Abstract
Through an investigator-administered questionnaire that follows the standard protocol of the International Study of Allergy and Asthma in Childhood, data on symptomatic histories of eczema and dietary habits were collected from 11,494 young Chinese adults in Singapore/Malaysia. Allergic sensitization status was assessed through a skin prick test reactivity to common house dust mites. Using three dietary indices (dietary protein score, animal protein score, and plant protein score), the associations between atopic dermatitis, intrinsic eczema, allergic sensitization, and intake of various proteins were estimated. On average, most subjects frequently eat meat, vegetables, and rice in their diets. Through a multivariable logistic regression adjusted for age, sex, body mass index, and parental eczema, subjects with high dietary protein score (adjusted OR = 1.397; 95% confidence interval = 1.133–1.724; P < 0.003) and high animal protein score (adjusted OR = 1.353; 95% confidence interval = 1.106–1.682; P < 0.003) were associated with increased risk of atopic dermatitis. Interestingly, synergy factor analysis revealed that a higher intake of plant proteins than animal proteins in diets significantly reduced overall associated risks of atopic dermatitis and allergic sensitization but not those of intrinsic eczema. Most importantly, these associations are independent of dietary fat intake. Taken together, frequent adherence to diets rich in plant proteins reduced associated risks of atopic dermatitis in Singapore/Malaysia Chinese adults.
Introduction
Atopic dermatitis (AD) is a specific type of eczema that is marked with an elevated IgE level and associated with a personal and/or parental history of atopic diseases. This is in contrast to intrinsic eczema (IE), which has been suggested to be an inflammatory cutaneous disease that is not associated with an allergic background nor a family history of atopy (Brenninkmeijer et al., 2008; Karimkhani et al., 2015). Although AD often starts in childhood, it can persist into or develop for the first time in adulthood (Brenninkmeijer et al., 2008). In Asia, AD prevalence and its associated disease burden have been rising tremendously over the last few decades (Tsai et al., 2019). Patients with AD face a significant impact on their health-related QOL and socioeconomic well-being (Drucker et al., 2017). Despite most patients with AD suffering from similar clinical symptoms, including intense itching and recurrent inflamed rash, the severity of symptoms and underlying causes of the disease vary from person to person (Chovatiya and Silverberg, 2022). The disease pathogenesis of AD is complex and multifactorial, in which numerous synergized factors, including genetic, biological, environmental, lifestyles, and even dietary habits, interact to complicate AD development (Bauer, 2017; Brown et al., 2020; Ng and Chew, 2020). Recent findings have suggested a strong association between specific dietary patterns or nutrients and AD (Khan et al., 2022; Lim et al., 2022; Silverberg et al., 2016).
For instance, a high intake of fatty acids such as palmitoleic acid, arachidonic acid, and omega-3 fatty acids is critical in steering inflammatory responses to promote the development of allergic diseases (Venter et al., 2019). On the other hand, individuals with AD tend to practice food avoidance and eliminate certain foods in their diets to prevent further symptom aggravations (Nosrati et al., 2017). Common trigger foods for AD are thought to be mostly protein related, such as naturally occurring proteins in dairy products, eggs, peanuts, and fish (Kanny, 2005). Owing to this reason, current studies are heavily concentrated on the role of proteins as a common food allergen in worsening allergic symptoms in susceptible individuals (Domínguez et al., 2020). However, fewer studies have investigated the relationship between dietary protein intake and AD. Although there is existing evidence of an association between animal protein diets and inflammation and other chronic diseases, no studies have yet to demonstrate the association with the increased risks of allergic disorders. On the contrary, the nutritional role of plant-based protein diets in improving health outcomes is widely discussed and advocated. This includes lowering body mass index, inflammatory markers, and even the incidence of metabolic syndromes (Ahnen et al., 2019; Azzini et al., 2022; Markova et al., 2020). We have shown previously that eating vegetables and fruits frequently, as opposed to eating high-fat or high-glycaemic index (GI) foods, reduced the associated risks for severe AD (Lim et al., 2022).
At present, there are limited studies in a large adult population on the implication of AD and intake frequencies for animal- and plant-based proteins. Therefore, this study attempts to address this gap by ascertaining the association between animal- and plant-based dietary protein intake and AD. Utilizing the data obtained from the Singapore/Malaysia Cross-sectional Genetic Epidemiology Study cohort, we evaluated the intakes of animal- and plant-based protein foods in diets across young Chinese adults (aged 18–22 years). We also seek to examine how frequent intakes of these different protein sources modulate the associated risks for allergic sensitization, IE, and AD through the use of three dietary protein score (DPS) indices. These findings on various protein dietary intakes will be useful to guide future dietary interventions in patients with AD and provide valuable insights for AD-related clinical trials.
Results
Population demographics
Among 11,494 Chinese subjects, the prevalence of AD (n = 1,550; 13.5%) was higher than the prevalence of IE (n = 511, 4.45%), and a significant proportion of subjects tested positive for skin prick test (SPT) (n = 7,936; 69.0%) (Figure 1). There was no significant difference between the mean age (range = 21.92–23.26 years) and mean body mass index (20.96–21.04 kg/m2) among those with IE and AD (Table 1). Most subjects have a healthy body mass index range between 18.5 and 23.0 kg/m2, and there was a preponderance of female subjects (>50.0%) across the two groups. Interestingly, the proportion of positive parental history of eczema in those with IE (17.1%) was similar to the proportion in those with AD (17.9%). It was previously highlighted that a parental history of eczema is a major risk factor for AD, and thus, we have adjusted it alongside age, sex, and body mass index in our multivariable logistic regression analysis to control for potential confounding effects.
Figure 1.
Flowchart summarizing the classification of AD disease and allergic sensitization status among the SMCGES cohort. AD, atopic dermatitis; SMCGES, Singapore/Malaysia Cross-sectional Genetic Epidemiology Study; SPT, skin prick test.
Table 1.
Demographics of 11,494 Young Chinese Adults from the SMCGES Population
| Demographics | IE1 | AD2 | ||
|---|---|---|---|---|
| Total Cases | 511 | 1,550 | ||
| Age, y, mean ± SD | 23.26 ± 7.12 | 21.92 ± 5.18 | ||
| BMI, kg/m2, mean ± SD | 21.00 ± 3.02 | 21.04 ± 3.34 | ||
| n (%) | Chi-square P-value3 | n (%) | Chi-square P-value3 | |
| Sex | ||||
| Male | 125 (24.5%) | 5.862 × 10−2 | 669 (43.2%) | 2.200 × 10−16 |
| Female | 386 (75.5%) | 881 (56.8%) | ||
| BMI, Asian class (kg/m2) | ||||
| Normal (18.5–23.0) | 317 (62.0%) | 5.333 × 10−2 | 914 (59.0%) | 1.224 × 10−3 |
| Underweight (<18.5) | 57 (11.2%) | 200 (12.9%) | ||
| Overweight (>23.0) | 97 (19.0%) | 295 (19.0%) | ||
| NA | 40 | — | 141 | — |
| Parental History of Eczema | ||||
| None | 246 (48.1%) | 2.200 × 10−16 | 725 (46.8%) | 2.200 × 10−16 |
| At least one | 79 (15.5%) | 251 (16.2%) | ||
| Both | 8 (1.57%) | 27 (1.70%) | ||
| NA | 178 | — | 547 | — |
Abbreviations: AD, atopic dermatitis; BMI, body mass index; IE, intrinsic eczema; NA, not available; SMCGES, Singapore/Malaysia Cross-sectional Epidemiology Study; SPT, skin prick test.
IE case is defined to be a subject with a recurrent itchy rash in flexural areas but having a negative SPT response.
AD case is defined to be a subject with positive SPT to either Blomia tropicalis or Dermatophagoides pteronyssinus and the presence of a recurrent itchy rash in flexural areas.
Chi-square test was performed for each indicated variable for IE presentation (IE cases vs. nonallergic noneczema controls) and AD presentation (AD cases vs. nonallergic noneczema controls). Chi-square P-value < 0.001 (adjusted by Bonferroni’s correction) is considered statistically significant and is written in bold.
Dietary intakes of animal and plant proteins
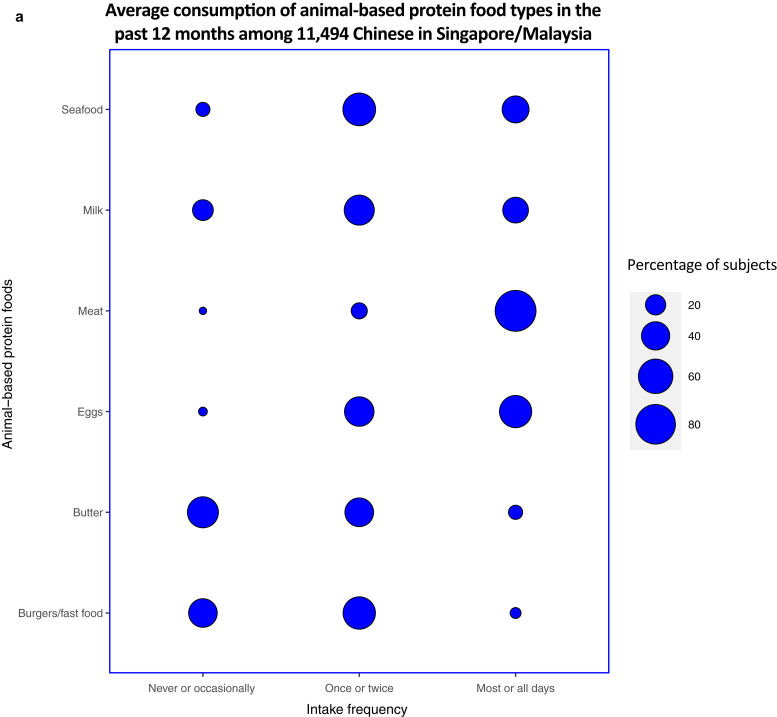
Most subjects (n = 9,932; 86.4%) reported meat consumption for most or all days, making meat the most consumed animal-based protein food in their diets in the past 12 months. The intake frequency of eggs on most or all days was reported to be the second highest for animal-based protein food at 53.8% (n = 6,183). Fewer subjects (at 11.5 and 6.84%, respectively) reported butter and burgers/fast food consumption frequently, making the contribution of these foods in terms of animal proteins low (Figure 2a).
Figure 2.
Average consumption of selected food types in the past 12 months among the diets of 11,494 young Chinese adults from the SMCGES cohort. The intake frequencies for each food type are represented in a balloon plot in terms of the percentage of subjects, with the food types classified on the basis of their origin source: (a) animal-based protein foods and (b) plant-based protein foods. SMCGES, Singapore/Malaysia Cross-sectional Genetic Epidemiology Study.
Regardless of protein source, rice was reported to be the most frequently consumed food group on most or all days among subjects at 87.8% (n = 10,096) (Figure 2b). This result is not unexpected because rice forms the staple food in the diets of most Chinese individuals residing in Asia (Muthayya et al., 2014). For other plant-based protein foods, the intake frequency of vegetables on most or all days stands the highest at 83.6% (n = 9,606), followed by fruits at 59.8% (n = 6,879), and cereals at 51.1% (n = 5,868). The findings were supported by the 2018 Singapore National Nutrition Survey, which indicated an overall improvement in the dietary patterns for higher fruit and vegetable intakes among most Singaporeans (Health Promotion Board, 2018). However, intake of pasta and nuts on most or all days was comparatively lower than that of other plant-based protein foods at 10.6% (n = 1,222) and 7.99% (n = 918), respectively. Overall, the average consumption of rice, meat, and vegetables dominated the diets of our Singapore/Malaysia Cross-sectional Genetic Epidemiology Study cohort.
Associations between various intake frequency of dietary protein and AD
We then examined the intake frequencies of various proteins, as determined by the indices, among our subjects (Table 2, Table 3, Table 4). A higher proportion of AD cases (29.5%) showed a high DPS than those of the nonallergic noneczema controls (22.0%), indicating that the intake frequency of high-protein foods in their diets, on average, was higher among patients with AD. Interestingly, there were more AD cases (37.9%) than nonallergic noneczema controls (30.4%) to have a high animal protein score (APS). However, the proportion of AD cases (33.7%) to have a high plant protein score (PPS) was slightly lower than those of nonallergic noneczema controls (35.7%). Chi-square analysis revealed that most differences between AD and nonallergic noneczema were statistically significant. Despite the trends being similar between IE cases and nonallergic noneczema controls, the differences were mostly statistically insignificant.
Table 2.
Distribution of AD Cases and Nonallergic Noneczema Controls Based on the Various Established Dietary Indices among 11,494 Young Chinese Adults in the SMCGES
| AD Presentation | AD Case (n = 1,550) | Nonallergic Noneczema Control (n = 2,978) | Chi-Square P-Value |
|---|---|---|---|
| DPS | |||
| Low total protein score | 508 (32.8%) | 1145 (38.4%) | 6.249 × 10−7 |
| Moderate total protein score | 584 (37.7%) | 1177 (39.5%) | |
| High total protein score | 458 (29.5%) | 656 (22.0%) | |
| APS | |||
| Low APS | 467 (30.1%) | 1041 (35.0%) | 1.553 × 10−6 |
| Moderate APS | 495 (37.9%) | 1031 (34.6%) | |
| High APS | 588 (37.9%) | 906 (30.4%) | |
| PPS | |||
| Low PPS | 504 (32.5%) | 856 (28.7%) | 3.167 × 10−2 |
| Moderate PPS | 523 (33.7%) | 1059 (35.6%) | |
| High PPS | 523 (33.7%) | 1063 (35.7%) | |
| Combined animal/plant score | |||
| High APS and low PPS | 100 (6.50%) | 109 (3.70%) | 6.029 × 10−9 |
| High APS and moderate PPS | 193 (12.5%) | 252 (8.50%) | |
| High APS and high PPS | 295 (19.0%) | 545 (18.3%) | |
| Moderate APS and low PPS | 179 (11.5%) | 306 (10.3%) | |
| Moderate APS and moderate PPS | 177 (11.4%) | 422 (14.2%) | |
| Moderate APS and high PPS | 139 (9.00%) | 303 (10.2%) | |
| Low APS and low PPS | 225 (14.5%) | 441 (14.8%) | |
| Low APS and moderate PPS | 153 (9.90%) | 385 (12.9%) | |
| Low APS and high PPS | 89 (5.70%) | 215 (7.20%) | |
| Combined dietary scores (DPS and DFS) | |||
| High PPS and low-fat score | 160 | 283 | 6.406 × 10−8 |
| High PPS and moderate-fat score | 212 | 502 | |
| High PPS and high-fat score | 178 | 333 | |
| Moderate PPS and low-fat score | 133 | 228 | |
| Moderate PPS and moderate-fat score | 151 | 400 | |
| Moderate PPS and high-fat score | 189 | 411 | |
| Low PPS and low-fat score | 183 | 248 | |
| Low PPS and moderate-fat score | 52 | 135 | |
| Low PPS and high-fat score | 292 | 438 | |
| Combined dietary scores (DPS and QDGIS) | |||
| High PPS and good QDGIS class | 185 | 327 | 1.147 × 10−1 |
| High PPS and moderate QDGIS class | 282 | 566 | |
| High PPS and poor QDGIS class | 172 | 384 | |
| Moderate PPS and good QDGIS class | 69 | 113 | |
| Moderate PPS and moderate QDGIS class | 195 | 429 | |
| Moderate PPS and poor QDGIS class | 204 | 414 | |
| Low PPS and good QDGIS class | 124 | 216 | |
| Low PPS and moderate QDGIS class | 98 | 161 | |
| Low PPS and poor QDGIS class | 221 | 368 | |
Abbreviations: AD, atopic dermatitis; APS, animal protein score; DPS, dietary protein score; PPS, plant protein score; QDGIS, quality of diet based on glycaemic index score; SMCGES, Singapore/Malaysia Cross-sectional Genetic Epidemiology Study.
A chi-square P-value < 0.001 (as adjusted by Bonferroni’s correction for multiple comparisons) is considered statistically significant and is written in bold.
Table 3.
Distribution of IE Cases and Nonallergic Noneczema Controls Based on the Various Established Dietary Indices among 11,494 Young Chinese Adults in the SMCGES Cohort
| IE Presentation | IE Case (n = 511) | Nonallergic Noneczema Control (n = 2,978) | Chi-Square P-Value |
|---|---|---|---|
| DQDPS | |||
| Low total protein score | 150 (29.4%) | 841 (28.2%) | 5.308 × 10−1 |
| Moderate total protein score | 176 (34.4%) | 1103 (37.0%) | |
| High total protein score | 185 (36.2%) | 1034 (34.7%) | |
| APS | |||
| Low APS | 182 (35.6%) | 1041 (35.0%) | 8.786 × 10−1 |
| Moderate APS | 158 (30.9%) | 906 (30.4%) | |
| High APS | 171 (33.5%) | 1031 (34.6%) | |
| PPS | |||
| Low PPS | 157 (30.7%) | 856 (28.7%) | 2.721 × 10−1 |
| Moderate PPS | 163 (31.9%) | 1059 (35.6%) | |
| High PPS | 191 (37.4%) | 1063 (35.7%) | |
| Combined APS/PPS | |||
| High PPS and low APS | 44 (8.60 %) | 215 (7.20 %) | 8.106 × 10−2 |
| High PPS and moderate APS | 65 (12.7%) | 303 (10.2%) | |
| High PPS and high APS | 82 (16.0%) | 545 (18.3%) | |
| Moderate PPS and low APS | 53 (10.4%) | 385 (12.9%) | |
| Moderate PPS and moderate APS | 60 (11.7%) | 422 (14.2%) | |
| Moderate PPS and high APS | 50 (9.80%) | 252 (8.50%) | |
| Low PPS and low APS | 85 (16.6%) | 441 (14.8%) | |
| Low PPS and moderate APS | 46 (9.00%) | 306 (10.3%) | |
| Low PPS and high APS | 26 (5.10%) | 109 (3.70%) | |
| Combined dietary scores (DPS and DFS) | |||
| High PPS and low-fat score | 103 (20.2%) | 502 (16.9%) | 3.815 × 10−1 |
| High PPS and moderate-fat score | 51 (10.0%) | 333 (11.2%) | |
| High PPS and high-fat score | 37 (7.20%) | 228 (7.70%) | |
| Moderate PPS and low-fat score | 58 (11.4%) | 400 (13.4%) | |
| Moderate PPS and moderate-fat score | 57 (11.2%) | 411 (13.8%) | |
| Moderate PPS and high-fat score | 48 (9.40%) | 248 (8.30%) | |
| Low PPS and low-fat score | 26 (5.10%) | 135 (4.50%) | |
| Low PPS and moderate-fat score | 54 (10.6%) | 283 (9.50%) | |
| Low PPS and high-fat score | 77 (15.1%) | 438 (14.7%) | |
| Combined dietary scores (DPS and QDGIS) | |||
| High PPS and good QDGIS class | 116 (22.7%) | 566 (19.0 %) | 6.260 × 10−2 |
| High PPS and moderate QDGIS class | 53 (10.4%) | 384 (12.9%) | |
| High PPS and poor QDGIS class | 22 (4.30%) | 113 (3.80%) | |
| Moderate PPS and good QDGIS class | 78 (15.3%) | 429 (14.4%) | |
| Moderate PPS and moderate QDGIS class | 56 (11.0%) | 414 (13.9%) | |
| Moderate PPS and poor QDGIS class | 29 (5.70%) | 216 (7.30%) | |
| Low PPS and good QDGIS class | 21 (4.10%) | 161 (5.40%) | |
| Low PPS and moderate QDGIS class | 71 (13.9%) | 327 (11.0%) | |
| Low PPS and poor QDGIS class | 65 (12.7%) | 368 (12.4%) | |
Abbreviations: APS, animal protein score; DPS, dietary protein score; DQDPS, diet quality based on dietary protein score; IE, intrinsic eczema; PPS, plant protein score; QDGIS, quality of diet based on glycaemic index score; SMCGES, Singapore/Malaysia Cross-sectional Genetic Epidemiology Study.
A chi-square P-value < 0.001 (as adjusted by Bonferroni’s correction for multiple comparisons) is considered statistically significant.
Table 4.
Distribution of Positive and Negative SPT Reactivity Based on the Various Established Dietary Indices among 11,494 Young Chinese Adults in the SMCGES Cohort
| SPT Reactivity to Common Mite Allergens | Positive SPT Response (n = 7,936) | Negative SPT Response (n = 3,489) | Chi-Square P-Value |
|---|---|---|---|
| DQDPS | |||
| Low total protein score | 1777 (22.4%) | 991 (28.4%) | 4.650 × 10−14 |
| Moderate total protein score | 2879 (36.3%) | 1279 (36.7%) | |
| High total protein score | 3280 (41.3 %) | 1219 (34.9%) | |
| APS | |||
| Low APS | 2341 (29.5%) | 1223 (35.1%) | 1.224 × 10−12 |
| Moderate APS | 2944 (37.1%) | 1064 (30.5%) | |
| High APS | 2651 (41.3%) | 1202 (34.5%) | |
| PPS | |||
| Low PPS | 2513 (31.7%) | 1013 (29.0%) | 1.703 × 10−2 |
| Moderate PPS | 2642 (33.3%) | 1222 (35.0%) | |
| High PPS | 2781 (35.0%) | 1254 (35.9%) | |
| Combined APS/PPS | |||
| High APS and low PPS | 429 (5.40%) | 135 (3.90%) | 3.023 × 10−16 |
| High APS and moderate APS | 924 (11.6%) | 302 (8.70%) | |
| High APS and high PPS | 1591 (20.0%) | 627 (18.0%) | |
| Moderate APS and low PPS | 904 (11.4%) | 352 (10.1%) | |
| Moderate APS and moderate PPS | 964 (12.1%) | 482 (13.8%) | |
| Moderate APS and high PPS | 783 (9.90%) | 368 (10.5%) | |
| Low APS and low PPS | 1180 (14.9%) | 526 (15.1%) | |
| Low APS and moderate PPS | 754 (9.50%) | 438 (12.6%) | |
| Low APS and high PPS | 407 (5.10%) | 259 (7.40%) | |
| Combined dietary scores (DPS and DFS) | |||
| High PPS and low-fat score | 1129 (14.2%) | 605 (17.3%) | 1.110 × 10−14 |
| High PPS and moderate-fat score | 940 (11.8%) | 384 (11.0%) | |
| High PPS and high-fat score | 712 (9.00%) | 265 (7.60%) | |
| Moderate PPS and low-fat score | 770 (9.70%) | 458 (13.1%) | |
| Moderate PPS and moderate-fat score | 994 (12.5%) | 468 (13.4%) | |
| Moderate PPS and high-fat score | 878 (11.1%) | 296 (8.50%) | |
| Low PPS and low-fat score | 301 (3.80%) | 161 (4.60%) | |
| Low PPS and moderate-fat score | 806 (10.2%) | 337 (9.70%) | |
| Low PPS and high-fat score | 1406 (17.7%) | 515 (14.8%) | |
| Combined dietary scores (DPS and QDGIS) | |||
| High PPS and good QDGIS class | 1446 (18.2%) | 682 (19.5%) | 4.569 × 10−4 |
| High PPS and moderate QDGIS class | 996 (12.6%) | 437 (12.5%) | |
| High PPS and poor QDGIS class | 339 (4.30%) | 135 (3.90%) | |
| Moderate PPS and good QDGIS class | 943 (11.9%) | 507 (14.5%) | |
| Moderate PPS and moderate QDGIS class | 1049 (13.2%) | 470 (13.5%) | |
| Moderate PPS and poor QDGIS class | 650 (8.20%) | 245 (7.00%) | |
| Low PPS and good QDGIS class | 453 (5.70%) | 182 (5.20%) | |
| Low PPS and moderate QDGIS class | 931 (11.7%) | 398 (11.4%) | |
| Low PPS and poor QDGIS class | 1129 (14.2%) | 433 (12.4%) | |
Abbreviations: APS, animal protein score; DPS, dietary protein score; DQDPS, diet quality based on dietary protein score; PPS, plant protein score; QDGIS, quality of diet based on glycaemic index score; SMCGES, Singapore/Malaysia Cross-sectional Genetic Epidemiology Study; SPT, skin prick test.
A chi-square P-value < 0.001 (as adjusted by Bonferroni’s correction for multiple comparisons) is considered statistically significant and is written in bold.
In both univariable and multivariable models, we confirmed that a frequent intake of high-protein foods in diets was only significantly associated with an increased risk of AD and allergic sensitization but not of IE (Table 5). Furthermore, there was a clear dose-dependent increase in the ORs and adjusted ORs (AORs) from a moderate to high DPS for AD and allergic sensitization. Thus, this altogether suggests a strong association between an intake frequency for high-protein foods and disease outcomes, and the association was largely dependent on the subjects’ allergic background. This was consistent with the associations of APS and PPS. For allergic sensitization, higher APSs were positively associated with allergic sensitization (AOR = 1.297) and AD (AOR = 1.353). Meanwhile, the association between having a high PPS, AD, and allergic sensitization was marginally insignificant in the multivariable model.
Table 5.
Association between Three Protein Dietary Indices (DPS, APS, and PPS) with IE, Allergic Sensitization, and AD Presentation among 11,494 Young Chinese adults from SMCGES Population
| Dietary Scores | IE Presentation1 |
Allergic Sensitization to HDMs2 |
AD Presentation3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value4 | OR | 95% CI | P-Value4 | OR | 95% CI | P-Value4 | |
| DPS, unadjusted | |||||||||
| Low total protein score | 1.000 | REF | — | 1.000 | REF | — | 1.000 | REF | — |
| Moderate total protein score | 0.895 | 0.707–1.133 | 0.354 | 1.255 | 1.134–1.390 | 1.210×10−5 | 1.130 | 0.962–1.328 | 0.137 |
| High total protein score | 1.003 | 0.794–1.268 | 0.979 | 1.501 | 1.355–1.661 | 5.510×10−15 | 1.493 | 1.276–1.749 | 6.130×10−7 |
| DPS, adjusted | |||||||||
| Low total protein score | 1.000 | REF | — | 1.000 | REF | — | 1.000 | REF | — |
| Moderate total protein score | 0.940 | 0.692–1.277 | 0.689 | 1.205 | 1.058–1.373 | 0.005 | 1.136 | 0.918–1.406 | 0.242 |
| High total protein score | 1.036 | 0.771–1.421 | 0.775 | 1.409 | 1.236–1.606 | 5.060×10−3 | 1.397 | 1.133–1.724 | 0.002 |
| APS, unadjusted | |||||||||
| Low animal protein score | 1.000 | REF | — | 1.000 | REF | — | 1.000 | REF | — |
| Moderate animal protein score | 0.949 | 0.757–1.189 | 0.649 | 1.152 | 1.046–1.270 | 0.004 | 1.070 | 0.918–1.247 | 0.384 |
| High animal protein score | 0.997 | 0.791–1.256 | 0.983 | 1.446 | 1.310–1.595 | 2.240×10−13 | 1.447 | 1.245–1.682 | 1.550×10−6 |
| APS, adjusted | |||||||||
| Low animal protein score | 1.000 | REF | — | 1.000 | REF | — | 1.000 | REF | — |
| Moderate animal protein score | 1.000 | 0.743–1.346 | 0.998 | 1.116 | 0.984–1.266 | 0.088 | 1.010 | 0.821–1.242 | 0.925 |
| High animal protein score | 1.048 | 0.773–1.420 | 0.761 | 1.297 | 1.142–1.474 | 6.540×10−5 | 1.353 | 1.106–1.655 | 0.003 |
| PPS, unadjusted | |||||||||
| Low plant protein score | 1.000 | REF | — | 1.000 | REF | — | 1.000 | REF | — |
| Moderate plant protein score | 0.839 | 0.662–1.064 | 0.147 | 0.872 | 0.789–0.963 | 0.007 | 0.839 | 0.721–0.976 | 0.023 |
| High plant protein score | 0.980 | 0.779–1.233 | 0.861 | 0.894 | 0.810–0.987 | 0.026 | 0.836 | 0.718–0.973 | 0.021 |
| PPS, adjusted | |||||||||
| Low plant protein score | 1.000 | REF | — | 1.000 | REF | — | 1.000 | REF | — |
| Moderate plant protein score | 0.755 | 0.549–1.039 | 0.084 | 0.856 | 0.752–0.975 | 0.020 | 0.830 | 0.676–1.020 | 0.076 |
| High plant protein score | 1.025 | 0.760–1.385 | 0.873 | 0.874 | 0.768–0.994 | 0.041 | 0.822 | 0.669–1.010 | 0.062 |
Abbreviations: AD, atopic dermatitis; APS, animal protein score; BMI, body mass index; CI, confidence interval; DPS, dietary protein score; HDM, house dust mite; IE, intrinsic eczema; PPS, plant protein score; REF, reference; SMCGES, Singapore/Malaysia Cross-sectional Genetic Epidemiology Study; SPT, skin prick test.
For results analyzed through multivariable logistic regression, the model adjusted for age, sex, BMI, and parental eczema, as confounding factors.
IE subject was defined to be positive for eczema symptoms if having a recurrent itchy rash that was coming and going for at least 6 months in flexural areas but testing negative for SPT reactivity to either one of the two common HDMs.
Allergic sensitization was compared between SPT-positive and SPT-negative subjects. SPT-positive subjects were determined by their reactivity to either one of the two common HDMs (Blomia tropicalis and Dermatophagoides pteronyssinus).
AD presentation was compared between an AD case and a nonallergic noneczema control. AD was defined in this study to be affirmative to positive SPT and having eczema symptoms.
Logistic regression P-value < 0.003 (after Bonferroni’s correction for multiple comparisons) is statistically significant and written in bold.
Antagonistic interactions between animal and plant protein in diets
There was clear initial evidence that an increased intake frequency of total proteins (AOR = 1.397), particularly animal-based protein foods (AOR = 1.353), was associated with increased odds for AD. However, most individuals maintain a typical diet that consists of both animal- and plant-based protein foods. Therefore, this study adopted a combined analysis with APS and PPS to understand the associations between intake frequencies of both animal- and plant-based protein foods in diets and AD. In the adjusted logistic model, there was an apparent dose–effect relationship between APS and PPS. Having a moderate plant-based protein with moderate animal-based proteins in diets was sufficient to confer a strong, significant protective association on AD (AOR = 0.482) (Figure 3). However, this was not observed for IE (Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13, Table 14, Table 15).
Figure 3.
Results obtained from the multivariable logistic regression analysis of protein dietary pattern in terms of the combined protein scores (APS and PPS) in relation to AD presentation (nonallergic noneczema controls vs. AD cases). Age, sex, BMI, and parental eczema were adjusted for in the model, with the values presented in AOR, 95% CI, and P-value. P-values < 0.003 are statistically significant (adjusted by Bonferroni’s correction). A reference dotted line is drawn at the interception point where AOR equals 1.000. The 95% CI for each combined protein class is represented by a single line that cuts the AOR. An SF analysis revealed that APS and PPS in diets interact in an antagonistic manner to reduce the associated odds of AD (SF value < 1.000). AD, atopic dermatitis; AOR, adjusted OR; APS, animal protein score; BMI, body mass index; CI, confidence interval; REF, reference; PPS, plant protein score; SF, synergy factor.
Table 6.
Multivariable Logistic Regression Analysis for Intrinsic Eczema and Allergic Sensitization Using Combined Protein Scores for PPS and APS
| Combined Protein Scores (PPS and APS) | Multivariable Logistic Regression (Adjusted for Age, Sex, BMI, and Parental Eczema) |
|||||
|---|---|---|---|---|---|---|
| Intrinsic Eczema |
Allergic Sensitization |
|||||
| AOR | 95% CI | P-Value1 | AOR | 95% CI | P-Value1 | |
| High PPS and low APS | 0.953 | 0.478–1.965 | 0.894 | 0.570 | 0.412–0.786 | 6.660×10−4 |
| High PPS and moderate APS | 1.093 | 0.544–2.070 | 0.911 | 0.756 | 0.558–1.018 | 0.068 |
| High PPS and high APS | 0.663 | 0.354–1.302 | 0.214 | 0.795 | 0.598–1.049 | 0.110 |
| Moderate PPS and low APS | 0.518 | 0.262–1.056 | 0.062 | 0.586 | 0.434–0.785 | 3.960×10−4 |
| Moderate PPS and moderate APS | 0.528 | 0.268–1.076 | 0.070 | 0.686 | 0.510–0.915 | 0.011 |
| Moderate PPS and high APS | 0.912 | 0.460–1.868 | 0.794 | 0.985 | 0.724–1.333 | 0.924 |
| Low PPS and low APS | 0.835 | 0.441–1.654 | 0.590 | 0.810 | 0.604–1.078 | 0.153 |
| Low PPS and moderate APS | 0.702 | 0.352–1.445 | 0.323 | 0.834 | 0.615–1.125 | 0.240 |
| Low PPS and high APS | 1.000 | REF | — | 1.000 | REF | — |
Abbreviations: AOR, adjusted OR; APS, animal protein score; BMI, body mass index; CI, confidence interval; PPS, plant protein score; REF, reference.
P < 0.003 (after Bonferroni correction for multiple comparisons) are statistically significant and written in bold.
Table 7.
SF Analysis for Intrinsic Eczema and Allergic Sensitization Using Combined Protein Scores for PPS and APS
| Combined Protein Scores (PPS and APS) | SF Analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Intrinsic Eczema |
Allergic Sensitization |
|||||||
| OR | SF Value | SF 95% CI | SF P-Value | OR | SF Value | SF 95% CI | SF P-Value | |
| Low PPS and low APS | 1.000 | 1.089 | 0.581–2.044 | 7.90 × 10−1 | 1.000 | 0.437 | 0.329–0.580 | 1.158 × 10−8 |
| Low PPS and high APS | 1.238 | 1.417 | ||||||
| High PPS and high APS | 0.781 | 1.131 | ||||||
| High PPS and low APS | 1.062 | 0.700 | ||||||
Abbreviations: APS, animal protein score; CI, confidence interval; PPS, plant protein score; SF, synergy factor.
P < 0.003 (after Bonferroni correction for multiple comparisons) are statistically significant and written in bold.
Table 8.
Multivariable Logistic Regression Analysis for Intrinsic Eczema, Allergic Sensitization, and Atopic Dermatitis Using PPS and DQDFS
| Combined Dietary Scores (PPS and DQDFS) | Multivariable Logistic Regression (Adjusted for Age, Sex, BMI, and Parental Eczema) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intrinsic Eczema |
Allergic Sensitization |
Atopic Dermatitis |
|||||||
| AOR | 95% CI | P-Value1 | AOR | 95% CI | P-Value1 | AOR | 95% CI | P-Value1 | |
| High PPS and low-fat score | 1.188 | 0.787–1.806 | 0.416 | 0.762 | 0.635–0.916 | 0.004 | 0.639 | 0.473–0.862 | 0.003 |
| High PPS and moderate-fat score | 0.932 | 0.575–1.500 | 0.772 | 0.831 | 0.680–1.016 | 0.070 | 0.821 | 0.600–1.122 | 0.218 |
| High PPS and high-fat score | 0.785 | 0.435–1.372 | 0.406 | 0.969 | 0.775–1.215 | 0.786 | 0.881 | 0.619–1.250 | 0.480 |
| Moderate PPS and low-fat score | 0.663 | 0.403–1.079 | 0.101 | 0.659 | 0.540–0.804 | 4.120×10−5 | 0.626 | 0.453–0.860 | 0.004 |
| Moderate PPS and moderate-fat score | 0.655 | 0.393–1.076 | 0.098 | 0.803 | 0.661–0.976 | 0.028 | 0.643 | 0.469–0.879 | 0.006 |
| Moderate PPS and high-fat score | 1.062 | 0.633–1.760 | 0.818 | 1.050 | 0.848–1.302 | 0.656 | 1.144 | 0.829–1.577 | 0.414 |
| Low PPS and low-fat score | 1.163 | 0.608–2.139 | 0.637 | 0.777 | 0.584–1.036 | 0.084 | 0.727 | 0.410–1.152 | 0.182 |
| Low PPS and moderate-fat score | 0.911 | 0.536–1.525 | 0.727 | 0.942 | 0.760–1.169 | 0.587 | 0.867 | 0.619–1.211 | 0.405 |
| Low PPS and high-fat score | 1.000 | REF | — | 1.000 | REF | — | 1.000 | REF | — |
Abbreviations: AOR, adjusted OR; BMI, body mass index; CI, confidence interval; DQDFS, diet quality based on dietary fat score; PPS, plant protein score; REF, reference.
P < 0.003 (after Bonferroni correction for multiple comparisons) are statistically significant and written in bold.
Table 9.
SF Analysis for Intrinsic Eczema, Allergic Sensitization, and Atopic Dermatitis Using PPS and DQDFS
| SF Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combined Protein Scores (PPS and DQDFS) | Intrinsic eczema |
Allergic Sensitization |
Atopic dermatitis |
|||||||||
| OR | SF Value | SF 95% CI | SF P-Value | OR | SF Value | SF 95% CI | SF P-Value | OR | SF Value | SF 95% CI | SF P-Value | |
| Low PPS and low-fat score | 1.000 | 1.385 | 0.736–2.608 | 3.130 × 10−1 | 1.000 | 0.476 | 0.361–0.627 | 1.384 × 10−7 | 1.000 | 0.418 | 0.269–0.651 | 1.129 × 10−4 |
| Low PPS and high-fat score | 0.913 | 1.460 | 1.731 | |||||||||
| High PPS and high-fat score | 0.843 | 1.437 | 1.514 | |||||||||
| High PPS and low-fat score | 1.065 | 0.998 | 1.096 | |||||||||
Abbreviations: CI, confidence interval; DQDFS, diet quality based on dietary fat score; PPS, plant protein score; SF, synergy factor.
P < 0.003 (after Bonferroni correction for multiple comparisons) are statistically significant and written in bold.
Table 10.
Multivariable Logistic Regression Analysis for Intrinsic Eczema, Allergic Sensitization, and Atopic Dermatitis Using PPS and QDGIS
| Combined Dietary Scores (PPS and QDGIS) | Multivariable Logistic Regression (Adjusted for Age, Sex, BMI, and Parental Eczema) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intrinsic Eczema |
Allergic Sensitization |
Atopic Dermatitis |
|||||||
| AOR | 95% CI | P-Value1 | AOR | 95% CI | P-Value1 | AOR | 95% CI | P-Value1 | |
| High PPS and good QDGIS class | 1.265 | 0.818–1.991 | 0.299 | 0.856 | 0.708–1.034 | 0.108 | 0.856 | 0.634–1.156 | 0.308 |
| High PPS and moderate QDGIS class | 0.835 | 0.493–1.411 | 0.490 | 0.878 | 0.712–1.083 | 0.225 | 0.709 | 0.505–0.992 | 0.045 |
| High PPS and poor QDGIS class | 1.058 | 0.517–2.072 | 0.873 | 0.898 | 0.670–1.209 | 0.476 | 0.897 | 0.558–1.426 | 0.650 |
| Moderate PPS and good QDGIS class | 0.960 | 0.596–1.562 | 0.869 | 0.717 | 0.585–0.878 | 0.001 | 0.719 | 0.520–0.993 | 0.045 |
| Moderate PPS and moderate QDGIS class | 0.661 | 0.381–1.137 | 0.136 | 0.907 | 0.738–1.114 | 0.352 | 0.880 | 0.640–1.212 | 0.434 |
| Moderate PPS and poor QDGIS class | 0.724 | 0.367–1.370 | 0.333 | 1.055 | 0.825–1.354 | 0.669 | 0.905 | 0.613–1.332 | 0.615 |
| Low PPS and good QDGIS class | 0.914 | 0.460–1.746 | 0.791 | 1.044 | 0.797v1.371 | 0.756 | 1.063 | 0.698–1.611 | 0.773 |
| Low PPS and moderate QDGIS class | 1.211 | 0.733–2.010 | 0.455 | 0.964 | 0.779–1.195 | 0.741 | 0.930 | 0.664–1.302 | 0.671 |
| Low PPS and poor QDGIS class | 1.000 | REF | — | 1.000 | REF | — | 1.000 | REF | — |
Abbreviations: AOR, adjusted OR; BMI, body mass index; CI, confidence interval; QDGIS, quality of diet based on glycemic index score; PPS, plant protein score; REF, reference.
P < 0.003 (after Bonferroni correction for multiple comparisons) are statistically significant and written in bold.
Table 11.
SF Analysis for Intrinsic Eczema, Allergic Sensitization, and Atopic Dermatitis Using PPS and QDGIS
| SF Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combined Dietary Scores (PPS and QDGIS) | Intrinsic Eczema |
Allergic Sensitization |
Atopic Dermatitis |
|||||||||
| OR | SF Value | SF 95% CI | SF P-Value | OR | SF Value | SF 95% CI | SF P-Value | OR | SF Value | SF 95% CI | SF P-Value | |
| Low PPS and poor QDGIS class | 1.000 | 1.426 | 0.691–2.942 | 3.374 × 10−1 | 1.000 | 0.806 | 0.597–1.088 | 1.587 × 10−1 | 1.000 | 0.641 | 0.415–0.990 | 4.478 × 10−2 |
| Low PPS and good QDGIS class | 0.738 | 1.048 | 0.987 | |||||||||
| High PPS and poor QDGIS class | 1.102 | 1.009 | 1.294 | |||||||||
| High PPS and good QDGIS class | 1.160 | 0.852 | 0.819 | |||||||||
Abbreviations: CI, confidence interval; PPS, plant protein score; QDGIS, quality of diet based on glycemic index score; SF, synergy factor.
P < 0.003 (after Bonferroni correction for multiple comparisons) are statistically significant and written in bold.
Table 12.
Multivariable Logistic Analysis for Intrinsic Eczema, Allergic Sensitization, and Atopic Dermatitis Using APS and DQDFS
| Combined Dietary Scores (APS and DQDFS) | Multivariable Logistic Regression (Adjusted for Age, Sex, BMI, and Parental Eczema) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intrinsic Eczema |
Allergic Sensitization |
Atopic Dermatitis |
|||||||
| AOR | 95% CI | P-Value1 | AOR | 95% CI | P-Value1 | AOR | 95% CI | P-Value1 | |
| Low APS and low-fat score | 0.884 | 0.602–1.300 | 0.529 | 0.624 | 0.529–0.735 | 1.940×10−8 | 0.588 | 0.454–0.761 | 5.520×10−5 |
| Low APS and moderate-fat score | 0.828 | 0.486–1.374 | 0.476 | 0.868 | 0.705–1.071 | 0.185 | 0.685 | 0.491–0.949 | 0.024 |
| Low APS and high-fat score | 1.156 | 0.587–2.153 | 0.659 | 0.794 | 0.600–1.057 | 0.110 | 0.804 | 0.516–1.237 | 0.326 |
| Moderate APS and low-fat score | 1.185 | 0.761–1.835 | 0.448 | 0.769 | 0.632–0.935 | 0.008 | 0.567 | 0.408–0.784 | 6.620×10−4 |
| Moderate APS and moderate-fat score | 0.763 | 0.487–1.183 | 0.230 | 0.744 | 0.623–0.890 | 0.001 | 0.649 | 0.491–0.856 | 0.002 |
| Moderate APS and high-fat score | 0.777 | 0.448–1.307 | 0.354 | 0.886 | 0.720–1.093 | 0.257 | 0.732 | 0.524–1.015 | 0.063 |
| High APS and low-fat score | 0.824 | 0.324–1.832 | 0.658 | 0.755 | 0.533–1.082 | 0.120 | 0.488 | 0.254–0.890 | 0.024 |
| High APS and moderate-fat score | 0.871 | 0.533–1.398 | 0.573 | 0.805 | 0.662–0.980 | 0.030 | 0.718 | 0.528–0.972 | 0.033 |
| High APS and high-fat score | 1.000 | REF | — | 1.000 | REF | — | 1.000 | REF | — |
Abbreviations: AOR, adjusted OR; APS, animal protein score; BMI, body mass index; DQDFS, diet quality based on dietary fat score; CI, confidence interval; REF, reference.
P < 0.003 (after Bonferroni correction for multiple comparisons) are statistically significant and written in bold.
Table 13.
SF Analysis for Intrinsic Eczema, Allergic Sensitization, and Atopic Dermatitis Using APS and DQDFS
| SF Analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intrinsic Eczema | Allergic Sensitization | Atopic Dermatitis | |||||||||||
| Combined Dietary Scores (APS and DQDFS) | OR | SF Value | SF 95% CI | SF P-Value | OR | SF Value | SF 95% CI | SF P-Value | OR | SF Value | SF 95% CI | SF P-Value | |
| Low APS and low-fat score | 1.000 | 1.106 | 0.489–2.498 | 8.094 × 10−1 | 1.000 | 1.002 | 0.708–1.420 | 9.902 × 10−1 | 1.000 | 1.200 | 0.697–2.066 | 5.113 × 10−1 | |
| Low APS and high-fat score | 1.086 | 1.430 | 1.335 | ||||||||||
| High APS and low-fat score | 0.982 | 1.267 | 1.175 | ||||||||||
| High APS and high-fat score | 1.179 | 1.816 | 1.882 | ||||||||||
Abbreviations: APS, animal protein score; CI, confidence interval; DQDFS, diet quality based on dietary fat score; SF, synergy factor.
Table 14.
Multivariable Logistic Regression Analysis for Intrinsic Eczema, Allergic Sensitization, and Atopic Dermatitis Using APS and QDGIS
| Combined Dietary Scores (APS and QDGIS) | Multivariable Logistic Regression (Adjusted for Age, Sex, BMI, and Parental Eczema) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intrinsic Eczema |
Allergic Sensitization |
Atopic Dermatitis |
|||||||
| AOR | 95% CI | P-Value1 | AOR | 95% CI | P-Value1 | AOR | 95% CI | P-Value1 | |
| Low APS and good QDGIS class | 1.371 | 0.715–2.761 | 0.357 | 0.638 | 0.482–0.840 | 0.002 | 0.624 | 0.408–0.955 | 0.030 |
| Low APS and moderate QDGIS class | 0.922 | 0.484–1.848 | 0.812 | 0.687 | 0.528–0.890 | 0.005 | 0.612 | 0.414–0.908 | 0.014 |
| Low APS and poor QDGIS class | 0.964 | 0.484–1.994 | 0.919 | 0.714 | 0.540–0.941 | 0.017 | 0.619 | 0.405–0.944 | 0.026 |
| Moderate APS and good QDGIS class | 1.162 | 0.619–2.305 | 0.652 | 0.624 | 0.478–0.810 | 4.560×10−4 | 0.542 | 0.362–0.812 | 0.003 |
| Moderate APS and moderate QDGIS class | 0.928 | 0.478–1.884 | 0.830 | 0.797 | 0.611–1.035 | 0.091 | 0.616 | 0.413–0.921 | 0.018 |
| Moderate APS and poor QDGIS class | 1.109 | 0.538–2.352 | 0.782 | 0.950 | 0.712–1.266 | 0.730 | 0.792 | 0.512–1.226 | 0.296 |
| High APS and good QDGIS class | 1.152 | 0.621–2.262 | 0.666 | 0.825 | 0.638–1.060 | 0.137 | 0.789 | 0.542–1.154 | 0.219 |
| High APS and moderate QDGIS class | 1.108 | 0.557–2.289 | 0.776 | 0.924 | 0.701–1.214 | 0.573 | 0.826 | 0.550–1.242 | 0.356 |
| High APS and poor QDGIS class | 1.000 | REF | — | 1.000 | REF | — | 1.000 | REF | — |
Abbreviations: AOR, adjusted OR; APS, animal protein score; BMI, body mass index; CI, confidence interval; QDGIS, quality of diet based on glycemic index score; REF, reference.
P < 0.003 (after Bonferroni correction for multiple comparisons) are statistically significant and written in bold.
Table 15.
SF Analysis for Intrinsic Eczema, Allergic Sensitization, and Atopic Dermatitis Using APS and QDGIS
| SF Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combined Dietary Scores (APS and QDGIS) | Intrinsic Eczema |
Allergic Sensitization |
Atopic Dermatitis |
|||||||||
| OR | SF Value | SF 95% CI | SF P-Value | OR | SF Value | SF 95% CI | SF P-Value | OR | SF Value | SF 95% CI | SF P-Value | |
| Low APS and poor QDGIS class | 1.000 | 0.739 | 0.390–1.399 | 3.353 × 10−1 | 1.000 | 0.889 | 0.680–1.165 | 3.952 ×10−1 | 1.000 | 0.605 | 0.404–0.907 | 1.489 × 10−2 |
| Low PPS and good QDGIS class | 1.377 | 0.810 | 1.104 | |||||||||
| High PPS and poor QDGIS class | 1.057 | 1.646 | 1.992 | |||||||||
| High APS and good QDGIS class | 1.076 | 1.186 | 1.332 | |||||||||
Abbreviations: APS, animal protein score; CI, confidence interval; QDGIS, quality of diet based on glycemic index score; SF, synergy factor.
P < 0.003 (after Bonferroni correction for multiple comparisons) are statistically significant and written in bold.
On the basis of the synergy factor (SF) analysis, there was an antagonistic interaction between APS and PPS to lower the risks of AD (Figure 3). This supported the earlier results that despite frequent intake of high-protein foods, particularly animal-based protein foods, the increased intake of plant-based protein foods in diets nullified the overall increased associated risks of AD. As previously reported, frequent intakes of high-fat and/or high-GI foods were positively associated with AD (Lim et al., 2022). Through an SF analysis using the combined dietary scores for fats and GI with PPS, a higher intake frequency of plant-based proteins in diets was also shown to significantly reduce the odds of AD imposed by high fat and high GI (Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13, Table 14, Table 15). Interestingly, a high animal protein diet was dependent on high-fat diets in terms of the associated AD risks (Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13, Table 14, Table 15).
Discussion
To the best of our knowledge, little is known about whether intake frequencies of different dietary protein sources could be associated with AD and potentially affect AD development through the modulation of various proinflammatory processes. This study found that a frequent intake of high-protein foods in diets, especially animal-based proteins, posed an increased associated risk of AD and allergic sensitization to common house dust mites (HDMs). Conversely, it also suggested that frequent consumption of high plant-based protein diets has a significant protective association on subjects with AD and allergic backgrounds.
During inflammation and tissue injury, it was recommended to have a higher protein intake than the usual requirement (10–35% calorie needs in a normal adult aged ≥18 years) (Guadagni and Biolo, 2009; Wolfe et al., 2017). However, protein consumption has been reported to exceed the recommended daily calorie needs in developed nations and can impose detrimental health effects (Bilsborough and Mann, 2006). Even in less developed countries, a dietary pattern characterized by a high protein, calorie, and saturated fat intake was found to be associated with metabolic syndrome among healthy adults (Atasi et al., 2022). Although findings from the Framingham Heart Study Offspring Cohort suggested that an increase in biomarkers of inflammation and oxidative stress was lower in those with higher protein intake, this was specific for plant protein and not animal protein (Hruby and Jacques, 2019). It was further indicated in a separate clinical trial with 20 patients with AD that a vegetarian diet improved skin inflammation by attenuating prostaglandin E2 synthesis in monocytes (Tanaka et al., 2001). Similarly, we showed that the type of protein source was important to impact the associated risks of disease outcomes.
The effects of high animal protein consumption on systemic inflammation are evident in several studies. A randomized, acute clinical intervention study demonstrated an upregulation of CCL5 in the postprandial blood serum after meals supplemented with animal proteins (Holmer-Jensen et al., 2011). In addition, diets high in chicken proteins were suggested to affect neuroinflammation by increasing IL-1β, IL-6, and TNF-ɑ (Zhang et al., 2020). In contrast, plant-based nutritional habits have been often attributed to decreased risks of chronic diseases associated with inflammation by lowering circulating inflammatory biomarkers and relieving inflammatory state (Craddock et al., 2019). In support of this view, an intervention trial revealed that high plant protein diets for 6 weeks lowered the expression of TGF-β1 and proinflammatory adipokines (chemerin and progranulin) in blood serum (Markova et al., 2020). Besides its immunomodulatory potential, TGF-β1 has a key role in wound epithelialization and skin homeostasis (Ramirez et al., 2014). A high exposure level of TGF-β1 in breast milk during the first month of life has been correlated with eczema later in life (Morita et al., 2018). Specifically, the TGF-β1–SMAD3 signaling pathway has been highlighted in AD murine models to influence the production of systemic IgE and promote allergen-induced skin inflammation (Anthoni et al., 2007). These results may partly explain the strongly associated protection between a frequent intake of high plant-based protein foods and AD.
In addition, the increased intake of mammal-derived protein-rich foods may lead to a change in the overall nutrition composition profiles, especially in the levels of total saturated fatty acids. Saturated fatty acids have been reported to be positively associated with increased prevalence of atopic diseases in developed countries (Black and Sharpe, 1997). High saturated fatty acids in diets were suggested to promote IgE synthesis and result in low-grade chronic systemic inflammation (Milanski et al., 2009). Data from the 2015 Canadian Community Health Survey - Nutrition highlighted that a dietary pattern of plant-based foods avoided excessive energy intake compared with red/processed meat dishes and minimized high saturated fatty acids, total cholesterol, and low-density lipoprotein cholesterol (Shafiee et al., 2022). Given the multiple benefits of plant-based foods to optimize human health, many international dietary guidelines encourage the incorporation of plant-based foods in diets (Liu, 2013; Rodriguez-Casado, 2016) As part of Singapore’s national efforts to promote healthy dietary habits, the Health Promotion Board recommended a mixture of animal-based and plant-based proteins from whole, fresh foods in meals to obtain a good source of essential nutrients and a healthy fat content (Health Hub, 2022).
Although we had examined the same association between various protein intakes and IE, there were no significant associations. This is consistent with a previous study in mice with psoriasis-like conditions whereby the feeding of high-protein diets exacerbated systemic inflammatory responses but had little effect on cutaneous conditions (Knopp et al., 2021). These results corroborated the idea that a frequent intake of high-protein foods in diets promoted the associated risks of AD by manifesting on top of the atopy predisposition. Thus, the influence of dietary proteins might be stronger in those with an allergic background and cutaneous hypersensitivity than in those without. Because AD forms a critical part of the classic triad of atopy, frequent intake of high-protein foods in diets may also influence and promote associated risks of other atopic diseases (Helm, 2004; Paller et al., 2019). More studies should be conducted to thoroughly assess the association between a diet of high protein intake and the development of other atopic diseases such as allergic rhinitis and asthma in addition to AD.
There is increasing evidence to suggest that dietary factors can affect microbiota diversity and composition to reduce the risk of AD development and severity in early life (Abrahamsson et al., 2012; Liu et al., 2022). In addition, studies highlighted that the source, concentration, and amino acid balance of dietary proteins drive the composition and function of gut microbes (Zhao et al., 2019). Protein digestion in the gut producing metabolites such as ammonia, amines, short-chain fatty acids, and hydrogen sulfide may trigger proinflammatory signaling pathways in epithelial cells and potentially contribute to AD pathogenesis (Abdallah et al., 2020). Apart from providing plant-based protein, a plant-based diet is rich in fibers, which act as a substrate to encourage anti-inflammatory effects and diverse growth of beneficial bacteria (Tomova et al., 2019). More studies are necessary to elucidate the specific mechanisms of gut microbiota in influencing AD with regard to plant-based food intake. Recent research has proposed targeting the gut microbiota on immune responses as a personalized dietary approach to improve AD symptoms (Alam et al., 2022).
One of the main study limitations lies within its cross-sectional design, which cannot be used to determine the causal direction between dietary habits of protein-based diets and AD. Furthermore, we cannot reveal the temporal sequence because the study was conducted retrospectively. There was also a lack of detailed information on cooking methods and a limited variety of food types in our dietary measurement. However, this study included a sufficiently large population with a high participant response rate, and the dietary indices used were carefully designed with established criteria and validated guidelines. Although there are concerns of potential recall bias in reporting diet, the effect of individual measurement errors is diluted, and the overall estimate of dietary intake frequencies is likely to be accurate with a large sample. Moreover, questionnaires were directly administered to subjects, and they were able to clarify doubts to reduce misunderstandings and errors. Although we have controlled for certain prominent confounding factors, including parental eczema, to address the genetic predisposition, we acknowledged that there might be other genetic and environmental factors not fully accounted for in our cross-sectional study. Therefore, prospective cohort studies can be conducted to provide valuable insights into the long-term relationships between dietary protein intake and AD/IE. Genetic and environmental information can be collected and controlled from subjects at baseline and throughout the follow-up period. This enables a more comprehensive assessment of gene–environment interactions that can help identify subgroups that may be more susceptible to the effects of dietary protein intake and better isolate the true association between dietary protein intake and AD/IE. At this stage, our findings provide strong initial evidence to improve the understanding of the relationship between dietary protein intake and AD/IE. Future research should be undertaken to thoroughly investigate the reliability of our dietary indices by cross-validating them in other independent cohorts but with more rigorous measures of dietary intake. This can involve the use of a detailed food frequency questionnaire that is more culturally based, in terms of food types/amount, culinary use, and nutritional compositions. Visual aids relating to standard portion sizes can also be employed to improve participants’ responses. By doing so, we seek to contextualize our results in relation to established metrics for chronic diseases such as the Alternative Healthy Eating Index, which holds significant importance in public health research (Morze et al., 2020).
Overall, the results in this study broadly support the work of other studies in this area linking dietary habits with chronic diseases and corroborate the ideas of how animal-based protein in diets may impose a possible detrimental risk to disease development, whereas plant-based protein may have associative protection against diseases. These data provide some valuable insights into how plant-based protein diets can be considered for nutritional treatment and clinical intervention in those with an allergic background and AD disease.
Materials and Methods
Study cohort
A cross-sectional study was conducted from 2005 to 2019 to investigate the epidemiology of allergy and atopic disease. A nonbias and random volunteer sample of staff and students was consecutively taken from universities in both Singapore (National University of Singapore, Singapore, Singapore) and Malaysia (Universiti Tunku Abdul Rahman, Perak, Malaysia and Sunway University, Selangor, Malaysia). Data on the personal and family symptomatic histories for eczema, lifestyles, and dietary habits were collected through an investigator-administered questionnaire that was validated by the International Study of Asthma and Allergy in Childhood protocol. The overall combined cohort of the Singapore/Malaysia Cross-sectional Genetic Epidemiology Study consists of 16,336 subjects. We first excluded 2,547 subjects who had missing responses for dietary habits, age, and sex. Owing to the unequal percentages of the three main races in Singapore and Malaysia, there was a lower probability of sampling among other ethnic groups, and the results obtained from the epidemiological investigation might not be fully representative of these ethnic groups (Department of Statistics Singapore, 2020). Thus, we have selected the major ethnic group Chinese (n = 11,494) to minimize ascertainment bias and the loss of statistical empowerment in our analysis (Figure 1).
Disease definitions
As a genetic predisposition toward the development of immediate hypersensitivity against environmental allergens, allergic sensitization can be assessed experimentally by the presence of allergen-specific IgE or positive SPT to common allergens (Cohen et al., 2003). Among the local HDM fauna, Blomia tropicalis and Dermatophagoides pteronyssinus were found to be the most common yet important source of allergens in Singapore and the tropics (Chew et al., 1999a, 1999b). Past allergen studies have established that IgE sensitization was highly associated with HDM allergens, with >80% of individuals being HDM serum IgE positive (Andiappan et al., 2014). Thus, we objectively assessed all 11,494 Chinese subjects for their allergic sensitization status through an SPT for their reactivity to common HDMs, B. tropicalis and D. pteronyssinus. Subjects who had taken antihistamines or any related allergy medications within 3 days before the study were declined to ensure reliability in SPT. Those who tested positive for either one of the HDMs will have a wheal diameter ≥3 mm compared with the negative saline control. Subjects who were SPT negative did not show any elevation of allergen-specific IgE and did not have a wheal diameter ≥3 mm when tested with either HDM.
In this study, skin disease was historically defined. Per validated guidelines from the United Kingdom Working Party Criteria (Williams et al., 1994) and Hanifin and Rajka Criteria (Hanifin and Rajka, 1980), we defined eczema symptoms to be having a recurrent itchy rash for at least 6 months that was in the flexural regions, including under the buttocks and behind the neck, cheeks, ears, or eyes. During the collection, our trained personnel also concurrently assessed for the presence of an itchy rash. The assessments by our trained personnel were cross-validated periodically by a dermatologist and found to be concordant. The incorporation of SPT to HDMs alongside symptomatic histories in defining AD showed high sensitivity and specificity in a previous study compared with the clinical standard of having allergic comorbidities (Lim et al., 2022). For the purpose of this study, AD cases were defined as having eczema symptoms and a positive SPT response as described earlier. IE cases were defined as having eczema symptoms and an SPT-negative response (Brenninkmeijer et al., 2008; Karimkhani et al., 2015). Nonallergic noneczema control subjects were defined by not having eczema symptoms and a negative SPT response. Figure 1 shows the population distribution of SPT reactivity, eczema symptoms, and disease cases and controls. For more information, refer to Lim et al. (2022) on the Singapore/Malaysia Cross-sectional Genetic Epidemiology Study cohort demographics and various AD phenotypes.
Dietary intake assessment and dietary indices
A section adapted from the International Study of Asthma and Allergy in Childhood phase III study was included to assess the dietary habits of 16 food types over the past 12 months (Ellwood et al., 2005). We asked the question “In the past 12 months, how often, on average, did you eat or drink the following?” for meat (e.g., beef, lamb, chicken, or pork), seafood (including fish), fruits, vegetables (green and root), pulses (pea, beans, lentils), cereals (including bread), pasta, rice, butter, margarine, nuts, potatoes, milk, egg, burgers/fast food, and yakult/vitagen/similar yogurt drinks. Subjects reported their intake frequencies as either “never or only occasionally,” “once or twice per week,” or “most or all days.”
On the basis of the intake frequency of selected food types, a specific score was assigned to the corresponding intake frequencies—0 (never or only occasionally), 2 (once or twice per week), and 7 (most or all days)—following the validated rubric by Manousos et al. (1983). We first derived DPS to assess the total protein intake in diets. A total of 12 food types were categorized according to their total estimated protein amount, for a usual serving size of 100 g per edible portion, from the nutrient composition database by the Health Promotion Board (Singapore, Singapore) (Tables 16 and 17). Food types with high total estimated protein comprised meat, seafood, nuts, eggs, burgers/fast food, and cereals, whereas food types with low total estimated protein comprised milk, rice, pulses, vegetables, potatoes, and fruits. A positive score was prepended to high-protein foods, whereas a negative score was prepended to low-protein foods. The summation of assigned scores for all 12 food types resulted in DPS (Figure 4a). A one-of-three cut off was selected to categorize subjects into their respective groups for all dietary indices. Adequate sensitivity analyses based on the preliminary population were performed to ensure robustness and reliability in designing the dietary indices and choosing the cut offs. A total of 3,941 subjects were grouped into low DPS (score −6); 4,588 were grouped into moderate DPS (score −5 to 0); and 2,965 were grouped into high DPS (score >0). A subject with a low DPS represents a low intake frequency of high-protein foods (i.e., frequently consumes more low-protein foods than high-protein foods) and vice versa.
Table 16.
Information on the Estimated Total Protein Amount, Based on the Usual Serving Size for the 16 Food Types, Retrieved from the HPB Database: The Average Total Protein Amount (Grams per 100 g Edible Portion) for Each Food Type
| Food Types Analyzed | Grams per 100 g Edible Portion |
|---|---|
| High protein foods | |
| Meat (e.g., beef, lamb, chicken, pork) | 25.06 |
| Seafood (including fish) | 22.73 |
| Nuts | 21.40 |
| Eggs | 12.20 |
| Burgers/fast food | 10.50 |
| Cereals (including bread) | 9.68 |
| Low protein foods | |
| Pasta | 6.60 |
| Milk | 3.97 |
| Rice | 2.56 |
| Pulses (peas, beans, lentils) | 2.48 |
| Vegetables (green and root) | 2.15 |
| Potatoes | 1.98 |
| Margarine | 1.20 |
| Fruits | 0.65 |
| Butter | 0.65 |
| Probiotic drinks | 0.35 |
Abbreviation: HPB, Singapore Health Promotion Board.
The estimated total protein amount was used as a reference to categorize the 16 food types into high-protein foods and low-protein foods. Information on estimated total protein amount (grams per 100 g edible portion) was retrieved from the Singapore HPB database at https://focos.hpb.gov.sg/eservices/ENCF/. The data were accessed in September 2022. The estimated total protein amount values were calculated on the basis of the list of common foods for each food type.
Table 17.
Information on the Estimated Total Protein Amount, Based on the Usual Serving Size for the 16 Food Types, Retrieved from the Singapore HPB Database: List of Specific Foods Adopted for the Averaging of Total Protein Amount (Grams per 100 g Edible Portion) for Each Food Type
| List of Common Foods Adopted for Each Food Type | Grams per 100 g Edible Portion |
|---|---|
| Animal-based protein foods | |
| Meat (e.g., beef, lamb, chicken, pork) | |
| Beef, boneless, unspecified cut, cooked, lean only | 31.3 |
| Pork (pork, boneless, unspecified cut, cooked, lean, and fat) | 25.5 |
| Chicken (chicken, broilers or fryers, drumstick, meat and skin, cooked, stewed) | 25.3 |
| Lamb (mutton cooked) | 23.2 |
| Beef, boneless, unspecified cut, raw, lean and fat | 20.0 |
| Seafood (including fish) | |
| Cuttlefish, cooked | 32.5 |
| Prawn, king, cooked | 23.7 |
| Steamed fish, unspecified | 20.2 |
| Crab, freshwater, boiled | 19.2 |
| Molluscs, scallop, mixed species, cooked, breaded, and fried | 18.1 |
| Eggs | |
| Egg, hen, whole, raw | 12.9 |
| Egg, duck, whole, raw | 12.6 |
| Egg, goose, whole, raw | 11.1 |
| Burgers/fast food | |
| Drumstick, original recipe, KFC | 26.0 |
| Winglets, KFC | 14.0 |
| Hamburger, Burger King | 14.0 |
| Hawaiian 12” Large Pan Pizza, Pizzahut | 13.0 |
| McSpicy, Mcdonald’s | 11.0 |
| Fillet-o-fish, McDonald’s | 10.0 |
| McChicken, McDonald’s | 8.0 |
| Onion rings, small, Burger King | 4.0 |
| KFC cheese fries | 3.0 |
| Apple pie, McDonald’s | 2.0 |
| Milk | |
| Milk, whole, evaporated, canned | 7.6 |
| Low-fat milk | 3.9 |
| UHT chocolate milk | 3.3 |
| Full cream milk | 3.2 |
| Soya bean milk, with sugar (hawker centre) | 3.2 |
| Milk, white | 3.2 |
| Butter | |
| Butter, salted | 0.7 |
| Butter, unsalted | 0.6 |
| Plant-based protein foods | |
| Nuts | |
| Nuts, peanut, with shell, dry roasted | 29.0 |
| Nuts, almonds | 21.3 |
| Nuts, cashew nut, raw | 21.2 |
| Nuts, pistachio nuts, raw | 20.6 |
| Nuts, hazelnuts | 15.0 |
| Cereals (including bread) | |
| Bread, French | 11.8 |
| Bread, brown | 9.7 |
| Bread, white | 9.7 |
| Breakfast cereal, plain, contains whole grains | 7.5 |
| Pasta | |
| Pasta, egg, dried raw | 12.7 |
| Pasta, marinara | 9.5 |
| Creamy chicken pasta | 6.5 |
| Meatball pasta | 6.3 |
| Plain Pesto pasta | 6.0 |
| Pasta, wholemeal, boiled | 5.8 |
| Crayfish pasta | 5.6 |
| Noodles, instant, dry, with seasoning, cooked | 5.2 |
| Pasta, white, boiled | 4.2 |
| Creamy mushroom pasta | 4.2 |
| Rice | |
| Brown rice cooked | 3.1 |
| White rice cooked | 2.8 |
| Rice, glutinous, white, cooked | 2.0 |
| Pulses (peas, beans, lentils) | |
| Long beans, boiled | 2.5 |
| Snow peas, raw | 2.5 |
| Beans, French, raw | 2.4 |
| Vegetables (green and root) | |
| Vegetables, dark green non-leafy, unspecified, boiled | 2.8 |
| Onions, ginger, garlic | 2.5 |
| Vegetable, dark green leafy, unspecified, boiled, drained | 2.2 |
| Carrot, boiled | 1.1 |
| Potatoes | |
| Potato, old, baked, flesh and skin | 2.3 |
| Sweet potato, boiled, without skin | 1.7 |
| Fruits | |
| Banana, raw, unspecified type | 1.1 |
| Orange | 1.0 |
| Watermelon | 0.8 |
| Papaya | 0.4 |
| Pear, green, raw with peel | 0.3 |
| Apples, raw, without skin | 0.3 |
| Others | |
| Probiotic drinks | |
| Yogurt drink, flavored, nonfat | 1.2 |
| Margarine | |
| Margarine spread, polysaturated | 0.4 |
| Margarine spread, monounsaturated | 0.4 |
| Margarine spread, polyunsaturated, reduced fat, reduced salt | 0.3 |
| Margarine spread, monounsaturated, reduced fat, reduced salt | 0.3 |
Abbreviations: HPB, Singapore Health Promotion Board; KFC, Kentucky Fried Chicken; UHT, ultra heat treatment.
The estimated total protein amount was used as a reference to categorize the 16 food types into high-protein foods and low-protein foods. Information on estimated total protein amount (grams per 100 g edible portion) was retrieved from the Singapore HPB database at https://focos.hpb.gov.sg/eservices/ENCF/. The data were accessed in September 2022. The estimated total protein amount values were calculated on the basis of the list of common foods for each food type.
Figure 4.
Flowchart illustrating the design of various dietary indices. (a) Dietary protein score to estimate the total protein intake in diets, (b) animal protein score and plant protein score to estimate the animal and plant-based protein intake in diets, respectively.
Next, we derived APS and PPS to assess the intake frequency of animal-derived protein and plant-derived protein in diets, respectively. Animal-derived protein foods were meat, seafood, milk, eggs, burgers/fast food, and butter, whereas plant-derived protein foods were cereals, rice, pulses, vegetables, potatoes, fruits, nuts, and pasta. For APS and PPS, only positive scores were allocated to their respective intake frequencies of various animal-derived protein and plant-derived protein foods. Similarly, the summation of the assigned score for animal food types yielded APS, and the summation of the assigned score for plant food types yielded PPS (Figure 4b). For APS, 3,588 subjects had a low APS (score <18); 3,875 had a moderate APS (score = 18–24); and 4,031 had a high APS (score > 24). For PPS, 3,551 subjects had a low PPS (score < 26); 3,883 subjects had a moderate PPS (score = 26–33); and 4,060 had a high PPS (score > 33). A low APS and PPS represent low intake frequency of animal-derived protein foods and plant-derived protein foods, respectively. Information on the design of various dietary indices is presented in Figure 4. We have previously described the use of a dietary fat score (diet quality based on dietary fat score) (Lim et al., 2022) and quality of diet based on GI score (Say et al., 2022) to assess the intake of fat and GI, respectively. In this study, we evaluated these scores in combination with the protein scores to determine the various associated risks for AD and IE. Kindly refer to the earlier studies for more information on the computation of diet quality based on dietary fat score and quality of diet based on GI score.
Statistical analysis
Logistic regression models were conducted to analyze the association between dietary indices and various outcomes. Outcomes of logistic regression were presented in ORs, 95% confidence intervals, and a P-value. The statistical significance of the results was defined as logistic regression P < 0.003 (as adjusted by Bonferroni’s correction for multiple comparisons), and the OR range did not cross 1.000. All statistical analyses and drawing of balloon plots were performed using R program, version 2021.09.0.351 (http://www.rstudio.com), whereas Microsoft Excel (http://office.microsoft.com/en-us/excel/) was used for data entry and drawing of bar plots. A chi-square test was performed to determine whether the distribution of a given categorical variable was different across different outcome groups. A chi-square P < 0.001 (as adjusted by Bonferroni’s correction) was considered statistically significant. SF is a statistical method to assess the potential binary interactions between risk factors in case-control studies, especially for study of complex diseases (Cortina-Borja et al., 2009). SF analysis is easy to use and clear to interpret. In SF analysis, we examine whether the combined effects of animal-derived and plant-derived protein intake in diets exhibit synergy (greater than additive effect) or antagonism (lesser than additive effects) in influencing the susceptibility of skin diseases. For a detailed explanation of SF methodology, interested readers can refer to the original work by Cortina-Borja et al. (2009). Understanding antagonistic interactions provides insights into the complex interplay between dietary proteins (of different sources) and potentially guides the development of dietary interventions in the future.
Ethics statement
This study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practices and in compliance with local regulatory requirements. Ethical approval was granted from the Institutional Review Board of the National University of Singapore (Singapore, Singapore) (National University of Singapore Institutional Review Board reference codes NUS-07-023, NUS-09-256, NUS-10-445, NUS-13-075, NUS-14-150, and NUS-18-036), the Scientific and Ethical Review Committee of Universiti Tunku Abdul Rahman (reference code U/SERC/03/2016), and Sunway University Research Ethics Committee (reference code SUREC 2019/029). Informed consent was obtained from all subjects before the study through a signed consent form. Parental consent was obtained for subjects below the age of 18 years.
Data availability statement
All data used and included in this study are available from the corresponding author (FTC).
ORCIDs
Jun Jie Lim: http://orcid.org/0000-0001-5485-1749
Kavita Reginald: http://orcid.org/0000-0003-1530-5934
Yee-How Say: http://orcid.org/0000-0003-2363-5239
Mei Hui Liu: http://orcid.org/0000-0001-7405-7008
Fook Tim Chew: http://orcid.org/0000-0003-1337-5146
Disclaimer
The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
FTC reports grants from the Singapore Ministry of Education Academic Research Fund, Singapore Immunology Network, National Medical Research Council, Biomedical Research Council, National Research Foundation, Singapore Food Agency, and the Agency for Science Technology and Research during the conduct of the study and has received consultancy fees from Sime Darby Technology Centre, First Resources, Genting Plantation, Olam International, and Syngenta Crop Protection, outside the submitted work. The remaining authors state no conflict of interest.
Acknowledgments
The authors would like to thank and acknowledge all our participants and their family members for being willing to participate in this study. All authors have read and consented to the publication of this manuscript. FTC received grants from the National University of Singapore (N-154-000-038-001), Singapore Ministry of Education Academic Research Fund (R-154-000-191-112, R-154-000-404-112, R-154-000-553-112, R-154-000-565-112, R-154-000-630-112, R-154-000-A08-592, R-154-000-A27-597, R-154-000-A91-592, R-154-000-A95-592, R154-000-B99-114), Biomedical Research Council (Singapore, Singapore) (BMRC/01/1/21/18/077, BMRC/04/1/21/19/315, BMRC/APG2013/108), Singapore Immunology Network (SIgN-06-006, SIgN-08-020), National Medical Research Council (Singapore, Singapore) (NMRC/1150/2008), National Research Foundation (Singapore, Singapore) (NRF-MP-2020-0004), Singapore Food Agency (SFS_RND_SUFP_001_04, W22W3D0006), and the Agency for Science Technology and Research (Singapore, Singapore) (H17/01/a0/008 and APG2013/108).
Author Contributions
Conceptualization: FTC; Data Curation: JJL; Formal Analysis: JJL; Funding Acquisition: FTC; Methodology: FTC; Project Administration: JJL, KR, Y-HS, LMH; Resources: JJL, KR, Y-HS, LMH; Supervision: FTC; Visualization: JJL; Writing - Original Draft Preparation: JJL; Writing - Review and Editing: LMH
accepted manuscript published online XXX; corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2023.100224
References
- Abdallah A., Elemba E., Zhong Q., Sun Z. Gastrointestinal interaction between dietary amino acids and gut microbiota: with special emphasis on host nutrition. Curr Protein Pept Sci. 2020;21:785–798. doi: 10.2174/1389203721666200212095503. [DOI] [PubMed] [Google Scholar]
- Abrahamsson T.R., Jakobsson H.E., Andersson A.F., Björkstén B., Engstrand L., Jenmalm M.C. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–440.e4402. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Ahnen R.T., Jonnalagadda S.S., Slavin J.L. Role of plant protein in nutrition, wellness, and health. Nutr Rev. 2019;77:735–747. doi: 10.1093/nutrit/nuz028. [DOI] [PubMed] [Google Scholar]
- Alam M.J., Xie L., Yap Y.A., Marques F.Z., Robert R. Manipulating microbiota to treat atopic dermatitis: functions and therapies. Pathogens. 2022;11:642. doi: 10.3390/pathogens11060642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andiappan A.K., Puan K.J., Lee B., Nardin A., Poidinger M., Connolly J., et al. Allergic airway diseases in a tropical urban environment are driven by dominant mono-specific sensitization against house dust mites. Allergy. 2014;69:501–509. doi: 10.1111/all.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthoni M., Wang G., Deng C., Wolff H.J., Lauerma A.I., Alenius H.T. Smad3 signal transducer regulates skin inflammation and specific IgE response in murine model of atopic dermatitis. J Invest Dermatol. 2007;127:1923–1929. doi: 10.1038/sj.jid.5700809. [DOI] [PubMed] [Google Scholar]
- Atasi M., Kammar-García A., Almendra-Pegueros R., Navarro-Cruz A.R. Dietary patterns and their association with metabolic syndrome and their components in middle-class adults from Damascus, Syria: a cross-sectional study. J Nutr Metab. 2022;2022 doi: 10.1155/2022/5621701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzini E., Peluso I., Intorre F., Barnaba L., Venneria E., Foddai M.S., et al. Total and plant protein consumption: the role of inflammation and risk of non-communicable disease. Int J Mol Sci. 2022;23:8008. doi: 10.3390/ijms23148008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S.M. Atopic eczema: genetic associations and potential links to developmental exposures. Int J Toxicol. 2017;36:187–198. doi: 10.1177/1091581817701075. [DOI] [PubMed] [Google Scholar]
- Bilsborough S., Mann N. A review of issues of dietary protein intake in humans. Int J Sport Nutr Exerc Metab. 2006;16:129–152. doi: 10.1123/ijsnem.16.2.129. [DOI] [PubMed] [Google Scholar]
- Black P.N., Sharpe S. Dietary fat and asthma: is there a connection? Eur Respir J. 1997;10:6–12. doi: 10.1183/09031936.97.10010006. [DOI] [PubMed] [Google Scholar]
- Brenninkmeijer E.E., Spuls P.I., Legierse C.M., Lindeboom R., Smitt J.H., Bos J.D. Clinical differences between atopic and atopiform dermatitis. J Am Acad Dermatol. 2008;58:407–414. doi: 10.1016/j.jaad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Brown S.J., Elias M.S., Bradley M. Genetics in atopic dermatitis: historical perspective and future prospects. Acta Derm Venereol. 2020;100 doi: 10.2340/00015555-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew F.T., Lim S.H., Goh D.Y., Lee B.W. Sensitization to local dust-mite fauna in Singapore. Allergy. 1999;54:1150–1159. doi: 10.1034/j.1398-9995.1999.00050.x. [DOI] [PubMed] [Google Scholar]
- Chew F.T., Zhang L., Ho T.M., Lee B.W. House dust mite fauna of tropical Singapore. Clin Exp Allergy. 1999;29:201–206. doi: 10.1046/j.1365-2222.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- Chovatiya R., Silverberg J.I. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21:172–176. doi: 10.36849/JDD.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Dworetzky M., Frick O.L. Coca and Cooke on the classification of hypersensitiveness. J Allergy Clin Immunol. 2003;111:205–210. doi: 10.1067/mai.2003.106. [DOI] [PubMed] [Google Scholar]
- Cortina-Borja M., Smith A.D., Combarros O., Lehmann D.J. The synergy factor: a statistic to measure interactions in complex diseases. BMC Res Notes. 2009;2:105. doi: 10.1186/1756-0500-2-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock J.C., Neale E.P., Peoples G.E., Probst Y.C. Vegetarian-based dietary patterns and their relation with inflammatory and immune biomarkers: a systematic review and meta-analysis. Adv Nutr. 2019;10:433–451. doi: 10.1093/advances/nmy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Statistics Singapore Census of population 2020 statistical release 1: demographic, characteristics, education, language and religion. 2020. https://www.singstat.gov.sg/-/media/files/publications/cop2020/sr1/findings.pdf;
- Domínguez O., Plaza A.M., Alvaro M. Relationship between atopic dermatitis and food allergy. Curr Pediatr Rev. 2020;16:115–122. doi: 10.2174/1573396315666191111122436. [DOI] [PubMed] [Google Scholar]
- Drucker A.M., Wang A.R., Li W.Q., Sevetson E., Block J.K., Qureshi A.A. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Ellwood P., Asher M.I., Beasley R., Clayton T.O., Stewart A.W., ISAAC Steering Committee The international study of asthma and allergies in childhood (ISAAC): phase three rationale and methods. Int J Tuberc Lung Dis. 2005;9:10–16. [PubMed] [Google Scholar]
- Guadagni M., Biolo G. Effects of inflammation and/or inactivity on the need for dietary protein. Curr Opin Clin Nutr Metab Care. 2009;12:617–622. doi: 10.1097/MCO.0b013e32833193bd. [DOI] [PubMed] [Google Scholar]
- Hanifin J., Rajka G. Diagnostic features of atopic eczema. Acta Derm Venereol. 1980;92:44–47. [Google Scholar]
- Health Hub My healthy plate. 2022. https://www.healthhub.sg/programmes/55/my-healthy-plate
- Health Promotion Board National Nutrition Survey 2018 shows gradual improvements in Singaporeans’ dietary habits. 2018. https://www.hpb.gov.sg/article/national-nutrition-survey-2018-shows-gradual-improvements-in-singaporeans-dietary-habits
- Helm R.M. Diet and the development of atopic disease. Curr Opin Allergy Clin Immunol. 2004;4:125–129. doi: 10.1097/00130832-200404000-00010. [DOI] [PubMed] [Google Scholar]
- Holmer-Jensen J., Karhu T., Mortensen L.S., Pedersen S.B., Herzig K.H., Hermansen K. Differential effects of dietary protein sources on postprandial low-grade inflammation after a single high fat meal in obese non-diabetic subjects. Nutr J. 2011;10:115. doi: 10.1186/1475-2891-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby A., Jacques P.F. Dietary protein and changes in biomarkers of inflammation and oxidative stress in the Framingham heart study offspring cohort. Curr Dev Nutr. 2019;3:nzz019. doi: 10.1093/cdn/nzz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanny G. [Atopic dermatitis in children and food allergy: combination or causality? Should avoidance diets be initiated?] Ann Dermatol Venereol. 2005;132 Special No 1:1S90–103. [PubMed] [Google Scholar]
- Karimkhani C., Silverberg J.I., Dellavalle R.P. Defining intrinsic vs. extrinsic atopic dermatitis. Dermatol Online J. 2015;21 13030/qt14p8p404. [PubMed] [Google Scholar]
- Khan A., Adalsteinsson J., Whitaker-Worth D.L. Atopic dermatitis and nutrition. Clin Dermatol. 2022;40:135–144. doi: 10.1016/j.clindermatol.2021.10.006. [DOI] [PubMed] [Google Scholar]
- Knopp T., Bieler T., Jung R., Ringen J., Molitor M., Jurda A., et al. Effects of dietary protein intake on cutaneous and systemic inflammation in mice with acute experimental psoriasis. Nutrients. 2021;13:1897. doi: 10.3390/nu13061897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.J., Lim Y.Y.E., Ng J.Y., Malipeddi P., Ng Y.T., Teo W.Y., et al. An update on the prevalence, chronicity, and severity of atopic dermatitis and the associated epidemiological risk factors in the Singapore/Malaysia Chinese young adult population: a detailed description of the Singapore/Malaysia Cross-Sectional Genetics Epidemiology Study (SMCGES) cohort. World Allergy Organ J. 2022;15 doi: 10.1016/j.waojou.2022.100722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.H. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4:384S–392S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Du X., Zhai S., Tang X., Liu C., Li W. Gut microbiota and atopic dermatitis in children: a scoping review. BMC Pediatr. 2022;22:323. doi: 10.1186/s12887-022-03390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manousos O., Day N.E., Trichopoulos D., Gerovassilis F., Tzonou A., Polychronopoulou A. Diet and colorectal cancer: a case-control study in Greece. Int J Cancer. 1983;32:1–5. doi: 10.1002/ijc.2910320102. [DOI] [PubMed] [Google Scholar]
- Markova M., Koelman L., Hornemann S., Pivovarova O., Sucher S., Machann J., et al. Effects of plant and animal high protein diets on immune-inflammatory biomarkers: a 6-week intervention trial. Clin Nutr. 2020;39:862–869. doi: 10.1016/j.clnu.2019.03.019. [DOI] [PubMed] [Google Scholar]
- Milanski M., Degasperi G., Coope A., Morari J., Denis R., Cintra D.E., et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Campos-Alberto E., Yamaide F., Nakano T., Ohnisi H., Kawamoto M., et al. TGF-β concentration in breast milk is associated with the development of eczema in infants. Front Pediatr. 2018;6:162. doi: 10.3389/fped.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morze J., Danielewicz A., Hoffmann G., Schwingshackl L. Diet quality as assessed by the healthy eating index, alternate healthy eating index, Dietary Approaches to Stop Hypertension score, and health outcomes: a second update of a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2020;120:1998–2031.e15. doi: 10.1016/j.jand.2020.08.076. [DOI] [PubMed] [Google Scholar]
- Muthayya S., Sugimoto J.D., Montgomery S., Maberly G.F. An overview of global rice production, supply, trade, and consumption. Ann N Y Acad Sci. 2014;1324:7–14. doi: 10.1111/nyas.12540. [DOI] [PubMed] [Google Scholar]
- Ng Y.T., Chew F.T. A systematic review and meta-analysis of risk factors associated with atopic dermatitis in Asia. World Allergy Organ J. 2020;13 doi: 10.1016/j.waojou.2020.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosrati A., Afifi L., Danesh M.J., Lee K., Yan D., Beroukhim K., et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523–538. doi: 10.1080/09546634.2016.1278071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller A.S., Spergel J.M., Mina-Osorio P., Irvine A.D. The atopic march and atopic multimorbidity: many trajectories, many pathways. J Allergy Clin Immunol. 2019;143:46–55. doi: 10.1016/j.jaci.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Ramirez H., Patel S.B., Pastar I. The role of TGFβ signaling in wound epithelialization. Adv Wound Care (New Rochelle) 2014;3:482–491. doi: 10.1089/wound.2013.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Casado A. The health potential of fruits and vegetables phytochemicals: notable examples. Crit Rev Food Sci Nutr. 2016;56:1097–1107. doi: 10.1080/10408398.2012.755149. [DOI] [PubMed] [Google Scholar]
- Say Y.H., Sio Y.Y., Heng A.H.S., Ng Y.T., Matta S.A., Pang S.L., et al. Golgin A7 family member B (GOLGA7B) is a plausible novel gene associating high glycaemic index diet with acne vulgaris. Exp Dermatol. 2022;31:1208–1219. doi: 10.1111/exd.14575. [DOI] [PubMed] [Google Scholar]
- Shafiee M., Islam N., Ramdath D.D., Vatanparast H. Most frequently consumed red/processed meat dishes and plant-based foods and their contribution to the intake of energy, protein, and nutrients-to-limit among Canadians. Nutrients. 2022;14:1257. doi: 10.3390/nu14061257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg N.B., Lee-Wong M., Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227–232. [PubMed] [Google Scholar]
- Tanaka T., Kouda K., Kotani M., Takeuchi A., Tabei T., Masamoto Y., et al. Vegetarian diet ameliorates symptoms of atopic dermatitis through reduction of the number of peripheral eosinophils and of PGE2 synthesis by monocytes. J Physiol Anthropol Appl Human Sci. 2001;20:353–361. doi: 10.2114/jpa.20.353. [DOI] [PubMed] [Google Scholar]
- Tomova A., Bukovsky I., Rembert E., Yonas W., Alwarith J., Barnard N.D., et al. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr. 2019;6:47. doi: 10.3389/fnut.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T.F., Rajagopalan M., Chu C.Y., Encarnacion L., Gerber R.A., Santos-Estrella P., et al. Burden of atopic dermatitis in Asia. J Dermatol. 2019;46:825–834. doi: 10.1111/1346-8138.15048. [DOI] [PubMed] [Google Scholar]
- Venter C., Meyer R.W., Nwaru B.I., Roduit C., Untersmayr E., Adel-Patient K., et al. EAACI position paper: influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy. 2019;74:1429–1444. doi: 10.1111/all.13764. [DOI] [PubMed] [Google Scholar]
- Williams H.C., Burney P.G., Hay R.J., Archer C.B., Shipley M.J., Hunter J.J., et al. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol. 1994;131:383–396. doi: 10.1111/j.1365-2133.1994.tb08530.x. [DOI] [PubMed] [Google Scholar]
- Wolfe R.R., Cifelli A.M., Kostas G., Kim I.Y. Optimizing protein intake in adults: interpretation and application of the recommended dietary allowance compared with the acceptable macronutrient distribution range. Adv Nutr. 2017;8:266–275. doi: 10.3945/an.116.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Song S., Zhao D., Shi J., Xu X., Zhou G., et al. High intake of chicken and pork proteins aggravates high-fat-diet-induced inflammation and disorder of hippocampal glutamatergic system. J Nutr Biochem. 2020;85 doi: 10.1016/j.jnutbio.2020.108487. [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhang X., Liu H., Brown M.A., Qiao S. Dietary protein and gut microbiota composition and function. Curr Protein Pept Sci. 2019;20:145–154. doi: 10.2174/1389203719666180514145437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used and included in this study are available from the corresponding author (FTC).