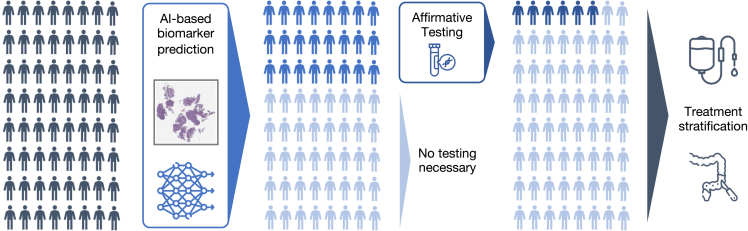
Summary
Deep learning (DL) can accelerate the prediction of prognostic biomarkers from routine pathology slides in colorectal cancer (CRC). However, current approaches rely on convolutional neural networks (CNNs) and have mostly been validated on small patient cohorts. Here, we develop a new transformer-based pipeline for end-to-end biomarker prediction from pathology slides by combining a pre-trained transformer encoder with a transformer network for patch aggregation. Our transformer-based approach substantially improves the performance, generalizability, data efficiency, and interpretability as compared with current state-of-the-art algorithms. After training and evaluating on a large multicenter cohort of over 13,000 patients from 16 colorectal cancer cohorts, we achieve a sensitivity of 0.99 with a negative predictive value of over 0.99 for prediction of microsatellite instability (MSI) on surgical resection specimens. We demonstrate that resection specimen-only training reaches clinical-grade performance on endoscopic biopsy tissue, solving a long-standing diagnostic problem.
Keywords: deep learning, biomarker, colorectal cancer, artificial intelligence, transformer, microsatellite instability, multiple instance learning
Graphical abstract
Highlights
-
•
AI-based prediction of biomarkers (MSI, BRAF, and KRAS) using transformers
-
•
MSI prediction reaches clinical-grade performance on biopsies of colorectal cancer
-
•
Transformer-based biomarker prediction generalizes better and is more data efficient
-
•
Large-scale multi-cohort evaluation on over 13,000 patients from 16 cohorts
Wagner et al. show that transformer-based prediction of biomarkers from histology substantially improves the performance, generalizability, data efficiency, and interpretability as compared with current state-of-the-art algorithms. The method significantly outperforms existing approaches for microsatellite instability detection in surgical resections and reaches clinical-grade performance on biopsies of colorectal cancer, solving a long-standing diagnostic problem.
Introduction
Precision oncology in colorectal cancer (CRC) requires the evaluation of genetic biomarkers, such as microsatellite instability (MSI)1,2,3,4,5,6,7,8 and mutations in the BRAF4,7 and NRAS/KRAS9 genes. These biomarkers are typically assessed by polymerase chain reaction (PCR), sequencing, or immunohistochemical assays. Biomarker identification in patients with CRC is an important step in providing treatment as recommended by various medical guidelines, such as those in the USA (NCCN guideline),10 UK (NICE guideline),11 and EU (ESMO guideline).12 Increasingly, genetic biomarkers such as MSI are also used in earlier tumor stages of CRC.13 In the future, the importance of biomarker-stratified therapy will likely increase.14 The presence of MSI should also trigger additional diagnostic processes for a possible diagnosis of Lynch syndrome, one of the most prevalent hereditary cancer syndromes. However, genetic diagnostic assays have several disadvantages. For many patients in low- and middle-income countries, genetic biomarkers are not routinely available due to the prohibitive costs and complex infrastructure required for testing. Even in high-income countries with universal healthcare coverage where genetic biomarkers may be routinely available, their utilization is not without its drawbacks. In such contexts, biomarker assessment can take several days to weeks delaying therapy decisions.15
The diagnosis of CRC requires a pathologist’s histopathological evaluation of tissue sections. Thus, tissue sections stained with hematoxylin and eosin (H&E) are routinely available for all patients with CRC. Since 2019, dozens of studies have shown that deep learning (DL) can predict genetic biomarkers directly from digitized H&E-stained CRC tissue sections.1,3,7,8,16,17 Based on these studies, the first commercial DL algorithm for biomarker detection from H&E images has been approved for routine clinical use in Europe in 2022 (MSIntuit, Owkin, Paris/New York).18 When evaluated in external patient cohorts, the state-of-the-art approaches reach a sensitivity and specificity of 0.95 and 0.46, respectively.19 Increasing the specificity would be a way to improve these established approaches. Another clinically significant limitation of current approaches is the poor performance on endoscopic biopsy tissue. Recent clinical trials (NICHE20 and NICHE-213) show high efficacy of neoadjuvant immunotherapy for patients with MSI CRC. These findings imply that in the future every patient with CRC should be tested for MSI on the initial biopsy tissue, although not all current medical guidelines reflect this. Among previous DL-based studies for MSI detection, only Echle et al.3 determine the performance of DL-based biomarker prediction on CRC biopsy tissue in a multicentric setting. They report a much lower performance on biopsy tissue than on surgical resection tissue sections (biopsy AUROC: 0.78; resection AUROC of 0.96). Current clinically approved commercial products for MSI detection in CRC from histopathology are only applicable to surgical resection tissue. Therefore, DL-based MSI testing of biopsies is a clinical need.
The technology underlying these algorithms in literature is based on weakly supervised learning, consisting of two components: the feature extractor and the aggregation module.21 The feature extractor is mostly based on a convolutional neural network (CNN), which processes multiple small tissue regions called tiles or patches.22 The CNN-based representations obtained from these tiles are subsequently aggregated to obtain a single prediction for the patient. Between 2019 and 2021, most studies used simple heuristics, such as taking the maximum (max pooling) or averaging (average pooling), as an aggregation module. Since then, variations of multiple instance learning (MIL) have become the new standard for this task, particularly for the prediction of genetic alterations from pathology slides.6,23,24 The most common approach replaces the pooling aggregation with a small two-layer network to learn the patch-level weighting of the embeddings.23 However, current MIL approaches univariately consider a single tile during aggregation and do not place it in context with other tiles even though local and global contexts are crucial for medical diagnosis.
In many non-medical and medical image-processing tasks, transformer neural networks have recently been adopted for computer vision tasks,25,26,27 replacing CNNs because of their improved performance and robustness.28 Originally proposed for sequencing tasks such as natural language processing, transformer networks show impressive capabilities of learning long-range dependencies and contextualizing concepts in long sequences. In computational pathology, transformers have therefore been proposed as potentially superior feature extractors29 or aggregation models,30,31,32,33 though these proposals still lack empirical evidence from large-scale analyses.
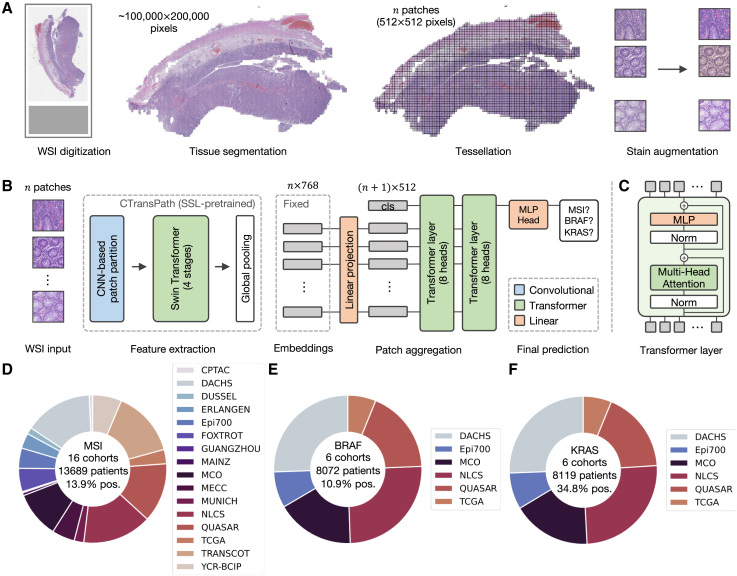
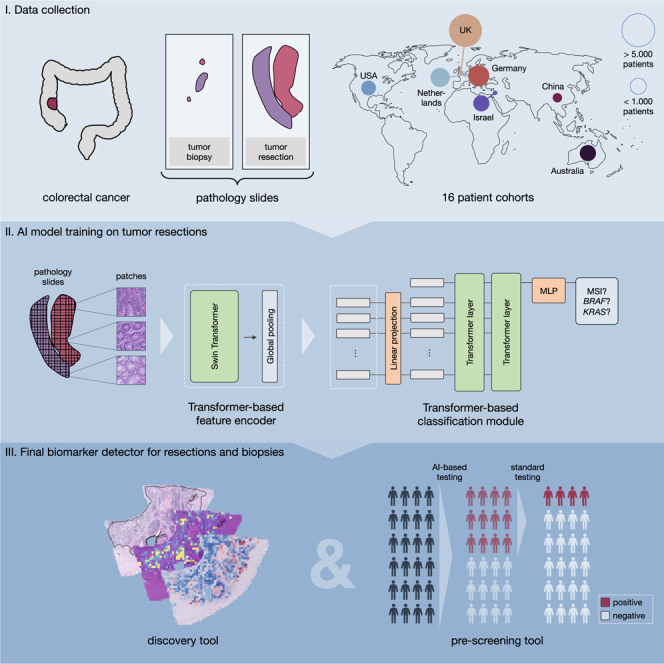
In this work, we first aim to enhance the performance of DL-based biomarker detection from pathology slides. Thereafter, in order to provide large-scale evidence of the performance on clinically relevant tasks, we investigate the use of a fully transformer-based workflow in CRC. Here, we present a new method derived from a transformer-based feature extractor and a transformer-based aggregation model (Figure 1A-C), which we evaluate in a large multi-centric study of 15 cohorts with resection specimen slides from over 13,000 patients with CRC worldwide, as well as two cohorts of CRC biopsies from over 1,500 patients in total (Figure 1D-F).
Figure 1.
Workflow overview with pre-processing and model architecture and cohort overview
(A) The data pre-processing pipeline with the steps whole slide image (WSI) digitization, tissue segmentation, WSI tessellation into patches, and stain augmentation, (B) the model architecture including the pre-trained feature extractor CTransPath and our transformer-based aggregation module, and (C) details of the transformer layer architecture.
(D) Overview of the 16 cohorts of CRC resections and biopsies with MSI/dMMR status, which were used in this study and the subsets of six cohorts with (E) BRAF and (F) KRAS ground truth data, respectively.
Results
Transformer-based MSI prediction outperforms the state-of-the-art
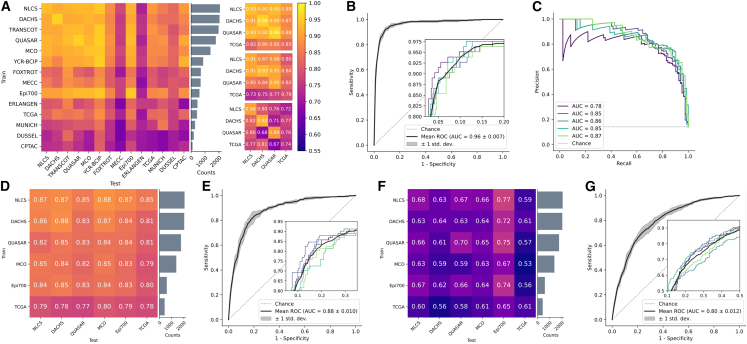
We tested our pipeline on MSI prediction in surgical resection cohorts of patients with CRC (Figure 1) in two ways: First, we trained the model on a single cohort and tested it on a held-out test set (in-domain) and on all other cohorts (external). We found that large cohorts, e.g., DACHS, QUASAR, or NLCS, achieved in-domain test AUROCs around 0.95 (Figure 2A). The model also achieved high performance close to 0.9 AUROC for early onset CRC, i.e., CRC in patients younger than 50 years (Figure S2B). We compared this performance to the work by Echle et al.3 which updated the CNN-based feature extractor during training and used mean pooling as their patch aggregation function. Our approach outperformed the CNN-based approach on all four cohorts. Further, we also evaluated AttentionMIL by Ilse et al.23 with CTransPath as a feature extractor yielding higher performance than the CNN-based approach on the large cohorts but partly lower results on the external validation trained on the smaller cohort TCGA. Overall, we observed the tendency of higher performance and better generalization for models trained on datasets with more than 1,000 patients. However, factors such as differing population genetics (e.g., for MECC) or the type of slide scanners (e.g., for ERLANGEN) influence the generalization capabilities beyond the training dataset size.
Figure 2.
Evaluation of the prediction performance for the biomarkers MSI, BRAF, and KRAS in single cohort and large-scale multi-centric experiments
Experimental results for MSI-high (A–C), BRAF (D,E), and KRAS (F,G) prediction. All values represent the mean of 5-fold cross-validation: (A) AUROC scores for single cohort experiments for all CRC cohorts ordered by size of the cohort. Each row shows the test performance of training on one cohort with the in-domain test results in the diagonal. Results for our transformer approach, AttentionMIL, and CNN approach (results taken from Echle et al.) are visualized separately. Note that compared to AttentionMIL and CNN, our transformer not only shows higher overall prediction accuracy but also better model generalizability, demonstrated by a smaller gap between internal and external testing cohorts. Raw data for the heatmap in Table S5.
(B) Receiver operator curve (ROC) for the model trained on all resection cohorts except YCR-BCIP, tested on YCR-BCIP.
(C) Precision recall curve (PRC) for the model trained on all resection cohorts except YCR-BCIP, tested on YCR-BCIP.
(D) AUROC scores for single cohort experiments.
(E) ROC for the model trained on all BRAF cohorts except Epi700, tested on Epi700.
(F) AUROC scores for single cohort experiments.
(G) ROC for the model trained on all KRAS cohorts except Epi700, tested on Epi700.
Second, we trained our model on all cohorts of CRC resections except YCR-BCIP and evaluated it on the external cohort YCR-BCIP (Figure 2B). In particular, we obtained a sensitivity of 0.99 with a negative predictive value of over 0.99 (Figure S2F, and Table S1). Analyzing the ROCs of patients with different clinicopathological properties showed that the model performs consistently well on all of these subgroups. Only on left-sided tumors the performance slightly dropped to 0.93 AUROC (Figure S2D). Moreover, a high-mean AUPRC score of 0.86 showed that the transformer-based model achieved high sensitivity with high precision despite a strong class imbalance of 12.9% MSI-high samples on average across all cohorts (Figure 2C). In parallel to our findings mentioned previously, we observed a generalization gap when intrinsic biological factors, such as ethnicity, change. However, the performance of our model on a cohort of MSI-high patients from Guangzhou, China, was still high with a sensitivity of 0.9. For a better comparison to state-of-the-art, we also mirrored the experimental setup of Echle et al.3 We trained AttentionMIL and our fully transformer-based model on the four large cohorts (DACHS, NLCS, QUASAR, and TCGA) using the same feature extractor CTransPath. The CNN-based approach from Echle et al. achieved an AUROC of 0.96, AttentionMIL yielded an AUROC of 0.96, and the fully transformer-based approach performed slightly better with an AUROC of 0.97.
The classical patch-based approach by Echle et al.3 suffered from severe losses in performance upon external testing. The largest performance drop in the AUROC of 0.21 was observed by a model trained on the DACHS and tested on the QUASAR cohort. Our transformer model, however, reduced the performance loss for external testing to a maximum of 0.09 for training on the NLCS and testing on the TCGA cohort (Figure 2). In addition, AttentionMIL trained with the same transformer-based feature extractor also demonstrated better generalization capabilities compared to the classical patch-based approach with mean pooling. This suggests that self-supervised pretraining on histology data contributes positively toward better generalization.
In summary, these results show that a fully transformer-based approach yields a higher performance for biomarker prediction both on large cohorts (DACHS, QUASAR, and NLCS) as well as on smaller cohorts (TCGA). Perhaps more importantly from a clinical perspective, the transformer-based approach resulted in a better generalization performance and more reliable results, as the deviation between the external cohorts was smaller. We published all trained models for reuse and further fine-tuning if needed.
The transformer-based model predicts multiple biomarkers in CRC
Next, we investigated whether the fully transformer-based model yields a similar high performance in other biomarker-prediction tasks. Following the experimental setup for MSI prediction, we trained the model first on single cohorts evaluated on all other external cohorts and second on one fully merged multi-center cohort excluding only one cohort from the training set to constitute an external test set. In clinical routine workup for CRC, the biomarkers BRAF and KRAS are determined in addition to MSI. We tested whether and how well these were predictable on the DACHS, QUASAR, MCO, NLCS, TCGA, and Epi700 cohorts, where the Epi700 cohort served as an external test set in the multi-centric run.
In the case of the largest cohorts, DACHS and NLCS, single cohort training was already capable of achieving good results, with AUROCs of 0.88 and 0.87, respectively (Figure 2D). The smaller cohorts achieved slightly poorer results with 0.83–0.85 AUROC and 0.78 for TCGA. Nonetheless, the AUROC for the in-domain test using TCGA by far outperformed previous approaches with AUROCs of 0.57,63 0.66,64 and 0.7333 in a more recent transformer-based method. The large multi-centric cohort yielded an AUROC of 0.88, almost reaching clinical-grade performance (Figure 2E). Furthermore, we observed that the generalization gap from the internal test set to external cohorts was consistently small with the largest internal-to-external gap of 0.03 drop in AUROC. This was also the case in multi-centric evaluation, where the performance did not decrease from the internal to the external test cohort.
We observed similar results regarding the generalization when investigating KRAS as a target (Figures 2F and 2G), with an AUROC of 0.80 when trained on the multi-centric cohort outperforming state-of-the-art methods. The AUROCs of the single cohort training ranged from 0.53 to 0.77 for single cohorts, in line with or higher than state-of-the-art results.33,63,64 While DL-based prediction performance for KRAS is still relatively low compared to MSI or BRAF, the results show that performance profits substantially from multi-cohort training and larger training cohorts.
Overall, these findings show that our model can predict multiple biomarkers that are relevant for routine diagnostics in CRC while highlighting the importance of large training cohorts to reach clinically relevant performance even in biomarkers such as KRAS which are notoriously difficult to predict from pathology images alone.
Transformer-based workflows are explainable
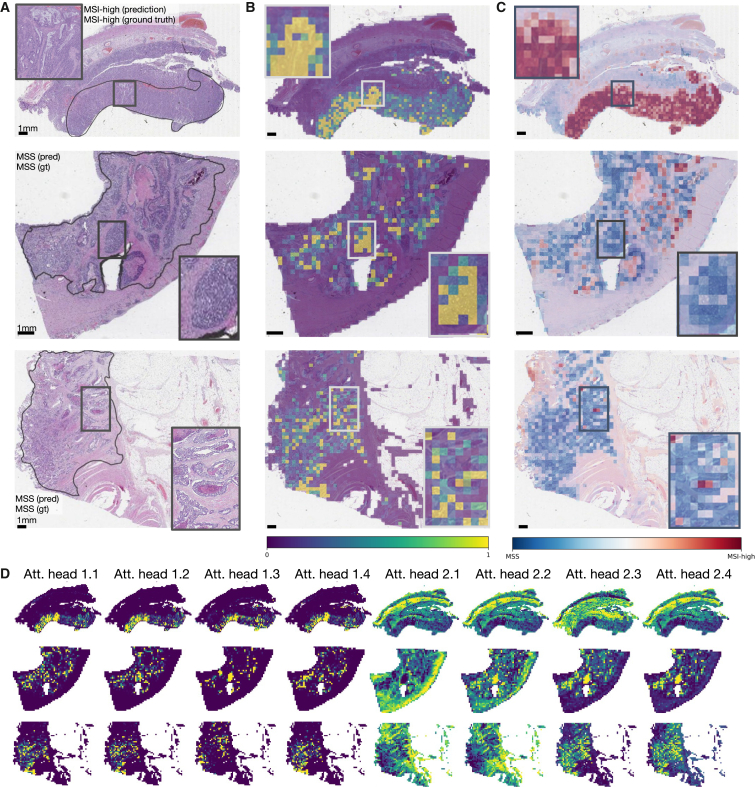
Ideally, DL-based biomarker predictions should be explainable to domain experts. To this end, we visualized how much each patch contributed to the final classification via attention rollout as well as whether it contributes toward a positive or negative classification (Figures 3A–3C, and S3).
Figure 3.
Attention and class score visualization for better model interpretability
(A) Resection specimen from the external cohort YCR-BCIP. The three depicted slides are the same as in Echle et al.3 Tumor regions are outlined in black.
(B) Attention rollout per patch for our trained transformer-based feature aggregation model. Large values (yellow) signify a high contribution to the model’s prediction, small values (purple) a low contribution.
(C) MSI classification scores per patch, where MSI-high is the positive class and MS-stable is the negative class.
(D) The attention heatmaps from eight heads, four of the first and four of the second layer. The model weights are taken from the best-performing fold of the multi-centric training on all cohorts except YCR-BCIP.
For better comparability, we used the same WSIs from the external cohort YCR-BCIP as had been used in a previous study3 for these visualizations (Figure 3A). For all three cases, the majority of highly contributing patches originate from tumor regions. In the MSI-high case, the mucinous region that is morphologically linked to MSI is correctly identified as important by high scores in the attention rollout as well as the patch-level classification (see the boxes in the first row of Figure 3). The MS-stable case in the second row of Figures 3A–3C attributes high contributions to the model’s prediction to carcinoma regions. At the same time, these patches all receive low-classification scores yielding the correct classification result. Similarly, the second MS-stable case in the third row had highly contributing scores in the tumor region while having only low-classification scores for all patches. Further tissue details that are morphologically related to MSI, such as solid growth pattern, poor differentiation, or tumor-infiltrating lymphocytes (Figure S3A) are highlighted in the attention heads of the last layer together with healthy tissue structures, such as the colon wall including muscle tissue or vessels (Figure 3D). The two cases with MSI-high ground truth predicted as MSS also show that relevant regions are identified and contribute to the prediction but the combination of potentially false attentions and associated classification scores of these attentive patches infer a wrong prediction (Figure S3B).
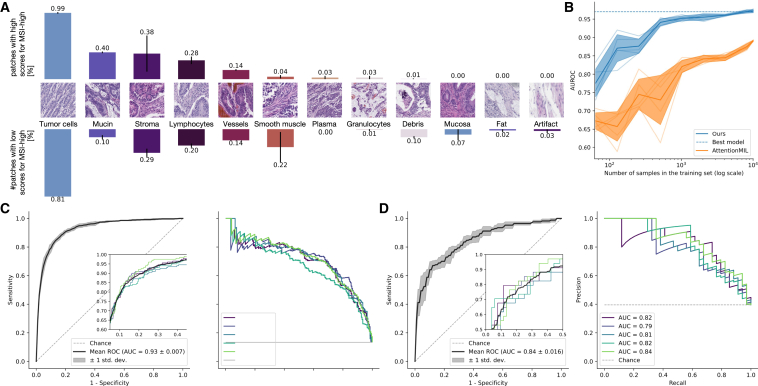
We quantified the morphological patterns occurring in high-attention regions in a small user study, where two pathologists annotated the patterns in 160 patches of 40 patients that the model attributes high attention to. We chose the 10 patients with the lowest and highest classification score for each MSS and MSI-high ground truth. For every patient, we chose the two patches associated with the highest and lowest class scores of the top 100 attention tiles. Our study showed that the majority of tiles belong to the tumor region (0.99% for high and 0.81% for low-classification scores) and cell types that are important for the prediction of MSI-high, such as lymphocytes occur in both tiles with low- and high-classification score in a similar ratio (0.28% vs. 0.2%, Figure 4A). Furthermore, morphological patterns related only to MSI-high, such as mucinous regions, occur more often in tiles with high-classification scores (0.4% vs. 0.1%). A chi-square test for independence shows that the underlying distributions of tiles with high- and low-classification scores are likely to be independent (p value = 5.6·10−6 < 5·10−5).
Figure 4.
Analysis of the quantitative user study on high-attention tiles, data efficiency, and model generalization to biopsies
(A) Prevalence of 12 histological patterns in 160 patches of high attention regions from 40 patients split by low and high patch-wise classification scores.
(B) AUROC scores on YCR-BCIP depending on the number of patients available for training. The samples were randomly drawn from all resection cohorts except YCR-BCIP.
(C) ROC and PRC for testing our model on YCR-BCIP-biopsies, trained on resections from all cohorts except YCR-BCIP.
(D) ROC and PRC for testing our model on biopsies of the cohort MAINZ, trained on resections from all cohorts except YCR-BCIP.
These examples showed that the model learns concepts relevant to MSI-high prediction and thus possesses a high degree of explainability. Visualizing the attention rollout together with the classification scores demonstrates that relevant regions can receive high-attention scores while the model can learn to ignore non-relevant regions or give them low-classification scores.
Transformer-based workflows are more data efficient
A long-standing problem in computational pathology is to determine the number of samples required for a given prediction task. This is primarily due to two reasons. First, it is unclear what the minimum required sample size is, and second, it is unclear if adding more samples improves performance, and if so, up to what point. To investigate this, we varied the number of patients in the training set and analyzed its impact on the test performance. Specifically, we merged all cohorts except for an external validation cohort, YCR-BCIP, resulting in a training set with 8,181 patients from nine cohorts. We trained models using a fixed number of epochs and randomly sampled patients from the training set. All experiments were repeated five times, and we reported the means and standard deviations of the results.
Our fully transformer-based model architecture achieved a mean testing AUROC value above 0.9 with 250 patients (in particular, an AUROC value of 0.92), while the AttentionMIL model exceeded an AUROC of 0.9 only with 4,000 patients (Figure 4A). In a similar vein, our model surpassed the 0.95 mean testing AUROC with already 1,500 patients, while AttentionMIL did not reach this performance. Hence, the transformer-based aggregation module helped the model to learn from data in a more efficient way than the attention mechanism. This may be due to the attention mechanism not contextualizing information from all input patches. Of note, above 1,000 patients, the performance of the transformer-based model seems to slowly saturate, while the attention mechanism continues to increase in performance with more patients but on a lower level.
Our fully transformer-based approach yielded high performance with a small sample size. Compared to an AttentionMIL-based approach, our approach is more data efficient in the regime of small numbers of patients. Looking at larger training numbers, we observed that performance increase is directly proportional to the number of patients for both approaches, but the fully transformer-based approach reaches equivalent performance already with much smaller datasets.
Transformer-based workflows result in clinical-grade performance on biopsies
Virtually all previous studies on biomarker prediction in CRC were performed using surgical resection slides. For this reason, commercially available MSI detection algorithms are intended to be used only with resection slides. However, recent clinical evidence shows that MSI-positive patients with CRC require immunotherapy prior to surgery13,65 and hence need to be tested for MSI on biopsy material. We addressed this problem by training our model on resections from all cohorts except YCR-BCIP and evaluating it on biopsies from 1,592 patients with CRC of the YCIP-BCIP.
Our model yielded a mean AUROC score of 0.92 and 0.86 when validated on biopsies from two external cohorts, YCR-BCIP and MAINZ, respectively (Figure 4B). It is worth mentioning that the MSI-high ratio in the MAINZ biopsy cohort was higher than in the training cohorts. We outperformed existing approaches (0.78 by Echle et al.3) by far and achieved clinical-grade performance on biopsies after model training on resection specimens (Figure S4). The mean AUPRC score of 0.69 and 0.82, respectively, however, was lower than that of the external cohort YCR-BCIP for resections (0.86) (Figure 4C). Hence, choosing a classification threshold with high sensitivity, the ratio of correctly MSI positive predicted cases from all positive predicted cases was lower on biopsies compared to resections. Still, with a classification threshold fixed on the in-domain test set of resections, our model obtains sensitivity scores of 0.98 and 0.91, respectively, with negative predictive values of 0.99 and 0.9. Of note, these values are higher than (for the cohort YCR-BCIP) and close to (for the MAINZ cohort) the clinically approved DL algorithm for resections,19 suggesting that our algorithm has potential for clinical usage for biopsies.
Our intended clinical use of this workflow is as follows (Figure 5): First, a patient attends a clinic either with suspected CRC or for routine CRC screening. A colonoscopy shows a suspicious tumor, which is evaluated histologically and found to be an adenocarcinoma. In many countries, this biopsy will then be tested for MSI/MMR status and BRAF and/or RAS mutation status. In practice, these procedures may take several days to even weeks. However, in low- or middle-income countries, this might not happen at all. Based on the MSI, BRAF, and RAS status, the most suitable treatment approach will be chosen for the patient. For example, in patients with early (non-metastatic) CRC, the presence of MSI could qualify a patient for neoadjuvant immunotherapy followed by surgery with curative intent. Similarly, in the metastatic disease setting, the presence of MSI in the biopsy tissue would qualify a patient for palliative immunotherapy. Because of its high sensitivity, our algorithm could serve as a filtering step followed by affirmative testing for MSI-high predicted cases. Applying AI-based biomarker prediction would reduce the additional testing burden and therefore speed up the step between taking the biopsy and the molecular determination of MSI-high status, thus enabling an earlier treatment with immunotherapy if indicated.
Figure 5.
Envisioned clinical workflow for the proposed MSI-high classifier on biopsies
This assumes the system reaches a sufficient performance in additional external validation and is approved as a medical device. This workflow would only apply to non-metastatic disease. Neoadjuvant immunotherapy is not yet recommended by medical guidelines but is backed up by Phase-II clinical trials. Not shown: tissue preprocessing and scanning pipelines and confirmatory tests of MSI-high after a positive deep learning-based pre-screening.
In summary, to the best of our knowledge, we developed a DL-based MSI-high predictor for biopsies that achieves clinical-grade performance. In particular, this high performance was also observed for external tests and could therefore improve clinical routine and speed up treatment decisions.
Discussion
The rollout of precision oncology to patients with CRC promises gains in life expectancy.66 Unfortunately, however, its implementation still remains slow and patchy. One reason for this is that precision oncology biomarkers are complex, costly, and require intricate instrumentation and expertise. DL is emerging as a possible solution for this problem.22,67 DL can extract biomarker information directly from routinely available material, thereby potentially providing cost savings.1 Using DL-based analysis of histopathology slides to extract biomarkers for oncology has become a common approach in the research setting in 2018.68 In turn, this has recently led to regulatory approval of multiple algorithms for clinical use. Some of these examples include a breast cancer survival prediction algorithm by Paige (New York, NY, USA), a method to predict survival in CRC by DoMore Diagnostics (Oslo, Norway), a method to predict MSI status in CRC by Owkin (Paris, France, and New York, NY, USA), among others.18,69 However, existing DL biomarkers have some key limitations: it is debated whether or not their performance is sufficient for large-scale use, they do not necessarily generalize to any patient population, and finally, they are not approved for use on biopsy material, as the application of DL algorithms to biopsies typically results in much lower performance compared to application to surgical specimens.3
A key reason for the limited performance of existing DL systems could be the fundamental limitations of the technology employed. Most studies between 2018 and 2020 used convolutional neural networks (CNNs) as their DL backbone,31 using publicly available information. Commercial products in the DL biomarker space are based on the same technology.19,69,70 However, a new class of neural networks has recently started to replace CNNs: transformers. Originating from the field of natural language processing, transformers are a powerful tool to process sequences and leverage the potential of large amounts of data. Also in computer vision, transformers yield a higher accuracy for image classification in non-medical tasks,25,26 are more robust to distortions in the input data28 and provide more detailed explainability.30 These advantages of transformers compared to CNNs have the potential to translate into more accurate and more generalizable clinical biomarkers, but there is currently no evidence to support this.
In the present study, we developed a transformer-based approach for biomarker prediction on whole-slide images of H&E-stained CRC tissue sections. Our model consists of a transformer-based feature extractor that was pretrained on histopathology images and a transformer-based aggregation module. In contrast to the state-of-the-art attention-based MIL approaches, the contribution of each patch was not only determined according to its feature embeddings but also contextualized with the feature embeddings of all other patches in the WSI via self-attention layers. Further, we presented a large-scale evaluation of transformers in biomarker prediction on WSIs. We demonstrated that transformer-based approaches learned better from small amounts of data and were therefore more data efficient than attention-based MIL approaches. At the same time, the performance increased proportionally with the number of training samples. Even though the performance seemed to plateau for MSI prediction, this suggests that larger training cohorts could lead to higher performance-approaching clinical application, also for more challenging tasks such as the prediction of the BRAF and KRAS mutational status. Our large-scale evaluation also showed that MIL and in particular transformer-based approaches generalize much better than the existing CNN approaches. We proved this by training the model on single cohorts and testing the generalization on all other cohorts. These experiments showed that the transformer-based approach reduced the drop in AUROC to under 0.09, while the CNN-based approach dropped by more than 0.21 in some cohorts. Most importantly, our approach trained on resections did not only generalize well to external cohorts of resections from geographically distinct regions but also to biopsies with a clinical-grade performance of 0.98 sensitivity on the YCR-BCIP biopsy cohort and 0.91 on the MAINZ cohort.
A caveat of our observations is that the ground truth might not be perfect. Potentially, the DL model is performing better than stated in the paper because dMMR and MSI only agree around 92% of the time and neither are 100% sensitive.57,71 Also, a small subset of CRCs have POLD1 and POLE mutations with a high-mutation burden that behaves clinically similar to MSI and might have a similar phenotype but are not detected by established MSI detection assays.72 Similarly, gene sequencing does not detect all mutations in KRAS/NRAS/BRAF depending on the sensitivity of the technology used and the presence of smaller clonal mutations. Current DL tests are at such high levels of performance that these nuanced subpopulations may be important. Our study has additional limitations: The focus of this study was to investigate the effect of handling the data with fully transformer-based approaches, especially in the context of large-scale multi-institutional data. Therefore, we did not exhaustively optimize every single hyperparameter. Points for optimization in this direction would be finding a fitting positional encoding and tuning the architecture of the transformer network and attention mechanisms. Additionally, collecting biopsy samples from different hospitals, for multi-cohort training directly on biopsy data could potentially improve the performance of our model on biopsy material. This would also hold for the prediction of BRAF mutation status and, in particular, of RAS mutation status, where we observed the largest potential for improvement. In both targets, the performance was higher on the larger cohorts with around 2,000 patients and increased dramatically by training on multiple cohorts. Further, we acknowledge that achieving an even higher specificity would be desirable. Choosing the final classification threshold is always a trade-off between sensitivity and specificity, where clinical application prefers a higher sensitivity, especially for pre-screening test as proposed in this study. Our method’s performance on biopsies is in the same range as current clinically approved assays on resections, but unlike these assays, our method also works on biopsies.
In summary, to the best of our knowledge, we presented a fully transformer-based model to predict MSI on WSI from CRC with an AUROC of 0.97 on resections and 0.92 and 0.86 on biopsies on external validation cohorts. Our model generalized better to unseen cohorts and was more data efficient compared to existing state-of-the-art MIL or CNN approaches. By publishing all trained models, we enable researchers and clinicians to apply the automated MSI prediction tool for research purposes, which we expect to bring the field of DL-based biomarkers a step closer to large-scale integration in the clinical workflow.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| The Cancer Genome Archive (TCGA) | https://portal.gdc.cancer.gov/ | RRID:SCR_003193 |
| Clinical Proteomic Tumor Analysis Consortium (CPTAC) | https://proteomic.datacommons.cancer.gov/pdc/ | RRID:SCR_017135 |
| Software and algorithms | ||
| Framework for experiments and model implementation | This manuscript; https://github.com/peng-lab/HistoBistro | https://zenodo.org/badge/latestdoi/613444008 |
| The model has also been implemented in this framework | https://github.com/KatherLab/marugoto | |
Resource availability
Lead contact
Further information and requests regarding this manuscript should be sent to and will be fulfilled by the lead investigator, Jakob Nikolas Kather (jakob_nikolas.kather@tu-dresden.de).
Materials availability
We release all multi-cohort model weights created in this study under an open-source license. More specifically, the model for MSI high, BRAF, and KRAS detection.
Experimental model and study participant details
Ethics statement
In this study, we retrospectively analyzed anonymized patient samples from multiple academic institutions. At each of the following sites, the respective ethics board has given consent to this analysis: DACHS, Epi700, ERLANGEN, MAINZ, MECC, MUNICH, NLCS, QUASAR, FOxTROT, TRANSCOT, MCO. At the following sites, specific ethics approval was not required for a retrospective analysis of anonymized samples: CPTAC, DUSSEL, TCGA, GUANGZHOU, and YCR-BCIP. Our study adheres to STARD (Table S3).
Cohort description
Through coordination by the MSIDETECT consortium (www.msidetect.eu), we have collected over 20,000 H&E tissue sections of 13,689 patients with CRC from 16 patient cohorts in total, including two public databases (Figures 1D–1F). The cohorts obtained are as follows:
-
1.
The public database “The Clinical Proteomic Tumor Analysis Consortium”, CPTAC (publicly available at https://pdc.cancer.gov/pdc/, USA)37,38 which includes tumors of any stage;
-
2.
DACHS (Darmkrebs: Chancen der Verhütung durch Screening, Southwest Germany),39,40 a large population-based case-control and patient cohort study on CRC, including samples of patients with stages I-IV from different laboratories in southwestern Germany coordinated by the German Cancer Research Center (Heidelberg, Germany);
-
3.
The DUSSEL (DUSSELdorf, Germany) cohort, a case series of CRC tumors resected with curative intent and collected at the Marien-Hospital in Duesseldorf, Germany, between January 1990 and December 199541;
-
4.
Epi700 (Belfast, N. Ireland, UK),42,43 a population-based cohort of stage II and III colon cancers treated by surgical resection between 2003 and 2008;
-
5.
The ERLANGEN cohort, a CRC cohort collected at the Uniklinikum Erlangen in Germany between 2002 and 2010.
-
6.
The “Fluoropyrimidine, Oxaliplatin, and Targeted Receptor pre-Operative Therapy for colon cancer cohort” (FOxTROT)44 including pre-therapeutic biopsy and post-therapeutic resection tumors from UK sites;
-
7.
The GUANGZHOU cohort, a small CRC case series of MSI-high cases collected in The Second Affiliated Hospital of Guangzhou Medical University, China;
-
8.
The MAINZ cohort, a small CRC case series of biopsies collected in the University Medical Center Mainz in Germany.
-
9.
The Molecular and Cellular Oncology Study (MCO) cohort45,46,47,48 from the University of New South Wales, Australia;
-
10.
MECC (Molecular Epidemiology of Colorectal Cancer, Israel),49 a population-based case-control study in northern Israel;
-
11.
The MUNICH (Munich, Germany) CRC series, a case series collected at the Technical University of Munich in Germany.
-
12.
The NLCS (Netherlands Cohort Study, The Netherlands)50,51 cohort, which contains tissue samples obtained from patients with any tumor stage as part of the Rainbow-TMA consortium study;
-
13.
QUASAR, the “Quick and Simple and Reliable” trial investigating survival benefit of adjuvant chemotherapy in patients from the United Kingdom with mostly stage II tumors52,53;
-
14.
The public repository “The Cancer Genome Atlas”, TCGA (publicly available at https://portal.gdc.cancer.gov/, USA)54,55 which includes tumors of any stage;
-
15.
The TransSCOT cohort, the translational arm of the SCOT trial, an “international, randomised, phase 3, non-inferiority trial” involving adult patients with high-risk stage II or stage III CRC56;
-
16.
The YCR-BCIP (Yorkshire Cancer Research Bowel Cancer Improvement Program, Yorkshire, United Kingdom [UK]), a population-based register of bowel cancer patients in Yorkshire, UK,57,58 for which surgical resections and biopsies were available as separate cohorts.
Detailed clinicopathological variables are shown in Table S4. In all cohorts, formalin-fixed paraffin-embedded (FFPE) tissue was used. Slides have been scanned at their respective centers. For each patient, either an MSI status or an MMR status, obtained by PCR or IHC, respectively, is available. Although MSI status and MMR status are not fully concordant,57 they are used interchangeably in clinical routine and grouped as a single category in this study. KRAS and BRAF mutational status are available for the cohorts DACHS, Epi700, NLCS, QUASAR, and TCGA.
Method details
Model description
Our biomarker prediction pipeline consists of three steps (Figure 1): i) the data pre-processing pipeline (Figure 1A), ii) the transformer-based feature extractor, and iii) the transformer-based aggregation module that yields the final prediction from the embeddings of all patches of a whole-slide image (WSI) (Figure 1B).
In the pre-processing pipeline, tissue regions are segmented using RGB thresholding and Canny edge detection34 to detect background and blurry regions. We include all tiles from a WSI, i.e., both tumor and healthy tissue tiles, thus reducing the burden of manual annotations when applying the algorithm. Subsequently, the WSI is tessellated into tiles of size 512 512 pixels at 20 magnification with a resolution of 0.5 microns per pixel. To reduce the impact of the staining color on the model generalization, the tiles are stain-color augmented using a structure-preserving GAN trained on TCGA.35
We extract feature representations of dimension 768 for every tile using the CTransPath model.29 (Figure 1B). The model architecture is based on a Swin Transformer26 that combines the hierarchical structure of CNNs with the global self-attention modules of transformers by computing self-attention in a sliding-window fashion. Similar to CNNs, these are stacked to increase the receptive field in every stage. CTransPath consists of three convolutional layers at the beginning to facilitate local feature extraction and improve training stability,29 followed by four Swin Transformer stages. Wang et al. trained the network using an unsupervised contrastive loss on data from TCGA and PAIP36 from multiple organs and provided the weights for public use. The embeddings for each tile are stored for the subsequent training procedure.
The final part of the model takes all patches of a WSI as input and predicts one biomarker for all input patches in a weakly supervised manner (Figure 1B). Common attention-based MIL approaches23 use a small neural network, which mostly consists of two layers, to compute patch importance based on the embeddings. Each weight is computed based on one patch and finally, all weights are normalized over the input elements. In contrast to this, in our model, the patch embeddings are passed into a transformer network using multi-headed self-attention that considers the patch embeddings as a sequence and relates each element to every other element. In particular, assuming that is the input sequence representing a WSI with patch embeddings of dimension , the self-attention layer computes a query-key product in the following way
where queries , keys , and values .These are computed from the input sequence by
where , , and are learnable parameters. Multi-headed self-attention applies self-attention in every head and concatenates the heads in a weighted manner:
where for
and is learnable. We choose a small transformer network architecture consisting of two layers with each eight heads (), a latent dimension of 512, and the same dimension for query, keys, and values. Therefore, the latent dimension of each head is , such that .
Assuming that is the number of patches per WSI, the embeddings of each patch are stacked to a sequence of dimension and are passed through a linear projection layer followed by the non-linear activation ReLU to reduce the dimension from 768 to 512. Subsequently, a class token is concatenated to the input, similar to the usage in vision transformers,25 yielding an input of dimension that is passed to the transformer layer. In each transformer layer, a block of layer normalization and multi-headed self-attention is followed by a block of layer normalization and a multi-layer perceptron (MLP), with skip connections applied across each block (Figure 1C).
After the two transformer layers, the class token of size is passed into an MLP head. Depending on the number of class tokens used, this enables single-target or multi-target binary prediction. Instead of attaching a class token, all sequence elements could be averaged to a single sequence element of size and passed into the MLP head. The averaging approach achieves similar performance to the class token version (Table S2), but we decided to use the class token for better interpretability of the attention heads. We also compared our model architecture to the existing transformer-based aggregation module TransMIL32 (Table S2).
Experimental setup and implementation details
We performed all experiments using 5-fold cross-validation with in-domain validation and testing. In this cross-validation variant, in-domain validation and test set are split off the full dataset on patient level, leaving 3-folds for training. By also cycling the in-domain test set through the complete dataset, we evaluated our model on more representative test sets than when fixing one smaller set for the dataset. During training, the validation set was used to determine the best model, which was finally evaluated on the test set. We further evaluated our models on external cohorts outside the dataset for out-of-domain testing.
The transformer models were trained with the AdamW59 optimizer using weight decay and learning rate both of . All models were trained for eight epochs with batch size of one for two reasons: first, the sequences of embeddings had different lengths due to the variable number of tiles per WSI and could thus not be stacked to mini-batches of equal length inputs; second, limits in GPU capacity (32GB) because of the quadratic complexity of the self-attention mechanism and the large number of tiles per slide (up to 12,000, Figure S1). To account for the varying cohort size, we evaluated the models every 500 iterations for runs on single cohorts, and every 1000 iterations for runs on multiple cohorts.
For comparison, we implemented the attention-based MIL approach from Ilse et al.,23 referred to as AttentionMIL. It provided the best results with Adam60 optimizer, as weight decay value, along with the fit-one-cycle learning rate scheduling policy61 with a maximum learning rate of , trained over 32 epochs, and the first 25% of the cycle with increasing learning rate.
Visualization and explainability
The final prediction is retrieved via the class token that is attached to the input sequence. To visualize the contribution of each input patch, we employed attention rollout as introduced by Abnar and Zuidema.62 To obtain the attention at the class token in the final layer, the attention maps of the preceding layers are multiplied recursively. Attention rollout thus quantifies to which extent each patch contributes to the final prediction in the class score. Additionally, we visualized the attention scores for each head in the transformer by taking the class token’s self-attention, i.e., the query and key product. All presented attention scores were normalized to the range and clamped to the lower and higher 5%-quantiles, respectively, for better visual interpretability.
To visualize whether a patch contributed toward a positive or negative classification outcome, we fed the patches one-by-one through the transformer and visualized the resulting classification scores of the model. These scores were naturally in the range and can thus be directly visualized without further normalizing or clamping of values.
Quantification and statistical analysis
We used the area under the receiver operator curve (AUROC) as our main evaluation metric. Since our data are naturally highly imbalanced with respect to the target variables MSI, BRAF, and KRAS (Figures 1D and 1E), we further used the area under the precision-recall curve (AUPRC) as a metric as this metric accounts better for class imbalances than the AUROC metric. The precision-recall curve relates the recall or specificity, i.e., the ratio of correctly positive predicted samples to all positive samples, to the precision, i.e., the ratio of correctly positive predicted samples to all positive predicted samples. For every experiment, we reported the mean and the standard deviation of respective 5-fold cross-validation’s model in-domain and external test performances. We split the dataset into patient-wise training, validation, and internal test sets stratified by the target label, thus ensuring that every patient can only occur in one of these sets. The external test sets always consisted of different cohorts to better quantify the generalization properties of our algorithms.
Consortia
We acknowledge the support of the Rainbow-TMA Consortium, especially the project group: PA van den Brandt, A zur Hausen, HI Grabsch, M van Engeland, LJ Schouten, J Beckervordersandforth; PHM Peeters, PJ van Diest, HB Bueno de Mesquita; J van Krieken, I Nagtegaal, B Siebers, B Kiemeney; FJ van Kemenade, C Steegers, D Boomsma, GA Meijer; FJ van Kemenade, B Stricker; L Overbeek, A Gijsbers; and Rainbow-TMA collaborating pathologists, among others: A de Bruïne; JC Beckervordersandforth; J van Krieken, I Nagtegaal; W Timens; FJ van Kemenade; MCH Hogenes; PJ van Diest; RE Kibbelaar; AF Hamel; ATMG Tiebosch; C Meijers; R Natté; GA Meijer; JJTH Roelofs; RF Hoedemaeker; S Sastrowijoto; M Nap; HT Shirango; H Doornewaard; JE Boers; JC van der Linden; G Burger; RW Rouse; PC de Bruin; P Drillenburg; C van Krimpen; JF Graadt van Roggen; SAJ Loyson; JD Rupa; H Kliffen; HM Hazelbag; K Schelfout; J Stavast; I van Lijnschoten; and K Duthoi.
Acknowledgments
SJW and DR are supported by the Helmholtz Association under the joint research school “Munich School for Data Science - MUDS” and SJW is supported by the Add-on Fellowship of the Joachim Herz Foundation. JNK is supported by the German Federal Ministry of Health (DEEP LIVER, ZMVI1-2520DAT111), the Max-Eder-Programme of the German Cancer Aid (grant #70113864), the German Federal Ministry of Education and Research (CAMINO, 01EO2101; SWAG, 01KD2215A; TRANSFORM LIVER, 031L0312A; TANGERINE, 01KT2302 through ERA-NET Transcan), the German Academic Exchange Service (SECAI, 57616814), the German Federal Joint Committee (Transplant.KI, 01VSF21048) and the European Union’s Horizon Europe and innovation program (ODELIA, 101057091; GENIAL, 101096312). JNK and MH are funded by the German Federal Ministry of Education and Research (PEARL, 01KD2104C). PQ, NW, SD, and GH are supported by Yorkshire Cancer Research grants L386 and L394. PQ, HG, NW, JNK, and SD are supported in part by the National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Center. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. PQ is also supported by an NIHR Senior Investigator award. FOxTROT was funded by Cancer Research UK (grant reference: C551/A8283; recipient: D.M.). Additional support was provided by the Birmingham and Leeds ECMC network, the RCS Eng and Rosetrees Trust, and the Swedish Cancer Society. Panitumumab was provided free of charge by Amgen, who also supported RAS testing and additional CT scans (recipient: D.M.). P.Q., N.W., and M.S. are supported by Yorkshire Cancer Research, R.G. by the Medical Research Council. Tumor tissue collection in the NLCS was done in the Rainbow-TMA study, which was financially supported by BBMRI-NL, a Research Infrastructure financed by the Dutch government (NWO 184.021.007 to PvdB). The analyses of MSI, BRAF, and KRAS in the NLCS were funded by The Dutch Cancer Society (KWF 11044 to PvdB). The DACHS study (HB, JCC, and MH) was supported by the German Research Council (BR 1704/6-1, BR 1704/6-3, BR 1704/6-4, CH 117/1-1, HO 5117/2-1, HO 5117/2-2, HE 5998/2-1, HE 5998/2-2, KL 2354/3-1, KL 2354/3-2, RO 2270/8-1, RO 2270/8-2, BR 1704/17-1 and BR 1704/17-2), the Interdisciplinary Research Program of the National Center for Tumor Diseases (NCT; Germany) and the German Federal Ministry of Education and Research (01KH0404, 01ER0814, 01ER0815, 01ER1505A and 01ER1505B). The study was further supported by project funding for the Pearl consortium from the German Federal Ministry of Education and Research (01KD2104A). SF is supported by the Deutsche Forschungsgemeinschaft (DFG) (FO 942/2-1), the German Federal Ministry of Education and Research (SWAG, 01KD2215A), the Mainz Research School of Translational Biomedicine (TransMed) and the Manfred-Stolte-Foundation. CM is supported by the Interdisciplinary Center for Clinical Research (IZKF) at the University Hospital of the University of Erlangen-Nuremberg (Junior Project J101). This work was supported in part by NIH R01 CA263318 (SG).
DACHS study: The authors thank the hospitals recruiting patients for the DACHS study and the cooperating pathology institutes. We thank the National Center for Tumor Diseases (NCT) Tissue Bank, Heidelberg, Germany, for managing, archiving, and processing tissue samples in the DACHS study.
The SCOT trial was funded by the Medical Research Council (transferred to NETSCC - Efficacy and Mechanism Evaluation) (Grant Ref: G0601705), NIHR Health Technology Assessment (Grant ref. 14/140/84), Cancer Research UK Core CTU Glasgow Funding (Funding Ref: C6716/A9894), and the Swedish Cancer Society. The TransSCOT sample collection was funded by a Cancer Research UK Clinical Trials Awards and Advisory Committee – Sample Collection (Grant Ref: C6716/A13941).
Molecular analysis of the SCOT samples were funded by the Oxford NIHR Comprehensive Biomedical Research Centre (BRC), a Cancer Research UK (CRUK) Advanced Clinician Scientist Fellowship (C26642/A27963) to DNC, CRUK award A25142 to the CRUK Glasgow Center. V.H.K. acknowledges funding by the Preeclampsia Foundation (F-87701-41-01). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health.
We furthermore acknowledge all collaborators in the YCR-BCIP and FOxTROT studies, as well as all other collaborators for all other cohorts included in this study.
Author contributions
S.J.W., M.B., T.P., and J.N.K. designed the concept of the study, S.J.W., J.M.N., and J.Z. prepared the data for this study, S.J.W. implemented the methods and ran all experiments and evaluations; S.F., C.M., C.P., M.B., and G.P.V. supported with domain-related advice; D.R., S.J.W., M.B., and T.P. developed and evaluated a preliminary study, where D.R. implemented the methods; all authors provided clinical and histopathological data and expertise; all authors provided clinical expertise and contributed to the interpretation of the results. S.J.W. wrote the manuscript with J.N.K. and input from all other authors.
Declaration of interests
J.N.K. reports consulting services for Owkin, France, Panakeia, UK, and DoMore Diagnostics, Norway and has received honoraria for lectures by M.S.D., Eisai, and Fresenius. N.W. has received fees for advisory board activities with BMS, Astellas, GSK, and Amgen, not related to this study. N.W. has received fees for advisory board activities with BMS, Astellas, and Amgen, not related to this study. P.Q. has received fees for advisory board activities with Roche and AMGEN and research funding from Roche through an Innovate UK National Pathology Imaging Consortium grant. H.I.G. has received fees for advisory board activities by AstraZeneca and BMS, not related to this study. M.S.T. is a scientific advisor to Mindpeak and Sonrai Analytics, and has received honoraria recently from BMS, MSD, Roche, Sanofi, and Incyte. He has received grant support from Phillips, Roche, MSD, and Akoya. None of these disclosures are related to this work. D.N.C. has participated in advisory boards for MSD and has received research funding on behalf of the TransSCOT consortium from HalioDx for analyses independent of this study. V.H.K. has served as an invited speaker on behalf of Indica Labs and has received project-based research funding from The Image Analysis Group and Roche outside of the submitted work. No other potential disclosures are reported by any of the authors.
Published: August 30, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ccell.2023.08.002.
Contributor Information
Tingying Peng, Email: tingying.peng@helmholtz-munich.de.
Jakob Nikolas Kather, Email: jakob_nikolas.kather@tu-dresden.de.
TransSCOT consortium:
David Church, Enric Domingo, Joanne Edwards, Bengt Glimelius, Ismail Gogenur, Andrea Harkin, Jen Hay, Timothy Iveson, Emma Jaeger, Caroline Kelly, Rachel Kerr, Noori Maka, Hannah Morgan, Karin Oien, Clare Orange, Claire Palles, Campbell Roxburgh, Owen Sansom, Mark Saunders, and Ian Tomlinson
Supplemental information
Data and code availability
Some of the data that support the findings of this study are publicly available, and some are proprietary datasets provided under collaboration agreements. All data (including histological images) from the TCGA database are available at https://portal.gdc.cancer.gov/. All data from the CPTAC cohort are available at https://proteomic.datacommons.cancer.gov/. All molecular data for patients in the TCGA and CPTAC cohorts are available at https://cbioportal.org/. Data access for the Northern Ireland Biobank can be requested at http://www.nibiobank.org/for-researchers. Data access for the MCO cohort can be requested at https://researchdata.edu.au/mco-study-tumour-collection/1957427. All other data are under controlled access according to the local ethical guidelines and can only be requested directly from the respective study groups that independently manage data access for their study cohorts.
All code was implemented in Python using the DL framework PyTorch. All source codes to reproduce the experiments of this paper are available under an open-source license at https://github.com/peng-lab/HistoBistro (https://doi.org/10.5281/zenodo.8208791). The model is also implemented in the DL pipeline https://github.com/KatherLab/marugoto/tree/transformer. We release all model weights under an open-source license.
References
- 1.Kather J.N., Pearson A.T., Halama N., Jäger D., Krause J., Loosen S.H., Marx A., Boor P., Tacke F., Neumann U.P., et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat. Med. 2019;25:1054–1056. doi: 10.1038/s41591-019-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao R., Yang F., Ma S.-C., Liu L., Zhao Y., Li Y., Wu D.-H., Wang T., Lu W.-J., Cai W.-J., et al. Development and interpretation of a pathomics-based model for the prediction of microsatellite instability in Colorectal Cancer. Theranostics. 2020;10:11080–11091. doi: 10.7150/thno.49864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Echle A., Grabsch H.I., Quirke P., van den Brandt P.A., West N.P., Hutchins G.G.A., Heij L.R., Tan X., Richman S.D., Krause J., et al. Clinical-Grade Detection of Microsatellite Instability in Colorectal Tumors by Deep Learning. Gastroenterology. 2020;159:1406–1416.e11. doi: 10.1053/j.gastro.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilal, M., Ahmed Raza, S.E., Azam, A., Graham, S., Ilyas, M., Cree, I.A., Snead, D., Minhas, F., and Rajpoot, N.M. Novel Deep Learning Algorithm Predicts the Status of Molecular Pathways and Key Mutations in Colorectal Cancer from Routine Histology Images. 10.1101/2021.01.19.21250122 [DOI] [PMC free article] [PubMed]
- 5.Lee S.H., Song I.H., Jang H.-J. Feasibility of deep learning-based fully automated classification of microsatellite instability in tissue slides of colorectal cancer. Int. J. Cancer. 2021;149:728–740. doi: 10.1002/ijc.33599. [DOI] [PubMed] [Google Scholar]
- 6.Schirris Y., Gavves E., Nederlof I., Horlings H.M., Teuwen J. DeepSMILE: Contrastive self-supervised pre-training benefits MSI and HRD classification directly from H&E whole-slide images in colorectal and breast cancer. Med. Image Anal. 2022;79 doi: 10.1016/j.media.2022.102464. [DOI] [PubMed] [Google Scholar]
- 7.Schrammen P.L., Ghaffari Laleh N., Echle A., Truhn D., Schulz V., Brinker T.J., Brenner H., Chang-Claude J., Alwers E., Brobeil A., et al. Weakly supervised annotation-free cancer detection and prediction of genotype in routine histopathology. J. Pathol. 2022;256:50–60. doi: 10.1002/path.5800. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita R., Long J., Longacre T., Peng L., Berry G., Martin B., Higgins J., Rubin D.L., Shen J. Deep learning model for the prediction of microsatellite instability in colorectal cancer: a diagnostic study. Lancet Oncol. 2021;22:132–141. doi: 10.1016/S1470-2045(20)30535-0. [DOI] [PubMed] [Google Scholar]
- 9.Jang H.-J., Lee A., Kang J., Song I.H., Lee S.H. Prediction of clinically actionable genetic alterations from colorectal cancer histopathology images using deep learning. World J. Gastroenterol. 2020;26:6207–6223. doi: 10.3748/wjg.v26.i40.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson A.B., Venook A.P., Al-Hawary M.M., Cederquist L., Chen Y.-J., Ciombor K.K., Cohen S., Cooper H.S., Deming D., Engstrom P.F., et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018;16:359–369. doi: 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence Colorectal cancer [NICE Guideline No. 151] 2020. https://www.nice.org.uk/guidance/ng151 [PubMed]
- 12.Schmoll H.J., Van Cutsem E., Stein A., Valentini V., Glimelius B., Haustermans K., Nordlinger B., van de Velde C.J., Balmana J., Regula J., et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann. Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 13.Chalabi M., Verschoor Y.L., van den Berg J., Sikorska K., Beets G., Lent A.V., Grootscholten M.C., Aalbers A., Buller N., Marsman H., et al. LBA7 Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: The NICHE-2 study. Ann. Oncol. 2022;33:S1389. [Google Scholar]
- 14.Vacante M., Borzì A.M., Basile F., Biondi A. Biomarkers in colorectal cancer: Current clinical utility and future perspectives. World J. Clin. Cases. 2018;6:869–881. doi: 10.12998/wjcc.v6.i15.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim C., Tsao M.S., Le L.W., Shepherd F.A., Feld R., Burkes R.L., Liu G., Kamel-Reid S., Hwang D., Tanguay J., et al. Biomarker testing and time to treatment decision in patients with advanced nonsmall-cell lung cancer. Ann. Oncol. 2015;26:1415–1421. doi: 10.1093/annonc/mdv208. [DOI] [PubMed] [Google Scholar]
- 16.Niehues J.M., Quirke P., West N.P., Grabsch H.I., van Treeck M., Schirris Y., Veldhuizen G.P., Hutchins G.G.A., Richman S.D., Foersch S., et al. Generalizable biomarker prediction from cancer pathology slides with self-supervised deep learning: A retrospective multi-centric study. Cell Rep. Med. 2023;4 doi: 10.1016/j.xcrm.2023.100980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilal M., Raza S.E.A., Azam A., Graham S., Ilyas M. Development and validation of a weakly supervised deep learning framework to predict the status of molecular pathways and key mutations in colorectal …. The Lancet Digital. 2021 doi: 10.1016/S2589-7500(21)00180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saillard C., Dubois R., Tchita O., Loiseau N., Garcia T., Adriansen A., Carpentier S., Reyre J., Enea D., Kamoun A., et al. Blind validation of MSIntuit, an AI-based pre-screening tool for MSI detection from histology slides of colorectal cancer. medRxiv. 2022 doi: 10.1038/s41467-023-42453-6. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svrcek M., Saillard C., Dubois R., Loiseau N., Mespoulhe P., Brulport F., Guillon J., Auffret M., Sefta M., Kamoun A., et al. 920P Blind validation of MSIntuit, an AI-based pre-screening tool for MSI detection from colorectal cancer H&E slides. Ann. Oncol. 2022;33:S967. [Google Scholar]
- 20.Chalabi M., Fanchi L.F., Dijkstra K.K., Van den Berg J.G., Aalbers A.G., Sikorska K., Lopez-Yurda M., Grootscholten C., Beets G.L., Snaebjornsson P., et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020;26:566–576. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 21.Bilal M., Jewsbury R., Wang R., AlGhamdi H.M., Asif A., Eastwood M., Rajpoot N. An Aggregation of Aggregation Methods in Computational Pathology. arXiv. 2022 doi: 10.1016/j.media.2023.102885. Preprint at. [cs.CV] [DOI] [PubMed] [Google Scholar]
- 22.Shmatko A., Ghaffari Laleh N., Gerstung M., Kather J.N. Artificial intelligence in histopathology: enhancing cancer research and clinical oncology. Nat. Can. (Ott.) 2022;3:1026–1038. doi: 10.1038/s43018-022-00436-4. [DOI] [PubMed] [Google Scholar]
- 23.Ilse, M., Tomczak, J., and Welling, M. (10--15 Jul 2018) Attention-based Deep Multiple Instance Learning. In Proceedings of the 35th International Conference on Machine Learning Proceedings of Machine Learning Research., J. Dy and A. Krause, eds. (PMLR), pp. 2127–2136
- 24.Saldanha O.L., Loeffler C.M.L., Niehues J.M., van Treeck M., Seraphin T.P., Hewitt K.J., Cifci D., Veldhuizen G.P., Ramesh S., Pearson A.T., Kather J.N. Self-supervised attention-based deep learning for pan-cancer mutation prediction from histopathology. npj Precis. Oncol. 2023;7:35. doi: 10.1038/s41698-023-00365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dosovitskiy A., Beyer L., Kolesnikov A., Weissenborn D., Zhai X., Unterthiner T., Dehghani M., Minderer M., Heigold G., Gelly S., et al. An Image is Worth 16x16 Words: Transformers for Image Recognition at Scale. arXiv. 2020 Preprint at. [cs.CV] [Google Scholar]
- 26.Liu Z., Lin Y., Cao Y., Hu H., Wei Y., Zhang Z., Lin S., Guo B. Proceedings of the IEEE/CVF International Conference on Computer Vision. 2021. Swin transformer: Hierarchical vision transformer using shifted windows; pp. 10012–10022. [Google Scholar]
- 27.He K., Gan C., Li Z., Rekik I., Yin Z., Ji W., Gao Y. Transformers in medical image analysis: A review. arXiv. 2022 Preprint at. [Google Scholar]
- 28.Ghaffari Laleh N., Truhn D., Veldhuizen G.P., Han T., van Treeck M., Buelow R.D., Langer R., Dislich B., Boor P., Schulz V., Kather J.N. Adversarial attacks and adversarial robustness in computational pathology. Nat. Commun. 2022;13:5711. doi: 10.1038/s41467-022-33266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Yang S., Zhang J., Wang M., Zhang J., Yang W., Huang J., Han X. Transformer-based unsupervised contrastive learning for histopathological image classification. Med. Image Anal. 2022;81 doi: 10.1016/j.media.2022.102559. [DOI] [PubMed] [Google Scholar]
- 30.Chen R.J., Chen C., Li Y., Chen T.Y., Trister A.D., Krishnan R.G., Mahmood F. Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition. 2022. Scaling Vision Transformers to Gigapixel Images via Hierarchical Self-Supervised Learning; pp. 16144–16155. [Google Scholar]
- 31.Ghaffari Laleh N., Muti H.S., Loeffler C.M.L., Echle A., Saldanha O.L., Mahmood F., Lu M.Y., Trautwein C., Langer R., Dislich B., et al. Benchmarking weakly-supervised deep learning pipelines for whole slide classification in computational pathology. Med. Image Anal. 2022;79 doi: 10.1016/j.media.2022.102474. [DOI] [PubMed] [Google Scholar]
- 32.Shao Z., Bian H., Chen Y., Wang Y., Zhang J., Ji X., Zhang Y. Advances in Neural Information Processing Systems. 2021. TransMIL: Transformer based correlated multiple instance learning for whole slide image classification; pp. 2136–2147. [Google Scholar]
- 33.Reisenbüchler D., Wagner S.J., Boxberg M., Peng T. Local Attention Graph-based Transformer for Multi-target Genetic Alteration Prediction. arXiv. 2022 Preprint at. [cs.CV] [Google Scholar]
- 34.Canny J. A computational approach to edge detection. IEEE Trans. Pattern Anal. Mach. Intell. 1986;8:679–698. [PubMed] [Google Scholar]
- 35.Wagner S.J., Khalili N., Sharma R., Boxberg M., Marr C., de Back W., Peng T. Medical Image Computing and Computer Assisted Intervention – MICCAI 2021. Springer International Publishing); 2021. Structure-Preserving Multi-domain Stain Color Augmentation Using Style-Transfer with Disentangled Representations; pp. 257–266. [Google Scholar]
- 36.Kim Y.J., Jang H., Lee K., Park S., Min S.-G., Hong C., Park J.H., Lee K., Kim J., Hong W., et al. PAIP 2019: Liver cancer segmentation challenge. Med. Image Anal. 2021;67 doi: 10.1016/j.media.2020.101854. [DOI] [PubMed] [Google Scholar]
- 37.Edwards N.J., Oberti M., Thangudu R.R., Cai S., McGarvey P.B., Jacob S., Madhavan S., Ketchum K.A. The CPTAC Data Portal: A Resource for Cancer Proteomics Research. J. Proteome Res. 2015;14:2707–2713. doi: 10.1021/pr501254j. [DOI] [PubMed] [Google Scholar]
- 38.Vasaikar S., Huang C., Wang X., Petyuk V.A., Savage S.R., Wen B., Dou Y., Zhang Y., Shi Z., Arshad O.A., et al. Proteogenomic Analysis of Human Colon Cancer Reveals New Therapeutic Opportunities. Cell. 2019;177:1035–1049.e19. doi: 10.1016/j.cell.2019.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmeister M., Jansen L., Rudolph A., Toth C., Kloor M., Roth W., Bläker H., Chang-Claude J., Brenner H. Statin use and survival after colorectal cancer: the importance of comprehensive confounder adjustment. J. Natl. Cancer Inst. 2015;107:djv045. doi: 10.1093/jnci/djv045. [DOI] [PubMed] [Google Scholar]
- 40.Brenner H., Chang-Claude J., Seiler C.M., Hoffmeister M. Long-term risk of colorectal cancer after negative colonoscopy. J. Clin. Oncol. 2011;29:3761–3767. doi: 10.1200/JCO.2011.35.9307. [DOI] [PubMed] [Google Scholar]
- 41.Grabsch H., Dattani M., Barker L., Maughan N., Maude K., Hansen O., Gabbert H.E., Quirke P., Mueller W. Expression of DNA double-strand break repair proteins ATM and BRCA1 predicts survival in colorectal cancer. Clin. Cancer Res. 2006;12:1494–1500. doi: 10.1158/1078-0432.CCR-05-2105. [DOI] [PubMed] [Google Scholar]
- 42.Gray R.T., Loughrey M.B., Bankhead P., Cardwell C.R., McQuaid S., O’Neill R.F., Arthur K., Bingham V., McGready C., Gavin A.T., et al. Statin use, candidate mevalonate pathway biomarkers, and colon cancer survival in a population-based cohort study. Br. J. Cancer. 2017;116:1652–1659. doi: 10.1038/bjc.2017.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gray R.T., Cantwell M.M., Coleman H.G., Loughrey M.B., Bankhead P., McQuaid S., O’Neill R.F., Arthur K., Bingham V., McGready C., et al. Evaluation of PTGS2 Expression, PIK3CA Mutation, Aspirin Use and Colon Cancer Survival in a Population-Based Cohort Study. Clin. Transl. Gastroenterol. 2017;8:e91. doi: 10.1038/ctg.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton D., Seymour M., Magill L., Handley K., Glasbey J., Glimelius B., Palmer A., Seligmann J., Laurberg S., Murakami K., et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J. Clin. Oncol. 2023;41:1541–1552. doi: 10.1200/JCO.22.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawkins N. 2011. MCO Study Tumour Collection. [Google Scholar]
- 46.Jonnagaddala J., Croucher J.L., Jue T.R., Meagher N.S., Caruso L., Ward R., Hawkins N.J. Integration and Analysis of Heterogeneous Colorectal Cancer Data for Translational Research. Stud. Health Technol. Inf. 2016;225:387–391. [PubMed] [Google Scholar]
- 47.Ward, R., and Hawkins, N. Molecular and Cellular Oncology (MCO) Study Data. UNSW Australia. doi
- 48.(2015). MCO Study Whole Slide Image Collection.
- 49.Shulman K., Barnett-Griness O., Friedman V., Greenson J.K., Gruber S.B., Lejbkowicz F., Rennert G. Outcomes of Chemotherapy for Microsatellite Instable-High Metastatic Colorectal Cancers. JCO Precis. Oncol. 2018;2:1–10. doi: 10.1200/PO.17.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Brandt P.A., Goldbohm R.A., van ’t Veer P., Volovics A., Hermus R.J., Sturmans F. A large-scale prospective cohort study on diet and cancer in The Netherlands. J. Clin. Epidemiol. 1990;43:285–295. doi: 10.1016/0895-4356(90)90009-e. [DOI] [PubMed] [Google Scholar]
- 51.Offermans K., Jenniskens J.C., Simons C.C., Samarska I., Fazzi G.E., Smits K.M., Schouten L.J., Weijenberg M.P., Grabsch H.I., van den Brandt P.A. Expression of proteins associated with the Warburg-effect and survival in colorectal cancer. J. Pathol. Clin. Res. 2022;8:169–180. doi: 10.1002/cjp2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quirke P., Morris E. Reporting colorectal cancer. Histopathology. 2007;50:103–112. doi: 10.1111/j.1365-2559.2006.02543.x. [DOI] [PubMed] [Google Scholar]
- 53.Quasar Collaborative Group. Gray R., Barnwell J., McConkey C., Hills R.K., Williams N.S., Kerr D.J. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 54.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isella C., Cantini L., Bellomo S.E., Medico E. 2014. TCGA CRC 450 Dataset. [Google Scholar]
- 56.Iveson T.J., Kerr R.S., Saunders M.P., Cassidy J., Hollander N.H., Tabernero J., Haydon A., Glimelius B., Harkin A., Allan K., et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2018;19:562–578. doi: 10.1016/S1470-2045(18)30093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West N.P., Gallop N., Kaye D., Glover A., Young C., Hutchins G.G.A., Brockmoeller S.F., Westwood A.C., Rossington H., Quirke P., Yorkshire Cancer Research Bowel Cancer Improvement Programme Group Lynch syndrome screening in colorectal cancer: results of a prospective two-year regional programme validating the NICE diagnostics guidance pathway across a 5.2 million population. Histopathology. 2021;79:690–699. doi: 10.1111/his.14390. [DOI] [PubMed] [Google Scholar]
- 58.Taylor J., Wright P., Rossington H., Mara J., Glover A., West N., Morris E., Quirke P., YCR BCIP study group Regional multidisciplinary team intervention programme to improve colorectal cancer outcomes: study protocol for the Yorkshire Cancer Research Bowel Cancer Improvement Programme (YCR BCIP) BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loshchilov I., Hutter F. Decoupled Weight Decay Regularization. arXiv. 2017 Preprint at. [cs.LG] [Google Scholar]
- 60.Kingma D.P., Ba J. Adam: A Method for Stochastic Optimization. arXiv. 2014 Preprint at. [cs.LG] [Google Scholar]
- 61.Smith L.N., Topin N. Artificial Intelligence and Machine Learning for Multi-Domain Operations Applications. SPIE); 2019. Super-convergence: very fast training of neural networks using large learning rates; pp. 369–386. [Google Scholar]
- 62.Abnar S., Zuidema W. Quantifying Attention Flow in Transformers. arXiv. 2020 Preprint at. [cs.LG] [Google Scholar]
- 63.Fu Y., Jung A.W., Torne R.V., Gonzalez S., Vöhringer H., Shmatko A., Yates L., Jimenez-Linan M., Moore L., Gerstung M. 2020. Pan-cancer Computational Histopathology Reveals Mutations, Tumor Composition and Prognosis. [DOI] [PubMed] [Google Scholar]
- 64.Kather J.N., Heij L.R., Grabsch H.I., Loeffler C., Echle A., Muti H.S., Krause J., Niehues J.M., Sommer K.A.J., Bankhead P., et al. Pan-cancer image-based detection of clinically actionable genetic alterations. Nat. Can. (Ott.) 2020;1:789–799. doi: 10.1038/s43018-020-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cercek A., Lumish M., Sinopoli J., Weiss J., Shia J., Lamendola-Essel M., El Dika I.H., Segal N., Shcherba M., Sugarman R., et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022;386:2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hendricks A., Amallraja A., Meißner T., Forster P., Rosenstiel P., Burmeister G., Schafmayer C., Franke A., Hinz S., Forster M., Williams C.B. Stage IV Colorectal Cancer Patients with High Risk Mutation Profiles Survived 16 Months Longer with Individualized Therapies. Cancers. 2020;12 doi: 10.3390/cancers12020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bera K., Schalper K.A., Rimm D.L., Velcheti V., Madabhushi A. Artificial intelligence in digital pathology - new tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019;16:703–715. doi: 10.1038/s41571-019-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coudray N., Ocampo P.S., Sakellaropoulos T., Narula N., Snuderl M., Fenyö D., Moreira A.L., Razavian N., Tsirigos A. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat. Med. 2018;24:1559–1567. doi: 10.1038/s41591-018-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kleppe A., Skrede O.-J., De Raedt S., Hveem T.S., Askautrud H.A., Jacobsen J.E., Church D.N., Nesbakken A., Shepherd N.A., Novelli M., et al. A clinical decision support system optimising adjuvant chemotherapy for colorectal cancers by integrating deep learning and pathological staging markers: a development and validation study. Lancet Oncol. 2022;23:1221–1232. doi: 10.1016/S1470-2045(22)00391-6. [DOI] [PubMed] [Google Scholar]
- 70.Campanella G., Hanna M.G., Geneslaw L., Miraflor A., Werneck Krauss Silva V., Busam K.J., Brogi E., Reuter V.E., Klimstra D.S., Fuchs T.J. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat. Med. 2019;25:1301–1309. doi: 10.1038/s41591-019-0508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.National Institute for Health and Care Excellence (NICE) Molecular Testing Strategies for Lynch Syndrome in People with Colorectal Cancer. NICE Guidance; 2020. https://www.nice.org.uk/guidance/ng151 [PubMed] [Google Scholar]
- 72.Liu Y., Sethi N.S., Hinoue T., Schneider B.G., Cherniack A.D., Sanchez-Vega F., Seoane J.A., Farshidfar F., Bowlby R., Islam M., et al. Comparative Molecular Analysis of Gastrointestinal Adenocarcinomas. Cancer Cell. 2018;33:721–735.e8. doi: 10.1016/j.ccell.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Some of the data that support the findings of this study are publicly available, and some are proprietary datasets provided under collaboration agreements. All data (including histological images) from the TCGA database are available at https://portal.gdc.cancer.gov/. All data from the CPTAC cohort are available at https://proteomic.datacommons.cancer.gov/. All molecular data for patients in the TCGA and CPTAC cohorts are available at https://cbioportal.org/. Data access for the Northern Ireland Biobank can be requested at http://www.nibiobank.org/for-researchers. Data access for the MCO cohort can be requested at https://researchdata.edu.au/mco-study-tumour-collection/1957427. All other data are under controlled access according to the local ethical guidelines and can only be requested directly from the respective study groups that independently manage data access for their study cohorts.
All code was implemented in Python using the DL framework PyTorch. All source codes to reproduce the experiments of this paper are available under an open-source license at https://github.com/peng-lab/HistoBistro (https://doi.org/10.5281/zenodo.8208791). The model is also implemented in the DL pipeline https://github.com/KatherLab/marugoto/tree/transformer. We release all model weights under an open-source license.