk of the disease and are often very high, especially in school-age populations, which lead to problems in regard to physical and mental performance, and health (Geletaw et al., 2021).
In recent times, the use of ivermectin has expanded globally, primarily due to numerous information sources promoting its potential as a treatment for SARS-CoV-2, the virus responsible for COVID-19 (Castañeda-Marín et al., 2020). As the pandemic unfolded, researchers and healthcare professionals sought to identify existing drugs that could be repurposed to combat the virus. Ivermectin, with its known antiparasitic and anti-inflammatory properties, emerged as a candidate for further investigation. Consequently, the widespread dissemination of this information, through various channels such as scientific articles, news reports, and social media, has contributed to the increased global interest in and utilization of ivermectin as a potential treatment option for COVID-19.
In Peru, ivermectin was administered to approximately 90% of hospitalized patients suffering from SARS-CoV-2 infections (Vasquez-Elera et al., 2022). Amid the COVID-19 pandemic, healthcare professionals sought potential treatment options to alleviate the burden on patients and healthcare systems. Ivermectin, with its known antiparasitic properties and suggested potential against SARS-CoV-2, became a widely used treatment option in Peru. This widespread adoption can be attributed to the urgent need for effective therapies and the desire to explore available drugs that could potentially improve patient outcomes during the pandemic.
Due to the recent interest in the use of ivermectin for COVID-19 treatment and prevention, we conducted a comprehensive review of the scientific literature on the use of ivermectin as an antiparasitic agent worldwide. The objective of this study is to conduct a comprehensive literature review of the use of ivermectin in the treatment of parasitic infections, including its efficacy and safety, with a specific focus on the prevalence of parasitic infections in Peru and the potential for the use of ivermectin in mass treatment campaigns.
2. Frequency of parasitic diseases in Peru
In Peru, parasitic infections are a significant public health concern, particularly in rural and economically disadvantaged areas (Chosidow et al., 2010). The prevalence of these infections can vary considerably depending on factors such as region, age, socioeconomic status, and access to clean water and sanitation facilities.
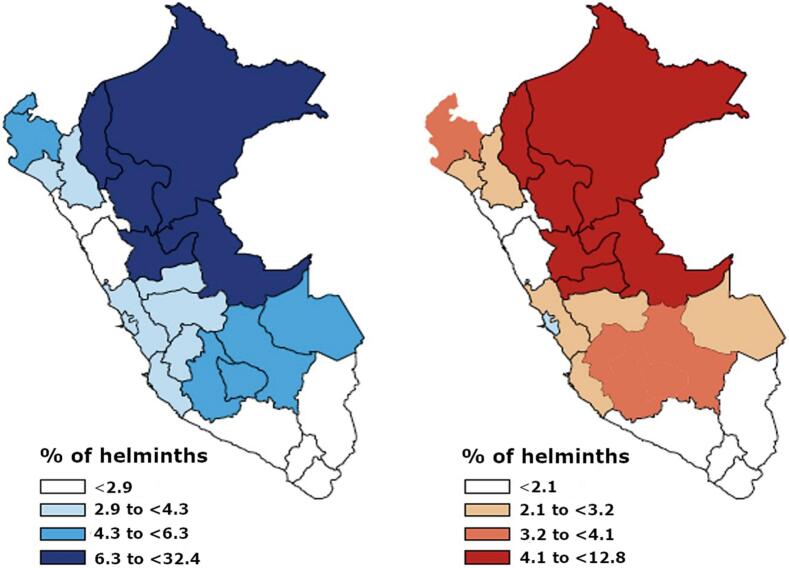
In Peru, the distribution of parasitic diseases has been documented in populations treated between 2010 and 2017 (Vidal-Anzardo et al., 2020). Nationally, the prevalence of overall parasitic diseases and helminth infections was 4.9% and 3.3%, respectively. Furthermore, 5.3% of children aged 12 to 17 years were found to have a parasitic infection. The most affected regions included the northeastern areas with tropical climates and the high Andean regions (Vidal-Anzardo et al., 2020) (Fig. 1). These regions are known for their low socioeconomic status in Peru (León Mendoza, 2019). Additionally, the prevalence of parasitic diseases varied across age groups: 9.5% among children aged 0 to 11 years, 5.3% among adolescents aged 12 to 17 years, 2.2% among young adults aged 18 to 29 years, and lower frequencies among other age groups.
Fig. 1.
Spatial distribution of parasitic diseases in Peru (Vidal-Anzardo et al., 2020). In the map, blue represents the overall prevalence of parasitic diseases, while red indicates the prevalence of helminth infections. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At the regional level, the departments with the highest parasite burden in Peru were Loreto (32.4%), San Martín (12.0%), and Ucayali (9.5%). The remaining regions exhibited a prevalence ranging from 4.3% to 6.3%. As for helminth infections, the results showed a similar pattern, with an annual prevalence of 3.3%: Loreto (12.8%), San Martín (8.9%), Pasco (8.0%), and Ucayali (7.6%) (Fig. 1) (Vidal-Anzardo et al., 2020).
In the Amazon region of Peru, for example, intestinal parasites are highly prevalent due to factors like inadequate sanitation, contaminated water sources, and limited access to healthcare. Ascaris lumbricoides, Trichuris trichiura, and hookworms are among the most common helminths in these areas, while protozoan parasites like Giardia lamblia and Entamoeba histolytica are also frequently observed. The prevalence of these infections is generally higher in children, as they are more susceptible to acquiring parasites through play and contact with contaminated soil or water.
In the highlands and coastal regions, other parasites such as Fasciola hepatica (liver fluke) and Echinococcus granulosus (causing cystic echinococcosis) may be more prevalent due to specific environmental conditions, such as the presence of intermediate hosts or livestock farming practices. The transmission of these parasites can be influenced by factors like close contact with infected animals and consumption of contaminated water or undercooked food.
In Peru, the prevalence of specific parasite species varies across different regions, environments, and age groups. Several factors, including climate, sanitation, and access to clean water, contribute to the distribution and prevalence of these parasites. Some of the most common parasitic infections in Peru include:
-
1.
Ascaris lumbricoides: This is a type of roundworm and is one of the most prevalent parasitic infections in Peru, particularly in rural areas (Choi & Kim, 2017). It is commonly found in children, who are more prone to ingesting the eggs through contaminated soil or food.
-
2.
Trichuris trichiura: Also known as whipworm, this parasite is frequently observed in rural and impoverished areas with poor sanitation. Like Ascaris, children are often more susceptible to this infection due to their exposure to contaminated environments.
-
3.
Hookworms: Necator americanus and Ancylostoma duodenale are two species of hookworms commonly found in Peru, especially in regions with warm, moist soil. These parasites can penetrate the skin, typically affecting people who walk barefoot or come into contact with contaminated soil.
-
4.
Giardia lamblia: This protozoan parasite is prevalent in areas with inadequate water treatment and sanitation facilities. It can affect all age groups but is more common in children due to their increased likelihood of ingesting contaminated water or food.
-
5.
Entamoeba histolytica: Another protozoan parasite, it is responsible for causing amoebic dysentery and liver abscesses. It is more prevalent in areas with poor sanitation and contaminated water sources, affecting both children and adults.
-
6.
Fasciola hepatica: Commonly known as liver fluke, this parasite is more prevalent in highland and coastal regions where livestock farming is practiced (Espinoza et al., 2010). The transmission occurs through the consumption of contaminated water or undercooked food, affecting people of all age groups.
-
7.
Echinococcus granulosus: This tapeworm is responsible for causing cystic echinococcosis, a zoonotic disease primarily affecting livestock and humans in close contact with infected animals (Moro et al., 2004). It is more prevalent in rural areas where people engage in livestock farming or animal husbandry practices.
In addition to the primary parasitic infections prevalent in Peru, other common parasites and parasitic interactions have been identified, with their prevalence varying based on specific regions within the country. For instance, the prevalence of Strongyloides stercoralis ranges from 0.8% to 19.5% depending on the region (Silva-Díaz et al., 2018). Infections such as cutaneous and visceral larva migrans have been found, with a prevalence of 7.3% in Lima and 32.4% in Morrope. Myiasis cases have been reported sporadically (1 per 100,000 people) in Lambayeque, located in northern Peru (Failoc-Rojas et al., 2018). Furthermore, a study investigating the distribution of parasitic diseases among children under 11 years old in Cajamarca found that Giardia lamblia was the most prevalent, affecting 27.2% of the population, followed by Ascaris lumbricoides at 19.5%. Co-infections of both types of parasites occurred in 3.3% of cases (Ipanaque-Chozo et al., 2018).
3. Safety of ivermectin
Safety of ivermectin has been assessed in several scientific studies and it has been found that the effective dose of ivermectin for treating onchocerciasis, strongyloidiasis, and enterobiasis is between 150 and 200 μg/kg (Juarez et al., 2018). For other diseases such as lymphatic filariasis the used dose is 400 μg/kg.
Higher doses than 400 μg/kg are still effective, but increase the number of cases of ocular adverse events, especially in onchocerciasis patients (Aziz et al., 1982). It is stated that ivermectin generates low levels of toxicity because its effect does not reach the central nervous system, and toxicity depends on immune and inflammatory responses against parasites, such as fever, pruritus, skin rashes, and malaise (Chandler, 2018).
In children under two years of age with less than 15 kg, not using ivermectin was suggested; however, there exists solid scientific evidence that the use of ivermectin can be safe and effective in children with less than 15 kg. In this regard, a systematic review analyzed the efficacy and safety of ivermectin in children under 15 kg, in which it was found that ivermectin was used in 1088 children that weighed less than 15 kg. The medical indication for this age group was to use this antiparasitic agent for treating infections due to strongyloidiasis, scabies, lice infestations, gnathostomiasis, myiasis, and for helminths reduction transmitted through soil, for which this drug was useful for the control of the above mentioned diseases (Jittamala et al., 2021). In the mentioned study, 18 possible adverse events were recorded; the most frequent were four (4/1088) due to diarrhea (0,4%).
Ivermectin shows promise as a treatment option for pregnant women as studies have shown that it can effectively reduce rates of anemia and parasitic infections in this population. Additionally, it has had good perinatal outcomes in neonates. However, it has not been approved globally yet, and clinical trials are still being conducted (Nicolas et al., 2020).
4. Ivermectin indications
The purpose of the search described in this section was to gather scientific evidence regarding the indications and uses of ivermectin, excluding its potential application for COVID-19 or SARS-related infections. The search was conducted using the PubMed database, which includes studies from various countries around the world. The search terms “Mesh” and “Tiab” were used in combination with specific keywords related to ivermectin (such as “Ivermectin”, “Mectizan”, “Stromectol”, “Eqvalan”, and “Ivomec”) and excluding terms related to COVID-19 or SARS (such as “Covid*” and “sars*”). To refine the search results, filters for “subject: Human” and “year: 2010–2022” (until July 2022) were applied.
In addition to searching PubMed, we also reviewed clinical practice guidelines and summaries from major institutions to gather further evidence on ivermectin's indications.
Out of the 1697 studies identified in the search, 14 were selected for their high level of scientific evidence (including systematic reviews, clinical trials, and observational studies) and good methodological quality. These studies have used ivermectin for various indications, as detailed in Table 1.
Table 1.
Evidence summary of ivermectin indications in the treatment of parasitic infections.
| Indication | Commentaries | Evidence |
|---|---|---|
| Lymphatic filariasis | A single oral dose of IVM at 200 μg/kg (Kimberlin, 2015). IVM reduces 60% of microfilariae on the first day and 85% the 30th day (Pion et al., 2019). |
Systematic reviews with meta-analysis: Pion et al., 2019 Siewe Fodjo et al., 2020 |
| Onchocerciasis | A single oral dose of IVM at 150 μg/kg, which can be repeated every six or twelve months until being asymptomatic. For the prevention of blindness due to ocular onchocerciasis, the dosage must be repeated every three to 12 months (Kimberlin, 2015). |
Systematic reviews with meta-analysis: Siewe Fodjo et al., 2020 |
| Soil-transmitted helminthiasis (ancylostomiasis- ascariasis, trichuriasis) and taeniasis in the duodenum, taenia solium, etc.) | A single dose of IVM at 200 μg/kg for two days is effective (Kimberlin, 2015). IVM combined with albendazole is able to halve helminths in compared to albendazole alone. (RR: 0.44; 95% IC: 0.31 a 0.62)(Palmeirim et al., 2018) | Systematic reviews with meta-analysis: Palmeirim et al., 2018 |
| Ectoparasites (lice infestations, scabies, mites, fleas, etc.). | Oral dose of 400 μg/kg on the first and eighth day, or 200 μg/kg, it has to repeated after 10 days (Kimberlin, 2015). Oral intake of IVM has a higher effectivity than topical IVM for the treatment of scabies (Rosumeck et al., 2018). |
Systematic reviews with meta-analysis: Rosumeck et al., 2018 |
| Strongyloidiasis | Oral dose of 200 μg/kg twice a day for two days. When treating parasitic diseases, IVM proved to be more effective than albendazole (RR 1,79; IC 95%: 1,55 to 2,08), but similar to thiabendazole (RR 0,80; 95% IC: 0,59 a 1,09); however, the adverse events were fewer using IVM (Henriquez-Camacho et al., 2016). |
Systematic reviews with meta-analysis: Henriquez-Camacho et al., 2016 |
| Myiasis | Oral administration with a single dose of 250 μg/kg | Observational studies: Calvopina et al., 2020 |
| Larva migrans | Oral administration of a daily single dose of 200 μg/kg for two days | Observational studies: Gerbig and Kempf, 2020 |
| Pediculus humanus capitis | Oral dose of 400 μg/kg on the first and eighth day, or 200 μg/kg with a repeated dose after 10 days | Systematic review with meta-analysis: Jones and English 3rd., 2003 Randomized clinical trial: Chosidow et al., 2010 |
| Rosacea (due to mite infestations, Demodex folliculorum) | Topical 1% IVM demonstrated that ivermectin can control papulopustular rosacea and improve life quality. | Systematic review and meta-analysis: Husein-ElAhmed and Steinhoff, 2020 |
| Malaria | There exists evidence of reduction in malaria indexes and sporozoites by 77% when mass IVM is used (Alout et al., 2014). | Epidemiological study: Alout et al., 2014 |
RR: Relative risk. CI: Confidence interval. IVM: Ivermectin.
The aim of this search and subsequent analysis was to synthesize the most recent and relevant information on ivermectin's use and effectiveness across different settings and populations, thereby contributing to a comprehensive understanding of the drug's applications and potential benefits.
5. Ivermectin mass drug administration
The implementation of mass ivermectin administration has proven successful in various countries for controlling and eliminating specific parasites, as supported by scientific evidence. In this section of the study, we have compiled and documented the applications of ivermectin as described in the scientific literature (Table 2).
Table 2.
Use of mass ivermectin and its efficacy against parasitic diseases.
| Disease | Population | Basal % of parasitic diseases | % of parasitic diseases after one month | % of parasitic diseases after nine to 12 months |
|---|---|---|---|---|
| Ancylostomiasis (Necator americanus and Ancylostoma duodenale) | Northeast Brazil, median age of 26.4, 48,4% were male. A total of 516 people (Heukelbach et al., 2004) (Heukelbach et al., 2004) | 28.5 | 16.4 | 7.7 |
| Ascariasis | Northeast Brazil, median age of 26.4, 48,4% were male. A total of 516 people (Heukelbach et al., 2004) (Heukelbach et al., 2004) | 17.1 | 0.4 | 0.4 |
| Trichuriasis | Northeast Brazil, median age of 26.4, 48.4% were male. A total of 516 people (Heukelbach et al., 2004) (Heukelbach et al., 2004) | 16.5 | 3.4 | 9.4 |
| Strongyloidiasis | Northeast Brazil, median age of 26.4, 48.4% were male. A total of 516 people (Heukelbach et al., 2004) (Heukelbach et al., 2004) | 11.0 | 0.6 | 0.7 |
| Lice infestation (Brazil) | Northeast Brazil, median age of 26.4, 48.4% were male. A total of 548 people (Heukelbach et al., 2004) (Heukelbach et al., 2004) | 28.1 | 21.1 | 13.5 |
| Scabies (Brazil) | Northeast Brazil, median age of 26.4, 48.4% were male. A total of 548 people (Heukelbach et al., 2004) (Heukelbach et al., 2004) | 3.8 | 1.0 | 1.5 |
| Scabies (Japan) | Fuji, a population of all the ages, with 716 people | 32.1 | 1.9 | |
| Cutaneous larva migrans | Northeast Brazil, median age of 26.4, 48.4% were male. A total of 548 people (Heukelbach et al., 2004) (Heukelbach et al., 2004) | 0.7 | 0.0 | 0.0 |
Peru, although it is a country with a high load of intestinal parasitic diseases and ectoparasites, does not have scientific evidence of the use and mass administration of ivermectin. It is suggested that this drug should be administrated to decrease the load of this disease, as well as to evaluate the usefulness of this antiparasitic agent.
Upon implementing these strategies, it is essential to conduct thorough pharmacovigilance to assess the efficacy of the intervention effectively.
6. Conclusions
Ivermectin is an effective antiparasitic agent that acts against many intestinal parasites (strongyloidiasis, ascariasis, trichuriasis, ancylostomiasis, among others), ectoparasites (scabies, larva migrans, myiasis, etc.), as well as filariasis and onchocerciasis. Ivermectin has a good safety profile. The dose of 200 μg/kg to 400 μg/kg is safe, depending on the treated pathology, age, and weight/height of the patient. Peru is still, because of its social and environmental conditions, a country with a high load of intestinal parasites and ectoparasites, reported by several research studies. The use of ivermectin to treat parasitic diseases and against ectoparasites is feasible, and it can be indicated for welfare activities in children with a suspected or diagnosed parasitic disease. Welfare initiatives involving mass ivermectin administration should be carried out with careful consideration of the drug's safety for individuals aged two years and older. However, it is recommendable that studies develop measurements of the impact of these activities in different regions.
Funding
No funding was received.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Thanks to the Health Technology Assessment and Research Institute of ESSALUD.
References
- Alout H., Krajacich B.J., Meyers J.I., Grubaugh N.D., Brackney D.E., Kobylinski K.C.…Foy B.D. Evaluation of ivermectin mass drug administration for malaria transmission control across different west African environments. Malar. J. 2014;13:417. doi: 10.1186/1475-2875-13-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M., Diop I., Diallo S., Lariviere M., Porta M. Efficacy and tolerance of ivermectinin human onchocerciasis. Lancet. 1982;320(8291):171–173. doi: 10.1016/s0140-6736(82)91026-1. [DOI] [PubMed] [Google Scholar]
- Calvopina M., Ortiz-Prado E., Castañeda B., Cueva I., Rodriguez-Hidalgo R., Cooper P.J. Human myiasis in Ecuador. PLoS Negl. Trop. Dis. 2020;14(2) doi: 10.1371/journal.pntd.0007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda-Marín E., Gonzalez-Gonzalez A., Grau-Bocanegra R., Caballero-Alvarado J. Uso de ivermectina en pacientes con la COVID-19: Una revision narrativa. Rev. Cuerpo Méd. Hospit. Nacional Almanzor Aguinaga Asenjo. 2020;13(4):440–445. [Google Scholar]
- Chandler R.E. Serious neurological adverse events after ivermectin—do they occur beyond the indication of onchocerciasis? Am. J. Trop. Med. Hyg. 2018;98(2):382. doi: 10.4269/ajtmh.17-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chosidow O., Giraudeau B., Cottrell J., Izri A., Hofmann R., Mann S.G., Burgess I. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N. Engl. J. Med. 2010;362(10):896–905. doi: 10.1056/NEJMoa0905471. [DOI] [PubMed] [Google Scholar]
- Duthaler U., Suenderhauf C., Karlsson M.O., Hussner J., Meyer zu Schwabedissen H., Krähenbühl S., Hammann F. Population pharmacokinetics of oral ivermectin in venous plasma and dried blood spots in healthy volunteers. Br. J. Clin. Pharmacol. 2019;85(3):626–633. doi: 10.1111/bcp.13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failoc-Rojas V.E., Molina-Ayasta C., Salazar-Zuloeta J., Samamé A., Silva-Díaz H. Case report: Myiasis due to Cochliomyia hominivorax and Dermatobia hominis: clinical and pathological differences between two species in northern Peru. American J. Trop. Med. Hyg. 2018;98(1):150. doi: 10.4269/ajtmh.16-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L.M. Ivermectin: uses and impact 20 years on. Curr. Opin. Infect. Dis. 2006;19(6):588–593. doi: 10.1097/QCO.0b013e328010774c. [DOI] [PubMed] [Google Scholar]
- Geletaw A., Egata G., Weldegebreal F., Kibr G., Semaw M. Nutritional status and associated factors among primary schoolchildren from pastoral communities, Mieso-Mulu District, Sitti Zone, Somali Regional State, Eastern Ethiopia: institution-based cross-sectional study. J. Nutrit. Metabol. 2021:2021. doi: 10.1155/2021/6630620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbig A.W., Kempf W. Topical treatment of cutaneous larva migrans with ivermectin 1% Int. J. Dermatol. 2020;59(1):e21–e22. doi: 10.1111/ijd.14673. [DOI] [PubMed] [Google Scholar]
- González Canga A., Sahagún Prieto A.M., Diez Liébana M.J., Fernández Martínez N., Sierra Vega M., García Vieitez J.J. The pharmacokinetics and interactions of ivermectin in humans--a mini-review. AAPS J. 2008;10(1):42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez-Camacho C., Gotuzzo E., Echevarria J., White A.C., Jr., Terashima A., Samalvides F.…Plana M.N. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst. Rev. 2016;2016(1):Cd007745. doi: 10.1002/14651858.CD007745.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukelbach J., Winter B., Wilcke T., Muehlen M., Albrecht S., de Oliveira F.A.…Feldmeier H. Selective mass treatment with ivermectin to control intestinal helminthiases and parasitic skin diseases in a severely affected population. Bull. World Health Organ. 2004;82(8):563–571. [PMC free article] [PubMed] [Google Scholar]
- Husein-ElAhmed H., Steinhoff M. Efficacy of topical ivermectin and impact on quality of life in patients with papulopustular rosacea: a systematic review and meta-analysis. Dermatol. Ther. 2020;33(1) doi: 10.1111/dth.13203. [DOI] [PubMed] [Google Scholar]
- Ipanaque-Chozo J., Claveri-Cesar I., Tarrillo-Díaz R., Silva-Díaz H. Parasitosis intestinal en niños atendidos en un establecimiento de salud rural de Cajamarca, Perú. Rev. Experien. Med. Hospit. Reg. Lambayeque. 2018;4(1):15–18. [Google Scholar]
- Jittamala P., Monteiro W., Smit M.R., Pedrique B., Specht S., Chaccour C.J.…Maruani A. A systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: is it time to reconsider the current contraindication? PLoS Negl. Trop. Dis. 2021;15(3) doi: 10.1371/journal.pntd.0009144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.N., English J.C., 3rd. Review of common therapeutic options in the United States for the treatment of pediculosis capitis. Clin. Infect. Dis. 2003;36(11):1355–1361. doi: 10.1086/374840. [DOI] [PubMed] [Google Scholar]
- Juarez M., Schcolnik-Cabrera A., Dueñas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 2018;8(2):317. [PMC free article] [PubMed] [Google Scholar]
- Kimberlin D.W. In: FAAP, American Academy of Pediatrics. David W., Kimberlin M.D., editors. American academy of pediatrics; 2015. RED BOOK, 2015 Report of the Committee on Infectious Diseases. [Google Scholar]
- León Mendoza J.C. Capital humano y pobreza regional en Perú. Reg. y Soci. 2019;31 [Google Scholar]
- Nicolas P., Maia M.F., Bassat Q., Kobylinski K.C., Monteiro W., Rabinovich N.R.…Chaccour C. Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis. Lancet Glob. Health. 2020;8(1):e92–e100. doi: 10.1016/S2214-109X(19)30453-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeirim M.S., Hürlimann E., Knopp S., Speich B., Belizario V., Jr., Joseph S.A.…Keiser J. Efficacy and safety of co-administered ivermectin plus albendazole for treating soil-transmitted helminths: a systematic review, meta-analysis and individual patient data analysis. PLoS Negl. Trop. Dis. 2018;12(4) doi: 10.1371/journal.pntd.0006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pion S.D., Tchatchueng-Mbougua J.B., Chesnais C.B., Kamgno J., Gardon J., Chippaux J.P.…Boussinesq M. Effect of a single standard dose (150-200 μg/kg) of Ivermectin on Loa loa Microfilaremia: systematic review and Meta-analysis. Open Forum. Infect. Dis. 2019;6(4):ofz019. doi: 10.1093/ofid/ofz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosumeck S., Nast A., Dressler C. Ivermectin and permethrin for treating scabies. Cochrane Database Syst. Rev. 2018;4(4):Cd012994. doi: 10.1002/14651858.cd012994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J.D., Coulibaly J.T., Schindler C., Wimmersberger D., Keiser J. Pharmacokinetics of ascending doses of ivermectin in Trichuris trichiura-infected children aged 2–12 years. J. Antimicrob. Chemother. 2019;74(6):1642–1647. doi: 10.1093/jac/dkz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewe Fodjo J.N., Remme J.H.F., Preux P.M., Colebunders R. Meta-analysis of epilepsy prevalence in West Africa and its relationship with onchocerciasis endemicity and control. Int. Health. 2020;12(3):192–202. doi: 10.1093/inthealth/ihaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Díaz H., Reynoso-Mego A., León-Jiménez F., Failoc-Rojas V.E. Características clínicas y epidemiológicas de la estrongiloidiasis en un hospital del Norte del Perú. Infectio. 2018;22:131–135. [Google Scholar]
- Vasquez-Elera L.E., Failoc-Rojas V.E., Martinez-Rivera R.N., Morocho-Alburqueque N., Temoche-Rivas M.S., Valladares-Garrido M.J. Self-medication in hospitalized patients with COVID-19: a cross-sectional study in northern Peru. Germs. 2022;12(1):46–53. doi: 10.18683/germs.2022.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Anzardo M., Yagui Moscoso M., Beltrán Fabian M. Parasitosis intestinal: Helmintos. Prevalencia y análisis de la tendencia de los años 2010 a 2017 en el Perú. Anales de la Facultad de Medicina. 2020;81:26–32. [Google Scholar]