Abstract
Background
In a previous meta-analysis, the use of serotonin1A(5-HT1A) receptor partial agonists of the azapirone class as an add-on therapy was associated with beneficial effects on positive symptoms and attention/processing speed in schizophrenia patients. This meta-analysis builds on that study by examining the effects of adjunctive treatment with 5-HT1A partial agonists in improving other domains of neurocognitive function in schizophrenia patients.
Methods
A literature search was performed from 1987 to May 2023 to identify randomized controlled trials. The standardized mean difference (SMD) with 95 % confidence intervals (CI) was calculated when there were two or more studies. Four studies, involving 313 patients, met the inclusion criteria and were used in the analysis.
Results
5-HT1A partial agonists (buspirone or tandospirone) did not have a significant effect on verbal learning (SMD = 0.08, 95 % CI = −0.31 to 0.47) or working memory (SMD = 0.15, 95 % CI = −0.09 to 0.39). Regarding executive functions (Wisconsin Card Sorting Test), positive but non-significant results were seen with the category number (SMD = 0.26, 95 % CI = −0.81 to 1.32), while non-significant effects were noted for percent preservation errors (SMD = −0.10, 95 % CI = −0.53 to 0.33).
Conclusions
The absence of any significant benefits in the cognitive domains studied here may have been due to the variance in the concomitant medication (typical vs atypical antipsychotic drugs), the level of cognition at baseline, or other factors. Further studies with various types of 5-HT1A agonists are warranted to examine the potential cognitive efficacy of stimulating these receptors.
Keywords: 5-HT1A receptor partial agonist, Azapirone derivative, Cognitive dysfunction, Atypical antipsychotic drugs, Schizophrenia
Highlights
-
•
Effect of adjunctive treatment with 5-HT1A receptor partial agonists were examined based on data from 313 patients with schizophrenia.
-
•
Augmentation therapy with buspirone or tandospirone was found to show limited effects on verbal learning, working memory, and executive function.
-
•
Further studies to evaluate cognitive benefits of other types of 5-HT1A agonists are warranted.
1. Introduction
Cognitive impairment is one of the core symptoms in patients with schizophrenia. It is estimated that up to 85 % of these patients experience impairments in several domains of cognitive function, such as verbal memory, working memory and executive functions (Jauhar et al., 2022; Meltzer and Sumiyoshi, 2008). Importantly, cognitive deficits have been shown to play a central role in predicting vocational and social outcomes in schizophrenia patients (Galderisi et al., 2020; Green, 1996; Heaton et al., 2001; Keefe et al., 2005; Sumiyoshi et al., 2016; Wittorf et al., 2008). Therefore, developing treatment approaches that target cognitive function in schizophrenia has been a priority for researchers.
Atypical antipsychotic drugs (AAPDs), with clozapine as the prototype, have been reported to be more effective than typical antipsychotic drugs (TAPDs) in treating psychotic and mood symptoms, as well as cognitive impairment in patients with schizophrenia (Meltzer, 2013, Meltzer, 2017; Meltzer and Gadaleta, 2021). The distinctive properties of AAPDs have been discussed in relation to their high affinity, either as an agonist or antagonist, for several serotonin (5-hydroxytryptamine; 5-HT) receptor subtypes (Meltzer and Gadaleta, 2021; Sumiyoshi, 2008). For example, a relatively high affinity for 5-HT2A receptors versus dopamine (DA)-D2 receptors may be related to the difference between AAPDs and TAPDs (Meltzer and Massey, 2011). This notion has been supported by in vivo experiments with rodents (Stockmeier et al., 1993; Sumiyoshi et al., 1994a, Sumiyoshi et al., 1994b; Sumiyoshi et al., 1993; Sumiyoshi et al., 1995), and may describe the mechanisms of some of the AAPDs currently used, e.g., risperidone, olanzapine, and quetiapine (Araki et al., 2006; Meltzer et al., 2003; Sumiyoshi et al., 2006; Sumiyoshi et al., 2003a, Sumiyoshi et al., 2003b).
5-HT1A receptors have also been thought to mediate the efficacy of several AAPDs (e.g., aripiprazole, lurasidone, brexpiprazole, risperidone, olanzapine) (Lemoine et al., 2012; Meltzer and Sumiyoshi, 2008; Newman-Tancredi and Albert, 2012; Sumiyoshi, 2020). 5-HT1A receptors are located widely in the areas of the brain governing cognitive and emotional processes, e.g., in the frontal cortex, hippocampus, and amygdala (Le François et al., 2008). The 5-HT1A partial agonist actions of AAPDs provide a preferential increase in extracellular concentrations of DA and acetylcholine in the prefrontal cortex relative to subcortical areas (Ichikawa et al., 2001; Li et al., 1998; Masana et al., 2011). As enhancement of prefrontal DA transmissions is thought to regulate the activities of mesolimbic DA neurons (Davis et al., 1991; Deutch, 1992), 5-HT1A partial agonism may alleviate psychotic symptoms, and cognitive impairment in schizophrenia patients (Ichikawa et al., 2001; Meltzer and Sumiyoshi, 2008; Newman-Tancredi and Albert, 2012; Newman-Tancredi and Kleven, 2011; Sumiyoshi, 2020; Yamada et al., 2023). However, the cognitive benefit of AAPDs has been found to be only small to moderate (Désaméricq et al., 2014; Keefe et al., 2007; Woodward et al., 2005; Woodward et al., 2007). Therefore, additional means are needed to develop novel strategies to improve cognitive impairment in schizophrenia (Leucht et al., 2009; Meltzer, 2013; Meltzer et al., 2012).
Azapirone derivatives, such as buspirone and tandospirone exhibit potent binding affinities for 5-HT1A receptors, with Ki values of 20 nM and 27 nM, while their affinities for D2 receptors are 240 nM and 1700 nM (Hamik et al., 1990; Matheson et al., 1994; Newman-Tancredi and Kleven, 2011). Several clinical trials have been conducted to determine whether add-on therapy with buspirone or tandospirone can improve cognitive functioning (PišKulić et al., 2009; Sumiyoshi et al., 2001b; Sumiyoshi et al., 2007; Wang et al., 2019), including studies reporting negative findings (PišKulić et al., 2009). In a recent meta-analysis we conducted, the addition of buspirone or tandospirone to ongoing treatment with antipsychotic drugs was found to improve attention/processing speed, in addition to positive symptoms in patients with schizophrenia (Yamada et al., 2023). Since attention/processing speed is thought to represent the construct of overall cognitive impairment of schizophrenia (Reichenberg, 2010), the results from our previous meta-analysis (Yamada et al., 2023) support the interpretation that 5-HT1A receptor stimulation produces cognitive benefits.
In spite of the above evidence, further consideration is necessary to determine whether augmentation therapy with 5-HT1A partial agonists produce benefits for the individual domains of cognitive function. This idea is particularly relevant not only to verbal learning memory that has been thought to largely affect functional outcomes (Green et al., 2000), but also cognitive domains associated with the function of the prefrontal cortex, e.g., working memory and executive function (Sheffield and Barch, 2016; Sheffield et al., 2015). These types of cognitive function have been targeted in pivotal studies of pharmacological cognitive enhancement, by means of augmentation therapy with tandospirone, which report improvement in verbal learning memory and its organization (Sumiyoshi et al., 2001a; Sumiyoshi et al., 2001b), as well as executive function (Sumiyoshi et al., 2001b) in patients with schizophrenia. These cognitive benefits are consistent with the data from animal studies where the systemic administration of 5-HT1A partial agonists increased extracellular DA concentrations in the prefrontal cortex. Importantly, all of the above cognitive domains have been considered to predict vocational and social outcomes (Meltzer and Sumiyoshi, 2008; Sumiyoshi et al., 2001b; Green et al., 2000).
In view of the continued uncertainty regarding the cognitive benefits of stimulation of 5-HT1A receptors (PišKulić et al., 2009; Yasuno et al., 2003), we conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to examine the possible efficacy of 5-HT1A receptor partial agonists of the azapirone class for improving specific domains of cognitive function. In line with the above discussions, verbal learning, working memory, and executive function were a focus of this study. We hypothesized that the addition of buspirone or tandospirone to ongoing treatment with antipsychotic drugs would be beneficial for improving specific neurocognitive functions in patients with schizophrenia.
2. Methods
2.1. Inclusion criteria and search strategies
We conducted the current meta-analysis on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The inclusion criteria were as follows: (1) RCTs, (2) human studies; studies that (3) targeted patients with schizophrenia or schizoaffective disorder, (4) evaluated the effect of cognitive functions, (5) provided sufficient data to evaluate effect sizes, (6) were written in English, (7) had a duration where the drug administration was 4 weeks or longer. Studies focusing on schizophrenia or schizoaffective disorder were included, based on previous considerations (Keepers et al., 2020; Yamada et al., 2023).
RY and AW independently conducted a literature search using PubMed, the Cochrane Library, and PsycINFO databases from 1987 until 23 May 2023 using the following keywords: “alnespirone” OR” binospirone” OR “buspirone” OR “enilospirone” OR “eptapirone” OR “gepirone” OR “ipsapirone” OR “revospirone” OR “tandospirone” OR “zalospirone” AND “schizophrenia”. Additional studies were obtained by scanning the reference lists of the included studies and previous reviews. TS approved the final list of included studies.
2.2. Data extraction and quality assessment
The information for each study was independently extracted by RY and AW with coding discrepancies resolved by TS. When the data were not fully described in the published article, the corresponding authors were contacted and asked to provide additional information. If there was no response to our queries, we tried to obtain the necessary information by measuring the length of graphs showing non-tabulated results. If none of these methods proved feasible, then the studies were excluded from the analysis. The outcome measures were classified into verbal learning, working memory, and executive function. The Cochrane risk of bias tool was used to evaluate the methodological quality of each RCT (Higgins et al., 2011).
RY and AW independently assessed the following characteristics of each trial: (i) random sequence generation, (ii) blinding of subjects and personnel, (iii) blinding of outcome assessment, (iv) incomplete outcome data, (v) selective reporting, and (vi) other potential sources of bias. The assessment was done by evaluating what was reported in the selected articles and accessing and evaluating the study protocols where available. If necessary, any disagreements were thereafter resolved by TS.
2.3. Statistical analysis
We based the analyses on intent-to-treat (ITT) or modified ITT data (i.e., at least one dose or at least one follow-up assessment); no data from observed cases analysis were included. Statistical analyses were performed using Review Manager 5.3 for Windows. The effect size was calculated based on the difference in the change of baseline scores, between the experimental vs. control conditions. When no data on the mean change from the baseline were available, we calculated the mean change and standard deviation based on the assumption that the correlation between the scores at follow-up and those at the baseline was 0.5. For continuous data, the standardized mean difference (SMD = Hedges' g as an effect size measure) was used, considering the correction for small sample bias (Lakens, 2013). If data for two or more outcome measures were provided, we selected a single outcome based on the focus of the meta-analysis (Scammacca et al., 2014). We used a fixed-effects model if homogeneity (p≧0.05) was found and a random-effects model (DerSimonian and Laird, 1986) if not (p < 0.05). Because a meta-analysis requires at least two studies in theory (Pigott, 2012), meta-analyses were performed when the mean effect was evaluated in at least two studies. Finally, funnel plots were inspected visually to explore the possibility of publication bias.
3. Results
3.1. Systematic review
Fig. 1 shows the PRISMA study selection flowchart. The initial search yielded 269 potential articles. After removing duplicates, 185 articles were screened. The four studies included in the systematic review included 313 patients (the experimental group n = 159; the control group n = 154). Characteristics of the included studies are shown in Table 1. These studies were conducted in the USA, Australia, Japan, and China. Three of the four studies were on outpatients. Sample sizes ranged from 9 to 99 and 9–97 participants in each of the groups. Three studies included only schizophrenia patients, while one study (PišKulić et al., 2009) included both schizophrenia (89.5 %) and schizoaffective disorder (10.5 %) patients. The mean age ranged from 27.8 to 43.4 years (in the experimental group), and from 31.8 to 39.7 (in the control group). The proportion of men ranged from 56.2 % to 77.8 %. The mean duration of illness ranged from 6.3 to 19.0 years (in the experimental group), and from 7.5 to 19.0 years (in the control group). The daily dose of 5-HT1A partial agonists (buspirone or tandospirone) ranged from 21.6 to 30 mg/day, while the mean duration of its use ranged from 6 to 24 weeks. As concomitant antipsychotic drugs, AAPDs were used in three studies (Sumiyoshi et al., 2007; PišKulić et al., 2009; Wang et al., 2019). More specifically, they were risperidone (Sumiyoshi et al., 2007; PišKulić et al., 2009; Wang et al., 2019), olanzapine (Sumiyoshi et al., 2007; PišKulić et al., 2009; Wang et al., 2019), clozapine (Sumiyoshi et al., 2007; PišKulić et al., 2009; Wang et al., 2019), quetiapine (PišKulić et al., 2009; Wang et al., 2019), ziprasidone (Sumiyoshi et al., 2007; Wang et al., 2019), amisulpride (PišKulić et al., 2009), and aripiprazole (Wang et al., 2019). Treatment with TAPDs (haloperidol, sulpiride, or pimozide) was ongoing in one study (Sumiyoshi et al., 2001b). The mean symptom severity score ranged from 48.03 to 52.8(in the experimental group), and 47.49 to 55.4(in the control group), as measured by the Positive and Negative Syndrome Scale, or from 16.8 to 20.6 (in the experimental group), and from 18.9 to 20.0 (in the control group), as measured by the Brief Psychiatric Rating Scale (Table 1). Table 2 shows neurocognitive outcomes used in the selected studies (verbal learning, working memory, and executive function).
Fig. 1.
PRISMA study selection flowchart.
Table 1.
Selected characteristics of the included studies.
| Study, country | Total | Men/Women | Drug | N | Age | Duration | Dose | Out/Inpatient | Duration of illness | Symptom severity | Concomitant txt | Neurocognitive outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | (%) | (mean ± SD. year) | (week) | (mg/day) | (year) | (BPRS or PANSS) | |||||||
| 1 | Sumiyoshi et al. | 26 | 57.7/42.3 | 6 | 30 (fixed) | Outpatient | BPRS | Typical antipsychotic | WMS-R(Revised verbal memory composite) Wisconsin Card Sorting Test |
||||
| (2001) Japan | Tan | 15 | Tan: 27.8 ± 6.30, | Tan: 6.3 ± 4.30, | Tan: 16.8 ± 9.00 | ||||||||
| Pbo | 11 | Pbo: 31.8 ± 9.40 | Pbo: 7.5 ± 5.40 | Pbo: 18.9 ± 8.70 | |||||||||
| 2 | Sumiyoshi et al. | 73 | 56.2/43.8 | 24 | 30 (fixed) | Outpatient | BPRS | Atypical antipsychotic | Auditory Consonants Trigram California Verbal Learning Test Wisconsin Card Sorting Test |
||||
| (2007) USA | Bus | 36 | Bus: 40.5 ± 11.80, | Bus: 19.0 ± 11.20 | Bus: 20.6 ± 8.00 | ||||||||
| Pbo | 37 | Pbo: 39.7 ± 12.50 | Pbo: 19.0 ± 13.50 | Pbo: 20.0 ± 8.60 | |||||||||
| 3 | PišKulić et al. | 18 | 77.8/22.2 | 6 | 21.6 ± 3.75 | Outpatient | PANSS | Atypical antipsychotic | Hopkins verbal learning test The n-back component of the task |
||||
| (2009) Australia | Bus | 9 | Bus: 43.4 ± 10.30 | Bus: 15.2 ± 10.20 | Bus:52.8 ± 9.30 | ||||||||
| Pbo | 9 | Pbo: 37.2 ± 13.70 | Pbo: 11.7 ± 9.40 | Pbo:55.4 ± 14.90 | |||||||||
| 4 | Wang et al. | 196 | 70.9/29.1 | 24 | 30 (fixed) | PANSS | Atypical antipsychotic | WAIS-RC (digital span test, digital symbols) | |||||
| (2019) China | Bus | 99 | Bus: 39.81 ± 10.11 | – | Bus: 12.01 ± 8.87 | Bus: 48.03 ± 12.95 | |||||||
| Con | 97 | Con: 39.02 + 9.56 | Con: 11.24 + 8.28 | Con: 47.49 + 12.32 |
BPRS, Brief Psychiatric Rating Scale; PANSS, Positive and Negative Syndrome Scale; Bus, buspirone; Pbo, placebo; Tan, tandospirone; Con, Control; txt, treatment.
Table 2.
Neurocognitive outcomes used in the selected studies.
| Cognitive domain | Cognitive variable |
|---|---|
| Verbal learning | Wechsler Memory Scale—Revised verbal memory composite |
| California Verbal Learning Test | |
| Hopkins verbal learning test | |
| Working memory | Auditory Consonants Trigram |
| digital span test (WAIS-R) | |
| The n-back component of the task | |
| Executive function | Wisconsin Card Sorting |
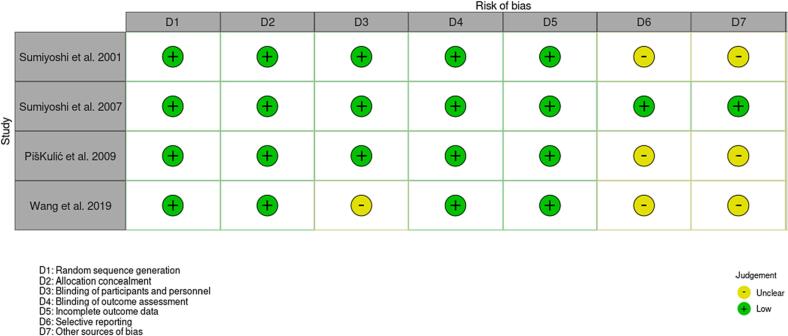
The summary for the risk of bias is shown in Fig. 2. Three studies had an unclear risk of bias, in relation to either the blinding of the participants and personnel, selective reporting and other sources of bias. We found little indication of publication bias for the outcomes. Visual inspection of the funnel plots for verbal learning, working memory, and executive function suggested symmetry (Fig. 3(A), (B), (C), (D)).
Fig. 2.
Risk of bias.
Fig. 3.
Funnel Plot for Verbal learning (A), working memory (B), Wisconsin Card Sorting Test (category number) (C), and Wisconsin Card Sorting Test (D)
3.2. Meta-analysis
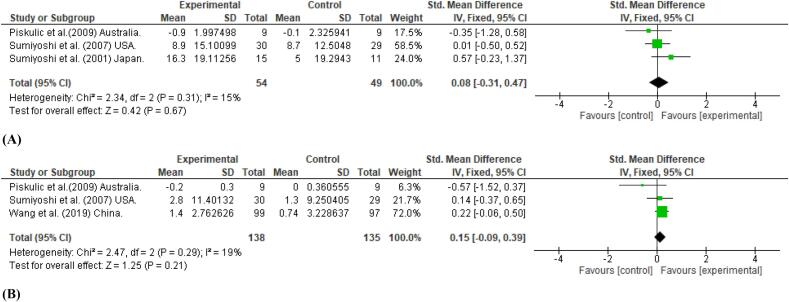
We performed meta-analyses on verbal learning, working memory, and executive function (Wisconsin Card Sorting Test (WCST), as these cognitive domains were examined in two or more studies. The use of 5-HT1A partial agonists did not have a statistically significant effect on verbal learning memory (SMD = 0.08, 95 % CI = −0.31 to 0.47), or working memory (SMD = 0.15, 95 % CI = −0.09 to 0.39) (Fig. 4(A), (B)). In terms of executive function, as measured by the WCST, positive but non-significant result was seen with the category number (SMD = 0.26, 95 % CI = −0.81 to 1.32), while a non-significant effect was noted for percent preservation errors (SMD = −0.10, 95 % CI = −0.53 to 0.33) (Fig. 4 (C), (D)).
Fig. 4.
Mean effect of 5-HT1A receptor partial agonists of the azapirone class as an add-on therapy on Verbal learning (A); working memory (B); Wisconsin Card Sorting Test (category number) (C); and Wisconsin Card Sorting Test (percent preservations) (D).
4. Discussion
This study examined the potential benefits of the adjunctive use of the azapirone derivatives buspirone or tandospirone, for potentiating the treatment of cognitive impairment in patients with schizophrenia. Our initiative was based on the ability of these 5-HT1A partial agonists to enhance attention/processing speed, a central construct of cognitive function, as shown in a recent meta-analysis (Yamada et al., 2023). However, contrary to those results, the add-on therapy was not found to improve verbal memory, working memory, or executive function.
The absence of a significant positive influence on the cognitive function domains examined in this study might be explained in several ways. First, the current meta-analysis included studies in which the patients who participated had already been treated with AAPDs, i.e., clozapine, risperidone, olanzapine, or ziprasidone, (Sumiyoshi et al., 2007; PišKulić et al., 2009; Wang et al., 2019) for more than three months. It is possible that the intrinsic 5-HT1A agonist activity of the AAPDs, by causing an increase in DA and/or Ach release, may have limited the ability of augmentation therapy with buspirone to improve specific cognitive domains. In support of this consideration, the 5-HT1A antagonist WAY 100635 inhibits the increase in DA release produced by AAPDs, such as clozapine and ziprasidone which are 5-HT1A partial agonists (Ichikawa et al., 2001; Chung et al., 2004) by themselves, as well as olanzapine and risperidone, which do not directly interact with 5-HT1A receptors (Díaz-Mataix et al., 2005; Ichikawa et al., 2001).
Second, none of the RCTs included in this meta-analysis set any criteria for the baseline scores on the cognitive function. The distribution of cognitive performance at baseline would obviously be expected to influence the results of the RCTs. In fact, some RCTs with schizophrenia patients have used inclusion criteria for the cognitive function scores e.g., by setting a cutoff point (Narita et al., 2017). Further RCTs are thus needed to examine if augmentation with 5-HT1A partial agonists is beneficial for improving multiple neurocognitive functions in patients with substantial cognitive decline.
Based on the results of this study, the future prospects of 5-HT1A partial agonists as a cognitive enhancer deserve further consideration. The benefits of adjunct treatment also depend on the properties (e.g., receptor affinity) of antipsychotic drugs administered, which may itself have direct- or indirect-acting 5-HT1A agonist properties. It is possible that the putative pro-cognitive effect of 5-HT1A partial agonists examined here (buspirone, tandospirone) may be more pronounced when added to treatment with an antipsychotic drug with a high affinity for 5-HT1A receptors, such as lurasidone. In fact, lurasidone by itself has been reported to improve working memory and executive function in patients with schizophrenia (Corponi et al., 2019; Meltzer et al., 2020). Therefore, augmentation with 5-HT1A partial agonists of the azapirone class may be beneficial when given to patients who can tolerate only to a limited dose of lurasidone due to adverse events (e.g., parkinsonism, insomnia) at a higher dose of the drug. This concept may be relevant to the clinical observation that clozapine, with higher 5-HT1A/D2 ratios of binding affinity than that of lurasidone (Ishibashi et al., 2010; Meltzer and Gadaleta, 2021), is associated with minimal incidence of extrapyramidal side effects (Miller, 2000).
It is also worthwhile to note that there are only two partial agonists currently available, i.e., buspirone and tandospirone. These compounds are structurally related azapirones, and are known to be rapidly metabolized to 1-PP, an alpha2 adrenoceptor antagonist (Gower and Tricklebank, 1988; Huang et al., 2017), which could have off-target effects. Thus, the potential benefits of targeting 5-HT1A receptors as a mechanism for improving therapy of cognitive deficits deserve additional examinations. In this line, NLX-101, a selective biased agonist acting on post-synaptic cortical neurons (Depoortère et al., 2010; Depoortere et al., 2021; van Goethem et al., 2015) is drawing attention. Previous studies have shown that NLX-101 provides a beneficial influence on working memory, as evaluated by the delayed non-matching to position task representing cholinergic tone (Depoortere et al., 2021). The drug has also been reported to alleviate cognitive impairment induced by N-methyl-d-aspartate receptor blockade, as demonstrated by the hole-board test (Depoortère et al., 2010) and the pattern separation test (van Goethem et al., 2015). These findings may encourage clinical trials of novel 5-HT1A receptor-related compounds for cognitive enhancement in patients with schizophrenia.
This study has several limitations. First, the number of studies included in the meta-analysis was small. To fully understand the potential merit of 5-HT1A partial agonists for individual cognitive domains, further studies are needed. Second, most studies included in this meta-analysis focused on short-term (6–8 weeks) outcomes, possibly underestimating the potential long-term benefits of the 5-HT1A partial agonists used in these studies. Third, as this meta-analysis aimed to examine the effect of 5-HT1A receptor partial agonists of the azapirone class, other 5-HT1A partial agonists, such as NLX-101, also need to be tested as an add-on agent.
5. Conclusions
The results of this meta-analysis did not show that augmentation with 5-HT1A partial agonists of the azapirone class produce sufficient effects on some domains of cognitive function in patients with schizophrenia. Further studies with other compounds and more refined protocols are warranted to examine the potential cognitive efficacy of stimulation of 5-HT1A receptors.
CRediT authorship contribution statement
RY conducted the statistical analyses, reviewed the literature, and wrote the first and subsequent drafts of the manuscript. RY, AW, and TS developed the study concept and hypothesis, managed the data collection, contributed to the interpretation of the results, and assisted in writing the manuscript. TS, YY and AS contributed to the interpretation of the results, revised drafts of the manuscript, and provided feedback with particular expertise. All authors contributed to and have approved the final version of the manuscript.
Funding sources
This study was supported by the Japan Society for the Promotion of Science KAKENHI (No. 20H03610, 23K18998), Intramural Research Grant (3-1, 5-3) for Neurological and Psychiatric Disorders of the National Center of Neurology and Psychiatry, the Japan Health Research Promotion Bureau Grants (2021-B-01) and AMED Grants (22dk0307114).
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: TS has received manuscript fees, speaker's honoraria, consultation fees, and/or grant support from Janssen Pharmaceuticals, Meiji Seika Pharma, Takeda Pharmaceutical, Otsuka Pharmaceutical, Sumitomo Pharma, Shionogi, Lundbeck Japan, Boehringer Japan, and VeraSci.
Acknowledgements
Not applicable.
References
- Araki T., Yamasue H., Sumiyoshi T., Kuwabara H., Suga M., Iwanami A., Kato N., Kasai K. Perospirone in the treatment of schizophrenia: effect on verbal memory organization. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(2):204–208. doi: 10.1016/j.pnpbp.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Chung Y.C., Li Z., Dai J., Meltzer H.Y., Ichikawa J. Clozapine increases both acetylcholine and dopamine release in rat ventral hippocampus: role of 5-HT1A receptor agonism. Brain Res. 2004;1023(1):54–63. doi: 10.1016/j.brainres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Corponi F., Fabbri C., Bitter I., Montgomery S., Vieta E., Kasper S., Pallanti S., Serretti A. Novel antipsychotics specificity profile: a clinically oriented review of lurasidone, brexpiprazole, cariprazine and lumateperone. Eur. Neuropsychopharmacol. 2019;29(9):971–985. doi: 10.1016/j.euroneuro.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Davis K.L., Kahn R.S., Ko G., Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am. J. Psychiatry. 1991;148(11):1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Depoortère R., Auclair A.L., Bardin L., Colpaert F.C., Vacher B., Newman-Tancredi A. F15599, a preferential post-synaptic 5-HT1A receptor agonist: activity in models of cognition in comparison with reference 5-HT1A receptor agonists. Eur. Neuropsychopharmacol. 2010;20(9):641–654. doi: 10.1016/j.euroneuro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Depoortere R.Y., Auclair A.L., Newman-Tancredi A. NLX-101, a cortical 5-HT(1A) receptor biased agonist, reverses scopolamine-induced deficit in the delayed non-matching to position model of cognition. Brain Res. 2021;1765 doi: 10.1016/j.brainres.2021.147493. [DOI] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Désaméricq G., Schurhoff F., Meary A., Szöke A., Macquin-Mavier I., Bachoud-Lévi A.C., Maison P. Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur. J. Clin. Pharmacol. 2014;70(2):127–134. doi: 10.1007/s00228-013-1600-y. [DOI] [PubMed] [Google Scholar]
- Deutch A.Y. The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J. Neural Transm. Suppl. 1992;36:61–89. doi: 10.1007/978-3-7091-9211-5_5. [DOI] [PubMed] [Google Scholar]
- Díaz-Mataix L., Scorza M.C., Bortolozzi A., Toth M., Celada P., Artigas F. Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J. Neurosci. 2005;25(47):10831–10843. doi: 10.1523/jneurosci.2999-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi S., Rucci P., Mucci A., Rossi A., Rocca P., Bertolino A., Aguglia E., Amore M., Bellomo A., Bozzatello P., Bucci P., Carpiniello B., Collantoni E., Cuomo A., Dell’Osso L., Di Fabio F., di Giannantonio M., Gibertoni D., Giordano G.M.…Maj M. The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World Psychiatry. 2020;19(1):81–91. doi: 10.1002/wps.20700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower A.J., Tricklebank M.D. Alpha 2-adrenoceptor antagonist activity may account for the effects of buspirone in an anticonflict test in the rat. Eur. J. Pharmacol. 1988;155(1–2):129–137. doi: 10.1016/0014-2999(88)90410-4. [DOI] [PubMed] [Google Scholar]
- Green M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Braff D.L., Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr. Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Hamik A., Oksenberg D., Fischette C., Peroutka S.J. Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites. Biol. Psychiatry. 1990;28(2):99–109. doi: 10.1016/0006-3223(90)90627-e. [DOI] [PubMed] [Google Scholar]
- Heaton R.K., Gladsjo J.A., Palmer B.W., Kuck J., Marcotte T.D., Jeste D.V. Stability and course of neuropsychological deficits in schizophrenia. Arch. Gen. Psychiatry. 2001;58(1):24. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Yang J., Yang S., Cao S., Qin D., Zhou Y., Li X., Ye Y., Wu J. Role of tandospirone, a 5-HT1A receptor partial agonist, in the treatment of central nervous system disorders and the underlying mechanisms. Oncotarget. 2017;8(60):102705–102720. doi: 10.18632/oncotarget.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J., Ishii H., Bonaccorso S., Fowler W.L., O’Laughlin I.A., Meltzer H.Y. 5-HT2A and D2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J. Neurochem. 2001;76(5):1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Horisawa T., Tokuda K., Ishiyama T., Ogasa M., Tagashira R., Matsumoto K., Nishikawa H., Ueda Y., Toma S., Oki H., Tanno N., Saji I., Ito A., Ohno Y., Nakamura M. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J. Pharmacol. Exp. Ther. 2010;334(1):171–181. doi: 10.1124/jpet.110.167346. [DOI] [PubMed] [Google Scholar]
- Jauhar S., Johnstone M., McKenna P.J. Schizophrenia. Lancet. 2022;399(10323):473–486. doi: 10.1016/s0140-6736(21)01730-x. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Eesley C.E., Poe M.P. Defining a cognitive function decrement in schizophrenia. Biol. Psychiatry. 2005;57(6):688–691. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Bilder R.M., Davis S.M., Harvey P.D., Palmer B.W., Gold J.M., Meltzer H.Y., Green M.F., Capuano G., Stroup T.S., McEvoy J.P., Swartz M.S., Rosenheck R.A., Perkins D.O., Davis C.E., Hsiao J.K., Lieberman J.A. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch. Gen. Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Keepers G.A., Fochtmann L.J., Anzia J.M., Benjamin S., Lyness J.M., Mojtabai R., Servis M., Walaszek A., Buckley P., Lenzenweger M.F., Young A.S., Degenhardt A., Hong S.H. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am. J. Psychiatry. 2020;177(9):868–872. doi: 10.1176/appi.ajp.2020.177901. [DOI] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le François B., Czesak M., Steubl D., Albert P.R. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55(6):977–985. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Lemoine L., Becker G., Vacher B., Billard T., Lancelot S., Newman-Tancredi A., Zimmer L. Radiosynthesis and preclinical evaluation of 18F-F13714 as a fluorinated 5-HT1A receptor agonist radioligand for PET neuroimaging. J. Nucl. Med. 2012;53(6):969–976. doi: 10.2967/jnumed.111.101212. [DOI] [PubMed] [Google Scholar]
- Leucht S., Corves C., Arbter D., Engel R.R., Li C., Davis J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41. doi: 10.1016/s0140-6736(08)61764-x. [DOI] [PubMed] [Google Scholar]
- Li X.-M., Perry K.W., Wong D.T., Bymaster F.P. Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology. 1998;136(2):153–161. doi: 10.1007/s002130050551. [DOI] [PubMed] [Google Scholar]
- Masana M., Bortolozzi A., Artigas F. Selective enhancement of mesocortical dopaminergic transmission by noradrenergic drugs: therapeutic opportunities in schizophrenia. Int. J. Neuropsychopharmacol. 2011;14(1):53–68. doi: 10.1017/s1461145710000908. [DOI] [PubMed] [Google Scholar]
- Matheson G.K., Pfeifer D.M., Weiberg M.B., Michel C. The effects of azapirones on serotonin1A neurons of the dorsal raphe. Gen. Pharmacol. 1994;25(4):675–683. doi: 10.1016/0306-3623(94)90245-3. [DOI] [PubMed] [Google Scholar]
- Meltzer H.Y. Update on typical and atypical antipsychotic drugs. Annu. Rev. Med. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- Meltzer H.Y. New trends in the treatment of schizophrenia. CNS Neurol. Disord. Drug Targets. 2017;16(8):900–906. doi: 10.2174/1871527316666170728165355. [DOI] [PubMed] [Google Scholar]
- Meltzer H.Y., Gadaleta E. Contrasting typical and atypical antipsychotic drugs. Focus (Am. Psychiatr. Publ.) 2021;19(1):3–13. doi: 10.1176/appi.focus.20200051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer H.Y., Massey B.W. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 2011;11(1):59–67. doi: 10.1016/j.coph.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Meltzer H.Y., Sumiyoshi T. Does stimulation of 5-HT(1A) receptors improve cognition in schizophrenia? Behav. Brain Res. 2008;195(1):98–102. doi: 10.1016/j.bbr.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Meltzer H.Y., Li Z., Kaneda Y., Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(7):1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Meltzer H.Y., Massey B.W., Horiguchi M. Serotonin receptors as targets for drugs useful to treat psychosis and cognitive impairment in schizophrenia. Curr. Pharm. Biotechnol. 2012;13(8):1572–1586. doi: 10.2174/138920112800784880. [DOI] [PubMed] [Google Scholar]
- Meltzer H.Y., Share D.B., Jayathilake K., Salomon R.M., Lee M.A. Lurasidone improves psychopathology and cognition in treatment-resistant schizophrenia. J. Clin. Psychopharmacol. 2020;40(3):240–249. doi: 10.1097/jcp.0000000000001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.D. Review and management of clozapine side effects. J. Clin. Psychiatry. 2000;61 Suppl 8:14–17. (discussion 18-19) [PubMed] [Google Scholar]
- Narita Z., Inagawa T., Maruo K., Sueyoshi K., Sumiyoshi T. Effect of transcranial direct current stimulation on functional capacity in schizophrenia: a study protocol for a randomized controlled trial. Front. Psych. 2017;8:233. doi: 10.3389/fpsyt.2017.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A., Albert R.P. In: Schizophrenia Research: Recent Advance. Sumiyoshi T., editor. Nova Science Publishers, Inc.; 2012. Gene polymophism at serotonin 5-HT1A receptors. [Google Scholar]
- Newman-Tancredi A., Kleven M.S. Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties. Psychopharmacology (Berl) 2011;216(4):451–473. doi: 10.1007/s00213-011-2247-y. [DOI] [PubMed] [Google Scholar]
- Pigott T. 2012. Advances in Meta-analysis; pp. 79–107. [DOI] [Google Scholar]
- PišKulić D., Olver J.S., Maruff P., Norman T.R. Treatment of cognitive dysfunction in chronic schizophrenia by augmentation of atypical antipsychotics with buspirone, a partial 5-HT<sub>1A</sub>receptor agonist. Hum. Psychopharmacol. Clin. Exp. 2009;24(6):437–446. doi: 10.1002/hup.1046. [DOI] [PubMed] [Google Scholar]
- Reichenberg A. The assessment of neuropsychological functioning in schizophrenia. Dialogues Clin. Neurosci. 2010;12(3):383–392. doi: 10.31887/DCNS.2010.12.3/areichenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammacca N., Roberts G., Stuebing K.K. Meta-analysis with complex research designs: dealing with dependence from multiple measures and multiple group comparisons. Rev. Educ. Res. 2014;84(3):328–364. doi: 10.3102/0034654313500826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield J.M., Barch D.M. Cognition and resting-state functional connectivity in schizophrenia. Neurosci. Biobehav. Rev. 2016;61:108–120. doi: 10.1016/j.neubiorev.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield J.M., Repovs G., Harms M.P., Carter C.S., Gold J.M., MacDonald A.W., 3rd, Daniel Ragland J., Silverstein S.M., Godwin D., Barch D.M. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. doi: 10.1016/j.neuropsychologia.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier C.A., DiCarlo J.J., Zhang Y., Thompson P., Meltzer H.Y. Characterization of typical and atypical antipsychotic drugs based on in vivo occupancy of serotonin2 and dopamine2 receptors. J. Pharmacol. Exp. Ther. 1993;266(3):1374–1384. [PubMed] [Google Scholar]
- Sumiyoshi T. Possible dose-side effect relationship of antipsychotic drugs: relevance to cognitive function in schizophrenia. Expert. Rev. Clin. Pharmacol. 2008;1(6):791–802. doi: 10.1586/17512433.1.6.791. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T. Cognitive enhancement in schizophrenia by buspirone: role of serotonin(1A) receptor agonism. Schizophr. Res. 2020;215:455–456. doi: 10.1016/j.schres.2019.09.010. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T., Kido H., Sakamoto H., Urasaki K., Suzuki K., Yamaguchi N., Mori H., Shiba K., Yokogawa K., Ichimura F. Time course of dopamine-D2 and serotonin-5-HT2 receptor occupancy rates by haloperidol and clozapine in vivo. Jpn. J. Psychiatry Neurol. 1993;47(1):131–137. doi: 10.1111/j.1440-1819.1993.tb02041.x. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T., Kido H., Sakamoto H., Urasaki K., Suzuki K., Yamaguchi N., Mori H., Shiba K. Time course of dopamine1,2 and serotonin2 receptor binding of antipsychotics in vivo. Pharmacol. Biochem. Behav. 1994;49(1):165–169. doi: 10.1016/0091-3057(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T., Kido H., Sakamoto H., Urasaki K., Suzuki K., Yamaguchi N., Mori H., Shiba K., Yokogawa K. In vivo dopamine-D2 and serotonin-5-HT2 receptor binding study of risperidone and haloperidol. Pharmacol. Biochem. Behav. 1994;47(3):553–557. doi: 10.1016/0091-3057(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T., Suzuki K., Sakamoto H., Yamaguchi N., Mori H., Shiba K., Yokogawa K. Atypicality of several antipsychotics on the basis of in vivo dopamine-D2 and serotonin-5HT2 receptor occupancy. Neuropsychopharmacology. 1995;12(1):57–64. doi: 10.1038/sj.npp.1380239. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T., Jayathilake K., Meltzer H.Y. A comparison of two doses of melperone, an atypical antipsychotic drug, in the treatment of schizophrenia. Schizophr. Res. 2003;62(1–2):65–72. doi: 10.1016/s0920-9964(02)00351-1. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T., Jayathilake K., Meltzer H.Y. The effect of melperone, an atypical antipsychotic drug, on cognitive function in schizophrenia. Schizophr. Res. 2003;59(1):7–16. doi: 10.1016/s0920-9964(01)00329-2. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi C., Sumiyoshi T., Roy A., Jayathilake K., Meltzer H.Y. Atypical antipsychotic drugs and organization of long-term semantic memory: multidimensional scaling and cluster analyses of category fluency performance in schizophrenia. Int. J. Neuropsychopharmacol. 2006;9(6):677–683. doi: 10.1017/s1461145705006310. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T., Park S., Jayathilake K., Roy A., Ertugrul A., Meltzer H.Y. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr. Res. 2007;95(1–3):158–168. doi: 10.1016/j.schres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T., Matsui M., Nohara S., Yamashita I., Kurachi M., Sumiyoshi C., Jayathilake K., Meltzer H.Y. Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment. Am. J. Psychiatry. 2001;158(10):1722–1725. doi: 10.1176/appi.ajp.158.10.1722. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T., Matsui M., Yamashita I., Nohara S., Kurachi M., Sumiyoshi S., Sumiyoshi C., Meltzer H.Y. The effect of tandospirone, a serotonin1A agonist, on memory function in schizophrenia. Biol. Psychiatry. 2001;49(10):861–868. doi: 10.1016/s0006-3223(00)01025-8. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T., Nishida K., Niimura H., Toyomaki A., Morimoto T., Tani M., Inada K., Ninomiya T., Hori H., Manabe J., Katsuki A., Kubo T., Koshikawa Y., Shirahama M., Kohno K., Kinoshita T., Kusumi I., Iwanami A., Ueno T.…Nakagome K. Cognitive insight and functional outcome in schizophrenia; a multi-center collaborative study with the specific level of functioning scale-Japanese version. Schizophr. Res. Cogn. 2016;6:9–14. doi: 10.1016/j.scog.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goethem N.P., Schreiber R., Newman-Tancredi A., Varney M., Prickaerts J. Divergent effects of the ‘biased’ 5-HT1 A receptor agonists F15599 and F13714 in a novel object pattern separation task. Br. J. Pharmacol. 2015;172(10):2532–2543. doi: 10.1111/bph.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang X., Song X., Zhao L., Wei J., Wang J., Tian H., Zheng C., Wei M., Wang Q., Guo W., Deng W., Li T., Ma X. Co-treatment of buspirone with atypical antipsychotic drugs (AAPDs) improved neurocognitive function in chronic schizophrenia. Schizophr. Res. 2019;209:135–140. doi: 10.1016/j.schres.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Wittorf A., Wiedemann G., Buchkremer G., Klingberg S. Prediction of community outcome in schizophrenia 1 year after discharge from inpatient treatment. Eur. Arch. Psychiatry Clin. Neurosci. 2008;258(1):48–58. doi: 10.1007/s00406-007-0761-z. [DOI] [PubMed] [Google Scholar]
- Woodward N.D., Purdon S.E., Meltzer H.Y., Zald D.H. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int. J. Neuropsychopharmacol. 2005;8(3):457–472. doi: 10.1017/s146114570500516x. [DOI] [PubMed] [Google Scholar]
- Woodward N.D., Purdon S.E., Meltzer H.Y., Zald D.H. A meta-analysis of cognitive change with haloperidol in clinical trials of atypical antipsychotics: dose effects and comparison to practice effects. Schizophr. Res. 2007;89(1–3):211–224. doi: 10.1016/j.schres.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Yamada R., Wada A., Stickley A., Yokoi Y., Sumiyoshi T. Effect of 5-HT1A receptor partial agonists of the azapirone class as an add-on therapy on psychopathology and cognition in schizophrenia: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 2023;26(4):249–258. doi: 10.1093/ijnp/pyad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno F., Suhara T., Nakayama T., Ichimiya T., Okubo Y., Takano A., Ando T., Inoue M., Maeda J., Suzuki K. Inhibitory effect of hippocampal 5-HT1A receptors on human explicit memory. Am. J. Psychiatry. 2003;160(2):334–340. doi: 10.1176/appi.ajp.160.2.334. [DOI] [PubMed] [Google Scholar]