Highlights
-
•
Full-length genomes of DHAV-1 worldwide were sorted into seven sub-groups.
-
•
Full-length genomes of DHAV-3 worldwide were sorted into five sub-groups.
-
•
DHAVs identified in China displayed great genetic diversity.
-
•
Recombination hotspot of DHAV genome is at the 5′ end and upstream of the capsid coding region.
-
•
The highest variability of DHAV polyprotein is at the upstream region of n terminus P-loop.
Keywords: Duck hepatitis a virus, Diversity, Phylogenetics, Phylogeographic, Recombination
Abstract
Duck hepatitis A virus (DHAV) is one of key pathogens for duck viral hepatitis, especially in Asian duck industry. Currently, two main genotypes (DHAV-1 and -3) exist. To explore insightfully the evolutionary character, we assessed the available 141 full-length genome sequences of DHAV isolated in 1986–2020 globally and divided DHAV-1 and DHAV-3 into further seven (DHAV-1 a-g) and five (DHAV-3 a-e) sub-clades, respectively. Phylogenetic and phylogeographic network analyses indicated great genetic diversity of DHAV identified in China, where the DHAV-1 cluster and DHAV-3 cluster were linked by virus strain HDHV1-BJ (GenBank ID: FJ157172.1) and Du_CH_LSD_090612 (GenBank ID: JF828995.1) via a long mutational branch and intermediate strains. Several strains previously identified as DHAV-1 according to the partial gene sequences were actually clustered within DHAV-3 in full-length genome-based analysis. Furthermore, we identified 32 recombination events across virus genome with the recombination hotspot at the 5′ end and upstream of the capsid coding region. The highest variability of DHAV polyprotein was shown at the upstream region of the N terminus P-loop region, e.g., amino acids 672–716, followed by the aa 334–359 in the Capsid encoding region. The results presented here provides a robust insight into the genetic exchange patterns of DHAV genomes during the past decades, which may be used to map the evolutionary history and facilitate preventive measures of DHAVs.
Duck viral hepatitis (DVH) is an acute infectious disease for young ducklings, which is usually divided into three types I, II and III. DVH type I is caused by duck hepatitis A virus (DHAV) (Yugo et al., 2016). DHAV is a member of the genus Avihepatovirus, family Picornaviridae and is the only species in this genus (ICTV). DHAV was initially divided into three serotypes or genotypes, designated as DHAV-1, DHAV-2, and DHAV-3 (Funk et al., 2007; Kim et al., 2008; Tseng et al., 2007; Tseng and Tsai, 2007). Recently however, DHAV-2 was included within the DHAV-3, while the DHAV-1 as a separate clade (Wen et al., 2014a). In natural infection, younger ducklings present with significant manifestations including lethargy, ataxia, and opisthotonos, or even sudden death, whereas the adult ducks do not display overt clinical signs (Yugo et al., 2016).
DHAV virion is non-enveloped and contains a positive-sense, single-stranded RNA genome of about 7.7 kbs in length (Yun et al., 2010). The genome of DHAV contains a single, open reading frame (ORF) that is flanked by a 5′-untranslated region (UTR) and a 3′-UTR with a poly(A) tail. The ORF encodes a large polyprotein of about 2249 amino acids. This protein is processed into three subunits P1, P2, and P3 during virus replication (Ding and Zhang, 2007; Kim et al., 2006). P1 is further processed into three structural capsid proteins VP0, VP1 and VP3. P2 is processed into non-structural proteins 2A1, 2A2, 2A3, 2B, 2C, while P3 into non-structural proteins 3A, 3B, 3C and 3D (Kloc et al., 2018; Sun et al., 2017; Yang et al., 2017; Zell et al., 2017). These non-structural proteins are responsible for the replication of virus and modulation of cell host activities (Cao et al., 2016; Lai et al., 2019; Li et al., 2022a, 2022b; Liu et al., 2021, 2022; Sun et al., 2019; Yang et al., 2018; Zhang et al., 2017b). The VP0, VP1 and VP3 as capsid proteins contain the major specific antigen epitopes (Tseng and Tsai, 2007), thus also serve as the targets in vaccine development (Zou et al., 2016).
The non-structural proteins and the UTRs of DHAV regulate the transcription, translation, and replication of viral genome. For example, 2C has the nucleoside triphosphatase activity (Li et al., 2022b), whereas the 3D is an RNA-dependent RNA polymerase (RdRp) (Zhang et al., 2017a). The 3′-UTR interacts with cellular insulin-like growth factor-2 mRNA-binding protein-1 to increase the internal ribosome-entry site (IRES)-mediated translation efficiency (Chen et al., 2019). 3CD reduces the expression of IRF7 (interferon (IFN) regulatory factor 7) and RIG-1 (retinoic acid-inducible gene I) to inhibit the antiviral innate immunity during virus replication (Xia et al., 2023).
Globally, DHAV has been identified in a number of countries in America, Europe, Africa and Asia, such as the United States, England, Poland, Egypt, South Korea, and Vietnam et al. In China, DHAV-1 was first identified in 1963 (Guo and Pan, 1984). In 2013, DHAV-3 emerged and always co-infected with DHAV-1 in China ducks (Lin et al., 2016; Wen et al., 2018b). DHAV-3 was also frequently identified in ducks in Egypt from 2016 to 2018 (Hassan et al., 2020; Rohaim et al., 2021b; Yehia et al., 2021). The co-infection of DHAV with other viral pathogens such as avian influenza virus (AIV) has been observed (Mansour et al., 2018). Thus, the control of DVH is a complex challenge.
DHAV can be transmitted horizontally and vertically (Stoute et al., 2020; Yang et al., 2021; Zhang et al., 2021). The infected adult duck can shed virus as one of the main sources of infection (Zhang et al., 2018). The exosomes seem to play a critical role in transmitting virus as the complete DHAV-1 genomic RNA was identified in the exosomes of duck embryo fibroblasts (DEFs) (Xu et al., 2023).
Like all animal infectious diseases, strict biosecurity measures combined with effective vaccination programmes are fundamental for the prevention and control of DHAV (Yang et al., 2021; Zhang et al., 2023). The effective vaccine is critical for the control of DHAV (Yang et al., 2021; Zhang et al., 2023). However, DHAV-1 vaccine could not provide complete protection (Lu et al., 1993; Xu et al., 2023). In addition, the existing DHAV-1 vaccine has no sufficient immunological cross-reactivity with DHAV-3, against which currently no licensed vaccines are available. In addition, high titers of DHAV-1-specific NAbs could not block the exosome-mediated virus transmission (Xu et al., 2023). As a potential gene therapy, DNAzymes were also tested to specifically cleaved DHAV-1 genome to inhibit virus replication (Li et al., 2022c). DNAzymes were tested to specifically cleaved 300–618 nt of the DHAV-1 5′ UTR to inhibit the translation and replication of DHAV-1, providing theoretical support for gene therapy of duck viral hepatitis (Li et al., 2022c). Thus, there is need of in-depth molecular and genetic investigations of DHAVs in order to design effective strategies against all the DHAVs.
To control effectively and efficiently the infection of DHAV, the extent of infection and genetic variation worldwide need to be well known. Herein, we analyzed comprehensively the phylogenetic relatedness among the DHAV strains isolated worldwide from 1986 to 2020 by using the full-length genomic sequences and mapped the phylogeographic network of the virus to better define the previous evolutionary characteristics and phylogeographic distribution of DHAV strains and provide helpful hints for the genetic basis of DHAV emergence and re-emergence.
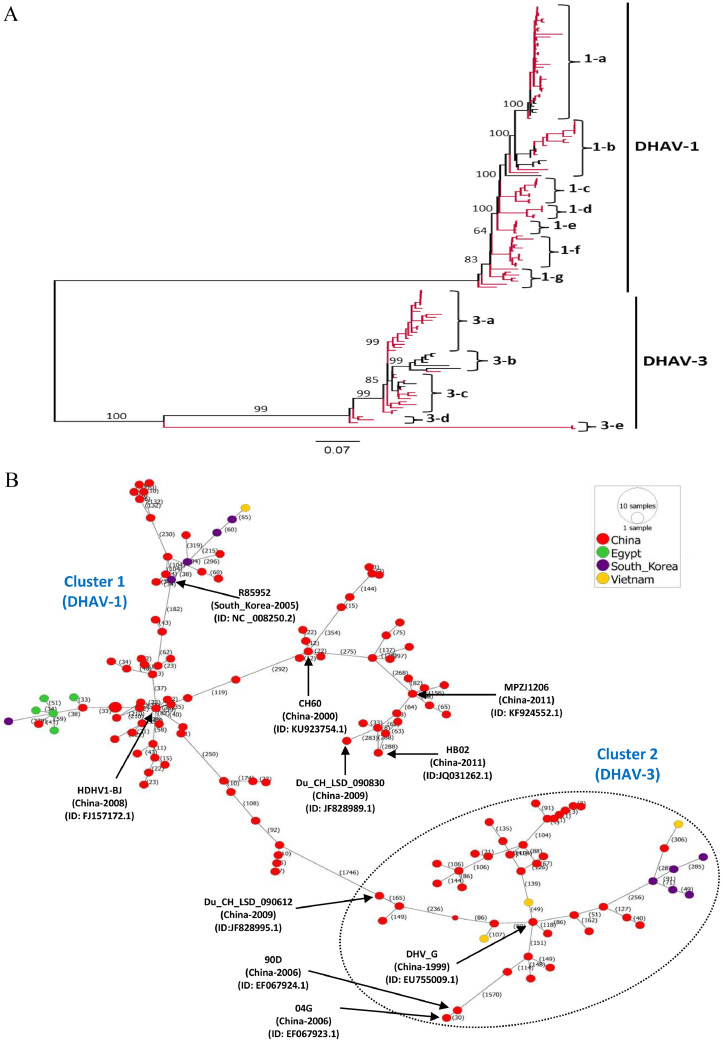
The previous studies have classified DHAV genotypes based on the phylogenetic analysis of the VP1 gene (Doan et al., 2016b; Gao et al., 2012; Ma et al., 2015a; Wen et al., 2014b, 2018a). However, multiple studies have reported divergence and variations in the VP1 gene locations (Doan et al., 2016b; Fehér et al., 2021; Li et al., 2013; Ma et al., 2015a), suggesting the whole-genome-based analysis of DHAV strains is to better understand the emergence of novel strains and their geographical dispersal (Fehér et al., 2021). To comprehensively understand the molecular epidemiology of DHAV strains circulating worldwide, we used full-length genomic sequences and a new approach for typing of DHAV to reveal the genetic characteristics. The available (n = 141) full-length genome sequences of DHAV in NCBI GenBank database were retrieved, aligned, and then subjected to the maximum likelihood (ML) phylogenetic analysis based on 1000 bootstrap replications and best-fitting model TIM2+F + I + G4 using the IQ-TREE multicore version 1.6.12 (Trifinopoulos et al., 2016). These full-length genome sequences were isolated between 1986 and 2020 in four different countries, where the highest number of sequences were from China (122 sequences) followed by South Korea (10), Egypt (5) and Vietnam (4) (Supplementary Table S1). As shown in Fig. 1A and Supplementary Fig. S1, DHAV full-length genome sequences are classified into two major clades, e.g., DHAV-1 and DHAV-3, where the DHAV-1 clade is further divided into seven (1-a to 1-g), while DHAV-3 into five (3-a to 3-e) sub-clades. All the attenuated vaccine strains belong to DHAV-1 clade, including China vaccine strains DHAV-1 X (GenBank ID: FJ496343) (Gao et al., 2012) and AV2111-A (GenBank ID: EU395440) (Gao et al., 2012; Ma et al., 2009) in 1-a; strains H (GenBank ID: DQ249300) (Tseng et al., 2007) and 5886 (GenBank ID: DQ249301) (Tseng et al., 2007) in 1-b; A66 (GenBank ID: DQ886445) (Huang et al., 2009) and CH60 (GenBank ID: KU923754.1) (Ou et al., 2017) in 1-e, and the Vietnam vaccine strain VXXT (GenBank ID: JF914945.1) (Doan et al., 2016a) in 1-b.
Fig. 1.
Phylogenetic and phylogeographic analyses. (A) The phylogenetic tree based on the full-length genome sequences of DHAV, 1986–2020. The unrooted ML phylogenetic tree of 141 full-length DHAVs genome classifying all the strains into two major clades (DHAV-1 and DHAV-3). DHAV-1 can be further classified into seven sub-clades (1a-1 g) and DHAV-3 into five sub-clades (3a-e). The major clades and sub-clades of the DHAV are indicated. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are indicated at each node. The evolutionary distances were computed using the best-fit substitution model (GTR+F + I + G4) in the IQ-TREE multicore version 1.6.12. The red color clades represent the viruses identified in China (n = 122) and black color represents viruses identified in South Korea (n = 10), Egypt (n = 5), and Vietnam (n = 4). The tree was visualized and modified using FigTree v1.4. The detailed information about each virus is seen in Supplementary Fig. S1, where the viruses were identified using their GenBank ID, virus name, country, and year of isolation, e.g., ID-virus name-country-year. (B) Phylogeographic network analysis of the full-length DHAV sequences, 1986–2020. The phylogenetic network of 141 full-length genomes of DHAVs was inferred using the MSN network implemented by PopArt v1.7. Both the two clusters are linked by the DHAV-1_HDHV1-BJ_China-2008 (GenBank ID: FJ157172.1) and DHAV-1_Du_CH_LSD_090612_China-2009 (GenBank ID: JF828995.1) respectively through a long mutational branch and intermediate strains. The DHV1_04G_China-2006 (GenBank ID: EF067923.1) and DHV1_90D_China-2006 (GenBank ID: EF067924.1) strains that clustered as a separate clade (DHAV 3-e) within the phylogenetic tree were identified to be evolved and clustered within the DHAV-3 cluster (Cluster 2) within the phylogeographic network. Similarly, the two distanced strains within the DHAV-1 clade within the 1-g sub-clade were showed to be clustering separately and connected to the DHAV-1_MPZJ1206_China-2011 (GenBank ID: KF924552.1) strain within the DHAV-1 cluster (Cluster 1). Numbers represent the number of mutations. Each color represents a different country.
The strains identified in China were the most dominant and spread across the clades and sub-clades. Our results indicate a great diversity and potential genetic exchange among the DHAV strains isolated in China. Earlier observations suggested that the European DHAV-1 strains are closely related to China strains (Feher et al., 2021). In China, DHAV-1 field isolates were previously classified into two groups by phylogenetic analyses based on the VP1 coding sequences: one group (DHAV-1) and another group (DHAV-3), which possess large genetic and serological differences (Wen et al., 2018b).
The phylogenetic analysis also indicated that some of the strains isolated in China are more distanced from all the other strains. For example, virus strains 90D (GenBank ID: EF067924.1) and 04 G (GenBank ID: EF067923.1) are sorted into an independent sub-clade 3-e of DHAV-3, genetically far distant from the remaining sub-clades of DHAV-3. Both were identified from DHAV-1 vaccinated ducklings in Taiwan region, which are antigenically unrelated to DHAV-1 (Tseng and Tsai, 2007). These strains were initially designated as DHAV-2 and were clustered along with the DHAV-1 clade (Cha et al., 2013), while were placed within the DHAV-3 later on (Wen et al., 2014a). The Du_CH_LJS_090905 (GenBank ID: JF828996.1), DHAV-1_Du_CH_LGD_090818 (GenBank ID: JF828994.1), and Du_CH_LSD_090612 (GenBank ID: JF828995.1) that were isolated from the duck liver in China in 2009 and classified as DHAV-1 in GenBank database actually fall into sub-clade 3-d of DHAV-3. Similarly, the Du/DH/LSD120220, Du/DH/LSD120223 and Du/DH/LSD120225 strains (GenBank IDs: MK371022.1, MK371023.1 and MK371024.1 respectively) isolated in China during 2012 that were classified as DHAV-1 (Liu and Kong, 2019) also fall in 3-a sub-clade of DHAV-3 (Fig. 1A and Supplementary Fig. S1).
The lineage of DHAV-3 was suggested previously to originate in South Korea and then spread to China and Vietnam (Ma et al., 2015b). Using the P1 nucleotide and amino acid sequences, respectively, all of the 44 DHAV-3 strains were clustered into two distinct genotypes: all Chinese isolates except B63 strain (GenBank ID: EU747874.1) were included in one genotype (referred to as Chinese genotype, CH genotype), while B63 strain and all the Korean and Vietnam strains belonged to the other genotype (referred to as Korean and Vietnamese genotype, KV genotype) (Zhang et al., 2017a). However, in our study, the DHAV-3 clade can be divided into five (3-a to 3-e) sub-clades based on full-length genome sequences. All Korean strains, one China strain B63 and one Vietnam strain DN2 (GenBank ID: JF914944.1) were clustered into 3-b, while another two Vietnamese strains NT (GenBank ID: KU860090.1) and NC (GenBank ID: KU860089.1) (Doan et al., 2016a) and some Chinese isolates were clustered into 3-c.
All the strains identified in Egypt (n = 5, Egyptian isolates under current study) clustered together within the 1-a, while the strains isolated in South Korea and Vietnam clustered closer to each other in the 1-b and 3-b along with the strains from China with the exception of two Vietnam viruses (GenBank ID: KU860090.1 and KU860089.1) in 3-c. Consistently, the phylogenetic analysis of the Egypt DHAV-1 isolates based on the 3D gene, 5′UTR, VP1 gene, and complete genome all show those viruses fall into the same subgroup (Rohaim et al., 2021a).
Phylogeographic network analysis was performed to further visualize the genetic relationship and regional level spread of DHAVs using the Minimum Spanning Network (MSN) phylogeographic method implemented by the PopArt version 1.7 (Leigh and Bryant, 2015). The phylogeographic network analysis also revealed great genetic diversity among the DHAVs, having multiple mutational branches (Fig. 1B) in two major clusters in relation to the DHAV-1 and DHAV-3 clades. Both the clusters are linked by the virus strains HDHV1-BJ (GenBank ID: FJ157172.1) and Du_CH_LSD_090612 (GenBank ID: JF828995.1) respectively through a long mutational branch and intermediate strains. In addition, virus strains 04 G (GenBank ID: EF067923.1) and 90D (GenBank ID: EF067924.1) that clustered as a separate clade 3-e in the phylogenetic tree were identified to be evolved and clustered within the DHAV-3 cluster (Cluster 2) within the phylogeographic network. Similarly, the two genetically distanced strains Du_CH_LSD_090830 (GenBank ID: JF828989.1) and HB02 (GenBank ID: JQ031262.1) in the 1-g sub-clade of DHAV-1 were shown to be clustering separately and connected to the MPZJ1206 (GenBank ID: KF924552.1) via two intermediate strains within the DHAV-1 cluster (Cluster 1) (Fig. 1B). These results suggest the extensive genetic exchange among the DHAVs.
Genomic recombination has been found to serve as force driving the evolution of DHAVs (Feher et al., 2021; Rohaim et al., 2021a). Previously, genetic recombination events are reported in the VP0 region of DHAVs (Fehér et al., 2021). Herein, we explored this issue using our dataset and Recombination Detection Program 4 software (RDP4) (Martin et al., 2015), in which seven methods including RDP, GENECONV, MaxChi, Bootscan, SiScan, Chimaera, and 3seq were used to identify the potential recombination events, and a recombination event confirmed by at least four of these methods was accepted. Although, traces of recombination have previously been described (Feher et al., 2021; Kim et al., 2007), the increased number of sequences with the improved methodology will provide comprehensive and more accurate results. Our analysis identified 32 recombination events, affecting both DHAV-1 and DHAV-3, where one event was inter-genotypic (Event 1) and the remaining (Events 2–32) were intra-genotypic (Supplementary Table S2). Both field isolates and the attenuated vaccine strains (such as A66 in Event 6 and VXXT in Event 18) were involved in the recombination. The recombination was seen throughout the whole genome, but the majority occurred at the 5′-end and upstream of the capsid coding region (Supplementary Fig. S2). There are four events at the RdRp coding region (Events 7, 9, 30 and 31), three at the P-loop NTPase coding region (Events 4, 12, 27), two in the capsid coding region (Events 18 and 26), two at Peptidase C3 coding region (Events 19 and 21), while one event occurred at the SF3 helicase coding region (Event 17) (Supplementary Fig. S2). In addition, two of the events encompassed both the P-loop NTPase and SF3 helicase coding regions (Events 2 and 27), one event encompassed SF3 helicase and Peptidase C3 coding regions (Event 25) (Supplementary Fig. S2).
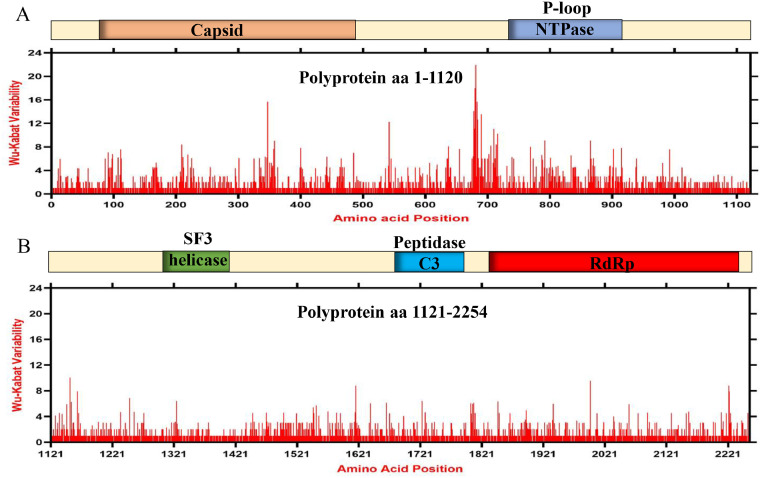
As the phylogenetic and phylogeographic analyses indicated great genetic diversity and mutations among the DHAVs, we evaluated its effect on the amino acid variability of the polyprotein. Thus, a consensus amino acids sequence and the protein variability were determined using the Wu-Kabat variability coefficient implemented by the Protein Variability Server (PVS) (Garcia-Boronat et al., 2008). The consensus polyprotein sequence of DHAV was 2254 amino acids long. The Wu-Kabat variability coefficient showed high variations in the amino acids across the full-length protein, where several positions showed the values more than the estimation limit 1.00 (Fig. 2). The highest variability was shown by upstream region of the N terminus P-loop region, e.g., amino acids 672–716 (highest value 21) (Fig. 2A), followed by the aa 334–359 in the Capsid protein encoding region. The amino acids position 1200–2254 (Fig. 2B) was found to be relatively conserved region of the DHAV polyprotein.
Fig. 2.
Amino acids variability landscape of DHAV polyprotein, 1986–2020. The plot represents amino acids variations in polyprotein at aa 1–1120 (A) and 1121–2254 (B). The ORF nucleotide sequences were used to acquire their consensus amino acids sequence and the Wu-Kabat variability coefficient was determined by PVS (Garcia-Boronat et al., 2008). The Wu-Kabat variability coefficient represents the susceptibility of an amino acid position to the mutations and is calculated using the following equation: variability coefficient = N*k/n (N is the number of sequences in the alignment, k is the number of kinds of amino acids at a given position and n is the frequency of the most common amino acid at that position. Y axes represents the Wu-Kabat variability coefficient values, where the estimation limit is 1. Above the limit 1 represents variations. X axes represents the amino acids positions.
In summary, this report provides both phylogeographic and phylogenetic relatedness of DHAV-1 and DHAV-3 worldwide. The data indicates that DHAV-1 and DHAV-3 field strains isolated from 1986 to 2020 can be sorted into seven and five sub-clades, respectively, according to the full-length genome sequences. The extensive recombination, especially between DHAV-1 and DHAV-3, may predict an emerging threat to the containing of virus infection. The updated information on the genetic features of DHAV strains will facilitate DHAV surveillance as well as re-evaluation for currently used vaccines.
Ethical approval
For this retrospective type of study, formal consent is not required.
Funding
This work is supported by Four “batches” innovation project of invigorating medical through science and technology of Shanxi province (2023XM015, 2023XM028), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2019L0102), Natural Science Foundation of Shanxi Province (201901D211135, 202203021221300), and the Programme of Introducing Talents of Discipline to Universities (D21004).
CRediT authorship contribution statement
Caiting Yang: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Pir Tariq Shah: Formal analysis, Writing – review & editing. Amina Nawal Bahoussi: Writing – original draft, Writing – review & editing. Changxin Wu: Project administration. Li Wang: Project administration. Li Xing: Conceptualization, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all the persons who contributed to the collection and production of Duck Hepatitis A virus genome sequences in NCBI GenBank.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199216.
Appendix. Supplementary materials
Data availability
The data has been shown in the supplementary materials.
References
- Cao J., Ou X., Zhu D., Ma G., Cheng A., Wang M., Chen S., Jia R., Liu M., Sun K., Yang Q., Wu Y., Chen X. The 2A2 protein of duck hepatitis a virus type 1 induces apoptosis in primary cell culture. Virus Genes. 2016;52(6):780–788. doi: 10.1007/s11262-016-1364-4. [DOI] [PubMed] [Google Scholar]
- Cha S.Y., Roh J.H., Kang M., Kim B., Jang H.K. Isolation and characterization of a low pathogenic duck hepatitis a virus 3 from South Korea. Vet. Microbiol. 2013;162(1):254–258. doi: 10.1016/j.vetmic.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Chen J.H., Zhang R.H., Lan J.J., Lin S.L., Li P.F., Gao J.M., Wang Y., Xie Z.J., Li F.C., Jiang S.J. IGF2BP1 significantly enhances translation efficiency of duck hepatitis a virus type 1 without affecting viral replication. Biomolecules. 2019;9(10) doi: 10.3390/biom9100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C., Zhang D. Molecular analysis of duck hepatitis virus type 1. Virology. 2007;361(1):9–17. doi: 10.1016/j.virol.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Doan H.T., Le X.T., Do R.T., Hoang C.T., Nguyen K.T., Le T.H. Molecular genotyping of duck hepatitis a viruses (DHAV) in Vietnam. J. Infect Dev. Ctries. 2016;10(9):988–995. doi: 10.3855/jidc.7239. [DOI] [PubMed] [Google Scholar]
- Doan H.T.T., Le X.T.K., Do R.T., Hoang C.T.M., Nguyen K.T., Le T.H. Molecular genotyping of duck hepatitis a viruses (DHAV) in Vietnam. J. Infect. Dev. Ctries. 2016;10(09):988–995. doi: 10.3855/jidc.7239. [DOI] [PubMed] [Google Scholar]
- Feher E., Jakab S., Bali K., Kaszab E., Nagy B., Ihasz K., Balint A., Palya V., Banyai K. Genomic epidemiology and evolution of duck hepatitis a virus. Viruses. 2021;13(8) doi: 10.3390/v13081592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehér E., Jakab S., Bali K., Kaszab E., Nagy B., Ihász K., Bálint Á., Palya V., Bányai K. Genomic epidemiology and evolution of duck hepatitis a virus. Viruses. 2021;13(8):1592. doi: 10.3390/v13081592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk A., Mhamdi M., Will H., Sirma H. Avian hepatitis B viruses: molecular and cellular biology, phylogenesis, and host tropism. World J. Gastroenterol. 2007;13(1):91–103. doi: 10.3748/wjg.v13.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Chen J., Si X., Xie Z., Zhu Y., Zhang X., Wang S., Jiang S. Genetic variation of the VP1 gene of the virulent duck hepatitis A virus type 1 (DHAV-1) isolates in Shandong province of China. Virol. Sin. 2012;27(4):248–253. doi: 10.1007/s12250-012-3255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Boronat M., Diez-Rivero C.M., Reinherz E.L., Reche P.A. PVS: a web server for protein sequence variability analysis tuned to facilitate conserved epitope discovery. Nucleic. Acids. Res. 2008;36(suppl_2):W35–W41. doi: 10.1093/nar/gkn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Pan W. Preliminary identifications of the duck hepatitis virus serotypes isolated in Beijing, China. Chin. J. Vet. Med. 1984;10(11):2–3. [Google Scholar]
- Hassan T.I.R., Eid A.A.M., Ghanem I.A.I., Shahin A.M., Adael S.A.A., Mohamed F.F. First report of duck hepatitis a virus 3 from duckling flocks of Egypt. Avian Dis. 2020;64(3):269–276. doi: 10.1637/aviandiseases-D-19-00158. [DOI] [PubMed] [Google Scholar]
- Huang X., Zhang X., Wei J., Li C., Liao J., Mao H. Molecular cloning and sequence analysis of full-length genome of A66 attenuated strain of DHV-Ⅰ. Chin. J. Vet. Med. 2009;45(2):8–10. [Google Scholar]
- ICTV, ICTV Report Chapters: Picornaviridae. https://ictv.global/report/chapter/picornaviridae. Accessed on Sept 7, 2023.
- Kim M.C., Kwon Y.K., Joh S.J., Kim S.J., Tolf C., Kim J.H., Sung H.W., Lindberg A.M., Kwon J.H. Recent Korean isolates of duck hepatitis virus reveal the presence of a new geno- and serotype when compared to duck hepatitis virus type 1 type strains. Arch. Virol. 2007;152(11):2059–2072. doi: 10.1007/s00705-007-1023-0. [DOI] [PubMed] [Google Scholar]
- Kim M.C., Kwon Y.K., Joh S.J., Kwon J.H., Lindberg A.M. Differential diagnosis between type-specific duck hepatitis virus type 1 (DHV-1) and recent Korean DHV-1-like isolates using a multiplex polymerase chain reaction. Avian Pathol. 2008;37(2):171–177. doi: 10.1080/03079450801918670. [DOI] [PubMed] [Google Scholar]
- Kim M.C., Kwon Y.K., Joh S.J., Lindberg A.M., Kwon J.H., Kim J.H., Kim S.J. Molecular analysis of duck hepatitis virus type 1 reveals a novel lineage close to the genus parechovirus in the family picornaviridae. J. Gen. Virol. 2006;87(Pt 11):3307–3316. doi: 10.1099/vir.0.81804-0. [DOI] [PubMed] [Google Scholar]
- Kloc A., Rai D.K., Rieder E. The roles of picornavirus untranslated regions in infection and innate immunity. Front. Microbiol. 2018;9:485. doi: 10.3389/fmicb.2018.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y., Zeng N., Wang M., Cheng A., Yang Q., Wu Y., Jia R., Zhu D., Zhao X., Chen S., Liu M., Zhang S., Wang Y., Xu Z., Chen Z., Zhu L., Luo Q., Liu Y., Yu Y., Zhang L., Huang J., Tian B., Pan L., Ur Rehman M., Chen X. The VP3 protein of duck hepatitis a virus mediates host cell adsorption and apoptosis. Sci. Rep. 2019;9(1):16783. doi: 10.1038/s41598-019-53285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J.W., Bryant D. POPART: full-feature software for haplotype network construction. Methods Ecol. Evol. 2015;6(9):1110–1116. [Google Scholar]
- Li J., Bi Y., Chen C., Yang L., Ding C., Liu W. Genetic characterization of duck hepatitis a viruses isolated in China. Virus Res. 2013;178(2):211–216. doi: 10.1016/j.virusres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Li J., Wang M., Zhou S., Cheng A., Ou X., Sun D., Wu Y., Yang Q., Gao Q., Huang J., Tian B., Mao S., Zhang S., Zhao X., Jia R., Liu M., Zhu D., Chen S., Liu Y., Yu Y., Zhang L., Pan L. The DHAV-1 protein VP1 interacts with PI3KC3 to induce autophagy through the PI3KC3 complex. Vet. Res. 2022;53(1):64. doi: 10.1186/s13567-022-01081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.H., Tang X.S., Wang M.S., Cheng A.C., Ou X.M., Mao S., Sun D., Yang Q., Wu Y., Zhang S.Q., Zhu D.K., Jia R.Y., Chen S., Liu M.F., Zhao X.X., Huang J., Gao Q., Tian B., Liu Y.Y., Yu Y.L., Zhang L., Pan L.C. The lysine at position 151 of the duck hepatitis a virus 1 2C protein is critical for its NTPase activities. Vet. Microbiol. 2022;264 doi: 10.1016/j.vetmic.2021.109300. [DOI] [PubMed] [Google Scholar]
- Li Y., Wei L., Cheng A., Wang M., Ou X., Mao S., Tian B., Yang Q., Wu Y., Zhang S., Huang J., Gao Q., Sun D., Zhao X., Jia R., Liu M., Zhu D., Chen S., Yu Y., Zhang L., Pan L. Specific DNAzymes cleave the 300-618nt of 5′UTR to inhibit DHAV-1 translation and replication. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1064612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.L., Cong R.C., Zhang R.H., Chen J.H., Xia L.L., Xie Z.J., Wang Y., Zhu Y.L., Jiang S.J. Circulation and in vivo distribution of duck hepatitis A virus types 1 and 3 in infected ducklings. Arch. Virol. 2016;161(2):405–416. doi: 10.1007/s00705-015-2648-z. [DOI] [PubMed] [Google Scholar]
- Liu X., Kong X. Isolation, identification and attenuation of a pathogenic duck hepatitis virus type 1 in China, and complete genomic sequence comparison between the embryo-passaged, attenuated derivatives and their parent. Pol. J. Vet. Sci. 2019;22(1):163–171. doi: 10.24425/pjvs.2018.125614. [DOI] [PubMed] [Google Scholar]
- Liu Y., Cheng A., Wang M., Mao S., Ou X., Yang Q., Wu Y., Gao Q., Liu M., Zhang S., Huang J., Jia R., Zhu D., Chen S., Zhao X., Yu Y., Liu Y., Zhang L., Tian B., Pan L. Duck hepatitis a virus type 1 induces eif2alpha phosphorylation-dependent cellular translation shutoff via PERK/GCN2. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.624540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.Z., Li Y.L., Wang M.S., Cheng A.C., Ou X.M., Mao S., Sun D., Wu Y., Yang Q., Jia R.Y., Tian B., Zhang S.Q., Zhu D.K., Chen S., Liu M.F., Zhao X.X., Huang J., Gao Q., Yu Y.L., Zhang L. Duck hepatitis a virus type 1 mediates cell cycle arrest in the S phase. Virol. J. 2022;19(1) doi: 10.1186/s12985-022-01839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.S., Lin D.F., Lee Y.L., Liao Y.K., Tsai H.J. Infectious bill atrophy syndrome caused by parvovirus in a co-outbreak with duck viral hepatitis in ducklings in Taiwan. Avian Dis. 1993;37(2):591–596. [PubMed] [Google Scholar]
- Ma X., Sheng Z., Huang B., Qi L., Li Y., Yu K., Liu C., Qin Z., Wang D., Song M. Molecular evolution and genetic analysis of the major capsid protein VP1 of duck hepatitis A viruses: implications for antigenic stability. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Sheng Z., Huang B., Qi L., Li Y., Yu K., Liu C., Qin Z., Wang D., Song M., Li F. Molecular evolution and genetic analysis of the major capsid protein VP1 of duck hepatitis a viruses: implications for antigenic stability. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Yu K., Wu J., Song M., Liao M., Xin C. Complete genome sequencing and variations analysis of seven isolated strains and one vaccine strain of duck hepatitis virus. Acta Vet. Zootech. Sin. 2009;40(8):1209–1214. [Google Scholar]
- Mansour S.M.G., Ali H., ElBakrey R.M., El-Araby I.E., Knudsen D.E.B., Eid A.A.M. Co-infection of highly pathogenic avian influenza and duck hepatitis viruses in Egyptian backyard and commercial ducks. Int. J. Vet. Sci. Med. 2018;6(2):301–306. doi: 10.1016/j.ijvsm.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D.P., Murrell B., Golden M., Khoosal A., Muhire B. RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1(1):vev003. doi: 10.1093/ve/vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Mao S., Cao J., Cheng A., Wang M., Zhu D., Chen S., Jia R., Liu M., Sun K., Yang Q., Wu Y., Chen X. Comparative analysis of virus-host interactions caused by a virulent and an attenuated duck hepatitis a virus genotype 1. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0178993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohaim M.A., El Naggar R.F., AbdelSabour M.A., Ahmed B.A., Hamoud M.M., Ahmed K.A., Zahran O.K., Munir M. Insights into the genetic evolution of duck hepatitis a virus in Egypt. Animals. 2021;11(9) doi: 10.3390/ani11092741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohaim M.A., Naggar R.F.E., AbdelSabour M.A., Ahmed B.A., Hamoud M.M., Ahmed K.A., Zahran O.K., Munir M. Insights into the genetic evolution of duck hepatitis a virus in Egypt. Animals. 2021;11(9) doi: 10.3390/ani11092741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoute S.T., Tsai H.J., Metwally S.A., Cheng A., Guérin J.L., Palya V.J. Viral infections of waterfowl. Dis. Poult. 2020:446–497. [Google Scholar]
- Sun D., Wang M., Wen X., Cheng A., Jia R., Sun K., Yang Q., Wu Y., Zhu D., Chen S., Liu M., Zhao X., Chen X. Cleavage of poly(A)-binding protein by duck hepatitis a virus 3C protease. Sci. Rep. 2017;7(1):16261. doi: 10.1038/s41598-017-16484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Wang M., Wen X., Mao S., Cheng A., Jia R., Yang Q., Wu Y., Zhu D., Chen S., Liu M., Zhao X., Zhang S., Chen X., Liu Y., Yu Y., Zhang L. Biochemical characterization of recombinant avihepatovirus 3C protease and its localization. Virol. J. 2019;16(1):54. doi: 10.1186/s12985-019-1155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifinopoulos J., Nguyen L.T., von Haeseler A., Minh B.Q. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic. Acids. Res. 2016;44(W1):W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.H., Knowles N.J., Tsai H.J. Molecular analysis of duck hepatitis virus type 1 indicates that it should be assigned to a new genus. Virus Res. 2007;123(2):190–203. doi: 10.1016/j.virusres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Tseng C.H., Tsai H.J. Molecular characterization of a new serotype of duck hepatitis virus. Virus Res. 2007;126(1–2):19–31. doi: 10.1016/j.virusres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Wen H., Han L., Zhang X., Lian C., Zhao L., Si C., Chen H. Duck hepatitis a virus (DHAV) genotype definition: comment on the article by Cha et al. Vet. Microbiol. 2014;170(3–4):462–464. doi: 10.1016/j.vetmic.2014.01.037. [DOI] [PubMed] [Google Scholar]
- Wen X., Cheng A., Wang M., Jia R., Zhu D., Chen S., Liu M., Liu F., Chen X. Detection, differentiation, and VP1 sequencing of duck hepatitis A virus type 1 and type 3 by a 1-step duplex reverse-transcription PCR assay. Poult. Sci. 2014;93(9):2184–2192. doi: 10.3382/ps.2014-04024. [DOI] [PubMed] [Google Scholar]
- Wen X., Zhu D., Cheng A., Wang M., Chen S., Jia R., Liu M., Sun K., Zhao X., Yang Q. Molecular epidemiology of duck hepatitis a virus types 1 and 3 in China, 2010–2015. Transbound. Emerg. Dis. 2018;65(1):10–15. doi: 10.1111/tbed.12741. [DOI] [PubMed] [Google Scholar]
- Wen X., Zhu D., Cheng A., Wang M., Chen S., Jia R., Liu M., Sun K., Zhao X., Yang Q., Wu Y., Chen X. Molecular epidemiology of duck hepatitis a virus types 1 and 3 in China, 2010-2015. Transbound. Emerg. Dis. 2018;65(1):10–15. doi: 10.1111/tbed.12741. [DOI] [PubMed] [Google Scholar]
- Xia X., Cheng A., Wang M., Ou X., Sun D., Zhang S., Mao S., Yang Q., Tian B., Wu Y., Huang J., Gao Q., Jia R., Chen S., Liu M., Zhao X.X., Zhu D., Yu Y., Zhang L. DHAV 3CD targets IRF7 and RIG-I proteins to block the type I interferon upstream signaling pathway. Vet. Res. 2023;54(1):5. doi: 10.1186/s13567-023-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Yan H., Zhu Y., Xie Z., Zhang R., Jiang S. Duck hepatitis a virus type 1 transmission by exosomes establishes a productive infection in vivo and in vitro. Vet. Microbiol. 2023;277 doi: 10.1016/j.vetmic.2022.109621. [DOI] [PubMed] [Google Scholar]
- Yang F., Liu P., Li X., Liu R., Gao L., Cui H., Zhang Y., Liu C., Qi X., Pan Q., Liu A., Wang X., Gao Y., Li K. Recombinant duck enteritis virus-vectored bivalent vaccine effectively protects against duck hepatitis a virus infection in ducks. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.813010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Cheng A., Wang M., Jia R., Sun K., Pan K., Yang Q., Wu Y., Zhu D., Chen S., Liu M., Zhao X.X., Chen X. Structures and corresponding functions of five types of picornaviral 2A proteins. Front. Microbiol. 2017;8:1373. doi: 10.3389/fmicb.2017.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Zeng Q., Wang M., Cheng A., Pan K., Zhu D., Liu M., Jia R., Yang Q., Wu Y., Chen S., Zhao X., Zhang S., Liu Y., Yu Y., Zhang L. DHAV-1 2A1 peptide - a newly discovered co-expression tool that mediates the ribosomal "skipping" function. Front. Microbiol. 2018;9:2727. doi: 10.3389/fmicb.2018.02727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia N., Erfan A.M., Omar S.E., Soliman M.A. Dual circulation of duck hepatitis a virus genotypes 1 and 3 in Egypt. Avian Dis. 2021;65(1):1–9. doi: 10.1637/aviandiseases-D-20-00075. [DOI] [PubMed] [Google Scholar]
- Yugo D.M., Hauck R., Shivaprasad H.L., Meng X.J. Hepatitis virus infections in poultry. Avian Dis. 2016;60(3):576–588. doi: 10.1637/11229-070515-Review.1. [DOI] [PubMed] [Google Scholar]
- Yun T., Ni Z., Liu G.Q., Yu B., Chen L., Huang J.G., Zhang Y.M., Chen J.P. Generation of infectious and pathogenic duck hepatitis virus type 1 from cloned full-length cDNA. Virus Res. 2010;147(2):159–165. doi: 10.1016/j.virusres.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Zell R., Delwart E., Gorbalenya A.E., Hovi T., King A.M.Q., Knowles N.J., Lindberg A.M., Pallansch M.A., Palmenberg A.C., Reuter G., Simmonds P., Skern T., Stanway G., Yamashita T., Ictv Report C. ICTV virus taxonomy profile: picornaviridae. J. Gen. Virol. 2017;98(10):2421–2422. doi: 10.1099/jgv.0.000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Chen J., Zhang J., Yang Y., Li P., Lan J., Xie Z., Jiang S. Novel duck hepatitis a virus type 1 isolates from adult ducks showing egg drop syndrome. Vet. Microbiol. 2018;221:33–37. doi: 10.1016/j.vetmic.2018.05.023. [DOI] [PubMed] [Google Scholar]
- Zhang R., Xia L., Chen J., Gong Y., Zhang L., Li P., Liu H., Xie Z., Jiang S. Molecular epidemiology and genetic diversity of duck hepatitis A virus type 3 in Shandong province of China, 2012-2014. Acta Virol. 2017;61(4):463–472. doi: 10.4149/av_2017_409. [DOI] [PubMed] [Google Scholar]
- Zhang R., Yang Y., Lan J., Xie Z., Zhang X., Jiang S. Evidence of possible vertical transmission of duck hepatitis A virus type 1 in ducks. Transbound. Emerg. Dis. 2021;68(2):267–275. doi: 10.1111/tbed.13708. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Cao Q.D., Wang M.S., Jia R.Y., Chen S., Zhu D.K., Liu M.F., Sun K.F., Yang Q., Wu Y., Zhao X.X., Chen X.Y., Cheng A.C. The 3D protein of duck hepatitis a virus type 1 binds to a viral genomic 3 ' UTR and shows RNA-dependent RNA polymerase activity. Virus Genes. 2017;53(6):831–839. doi: 10.1007/s11262-017-1476-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wu S., Liu W., Hu Z. Current status and future direction of duck hepatitis a virus vaccines. Avian Pathol. 2023:1–11. doi: 10.1080/03079457.2022.2162367. [DOI] [PubMed] [Google Scholar]
- Zou Z., Ma J., Huang K., Chen H., Liu Z., Jin M. Live attenuated vaccine based on duck enteritis virus against duck hepatitis a virus types 1 and 3. Front. Microbiol. 2016;7:1613. doi: 10.3389/fmicb.2016.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data has been shown in the supplementary materials.