This systematic review and meta-analysis investigates the association of mortality, poor functional outcomes, recurrent stroke, and dementia in patients with poststroke seizures compared with patients without poststroke seizures.
Key Points
Question
Are patients with poststroke seizures (PSSs) at a greater risk of mortality, poor functional outcomes, recurrent stroke, and dementia compared with patients without PSSs?
Findings
This systematic review and meta-analysis of 71 studies and 20 110 patients with PSS suggests that PSSs are associated with increased mortality risk, poor functional outcomes, disability, and dementia. This study also identifies limitations in existing PSS research, eg, the lack of common data elements, definitions of relevant outcomes, and reporting standards.
Meaning
The findings highlight that PSSs are a public health concern and warrant significant research efforts to prevent poststroke epileptogenesis.
Abstract
Importance
Published data about the impact of poststroke seizures (PSSs) on the outcomes of patients with stroke are inconsistent and have not been systematically evaluated, to the authors’ knowledge.
Objective
To investigate outcomes in people with PSS compared with people without PSS.
Data Sources
MEDLINE, Embase, PsycInfo, Cochrane, LILACS, LIPECS, and Web of Science, with years searched from 1951 to January 30, 2023.
Study Selection
Observational studies that reported PSS outcomes.
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist was used for abstracting data, and the Joanna Briggs Institute tool was used for risk-of-bias assessment. Data were reported as odds ratio (OR) and standardized mean difference (SMD) with a 95% CI using a random-effects meta-analysis. Publication bias was assessed using funnel plots and the Egger test. Outlier and meta-regression analyses were performed to explore the source of heterogeneity. Data were analyzed from November 2022 to January 2023.
Main Outcomes and Measures
Measured outcomes were mortality, poor functional outcome (modified Rankin scale [mRS] score 3-6), disability (mean mRS score), recurrent stroke, and dementia at patient follow-up.
Results
The search yielded 71 eligible articles, including 20 110 patients with PSS and 1 166 085 patients without PSS. Of the participants with PSS, 1967 (9.8%) had early seizures, and 10 605 (52.7%) had late seizures. The risk of bias was high in 5 studies (7.0%), moderate in 35 (49.3%), and low in 31 (43.7%). PSSs were associated with mortality risk (OR, 2.1; 95% CI, 1.8-2.4), poor functional outcome (OR, 2.2; 95% CI, 1.8-2.8), greater disability (SMD, 0.6; 95% CI, 0.4-0.7), and increased dementia risk (OR, 3.1; 95% CI, 1.3-7.7) compared with patients without PSS. In subgroup analyses, early seizures but not late seizures were associated with mortality (OR, 2.4; 95% CI, 1.9-2.9 vs OR, 1.2; 95% CI, 0.8-2.0) and both ischemic and hemorrhagic stroke subtypes were associated with mortality (OR, 2.2; 95% CI, 1.8-2.7 vs OR, 1.4; 95% CI, 1.0-1.8). In addition, early and late seizures (OR, 2.4; 95% CI, 1.6-3.4 vs OR, 2.7; 95% CI, 1.8-4.1) and stroke subtypes were associated with poor outcomes (OR, 2.6; 95% CI, 1.9-3.7 vs OR, 1.9; 95% CI, 1.0-3.6).
Conclusions and Relevance
Results of this systematic review and meta-analysis suggest that PSSs were associated with significantly increased mortality and severe disability in patients with history of stroke. Unraveling these associations is a high clinical and research priority. Trials of interventions to prevent seizures may be warranted.
Introduction
Cerebrovascular disease is the leading cause of new-onset epilepsy in older adults, accounting for nearly 50% of cases.1 Stroke mortality rates have decreased owing to advances in hyperacute stroke treatments, including intravenous thrombolysis or endovascular thrombectomy, patient care in a stroke unit, and risk management.2 As a result, these patients are living longer. The older adult population is expanding as well, resulting in an increase in stroke survivors.3 As stroke is the most common cause of new-onset epilepsy in older individuals, the burden of poststroke seizures (PSSs) is also likely to expand. PSSs impair quality of life because they require that patients take antiseizure medications with associated cognitive and other adverse effects, carry the risk of injury and sudden death from unexpected seizures, and impose restrictions on work, driving, and other aspects of daily life. Previous studies have indicated that epileptic seizures adversely affect the functional, neurologic, and cognitive outcomes of patients with stroke. However, the published data are inconsistent and have not, to our knowledge, been systematically evaluated. We therefore undertook a comprehensive systematic review and meta-analysis to investigate the association of outcomes, including mortality, poor functional outcome, disability, recurrent stroke, and dementia in patients with PSS compared with patients without PSS.
Methods
Search Strategy and Study Selection
We searched MEDLINE, Embase, PsycInfo, Cochrane, LILACS, LIPECS, and Web of Science databases for eligible studies from 1951 until January 30, 2023 (eAppendix in Supplement 1).
Our inclusion criteria included patients with history of stroke (ischemic, hemorrhagic, or both) and those aged 18 years or older presenting with either early or late PSS, which included desired outcome data in patients with and without PSS. We did not impose restrictions based on publication date, language, gender, or ethnicity. We included published patient data irrespective of their race and ethnicity. We, however, did not include information on race and ethnicity in our analyses as these data were not consistently available from all the studies.
Our study exclusion criteria included patients with a prior history of seizures before the index stroke, studies not in full text, studies without outcome data, duplicate publications, narrative or systematic reviews, conference proceedings, dissertations, ongoing research, and preprints. The protocol was preregistered on PROSPERO.4
Definition and Outcomes
The definition of seizures and poststroke epilepsy have varied over time. Until 2014, 2 or more seizures were termed poststroke epilepsy; however, after 2014, the International League Against Epilepsy updated the criteria for seizure classification. One seizure after stroke was enough to lead to the diagnosis of poststroke epilepsy.5 However, considering the lack of uniformity and reporting regarding the classification and definition of early and late-onset seizures, we accepted the definitions reported by individual studies. We provide the breakdown of early and late seizures in eTable 1 in Supplement 1.
Study outcomes were mortality, poor functional outcome (modified Rankin Scale [mRS] score 3-6), disability (mean mRS score), recurrent stroke, and dementia at patient follow-up.
Data Extraction
The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 reporting guidelines.6 We used Covidence software for the review and management of articles. We first assessed the titles and abstracts of the retrieved articles for eligibility. We carefully reviewed multiple publications from the same group to prevent duplicate data entry and included the most updated article in our meta-analysis. Seven reviewers (S. Misra, E.E., J.V., L.S.S., L.B.H., E.I.K., and S. Mohidat) independently screened the title and abstract. Subsequently, we screened the full-text articles for inclusion. We resolved conflicts via discussion with the corresponding author (N.K.M.). We extracted the following from each eligible study: first author; publication year; country; study design; sample size; patient age and sex; stroke subtype (ischemic and hemorrhagic); early and late seizures; disability on mRS; follow-up duration; the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes; vascular risk factors; thrombolysis; and hemorrhagic transformation.
Risk-of-Bias (Quality) Assessment
Two authors (S. Misra and E.E.) assessed the studies’ methodological quality using the Joanna Briggs Institute tool for cohort studies.7 The 11-item tool scored each item as No (0 point), Unclear (1 point), and Yes (2 points). Scores ranged from 0 (minimum) to 22 (maximum), and the studies were classified into high risk of bias (0-12), some concerns/moderate risk of bias (13-18), and low risk of bias (19-22).
Statistical Analysis
Dichotomous variables were reported as percentages, and continuous variables as mean and SD. A random-effects meta-analysis was performed if 2 or more studies were pooled. We determined the association of different outcome measures with the prognosis of PSS using pooled odds ratio (OR) or pooled standardized mean difference (SMD) along with 95% CIs. Heterogeneity was assessed using I2 and Cochrane Q values and categorized as low (I2 < 25%), moderate (I2 = 25%-75%), and high (I2 > 75%).8 We used the Sidik-Jonkman estimator for binary outcome data and the restricted maximum-likelihood estimator for continuous outcome data. We applied Knapp-Hartung adjustments to calculate the CI around the pooled effect. Publication bias was assessed using a funnel plot and quantitatively analyzed using the Egger regression test. We conducted a limit meta-analysis9 to adjust for small-study effects and provided adjusted-pooled OR and SMD. We further explored the source of heterogeneity by conducting meta-regression analysis (predictor variables: risk of bias, publication year, mean age, study design, and follow-up duration), outlier analysis, and sensitivity analysis using the leave-one-out method. Subgroup analyses were performed for stroke subtypes, early and late-onset seizures, and risk of bias. The meta-analysis was conducted using the meta, metafor, and dmetar packages in R, version 4.2.0 (R Project for Statistical Computing). All P values were 2-sided, and P values < .05 were considered significant. Data were analyzed from November 2022 to January 2023.
Results
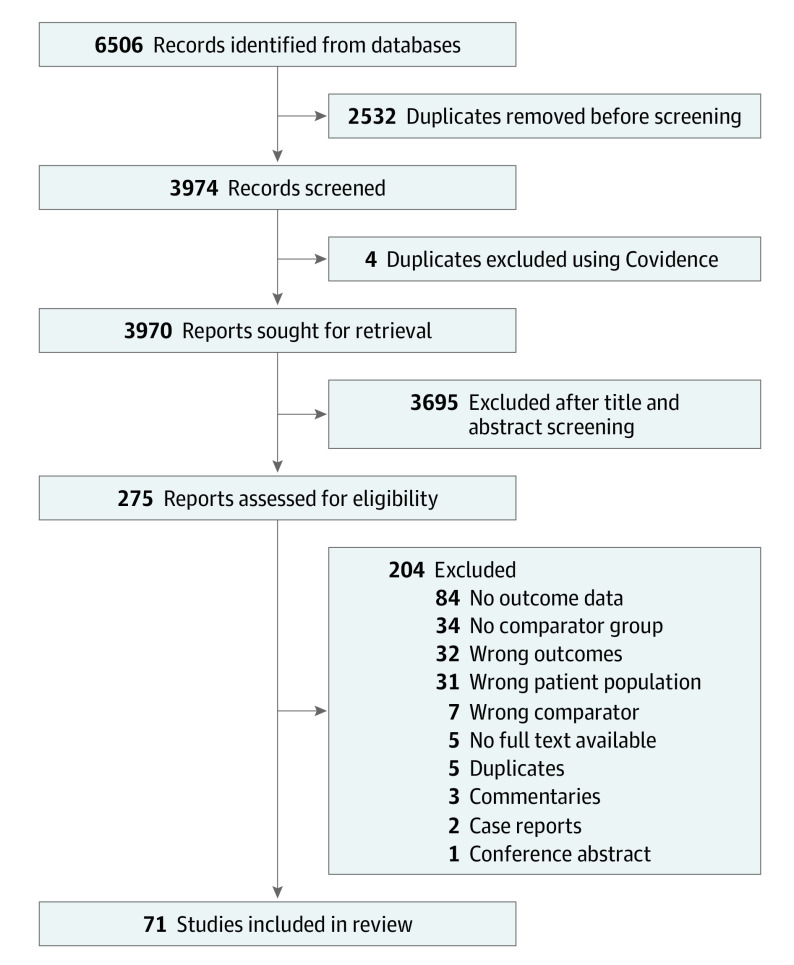
Our search yielded 71 studies (Figure 1).10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80 There was 1 ambispective,35 20 prospective,13,18,24,28,29,36,42,43,45,47,50,53,54,56,57,63,65,66,67,75 47 retrospective10,11,12,14,16,17,19,20,21,22,23,25,26,27,30,31,32,33,34,37,38,39,41,44,46,48,49,51,52,55,58,59,60,61,62,64,68,69,70,71,72,73,74,76,77,78,80 cohort studies, and 3 case-control15,40,79 studies. All studies were published in English, and we identified no studies in other languages. The follow-up duration ranged from hospital discharge to 26 years (eTable 2 in Supplement 1). The studies included patients from 31 countries (eFigure 1 in Supplement 1).
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses Flow Diagram.
The studies included 20 110 patients with PSS and 1 166 085 patients without PSS. The articles included 1967 patients (9.8%) with early seizures and 10 605 patients (52.7%) with late seizures after stroke; 7538 seizures (37.5%) were not classified as early or late. PSS were diagnosed according to clinical or medical chart data in 59 studies10,11,12,13,15,16,17,18,19,20,22,23,24,25,26,27,28,29,30,31,32,34,36,37,38,39,40,41,42,43,44,45,47,49,50,51,52,53,54,56,57,58,59,61,62,63,64,65,66,67,69,70,71,72,73,75,76,78,79 and using the ICD-10 codes in 12 studies14,21,33,35,46,48,55,60,68,74,77,80 (including 15 033 patients [74.8%]). Patients with PSS had a significantly higher history of ischemic heart disease (OR, 1.3; 95% CI, 1.1-1.6), prior cerebrovascular disease (OR, 1.3; 95% CI, 1.0-1.6), atrial fibrillation (OR, 1.2; 95% CI, 1.1-1.4), and the presence of hemorrhagic transformation (OR, 2.2; 95% CI, 1.6-3.0) than patients without PSS (eTable 3 in Supplement 1).
Risk-of-Bias (Quality) Assessment
Five studies (7.0%)17,19,40,49,64 had a high risk of bias, 35 studies (49.3%)10,12,13,14,15,22,23,25,27,29,31,33,34,36,37,38,42,47,48,50,51,52,53,55,57,66,68,69,72,73,74,75,77,79,80 presented a moderate risk of bias, and 31 studies (43.7%)12,16,18,20,21,24,26,28,30,32,35,39,41,43,44,45,46,54,56,58,59,60,61,62,63,65,67,70,71,76,78 had a low risk of bias (eTable 4 in Supplement 1).
Statistical analyses were appropriate in 44 studies (62%).11,13,14,16,18,20,21,24,25,26,27,28,29,30,32,33,34,35,38,39,43,46,47,48,50,53,54,56,58,59,60,62,63,65,67,68,69,71,72,74,76,77,78,80 Confounding factors were identified in 58 studies (81.7%)11,13,14,15,16,18,20,21,24,25,26,27,28,29,30,31,32,33,34,35,36,38,39,41,43,44,45,46,47,48,49,50,52,53,54,55,56,57,58,59,60,61,62,63,65,66,67,68,69,70,71,72,74,75,76,77,78,80 and were adjusted in the statistical analysis in 53 studies (74.6%).11,13,14,15,16,18,20,21,24,25,26,27,28,29,32,33,34,35,38,39,41,43,44,45,46,47,48,49,50,52,53,54,55,56,57,58,59,60,61,62,63,65,67,68,69,70,71,72,74,76,77,78,80 Only 37 studies (52.1%)10,11,12,16,19,21,22,25,28,29,30,31,32,33,35,37,41,42,44,46,50,52,53,56,59,64,68,70,71,72,73,74,75,76,77,78,80 provided complete follow-up data. The outcomes were measured validly and reliably in 53 studies (74.6%).10,11,13,14,16,17,18,20,21,22,23,24,26,27,29,32,34,37,38,39,40,41,43,44,45,46,49,50,51,52,53,54,55,56,57,59,61,62,63,64,67,68,69,70,71,72,73,75,76,77,78,79,80
Mortality (mRS 6)
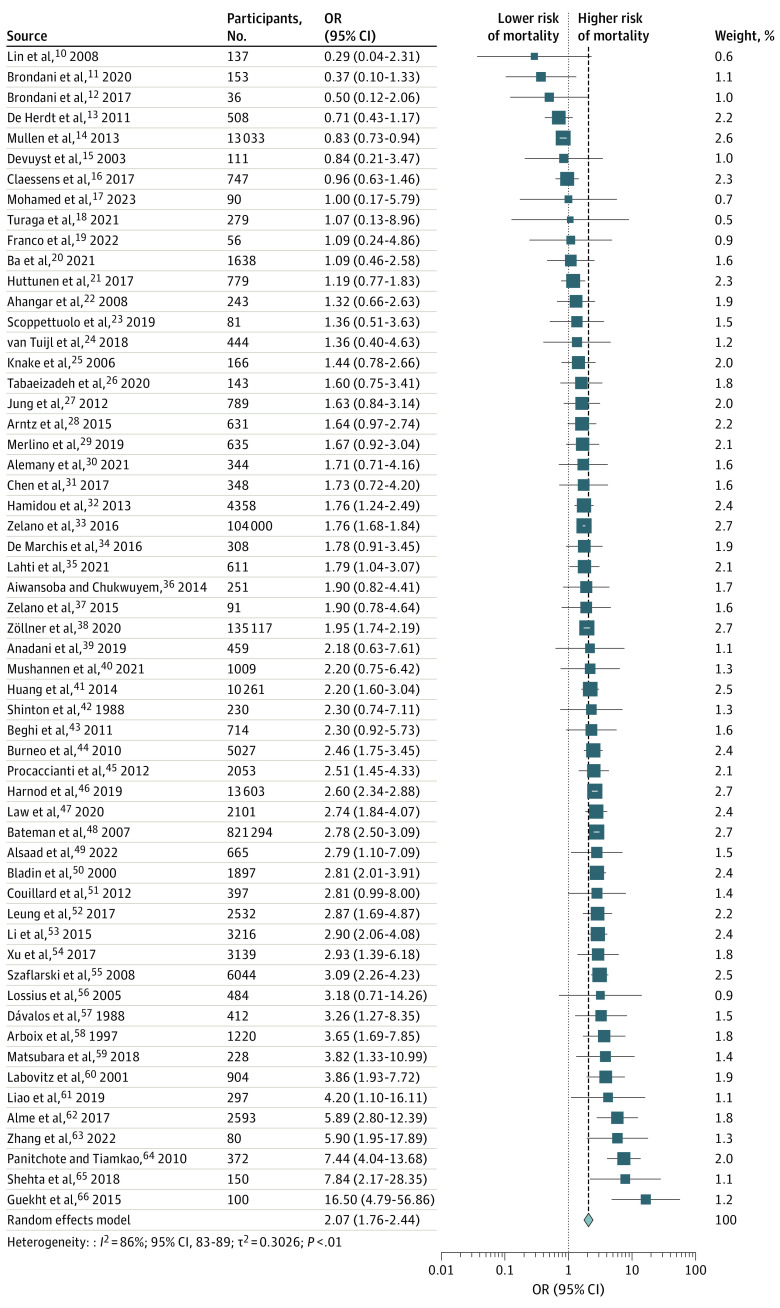
In 57 studies,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66 patients with PSS had approximately double the risk of death (OR, 2.1; 95% CI, 1.8 to 2.4; I2 = 86%) with substantial heterogeneity (Figure 2). The funnel plot was symmetric, and no publication bias was observed (Egger P value = 0.4) (eFigure 2A in Supplement 1). The association remained significant when adjusting for the small-study effect using limit meta-analysis (Table 1). We identified 8 outlier studies using the outlier analysis,11,13,14,16,48,62,64,66 and the significant association persisted after removing these studies with reduced heterogeneity (I2 = 59%) (Table 1). We conducted a sensitivity analysis using the leave-one-out method. We observed that the overall effect size remained unchanged, and heterogeneity reduced to I2 = 76% after removing the study by Mullen et al14 (eFigure 3 in Supplement 1). Meta-regression analyses showed that follow-up duration accounted for 5.5% heterogeneity, but this was not statistically significant.
Figure 2. Association of Poststroke Seizures With Mortality.
The vertical dashed line represents the overall meta-analyzed measure of effect, ie, the pooled odds ratio.
Table 1. Association of Mortality With Poststroke Seizures.
| Serial No. | Outcome measures | PSS (event/total) | No PSS (event/total) | No. of studies | OR (95% CI) | I2, % |
|---|---|---|---|---|---|---|
| Main analysis | ||||||
| 1 | Primary analysis | |||||
| PSS vs no PSS | 7556/17 252 | 181 227/1 130 356 | 57 | 2.07 (1.76-2.44)a | 86 | |
| Limit analysis by adjusting for small study effect | ||||||
| PSS vs no PSS | 7556/17 252 | 181 227/1 130 356 | 57 | 2.19 (1.71-2.82)a | 86 | |
| Outlier analysis (by removing eight outlier studies) | ||||||
| PSS vs no PSS | 6560/13 789 | 70 779/295 019 | 49 | 2.11 (1.87-2.39)a | 59 | |
| Subgroup analyses | ||||||
| 2 | Seizure types | |||||
| Early seizure | 474/1524 | 7862/42 910 | 30 | 2.35 (1.88-2.92)a | 53 | |
| Late seizure | 4461/8136 | 41 275/99 884 | 9 | 1.24 (0.76-2.00) | 58 | |
| 3 | Risk of bias | |||||
| Low | 1541/3101 | 12 402/48 238 | 26 | 2.18 (1.72-2.77)a | 63 | |
| Moderate | 5969/13 951 | 168 753/1 080 126 | 26 | 1.89 (1.47-2.43)a | 91 | |
| High | 46/200 | 122/1992 | 5 | 2.70 (0.97-7.57) | 64 | |
| 4 | ICD-10 codes | |||||
| No ICD-10 codes | 1320/4319 | 26 499/183 021 | 49 | 2.09 (1.74-2.52)a | 60 | |
| ICD-10 codes | 6236/12 933 | 154 778/947 335 | 8 | 1.97 (1.28-3.03)a | 97 | |
| 5 | Stroke subtypes | |||||
| Ischemic stroke | 1138/4227 | 93 426/879 801 | 23 | 2.23 (1.82-2.74)a | 76 | |
| Hemorrhagic stroke | 817/2548 | 40 659/123 464 | 15 | 1.35 (1.01-1.82)a | 85 | |
| Early and late seizures after ischemic stroke | ||||||
| Early PISS vs no PISS | 172/564 | 4090/22 828 | 12 | 2.41 (1.81-3.20)a | 34 | |
| Late PISS vs no PISS | 32/49 | 404/528 | 2 | 0.66 (0.27-1.61) | 0 | |
| Early and late seizures after hemorrhagic stroke | ||||||
| Early PHSS vs no PHSS | 95/251 | 938/3328 | 4 | 1.55 (0.71-3.40) | 86 | |
| Late PHSS vs no PHSS | 143/278 | 908/1827 | 3 | 1.23 (0.88-1.73) | 34 | |
| 6 | Status epilepticus | |||||
| PSSE vs no PSSE | 589/1879 | 106 786/821 302 | 7 | 2.54 (1.67-3.84)a | 34 | |
Abbreviations: ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Edition; OR, odds ratio; PHSS, posthemorrhagic stroke seizure; PISS, postischemic stroke seizure; PSS, poststroke seizure; PSSE, poststroke status epilepticus.
P < .05.
A subgroup analysis based on seizure subtypes identified that early seizures were associated with an increased mortality risk but not late seizures (OR, 2.4; 95% CI, 1.9-2.9 vs OR, 1.2; 95% CI, 0.8-2.0) (eFigure 4 in Supplement 1). The findings were the same in patients with ischemic and hemorrhagic stroke who subsequently developed seizures; both subtypes were associated with mortality (OR, 2.2; 95% CI, 1.8-2.7 vs OR, 1.4; 95% CI, 1.0-1.8) (eFigure 5 in Supplement 1). We also observed that early seizures after ischemic stroke were associated with mortality but not late seizures (OR, 2.4; 95% CI, 1.8-3.2 vs OR, 0.7; 95% CI, 0.3-1.6) (eFigure 6A in Supplement 1). We did not identify any significant association between early or late seizures and increased mortality risk after hemorrhagic stroke (eFigure 6B in Supplement 1). Additionally, the subgroup of patients with poststroke status epilepticus presented with increased mortality (OR, 2.5; 95% CI, 1.7-3.8) (eFigure 7 in Supplement 1 and Table 1). There was no significant difference in the subgroups for risk of bias.
Poor Functional Outcome (mRS 3-6)
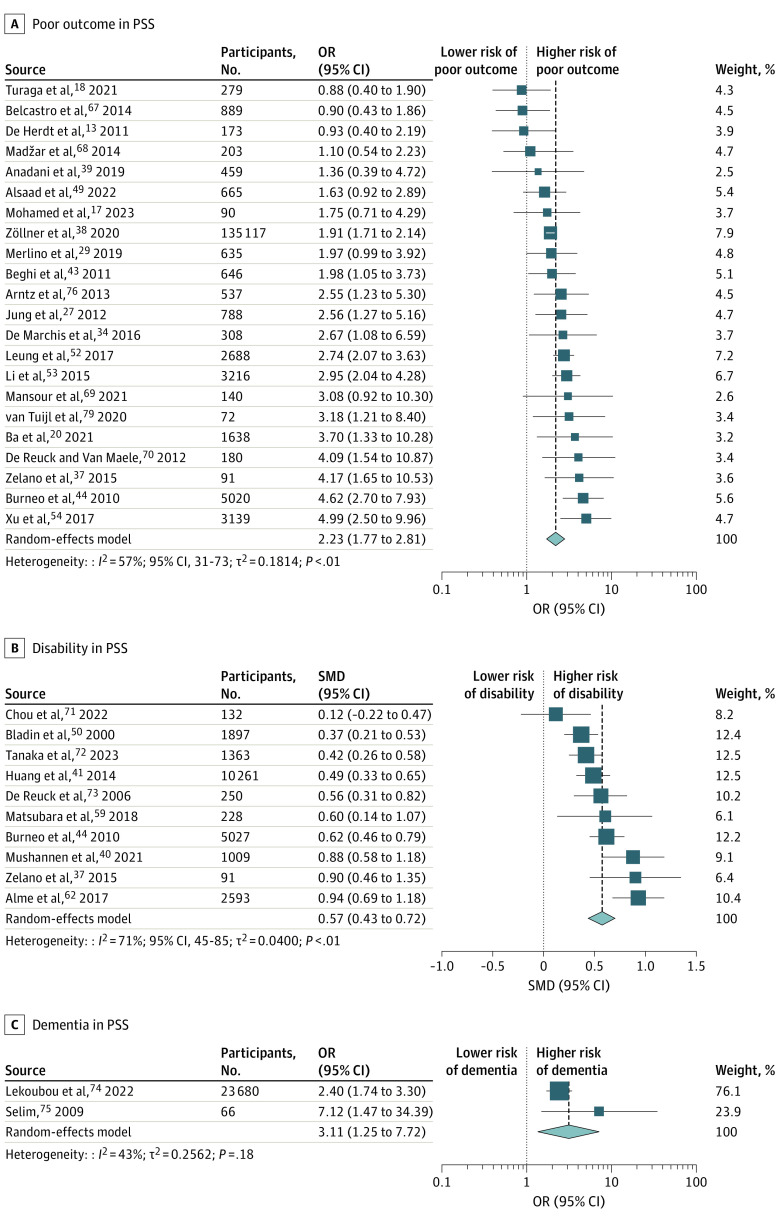
In 22 studies, patients with PSS had poorer outcomes than those without PSS (OR, 2.2; 95% CI, 1.8-2.8; I2 = 57%) (Figure 3A).13,17,18,20,27,29,34,37,38,39,43,44,49,52,53,54,67,68,69,70,76,79 The funnel plot was symmetric, and no publication bias was observed using the Egger test (eFigure 2B in Supplement 1). There were no variations in the pooled effect size after conducting a limit meta-analysis and the leave-one-out sensitivity analysis (eFigure 8 in Supplement 1). Meta-regression could not identify any predictor that significantly contributed to increased heterogeneity.
Figure 3. Association of Poststroke Seizures With Poor Functional Outcome, Disability, and Dementia.
Association of poststroke seizures with poor functional outcome (A), disability (B), and dementia (C).
We conducted subgroup analyses based on seizure subtypes (eFigure 9 in Supplement 1) and stroke subtypes (eFigure 10 in Supplement 1). We observed that early and late-onset seizures after stroke (OR, 2.4; 95% CI, 1.6-3.4 vs OR, 2.7; 95% CI, 1.8-4.1) and seizures after ischemic and hemorrhagic stroke (OR, 2.6; 95% CI, 1.9-3.7 vs OR, 1.9; 95% CI, 1.0-3.6) were significantly associated with poor outcomes. In patients with ischemic stroke, early seizures were associated with poor outcomes (eFigure 11A in Supplement 1), whereas neither early nor late seizures were associated with poor outcomes after hemorrhagic stroke (eFigure 11B in Supplement 1 and Table 2). A significant difference in heterogeneity was observed after performing a subgroup analysis by risk of bias (low, I2 = 70%; moderate, I2 = 50%; high, I2 = 0%; P for subgroup differences =.02).
Table 2. Association of Poor Outcome (Modified Rankin Scale Score 3-6) With Poststroke Seizures.
| Serial No. | Outcome measures | PSS (event/total) | No PSS (event/total) | No. of studies | OR (95% CI) | I2, % |
|---|---|---|---|---|---|---|
| Main analysis | ||||||
| 1 | Primary analysis | |||||
| PSS vs no PSS | 2167/2952 | 96 967/154 021 | 22 | 2.23 (1.77-2.81)a | 57 | |
| Limit analysis by adjusting for small study effect | ||||||
| PSS vs no PSS | 2167/2952 | 96 967/154 021 | 22 | 2.25 (1.43-3.53)a | 57 | |
| Subgroup analyses | ||||||
| 2 | Seizure types | |||||
| Early seizure | 276/424 | 4646/10 468 | 11 | 2.36 (1.64-3.39)a | 43 | |
| Late seizure | 154/273 | 1403/3940 | 6 | 2.73 (1.83-4.09)a | 0 | |
| 3 | Risk of bias | |||||
| Low | 304/436 | 6607/12 351 | 9 | 2.35 (1.38-4)a | 70 | |
| Moderate | 1822/2435 | 90 089/140 996 | 11 | 2.25 (1.71-2.96)a | 50 | |
| High | 41/81 | 271/674 | 2 | 1.66 (1.09-2.53)a | 0 | |
| 4 | ICD-10 codes | |||||
| No ICD-10 codes | 2143/2912 | 96 873/153 858 | 21 | 2.31 (1.84-2.91)a | 56 | |
| ICD-10 codes | 24/40 | 94/163 | 1 | 1.10 (0.54-2.23) | NA | |
| 5 | Stroke subtypes | |||||
| PISS vs no PISS | 1788/2383 | 93 644/147 608 | 12 | 2.60 (1.85-3.67)a | 64 | |
| PHSS vs no PHSS | 218/302 | 2397/4494 | 6 | 1.91 (1-3.63)a | 42 | |
| Early and late seizures after ischemic stroke | ||||||
| Early PISS vs no PISS | 279/439 | 4146/9266 | 8 | 2.97 (2.05-4.31)a | 11 | |
| Late PISS vs no PISS | 28/41 | 462/901 | 2 | 2.27 (0-4048.73) | 64 | |
| Early and late seizures after hemorrhagic stroke | ||||||
| Early PHSS vs no PHSS | 12/17 | 41/74 | 1 | 1.93 (0.62-6.04) | NA | |
| Late PHSS vs no PHSS | 0/10 | 5/29 | 1 | 0.21 (0.01-4.19) | NA | |
Abbreviations: ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Edition; NA, not applicable; OR, odds ratio; PHSS, posthemorrhagic stroke seizure; PISS, postischemic stroke seizure; PSS, poststroke seizure.
P < .05.
Disability (Mean mRS Score)
The mean mRS disability at follow-up was significantly higher in 10 studies37,40,41,44,50,59,62,71,72,73 in patients with PSS than in patients without PSS (SMD, 0.6; 95% CI, 0.4-0.7; I2 = 71%) (Figure 3B). No publication bias was detected (eFigure 2C in Supplement 1), and findings remained consistent in a sensitivity analysis using the leave-one-out method (eFigure 12 in Supplement 1). Meta-regression identified that age significantly accounted for 45.1% heterogeneity (estimate, −0.02; 95% CI, −0.04 to −0.003; P = .03). In subgroup analyses, early and late seizures were associated with higher disability (eFigure 13 in Supplement 1). Seizures after ischemic stroke were associated with a higher disability but not after hemorrhagic stroke (eFigure 14 in Supplement 1). No significant difference in heterogeneity was observed when stratified by risk of bias (eTable 5 in Supplement 1).
Recurrent Stroke and Dementia
Five studies49,71,77,78,80 found no association of recurrent stroke with PSS (OR, 1.3; 95% CI, 0.6-3.0) (eFigure 15 in Supplement 1). Two studies74,75 identified an increased dementia risk in patients with PSS (OR, 3.1; 95% CI, 1.3-7.7) (Figure 3c) (eTable 6 in Supplement 1).
Discussion
This was a comprehensive analysis of PSS outcome data obtained from 71 studies published through January 2023. In this systematic review and meta-analysis, results suggest that in patients with history of stroke, PSSs were associated with significantly increased risk of mortality, poor functional outcomes, higher disability, and increased risk of dementia compared with patients without PSS.
The main reason for death in PSS is known to be cardiovascular death, not seizure related.81 However, chronic epilepsy is associated with abnormal cardiac functions, including greater cardiovascular risk.82 The increased cardiovascular risk has been attributed to the interactions of antiseizure medications or cardiopathies due to chronic seizures, ie, “epileptic heart.”83,84,85
On the contrary, stroke severity is strongly related to early seizures; therefore, the mortality rate from the stroke itself may be higher in the early seizure group.86 We conducted subgroup analyses to determine outcomes in patients with early and late seizures. We found that patients with early seizures had a greater mortality risk. Because the studies did not consistently report stroke severity and raw data for cardiovascular risks, we could not test the interaction of these variables with PSS on influencing mortality risk.
We found that patients with late seizures also had a greater mortality risk; however, the CI for this subgroup failed to reach statistical significance. We speculate that this was probably because of a smaller sample size or other unknown mechanisms, eg, bias in the data (Table 1). We, therefore, classified patients based on the risk of bias in the included studies. We found that patients in the studies with high risk of bias were at statistically significant risk of poor outcomes and disability. Even though the point estimate suggested greater mortality risk in this subgroup, the 95% CI failed to reach statistical significance (Table 1). In contrast, patients in the studies with a low or moderate risk of bias had a significantly increased risk of mortality, poor outcome, and disability.
We conducted additional subgroup analyses examining outcomes in patients with ischemic and hemorrhagic strokes separately. We found that early but not late seizures were associated with mortality in the ischemic stroke subtype. We found no association of mortality for hemorrhagic stroke subtype with any seizure type (Table 1). Mortality in patients with stroke and late seizures may be determined by age, stroke severity, and vascular risk factors rather than by seizure development. Both seizure types and stroke subtypes were associated with poor outcomes. Early seizures after ischemic stroke subtype were associated with poor outcomes but not late seizures. Neither early nor late seizures were associated with poor outcomes in patients with the hemorrhagic stroke subtype (Table 2).
In addition to examining mortality and functional outcomes in patients with PSS, we decided to examine a priori the dementia risk and the risk of recurrent stroke in patients with PSS. We observed a 3-fold increased dementia risk in patients with PSS than in those without PSS (Figure 3C). This finding, however, was based on data pooled from only 2 studies (45 patients with PSS and dementia74 and 5 patients with PSS and dementia75). Patients with cerebrovascular disease are at an increased risk of vascular cognitive impairment and dementia.87 Chronic seizure activity may contribute to the dementia risk.88,89 To characterize the PSS-associated cognitive impairment and dementia as outcomes in these patients, longitudinally collected data are needed.
Patients with PSS may be at greater risk of recurrent stroke. This could be due to poor compliance with secondary stroke prevention medications and interaction of antiseizure medications with efficacy of secondary stroke prevention drugs. Risk factor management is crucial in patients with PSS to reduce the frequency and severity of seizures.90 Although due to a limited number of studies we found no association between recurrent stroke and PSS, some data indicate that people with epilepsy have a higher prevalence of cardiovascular risk factors, independent of stroke.91 Thus, patients with PSS may be at higher risk of recurrent stroke, which requires close monitoring of vascular risk factors in patients with PSS.
Our systematic review identified that 15 033 patients with PSS (74.8%) from 12 studies were diagnosed using ICD-10 codes. ICD-10 code–based research is prone to misclassification and may lead to errors in hospital admissions.92 However, when we stratified the data based on ICD-10 and no ICD-10 codes, the results were consistent for studies with no ICD-10 codes (Table 1, Table 2, and eTables 5 and 6 in Supplement 1).
Mechanisms of early and late seizures are very different and, not surprisingly, have very different clinical significance93; therefore, early and late seizures must be treated separately in future studies, including meta-analyses. Early seizures reflect acute reversible metabolic defects that might be life-threatening. In contrast, late seizures reflect structural changes that require time to develop and are not due to life-threatening underlying pathology.94 Also, efforts to reduce the mortality associated with early seizures involve efforts to prevent or correct acute metabolic defects than efforts to prevent seizures, which are just a symptom of the problem. Collaborative efforts to predict and prevent the structural and associated biological changes that lead to late seizures (ie, poststroke epileptogenesis and ictogenesis) can address the disability and mortality of these events.95
Strengths and Limitations
The strengths of our meta-analysis include its robust methodology on a large international sample of patients with PSS. This is the largest meta-analysis to date, to our knowledge, which allowed us to assess outcomes in relation to seizure subtypes, stroke subtypes, and study quality. We collected raw data and adjusted for multiple confounders using meta-regression analyses. We rigorously tested for publication bias. Furthermore, we conducted a limit analysis to account for bias due to small-study effects and provided adjusted estimates for each outcome measure. Our methods allowed us to include a larger number of studies (N = 71 vs N = 1096 and N = 1397) and avoid the confounder imbalance,98 which can be introduced by pooling adjusted ORs as was done in the 2 previous systematic reviews.96,97 We compared and contrasted our systematic review with these 2 previous systematic reviews in eTable 7 in Supplement 1.96,97
Despite adopting robust methods, we observed several challenges in pooling data in this meta-analysis. eTable 8 in Supplement 1 contains a list of challenges and our recommendations to tackle them.
The results of our meta-analysis must be considered in light of the following limitations. First, there are scarce data from prospective studies; most data came from retrospective cohorts. Second, few studies did not provide outcome data segregated by seizure type and stroke subtype. Third, due to a lack of consistent reporting of National Institutes of Health Stroke Scale data, we could not examine the effect of stroke severity on clinical outcomes after PSS, which is very likely a potential confounder. Fourth, we could not determine the effect of concurrent medications. Fifth, data on the cause of mortality were unavailable. Sixth, the authors of some studies used disparate definitions for seizures that might introduce some misclassification in our subgroup analyses (eTable 1 in Supplement 1). Seventh, outcomes were measured at variable time points ranging from hospital discharge to 26 years after stroke (eTable 2 in Supplement 1). We suggest that within-study comparisons should be valid if follow-up was the same for patients with and without PSS, but absolute values are not readily interpretable. An individual patient data analysis project is under way in which we will collect original, raw data from eligible studies and conduct meta-analysis by standardizing the common data elements, patient outcomes, and the duration of patient follow-up.99
Conclusions
Results of this systematic review and meta-analysis suggest that PSSs were associated with a doubled risk of death and severe disability and were thus an important burden of disease. PSS prevention is a high clinical and research priority. We also observed a significant variation in reporting standards in the published literature and propose future directions for PSS research. Collaborative scientific efforts should be directed toward addressing these challenges.95 The role of stroke severity and lesion location or volume also requires further investigation.
eAppendix. Detailed Search Strategy
eFigure 1. The List of Countries Representing the Number of Studies
eFigure 2. Funnel Plot to Test the Presence of Publication Bias
eFigure 3. Sensitivity Analysis Using the Leave-One-Out Method
eFigure 4. Subgroup Analysis Based on Seizure Subtypes
eFigure 5. Subgroup Analysis Based on Stroke Subtypes
eFigure 6. Subgroup Analysis Based on Seizure and Stroke Subtypes
eFigure 7. Subgroup Analysis on Patients With Status Epilepticus
eFigure 8. Sensitivity Analysis Using the Leave-One-Out Method
eFigure 9. Subgroup Analysis Based on Seizure Subtypes
eFigure 10. Subgroup Analysis Based on Stroke Subtypes
eFigure 11. Subgroup Analysis Based on Seizure and Stroke Subtypes
eFigure 12. Sensitivity Analysis Using the Leave-One-Out Method
eFigure 13. Subgroup Analysis Based on Seizure Subtypes
eFigure 14. Subgroup Analysis Based on Stroke Subtypes
eFigure 15. Association of Poststroke Seizures With Recurrent Stroke
eTable 1. Definition of Early and Late Seizures Used by the Studies
eTable 2. Baseline Characteristics of Studies
eTable 3. Risk Factors Associated With PSS
eTable 4. Risk of Bias (Quality) Assessment for the Studies
eTable 5. Association of Disability (Mean mRS Score) With Poststroke Seizures
eTable 6. Association of Recurrent Stroke and Dementia With Poststroke Seizures
eTable 7. Comparison of Our Meta-Analysis With Previously Published Meta-Analyses
eTable 8. Future Directions for PSS Studies
eReferences
Data Sharing Statement
References
- 1.Sen A, Jette N, Husain M, Sander JW. Epilepsy in older people. Lancet. 2020;395(10225):735-748. doi: 10.1016/S0140-6736(19)33064-8 [DOI] [PubMed] [Google Scholar]
- 2.Tanaka T, Ihara M. Poststroke epilepsy. Neurochem Int. 2017;107:219-228. doi: 10.1016/j.neuint.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 3.Wafa HA, Wolfe CDA, Emmett E, Roth GA, Johnson CO, Wang Y. Burden of stroke in Europe: thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life-years. Stroke. 2020;51(8):2418-2427. doi: 10.1161/STROKEAHA.120.029606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misra S, Khan E, Funaro M, Quinn T, Mishra NK. Clinical outcomes in patients who have poststroke epilepsy: a systematic review and meta-analysis. Accessed March 27, 2023. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=308648
- 5.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475-482. doi: 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 6.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moola SMZ, Tufanaru C, Aromataris E, et al. Systematic Reviews of Etiology and Risk. JBI Manual for Evidence Synthesis. JBI; 2020. [Google Scholar]
- 8.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rücker G, Schwarzer G, Carpenter JR, Binder H, Schumacher M. Treatment-effect estimates adjusted for small-study effects via a limit meta-analysis. Biostatistics. 2011;12(1):122-142. doi: 10.1093/biostatistics/kxq046 [DOI] [PubMed] [Google Scholar]
- 10.Lin YJ, Chang WN, Chang HW, et al. Risk factors and outcome of seizures after spontaneous aneurysmal subarachnoid hemorrhage. Eur J Neurol. 2008;15(5):451-457. doi: 10.1111/j.1468-1331.2008.02096.x [DOI] [PubMed] [Google Scholar]
- 11.Brondani R, de Almeida AG, Cherubini PA, et al. Risk factors for epilepsy after thrombolysis for ischemic stroke: a cohort study. Front Neurol. 2020;10:1256. doi: 10.3389/fneur.2019.01256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brondani R, Garcia de Almeida A, Abrahim Cherubini P, et al. High risk of seizures and epilepsy after decompressive hemicraniectomy for malignant middle cerebral artery stroke. Cerebrovasc Dis Extra. 2017;7(1):51-61. doi: 10.1159/000458730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Herdt V, Dumont F, Hénon H, et al. Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology. 2011;77(20):1794-1800. doi: 10.1212/WNL.0b013e31823648a6 [DOI] [PubMed] [Google Scholar]
- 14.Mullen MT, Kasner SE, Messé SR. Seizures do not increase in-hospital mortality after intracerebral hemorrhage in the nationwide inpatient sample. Neurocrit Care. 2013;19(1):19-24. doi: 10.1007/s12028-012-9791-0 [DOI] [PubMed] [Google Scholar]
- 15.Devuyst G, Karapanayiotides T, Hottinger I, Van Melle G, Bogousslavsky J. Prodromal and early epileptic seizures in acute stroke: does higher serum cholesterol protect? Neurology. 2003;61(2):249-252. doi: 10.1212/01.WNL.0000070410.68541.7E [DOI] [PubMed] [Google Scholar]
- 16.Claessens D, Bekelaar K, Schreuder FHBM, et al. Mortality after primary intracerebral hemorrhage in relation to post-stroke seizures. J Neurol. 2017;264(9):1885-1891. doi: 10.1007/s00415-017-8573-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed AT, El Rakawy MH, Abdelhamid YAE, Hazzou AM, Wahid el din MM. Stroke-related early seizures: clinical and neurophysiological study in a sample of Egyptian population. Egypt J Neurol Psychiat Neurosurg. 2023;59:3. doi: 10.1186/s41983-022-00603-5 [DOI] [Google Scholar]
- 18.Turaga SP, Chaithanya RL, Kohat AK, Alladi S, Kaul S. Frequency and predictors of early seizures following first acute stroke: data from a university hospital in South India. Neurol India. 2021;69(4):847-855. doi: 10.4103/0028-3886.325345 [DOI] [PubMed] [Google Scholar]
- 19.Franco AC, Fernandes T, Peralta AR, et al. Frequency of epileptic seizures in patients undergoing decompressive craniectomy after ischemic stroke. Seizure. 2022;101:60-66. doi: 10.1016/j.seizure.2022.07.011 [DOI] [PubMed] [Google Scholar]
- 20.Ba K, Casolla B, Caparros F, et al. Early epileptic seizures in ischaemic stroke treated by mechanical thrombectomy: influence of rt-PA. J Neurol. 2021;268(1):305-311. doi: 10.1007/s00415-020-10155-4 [DOI] [PubMed] [Google Scholar]
- 21.Huttunen J, Lindgren A, Kurki MI, et al. Epilepsy-associated long-term mortality after aneurysmal subarachnoid hemorrhage. Neurology. 2017;89(3):263-268. doi: 10.1212/WNL.0000000000004113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahangar AA, Hosseini S, Saghebi R. Clinical features of post stroke seizure in Babol, northern Iran. Neurosciences (Riyadh). 2008;13(1):88-90. [PubMed] [Google Scholar]
- 23.Scoppettuolo P, Gaspard N, Depondt C, Legros B, Ligot N, Naeije G. Epileptic activity in neurological deterioration after ischemic stroke, a continuous EEG study. Clin Neurophysiol. 2019;130(12):2282-2286. doi: 10.1016/j.clinph.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 24.van Tuijl JH, van Raak EPM, van Oostenbrugge RJ, Aldenkamp AP, Rouhl RPW. The occurrence of seizures after ischemic stroke does not influence long-term mortality; a 26-year follow-up study. J Neurol. 2018;265(8):1780-1788. doi: 10.1007/s00415-018-8907-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knake S, Rochon J, Fleischer S, et al. Status epilepticus after stroke is associated with increased long-term case fatality. Epilepsia. 2006;47(12):2020-2026. doi: 10.1111/j.1528-1167.2006.00845.x [DOI] [PubMed] [Google Scholar]
- 26.Tabaeizadeh M, Aboul Nour H, Shoukat M, et al. Burden of epileptiform activity predicts discharge neurologic outcomes in severe acute ischemic stroke. Neurocrit Care. 2020;32(3):697-706. doi: 10.1007/s12028-020-00944-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung S, Schindler K, Findling O, et al. Adverse effect of early epileptic seizures in patients receiving endovascular therapy for acute stroke. Stroke. 2012;43(6):1584-1590. doi: 10.1161/STROKEAHA.111.645358 [DOI] [PubMed] [Google Scholar]
- 28.Arntz RM, Rutten-Jacobs LC, Maaijwee NA, et al. Poststroke epilepsy is associated with a high mortality after a stroke at young age: follow-up of transient ischemic attack and stroke patients and unelucidated risk factor evaluation study. Stroke. 2015;46(8):2309-2311. doi: 10.1161/STROKEAHA.115.010115 [DOI] [PubMed] [Google Scholar]
- 29.Merlino G, Gigli GL, Bax F, Serafini A, Corazza E, Valente M. Seizures do not affect disability and mortality outcomes of stroke: a population-based study. J Clin Med. 2019;8(11):2006. doi: 10.3390/jcm8112006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alemany M, Nuñez A, Falip M, et al. Acute symptomatic seizures and epilepsy after mechanical thrombectomy—a prospective long-term follow-up study. Seizure. 2021;89:5-9. doi: 10.1016/j.seizure.2021.04.011 [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Churilov L, Koome M, et al. Poststroke seizures is associated with low alberta stroke program early CT score. Cerebrovasc Dis. 2017;43(5-6):259-265. doi: 10.1159/000458449 [DOI] [PubMed] [Google Scholar]
- 32.Hamidou B, Aboa-Eboulé C, Durier J, et al. Prognostic value of early epileptic seizures on mortality and functional disability in acute stroke: the Dijon Stroke Registry (1985-2010). J Neurol. 2013;260(4):1043-1051. doi: 10.1007/s00415-012-6756-3 [DOI] [PubMed] [Google Scholar]
- 33.Zelano J, Redfors P, Åsberg S, Kumlien E. Association between poststroke epilepsy and death: a nationwide cohort study. Eur Stroke J. 2016;1(4):272-278. doi: 10.1177/2396987316669000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Marchis GM, Pugin D, Meyers E, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86(3):253-260. doi: 10.1212/WNL.0000000000002281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahti AM, Huhtakangas J, Juvela S, Bode MK, Tetri S. Increased mortality after post-stroke epilepsy following primary intracerebral hemorrhage. Epilepsy Res. 2021;172:106586. doi: 10.1016/j.eplepsyres.2021.106586 [DOI] [PubMed] [Google Scholar]
- 36.Aiwansoba IF, Chukwuyem OW. Early postacute stroke seizures: clinical profile and outcome in a Nigerian stroke unit. Ann Afr Med. 2014;13(1):11-15. doi: 10.4103/1596-3519.126936 [DOI] [PubMed] [Google Scholar]
- 37.Zelano J, Lundberg RG, Baars L, Hedegärd E, Kumlien E. Clinical course of poststroke epilepsy: a retrospective nested case-control study. Brain Behav. 2015;5(9):e00366. doi: 10.1002/brb3.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zöllner JP, Misselwitz B, Kaps M, et al. National Institutes of Health Stroke Scale (NIHSS) on admission predicts acute symptomatic seizure risk in ischemic stroke: a population-based study involving 135,117 cases. Sci Rep. 2020;10(1):3779. doi: 10.1038/s41598-020-60628-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anadani M, Lekoubou A, Almallouhi E, et al. Incidence, predictors, and outcome of early seizures after mechanical thrombectomy. J Neurol Sci. 2019;396:235-239. doi: 10.1016/j.jns.2018.11.033 [DOI] [PubMed] [Google Scholar]
- 40.Mushannen T, Aleyadeh R, Siddiqui M, et al. Effect of reperfusion therapies on incidence of early poststroke seizures. Front Neurol. 2021;12:758181. doi: 10.3389/fneur.2021.758181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang CW, Saposnik G, Fang J, Steven DA, Burneo JG. Influence of seizures on stroke outcomes: a large multicenter study. Neurology. 2014;82(9):768-776. doi: 10.1212/WNL.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 42.Shinton RA, Gill JS, Melnick SC, Gupta AK, Beevers DG. The frequency, characteristics, and prognosis of epileptic seizures at the onset of stroke. J Neurol Neurosurg Psychiatry. 1988;51(2):273-276. doi: 10.1136/jnnp.51.2.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beghi E, D’Alessandro R, Beretta S, et al. ; Epistroke Group . Incidence and predictors of acute symptomatic seizures after stroke. Neurology. 2011;77(20):1785-1793. doi: 10.1212/WNL.0b013e3182364878 [DOI] [PubMed] [Google Scholar]
- 44.Burneo JG, Fang J, Saposnik G; Investigators of the Registry of the Canadian Stroke Network . Impact of seizures on morbidity and mortality after stroke: a Canadian multicentre cohort study. Eur J Neurol. 2010;17(1):52-58. doi: 10.1111/j.1468-1331.2009.02739.x [DOI] [PubMed] [Google Scholar]
- 45.Procaccianti G, Zaniboni A, Rondelli F, Crisci M, Sacquegna T. Seizures in acute stroke: incidence, risk factors, and prognosis. Neuroepidemiology. 2012;39(1):45-50. doi: 10.1159/000338374 [DOI] [PubMed] [Google Scholar]
- 46.Harnod T, Lin CL, Kao CH. Epilepsy is associated with higher subsequent mortality risk in patients after stroke: a population-based cohort study in Taiwan. Clin Epidemiol. 2019;11:247-255. doi: 10.2147/CLEP.S201263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Law ZK, England TJ, Mistri AK, et al. Incidence and predictors of early seizures in intracerebral haemorrhage and the effect of tranexamic acid. Eur Stroke J. 2020;5(2):123-129. doi: 10.1177/2396987320901391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bateman BT, Claassen J, Willey JZ, et al. Convulsive status epilepticus after ischemic stroke and intracerebral hemorrhage: frequency, predictors, and impact on outcome in a large administrative dataset. Neurocrit Care. 2007;7(3):187-193. doi: 10.1007/s12028-007-0056-2 [DOI] [PubMed] [Google Scholar]
- 49.Alsaad F, Alkeneetir N, Almatroudi M, et al. Early seizures in stroke—frequency, risk factors, and effect on patient outcomes in a tertiary center in Saudi Arabia. Neurosciences (Riyadh). 2022;27(2):104-110. doi: 10.17712/nsj.2022.2.20210144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol. 2000;57(11):1617-1622. doi: 10.1001/archneur.57.11.1617 [DOI] [PubMed] [Google Scholar]
- 51.Couillard P, Almekhlafi MA, Irvine A, et al. Subacute seizure incidence in thrombolysis-treated ischemic stroke patients. Neurocrit Care. 2012;16(2):241-245. doi: 10.1007/s12028-011-9657-x [DOI] [PubMed] [Google Scholar]
- 52.Leung T, Leung H, Soo YO, Mok VC, Wong KS. The prognosis of acute symptomatic seizures after ischaemic stroke. J Neurol Neurosurg Psychiatry. 2017;88(1):86-94. doi: 10.1136/jnnp-2015-311849 [DOI] [PubMed] [Google Scholar]
- 53.Li Z, Zhao X, Wang Y, et al. Association between seizures and outcomes among intracerebral hemorrhage patients: the China National Stroke Registry. J Stroke Cerebrovasc Dis. 2015;24(2):455-464. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 54.Xu Y, Hackett ML, Chalmers J, et al. ; ENCHANTED Study Group . Frequency, determinants, and effects of early seizures after thrombolysis for acute ischemic stroke: The ENCHANTED trial. Neurol Clin Pract. 2017;7(4):324-332. doi: 10.1212/CPJ.0000000000000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szaflarski JP, Rackley AY, Kleindorfer DO, et al. Incidence of seizures in the acute phase of stroke: a population-based study. Epilepsia. 2008;49(6):974-981. doi: 10.1111/j.1528-1167.2007.01513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lossius MI, Rønning OM, Slapø GD, Mowinckel P, Gjerstad L. Poststroke epilepsy: occurrence and predictors--a long-term prospective controlled study (Akershus Stroke Study). Epilepsia. 2005;46(8):1246-1251. doi: 10.1111/j.1528-1167.2005.57904.x [DOI] [PubMed] [Google Scholar]
- 57.Dávalos A, Cendra E, Genís D, López-Pousa S. The frequency, characteristics, and prognosis of epileptic seizures at the onset of stroke. J Neurol Neurosurg Psychiatry. 1988;51(11):1464. doi: 10.1136/jnnp.51.11.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arboix A, García-Eroles L, Massons JB, Oliveres M, Comes E. Predictive factors of early seizures after acute cerebrovascular disease. Stroke. 1997;28(8):1590-1594. doi: 10.1161/01.STR.28.8.1590 [DOI] [PubMed] [Google Scholar]
- 59.Matsubara S, Sato S, Kodama T, et al. Nonconvulsive status epilepticus in acute intracerebral hemorrhage. Stroke. 2018;49(7):1759-1761. doi: 10.1161/STROKEAHA.118.021414 [DOI] [PubMed] [Google Scholar]
- 60.Labovitz DL, Hauser WA, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology. 2001;57(2):200-206. doi: 10.1212/WNL.57.2.200 [DOI] [PubMed] [Google Scholar]
- 61.Liao HC, Chen SH, Yang CD, Chen YW. Clinical profile and outcomes of early seizures in asian patients with acute intracerebral hemorrhage. J Acute Med. 2019;9(4):172-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alme KN, Engelsen BA, Naik M, Naess H. Identifying patients at risk of acute symptomatic seizure after ischemic stroke. Acta Neurol Scand. 2017;136(3):265-271. doi: 10.1111/ane.12721 [DOI] [PubMed] [Google Scholar]
- 63.Zhang L, Zheng W, Chen F, et al. Associated factors and prognostic implications of nonconvulsive status epilepticus in ischemic stroke patients with impaired consciousness. Front Neurol. 2022;12:795076. doi: 10.3389/fneur.2021.795076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panitchote A, Tiamkao S. Prevalence of poststroke seizures in Srinagarind hospital. J Med Assoc Thai. 2010;93(9):1037-1042. [PubMed] [Google Scholar]
- 65.Shehta N, Fahmi RM, Ramadan BM, Emad EM, Elsaid AF. Early poststroke seizures in a sample of Egyptian patients with first-ever stroke. Neurol India. 2018;66(4):1031-1035. doi: 10.4103/0028-3886.236973 [DOI] [PubMed] [Google Scholar]
- 66.Guekht A, Mizinova M, Ershov A, et al. In-hospital costs in patients with seizures and epilepsy after stroke. Epilepsia. 2015;56(8):1309-1313. doi: 10.1111/epi.13062 [DOI] [PubMed] [Google Scholar]
- 67.Belcastro V, Vidale S, Gorgone G, et al. Nonconvulsive status epilepticus after ischemic stroke: a hospital-based stroke cohort study. J Neurol. 2014;261(11):2136-2142. doi: 10.1007/s00415-014-7471-z [DOI] [PubMed] [Google Scholar]
- 68.Madžar D, Kuramatsu JB, Gollwitzer S, et al. Seizures among long-term survivors of conservatively treated ICH patients: incidence, risk factors, and impact on functional outcome. Neurocrit Care. 2014;21(2):211-219. doi: 10.1007/s12028-014-9968-9 [DOI] [PubMed] [Google Scholar]
- 69.Mansour S, Youness M, Cherri S, et al. Assessment of the incidence and risk factors of early poststroke seizures in Lebanese patients. Brain Behav. 2021;11(11):e02204. doi: 10.1002/brb3.2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Reuck JL, Van Maele G. Seizures and epilepsy in patients with a posterior circulation infarct. J Stroke Cerebrovasc Dis. 2012;21(1):1-4. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 71.Chou CC, Shih YC, Chiu HH, et al. Strategic infarct location for poststroke seizure. Neuroimage Clin. 2022;35:103069. doi: 10.1016/j.nicl.2022.103069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka T, Fukuma K, Abe S, et al. Association of cortical superficial siderosis with poststroke epilepsy. Ann Neurol. 2023;93(2):357-370. doi: 10.1002/ana.26497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Reuck J, De Clerck M, Van Maele G. Vascular cognitive impairment in patients with late-onset seizures after an ischemic stroke. Clin Neurol Neurosurg. 2006;108(7):632-637. doi: 10.1016/j.clineuro.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 74.Lekoubou A, Ba DM, Nguyen C, et al. Poststroke seizures and the risk of dementia among young stroke survivors. Neurology. 2022;99(4):e385-e392. doi: 10.1212/WNL.0000000000200736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Selim H. Prestroke cognitive decline and early epileptic seizures after stroke as predictors of new onset dementia. Egypt J Neurol Psychiat Neurosurg. 2009;46(2):579-587. [Google Scholar]
- 76.Arntz RM, Maaijwee NAM, Rutten-Jacobs LCA, et al. Epilepsy after TIA or stroke in young patients impairs long-term functional outcome: the FUTURE Study. Neurology. 2013;81(22):1907-1913. doi: 10.1212/01.wnl.0000436619.25532.f3 [DOI] [PubMed] [Google Scholar]
- 77.Redfors P, Holmegaard L, Pedersen A, Jern C, Malmgren K. Long-term follow-up of poststroke epilepsy after ischemic stroke: room for improved epilepsy treatment. Seizure. 2020;76:50-55. doi: 10.1016/j.seizure.2020.01.009 [DOI] [PubMed] [Google Scholar]
- 78.So EL, Annagers JF, Hauser WA, O’Brien PC, Whisnant JP. Population-based study of seizure disorders after cerebral infarction. Neurology. 1996;46(2):350-355. doi: 10.1212/wnl.46.2.350 [DOI] [PubMed] [Google Scholar]
- 79.van Tuijl JH, van Raak EPM, van Oostenbrugge RJ, Aldenkamp AP, Rouhl RPW. Cognition and quality of life in patients with poststroke epilepsy: a case-control study. Epilepsy Behav. 2020;104(Pt B):106444. doi: 10.1016/j.yebeh.2019.106444 [DOI] [PubMed] [Google Scholar]
- 80.Do PT, Chen LY, Chan L, Hu CJ, Chien LN. Risk factors for postischemic stroke epilepsy in young adults: a nationwide population-based study in Taiwan. Front Neurol. 2022;13:880661. doi: 10.3389/fneur.2022.880661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hansen J, Åsberg S, Kumlien E, Zelano J. Cause of death in patients with poststroke epilepsy: results from a nationwide cohort study. PLoS One. 2017;12(4):e0174659. doi: 10.1371/journal.pone.0174659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Terman SW, Aubert CE, Hill CE, Skvarce J, Burke JF, Mintzer S. Cardiovascular disease risk, awareness, and treatment in people with epilepsy. Epilepsy Behav. 2021;117:107878. doi: 10.1016/j.yebeh.2021.107878 [DOI] [PubMed] [Google Scholar]
- 83.Verrier RL, Pang TD, Nearing BD, Schachter SC. The epileptic heart: concept and clinical evidence. Epilepsy Behav. 2020;105:106946. doi: 10.1016/j.yebeh.2020.106946 [DOI] [PubMed] [Google Scholar]
- 84.Lee-Lane E, Torabi F, Lacey A, et al. Epilepsy, antiepileptic drugs, and the risk of major cardiovascular events. Epilepsia. 2021;62(7):1604-1616. doi: 10.1111/epi.16930 [DOI] [PubMed] [Google Scholar]
- 85.Pang T, Yu C, Rodriguez Y, et al. Detection of the epileptic heart with established cardiac markers in EMU admission 12-lead ECGs. Accessed July 13, 2023. https://aesnet.org/abstractslisting/detection-of-the-epileptic-heart-with-established-cardiac-markers-in-emu-admission-12-lead-ecgs
- 86.Galovic M, Döhler N, Erdélyi-Canavese B, et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol. 2018;17(2):143-152. doi: 10.1016/S1474-4422(17)30404-0 [DOI] [PubMed] [Google Scholar]
- 87.Verdelho A, Biessels GJ, Chabriat H, et al. Cerebrovascular disease in patients with cognitive impairment: a white paper from the ESO dementia committee—a practical point of view with suggestions for the management of cerebrovascular diseases in memory clinics. Eur Stroke J. 2021;6(2):111-119. doi: 10.1177/2396987321994294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer disease: causes and clinical relevance. Lancet Neurol. 2017;16(4):311-322. doi: 10.1016/S1474-4422(17)30044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cordonnier C, Hénon H, Derambure P, Pasquier F, Leys D. Early epileptic seizures after stroke are associated with increased risk of new-onset dementia. J Neurol Neurosurg Psychiatry. 2007;78(5):514-516. doi: 10.1136/jnnp.2006.105080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kwon SY, Obeidat AZ, Sekar P, et al. Risk factors for seizures after intracerebral hemorrhage: Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) Study. Clin Neurol Neurosurg. 2020;192:105731. doi: 10.1016/j.clineuro.2020.105731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Husein N, Josephson CB, Keezer MR. Understanding cardiovascular disease in older adults with epilepsy. Epilepsia. 2021;62(9):2060-2071. doi: 10.1111/epi.16991 [DOI] [PubMed] [Google Scholar]
- 92.Kuohn LR, Herman AL, Soto AL, et al. Hospital revisits for post-ischemic stroke epilepsy after acute stroke interventions. J Stroke Cerebrovasc Dis. 2022;31(1):106155. doi: 10.1016/j.jstrokecerebrovasdis.2021.106155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zelano J, Holtkamp M, Agarwal N, Lattanzi S, Trinka E, Brigo F. How to diagnose and treat poststroke seizures and epilepsy. Epileptic Disord. 2020;22(3):252-263. doi: 10.1684/epd.2020.1159 [DOI] [PubMed] [Google Scholar]
- 94.Myint PK, Staufenberg EF, Sabanathan K. Poststroke seizure and poststroke epilepsy. Postgrad Med J. 2006;82(971):568-572. doi: 10.1136/pgmj.2005.041426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mishra NK, Engel J Jr, Liebeskind DS, et al. ; for International Post Stroke Epilepsy Research Consortium (IPSERC) . International Post Stroke Epilepsy Research Consortium (IPSERC): a consortium to accelerate discoveries in preventing epileptogenesis after stroke. Epilepsy Behav. 2022;127:108502. doi: 10.1016/j.yebeh.2021.108502 [DOI] [PubMed] [Google Scholar]
- 96.Ren Z, Wen Q, Yan X, Wang Y, Zhang Y. Poststroke epilepsy and risk of all-cause mortality: a systematic review and meta-analysis of cohort studies. Clin Neurol Neurosurg. 2022;220:107362. doi: 10.1016/j.clineuro.2022.107362 [DOI] [PubMed] [Google Scholar]
- 97.Lin HY, Wei QQ, Huang JY, et al. Relationship between mortality and seizures after intracerebral hemorrhage: a systematic review and meta-analysis. Front Neurol. 2022;13:922677. doi: 10.3389/fneur.2022.922677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ray D, Muñoz A, Zhang M, et al. Meta-analysis under imbalance in measurement of confounders in cohort studies using only summary-level data. BMC Med Res Methodol. 2022;22(1):143. doi: 10.1186/s12874-022-01614-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Misra S, Quinn TJ, Kwan P, Mishra NK. An individual patient data analysis protocol to characterize poststroke epilepsy population and their outcomes. Accessed April 10, 2023. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=410055
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Detailed Search Strategy
eFigure 1. The List of Countries Representing the Number of Studies
eFigure 2. Funnel Plot to Test the Presence of Publication Bias
eFigure 3. Sensitivity Analysis Using the Leave-One-Out Method
eFigure 4. Subgroup Analysis Based on Seizure Subtypes
eFigure 5. Subgroup Analysis Based on Stroke Subtypes
eFigure 6. Subgroup Analysis Based on Seizure and Stroke Subtypes
eFigure 7. Subgroup Analysis on Patients With Status Epilepticus
eFigure 8. Sensitivity Analysis Using the Leave-One-Out Method
eFigure 9. Subgroup Analysis Based on Seizure Subtypes
eFigure 10. Subgroup Analysis Based on Stroke Subtypes
eFigure 11. Subgroup Analysis Based on Seizure and Stroke Subtypes
eFigure 12. Sensitivity Analysis Using the Leave-One-Out Method
eFigure 13. Subgroup Analysis Based on Seizure Subtypes
eFigure 14. Subgroup Analysis Based on Stroke Subtypes
eFigure 15. Association of Poststroke Seizures With Recurrent Stroke
eTable 1. Definition of Early and Late Seizures Used by the Studies
eTable 2. Baseline Characteristics of Studies
eTable 3. Risk Factors Associated With PSS
eTable 4. Risk of Bias (Quality) Assessment for the Studies
eTable 5. Association of Disability (Mean mRS Score) With Poststroke Seizures
eTable 6. Association of Recurrent Stroke and Dementia With Poststroke Seizures
eTable 7. Comparison of Our Meta-Analysis With Previously Published Meta-Analyses
eTable 8. Future Directions for PSS Studies
eReferences
Data Sharing Statement