Abstract
Background
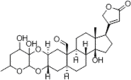
Calotropin, a cardiac glycoside obtained from the plant Calotropis gigantea, has demonstrated promising potential as an anti-tumorigenesis compound.
Objective
The main objective of this study was to investigate the potential anti-cancer properties of calotropin against HSC-3 oral squamous cancer cells and to elucidate the underlying mechanisms involved in its action.
Material and method
Calotropin were treated in HSC-3 to evaluate cell viability by MTT assay. Flow cytometry analysis divulged that calotropin G0/G1 phase cell cycle arrest and apoptosis in HSC-3 cells. Calotropin displayed inhibitory properties against aerobic glycolysis, a metabolic alteration using glucose uptaken, lactose production and LDHA activity assays. Furthermore, migration and invasion assays help that calotropin has ability to reduce the migratory and invasive of HSC-3 cells, using transwell and Matrigel assay. Validation of mRNA expression through RT-PCR. Molecular docking was implemented to validate the binding association of calotropin with apoptosis and metastatic regulating targets.
Result
The results exemplify that increasing doses of calotropin effectively hold back the HSC-3 cell progression. Migration and invasion assays help that calotropin has ability to reduce the migratory and invasive of HSC-3 cells, indicating its potential to inhibit cancer metastasis. These results imply that calotropin may influence genes linked to metastasis and apoptosis in order to achieve its beneficial effects on cancer. Docking results provided further support, showing a high binding energy between calotropin and metastasis-mediated pathways.
Conclusion
Overall, our findings shed an experimental evidence on how calotropin inhibits the HSC-3 oral squamous cancer cell growth, highlighting the drug's potential as a treatment for oral cancer. Further, investigation on in-vivo experiment is warranted to explore its potential mechanism of action and to develop a novel drug towards clinical trial.
Keywords: Calotropin, Oral cancer, Cell growth, Apoptosis, Metastatic property, Therapeutic targets
Graphical abstract
1. Introduction
Cancer, known as the “king of all maladies,” is rapidly becoming the most common non-communicable disease worldwide.1 It is triggered by a mix of internal and external stimuli, leading in the successive accumulation of genetic changes, a process known as multi-step oncogenesis. Oral squamous cell carcinoma (OSCC) secure 12th rank in Asia and 8th preponderance leading cancer in men. In 2018, the global occurrence of oral cancer was 354,864 cases, with 246,420 new cases in men and 108,444 in women. In India, according to GLOBOCAN, oral cancer accounted for 16.1% among cancers in men, making it the familiar cancer type, and 4.8% in women, ranking as the 4th most common cancer.2 Another study by Rajkumar et al. on the South Indian population found that a low body mass index (BMI) was connected to a higher incidence of oral cancer. Furthermore, they found that eating fruits and vegetables, both raw and cooked, was linked to a lower incidence of oral cancer.3 While surgical therapy for oral cancer is life-saving, it can result in disfigurement due to the need for wide excisional removal. Chemotherapy, contrasted with, has various adverse activity on cells and the body as a whole. Therefore, there is a rising demand to find new, potent, trustworthy phytochemicals for the cancer drug development. Plant-based natural remedies have the potential to provide therapies with fewer adverse reactions and equivalent or greater efficacy.4,5 Medicinal plants have long been used in treating various diseases, including cancer, and they remain an essential resource for finding new drugs.6 Despite their potential, many plants have yet to be thoroughly investigated for their anti-cancer activity specifically against oral squamous cell carcinoma.
Among them, one of the potent anti-cancer agent calotropin, has been explored. Calotropin is a cardiac glycoside derived from Calotropis gigantea, also known as giant milkweed possesses anti-proliferative effects against various types of cancer cells, as well as lung, breast, prostate, leukemia and colon.7, 8, 9 According to reports, it causes reduction of cisplatin-resistant cell growth in lung cancer cel.10 Calotropin inhibits the Na+/K+-ATPase to the alpha-subunit of the enzyme, which causes a rise in intracellular calcium levels and the breakdown of ion homeostasis in cancer cells as one of its main modes of action as a cancer prevention drug.11 Moreover, it can inhibit the PI3K/Akt/mTOR pathway activation, which are often dysregulated in cancer cells, leading to uncontrolled cell growth and survival.12 In animal studies, calotropin has shown promising anti-tumor effects. It has been found to prevent tumor growth, reduce tumor size, and prolong survival in various cancer models.13
The study mainly gets attention on exploring the anti-tumorigenic property of calotropin on HSC-3 cell lines. These findings showed that calotropin caused G0/G1 phase cell cycle arrest and curbed cell growth in a manner that was dose-dependent, indicating its potential to impede cancer cell proliferation. It also triggered apoptosis, inhibited aerobic glycolysis, reduced migration and invasion capabilities, and modulated genes associated with apoptosis and metastasis. Given the limitations and side effects of current cancer therapies, the discovery of a natural compound like calotropin with anti-cancer properties would hold significant clinical importance. Ultimately, this study's findings could contribute to the advancement of oral cancer research and pave the way for future investigations and clinical trials exploring calotropin's potential as a complementary or alternative therapy for oral squamous cancer patients.
2. Materials and methods
2.1. Reagents
Dulbecco's Modified Eagle's Medium (DMEM), Fetal Bovine Serum (FBS), penicillin/streptomycin antibiotics, Propidium Iodide (PI) stain, Dimethyl sulfoxide (DMSO), RNase, metabolic assay kits (Glucose uptaken and Lactose production) were purchased from HiMedia and abcam. Calotropin was purchased from Sigma Aldrich, India. The total RNA isolation kit was provided by Invitrogen, USA. The primers for BAX, BCL-2, CDH1, CDH2, SNAI1, SNAI2, CLDN-1, and VIM were provided by Eurofins Genomics India Pvt. Ltd, Bangalore, India.
2.2. Cell lines
A cell line for human oral squamous carcinoma (HSC-3) was procured from the American Type Culture Collection (ATCC), Manassas, Virginia, USA. The cells were maintained and grown in a controlled environment inside a CO2 incubator set at a temperature of 37 °C. DMEM, together with 10% FBS and 1% penicillin-streptomycin antibiotics, was the culture media utilized for cell growth.
2.3. Cell viability assay
In this study, the cytotoxicity of the natural compound calotropin against the HSC-3 cell line was investigated by the MTT colorimetric test. Briefly, 1 × 104 cells of HSC-3 were seeded and concentrations ranging from 10 to 100 μM were prepared in serum-free media. The wells containing the HSC-3 cells received the diluted calotropin samples, which were then incubated in a CO2 incubator for either 24 or 48 h. Following the period of treatment, the supernatant was discarded and then filled with MTT solution for an hour, DMSO was used to dissolve the formazan crystals and used to detect the absorbance at 590 nm.
2.4. Trypan blue exclusion assay
This study used the trypan blue exclusion test, which was previously reported14 to regulate the effect of calotropin on viable cells. Briefly 2 × 104 of HSC-3 cells were sown into each well of a 96-well plate and treatment underwent with calotropin, ranging from 20 to 100 μM, for either 24 or 48 h. An equivalent amount of a 0.4% trypan blue solution in 1xPBS was added to the cell suspension after it had formed. The combination was then let to keep at room temperature for an additional 5 min so that the dye could stain any non-viable cells.
2.5. Metabolic assays
1 × 105 HSC-3 cells were sown in each well of 96-well plates, and grown for 24 h and treated for 24 and 48 h. The glucose uptake test colorimetric kit was used in accordance with the directions provided by the manufacturer to measure glucose uptake. A pre-treatment with 5 mM and 10 mM d-glucose (2-DG) was given to HSC-3 cells at a population of 1 × 105 cells for 6 h before the intended drug treatment. Following the directions provided by the company, colorimetric kits were used to test lactate levels. HSC-3 cells were planted at a population density of 1 × 105 cells to measure LDH enzyme activity after being exposed to calotropin for 24 and 48 h. After extracting the cell lysates and incubating them for an hour with the LDH enzymatic mix together, the samples were assessed at an optical density (OD) of 450 nm.
2.6. Cell cycle arrest
Using the methods outlined by flow cytometric analysis was used to examine the circulation of DNA content in cell cycle phases. Around 1 × 106 cells in each well, HSC-3 cells were seeded and treated with calotropin for 24 and 48 h following a 24-h incubation period, the cells were fixed in 70% ice-cold ethanol. Propidium iodide (PI) dye was then used to stain the fixed cells after they had been treated with RNase A at a dosage of 10 mg/mL. Using a flow cytometer (BD FACS Calibur, Becton Dickinson, USA), the fluorescence that the PI-labelled nuclei produced was quantified.
2.7. Analysis of apoptosis by annexin V-FITC/PI double stain
To evaluate apoptotic cells, Annexin V-FITC Apoptosis Kit was utilized following the manufacturer's protocol.15 HSC-3 cells were first incubated for 24- and 48-h following administration with the IC50 dose of calotropin. Subsequently, Propidium iodide (PI) and Annexin V-FITC were used to stain the cells for 15 min at 25 °C in dark conditions. To analyze the apoptotic index, flow cytometry was used by BD FACS Calibur and data interpreted using Cell Quest Pro V 3.2.1 (Becton Dickinson, USA).
2.8. Drug-protein interacting network analysis
To better comprehend the interactions between proteins and drugs, computational methods have emerged as a valuable tool for predicting protein targets specific to drug molecules.16 To facilitate this investigation, the STITCH database, developed by the European Molecular Biology Laboratory (EMBL), serves as a valuable resource for both known and predicted drug-protein interactions by inputting target genes into the STITCH database, it becomes possible to construct a drug-protein interaction network, providing insights into the relationships between drugs and their respective protein targets.
2.9. Migration and invasion assay
At a population density of 2 × 104 cells per well, HSC-3 cells were plated in 6-well plates. Following the initial incubation period, the cells were exposed to calotropin treatment for 24- and 48-h’ time interval. Subsequently, the cell suspension was filled to the upper chamber of a transwell insert, which may have been previously coated with Matrigel, to evaluate the cells' migration and invasion abilities. After, a culture plate with full media was used to hold the transwell inserts, and they were cultured for an additional 24 or 48 h. Crystal violet was used to stain the cells that had successfully penetrated the membrane and attached to the lower surface. After staining, the invaded cells were visualized using an inverted microscope (4×).
2.10. mRNA expression analysis
HSC-3 cells were planted at a density of 5 × 104 cells per well. Calotropin in serum-free media was applied to the cells after an incubation period, utilizing IC50 concentrations for both 48 and 24 h of treatment. After harvesting the HSC-3 cells, RNA was extracted using the TRIR kit (Ab gene, UK). The concentration of the isolated RNA was determined through spectrometric quantification.17 Forward and reverse primers were specifically designed, and their patterns, as well as the reaction conditions, can be found in Table 1. For this procedure, the CFX96 Touch Real-Time PCR detection equipment was used.
Table 1.
Real-time PCR primers.
| Gene(s) | Primer 5′-3′ | References |
|---|---|---|
| BAX | F-TTCTGACGGCAACTTCAACTG | Chen S et al., 2015 |
| R-TGAGGAGTCTCACCCAACCA | ||
| BCL2 | F-GACGCTTTGCCACGGTGGTG | Chen S et al., 2015 |
| R-GGGGCAGGCATGTTGACTTCAC | ||
| CDH1 | F-CACCTGGAGAGAGGCCATGT | Guo W et al., 2012 |
| R-TGGGAAACATGAGCAGCTCT | ||
| CDH2 | F—ATGTGCCGGATAGCGGGAGC | Guo W et al., 2012 |
| R--TACACCGTGCCGTCCTCGTC | ||
| SNAI1 | F-CTCACCTCGGGAGCATACAG | Guo W et al., 2012 |
| R-GACTTACACGCCCCAAGGATG | ||
| SNAI2 | F-GAGCCGGGTGACTTCAGAG | Guo W et al., 2012 |
| R-GGCGTTGAAATGTTTCTTGA | ||
| CLDN1 | F-GGGGACAACATCGTGACCG | Guo W et al., 2012 |
| R-AGGAGTCGAAGACTTTGCACT | ||
| VIM | F-AGGAGTCGAAGACTTTGCACT | Guo W et al., 2012 |
| GTCAGGCTTGGAAACGTCC | ||
| β-actin | F-AACAAGATGAGATTGGCA | Chen S et al., 2015 |
| R-AGTGGGGTGGCTTTTAGGAT |
F- Forward; R- Reverse.
2.11. Molecular docking analysis
The binding interactions of calotropin (CID: 16142) with various apoptotic and metastatic regulating targets, including BAX (PDB ID: 2K7W), BCL-2 (PDB ID: ZW3L), CDH1 (PDB ID: 4ZT1), CDH2 (PDB ID: 3G2W), and VIM (PDB ID: 1GK4) crystal structures of these proteins were sourced from the Protein Data Bank (https://www.pdb.org/pdb). During the docking scrutiny, a grid box with dimensions of 90 Å × 90 Å × 90 Å and a grid spacing of 0.45 Å was used. The docking calculations were carried out using the Lamarckian genetic algorithm (LGA) with 100 genetic algorithm cycles using AutoDock 1.5.4. To visualize the outcomes of the 3D structured complex docking results, BIOVIA Discovery Studio software was utilized.
2.12. Statistical analysis
The GraphPad prism 8 software was used to evaluate the statistical data, which is presented as mean standard deviation (S.D.). The data were analyzed using the t-test, and statistically significant p-values of less than 0.05, 0.01, and 0.001 were observed as demonstrating the presence of significant differences across groups.
3. Results
3.1. Effect of calotropin on the viability of HSC-3 cells
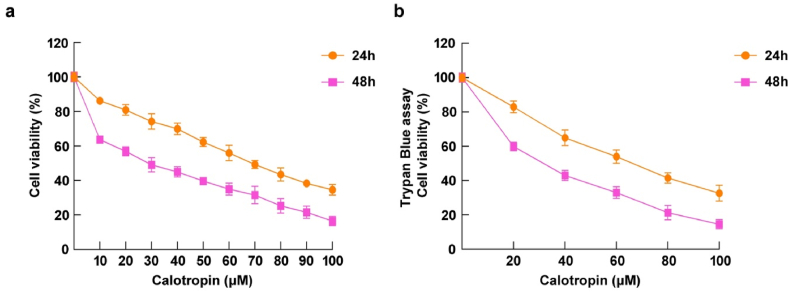
The cytotoxicity of calotropin on HSC-3 cells in oral cancer were evaluated by MTT assay. HSC-3 cells were exposed with an increasing concentration of calotropin from 10 to 100 μM for two-time interval. The results clearly demonstrated a significant dose-dependent reduction in cell viability upon exposure to calotropin (Fig. 1a). Notably, the cytotoxic effect of calotropin was more pronounced after 48 h of treatment compared to 24 h. The half-maximal inhibitory concentration (IC50) of calotropin was found to be 27.53 μM following 48 h of treatment and 61.17 μM after 24 h of treatment. These results were further validated by the Trypan blue assay (Fig. 1b), which confirmed a remarkable decrease in HSC-3 cell viability induced by calotropin. These findings underscore the potent cytotoxic properties of calotropin against HSC-3 cells, emphasising its curative value for the management of oral cancer.
Fig. 1.
Cytotoxicity of calotropin in HSC-3 cells. (a) HSC-3 administrated with 10–100 μM dose of calotropin for 24 h and 48 h cytotoxicity was analyzed by MTT assay. (b) Cell viability was evaluating by trypan blue assay (right). The software Graph Pad Prism8 was used to determine IC50 values. Data were shown as means ± S.D.
3.2. Identifying the potent role of calotropin by STITCH
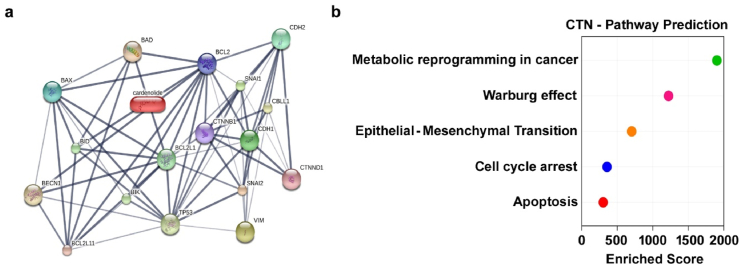
To further support the significant role of calotropin in oral tumorigenesis, we conducted an analysis using the STITCH database. In our study, we utilized the STITCH database to explore the role of calotropin. The results obtained from the STITCH database revealed that calotropin has an interaction with the regulation of metabolic reprogramming in cancer, specifically aerobic glycolysis (Warburg effect). Furthermore, Calotropin was discovered to an eminent role in number biological functions, notably epithelial-mesenchymal transition, cell cycle arrest, and apoptosis. The network clustering analysis highlights the interconnectedness of calotropin with targets involved in aerobic glycolysis, apoptosis, and metastasis-mediated signalling (Fig. 2 a and b). Overall our bioinformatics analysis utilizing the STITCH database strengthens the understanding of calotropin's potential mechanisms of action and its significance in oral cancer research.
Fig. 2.
Drug-protein interaction networking. (a) DPI network constructed by STITCH database. The link of calotropin with aerobic glycolysis, cell cycle, apoptosis, and metastatic associated genes were indicated. (b) Network of interactions between the high nodal strength targets with the calotropin were highlighted using Enrichr database.
3.3. Calotropin alters promotion of cell proliferation and aerobic glycolysis in HSC-3 cells
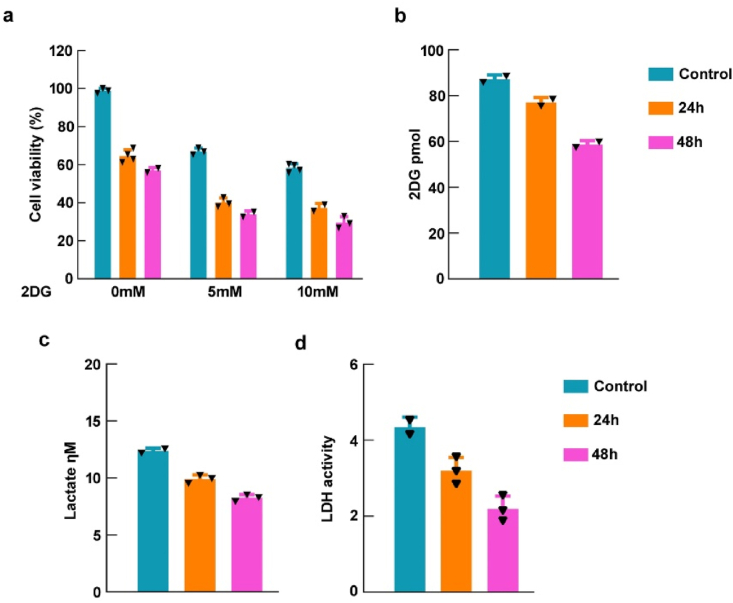
We performed a cell viability experiment in HSC-3 oral squamous carcinoma cells to establish if aerobic glycolysis serves an important part in cell proliferation in order to examine the biological relevance of calotropin in oral carcinogenesis. We employed the glycolytic inhibitor, namely, 2DG in combination with calotropin to examine its effect on cell proliferation of HSC-3 cells were significantly decreased when administered calotropin and 2DG (Fig. 3a. Additionally, we examined the function of calotropin in aerobic glycolysis and observed that it markedly decreased glucose uptake, lactate production, and LDHA activity in HSC-3 cells (Fig. 3b–d). These findings clearly demonstrate that calotropin effectively suppresses cell proliferation and aerobic glycolysis in HSC-3 cells.
Fig. 3.
Calotropin inhibits aerobic glycolysis regulation in HSC-3 cells. (a) HSC-3 cells were exposed with 2DG (5 mM, 10 mM) for 24 h and 48 h. Cell viability was measured. (b–d) Glucose uptaken, lactose production, and LDH activity were calculated at 24 h and 48 h treatment of calotropin in HSC-3 cells. Glucose uptake, lactate production, and LDH activity were counted. The t-test was used to evaluate the statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).
3.4. Calotropin inhibits cell cycle regulation in HSC-3 cells
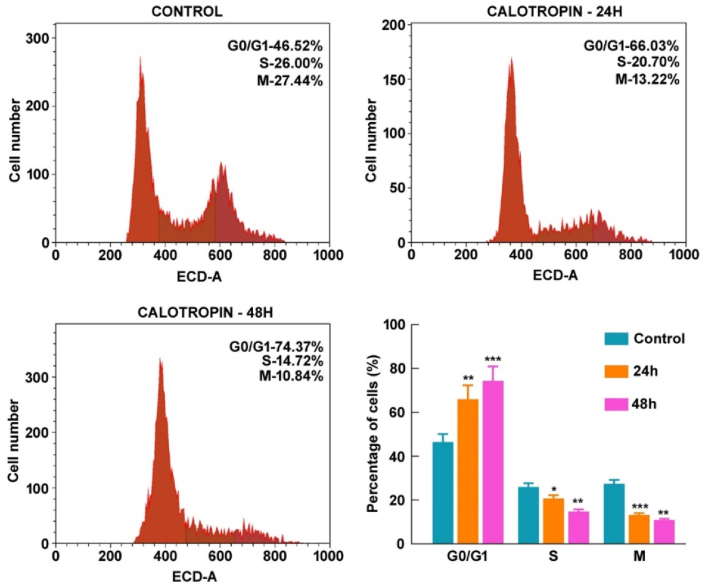
Previous research suggests the arrest of cell cycle progression at a specific phase is a recognized mechanism by which various anticancer agents exert their inhibitory effects on tumor growth.18 Building upon these findings, assessed the cell cycle distribution, flow cytometric analysis of DNA content was performed. The results indicate that after 48 h of calotropin administration, the cell percentage arrested in the G0/G1 phase raised to 74.37%, compared to 66.03% later 24 h of treatment. In contrast, the control group exhibited 46.52% of cells in the G0/G1 phase (Fig. 4). These results illustrate that calotropin induces cell cycle arrest in HSC-3 cells during the G0/G1 phase, supporting its potential as an agent for inhibiting cell proliferation in oral cancer.
Fig. 4.
Calotropin induces cell cycle arrest in HSC-3 cells. Human oral squamous carcinoma cell line, HSC-3 were analyzed for cell cycle distribution by flow cytometry. The t-test was used to investigate the statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).
3.5. Calotropin induces cell death in HSC-3 cells by annexin V-FITC/PI
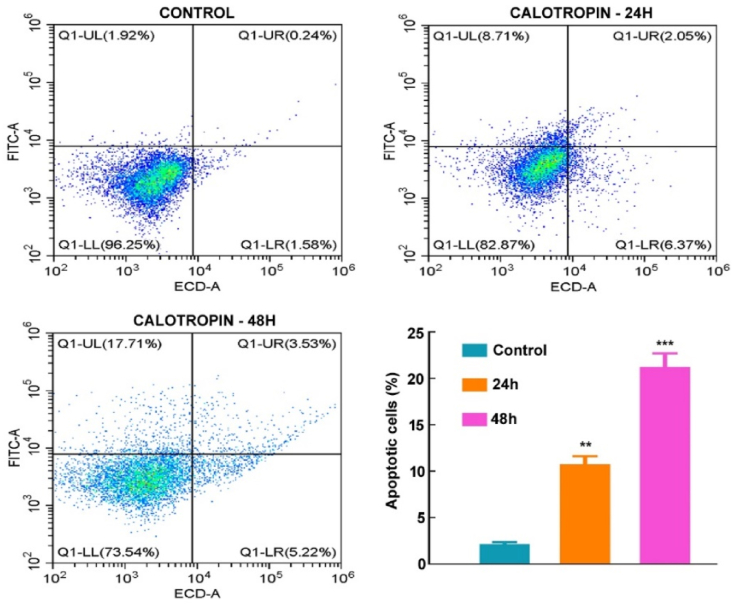
Using annexin V-FITC/PI double labelling, we analyzed cell death to find out more about whether calotropin affects oral cancer. Our outcomes showed that calotropin administration for 48 h significantly induced cell death, with an apoptotic rate of 21.24%. In comparison, the apoptotic rate was lower at 10.06% after 24 h of treatment, while the control group exhibited a basal level of apoptosis at 2.16% in HSC-3 cells (Fig. 5). These findings strongly indicate that calotropin treatment exerts potent anticancer activity in HSC-3 cancer cells by inducing apoptosis.
Fig. 5.
Calotropin induces cell death in HSC-3 cells. OSCC cell line, HSC-3 were analyzed for apoptotic rate using annexin V-FITC by flow cytometry. (LL-Live cells; UL-early apoptosis; UR-Late apoptosis; LR-Necrosis). The t-test was used to test the statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).
3.6. Calotropin inhibits migration and invasion in oral cancer cells
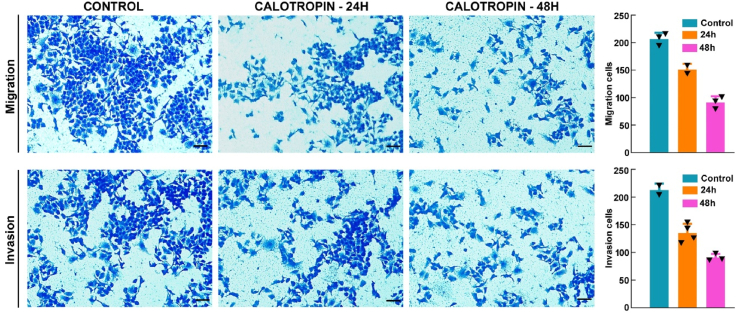
Calotropin is supposed to play a substantial part in cancer cells' ability to metastasize, according to earlier studies.19 To look into the inhibiting influence of calotropin on metastasis in HSC-3 cells, migration and invasion assays were conducted after treating the cells for 24 h and 48 h. The results demonstrate that calotropin treatment suppresses invasive and motility of oral cancer cells (Fig. 6). When compared to the untreated group, the quantification of the migratory and invaded cells clearly shows a decrease in cell counts. These findings further emphasize the potent anti-metastatic properties of calotropin in oral cancer cells.
Fig. 6.
Calotropin inhibits motility and invasive of oral cancer cells. The treatment of calotropin for 24 h and 48 h in HSC-3 shows inhibition of migration and invasion. Cell invasion was examined using Matrigel-coated Transwell chambers in the HSC-3 cells and treated with calotropin. Quantification of the migration and invasion were displayed as the percentage of migration and invasion representing the number of cells per field compared with the control. The t-test was used to determine the statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).
3.7. Effect of calotropin on the expression of apoptosis and metastatic-mediated genes
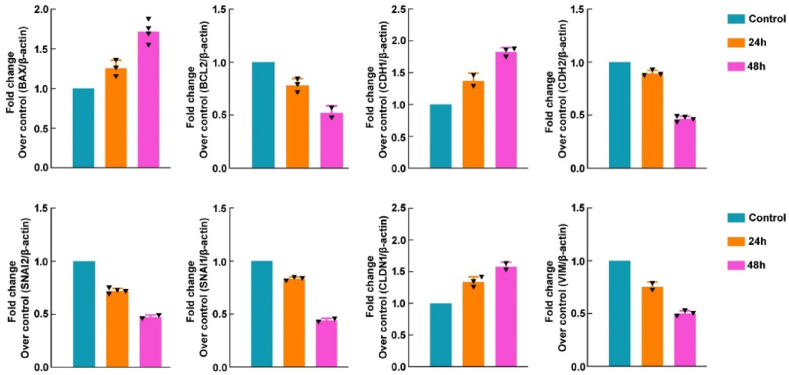
After establishing that calotropin can inhibit migration and invasion while inducing cell death in HSC-3 cells, our goal was to look into the underlying mechanistic processes. RT- PCR was used to evaluate the mRNA expression of genes implicated in apoptosis and metastasis in HSC-3 cells and the results revealed several significant findings. The pro-apoptotic gene metastatic regulating genes BAX, CDH1, and CLDN-1 mRNA expression increased in response to calotropin, indicating its role in promoting apoptosis and metastasis (Fig. 7). Furthermore, the anti-apoptotic gene Bcl-2, and CDH2, SNAI1, SNAI2, and VIM mRNA expression was reduced by calotropin treatment in HSC-3 cells suggesting its involvement in promoting cell death and inhibition of metastasis. Overall, these findings offer compelling proof that calotropin is a potent anti-cancer drug capable of repressing EMT in oral cancer cells.
Fig. 7.
Effect of calotropin in HSC-3 cells. The effect of calotropin on Apoptosis-Related Genes expression (Bax, BCL2, CDH1, CDH2, SNAI2, SNAI1, CLDN1, and VIM) in HSC-3 Cells. As previously mentioned, the expression capacities were measured using qRT-PCR. The relative level of each gene obtained by normalization with β-actin in the untreated group was set subjectively at 1, and Values are reported as the mean ± SEM of three independent experiments, with the level in other groups having been determined in accordance. (*p < 0.05, **p < 0.01; ***p < 0.001) with untreated groups.
3.8. Molecular docking studies
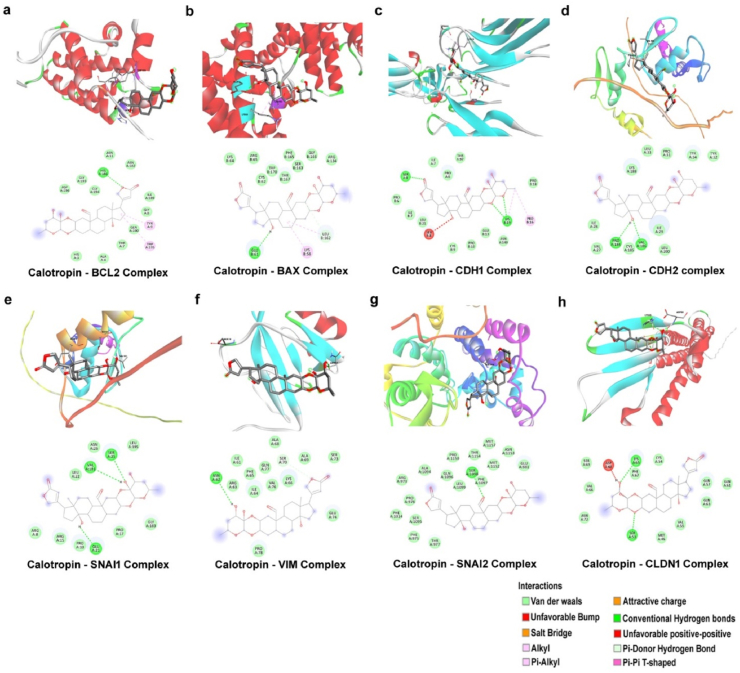
In order to examine the relationship between calotropin and apoptotic and metastasis-related proteins using molecular docking, and VIM. Specifically, calotropin exhibited higher binding energy with BAX (−8.8 kcal/mol), BCL-2 (−8.9 kcal/mol), CDH1 (−8.6 kcal/mol), CDH2 (−8.3 kcal/mol), SNAI1 (−8.1 kcal/mol), SNAI2 (−8.2 kcal/mol), CLDN1 (−7.5 kcal/mol), and VIM (−9.9 kcal/mol), suggesting a strong affinity for these proteins as mentioned in Table 2. Furthermore, the molecular docking studies revealed the creation of hydrogen bonds between calotropin and the active sites of various proteins, including BAX (GLU61), BCL-2 (HIS186), CDH1 (SER8, LYS19), CDH2 (SER1098), SNAI1 (VAL181, SER25, GLU21), SNAI2 (PRO184, VAL186), CLDN1 (LYS65, SER53), and VIM (ASN62). These hydrogen bonds are crucial for the stability of the protein-ligand complex and indicate that calotropin may disrupt the structure of these proteins, potentially inhibiting their activity (Fig. 8). The results obtained indicate that calotropin may interfere with the apoptosis and metastasis mediated signaling pathway, leading to cell death and the suppression of epithelial-mesenchymal transition. These molecular interactions confirm calotropin's promise as a therapeutic drug for cancer treatment and shed light on probable processes behind its anti-cancer activities.
Table 2.
Molecular docking analysis for calotropin with metastasis target genes.
| S. no | Drug | Protein | Binding energy (kcal/mol) | No. of H bonds involved | Amino acid residues |
|---|---|---|---|---|---|
| 1. |
 Calotropin (16142) |
BAX | −8.8 | 1 | GLU61 |
| 2. | BCL2 | −8.9 | 1 | HIS186 | |
| 3. | CDH1 | −8.6 | 2 | SER8, LYS19 | |
| 4. | CDH2 | −8.3 | 1 | SER1098 | |
| 5. | SNAI1 | −8.1 | 3 | VAL181, SER25, GLU21 | |
| 6. | SNAI2 | −8.2 | 2 | PRO184, VAL186 | |
| 7. | CLDN1 | −7.5 | 2 | LYS65, SER53 | |
| 8. | VIM | −9.9 | 1 | ASN62 |
Fig. 8.
Association of high binding energy in calotropin (a–h) The binding affinity of calotropin in the active docking site of apoptotic and metastatic regulating targets (BAX, BCL2, CDH1, CDH2, SNAI1, SNAI2, CLDN1, and VIM) the binding site is lined by important residues. The hydrogen bond lined with active residues. The molecular docking analysis were performed in Autodock 1.5.4 and visualized using Biovia Discovery tool.
4. Discussion
Cancer is a prevalent non-communicable disease worldwide, characterized by a multi-step process called carcinogenesis. During this process, genetic occurrences during this phase interfere with the common regular regulatory mechanisms that regulate dynamic biological processes including cell differentiation and cell death, eventually leading to the growth of invasive tumours.20 The generation of oxygen free radicals within cells has been implicated in the pathogenesis of cancer, as they contribute to DNA mutations and tumor progression.21 Several key genes have been identified to have significant involvement in the progression of oral cancer, including TP53, NOTCH1, EGFR, CDKN2A, STAT3, Cyclin D1, and Rb.22 These genes play critical roles in regulating crucial cellular function such as cell cycle control, apoptosis, proliferation, and differentiation. Dysregulation or mutations in these genes can result in uncontrolled cell growth and the development of tumours in the mouth.23,24 Oral squamous cell carcinoma frequently exhibits dysregulation of NOTCH1, which is important in cell differentiation.25 EGFR (epidermal growth factor receptor) is a cell surface receptor which regulates cell extension and division, and that oral cancer growth is linked to its overexpression.26 CDKN2A is a tumour suppressor gene that crucial role in cell cycle progression inhibition, and its inactivation contributes to oral cancer development.27
Traditional medicine, particularly phytomedicine, has a long-standing history and is still widely practiced today. India, in particular, possesses a vast wealth of medicinal plants, contributing to its rich traditional medicine heritage.28 In the pursuit of natural remedies for diseases, especially cancer, which is one of the most debilitating non-communicable diseases globally, researchers have extensively explored different plant parts for their therapeutic potential.29 By focusing on natural sources, such as medicinal plants, researchers aim to uncover bioactive compounds and substances that may possess anti-cancer attributes.30 The study of phytocompounds compounds for their medicinal value holds promise for developing novel cancer treatments.
The study focused on investigating the anti-cancer effects of calotropin on HSC-3 oral squamous cancer cells, shedding light on its potential as a therapeutic agent. The results revealed several significant findings that contribute to our understanding of calotropin's anti-cancer mechanisms. Firstly, calotropin exhibited potent anti-cancer properties by inhibiting cell proliferation in a concentration-dependent manner by cell viability assays. MTT and trypan blue assay indicates its ability to impede the multiplication of HSC-3 cancer cells. The half-maximal inhibitory concentration of calotropin is 27.53 μM following 48 h of treatment and 61.17 μM after 24 h of treatment. Moreover, using bioinformatics tools, we confirmed that calotropin having a strong association with apoptosis and metastatic mediated targets using STITCH database. And also, we predicted that calotropin having a huge part in regulation of aerobic glycolysis, epithelial-mesenchymal transition, cell cycle arrest and apoptosis. Furthermore, to validate the previous report, calotropin demonstrated inhibitory effects on aerobic glycolysis, a metabolic pathway often upregulated in cancer cells. By reducing glucose uptake, lactate production, and LDHA activity, calotropin disrupted the cancer cells' reliance on aerobic glycolysis for energy production. This highlights calotropin's ability to target metabolic reprogramming in cancer cells, potentially weakening their survival and development mechanisms in oral cancer cells.
Moreover, calotropin potent to cell cycle arrest at the G0/G1 phase, further supporting its role in inhibiting cell growth. This finding is important as arresting cell cycle progression at specific phases is a recognized mechanism through which anti-cancer agents inhibit tumor growth. Additionally, calotropin also triggered apoptosis, a process of programmed cell death which is vital to understanding preventing cancer progression.31 The significant induction of cell death observed after calotropin treatment suggests its potential as an apoptosis-inducing agent in HSC-3 cancer cells. This finding aligns with previous studies that have reported calotropin's role in promoting cell death in different cancer cell lines. Metastasis, the spread of cancer cells to distant sites, is a crucial element in cancer development and treatment resistance. By inhibiting migration and invasion, calotropin may help impede the metastatic potential of HSC-3 cancer cells, reducing their ability to spread and establish secondary tumours. The research also showed that calotropin inhibited the ability of HSC-3 cancer cells in the oral cavity to migrate and invade.
Moreover, the study looked at how calotropin administration affected the expression of genes related to apoptosis and metastasis. Calotropin treatment led to an upregulate the pro-apoptotic BAX expression, indicating its role in promoting apoptosis. Additionally, calotropin treatment resulted an increased expression of metastatic regulating genes such as CDH1 and CLDN-1, which are associated with the inhibition of metastasis. The findings revealed that reduced the target metastatic genes expression namely CDH2, SNAI1, SNAI2, and VIM in HSC-3 cells. This suggests that calotropin may directly influence the activity of these genes, further supporting its anti-cancer effects in oral cancer cells.
Molecular docking studies provided additional insights into the potential interactions between calotropin and key proteins involved in apoptosis and metastasis. The strong binding energies observed between calotropin and proteins such as BAX, BCL-2, CDH1, CDH2, SNAI1, SNAI2, CLDN1, and VIM indicate the potential for direct interaction and modulation of their apoptotic and metastatic activities. The hydrogen bonds are created between calotropin and active sites of these proteins further suggests the potential disruption of their structure and function by calotropin. Overall, the findings suggest that calotropin has the potential to disrupt the signaling pathway involved in apoptosis and metastasis, resulting in cell death and inhibition of the transition from epithelial to mesenchymal cells (Fig. 9). Consequently, a comprehensive understanding of the specific molecular mechanisms underlying calotropin's actions remains elusive. To address this limitation, future studies are needed to explore calotropin's targets and enriched pathways involved in the development of oral squamous cell carcinoma (OSCC). This additional research would provide valuable insights into the intricate interactions between calotropin and the molecular components of OSCC, potentially leading to the identification of promising therapeutic targets and pathways for more effective cancer treatment strategies.
Fig. 9.
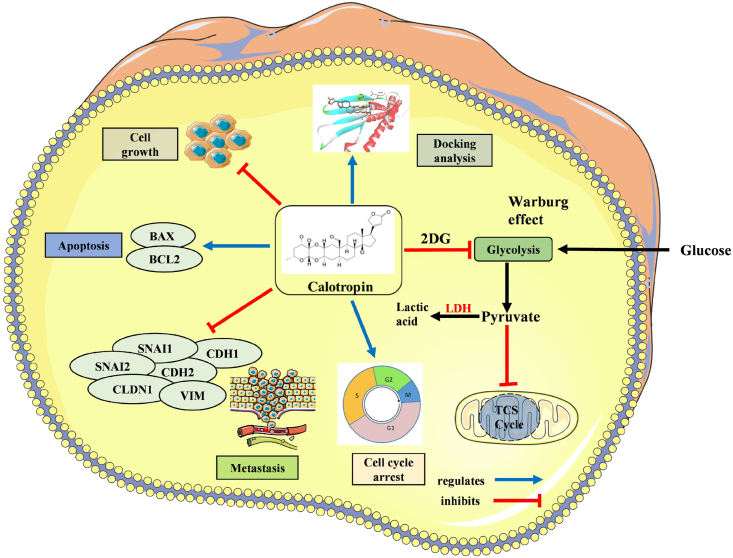
Diagrammatic illustration of calotropin role in oral cancer cells. Calotropin inhibits HSC-3 cell growth and suppresses metabolic regulations. The treatment of calotropin regulates G0/G1 phase cell cycle arrest and induces apoptosis in HSC-3 oral cancer cells. Calotropin inhibits the metastatic property in oral cancer cells. Molecular docking analysis revealed high binding energy shows strong association of calotropin with apoptotic and metastatic regulating targets.
5. Conclusion
This study provides a comprehensive evidence of anti-cancer effect of calotropin on HSC-3 oral squamous cancer cells. The findings highlight its potential as a therapeutic agent by inhibiting cell proliferation, inducing G0/G1 phase cell cycle arrest, triggering cell death, inhibiting aerobic glycolysis, and suppressing migration and invasion and further supports its anti-cancer mechanisms by gene expression studies. The molecular docking results provide insights into the potential interactions between calotropin and key proteins involved in the apoptosis and metastatic processes. These findings contribute to our understanding of calotropin's therapeutic potential in oral cancer treatment and warrant further investigation for its use for therapeutic approaches.
Declaration of competing interest
The authors declare no conflict of interest.
Contributor Information
Selvaraj Jayaraman, Email: selvarajj.sdc@saveetha.com.
Sathan Raj Natarajan, Email: sathan94speaks@gmail.com.
Vishnu Priya Veeraraghavan, Email: vishnupriyav@saveetha.com.
Sharmila Jasmine, Email: dr.sharmilajasmine@rajasdentalcollege.edu.in.
References
- 1.Swaminathan R., Shanta V., Ferlay J., Balasubramanian S., Bray F., Sankaranarayanan R. Trends in cancer incidence in Chennai city (1982–2006) and statewide predictions of future burden in Tamil Nadu (2007–16) Natl Med J India. 2011;24(2):72–77. [PubMed] [Google Scholar]
- 2.Rajkumar T., Sridhar H., Balaram P., et al. Oral Cancer in Southern India: the influence of body size, diet, infections and sexual practices. Eur J Cancer Prev. 2003;12(2):135–143. doi: 10.1097/00008469-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Devasagayam T.P.A., Sainis K.B. Immune system and antioxidants, especially those derived from Indian medicinal plants. Indian J Exp Biol. 2002;40:639–655. [PubMed] [Google Scholar]
- 4.Sanda K.A., Grema H.A., Geidam Y.A., Bukar-Kolo Y.M. Pharmacological aspects of Psidium guajava: an update. Int J Pharmacol. 2011:1–9. [Google Scholar]
- 5.Rajkovic Jovana, Novakovic Radmila, Grujic-Milanovic Jelica, et al. An updated pharmacological insight into calotropin as a potential therapeutic agent in cancer. Front Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1160616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma Rohit, Thakur Gulab S., Sanodiya Bhagwan S., et al. Therapeutic potential of Calotropis procera: a giant milkweed. ISOR J Pharm Bio Sci. 2012;4(no. 2):42–57. [Google Scholar]
- 7.Regassa Hailemeleak, Sourirajan Anuradha, Kumar Vikas, Pandey Sadanand, Kumar Deepak, Dev Kamal. A review of medicinal plants of the himalayas with anti-proliferative activity for the treatment of various cancers. Cancers. 2022;14(16):3898. doi: 10.3390/cancers14163898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mo En-Pan, Zhang Rong-Rong, Xu Jun, et al. Calotropin from Asclepias curasavica induces cell cycle arrest and apoptosis in cisplatin-resistant lung cancer cells. Biochem Biophys Res Commun. 2016;478(2):710–715. doi: 10.1016/j.bbrc.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Reddy Dhanasekhar, Kumavath Ranjith, Barh Debmalya, Azevedo Vasco, Ghosh Preetam. Anticancer and antiviral properties of cardiac glycosides: a review to explore the mechanism of actions. Molecules. 2020;25(16):3596. doi: 10.3390/molecules25163596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumavath Ranjith, Paul Sayan, Pavithran Honey, et al. "Emergence of cardiac glycosides as potential drugs: current and future scope for cancer therapeutics.". Biomolecules. 2021;11(9):1275. doi: 10.3390/biom11091275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharat Kiran R., Kharat Arun S. The Calotropis Gigantea methanolic extract induces apoptosis in human breast carcinoma cells. Iran J Med Sci. 2019;44(6):483. doi: 10.30476/ijms.2019.44966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian Lu, Xie Xiao-Hong, Zhu Ze-Hao. Calotropin regulates the apoptosis of non-small cell cancer by regulating the cytotoxic T-lymphocyte associated antigen 4-mediated TGF-β/ERK signaling pathway. Mol Med Rep. 2018;17(6):7683–7691. doi: 10.3892/mmr.2018.8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Zhenshi, Zhang Lanyue, Zheng Wan, et al. Atorvastatin and caffeine in combination regulates apoptosis, migration, invasion and tumorspheres of prostate cancer cells. Pathol Oncol Res. 2020;26:209–216. doi: 10.1007/s12253-018-0415-7. [DOI] [PubMed] [Google Scholar]
- 14.Nunez Rafael. DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol. 2001;3(3):67–70. [PubMed] [Google Scholar]
- 15.Crowley Lisa C., Brooke J. Marfell, Adrian P. Scott, Waterhouse Nigel J. Quantitation of apoptosis and necrosis by annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb Protoc. 2016;2016(11) doi: 10.1101/pdb.prot087288. pdb-prot087288. [DOI] [PubMed] [Google Scholar]
- 16.Li Yvonne Y., An Jianghong, Jones Steven JM. "A computational approach to finding novel targets for existing drugs.". PLoS Comput Biol. 2011;7(9) doi: 10.1371/journal.pcbi.1002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porichi Ourania, Nikolaidou Maria-Evangelia, Apostolaki Aikaterini, et al. BCL-2, BAX and P53 expression profiles in endometrial carcinoma as studied by real-time PCR and immunohistochemistry. Anticancer Res. 2009;29(10):3977–3982. [PubMed] [Google Scholar]
- 18.Mateen S., Tyagi A., Agarwal C., Singh R.P., Agarwal R. Silibinin inhibits human non-small cell lung cancer cell growth through cell-cycle arrest by modulating expression and function of key cell-cycle regulators. Mol Carcinog. 2010;49:247–258. doi: 10.1002/mc.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sferrazza Gianluca, Corti Marco, Brusotti Gloria, et al. Nature-derived compounds modulating Wnt/β-catenin pathway: a preventive and therapeutic opportunity in neoplastic diseases. Acta Pharm Sin B. 2020;10(10):1814–1834. doi: 10.1016/j.apsb.2019.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlberg Carsten, Velleuer Eunike, Carlberg Carsten, Velleuer Eunike. Introduction to cancer. Cancer Biol: How Sci Works. 2021:1–16. [Google Scholar]
- 21.Dreher Don, Junod Alain François. Role of oxygen free radicals in cancer development. Eur J Cancer. 1996;32(1):30–38. doi: 10.1016/0959-8049(95)00531-5. [DOI] [PubMed] [Google Scholar]
- 22.Curry Joseph M., Sprandio John, Cognetti David, et al. "Tumor microenvironment in head and neck squamous cell carcinoma.". Semin Oncol. 2014;41(2):217–234. doi: 10.1053/j.seminoncol.2014.03.003. WB Saunders. [DOI] [PubMed] [Google Scholar]
- 23.Khan Zakir, Bisen Prakash S. Oncoapoptotic signaling and deregulated target genes in cancers: special reference to oral cancer. Biochim Biophys Acta Rev Cancer. 2013;1836(1):123–145. doi: 10.1016/j.bbcan.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Molinolo Alfredo A., Amornphimoltham Panomwat, Squarize Cristiane H., Castilho Rogerio M., Patel Vyomesh, Silvio Gutkind J. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009;45(4-5):324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salameti Vasiliki, Bhosale Priyanka G., Ames-Draycott Ashley, Sipilä Kalle, Watt Fiona M. NOTCH1 signaling in oral squamous cell carcinoma via a TEL2/SERPINE1 axis. Oncotarget. 2019;10(63):6791. doi: 10.18632/oncotarget.27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro Flávia Andressa Pidone, Noguti Juliana, Celina Tizuko Fujiyama Oshima, Ribeiro Daniel Araki. Effective targeting of the epidermal growth factor receptor (EGFR) for treating oral cancer: a promising approach. Anticancer Res. 2014;34(4):1547–1552. [PubMed] [Google Scholar]
- 27.Ha Patrick K., Califano Joseph A. Promoter methylation and inactivation of tumour-suppressor genes in oral squamous-cell carcinoma. Lancet Oncol. 2006;7(1):77–82. doi: 10.1016/S1470-2045(05)70540-4. [DOI] [PubMed] [Google Scholar]
- 28.Meena Ajay Kumar, Bansal Parveen, Kumar Sanjiv. Plants-herbal wealth as a potential source of ayurvedic drugs. Asian J Tradit Med. 2009;4(4):152–170. [Google Scholar]
- 29.Makhoba Xolani H., Claudio Viegas Jr, Rebamang A. Mosa, Flávia PD Viegas, Ofentse J. Pooe. "Potential impact of the multi-target drug approach in the treatment of some complex diseases.". Drug Des Dev Ther. 2020:3235–3249. doi: 10.2147/DDDT.S257494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mgbeahuruike Eunice Ego, Yrjönen Teijo, Vuorela H., Holm Y. Bioactive compounds from medicinal plants: focus on Piper species. South Afr J Bot. 2017;112:54–69. [Google Scholar]
- 31.Park Hyun Young, Toume Kazufumi, Arai Midori A., Sadhu Samir K., Ahmed Firoj, Ishibashi Masami. Calotropin: a cardenolide from Calotropis gigantea that inhibits Wnt signaling by increasing casein kinase 1α in colon cancer cells. Chembiochem. 2014;15(6):872–878. doi: 10.1002/cbic.201300786. [DOI] [PubMed] [Google Scholar]