Key Points
Question
Is progression-free survival among patients with RAS wild-type metastatic colorectal cancer improved with maintenance therapy with cetuximab?
Findings
In this phase 2 randomized clinical trial of 139 patients receiving maintenance therapy with single-agent cetuximab or no treatment after induction with FOLFIRI plus cetuximab, the cetuximab group did not meet the primary end point of improvement in 6-month progression-free rate after randomization. However, meaningful progression-free survival and overall survival benefits in favor of cetuximab maintenance therapy were observed, especially for patients without a tumor-activating mutation in the mitogen-activated protein kinase (MAPK) pathway genes.
Meaning
This study suggests a benefit of single-agent anti–epidermal growth factor receptor in the maintenance setting, while the putative association of tumor-activating mutations within the MAPK pathway warrants further investigation.
Abstract
Importance
The optimal maintenance strategy after induction chemotherapy with anti–epidermal growth factor receptor antibody for patients with RAS wild-type metastatic colorectal cancer (mCRC) remains to be debated.
Objective
To evaluate the efficacy and safety of maintenance therapy with single-agent cetuximab after FOLFIRI (leucovorin [folinic acid], fluorouracil, and irinotecan) plus cetuximab induction therapy.
Design, Setting, and Participants
The TIME (Treatment After Irinotecan-Based Frontline Therapy: Maintenance With Erbitux]) (PRODIGE 28 [Partenariat de Recherche en Oncologie Digestive]–UCGI 27 [UniCancer GastroIntestinal Group]) phase 2 noncomparative, multicenter randomized clinical trial was conducted from January 15, 2014, to November 23, 2018, among 139 patients with unresectable RAS wild-type mCRC. The cutoff date for analysis was July 21, 2022.
Interventions
After first-line induction therapy with 8 cycles of FOLFIRI plus cetuximab, patients without disease progression were randomized (1:1) to biweekly maintenance with cetuximab or observation. On disease progression, the same induction regimen was recommended for 16 weeks followed by further maintenance with cetuximab or observation until disease progression under the full induction regimen.
Main Outcomes and Measures
The primary end point was the 6-month progression-free rate from randomization. Analysis was performed on an intention-to-treat basis. An exploratory biomolecular analysis, using next-generation sequencing, investigated the putative prognostic value of the tumor mutation profile.
Results
Of 214 patients enrolled (141 men [65.9%]; median age, 67 years [range, 23-85 years]), 139 were randomized to receive cetuximab (n = 67; 45 men [67.2%]; median age, 64 years [range, 34-85 years]) or to be observed (n = 72; 50 men [69.4%]; median age, 68 years [23-85 years]). The 6-month progression-free rate was 38.8% ([26 of 67] 95% CI, 27.1%-51.5%) in the cetuximab group and 5.6% ([4 of 72] 95% CI, 1.5%-13.6%) in the observation group. At a median follow-up of 40.5 months (95% CI, 33.6-47.5 months), median progression-free survival (PFS) from randomization was 5.3 months (95% CI, 3.7-7.4 months) in the cetuximab group and 2.0 months (95% CI, 1.8-2.7 months) in the observation group. Median overall survival (OS) was 24.8 months (95% CI, 18.7-30.4 months) in the cetuximab group and 19.7 months (95% CI, 13.3-24.4 months) in the observation group. In an exploratory multivariate analysis, any tumor-activating mutation in the mitogen-activated protein kinase (MAPK) pathway genes was associated with shorter PFS from randomization regardless of treatment group (hazard ratio, 1.63 [95% CI, 1.01-2.62]; P = .04). The most frequent grade 3 or 4 treatment-related toxic effect in the cetuximab group during maintenance therapy was rash (8 of 67 [11.9%]).
Conclusion and Relevance
The randomized clinical trial did not meet its primary end point but suggests clinically meaningful PFS and OS benefits associated with cetuximab maintenance therapy. However, maintenance cetuximab or treatment breaks after first-line combination FOLFIRI-cetuximab therapy seems inappropriate for patients with MAPK-mutated independently of the side of primary tumor. A more complete assessment of MAPK pathway mutations warrants further investigation to the refine treatment strategy for patients with RAS wild-type mCRC.
Trial Registration
ClinicalTrials.gov Identifier: NCT02404935
This randomized clinical trial evaluates the efficacy and safety of maintenance therapy with single-agent cetuximab after FOLFIRI (leucovorin [folinic acid], fluorouracil, and irinotecan irinotecan) plus cetuximab induction therapy for patients with RAS wild-type metastatic colorectal cancer (mCRC).
Introduction
For patients with metastatic colorectal cancer (mCRC) that is not amenable to curative-intent treatment and whose disease is controlled after induction chemotherapy, the optimal duration of the upfront combination of cytotoxic drugs with a targeted agent remains controversial. Phase 3 randomized trials have shown that de-escalation significantly reduces toxic effects and improves patients’ quality of life, whereas there is no benefit in terms of progression-free survival (PFS) or overall survival (OS) to continuing the full induction regimen until disease progression.1,2 However, there is still debate about the best option among de-escalation modalities, including fluoropyrimidine-based maintenance, chemotherapy-free intervals with a targeted agent alone, or planned off-therapy breaks.
According to current European Society for Medical Oncology (ESMO) guidelines,3 maintenance treatment with fluoropyrimidine plus bevacizumab or anti–epidermal growth factor receptor (EGFR) targeted therapy is recommended after oxaliplatin-based chemotherapy. Conversely, first-line FOLFIRI (leucovorin [folinic acid], fluorouracil, and irinotecan) should be continued until disease progression, while FOLFIRI plus targeted therapy is not considered.
A recent network meta-analysis confirmed the PFS benefit of maintenance therapy with fluoropyrimidine with or without bevacizumab vs observation; however, the OS benefit was not confirmed, leading to the conclusion that shared decision-making should include observation as an acceptable alternative.2 Although an anti-EGFR monoclonal antibody combined with doublet chemotherapy is currently recommended as a first-line treatment option for RAS wild-type mCRC (KRAS, OMIM 190070; and NRAS, OMIM 164790), the level of evidence in favor of anti-EGFR–based maintenance therapy is lower because only a few randomized studies (especially randomized comparisons of anti-EGFR–free maintenance therapy) investigated maintenance options after chemotherapy plus anti-EGFR monoclonal antibodies.3 Furthermore, anti-EGFR monoclonal antibodies (unlike bevacizumab) are active as a single-agent therapy in RAS wild-type mCRC and may allow chemotherapy-free intervals with fewer toxic effects.4 Therefore, the optimal duration of an anti-EGFR–based upfront treatment and maintenance strategy is still to be defined. The TIME (Treatment After Irinotecan-Based Frontline Therapy: Maintenance With Erbitux]) (PRODIGE 28 [Partenariat de Recherche en Oncologie Digestive]–UCGI 27 [UniCancer GastroIntestinal Group]) study was designed to evaluate the efficacy of single-agent cetuximab as maintenance therapy after induction chemotherapy with FOLFIRI plus cetuximab for patients with RAS wild-type mCRC.
Methods
Study Design and Participants
TIME was a multicenter, open-label, randomized, noncomparative phase 2 trial conducted from January 15, 2014, to November 23, 2018. Details of the protocol are described in Supplement 1. Eligible patients had histologically confirmed nonresectable mCRC, 1 or more measurable target lesions according to Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1),5 RAS wild-type status (locally assessed), Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, and adequate organ functions. Key exclusion criteria consisted of any prior chemotherapy except in the adjuvant setting if the last cycle was administered more than 6 months prior to inclusion, brain metastases, and concurrent active malignant neoplasms or clinically relevant comorbidities that could interfere with the conduct of the study or affect outcomes. All participants provided written informed consent before any study-related procedure. The study was conducted in accordance with International Conference for Harmonization guidelines and applicable national laws and regulations, as well as ethical principles derived from the Declaration of Helsinki.6 It was approved by a central ethics committee in April 2013 and by the French Regulatory Authority in May 2013. This article was prepared in accordance with the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Treatment Plan and Randomization
Induction chemotherapy consisted of 8 cycles of biweekly FOLFIRI (irinotecan, 180 mg/m2; leucovorin, 400 mg/m2, or L-leucovorin, 200 mg/m2; and fluorouracil, 400 mg/m2, intravenous bolus injection, then fluorouracil, 2400 mg/m2, continuous intravenous infusion over 46 hours) plus cetuximab (500 mg/m2, 1-hour infusion). After induction chemotherapy, patients with objective response or stable disease were randomized in a 1:1 ratio to receive biweekly cetuximab, 500 mg/m2, or to be observed. Randomization was performed using a minimization method that included a random element of 80%,7 taking into account the following stratification factors: center, tumor response after induction chemotherapy (objective response vs stable disease), baseline (before induction chemotherapy) carcinoembryonic antigen level (<100 vs ≥100 ng/mL [to convert to micrograms per liter, multiply by 1.0]), baseline platelet count (<400 vs ≥400 × 103/μL [to convert to ×109 per liter, multiply by 1.0]), and Köhne score8 (1 vs 2 vs 3).
At disease progression, randomized patients resumed treatment with 8 cycles of FOLFIRI plus cetuximab, followed by either cetuximab maintenance therapy or observation according to the randomization group. The cycling of treatment and maintenance therapy or complete breaks could be continued until progressive disease, toxic effects, or patient choice. If progression occurred during FOLFIRI plus cetuximab induction or rechallenge, second-line therapy containing oxaliplatin plus bevacizumab was recommended (eFigure 1 in Supplement 2). Preventive treatment of cetuximab-induced skin toxic effects with doxycycline was recommended.
Study Assessments
Computed tomography of the chest, abdomen, and pelvis was performed within 21 days prior to inclusion and repeated every 8 weeks throughout the study. Tumor response was evaluated using RECIST v1.1. Safety was assessed according to Common Terminology Criteria for Adverse Events, version 4.0 classification during the induction and maintenance phases by recording of adverse events, as well as clinical and biological examinations.9 Molecular profiling was performed on fixed tumor biopsies by targeted next-generation sequencing, using the ColonLung Hotspot V2 panel (Thermo Fisher Scientific Inc) (eMethods in Supplement 2).
Outcomes
The primary end point was the 6-month progression-free rate (PFR) from the start of maintenance therapy, defined as the percentage of patients alive without documented progression 6 months after randomization. Secondary end points included the objective response rate after induction chemotherapy, during maintenance, and after rechallenge with FOLFIRI plus cetuximab; median OS and PFS in the overall and randomized populations; and time to first-line strategy failure (defined as the time between randomization and progression during reintroduced FOLFIRI plus cetuximab) or first-line strategy discontinuation due to toxic effects, patient choice, or death.
Study Populations
Intention-to-treat (ITT) populations comprised all included patients (ITT1), all randomized patients (ITT2), and ITT2 patients with proven RAS and BRAF V600 (OMIM 164757) wild-type after centrally assessed molecular validation (ITT3). The safety population comprised all patients who received at least 1 dose of study treatments, analyzed according to the treatment they actually received.
Statistical Analysis
Based on a Fleming10 1-step design with 1-sided α of 5% and 80% power, selecting a 6-month PFR of 40% for the null hypothesis and 55% for the alternative hypothesis required 67 patients evaluable in the cetuximab group, leading to 134 evaluable randomized patients. Considering 30% of patients not eligible for randomization, 195 patients were to be included.
According to the Fleming rule,10 cetuximab maintenance therapy was to be deemed effective if 34 or more of 67 patients were alive without progression 6 months after randomization; this rule was applicable if 40% was included in the 95% CI of the 6-month PFR for the observation group. The trial was not powered for comparison between treatment groups.
Descriptive statistics were presented using median values and ranges for continuous parameters and frequencies and percentages for categorical variables. Median follow-up was estimated using the reverse Kaplan-Meier method. Time-to-event analyses (from randomization or inclusion) were performed using the Kaplan-Meier method; survival estimates at 6 months and 1 year were calculated with their associated 95% CIs. Missing data were not imputed.
Univariate and multivariate analyses were performed to identify a putative association of the tumor mutation profile with the MAPK pathway. Hazard ratios (HRs) with 95% CIs were estimated using a Cox proportional hazards regression model. The proportional hazards assumption was verified by the Schoenfeld residual method for covariates. All statistical analyses were performed using SAS, version 9.4 software (SAS Institute Inc). The cutoff date for analysis was July 21, 2022.
Results
Patients
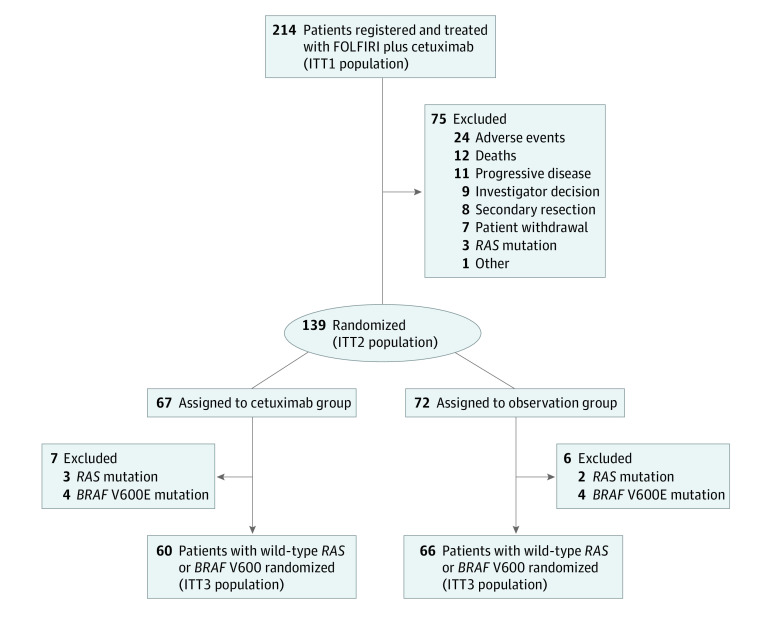
Of the 214 patients recruited from 35 French sites (eAppendix in Supplement 2) from January 15, 2014, to November 23, 2018, 141 were men (65.9%), 105 (50.5%) had an ECOG performance status of 0, and the median age at inclusion was 67 years (range, 23-85 years) (Table 1; eTable 1 in Supplement 2). Among these patients, 139 had an objective response or stable disease after induction chemotherapy and were randomly assigned to the cetuximab group (n = 67; 45 men [67.2%]; median age, 64 years [range, 34-85 years]) or the observation group (n = 72; 50 men [69.4%]; median age, 68 years [23-85 years]) (Figure 1). Patients not randomized were more frequently older than 75 years and were more likely than randomized patients to have a right-sided primary tumor (Table 1; eTable 1 in Supplement 2). Central next-generation sequencing detected tumors with BRAF V600E or RAS mutations including alterations at codon 12 and 13 in 19 of 214 patients (8.8%) and 11 of 214 patients (5.1%), respectively, in the ITT1 population and 8 of 139 patients (5.7%) and 5 of 139 patients (3.6%), respectively, in the ITT2 population, (Figure 1).
Table 1. Characteristics of Patients.
| Characteristic | Patients not randomized, No. (%) (n = 75) | Patients randomized, No. (%) | Patients registered, No. (%) (N = 214) | ||
|---|---|---|---|---|---|
| Cetuximab group (n = 67) | Observation group (n = 72) | Total (n = 139) | |||
| Age, median (range), y | 68 (43-83) | 64 (34-85) | 68 (23-85) | 66 (23-85) | 67 (23-85) |
| Aged >75 y | 18 (24.0) | 9 (13.4) | 11 (15.3) | 20 (14.4) | 38 (17.8) |
| Sex | |||||
| Male | 46 (61.3) | 45 (67.2) | 50 (69.4) | 95 (68.3) | 141 (65.9) |
| Female | 29 (38.7) | 22 (32.8) | 22 (30.6) | 44 (31.7) | 73 (34.1) |
| ECOG performance status | |||||
| 0 | 36 (48.6) | 36 (54.5) | 33 (48.5) | 69 (51.5) | 105 (50.5) |
| 1 | 31 (41.9) | 26 (39.4) | 28 (41.2) | 54 (40.3) | 85 (40.9) |
| 2 | 7 (9.5) | 4 (6.1) | 7 (10.3) | 11 (8.2) | 18 (8.7) |
| Missing information, No. | 1 | 1 | 4 | 5 | 6 |
| Site of primary tumor | |||||
| Right | 25 (33.3) | 16 (23.9) | 13 (18.3) | 29 (21.0) | 54 (25.4) |
| Left | 50 (66.7) | 51 (76.1) | 57 (80.3) | 108 (78.3) | 158 (74.2) |
| Both | 0 | 0 | 1 (1.4) | 1 (0.7) | 1 (0.5) |
| Missing information, No. | 0 | 0 | 1 | 1 | 1 |
| No. of metastatic sites | |||||
| 1 | 35 (47.3) | 39 (58.2) | 34 (47.9) | 73 (52.9) | 108 (50.9) |
| >1 | 39 (52.7) | 28 (41.8) | 37 (52.1) | 65 (47.1) | 104 (49.1) |
| Missing information, No. | 1 | 0 | 1 | 1 | 2 |
| Synchronous metastases | |||||
| Yes | 56 (74.7) | 46 (68.7) | 58 (80.6) | 104 (74.8) | 160 (74.8) |
| No | 19 (25.3) | 21 (31.3) | 14 (19.4) | 35 (25.2) | 54 (25.2) |
| Prior adjuvant chemotherapy | 17 (22.7) | 17 (25.4) | 10 (13.9) | 27 (19.4) | 44 (20.6) |
| Response to induction therapy, No. (% of evaluable patients) | |||||
| Objective response rate | 17 (43.6) | 50 (74.6) | 50 (69.4) | 100 (71.9) | 117 (65.7) |
| Stable disease | 10 (25.6) | 17 (25.4) | 22 (30.6) | 39 (28.1) | 49 (27.5) |
| Progressive disease | 12 (30.8) | NA | NA | NA | 12 (6.7) |
| Not evaluated, No. | 36 | NA | NA | NA | 36 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NA, not applicable.
Figure 1. Study Flow Diagram.
RAS and BRAF V600 were centrally assessed. FOLFIRI indicates fluorouracil, leucovorin (folinic acid), and irinotecan; ITT, intent-to-treat.
Among randomized patients, 63 of 139 (45.3%; 50.7% [34 of 67] in the cetuximab group and 40.3% [29 of 72] in the observation group) received 1 additional sequence of FOLFIRI plus cetuximab, 26 of 139 (18.7%; 13.4% [9 of 67] in the cetuximab group and 23.6% [17 of 72] in the observation group) received 2 additional sequences of FOLFIRI plus cetuximab, and 14 of 139 (10.1%; 7.5% [5 of 67] in the cetuximab group and 12.5% [9 of 72] in the observation group) received 3 or more additional sequences of FOLFIRI plus cetuximab (eTable 2 in Supplement 2).
Efficacy
At database lock, the median follow-up from randomization was 40.5 months (95% CI, 33.6-47.5 months). Among evaluable patients in the ITT1 population (178 of 214 [83.2%]), 2 patients (1.1%) had complete response, 115 (64.6%) had partial response, 49 (27.5%) had stable disease, and 12 (6.7%) had disease progression, for an objective response rate of 65.7% (117 of 178).
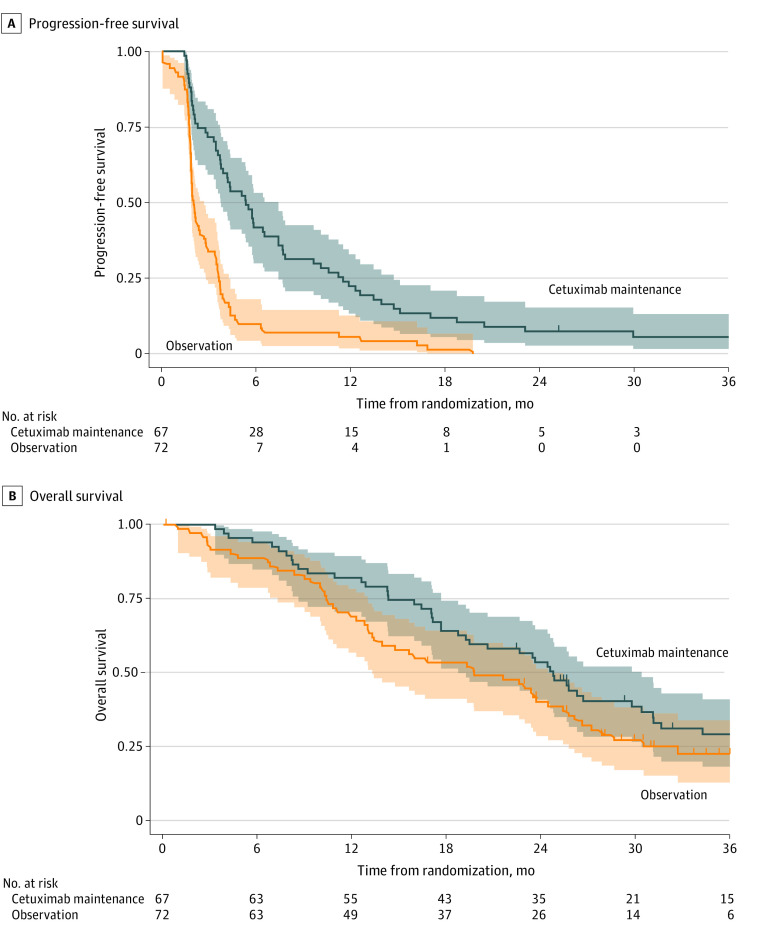
At 6 months after randomization, 26 patients in the cetuximab group (38.8%; 95% CI, 27.1%-51.5%) and 4 patients in the observation group (5.6%; 95% CI, 1.5%-13.6%) were alive without disease progression (Table 2). Thus, the 6-month PFR in the cetuximab group did not reach the predefined threshold of 34 successes, but the Fleming conclusion could not be applied because the upper limit of the 95% CI of the 6-month PFR rate in the observation group was below 40%. Median PFS from randomization was 5.3 months (95% CI, 3.7-7.4 months) in the cetuximab group and 2.0 months (95% CI, 1.8-2.7 months) in the observation group (Table 2 and Figure 2), and median PFS from inclusion was 9.0 months (95% CI, 7.8-10.8 months) in the cetuximab group and 6.2 months (95% CI, 5.7-6.8 months) in the observation group.
Table 2. Summary of Efficacy Outcomes Among Patients Randomized.
| Outcome | Cetuximab group (n = 67) | Observation group (n = 72) |
|---|---|---|
| Patients alive without progression at 6 mo, No. (%) [95% CI] | 26 (38.8) [27.1-51.5] | 4 (5.6) [1.5-13.6] |
| Progression, No. | 39 | 63 |
| Death, No. | 2 | 3 |
| Study discontinuation, No. | 0 | 1 |
| Nonevaluable, No. | 0 | 1 |
| PFS from randomization, median (95% CI), mo | 5.3 (3.7-7.4) | 2.0 (1.8-2.7) |
| PFS from inclusion, median (95% CI), mo | 9.0 (7.8-10.8) | 6.2 (5.7-6.8) |
| Time to first-line strategy failure, median (95% CI), mo | 8.7 (7.5-15.0) | 10.1 (7.3-10.9) |
| Response during first chemotherapy-free interval, No. (% of evaluable patients) | ||
| Complete response | 2 (3.2) | 1 (1.6) |
| Partial response | 9 (14.3) | 2 (3.2) |
| Stable disease | 34 (54.0) | 19 (30.2) |
| Progressive disease | 18 (28.6) | 41 (65.1) |
| Not evaluable, No. | 4 | 9 |
| Response to first FOLFIRI + cetuximab rechallenge, No. (% of evaluable patients) | ||
| Complete response | 0 | 0 |
| Partial response | 5 (10.9) | 14 (25.9) |
| Stable disease | 30 (65.2) | 33 (61.1) |
| Progressive disease | 11 (23.9) | 7 (13.0) |
| Not evaluable, No. | 21 | 18 |
| OS from randomization, median (95% CI), mo | 24.8 (18.7-30.4) | 19.7 (13.3-24.4) |
| OS from inclusion, median, (95% CI), mo | 28.6 (22.8-35.0) | 24.4 (17.2-28.6) |
Abbreviations: FOLFIRI, leucovorin [folinic acid], fluorouracil, and irinotecan; OS, overall survival; PFS, progression-free survival.
Figure 2. Progression-Free and Overall Survival.
Kaplan-Meier estimates of progression-free survival (A) and overall survival (B) from randomization by treatment group (intent-to-treat 2 population). Shaded areas indicate 95% CIs.
Objective response during maintenance and after first rechallenge was 17.5% (11 of 63) and 10.9% (5 of 46), respectively, in the cetuximab group, and 4.8% (3 of 63) and 25.9% (14 of 54), respectively, in the observation group (Table 2). Median time to strategy failure was 8.7 months (95% CI, 7.5-15.0 months) in the cetuximab group and 10.1 months (95% CI, 7.3-10.9 months) in the observation group.
At data cutoff, 74.8% of the randomized patients (104 of 139) had died, mainly from cancer-related causes (88.5% [92 of 104]). Median OS from randomization was 24.8 months (95% CI, 18.7-30.4 months) in the cetuximab ITT2 population and 19.7 months (95% CI, 13.3-24.4 months) in the observation ITT2 population, while median OS from inclusion was 28.6 months (95% CI, 22.8-35.0 months) in the cetuximab ITT2 population and 24.4 months (95% CI, 17.2-28.6 months) in the observation ITT2 population (eTable 3 in Supplement 2). An exploratory analysis of efficacy in the ITT3 population yielded similar outcomes.
Biomolecular Exploratory Analysis
Among patients with available tumor next-generation sequencing data in the ITT1 population (189 of 214 [88.3%]), 61.4% (116 of 189), 28.6% (54 of 189), and 19.1% (36 of 189) exhibited TP53 (OMIM 191170) mutations, activating mutation in the MAPK pathway (defined by at least 1 mutation in RAS, BRAF, MAP2K1 [OMIM 176872], or RTK genes [EGFR, OMIM 131550; ERBB2, OMIM 164870; MET, OMIM 164860; FGFR1, OMIM 136350; FGFR2, OMIM 176943; FGFR3, OMIM 134934; and ALK, OMIM 105590]), and activating mutations in the PIK3CA (OMIM 171834) pathway (defined by at least 1 mutation in PIK3CA, PTEN [OMIM 601728], AKT1 [OMIM 164730], or RTKs genes), respectively (eTable 4 in Supplement 3 and eTable 5 in Supplement 2). The MAPK and PIK3CA pathway mutations were more frequent in right-sided than in left-sided primary tumors (60.0% [27 of 45] vs 18.9% [27 of 143] and 40.0% [18 of 45] vs 13.2% [19 of 144], respectively; both P < .001). MAPK pathway mutations were more frequent among nonrandomized patients (40.9% [27 of 66] vs 22.0% [27 of 123]; P = .006).
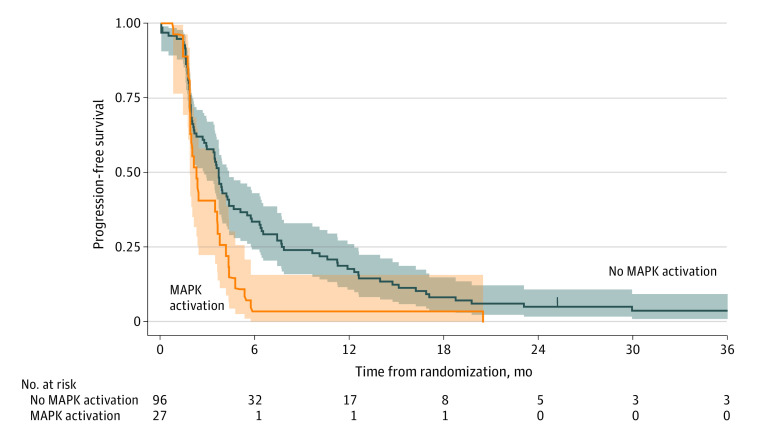
None of the mutations were significantly associated with the objective response rate in the ITT1 population. In the ITT2 population, any activating mutation in the MAPK pathway genes was associated with significantly lower median PFS from randomization (mutated, 2.3 months [95% CI, 1.8-3.6 months] vs wild-type, 3.7 months [95% CI, 2.8-4.3 months]; HR, 1.74 [95% CI, 1.12-2.71]; P = .02), respectively (Figure 3), even after exclusion of BRAF V600E and RAS mutations (mutated, 2.1 months [95% CI, 1.7-4.3 months] vs wild-type, 3.7 months [95% CI, 2.8-4.3 months]; HR, 1.91 [95% CI, 1.06-3.42]; P = .04). No significant differences were observed in terms of OS (eFigure 2 in Supplement 2).
Figure 3. Kaplan-Meier Estimates of Progression-Free Survival From Randomization According to Mitogen-Activated Protein Kinase (MAPK) Pathway Activation (Intent-to-Treat Population).
Shaded areas indicate 95% CIs.
In the MAPK wild-type ITT2 population, 6-month PFS was 53.2% (95% CI, 38.1%-66.2%) in the cetuximab group and 14.6% (95% CI, 6.4%-25.9%) in the observation group, with a median PFS of 6.5 months (95% CI, 3.9-9.6 months) in the cetuximab group and 2.1 (95% CI, 1.9-3.4 months) in the observation group (eFigure 3 in Supplement 2). In the exploratory multivariate analysis (eTable 6 in Supplement 2), cetuximab maintenance was independently associated with improved PFS (HR, 0.36 [95% CI, 0.24-0.53]; P < .001). Conversely, high platelet count (HR, 1.64 [95% CI, 1.03-2.63]; P = .04), intermediate Köhne score (HR, 1.52 [95% CI, 1.00-2.29]; P = .04), right-sided primary tumor (HR, 2.33 [95% CI, 1.39-3.92]; P = .001), and any MAPK-activating mutation (HR, 1.63 [95% CI, 1.01-2.62]; P = .04) were independently associated with shorter PFS. Factors significantly associated with impaired OS were high platelet count (HR, 1.66 [95% CI, 1.05-2.61]; P = .03) and right-sided primary tumor (HR, 2.13 [95% CI, 1.35-3.36]; P = .001). Interaction between MAPK status and treatment group was not significant.
Safety
Of the 208 patients in the safety population, 134 (64.4%) experienced at least 1 grade 3 or higher adverse event related or unrelated to study medication during induction chemotherapy (eTable 7 in Supplement 2). Seven patients died during induction therapy from overall worsening of health condition (n = 4), septic shock (n = 2), or myocardial infarction (n = 1).
During the first maintenance therapy, patients in the cetuximab group received a median number of 8 cetuximab cycles (range, 1-47 cetuximab cycles), 50% of patients (33 of 66) experienced at least 1 dose delay, and 16.7% (11 of 66) required at least 1 dose modification. The median relative dose intensity until first progression was 95% (range, 50%-100%). The reasons for cetuximab discontinuation were mainly disease progression (51 of 67 [76.1%]) and investigator decision (7 of 67 [10.4%]), with adverse events being involved in 3 of 67 cases (4.5%).
During the maintenance phase, 30 of 67 patients in the cetuximab group (44.8%) experienced at least 1 grade 3 or higher adverse event related or unrelated to study medication. The most frequent grade 3 or higher adverse events in the cetuximab group were rash (8 of 67 [11.9%]) and diarrhea (4 of 67 [6.0%]) (eTable 7 in Supplement 2).
Discussion
Although its primary end point was not met, our study is the first, to our knowledge, that included a randomized anti-EGFR–free group after irinotecan-based doublet induction chemotherapy suggesting a benefit of anti-EGFR maintenance therapy regarding PFS for patients with mCRC. Our results are in line with those of the COIN-B11 and PANAMA12 trials evaluating the efficacy of cetuximab vs observation and of fluorouracil plus panitumumab vs fluorouracil alone, respectively, both after oxaliplatin-based doublet induction chemotherapy.
According to current ESMO guidelines, de-escalation after oxaliplatin-based chemotherapy is advocated, but for patients who receive first-line FOLFIRI without targeted therapy, treatment should be continued until disease progression, while the situation of patients treated with FOLFIRI plus targeted therapy is not considered.3 However, several studies (most of them not including a randomized anti-EGFR–free group) have suggested that maintenance with anti-EGFR monoclonal antibodies alone or in combination with fluorouracil might be relevant maintenance options after anti-EGFR–based doublet induction chemotherapy.13,14,15,16 Although the ERMES phase 3 trial did not demonstrate noninferiority of maintenance with cetuximab monotherapy compared with FOLFIRI plus cetuximab until disease progression in terms of PFS, a higher incidence of grade 3 or higher toxic effects (coprimary end point) was reported in the combination group.16 Therefore, a chemotherapy-free control group may be justified.
In our study, the median PFS from randomization in the cetuximab group was close to that observed in the anti-EGFR monotherapy groups of COIN-B (5.3 and 5.7 months, respectively) but was shorter in the anti-EGFR–free groups of COIN-B and PANAMA (2.0, 3.1, and 8.8 months, respectively). Disease assessment was performed only every 3 months in COIN-B, and maintenance with fluorouracil was used in the control group of PANAMA.11,12
The short PFS observed in the control group is most likely the result of no further maintenance therapy. On the other hand, the lack of further maintenance therapy did not seem to have a clear effect on OS because the treatment group was not found to be significantly associated with OS in the multivariate analysis. If these results are consistent with those of the meta-analysis by Sonbol et al,2 which did not find significant OS benefit of maintenance therapy with fluoropyrimidine with or without bevacizumab compared with observation, this remains to be elucidated in the case of anti-EGFR–based maintenance treatment.
The originality of our exploratory molecular analysis lies in the significant prognostic value of the MAPK-activating mutation on PFS among patients with mCRC during chemotherapy-free intervals with or without cetuximab, even after exclusion of BRAF V600E and RAS mutations. In our ITT1 population selected according to RAS status (n = 190), we reported 24 tumors (12.6%) with MAPK gene alterations other than RAS-BRAF V600E mutations. The spectrum of MAPK pathway mutations with potential therapeutic involvement encompasses rare BRAF or RAS mutations and other alterations that are not considered in current recommendations. Recent pancancer data showed that patients with tumors with MAPK pathway alterations tended to globally have worse disease outcomes.17 The predictive value (ie, treatment-dependent effect) of MAPK-activating mutations (ie, significant benefit from cetuximab maintenance among patients with no MAPK-mutated tumor) could not be demonstrated, probably due to underpowered analysis, and should be assessed among larger patient populations. Nevertheless, our results suggest that patients with right-sided mCRC and/or MAPK activation may not be good candidates for cetuximab maintenance or treatment break. We believe that a more complete assessment of MAPK pathway mutations could refine treatment strategy decisions for patients with mCRC. No unexpected safety issues emerged, and few severe cetuximab-related adverse events were observed during the maintenance phase, probably because cetuximab dose adaptation and treatment discontinuation occurred during induction chemotherapy, before randomization.
Limitations
Our study has some limitations. Our hypothesis for 6-month PFR was overoptimistic. Progression-free survival may appear inappropriate because it does not consider the subsequent benefit of planned reintroduction of full first-line treatment at disease progression. However, the best parameter to assess the benefit of maintenance is PFS in the interval. At the time our study was designed, the prognostic and predictive effect of primary tumor site and BRAF V600E mutation status was unknown, so our population was not selected according to these criteria. We actually observed that patients with right-sided primary tumors exhibited shorter PFS and OS and that left localization significantly increased the probability of response during the induction phase. Current guidelines recommend first-line chemotherapy with anti-EGFR antibodies only for patients with left colon tumors.3,18,19 For the same reasons, we did not analyze the microsatellite status that now determines the choice of first-line immunotherapy for DNA mismatch repair deficient or microsatellite instability-high tumors.
Conclusions
Although this randomized clinical trial did not meet its primary end point, maintenance cetuximab after induction FOLFIRI plus cetuximab appeared feasible and was associated with longer PFS, OS, and chemotherapy-free intervals than observation. Our exploratory multivariate analysis including molecular alterations suggests that patients with right-sided primary tumors and/or any tumor-activating mutation in MAPK pathway genes may not benefit from maintenance treatment with cetuximab nor treatment breaks after first-line FOLFIRI plus cetuximab. Further studies will need to better clarify subgroups of patients who will benefit or not benefit from maintenance with anti-EGFR antibodies and to define the most appropriate maintenance regimens according to the tumors’ molecular characteristics.
Study Protocol and Data Analysis Plan
eAppendix. List of Recruiting Study Centers
eFigure 1. Study Design
eTable 1. Stratification Factors in Patients Randomized (ITT2 Population)
eTable 2. Number of FOLFIRI Plus Cetuximab Sequence Reintroductions in Patients Randomized (ITT2 Population)
eTable 3. Summary of Outcomes in RAS and BRAF V600 Wild-Type Randomized Patients (ITT3 Population)
eTable 5. Summary of TP53, MAPK or PIK3CA Pathway Activating Mutations (ITT1 Population)
eFigure 2. Kaplan-Meier Estimates of Overall Survival From Randomization According to MAPK Pathway Activation (ITT2 Population)
eFigure 3. Kaplan-Meier Estimates of Progression-Free Survival From Randomization According to MAPK Pathway Activation and to Treatment Arm (ITT2 Population)
eTable 6. Exploratory Multivariate Analysis: Factors Significantly Associated With Progression-Free (A) and Overall Survival (B) in Patients Randomized (ITT2 Population)
eTable 7. Adverse Events of Interest During Induction FOLFIRI Plus Cetuximab and During First Chemotherapy-Free Interval
eMethods. Sequencing
eTable 4. Detailed Tumor NGS Data Available in the ITT1 Population (n=189)
Data Sharing Statement
References
- 1.Ron DA, Vera R, Labandeira CM, et al. Maintenance treatment in metastatic colorectal cancer: in search of the best strategy. Clin Transl Oncol. 2020;22(8):1205-1215. doi: 10.1007/s12094-019-02267-9 [DOI] [PubMed] [Google Scholar]
- 2.Sonbol MB, Mountjoy LJ, Firwana B, et al. The role of maintenance strategies in metastatic colorectal cancer: a systematic review and network meta-analysis of randomized clinical trials. JAMA Oncol. 2020;6(3):e194489. doi: 10.1001/jamaoncol.2019.4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cervantes A, Adam R, Roselló S, et al. ; ESMO Guidelines Committee . Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10-32. doi: 10.1016/j.annonc.2022.10.003 [DOI] [PubMed] [Google Scholar]
- 4.Aparicio T, Ghiringhelli F, Boige V, et al. ; PRODIGE 9 Investigators . Bevacizumab maintenance versus no maintenance during chemotherapy-free intervals in metastatic colorectal cancer: a randomized phase III trial (PRODIGE 9). J Clin Oncol. 2018;36(7):674-681. doi: 10.1200/JCO.2017.75.2931 [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 6.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 7.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15(5):443-453. doi: 10.1002/cpt1974155443 [DOI] [PubMed] [Google Scholar]
- 8.Köhne CH, Cunningham D, Di Costanzo F, et al. Clinical determinants of survival in patients with 5-fluorouracil–based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13(2):308-317. doi: 10.1093/annonc/mdf034 [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute . Division of Cancer Treatment & Diagnosis. Cancer Therapy Evaluation Program (CTEP) Adverse Events/CTCAE. Accessed August 16, 2023. https://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm
- 10.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38(1):143-151. doi: 10.2307/2530297 [DOI] [PubMed] [Google Scholar]
- 11.Wasan H, Meade AM, Adams R, et al. ; COIN-B investigators . Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial. Lancet Oncol. 2014;15(6):631-639. doi: 10.1016/S1470-2045(14)70106-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modest DP, Karthaus M, Fruehauf S, et al. Panitumumab plus fluorouracil and folinic acid versus fluorouracil and folinic acid alone as maintenance therapy in RAS wild-type metastatic colorectal cancer: the randomized PANAMA trial (AIO KRK 0212). J Clin Oncol. 2022;40(1):72-82. doi: 10.1200/JCO.21.01332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aranda E, García-Alfonso P, Benavides M, et al. ; Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD) . First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: phase II randomised MACRO2 TTD study. Eur J Cancer. 2018;101:263-272. doi: 10.1016/j.ejca.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 14.Munemoto Y, Nakamura M, Takahashi M, et al. SAPPHIRE: a randomised phase II study of planned discontinuation or continuous treatment of oxaliplatin after six cycles of modified FOLFOX6 plus panitumumab in patients with colorectal cancer. Eur J Cancer. 2019;119:158-167. doi: 10.1016/j.ejca.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 15.Pietrantonio F, Morano F, Corallo S, et al. Maintenance therapy with panitumumab alone vs panitumumab plus fluorouracil-leucovorin in patients with RAS wild-type metastatic colorectal cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1268-1275. doi: 10.1001/jamaoncol.2019.1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto C, Orlandi A, Normanno N, et al. LBA22 phase III study with FOLFIRI/cetuximab versus FOLFIRI/cetuximab followed by cetuximab (Cet) alone in first-line therapy of RAS and BRAF wild-type (wt) metastatic colorectal cancer (mCRC) patients: the ERMES study. Ann Oncol. 2022;33:S1390. doi: 10.1016/j.annonc.2022.08.018 [DOI] [Google Scholar]
- 17.Sinkala M, Nkhoma P, Mulder N, Martin DP. Integrated molecular characterisation of the MAPK pathways in human cancers reveals pharmacologically vulnerable mutations and gene dependencies. Commun Biol. 2021;4(1):9. doi: 10.1038/s42003-020-01552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phelip JM, Tougeron D, Léonard D, et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis. 2019;51(10):1357-1363. doi: 10.1016/j.dld.2019.05.035 [DOI] [PubMed] [Google Scholar]
- 19.Morris VK, Kennedy EB, Baxter NN, et al. Treatment of metastatic colorectal cancer: ASCO guideline. J Clin Oncol. 2023;41(3):678-700. doi: 10.1200/JCO.22.01690 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol and Data Analysis Plan
eAppendix. List of Recruiting Study Centers
eFigure 1. Study Design
eTable 1. Stratification Factors in Patients Randomized (ITT2 Population)
eTable 2. Number of FOLFIRI Plus Cetuximab Sequence Reintroductions in Patients Randomized (ITT2 Population)
eTable 3. Summary of Outcomes in RAS and BRAF V600 Wild-Type Randomized Patients (ITT3 Population)
eTable 5. Summary of TP53, MAPK or PIK3CA Pathway Activating Mutations (ITT1 Population)
eFigure 2. Kaplan-Meier Estimates of Overall Survival From Randomization According to MAPK Pathway Activation (ITT2 Population)
eFigure 3. Kaplan-Meier Estimates of Progression-Free Survival From Randomization According to MAPK Pathway Activation and to Treatment Arm (ITT2 Population)
eTable 6. Exploratory Multivariate Analysis: Factors Significantly Associated With Progression-Free (A) and Overall Survival (B) in Patients Randomized (ITT2 Population)
eTable 7. Adverse Events of Interest During Induction FOLFIRI Plus Cetuximab and During First Chemotherapy-Free Interval
eMethods. Sequencing
eTable 4. Detailed Tumor NGS Data Available in the ITT1 Population (n=189)
Data Sharing Statement