Abstract
Natural variations in the 13C:12C ratio (carbon-13 isotopic abundance [δ13C]) of the food supply have been used to determine the dietary origin and metabolism of fatty acids, especially in the n-3 PUFA biosynthesis pathway. However, n-6 PUFA metabolism following linoleic acid (LNA) intake remains under investigation. Here, we sought to use natural variations in the δ13C signature of dietary oils and fatty fish to analyze n-3 and n-6 PUFA metabolism following dietary changes in LNA and eicosapentaenoic acid (EPA) + DHA in adult humans. Participants with migraine (aged 38.6 ± 2.3 years, 93% female, body mass index of 27.0 ± 1.1 kg/m2) were randomly assigned to one of three dietary groups for 16 weeks: 1) low omega-3, high omega-6 (H6), 2) high omega-3, high omega-6 (H3H6), or 3) high omega-3, low omega-6 (H3). Blood was collected at baseline, 4, 10, and 16 weeks. Plasma PUFA concentrations and δ13C were determined. The H6 intervention exhibited increases in plasma LNA δ13C signature over time; meanwhile, plasma LNA concentrations were unchanged. No changes in plasma arachidonic acid δ13C or concentration were observed. Participants on the H3H6 and H3 interventions demonstrated increases in plasma EPA and DHA concentration over time. Plasma δ13C-EPA increased in total lipids of the H3 group and phospholipids of the H3H6 group compared with baseline. Compound-specific isotope analysis supports a tracer-free technique that can track metabolism of dietary fatty acids in humans, provided that the isotopic signature of the dietary source is sufficiently different from plasma δ13C.
Supplementary key words: arachidonic acid, linoleic acid, EPA, fatty acid metabolism, isotope ratio MS, lipids, nutrition, omega-3 fatty acids, omega-6 fatty acids, human plasma
Compound-specific isotope analysis (CSIA) is a relatively simple and cost-effective method that has been used to analyze the metabolism and dietary origin of fatty acids by taking advantage of natural variations in the dietary 13C isotope composition of the food supply (1). 13C isotopic values of natural samples are universally expressed as the difference between the 13C:12C ratio of a metabolite and a reference material, identified as carbon-13 isotopic abundance (δ13C) and reported in milliUrey (mUr) (2). Each 1 mUr change demonstrates a one per mille (1 in 1,000, ‰) change in the 13C:12C ratio compared with the reference material. δ13C is referenced to the remains of a Cretaceous marine fossil (Belemnitella americana), having a higher δ13C signature than all living things (3). Therefore, all living things will have negative δ13C signatures because of the lower 13C:12C ratio compared to Belemnitella americana (4).
There are differences in carbon isotope composition throughout the food chain, starting with differences in photosynthetic processes and carbon fixation in plants (5). This variation results in plants being categorized into C3 or C4, where fatty acids isolated from C3 plants have a naturally lower 13C:12C ratio (−23 to −32 mUr) compared with C4 plants (−10 to −16 mUr) (4). In addition, aquatic organisms typically have more intermediate 13C:12C ratios (−16 to −23 mUr) (5). These natural differences in the δ13C signatures of the food supply have been utilized to answer questions relating to dietary assessment and metabolism. For example, high δ13C-arachidonic acid (ARA; 20:4n-6) and δ13C-docosahexaenoic acid (DHA, 22:6n-3) via formula fed to infants were used to estimate turnover rates of plasma phospholipid (PL) ARA and DHA (6), and feeding high δ13C corn oil to infants was used to estimate the percentage conversion of linoleic acid (LNA; 18:2n-6) to ARA (7). Our laboratory has also utilized these natural variations in the 13C:12C ratio of the food supply to determine the dietary origin of DHA in fat-1 mice using high δ13C content corn oil and low δ13C content safflower oil (8), or consumption of intermediate δ13C content DHA and low δ13C content alpha-linolenic acid (ALA; 18:3n-3) in BALB/c mice (1). In addition, natural variations in δ13C of the food supply have been used to analyze the n-3 PUFA biosynthesis pathway in rats (9) and humans (10). For example, natural variations in the δ13C of eicosapentaenoic acid (EPA, 20:5n-3) and DHA supplements were used to determine EPA and DHA metabolism in humans (10). Therefore, using δ13C signatures of fatty acids in the food supply can provide insight into in vivo fatty acid metabolism, especially in the n-3 PUFA biosynthesis pathway, by analyzing changes in the δ13C of tissue or plasma fatty acids following dietary intake compared with the δ13C signature of fatty acids from the diet.
In the past century, the Western diet has seen a significant increase in LNA intake (11), leading to concerns about increased inflammation because of the downstream proinflammatory effects of ARA. However, high dietary LNA intake is not associated with increased inflammatory markers (12, 13, 14, 15, 16), and ARA levels remain unchanged (17). Despite these findings, it is still debated why dietary LNA intake does not affect ARA concentration. Hence, natural δ13C differences in the food supply may also be a useful method to assess the metabolite fate of LNA. The present study is an exploratory secondary analysis using specimens from the Nutrition for Migraine Prevention trial: a double-blinded and randomized controlled trial where the primary end point analyzed the effectiveness of altering n-3 EPA + DHA and/or n-6 LNA on circulating lipid mediators in headache pathogenesis and headache impact in adults with migraine (18). We utilize the natural variations in the δ13C of dietary oils from this trial to explore 1) the metabolism of the n-3 PUFA biosynthesis pathway, specifically changes in plasma δ13C of EPA and DHA in response to altering dietary EPA + DHA and 2) the metabolism of the n-6 PUFA biosynthesis pathway, especially changes in plasma δ13C of ARA and LNA following changes in dietary LNA. We would expect to see the δ13C of plasma EPA and DHA shift toward the dietary δ13C signature of EPA and DHA in diets with increased consumption of EPA and DHA and the δ13C of plasma ARA and LNA change toward the dietary δ13C signature of LNA in diets with increased LNA intake.
Materials and methods
Participants and study design
We analyzed a randomly selected subset of 30 participants (aged 38.6 ± 2.3 years, 93% female, body mass index = 27.0 ± 1.1 kg/m2) from the original 141 study participants who completed participation in the Nutrition for Migraine Prevention trial. These participants were recruited from headache specialty clinics and community sources around the University of North Carolina. Eligible participants were required to be at least 18 years of age and met the 2004 International Classification of Headache Disorders criteria for migraine (5–20 migraine days per month). Exclusion criteria included regular use of dietary supplements containing fatty acids, food allergies resulting in a rash or dyspnea, aversion to eating seafood, pregnancy or a recent change in hormone use, marked depression, anxiety, or psychosis at the time of enrollment, active treatment for a major medical illness (e.g., autoimmune disorder, malignancy), recent substance abuse, or a history of head trauma, hemorrhage, hematoma, nervous system infections (e.g., meningitis, encephalitis), intracranial mass, clotting disorders, vasculitis, or cognitive dysfunction that would prevent informed consent. The study was registered at clinicaltrials.gov (NCT02012790) and was conducted from July 2014 to May 2018. Primary (plasma antinociceptive mediator 17-hydroxy-DHA and headache impact test) and secondary (headache frequency, patient-reported outcome measurement information system-29 profile, 17-hydroxy DHA trajectory) endpoints and sample size calculations are previously described (18).
All participants provided written informed consent. The study was approved by the Human Research Ethics Committee of the University of North Carolina (Institutional Review Board no.: 13-3284) and abided by the Declaration of Helsinki principles. In a three-armed, parallel-group, double-blinded, and randomized design, participants were randomly assigned to one of three dietary groups for a 16-week intervention: 1) a low n-3, high n-6 group (H6) consisting of the average US dietary intake of n-3 PUFAs and LNA (n = 10), 2) a high n-3, high n-6 group (H3H6) consisting of increased DHA and EPA intake with the average US dietary intake of LNA (n = 10), or 3) a high n-3, low n-6 group (H3) consisting of increased DHA and EPA with low LNA intake (n = 10). All participants had fasting blood withdrawn at baseline (week 0) and after 4, 10, and 16 weeks of diet exposure.
Dietary intervention
Detailed information regarding dietary interventions used for the three dietary groups, including dietary adherence, has been previously described (18, 19, 20). Foods and oils were carefully selected to achieve the nutrient intake levels of each diet group and were provided to study participants. The H3H6 diet was designed to increase EPA + DHA intake to 1.5 g/day while maintaining the average US intake of LNA (7.0% of food energy). The H3 diet was designed to increase EPA + DHA to 1.5 g/day while decreasing LNA intake to ≤1.8% of energy. The H6 diet was designed to maintain the average US intake of LNA (7.0% of energy) and EPA + DHA (<150 mg/day). The three diets were similar in composition, with the differences being in the study provided oil and butter formulations, as well as protein sources (e.g., fatty fish or low-fat fish and lean meats). The H6 and H3H6 groups consumed a high-LNA oil blend, consisting of 75% corn oil and 25% extra virgin olive oil, and a butter blend consisting of 50% corn oil and butter. The H3 group consumed a low-LNA oil blend containing 75% macadamia nut oil and 25% extra virgin olive oil in addition to butter (supplemental Table S1). These dietary oils were the primary source of fat and used in home cooking, the study provided salad dressing, and the study provided foods. Dietary EPA and DHA intakes were achieved in the H3 and H3H6 groups through the consumption of study-provided tuna, salmon, and sardines, whereas the H6 group consumed low-fat fish and lean meats. Participants in all dietary interventions were provided with a variety of low-LNA foods and were instructed to purchase other low-LNA foods to complement the study-provided foods.
Blood collection
Methods for blood collection have been previously described (19). Briefly, fasting blood was collected into EDTA tubes. Blood fractions were immediately separated by centrifugation at 2,960 rpm for 15 min, and the upper plasma layer was aliquoted. Samples were stored at the University of North Carolina Bioanalytical Core Laboratory at −80°C. Plasma samples were shipped to the University of Toronto on dry ice and stored at −80°C until lipid extraction in 2021.
Lipid extraction, separation by TLC, and transesterification
For the secondary analysis, ten participants from each dietary group who completed the study protocol were randomly selected by a computer-generated random number list for fatty acid analysis. Total lipids were extracted from 300 μl of plasma using a modified Folch method (21), as previously described (22, 23). Lipid extraction was performed with 2:1 chloroform:methanol in the presence of 10 μg of unesterified heptadecanoic acid, cholesteryl ester (CE), triglyceride (TG), and phosphatidylcholine (17:0 FFA, CE, TG; Nu-Chek Prep, Inc; phosphatidylcholine; Avanti Polar Lipids) as the internal standard. Potassium chloride (0.88% [w/v]) was added to separate phases. Aliquots of total lipid extracts were used for total lipid quantification and neutral lipid separation using TLC. Lipid class separation was performed using TLC-G plates (Miles Scientific) with heptane:diethyl ether:glacial acetic acid (60:40:2, by volume) as the migration solvent. Lipids were visualized by application of a 0.1% w/v solution of 8-anilino-1-napthalene sulfonic acid in methanol under UV light. The PL, CE, TG, and FFA fractions were collected. Total lipid extract and TLC scrapes containing internal standards were transesterified to fatty acid methyl esters (FAMEs) with 14% boron trifluoride in methanol. For the dietary oils described above (75% corn/25% extra virgin olive oil, 75% macadamia nut/25% extra virgin olive oil, corn oil, macadamia nut oil, and extra virgin olive oil), total lipids were extracted and methylated in triplicate from 10 μl of oil using the same procedure for total lipid quantification. FAMEs were analyzed by GC-flame ionization detection (GC-FID).
GC-FID
FAMEs were analyzed on a Varian 430 Gas Chromatograph (Scion Instruments) equipped with a 30 m length × 0.25 mm diameter × 0.20 μm film thickness DB-FFAP column with helium as the carrier gas and nitrogen as the make-up gas (Agilent Technologies). Samples were introduced by a Varian CP-8400 autosampler (Scion Instruments) in the injector heated to 250°C at a split ratio of 30:1. The initial temperature was 50°C with a 1 min hold followed by a 30°C/min ramp to 130°C, a 10°C/min ramp to 175°C, a 5°C ramp to 230°C, and a 9.5 min hold and a 50°C/min ramp to 240°C with an 11.1 min hold for a total run time of 40 min. The FID temperature was 300°C with air and helium makeup gas flow rates of 300 and 25 ml/min, respectively, and a sampling frequency of 40 Hz. Identification of peaks was performed by retention time comparison to external FAME reference standard mixture (GLC-569; Nu-Chek Prep, Inc). Chromatograms were analyzed with CompassCDS (version 3.0.0.68; Scion Instruments). FAMEs were quantified by comparing the concentration of each fatty acid to the known amount of 17:0 internal standard present in all the samples. After GC-FID analysis, vials were recapped and stored at −20°C until analysis by GC-isotope ratio MS (GC-IRMS) in 2022 and 2023.
Isotopic analysis
The plasma and dietary oil δ13C of FAMEs were determined by GC-IRMS. FAMEs were separated using Trace 1310 Gas Chromatograph (Thermo Fisher Scientific) equipped with TriPlus RSH autosampler, as previously described (24). The GC was equipped with a fused silica capillary column (100 mm × 0.25 mm × 0.2 μm film thickness; Supelco, Sigma-Aldrich; catalog no.: 24056). Complete fatty acid separation was achieved with the following program: initial temperature of 60°C with an immediate ramp of 15°C/min to 180°C with no hold, followed by a 1.5°C/min ramp to 240°C with an 18-min hold for a total run time of 66 min. The carrier flow rate was set to 1.2 ml/min. Isolated fatty acids were combusted by GC-Isolink II held at 1,000°C and interfaced to MAT 253 IRMS (Thermo Fisher Scientific) via a continuous-flow ConFlo IV (Thermo Fisher Scientific). Carbon dioxide was dried via flowing gas through a Nafion dryer before entering the MAT 253 IRMS ion source for isotope detection. Peaks for FAMEs were identified by retention time relative to injections of reference material (GLC-462; Nu-Check Prep, Inc) and integrated with peak detection parameters of start and stop slopes of 0.2 mV/s and 1.4 mV/s, respectively, with individual background correction.
Isotopic normalization
δ13C of plasma PUFA and dietary oils collected by GC-IRMS were normalized and converted to the international carbon isotope reference scale, Vienna Peedee Belemnite, through multipoint linear normalization and reported in mUr, as described previously (1, 8, 9). Certified calibrated 20-carbon FAME reference materials (USGS70, USGS71, USGS72; Reston Stable Isotope Laboratory, US Geological Survey) were injected periodically during the sequences. Linear regression of measured values compared with true values (−30.53 ± 0.04, −10.50 ± 0.03, and −1.54 ± 0.03 mUr for USGS70, USGS71, and USGS72, respectively) was used to generate the normalizing equation to report δ13C values for all data. R2 values for all normalizing equations were >0.9997.
Methyl correction
To account for the contribution of carbon from the derivatized methyl group, a correction calculation was performed by elemental analyzer as previously described (1, 9). For the correction, we compared the isotopic signatures of heptadecanoic acid (17:0, Nu-Chek Prep, Inc) and heptadecanoic acid methyl esters prepared using the same stock solution of 14% boron trifluoride in methanol.
Data analyses
All statistical analyses were performed with GraphPad Prism (version 9.3.1; GraphPad Software, Inc). Differences in plasma fatty acid concentration, relative percentage, and δ13C signature between baseline, 4-week, 10-week, and 16-week time points for each dietary intervention group for total lipids, PL, CE, TG, and FFA were determined by one-way repeated-measures ANOVA. Significant results were followed up with Fisher’s least significant difference test to compare differences from each time point (4 weeks, 10 weeks, and 16 weeks) to baseline. Carbon isotope ratio correlations between lipid fractions and total lipids and carbon isotope ratio correlations between specific fatty acids were determined by Pearson correlations. The significance for all statistical analyses was determined at P < 0.05. All data are presented as mean ± SEM. Additional analyses for differences in plasma fatty acid concentration and δ13C signature adjusted for participant baseline values are provided in supplemental Figs. S1–S5.
Results
Participants
Final analyses for plasma fatty acid concentrations and δ13C included randomly selected participants from the 141 who completed the full study protocol: ten participants in the H6 dietary group, ten in the H3H6 dietary group, and ten in the H3 dietary group. No between-group differences in adverse effects were observed during the intervention. Baseline participant characteristics for each dietary group, including age (y), body mass index (kg/m2), sex, race, relationship status, education, and annual household income are reported in supplemental Table S2.
Fatty acid composition and δ13C of dietary oils
The fatty acid compositions and δ13C of selected fatty acids of each dietary oil are reported in Table 1. The 75% corn/25% olive oil blend (high-LNA oil blend) contained 44.6% ± 0.06% of its total fatty acids as LNA, with a δ13C-LNA signature of −16.1 ± 0.04 (mUr ± SEM), whereas the 75% macadamia/25% olive oil blend (low-LNA oil blend) contained 4.46% ± 0.26% of its total fatty acids as LNA, with a δ13C-LNA signature of −28.3 ± 0.69.
Table 1.
Fatty acid composition and δ13C of selected fatty acids contained in dietary oils
| Fatty acid | Dietary oil |
||||
|---|---|---|---|---|---|
| 75% Macadamia/25% olive | 75% Corn/25% olive | Macadamia | Corn | Olive | |
| 16:0 | 9.21 ± 0.02 | 11.6 ± 0.02 | 8.60 ± 0.03 | 11.5 ± 0.06 | 12.2 ± 0.04 |
| 18:0 | 2.97 ± 0.004 | 1.79 ± 0.003 | 3.11 ± 0.004 | 1.36 ± 0.007 | 2.35 ± 0.005 |
| 20:0 | 1.71 ± 0.004 | 0.35 ± 0.0006 | 2.09 ± 0.02 | 0.28 ± 0.005 | 0.35 ± 0.0008 |
| 22:0 | 0.43 ± 0.002 | 0.10 ± 0.0005 | 0.52 ± 0.006 | 0.08 ± 0.002 | 0.09 ± 0.0008 |
| Saturated fatty acids | 15.0 ± 0.02 | 14.0 ± 0.02 | 15.2 ± 0.003 | 13.3 ± 0.05 | 15.1 ± 0.04 |
| 16:1n-7 | 14.9 ± 0.14 | 0.34 ± 0.0009 | 19.7 ± 0.05 | 0.19 ± 0.04 | 0.85 ± 0.01 |
| 18:1n-7 | 2.90 ± 0.02 | 0.90 ± 0.006 | 3.32 ± 0.03 | 0.49 ± 0.01 | 1.52 ± 0.03 |
| 18:1n-9 | 59.5 ± 0.11 | 38.4 ± 0.01 | 55.4 ± 0.10 | 26.0 ± 0.05 | 73.1 ± 0.34 |
| 20:1n-9 | 1.78 ± 0.006 | 0.32 ± 0.0003 | 2.18 ± 0.02 | 0.25 ± 0.006 | 0.33 ± 0.002 |
| 22:1n-9 | 0.15 ± 0.0006 | 0.04 ± 0.002 | 0.19 ± 0.002 | 0.04 ± 0.0005 | 0.006 ± 0.0003 |
| MUFAs | 79.3 ± 0.21 | 40.1 ± 0.008 | 80.9 ± 0.04 | 27.0 ± 0.10 | 76.0 ± 0.35 |
| 18:2n-6 | 4.46 ± 0.26 | 44.6 ± 0.06 | 2.57 ± 0.04 | 57.9 ± 0.12 | 7.71 ± 0.39 |
| 20:2n-6 | 0.003 ± 0.0001 | 0.02 ± 0.0003 | 0.01 ± 0.0007 | 0.02 ± 0.0002 | 0.003 ± 0.0004 |
| n-6 PUFA | 4.46 ± 0.26 | 44.6 ± 0.06 | 2.57 ± 0.03 | 57.9 ± 0.11 | 7.71 ± 0.39 |
| 18:3n-3 | 0.35 ± 0.003 | 0.83 ± 0.002 | 0.23 ± 0.004 | 0.95 ± 0.002 | 0.75 ± 0.002 |
| 20:3n-3 | 0.005 ± 0.0003 | 0.008 ± 0.0009 | 0.004 ± 0.0004 | 0.002 ± 0.00004 | 0.002 ± 0.0004 |
| n-3 PUFA | 0.35 ± 0.003 | 0.84 ± 0.001 | 0.23 ± 0.004 | 0.95 ± 0.002 | 0.75 ± 0.002 |
| δ13C-18:2n-6 | −28.3 ± 0.69 | −16.1 ± 0.04 | −29.6 ± 0.03 | −15.6 ± 0.08 | −28.5 ± 0.70 |
| δ13C-18:3n-3 | −29.8 ± 0.02 | −20.0 ± 0.67 | −29.9 ± 0.03 | −16.2 ± 0.13 | −30.0 ± 0.25 |
Data are means ± SEM, n = 3. Fatty acid composition expressed as the relative percentage of fatty acid in total fatty acids.
Plasma LNA and ARA concentrations and δ13C following intervention
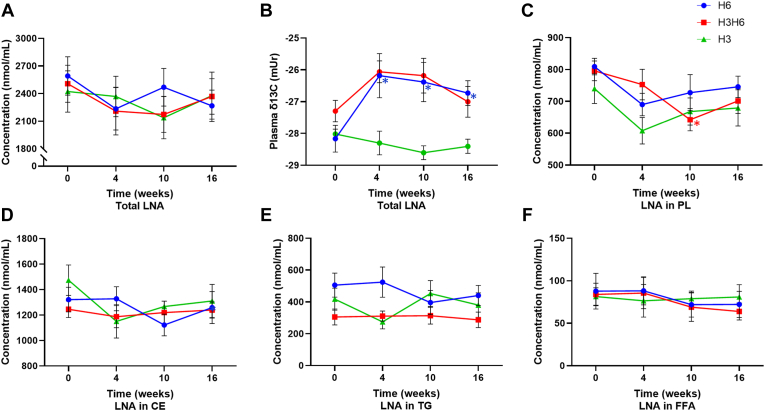
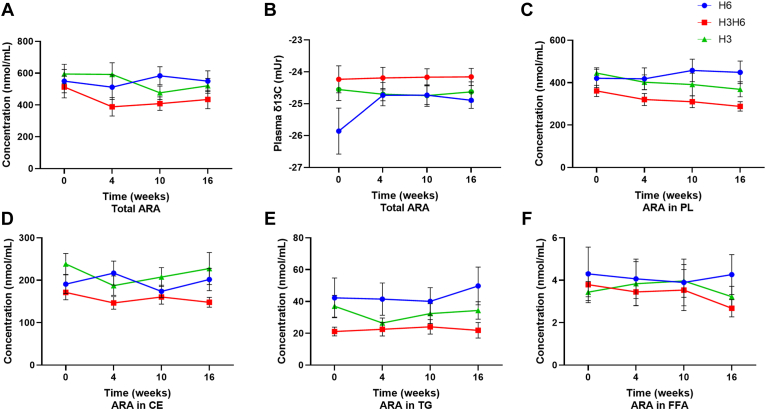
H6 dietary intervention did not affect the plasma concentration of LNA for the total, PL, CE, TG, and FFA lipid pools (Fig. 1A, C, D, E, and F). The H6 intervention increased plasma total lipid δ13C of LNA at 4, 10, and 16 weeks from −28.2 ± 0.42 (mUr ± SEM) to −26.2 ± 0.73 at 4 weeks, −26.4 ± 0.65 at 10 weeks, and −26.7 ± 0.36 at 16 weeks (Fig. 1B, P < 0.05). Plasma PL δ13C of LNA increased at 4 weeks (P < 0.05), though there was no effect at 10 or 16 weeks or at any time point in the CE, TG, and FFA lipid fractions (supplemental Fig. S6). There was no effect on the plasma concentration of ARA for total lipids and all other lipid fractions (Fig. 2A, C, D, E, and F). Furthermore, H6 intervention did not affect plasma total lipid δ13C of ARA (−25.9 ± 0.72 at baseline to −24.7 ± 0.17 at 4 weeks, −24.7 ± 0.29 at 10 weeks, and −24.9 ± 0.25 at 16 weeks) (Fig. 2B) or plasma PL, CE, TG, and FFA δ13C of ARA (supplemental Fig. S7).
Fig. 1.
Changes in plasma LNA (A) concentration of total lipids, (B) total lipid δ13C content, (C) concentration of PLs, (D) concentration of CEs, (E) concentration of TGs, and (F) concentration of FFAs from baseline (week 0) to 4, 10, and 16 weeks for low n-3, high n-6 (H6; blue), high n-3, high n-6 (H3H6; red), and high n-3, low n-6 (H3; green) dietary groups. ∗ represents a significant effect of diet at the respective time point compared with baseline, as determined by one-way repeated-measures ANOVA with Fisher’s least significant difference post hoc test, P < 0.05, n = 10, means ± SEM.
Fig. 2.
Changes in plasma ARA (A) concentration of total lipids, (B) total lipid δ13C content, (C) concentration of PLs, (D) concentration of CEs, (E) concentration of TGs, and (F) concentration of FFAs from baseline (week 0) to 4, 10, and 16 weeks for low n-3, high n-6 (H6; blue), high n-3, high n-6 (H3H6; red), and high n-3, low n-6 (H3; green) dietary groups. Statistically significant effects were determined by one-way repeated-measures ANOVA, P > 0.05, n = 10, means ± SEM.
H3H6 dietary intervention revealed no effect on the plasma concentration of LNA for total, CE, TG, and FFA pools (Fig. 1A, D, E, and F), though there was a decrease of 19.3% in LNA concentration at 10 weeks in the PL fraction (Fig. 1C, P < 0.05). There was no H3H6 effect on plasma total lipid δ13C of LNA (−27.3 ± 0.42 at baseline to −26.1 ± 0.34 at 4 weeks, −26.2 ± 0.54 at 10 weeks, and −27.0 ± 0.48 at 16 weeks) (Fig. 1B) or plasma PL, CE, TG, and FFA δ13C of LNA (supplemental Fig. S6). The H3H6 intervention did not affect the plasma concentration of ARA for total lipids and all other lipid fractions as well as the plasma δ13C-ARA (Fig. 2 and supplemental Fig. S7).
H3 dietary intervention yielded no effect on plasma total lipid δ13C of LNA and concentration of LNA for the total, PL, CE, TG, and FFA pools (Fig. 1 and supplemental Fig. S6). Similarly, the H3 dietary intervention yielded no effect on the plasma concentration of ARA for the total, PL, CE, TG, and FFA pools as well as plasma total lipid δ13C of ARA (Fig. 2 and supplemental Fig. S7). All dietary interventions did not affect plasma δ13C of gamma-linolenic acid (18:3n-6) and dihomo-γ-linolenic acid (20:3n-6) (supplemental Fig. S8). Data were similar when comparing the plasma δ13C of n-6 PUFAs in the H6 and H3H6 dietary interventions compared with the H3 dietary group (data not shown).
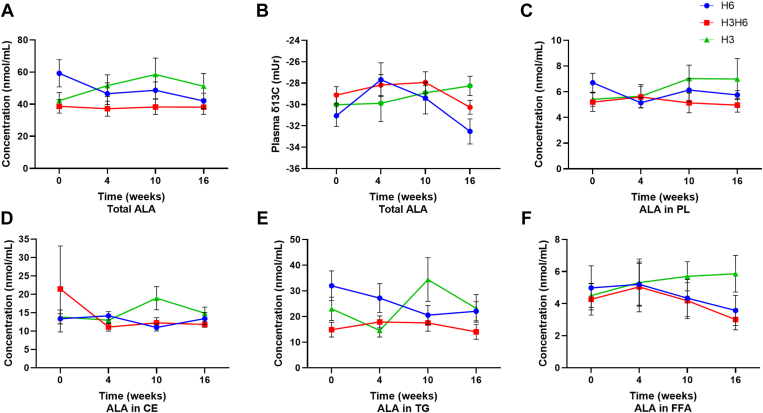
Plasma EPA, DHA, and ALA concentrations and δ13C following intervention
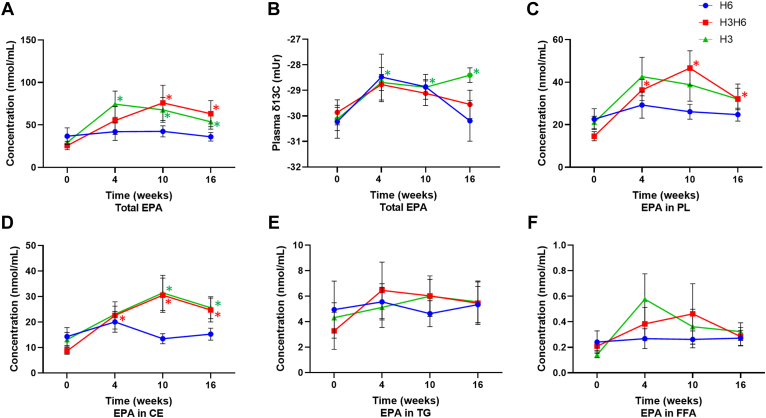
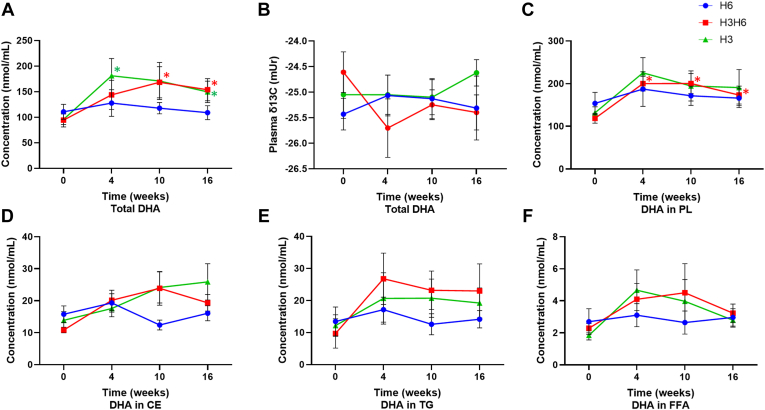
H3 dietary intervention revealed an increase in plasma total lipid concentration of EPA at 4, 10, and 16 weeks, by 154%, 131%, and 83.2%, respectively (Fig. 3A, P < 0.05). There was an increase in the plasma concentration of EPA for the CE lipid fraction at 10 and 16 weeks (Fig. 3D, P < 0.05), though there was no effect for the PL, TG, or FFA fractions (Fig. 3C, E, and F). The H3 intervention demonstrated an increase in the plasma total lipid δ13C of EPA at all time points from −30.1 ± 0.46 (mUr ± SEM) at baseline to −28.7 ± 0.31 at 4 weeks, −28.9 ± 0.29 at 10 weeks, and −28.4 ± 0.29 at 16 weeks (Fig. 3B, P < 0.05), while there was no effect in the PL and CE lipid fractions (supplemental Fig. S9). H3 dietary intervention increased the plasma DHA concentration in the total lipid pool at 4 and 16 weeks, by 89.0% and 56.1%, respectively (Fig. 4A, P < 0.05). However, there was no effect on plasma DHA concentration in the PL, CE, TG, and FFA fractions (Fig. 4C–F). There was no effect on the δ13C of DHA (Fig. 4B and supplemental Fig. S10).
Fig. 3.
Changes in plasma EPA (A) concentration of total lipids, (B) total lipid δ13C content, (C) concentration of PLs, (D) concentration of CEs, (E) concentration of TGs, and (F) concentration of FFAs from baseline (week 0) to 4, 10, and 16 weeks for low n-3, high n-6 (H6; blue), high n-3, high n-6 (H3H6; red), and high n-3, low n-6 (H3; green) dietary groups. ∗ represents a significant effect of diet at the respective time point compared with baseline, as determined by one-way repeated-measures ANOVA with Fisher’s least significant difference post hoc test, P < 0.05, n = 10, means ± SEM.
Fig. 4.
Changes in plasma DHA (A) concentration of total lipids, (B) total lipid δ13C content, (C) concentration of PLs, (D) concentration of CEs, (E) concentration of TGs, and (F) concentration of FFAs from baseline (week 0) to 4, 10, and 16 weeks for low n-3, high n-6 (H6; blue), high n-3, high n-6 (H3H6; red), and high n-3, low n-6 (H3; green) dietary groups. ∗ represents a significant effect of diet at the respective time point compared with baseline, as determined by one-way repeated-measures ANOVA with Fisher’s least significant difference post hoc test, P < 0.05, n = 10, means ± SEM.
The H3H6 intervention significantly increased plasma total lipid concentration of EPA at 10 and 16 weeks, by 196% and 146%, respectively (Fig. 3A, P < 0.05). Similarly, there was an increase in the plasma concentration of EPA in the PL and CE lipid fractions at all time points compared with baseline (Fig. 3C, D, P < 0.05). However, there was no effect on the plasma concentration of EPA in the TG and FFA lipid fractions (Fig. 3E, F). There was no effect on the plasma total lipid δ13C of EPA (Fig. 3B), though there was an increase in the plasma PL δ13C-EPA at 4 and 16 weeks (supplemental Fig. S9, P < 0.05). H3H6 intervention showed an increase in the plasma total lipid concentration of DHA at 10 and 16 weeks, by 78.2% and 62.9%, respectively (Fig. 4A, P < 0.05). In addition, the PL pool demonstrated an increase (P < 0.05) in plasma DHA at 4, 10, and 16 weeks compared with baseline (Fig. 4C, P < 0.05), though there was no effect on plasma DHA in the CE, TG, and FFA fractions (Fig. 4D–F). There was no effect on the plasma δ13C of DHA (Fig. 4B and supplemental Fig. S10).
H6 dietary intervention did not affect plasma δ13C of EPA and DHA and did not affect EPA and DHA concentration for the total lipid, PL, CE, TG, and FFA pools (Figs. 3 and 4; supplemental Figs. S8 and S9). All dietary interventions did not affect plasma concentration of ALA for the total, PL, CE, TG, and FFA pools (Fig. 5A, C, D, E, and F) and there was no effect of dietary intervention on the plasma δ13C-ALA (Fig. 5B and supplemental Fig. S11). Data were similar when comparing the plasma δ13C of n-3 PUFAs in the H6 and H3H6 dietary interventions compared with the H3 dietary group (data not shown).
Fig. 5.
Changes in plasma ALA (A) concentration of total lipids, (B) total lipid δ13C content, (C) concentration of PLs, (D) concentration of CEs, (E) concentration of TGs, and (F) concentration of FFAs from baseline (week 0) to 4, 10, and 16 weeks for low n-3, high n-6 (H6; blue), high n-3, high n-6 (H3H6; red), and high n-3, low n-6 (H3; green) dietary groups. Statistically significant effects were determined by one-way repeated-measures ANOVA, P > 0.05, n = 10, means ± SEM.
Plasma fatty acid concentration of other fatty acids
No dietary intervention affected plasma concentrations or relative percentage of 14:0, 16:0, 18:0, 20:0, 24:0, 16:1n-7, 16:1n-9, 18:1n-7, 18:1n-9, 20:1n-9, 22:1n-9, 24:1n-9, 18:3n-6, 20:2n-6, 20:3n-6, 22:4n-6, 20:3n-3, 22:5-3, total saturated fatty acid, total MUFA, total n-6 PUFA, total PUFA, or total fatty acids (supplemental Tables S3 and S4). The H3H6 dietary intervention yielded decreases in the plasma concentration of 22:0 and percentage of 22:5n-6 at 4 and 10 weeks compared with baseline (supplemental Tables S3 and S4, P < 0.05). Furthermore, H3H6 dietary intervention increased the plasma concentration of total n-3 PUFA at 10 and 16 weeks (supplemental Table S3, P < 0.05) and increased the relative percentage of total n-3 PUFA at 4, 10, and 16 weeks compared with baseline (supplemental Table S4, P < 0.05).
The H3 dietary intervention decreased the plasma concentration of 22:0 at 4 weeks (supplemental Table S3, P < 0.05) and decreased the relative percentage of 22:5n-6 at 4 weeks (supplemental Table S4, P < 0.05). There was an increase in the relative percentage of total n-3 PUFA at 4, 10, and 16 weeks (supplemental Table S4, P < 0.05). However, no effect was observed on the plasma concentration of total n-3 PUFA.
Comparison of plasma δ13C in total lipids with plasma δ13C of lipid fractions
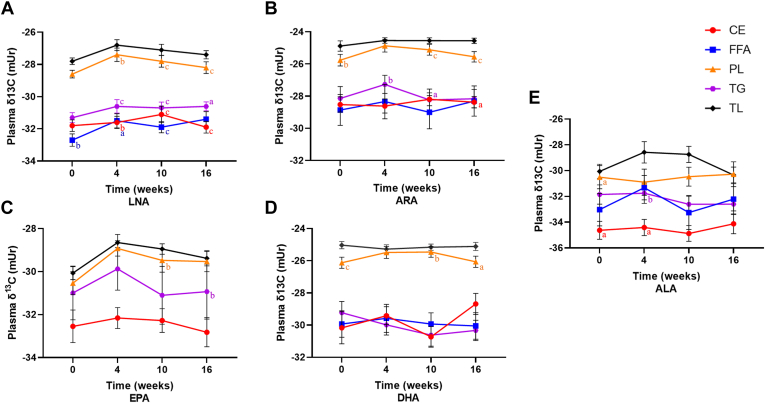
The PL lipid fraction was correlated with total lipids, particularly in δ13C-LNA at week 4 (r = 0.51, P < 0.01), week 10 (r = 0.85, P < 0.001), and week 16 (r = 0.57, P < 0.001) (Fig. 6A), in δ13C-ARA at week 0 (r = 0.49, P < 0.01), week 10 (r = 0.65, P < 0.001), and week 16 (r = 0.57, P < 0.001) (Fig. 6B), and δ13C-DHA at week 0 (r = 0.60, P < 0.001), week 10 (r = 0.56, P < 0.01), and week 16 (r = 0.40, P < 0.05) (Fig. 6E). The δ13C-LNA of the CE lipid fraction was positively correlated with total lipids at week 4 (r = 0.52, P < 0.01), week 10 (r = 0.68, P < 0.001), and week 16 (r = 0.65, P < 0.001). There was a positive correlation between the δ13C-LNA of the TG lipid fraction and total lipids at week 4 (r = 0.68, P < 0.001), week 10 (r = 0.74, P < 0.001), and week 16 (r = 0.37, P < 0.05), as well as between the FFA lipid fraction and total lipids at week 0 (r = 0.52, P < 0.01), week 4 (r = 0.42, P < 0.05), and week 10 (r = 0.68, P < 0.001). Pearson correlation coefficients measured between plasma δ13C of LNA, ARA, EPA, DHA, and ALA in total lipids versus PL, CE, TG, and FFA lipid fractions at weeks 0, 4, 10, and 16 for the combination of all dietary interventions are available in supplemental Tables S5–S9.
Fig. 6.
Comparison of the δ13C of (A) LNA, (B) ARA, (C) EPA, (D) DHA, (E) ALA between total lipids (TLs; black), PLs (orange), CEs (red), TGs (purple), and FFAs (blue) at weeks 0, 4, 10, and 16 for the combination of all dietary interventions. Superscripts represent a significant correlation between the respective lipid fraction and total lipids at the indicated time point as determined by Pearson correlation, aP < 0.05, bP < 0.01, cP < 0.001, n = 5–30, means ± SEM.
Correlation of plasma δ13C of LNA with plasma δ13C of ARA
Pearson correlation coefficients between plasma δ13C-LNA and plasma δ13C-ARA at weeks 0, 4, 10, and 16 are presented in Table 2. In the H6 dietary intervention, δ13C-LNA was positively correlated with δ13C-ARA at week 0 (r = 0.36, P < 0.05), week 4 (r = 0.70, P < 0.001), week 10 (r = 0.56, P < 0.001), and week 16 (r = 0.51, P < 0.001). δ13C-LNA in the H3H6 dietary intervention was positively correlated with δ13C-ARA at week 4 (r = 0.68, P < 0.001), week 10 (r = 0.47, P < 0.01), and week 16 (r = 0.38, P < 0.05). In the H3 dietary intervention, δ13C-LNA was positively correlated with δ13C-ARA at week 0 (r = 0.71, P < 0.001), week 4 (r = 0.65, P < 0.001), week 10 (r = 0.55, P < 0.001), and week 16 (r = 0.46, P < 0.01).
Table 2.
Correlation coefficients measured between plasma δ13C-ARA versus δ13C-LNA of PL, CE, TG, and FFA lipid fractions at week 0, 4, 10, and 16
| ARA | LNA |
|||
|---|---|---|---|---|
| Week 0 | Week 4 | Week 10 | Week 16 | |
| H6 | ||||
| Week 0 | 0.36∗ | |||
| Week 4 | 0.70∗∗∗ | |||
| Week 10 | 0.56∗∗∗ | |||
| Week 16 | 0.51∗∗∗ | |||
| H3H6 | ||||
| Week 0 | 0.27 | |||
| Week 4 | 0.68∗∗∗ | |||
| Week 10 | 0.47∗∗ | |||
| Week 16 | 0.38∗ | |||
| H3 | ||||
| Week 0 | 0.71∗∗∗ | |||
| Week 4 | 0.65∗∗∗ | |||
| Week 10 | 0.55∗∗∗ | |||
| Week 16 | 0.46∗∗ | |||
Asterisks represent statistically significant correlations determined by Pearson correlation, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, df = 38.
Discussion
In this study, participants were assigned to the H6, H3H6, or H3 dietary intervention for 16 weeks. Plasma was collected at baseline, 4, 10, and 16 weeks to assess participant plasma δ13C content and concentration of n-6 and n-3 PUFAs. The H6 and H3H6 dietary groups consumed a high-LNA oil blend with naturally δ13C-enriched LNA (−16.1 ± 0.13 mUr) and a butter blend containing corn oil (−15.6 ± 0.28 mUr), which was higher than the δ13C of the participant’s plasma fatty acids at baseline. Meanwhile, the H3H6 and H3 dietary groups consumed fish that tend to have an intermediate δ13C signature (−16 to −23 mUr) higher than the δ13C of the participant’s plasma at baseline. We found that plasma δ13C-LNA increased toward the δ13C-LNA of dietary oils used in the H6 group in total lipids and PL, whereas plasma δ13C-EPA increased toward the expected δ13C-EPA of the dietary source consumed in the total lipids of the H3 group and PLs of the H3H6 group, suggesting turnover and replenishment from the dietary source.
Plasma δ13C-LNA increased toward the δ13C-LNA of dietary oils used in the H6 group, demonstrating metabolism and maintenance of plasma LNA with dietary LNA for the first time in adult humans without concurrent changes in LNA concentration. LNA consumption in the diet is relatively high, up to 10% of daily energy intake (13, 25), and plasma concentrations may already be at a plateau. In rats, this plateau has been demonstrated to start around 8% of daily energy intake as LNA (26). As the high n-6 PUFA groups in this study received a target daily energy intake of 7.0% from LNA, baseline concentrations of plasma LNA may have already reached saturation, making it unlikely to observe increases in LNA concentration following dietary intake. Despite the lack of change in total plasma concentrations of LNA, plasma δ13C-LNA increased toward the δ13C-LNA of the dietary oils used in the H6 group by 4 weeks, providing evidence for complete turnover of LNA and maintenance of total and PL plasma LNA concentrations from dietary intake.
The increase in plasma δ13C-EPA in the total lipids of the H3 group and PL of the H3H6 group toward the expected δ13C-EPA of the dietary source is consistent with a previous study showing an increase in δ13C-EPA toward δ13C-EPA of an EPA supplement with a δ13C signature of −23.5 mUr (10). It is important to note that we were unable to directly measure the dietary δ13C-EPA signature; however, the fatty fish consumed in this study, including tuna, salmon, and sardines, tend to yield more intermediate δ13C signatures of around −16 to −23 mUr (5). Hence, dietary δ13C-EPA is sufficiently different from participant plasma δ13C at baseline (total lipids: −30.1 ± 0.46; PL: −31.6 ± 1.0 mUr), and changes in δ13C-EPA were observed. Like LNA, δ13-EPA increased toward the expected δ13C-EPA of the dietary source used in the H3 and H3H6 groups by 4 weeks, suggesting complete EPA turnover. However, one limitation of our study is that the 4-week time point is the first time point measuring concentration and δ13C signatures following dietary intervention, and it appears that δ13C-EPA, as well as δ13C-LNA, have already plateaued by 4 weeks. It is unclear what might be occurring with metabolism and concentration before this time point, thus, future research could benefit from additional sampling at earlier time points.
The increase in plasma δ13C-LNA and δ13C-EPA toward the δ13C-LNA and δ13C-EPA of the dietary source supports the utility of δ13C to determine the dietary origin of fatty acids from the food supply and their metabolism. Natural variations in δ13C content of the food supply have previously been used to assess n-6 PUFA metabolism in infants (6, 7), particularly in one study showing that a formula-based diet with high δ13C-LNA (−16.4 mUr) corn oil could increase serum δ13C-LNA from −31.5 to −18.8 mUr and serum δ13C-ARA from −30.1 to −27.4 mUr after 4 days (7). In addition, previous studies in our laboratory have used δ13C to analyze the dietary origin and metabolism of n-3 PUFA (1, 8, 9, 10). EPA and DHA supplementation have been used in humans to show substantial conversion of EPA to DHA (10). These studies, including the present study, demonstrate the utility of CSIA to assess fatty acid metabolism and the dietary origin of plasma fatty acids in humans.
While changes in δ13C-LNA and δ13C-EPA were observed in PL, these changes were not observed in the CE, TG, and FFA lipid fractions. PL is generally the largest contributor to plasma total lipids; hence, they are a major pool for dietary PUFAs (27). In our study, δ13C of total lipids were most closely correlated with δ13C of PL, so it appears that changes in total lipid δ13C were driven by changes in δ13C of PL. In addition, there were technical limitations in the detection of fatty acid peaks with GC-IRMS, especially, but not limited to the FFA fraction. FFAs compromise a very small proportion of total lipids compared with PL and CE (28), so some peaks were below the minimal threshold for quantification by GC-IRMS. As a result, some samples were unable to be quantified, and the δ13C-EPA for FFA was unable to be reported. Because of these technical limitations and the sample size limitations of a secondary analysis, this study was underpowered to observe changes in δ13C in some fatty acids, particularly in the FFA fraction.
Multiple studies show that increasing LNA intake results in no effect on peripheral ARA concentrations in humans (17, 29, 30, 31, 32, 33, 34, 35). Decreasing LNA intake from as high as 20% of daily energy to as low as 0% has also not affected ARA concentrations in plasma or red blood cells (36, 37, 38, 39, 40). Our current study supports these findings as plasma ARA concentrations did not change throughout the intervention in any dietary group, including the H3 group with an LNA intake lowered to 3.2% of daily energy at 16 weeks of intervention (20). Furthermore, the δ13C of total plasma ARA did not change over time in dietary groups given δ13C-enriched LNA in dietary oils. This result is in contrast with previous studies analyzing serum δ13C-ARA, where the serum δ13C-AA shifted from baseline concentrations toward the resemblance of dietary δ13C (7, 41). However, the lack of change in δ13C-ARA in our study may be explained by the higher baseline δ13C-ARA in the H6 and H3H6 dietary groups (total lipids: −25.9 ± 0.72, −24.2 ± 0.42 mUr) who consumed δ13C-enriched LNA compared with baseline δ13C-LNA (total lipids: −28.2 ± 0.42, −27.3 ± 0.42 mUr). Plasma δ13C-ARA was already enriched at baseline, so it would be difficult to observe further increases throughout the intervention. δ13C-LNA was correlated with δ13C-ARA except at baseline in the H3H6 dietary intervention, which provides further evidence that changes in δ13C-ARA were unable to be observed because of its enrichment at baseline. In addition, we did not observe changes in plasma δ13C-DHA over time in high n-3 PUFA diets. Similarly, δ13C-DHA was more enriched at baseline in the H3H6 and H3 groups (total lipids: −24.6 ± 0.40, −25.0 ± 0.46 mUr) compared with baseline δ13C-EPA (total lipids: −29.9 ± 0.49, −30.1 ± 0.46 mUr) and may not be sufficiently different from the dietary source of DHA (−16 to −23 mUr). These results do not mean that dietary LNA and EPA were not being converted into ARA and DHA, respectively, or that the dietary DHA was not being incorporated into plasma but indicates an important limitation of our study. Being a secondary analysis of a randomized controlled trial, the δ13C of the dietary intervention was not originally designed to contain appreciably different δ13C content of any fatty acid compared with endogenous human plasma. In addition, it is unclear how much of the dietary oils or food the participants consumed, as there were other components of the participant’s diets that contributed to the dietary intervention. Future studies need to be designed a priori to contain δ13C of fatty acids that are adequately different from human plasma δ13C concentrations and the general food supply.
Although we could not observe changes in δ13C content of DHA in the H3H6 and H3 groups because of limitations of the secondary analysis, dietary interventions containing high n-3 PUFA intake (as EPA + DHA) demonstrated increases in plasma EPA and DHA concentrations. This result is consistent with published studies providing n-3 PUFA supplementation (10, 23, 42, 43) and confirms patient compliance and efficacy of the dietary intervention. It is important to note that 93% of the participants in this secondary analysis were female. Previous single oral dosing studies of 2H5-ALA in humans have demonstrated that more of this label is detected in downstream n-3 in PUFAs in females as compared to males (44, 45, 46). However, because of the nature of these studies, it is unclear if these differences are due to differences in rates or metabolic consumption of the 2H5-ALA. Nonetheless, there may be potential differences in synthesis rates between sexes, and a future study could address differences in synthesis rates.
Since the present study is a secondary analysis of a clinical trial conducted between July 2014 and May 2018, plasma samples were stored long-term until lipid analysis. Fatty acid stability has been analyzed previously, demonstrating that fatty acids are stable at −80°C for at least 10 years and that PL, TG, CE, and FFA lipid fractions are stable at −20°C for a minimum of 1 year without nitrogen (47). The samples used in the present study were stored at −80°C until lipid extraction and at −20°C while awaiting analyses by GC-FID or GC-IRMS, which is generally within these time parameters. Therefore, storage should not have greatly affected fatty acid concentrations. In this study, we used heptadecanoic acid as the internal standard for fatty acid quantification. Heptadecanoic acid is naturally present in butter and dairy products that were included in the dietary interventions, albeit in small amounts, with an average of 0.4–0.6% of the fatty acid composition in cheese, milk, and various types of butter (48, 49, 50, 51). However, this amount is relatively small in comparison to the known concentration of heptadecanoic acid used as the internal standard, and all dietary interventions consumed butter and at least one other type of dairy product. Hence, the impact of naturally present heptadecanoic acid in dairy products is relatively small and affects all dietary groups similarly. In addition, the plasma fatty acid concentrations and relative percentages presented in this study are similar to other studies presenting these concentrations, which suggests a minimal impact of sample storage and the choice to use a heptadecanoic acid internal standard (52, 53).
While the present study used natural variations in the dietary 13C isotope composition of the food supply, natural variations in the dietary 2H isotope composition may also be useful for fatty acid analysis either as a standalone or a dual isotope model with 13C (54). Deuterium is fractioned similarly to 13C but within the water cycle through different processes such as condensation across different latitudes and evaporation (54). 2H isotope composition has been used to track migration using precipitation gradients across geographical regions (55), though this method is rapidly advancing to assess food sources and metabolism of PUFA (56). 2H values may have a larger isotopic separation between diet sources because of larger relative mass differences (57), and could be use to study PUFA metabolism in humans in a future study. The present study used human plasma to determine changes in concentration and δ13C over time. While plasma was a suitable choice for dietary fatty acid incorporation and an indicator of the fatty acid profile at the respective time point (28), red blood cells, which have slower turnover than plasma and generally indicate long-term dietary compliance (58), could also be analyzed in a future study.
In conclusion, this study assessed the changes in plasma concentrations and δ13C of PUFAs in adult humans following dietary intervention, specifically a H6, H3H6, and H3 diet. We illustrated that changes in dietary LNA and EPA intake can be detected in plasma total lipid and PL δ13C-LNA and δ13C-EPA. These findings suggest that 1) there is complete turnover of plasma LNA and EPA within 4 weeks and 2) determining δ13C is an effective technique to measure turnover if the isotopic signature of the dietary source does not match the baseline plasma δ13C signature. Despite the limitations and lack of changes observed in plasma δ13C-ARA and δ13C-DHA, we demonstrate that determining δ13C of PUFAs in humans has the potential to track dietary intake patterns and provide insight into n-3 and n-6 PUFA metabolism. Future studies utilizing CSIA should a priori confirm that the isotopic signature of any dietary fatty acid intervention differs from plasma levels.
Data availability
All datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental data
This article contains supplemental data.
Conflict of interest
R. P. B. has received industrial grants, including those matched by the Canadian government, and/or travel support related to work on brain fatty acid uptake from Arctic Nutrition, Bunge Ltd, DSM, Fonterra, Mead Johnson, and Nestle, Inc. Moreover, R. P. B. is on the executive of the International Society for the Study of Fatty Acids and Lipids and held a meeting on behalf of Fatty Acids and Cell Signaling, both of which rely on corporate sponsorship. R. P. B. has given expert testimony in relation to supplements and the brain and holds the Canada Research Chair in Brain Lipid Metabolism. None of the other authors report a conflict of interest related to research presented in this article.
Acknowledgments
The authors thank Brinley Klievik of the Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Canada, for the insight into fatty acid turnover. This project was supported by the National Center for Complementary and Integrative Health (grant no.: 1R01AT007813-01A1) and the National Institutes of Health (NIH) (main funding source). Additional support was provided by the Intramural Programs of the National Institute on Aging and the National Institute on Alcohol Abuse and Alcoholism; the National Center for Complementary and Integrative Health T32 Research Fellowship Program (award #T32 AT003378); the Mayday Fund; and the University of North Carolina Nutrition Obesity Research Center, CHAI Core (National Institute of Diabetes and Digestive and Kidney Diseases, NIH (grant no.: DK056350). The authors acknowledge the database support of the NC Translational and Clinical Sciences Institute, which is supported by the National Center for Advancing Translational Sciences, NIH (grant no.: UL1TR002489). R. P. B. holds the Canada Research Chair in Brain Lipid Metabolism and received funds from the Canadian Institutes of Health Research.
Author contributions
D. K. C., A. H. M., C. E. R., K. R. F., B. M., D. Z., and R. P. B. conceptualization; D. K. C., A. H. M., C. E. R., M. H., K. R. F., B. M., D. Z., and R. P. B. methodology; D. K. C. validation; D. K. C. and C. P. formal analysis; D. K. C., A. H. M., K. R., and C. P. investigation; C. T. C., C. E. R., and R. P. B. resources; D. K. C. and M. H. data curation; D. K. C. writing–original draft; A. H. M., K. R., C. P., C. T. C., C. E. R., M. H., K. R. F., B. M., D. Z., and R. P. B. writing–review & editing; D. K. C. and M. H. visualization; A. H. M., C. T. C., K. R. F., and R. P. B. supervision; R. P. B. project administration; K. R. F., D. Z., and R. P. B. funding acquisition.
Funding and additional information
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplemental data
References
- 1.Lacombe R.J.S., Giuliano V., Colombo S.M., Arts M.T., Bazinet R.P. Compound specific isotope analysis resolves the dietary origin of docosahexaenoic acid (DHA) in the mouse brain. J. Lipid Res. 2017;58:2071–2081. doi: 10.1194/jlr.D077990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand W.A., Coplen T.B. Stable isotope deltas: tiny, yet robust signatures in nature. Isotopes Environ. Health Stud. 2012;48:393–409. doi: 10.1080/10256016.2012.666977. [DOI] [PubMed] [Google Scholar]
- 3.Brand W.A., Coplen T.B., Vogl J., Rosner M., Prohaska T. Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report) Pure Appl. Chem. 2014;86:425–467. [Google Scholar]
- 4.Smith B.N., Epstein S. Two categories of c/c ratios for higher plants. Plant Physiol. 1971;47:380–384. doi: 10.1104/pp.47.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura K., Schoeller D.A., Winkler F.J., Schmidt H.L. Geographical variations in the carbon isotope composition of the diet and hair in contemporary man. Biomed. Mass Spectrom. 1982;9:390–394. doi: 10.1002/bms.1200090906. [DOI] [PubMed] [Google Scholar]
- 6.Carnielli V.P., Simonato M., Verlato G., Luijendijk I., De Curtis M., Sauer P.J., et al. Synthesis of long-chain polyunsaturated fatty acids in preterm newborns fed formula with long-chain polyunsaturated fatty acids. Am. J. Clin. Nutr. 2007;86:1323–1330. doi: 10.1093/ajcn/86.5.1323. [DOI] [PubMed] [Google Scholar]
- 7.Demmelmair H., von Schenck U., Behrendt E., Sauerwald T., Koletzko B. Estimation of arachidonic acid synthesis in full term neonates using natural variation of 13C content. J. Pediatr. Gastroenterol. Nutr. 1995;21:31–36. doi: 10.1097/00005176-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano V., Lacombe R.J.S., Hopperton K.E., Bazinet R.P. Applying stable carbon isotopic analysis at the natural abundance level to determine the origin of docosahexaenoic acid in the brain of the fat-1 mouse. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863:1388–1398. doi: 10.1016/j.bbalip.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Metherel A.H., Chouinard-Watkins R., Trepanier M.O., Lacombe R.J.S., Bazinet R.P. Retroconversion is a minor contributor to increases in eicosapentaenoic acid following docosahexaenoic acid feeding as determined by compound specific isotope analysis in rat liver. Nutr. Metab. (Lond) 2017;14:75. doi: 10.1186/s12986-017-0230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metherel A.H., Irfan M., Klingel S.L., Mutch D.M., Bazinet R.P. Compound-specific isotope analysis reveals no retroconversion of DHA to EPA but substantial conversion of EPA to DHA following supplementation: a randomized control trial. Am. J. Clin. Nutr. 2019;110:823–831. doi: 10.1093/ajcn/nqz097. [DOI] [PubMed] [Google Scholar]
- 11.Blasbalg T.L., Hibbeln J.R., Ramsden C.E., Majchrzak S.F., Rawlings R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Innes J.K., Calder P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Fritsche K.L. Too much linoleic acid promotes inflammation-doesn’t it? Prostaglandins Leukot. Essent. Fatty Acids. 2008;79:173–175. doi: 10.1016/j.plefa.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L., Cherubini A., Bandinelli S., Bartali B., Corsi A., Lauretani F., et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 15.Johnson G.H., Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J. Acad. Nutr. Diet. 2012;112:1029–1041. doi: 10.1016/j.jand.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Pischon T., Hankinson S.E., Hotamisligil G.S., Rifai N., Willett W.C., Rimm E.B. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 17.Rett B.S., Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming western-type diets: a systematic review. Nutr. Metab. (Lond) 2011;8:36. doi: 10.1186/1743-7075-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsden C.E., Zamora D., Faurot K.R., MacIntosh B., Horowitz M., Keyes G.S., et al. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: randomized controlled trial. BMJ. 2021;374 doi: 10.1136/bmj.n1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann J.D., Faurot K.R., MacIntosh B., Palsson O.S., Suchindran C.M., Gaylord S.A., et al. A sixteen-week three-armed, randomized, controlled trial investigating clinical and biochemical effects of targeted alterations in dietary linoleic acid and n-3 EPA+DHA in adults with episodic migraine: study protocol. Prostaglandins Leukot. Essent. Fatty Acids. 2018;128:41–52. doi: 10.1016/j.plefa.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacIntosh B.A., Ramsden C.E., Honvoh G., Faurot K.R., Palsson O.S., Johnston A.D., et al. Methodology for altering omega-3 EPA+DHA and omega-6 linoleic acid as controlled variables in a dietary trial. Clin. Nutr. 2021;40:3859–3867. doi: 10.1016/j.clnu.2021.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 22.Chouinard-Watkins R., Calleja M., Bazinet R.P., Josse A.R. Dairy product consumption is associated with a lowering of linoleic acid within serum TAG in adolescent females with overweight or obesity: a secondary analysis. Br. J. Nutr. 2022;127:68–77. doi: 10.1017/S0007114521001677. [DOI] [PubMed] [Google Scholar]
- 23.Metherel A.H., Domenichiello A.F., Kitson A.P., Lin Y.H., Bazinet R.P. Serum n–3 tetracosapentaenoic acid and tetracosahexaenoic acid increase following higher dietary alpha-linolenic acid but not docosahexaenoic acid. Lipids. 2017;52:167–172. doi: 10.1007/s11745-016-4223-0. [DOI] [PubMed] [Google Scholar]
- 24.Lacombe R.J.S., Smith M.E., Perlman K., Turecki G., Mechawar N., Bazinet R.P. Quantitative and carbon isotope ratio analysis of fatty acids isolated from human brain hemispheres. J. Neurochem. 2022;164:44–56. doi: 10.1111/jnc.15702. [DOI] [PubMed] [Google Scholar]
- 25.Alashmali S.M., Hopperton K.E., Bazinet R.P. Lowering dietary n-6 polyunsaturated fatty acids: interaction with brain arachidonic and docosahexaenoic acids. Curr. Opin. Lipidol. 2016;27:54–66. doi: 10.1097/MOL.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 26.Høy C.E., Hølmer G. Dietary linoleic acid and the fatty acid profiles in rats fed partially hydrogenated marine oils. Lipids. 1988;23:973–980. doi: 10.1007/BF02536346. [DOI] [PubMed] [Google Scholar]
- 27.Risé P., Eligini S., Ghezzi S., Colli S., Galli C. Fatty acid composition of plasma, blood cells and whole blood: relevance for the assessment of the fatty acid status in humans. Prostaglandins Leukot. Essent. Fatty Acids. 2007;76:363–369. doi: 10.1016/j.plefa.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Furtado J.D., Beqari J., Campos H. Comparison of the utility of total plasma fatty acids versus those in cholesteryl ester, phospholipid, and triglyceride as biomarkers of fatty acid intake. Nutrients. 2019;11:2081. doi: 10.3390/nu11092081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson A., Nälsén C., Tengblad S., Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am. J. Clin. Nutr. 2002;76:1222–1229. doi: 10.1093/ajcn/76.6.1222. [DOI] [PubMed] [Google Scholar]
- 30.James M.J., Gibson R.A., D'Angelo M., Neumann M.A., Cleland L.G. Simple relationships exist between dietary linoleate and the n-6 fatty acids of human neutrophils and plasma. Am. J. Clin. Nutr. 1993;58:497–500. doi: 10.1093/ajcn/58.4.497. [DOI] [PubMed] [Google Scholar]
- 31.Johansson A.K., Korte H., Yang B., Stanley J.C., Kallio H.P. Sea buckthorn berry oil inhibits platelet aggregation. J. Nutr. Biochem. 2000;11:491–495. doi: 10.1016/s0955-2863(00)00105-4. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein A.H., Matthan N.R., Jalbert S.M., Resteghini N.A., Schaefer E.J., Ausman L.M. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am. J. Clin. Nutr. 2006;84:497–504. doi: 10.1093/ajcn/84.3.497. [DOI] [PubMed] [Google Scholar]
- 33.Thies F., Nebe-von-Caron G., Powell J.R., Yaqoob P., Newsholme E.A., Calder P.C. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am. J. Clin. Nutr. 2001;73:539–548. doi: 10.1093/ajcn/73.3.539. [DOI] [PubMed] [Google Scholar]
- 34.Vega-López S., Ausman L.M., Jalbert S.M., Erkkilä A.T., Lichtenstein A.H. Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am. J. Clin. Nutr. 2006;84:54–62. doi: 10.1093/ajcn/84.1.54. [DOI] [PubMed] [Google Scholar]
- 35.Wallace F.A., Miles E.A., Calder P.C. Comparison of the effects of linseed oil and different doses of fish oil on mononuclear cell function in healthy human subjects. Br. J. Nutr. 2003;89:679–689. doi: 10.1079/BJN1079/2002821. [DOI] [PubMed] [Google Scholar]
- 36.Angela Liou Y., Innis S.M. Dietary linoleic acid has no effect on arachidonic acid, but increases n-6 eicosadienoic acid, and lowers dihomo-gamma-linolenic and eicosapentaenoic acid in plasma of adult men. Prostaglandins Leukot. Essent. Fatty Acids. 2009;80:201–206. doi: 10.1016/j.plefa.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Liou Y.A., King D.J., Zibrik D., Innis S.M. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J. Nutr. 2007;137:945–952. doi: 10.1093/jn/137.4.945. [DOI] [PubMed] [Google Scholar]
- 38.Ramsden C.E., Faurot K.R., Zamora D., Suchindran C.M., MacIntosh B.A., Gaylord S., et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. 2013;154:2441–2451. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taha A.Y., Cheon Y., Faurot K.F., Macintosh B., Majchrzak-Hong S.F., Mann J.D., et al. Dietary omega-6 fatty acid lowering increases bioavailability of omega-3 polyunsaturated fatty acids in human plasma lipid pools. Prostaglandins Leukot. Essent. Fatty Acids. 2014;90:151–157. doi: 10.1016/j.plefa.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thijssen M.A., Mensink R.P. Small differences in the effects of stearic acid, oleic acid, and linoleic acid on the serum lipoprotein profile of humans. Am. J. Clin. Nutr. 2005;82:510–516. doi: 10.1093/ajcn.82.3.510. [DOI] [PubMed] [Google Scholar]
- 41.Rhee S.K., Reed R.G., Brenna J.T. Fatty acid carbon isotope ratios in humans on controlled diets. Lipids. 1997;32:1257–1263. doi: 10.1007/s11745-006-0161-6. [DOI] [PubMed] [Google Scholar]
- 42.Allaire J., Harris W.S., Vors C., Charest A., Marin J., Jackson K.H., et al. Supplementation with high-dose docosahexaenoic acid increases the Omega-3 Index more than high-dose eicosapentaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids. 2017;120:8–14. doi: 10.1016/j.plefa.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Schuchardt J.P., Ostermann A.I., Stork L., Kutzner L., Kohrs H., Greupner T., et al. Effects of docosahexaenoic acid supplementation on PUFA levels in red blood cells and plasma. Prostaglandins Leukot. Essent. Fatty Acids. 2016;115:12–23. doi: 10.1016/j.plefa.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Burdge G.C., Jones A.E., Wootton S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br. J. Nutr. 2002;88:355–363. doi: 10.1079/BJN2002662. [DOI] [PubMed] [Google Scholar]
- 45.Burdge G.C., Wootton S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002;88:411–420. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- 46.Pawlosky R., Hibbeln J., Lin Y., Salem N., Jr. n-3 fatty acid metabolism in women. Br. J. Nutr. 2003;90:993–995. doi: 10.1079/bjn2003985. [DOI] [PubMed] [Google Scholar]
- 47.Metherel A.H., Stark K.D. The stability of blood fatty acids during storage and potential mechanisms of degradation: a review. Prostaglandins Leukot. Essent. Fatty Acids. 2016;104:33–43. doi: 10.1016/j.plefa.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Månsson H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008 doi: 10.3402/fnr.v52i0.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paszczyk B., Łuczyńska J. The comparison of fatty acid composition and lipid quality indices in hard cow, sheep, and goat cheeses. Foods. 2020;9:1667. doi: 10.3390/foods9111667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pustjens A.M., Boerrigter-Eenling R., Koot A.H., Rozijn M., van Ruth S.M. Characterization of retail conventional, organic, and grass full-fat butters by their fat contents, free fatty acid contents, and triglyceride and fatty acid profiling. Foods. 2017;6:26. doi: 10.3390/foods6040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu E., Hu F.B. Dairy products, dairy fatty acids, and the prevention of cardiometabolic disease: a review of recent evidence. Curr. Atheroscler. Rep. 2018;20:24. doi: 10.1007/s11883-018-0724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdelmagid S.A., Clarke S.E., Nielsen D.E., Badawi A., El-Sohemy A., Mutch D.M., et al. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buchanan C.D.C., Lust C.A.C., Burns J.L., Hillyer L.M., Martin S.A., Wittert G.A., et al. Analysis of major fatty acids from matched plasma and serum samples reveals highly comparable absolute and relative levels. Prostaglandins Leukot. Essent. Fatty Acids. 2021;168 doi: 10.1016/j.plefa.2021.102268. [DOI] [PubMed] [Google Scholar]
- 54.Twining C.W., Taipale S.J., Ruess L., Bec A., Martin-Creuzburg D., Kainz M.J. Stable isotopes of fatty acids: current and future perspectives for advancing trophic ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020;375 doi: 10.1098/rstb.2019.0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hobson K.A. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia. 1999;120:314–326. doi: 10.1007/s004420050865. [DOI] [PubMed] [Google Scholar]
- 56.Pilecky M., Závorka L., Soto D.X., Guo F., Wassenaar L.I., Kainz M.J. Assessment of compound-specific fatty acid δ13C and δ2H values to track fish mobility in a small sub-alpine catchment. Environ. Sci. Technol. 2022;56:11051–11060. doi: 10.1021/acs.est.2c02089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soto D.X., Decru E., Snoeks J., Verheyen E., Van de Walle L., Bamps J., et al. Terrestrial contributions to afrotropical aquatic food webs: the Congo river case. Ecol. Evol. 2019;9:10746–10757. doi: 10.1002/ece3.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Q., Ma J., Campos H., Hankinson S.E., Hu F.B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007;86:74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.