Abstract

Herein we report a modular synthetic method for the preparation of diaryl-substituted cyclohexenone acids starting from phenyl pyruvate and suitable enones. When the reaction is carried out in alkaline tert-butanol or toluene solutions in microwave-assisted conditions mainly anti configuration products are obtained with up to 86% isolated yield. However, when the reaction is carried out in alkaline water, a mixture of products with anti and syn conformations is obtained with up to 98% overall isolated yield. Mechanistically the product with anti conformation forms by a hemiketal–oxy-Cope type [3,3]-sigmatropic rearrangement–intramolecular aldol condensation route and syn product by an intermolecular aldol condensation-electrocyclization (disrotatory type) route.
Introduction
Chiral cyclohexenones are core structures in many natural products and pharmaceuticals,1−7 and therefore important targets for synthetic chemistry.5−11 Commonly employed synthetic strategies for chiral cyclohexenones include Robinson annulations,12,13 Michael additions,14−16 and aldol condensations15,17 together with recent examples including homo-Nazarov cyclization18 and 1,3 protonic shift.19 [3,3]-sigmatropic rearrangements are a convenient way to achieve regio- and stereoselective C–C and C–X bond forming reactions in organic chemistry.20−24 Notably, the [3,3]-sigmatropic rearrangement approach is largely an unexplored option for cyclohexenone synthesis.
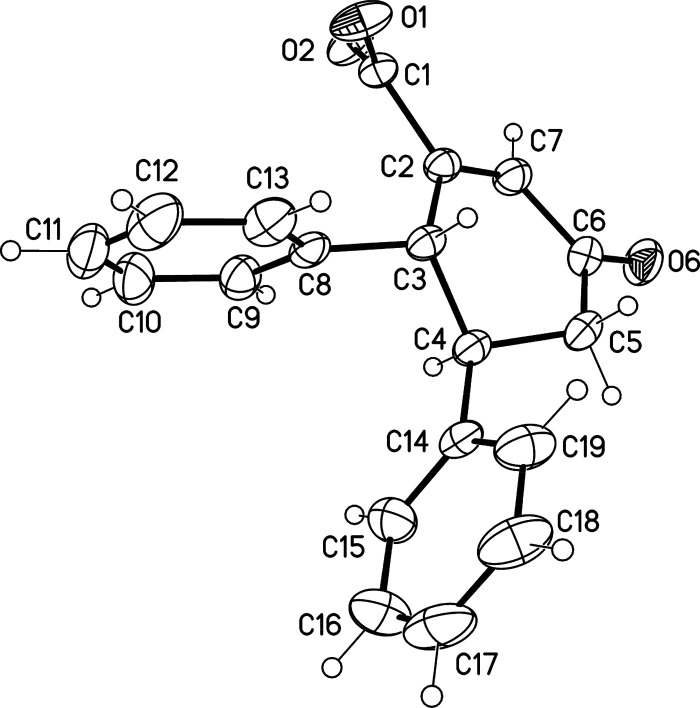
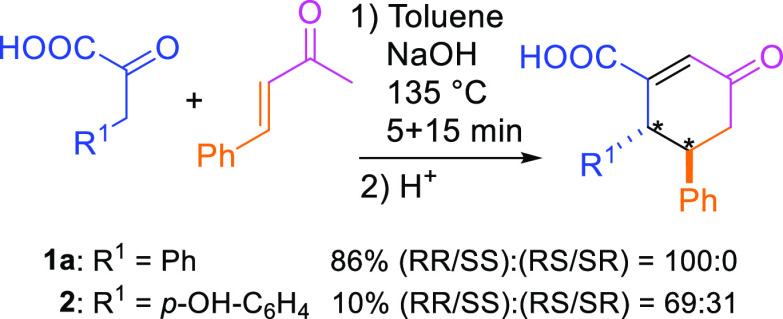
In our previous work,25 we successfully utilized [3,3]-sigmatropic rearrangement in dicarboxylic acid synthesis from phenylpyruvate and cinnamaldehyde under microwave-assisted conditions from alkaline alcohol solutions (Figure 1a). We report here that the substrate scope can be expanded toward aryl enones, and this subsequently opens a unique synthesis strategy toward substituted cyclohexanones. For example, a reaction between phenylpyruvate and 4-phenyl-3-buten-2-one generates 4,5-diphenyl-cyclohex-2-en-1-one-3-carboxylic acid (1) salt (Figure 1b). Regarding the product, there is only one preliminary report from 1956 by Krisensen-Reh, where its identification, let alone the reaction pathway, was not unambigous.26 Based on X-ray crystallography herein, the reaction proceeds in a diastereospecific manner; stereocenters in 1a are either RR or its enantiomer SS due to the centrosymmetry in the crystal structure (Figure 2) (CDCC 2193907). As shown earlier by us, the diastereospecificity in this new synthetic strategy toward chiral cyclohexenones arises from the elemental reaction step, [3,3]-sigmatropic rearrangement.25
Figure 1.
Utilization of [3,3]-sigmatropic rearrangement of phenylpyruvic acid under microwave-assisted conditions with (a) cinnamaldehyde into sodium salt of dicarboxylic acid.25 (b) 4-Phenyl-3-buten-2-one into sodium salt of cyclohexanone acid (1a) (this work).
Figure 2.
Molecular structure of the anion of the sodium salt of 1a (displacement parameters are drawn at the 50% probability level). Stereocenters are RR and its enantiomer SS due to centrosymmetry (CDCC 2193907).
Results and Discussion
In our previous work, phenylpyruvic acid was successfully used with aromatic enal (cinnamaldehyde) in the dicarboxylic acid synthesis. The reaction proceeds through a hemiacetal intermediate;25 however, it remained uncertain if sterically more demanding aromatic enones could also be utilized in the synthesis. We explored the reaction between phenylpyruvic acid and aryl enone (4-phenyl-3-buten-2-one) under different reaction conditions. When the alkaline toluene solution was heated under microwave-assisted conditions (135 °C, 15 min), a crystalline beige/yellowish powder was obtained after cooling. After acetone washing, a sodium salt of 1a (4,5-diphenyl-cyclohex-2-en-1-one-3-carboxylic acid) was isolated at 86% yield, and its solid-state structure was determined (Figure 2). Similarly, when 4-hydroxy-phenylpyruvic acid was used instead of phenylpyruvic acid, product 4-(para-phenol)-5-phenyl-cyclohex-2-en-1-one-3-carboxylic acid (2) is obtained at 10% isolated yield (racemic mixture (RR/SS):(RS/SR) = 69:31). The presence of para-phenol R1 in 2 indicates that the R1 substituent originates from the pyruvate acid and the second phenyl ring originates from the enone (Figure 3). This observation is in line with our previous work, giving R1 next to the acid group. Mechanistically the reaction is assumed to proceed through a hemiketal intermediate similar to our previous dicarboxylic acid synthesis.25
Figure 3.
Formation of cyclohexenone acids from aromatic pyruvates and presynthesized aromatic enone (4-phenyl-3-buten-2-one). Given isolated yields are calculated from pyruvic acid.
To further expand the substrate scope, we investigated a series of reactions in which aromatic enones were synthesized in situ from different benzaldehydes and acetone via aldol condensation (Figure 4a). When combined with phenylpyruvic acid, this approach grants a straightforward way to synthesize unprecedented cyclohexenone acid derivatives with moderate yields simply by altering the aromatic aldehyde (Figure 4b). The ease of the in situ process is offset by lower yields, for example, giving 31% isolated yield of 1a compared to 86% yield when using the presynthesized aryl enone.
Figure 4.
(a) In situ formation of aromatic enones from acetone and aromatic aldehydes. (b) Reaction conditions: α-ketoacid (2.0 mmol), aromatic aldehyde (2.0 mmol), acetone (2.0 mmol), NaOH (3 mmol, 10 M at H2O solution), tert-butanol (3 mL); microwave vial was heated to 135 °C in 5 min and kept at 135 °C for 30 min. Products emerge as an enantiomeric pair of RR and SS. Yields are isolated yields calculated from phenylpyruvic acid.
The reaction tolerates halogen functionalities including fluoride, chloride, and trifluoromethyl substitution resulting in, under in situ generated enone conditions, products 9, 10, and 11 with 10%, 20%, and 4% isolated yields, respectively. Sterically demanding substituents are also compatible within the reaction, which was demonstrated with 4-(benzyloxy)benzaldehyde, giving 4-phenyl-5-(4-benzyloxy)phenyl-cyclohex-2-en-1-one-3-carboxylic acid (12) with an isolated 42% ((RR/SS, anti):(RS/SR, syn) = 62:38 stereocenters) yield.
Mechanistically, the reaction commences when an enolate tautomer of phenylpyruvic acid attacks the aromatic enone and forms a hemiketal intermediate (i, Figure 5). The reaction continues via oxy-Cope type [3,3]-sigmatropic rearrangement followed by H+ transfer, generating a diketone intermediate ii, which bears pyruvate acid and ketone functionalities. This intermediate undergoes intramolecular aldol condensation (A) and, through the elimination of water, yields the desired cyclohexenone acid product as a sodium salt.
Figure 5.
Formation of cyclohexenone acid. Enolate tautomer of pheylpyruvic acid forms hemiketal i with an aromatic enone. Intermediate i undergoes oxy-Cope type [3,3]-sigmatropic rearrangement, and after H+ transfer, the intermediate ii is formed. Intermediate ii undergoes intramolecular aldol condensation (A) and after OH group elimination, the final cyclohexenone acid product is obtained as sodium salt.
Similarly to our previous work,25 the oxy-Cope type [3,3]-sigmatropic rearrangement is the source of diastereospecifity. The formation of certain diastereomer pairs is favored when the phenyls of phenylpyruvic acid and the aromatic enone do not overlap during the formation of intermediate i. Thus, when enolate tautomer of phenylpyruvic acid (E or Z) attacks the prochiral aromatic enone from either the re or si face, the hemiketal i with R configuration in the chiral center gives ii as an SS diastereomer with E enolate or RR diastereomer with Z enolate. Likewise, hemiketal i with S configuration gives ii as the RR diastereomer with E enolate or the SS diastereomer with Z enolate. Interestingly, when the aryl enone contains a large substituent such as a benzyloxy group in 12a/b, similar to compound 2 the stereochemical preference is disturbed and RS/SR diastereomers are also observed (38% ratio of total yield); see below.
When in situ synthesis of cyclohexenone acids is performed using 2-butanone instead of acetone, a methyl group at the α-position is introduced, and this opens further possibilities to tailor the cyclohexanone structures (13a, Figure 6, R3). For example, following this observation and replacing acetone with 2-pentanone, an ethyl group can be introduced at the α-position instead. Interestingly, the product 2-ethyl-1-hydroxy-3-oxo-5,6-diphenyl-cyclohexane-1-carboxylic acid 14A does not undergo the final dehydration step (Figure 5). The crystal structure of 14A shows the ethyl group at the α carbon in an equatorial position and the OH group at the β carbon in an axial position (Figure 7, 14A). Most likely the predicted OH-elimination cannot occur due to the increased steric hindrance introduced by the ethyl group. On the other hand, the remaining OH group in 14A is further evidence of the ring closure by the intramolecular aldol condensation reaction.
Figure 6.
Synthesis of Cyclohexenone Acids with extended ketone (R3). Reaction conditions: α-ketoacid (2.0 mmol), benzaldehyde (2.0 mmol), ketone (2.0 mmol), NaOH (3 mmol, 10 M at H2O solution), and tert-butanol (3 mL); microwave vial was heated to 135 °C in 5 min and kept at 135 °C for 30 min. aEntry 4: 2 mmol of 4-(benzyloxy)benzaldehyde instead of benzaldehyde.
Figure 7.
Molecular structures of 1st (a) and 2nd (b) crystallographic independent molecule of 14A (displacement parameters are drawn at 50% probability level) (CDCC 2193908, stereocenters: 1S, 2S, 5R, 6R and 1R, 2R, 5S, 6S) and molecular structure of 15a (solvent water omitted for clarity, displacement parameters are drawn at 50% probability level) (CDCC 2193909) with RRR and its enantiomer pair SSS.
The crystal structure of 14A also exhibits centrosymmetry. The results suggest that the intramolecular aldol condensation is stereospecific when the R3 group is suitably sterically demanding. A stark contrast is obtained when the synthesis is performed with 3-methyl-2-butanone. Two different enantiomeric pairs are obtained (RRR and SSS (15a)) and (1S, 5R, and 6R and 1R, 5S, and 6S (15b)) at nearly a 1:1 ratio. As the α carbon is missing a proton, OH group elimination cannot occur. Thus, products 15a and 15b also contain an OH group, as a result. We expected the stereochemistry of the β carbon to behave similarly to product 14A, whereby the carboxylic acid group would be at the equatorial position as it is a larger and more demanding functionality. However, the solid state crystal structure of product 15a clearly has the carboxylic acid group at the axial position (Figure 7, 15a). Evidently the carboxylic acid group does not provide enough driving force to guide the aldol condensation to be stereospecific in this reaction, when the α-position does not contain additional steric guidance.
Interestingly, when 3-methyl-2-butanone is reacted with the sterically demanding group 4-(benzyloxy)benzaldehyde (Figure 6 entry 4) the products 16a and 16b are obtained. Stereocenters of 16a and 16b at γ and δ carbons only exist in the anti position with respect to each other, and no RS/SR epimers are observed unlike with compounds 2 and 12. Instead the compounds follow the same stereocenter pattern as 15a and 15b.
We propose that, in product 12, the bulky side group -C6H4-O-Bn from 4-(benzyloxy)benzaldehyde makes the hemiketal formation i or [3,3]-sigmatropic rearrangement energetically more demanding. The in situ formed enone undergoes keto–enol tautomerization and opts for intermolecular aldol condensation with phenylpyruvic acid (Figure 8). Followed by elimination of water, intermediate iii is formed. Next, iii undergoes keto–enol tautomerization into intermediate iv. The reaction proceeds to an electrocyclization reaction in a disrotatory fashion. A similar reactivity pattern is supported by earlier work from Isoe et al.27,28 Disrotatory electrocyclization reaction forces the phenyl groups into the syn configuration. This explains formation of epimers RS/SR. As when 16a/b is synthesized the additional sterics from 3-methyl-2-butanone as the enone constituent, this makes intermolecular aldol condensation unfavorable. This pushes the reaction mechanism back to the [3,3]-sigmatropic rearrangement despite the sterically demanding side group. Similarly, in product 2 we suspect that the electron donating phenolic group encourages pyruvic acid to remain in the ketone form rather than in the enol/enolate form. This makes intermolecular aldol condensation more likely to take place and opens up the system to generate the syn (RS/SR) epimeric pairs as well.
Figure 8.
Alternative pathway for the formation of cyclohexenone acid (an example is for 12b). Enol form of aromatic enone undergoes aldol condensation with phenylpyruvic acid. After elimination, the intermediate iii undergoes keto–enol tautomerization into intermediate iv, followed up by electrocyclisation. Final product is obtained after keto–enol tautomerisation. As intermediate iv undergoes electrocyclization by a disrotatory pathway, the final product is obtained as a syn conformation.
Water is often used as a solvent in the aldol condensation reactions. When the synthesis of 1 (Table 1, entry 1) is performed in water instead of tert-BuOH or toluene, the overall yield of the reaction rises to near quantitative (96%). However, water as a reaction medium also promotes formation of 1b (RS/SR) having a syn-configuration with a partial yield of 36%. Usage of water opens the intermolecular aldol condensation-electrocyclization pathway described in Figure 8 even for examples that previously did not undergo it.
Table 1. Synthesis of Cyclohexenones in Water.
| Entry | Synthesis of | Conditionsa | Yield (%) (anti:syn) |
|---|---|---|---|
| 1 | 1b | H2O, 135 °C | 96 (64:36) |
| 2 | 13 | H2O, 135 °C | 98 (72:28) |
| 3 | 14 | H2O, 135 °C | 98 (75:25) |
| 4 | 15 | H2O, 135 °C | 90 (15a:15b 67:33), no syn products |
| 5 | 12 | H2O, 135 °C | 83 (62:38) |
| 6 | 1b | H2O, RTc | 96 (1A, 100:0) |
| 7 | 1b | Brine,d 135 °C | 94 (95:5) |
Reaction conditions: α-ketoacid (2.0 mmol), benzaldehyde (2.0 mmol), ketone (2.0 mmol), NaOH (3 mmol, 10 M at H2O solution), H2O (3 mL); microwave vial was heated to 135 °C and heat was maintained for 30 min.
4-Phenyl-3-buten-2-one (2 mmol) is used instead of benzaldehyde and ketone.
Reaction was stirred for 4 h at room temperature at 20 mL vial.
Brine solution (3 mL, saturated NaCl solution) was used instead of Milli-Q H2O.
The usage of water as a solvent also benefits the in situ formation of aromatic enones. When synthesis of 13 (Table 1, entry 2) is carried out in water, the overall isolated yield was near quantitative 98% and syn (RS/SR) product (13b) is obtained with 28% of overall yield. Interestingly, for the synthesis of 14 (Table 1, entry 3) in water, the reaction went into completion to 14a:14b (75:25) with 98% overall yield. Rather than stopping at stage 14A (Figure 6, entry 2) like in the above case, the reaction was carried out in tert-BuOH solution. For the synthesis of 15 (Table 1, entry 4) in water, high overall yields are obtained (90%; 15a:15b = 67:33). But, as observed previously the enone constituent with a terminal isopropyl group is unable to undergo the intermolecular aldol condensation–electocyclisation pathway, and thus, a syn product does not form. Curiously, when the synthesis of 12 (Table 1, entry 5) is carried out in water, a high yield is obtained (83%) but the ratio between anti (12a) and syn (12b) stayed the same (62:38) as when the synthesis was done at tert-BuOH (Figure 4). Product, which was already undergoing the syn formation pathway, did not get additional benefits from the water for this pathway.
Interestingly, the formation ratio of the syn product has a negative correlation with the stericity of the enone constituent (Table 1, entries 1–4). The least hindered enone constituent yields the highest syn ratio (36%), while the extreme example in the series, the isopropyl-terminated enone component, does not allow for the formation of the syn product (15) at all. Thus, it is clear that in water the syn products are coming from the intermolecular aldol condensation-electocyclization pathway described in Figure 8.
Curiously, when the synthesis of 1 is carried out at room temperature in water (Table 1, entry 6), the reaction stops at intermediate A (Figure 5) prior to final hydroxyl group elimination. The product 1A is obtained with near quantitative isolated yield (96%) and only as an anti (RR/SS) conformer. This result suggests that the hemiketal–[3,3]-sigmatropic rearrangement route proceeds through a lower energy pathway than the intermolecular aldol condensation–electrocyclization route. Interestingly, the result also suggests that the intramolecular aldol condensation (Figure 5) is stereospecific at room temperature. Accordingly, the carboxylic acid group directs itself to the equatorial position at β carbon and leaves the hydroxyl group to the axial position.
When the synthesis of 1 is carried out in a hot brine solution (Table 1, entry 7) high yields are still obtained (94%), but the syn product ratio dropped to 5%. Most likely, the increased Na+-ion concentration is stabilizing the enolate form of phenylpyruvic acid. This promotes hemiketal formation and the subsequent [3,3]-sigmatropic rearrangement reaction over the intermolecular aldol condensation–electrocyclization pathway even in the water.
Conclusions
To summarize, we have developed a new strategy for cyclohexenone synthesis by utilizing pyruvates with aromatic enones. Anti conformers of cyclohexenone acids are obtained via the hemiketal–oxy-Cope type [3,3]-sigmatropic rearrangement–intramolecular aldol condensation pathway and syn conformers via the intermolecular aldol condensation–electrocyclisation pathway. Products form in a diastereospecific manner, with typically two but up to four stereogenic centers. High yields can be obtained in H2O, but with a mixture of anti and syn conformers, in organic solvents the conditions favor anti conformer formation although with lower overall yields. As shown, the structure of cyclohexenone acids can be varied in a straightforward manner, by choice of pyruvate (R1), aromatic aldehyde (R2) and ketone (R3). The synthesis tolerates a variety of functional groups, and cyclohexenone acids contain a multitude of functionalities such as ketone, carboxylic acid, and double bonds variants. Thus, cyclohexenone acids reported here could be utilized as building blocks for further synthesis.
Experimental Section
General Information
All chemicals were obtained from commercial sources and used as such without further purification. 1H, 13C{1H}, 19F, HSQC, and HMBC NMR spectra were recorded with an Avance Neo (500 MHz, 25 °C) NMR spectrometer by Bruker. Recorded spectra were calibrated by solvent signals (acetone-d6: 1H 2.050 ppm, 13C 29.840 ppm; DMSO-d6: 1H 2.500 ppm, 13C 39.520 ppm; D2O: 1H 4.790 ppm). 19F NMR chemical shift axis was indirectly referenced via calculating 19F 0 ppm from the solvent lock frequency. All spectra were processed with MestReNova software. High-resolution mass spectra were measured by Bruker microTOF-MS in negative ion mode. Sodium formate was used as a calibrant. The samples were diluted to 1 ppm concentration with acetonitrile and filtered with syringe filters prior to measurements. IR spectra were measured with an Alpha ATR-FTIR by Bruker. The single-crystal X-ray diffraction study was carried out on a Bruker D8 Venture diffractometer with a PhotonII CPAD detector at 173(2) K (1a, 14A) or Rigaku XtaLAB Synergy-S with HyPIX-6000 detector at 123(2) K (15a) using Cu–Kα radiation (λ = 1.54178 Å). The synthesis of cyclohexenone acid salts were conducted with a microwave reactor Monowave 450 by Anton Paar. Experiments were conducted in G10 vials, which were capped by snap caps with Teflon-coated silicon septums. The temperature during synthesis was monitored by a surface IR sensor, which was calibrated by a Ruby thermometer—an internal temperature probe—frequently.
General Procedure for Cyclohexenone Acid Synthesis from Presynthesized Enone
Pyruvic acid (2 mmol), 4-phenyl-3-buten-2-one (2 mmol), NaOH (3 mmol, 300 μL, 10 M), and toluene (2 mL) were charged into a microwave vial (10 mL). The vial was capped and heated to 135 °C in 5 min with a microwave reactor. The temperature was maintained for 15 min. Then the vial was cooled to 50 °C using compressed air (see microwave heating profile at Figure S1). Following this, acetone (5 mL) was added to the vial to initiate the crystallization. The next day, the formed powder was collected by filtration, washed with acetone, and dried under air. The obtained product was dissolved with water, and HCl (1 M) was added until the formation of a white powder stopped. The powder was filtered, washed with water, and dried under vacuum.
General Procedure for Cyclohexenone Acid Synthesis from in situ Synthesized Enone
Pyruvic acid (2 mmol), benzaldehyde (2 mmol), ketone (2 mmol), NaOH (3 mmol), and tert-BuOH (3 mL) were added into a 10 mL microwave vial. The heating program was set to heat the microwave vial to 135 °C in 5 min and maintain the temperature for 30 min. The stirring speed was set to 600 rpm. Next, the microwave vial was cooled down to 50 °C with compressed air (see microwave heating profile at Figure S2). After the mixture cooled, acetone was added to initiate the crystallization overnight. The following day, the formed product salt was collected by filtration, washed with acetone, and dried under air. Then, the salt of the product was dissolved with water, and 1 M HCl was added until all the product had precipitated from the solution. The formed powder was filtered, washed with water, and dried under vacuum.
General Procedure for Cyclohexenone Acid Synthesis in Water
Phenylpyruvic acid (2 mmol), enone (2 mmol) (in case of in situ synthesized enone corresponding ketone (2 mmol) and aromatic aldehyde (2 mmol) was used instead), NaOH (3 mmol, 300 μL, 10 M), and milli-Q water (3 mL) were charged into a microwave vial (10 mL). The vial was capped and heated to 135 °C as fast as possible with a microwave reactor. The temperature was maintained for 30 min. Then the vial was cooled to 50 °C using compressed air (see microwave heating profile at Figure S3). Following this, HCl (1M, ca. 4 mL) was added into the microwave vial until precipitation formation stopped. The next day, the formed powder was collected by filtration, washed with water and n-hexane, and dried under vacuum.
4,5-Diphenyl-cyclohex-2-en-1-one-3-carboxylic Acid (1a and 1b) (Enantiomeric Pair of RR/SS (anti), RS/SR (syn))
The synthesis of a mixture of anti and syn products was carried out according to the general procedure for cyclohexenone acid synthesis in water using phenylpyruvic acid (2 mmol, 328.3 mg) and 4-phenyl-3-buten-2-one (2 mmol, 292.4 mg). After reaction, the products were obtained as a mixture of 1a and 1b as a beige powder 562 mg (96%). According to 1H NMR measurement, the ratio of 1a (RR/SS, anti):1b (RS/SR, syn) was 64:36.
Isolation of 1b
After the reaction was performed in water, the water dissolved salt mixture of 1a and 1b was transferred into a 20 mL vial. Acetone, ca. 10 mL, was added into the vial. In the following 5 days crystals formed in the vial; this salt (180 mg) was filtered and washed with acetone (ca. 5 mL), and this first fraction was recognized as the salt of 1a. Within the next week, another fraction of salt (60 mg) formed in the vial. This fraction was filtered and washed with acetone (ca. 5 mL). This fraction was identified as the 1b salt. The salt was dissolved into water and protonated with HCl, filtered, washed with water, and dried in vacuum.
Synthesis of 1a by the general procedure from presynthesized enone: The product was synthesized from phenylpyruvic acid (2 mmol, 328.3 mg) and from presynthesized enone 4-phenyl-3-buten-2-one (2 mmol, 292.4 mg). After reaction, the product was isolated as a brownish beige salt (541 mg, 86%). The product was then dissolved with water, and HCl (1 M) was added until formation of the white powder stopped. The powder was filtered, washed with water, and dried under vacuum. The isolated protonated yield was 425 mg (73%).
Synthesis of 1a by the general procedure for in situ synthesized enone phenylpyruvic acid (2 mmol, 328.3 mg), acetone (2 mmol, 150 μL), and benzaldehyde (2 mmol, 205 μL). Product was isolated as 182 mg (31%) of a white powder.
1a: 1H NMR (500 MHz, acetone-d6): δ 7.29–7.16 (m, 10H), 6.80 (d, J = 1.8 Hz, 1H), 4.41 (dd, J = 6.8, 1.6 Hz, 1H), 3.53 (ddd, J = 9.3, 6.8, 4.6 Hz, 1H), 2.91 (dd, J = 16.4, 9.3 Hz, 1H), 2.66 (dd, J = 16.4, 4.6 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 198.8, 167.7, 151.1, 143.2, 141.8, 133.3, 129.28, 129.25, 129.1 128.5, 127.7, 127.6, 49.3, 49.0, 42.5. IR (atr) cm–1: 2700–3200 (broad), 1362 (m) (R-COOH), 1721 (s) (C=C-ROOH), 1643 (s) (C=C-CO-R), 1452 (m) (R-CO-CH2-R), 1238 (s), 1216 (s) (R-CO-R), 762 (s), 695 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF) m/z: [1a-H]− calculated for C19H15O3 291.1016; found 291.1010. See the X-ray diffraction section for solved crystal structure of 1a (CCDC: 2193907).
1b: 1H NMR (500 MHz, acetone-d6): δ 7.21–7.17 (m, 3H), 7.17–7.14 (m, 1H), 7.14–7.09 (m, 2H), 6.98 (s, 1H), 6.90 (m, 2H), 6.72 (m, 2H), 4.40 (d, J = 4.9 Hz, 1H), 3.95 (dt, J = 15.1, 4.4 Hz, 1H), 2.95 (dd, J = 17.0, 15.0 Hz, 1H), 2.42 (dd, J = 16.9, 3.7 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 200.2, 167.1, 150.9, 141.9, 135.8, 134.0, 130.3, 128.82, 128.81, 128.7, 127.9, 127.7, 48.5, 45.0, 38.1. IR (atr) cm–1: 2800–3200 (broad), 1279 (s) (R-COOH), 1692 (s) (C=C-ROOH), 1672 (s) (C=C-CO-R), 1452 (m) (R-CO-CH2-R), 1253 (s) (R-CO-R), 772 (s), 696 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF) m/z: [1b-H]− calculated for C19H15O3 291.1016; found 291.1016.
1-Hydroxy-5-oxo-2,3-diphenylcyclohexane-1-carboxylic acid (1A) (Enantiomeric Pair of RRR and SSS)
The product was synthesized from 4-phenyl-3-buten-2-one (2 mmol, 292.4 mg), phenylpyruvic acid (2 mmol, 328.3 mg), water (3 mL), and NaOH (3 mmol). Reactants and solvents were placed in a 20 mL vial. The vial was stirred (600 rpm) at room temperature for 4 h. After reaction, 1 M HCl was added to the vial until the precipitation formation stopped. Product was filtered, washed with water, and dried under vacuum. Product was isolated as 596 mg (96%) of white powder (500 MHz, DMSO-d6): 7.24–7.20 (m, 2H), 7.20–7.16 (m, 2H), 7.11–7.07 (m, 2H), 7.05–7.01 (m, 2H), 7.00–6.95 (m, 2H), 5.35 (s (broad, 1H, OH proton), 3.83 (d, J = 12.2 Hz, 1H), 3.74 (td, J = 12.3, 4.4 Hz, 1H), 3.30 (d, J = 13.6 Hz, 1H), 2.95 (dd, J = 14.1, 12.5 Hz, 1H), 2.44 (dd, J = 13.7, 2.3 Hz, 1H), 2.37 (ddd, J = 14.3, 4.4, 2.3 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6): δ 206.8, 174.3, 143.1, 138.3, 129.8, 128.0, 127.8, 127.16, 126.17, 126.0, 78.56, 52.6, 51.1, 49.1, 44.1. IR (atr) cm–1: 3515 (m, sharp), 1075 (s) (Axial −OH), 2800–3200 (broad), 1721 (s), 932 (m) (R-COOH), 1680 (s), 1216 (s) (R-CO-R), 1454 (m) (R-CH2–R), 768 (s), 702 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF) m/z: [1A-H]− calculated for C19H17O4 309.1121; found 309.1123.
4-(para-Phenol)-5-phenyl-cyclohex-2-en-1-one-3-carboxylic Acid (2) (Mixture of Epimers RR/SS and RS/SR)
The product was synthesized according to the general procedure from presynthesized enone using 4-hydroxy phenylpyruvic acid (2 mmol, 360.3 mg) instead of phenylpyruvic acid. Purification: After the reaction, the remaining solvent in the microwave tube was evaporated. Next the crude powder was washed with hexane. The crude powder was then dissolved in water and filtered. The filtrate was protonated by adding HCl until the formation of a cloudy precipitate stops. The precipitate was difficult to filter; thus, it was dried under vacuum. The protonated powder was moved into a clean vial and dissolved with ethanol (ca. 3 mL). In the following weeks, each day a small amount of water (ca. 300 μL) was added each day to the vial. After the first week, mainly a brown-red powder was formed from the slow crystallization process. The brown-red powder was filtered out, and the crystallization process continues until colorless crystals are formed (see Figure S4 as reference picture). This crystallization must be done very slowly in order to obtain good high purity crystals. The crystals are filtered, washed with water, and dried under vacuum. Colorless crystals in an epimeric mixture were isolated with a 60 mg (10%) yield. Epimers RR/SS and RS/SR form in a 69% and 31% ratio respectively. 1H NMR (500 MHz, acetone-d6): δ 8.23 (broad, 1H, phenolic OH.), 7.29–7.16 (m, 5H), 7.03–6.99 (m, 1.5H), 6.94–6.90 (m, 1H), 6.75–6.71 (m, 2H), 6.64–6.50 (m, 1.7H), 4.32 (m, 1H), 3.87 (dt, J = 15.0, 4.2 Hz, 0.36H), 3.48 (ddd, J = 8.9, 6.5, 4.6 Hz, 0.81H), 2.94 (dd, J = 17.0, 14.9 Hz, 1.2H), 2.87 (dd, J = 16.4, 8.9 Hz, 1.4H), 2.67 (dd, J = 16.5, 4.7 Hz, 1H). 2.39 (dd, J = 16.9, 3.7 Hz, 0.38H). 13C{1H} NMR (125 MHz, acetone-d6): δ 200.4, 199.0, 167.8, 167.2, 157.2, 151.6, 151.1, 143.5, 142.2, 133.5, 132.8, 132.2, 131.4, 130.1, 129.3, 128.9, 128.8, 128.5, 127.6, 127.5, 126.0, 116.1, 115.6, 49.1, 48.5, 47.7, 45.1, 42.3, 38.2. IR (atr) cm–1: 2700–3200 (broad) (R-COOH), 3200–3500 (broad) (intermolecular hydrogen bonds), 1663 (s) (C=C-CO-R), 1610 (m) (R2-C=CH-R), 1446 (m) (R-CH2-R), 1361 (w), 1212 (s) (OH-Ph), 831 (s) (2 adjacent H (Ph)), 760 (s), 697 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF) m/z: [2-H]− calculated for C19H15O4 307.0965; found 307.0962.
4-Phenyl-5-(4-methylphenyl)-cyclohex-2-en-1-one-3-carboxylic acid (3) (Enantiomeric Pair of RR and SS)
The product was synthesized according to the general procedure for in situ synthesized enone using p-tolualdehyde (2 mmol, 235 μL) instead of benzaldehyde. Product was isolated as 121 mg (20%) of a white powder. 1H NMR (500 MHz, acetone-d6): δ 7.28–7.24 (m, 2H), 7.22–7.17 (m, 3H), 7.13–7.06 (m, 4H), 6.79 (d, J = 1.8 Hz, 1H), 4.39 (dd, J = 6.6, 1.6 Hz, 1H), 3.49 (ddd, J = 9.0, 6.6, 4.7 Hz, 1H), 2.87 (dd, J = 16.4, 9.1 Hz, 1H), 2.64 (dd, J = 16.4, 4.6 Hz, 1H), 2.26 (s, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 198.9, 167.7, 151.0, 141.8, 140.2, 137.0, 133.4, 129.9, 129.3, 129.1, 128.3, 127.7, 49.3, 48.6, 42.5, 21.0. IR (atr) cm–1: 2800–3200 (broad) (R-COOH), 1680 (s) (C=C-CO-R), 1419 (m) (R-CO-CH2-R), 1255 (s) (R-CO-R), 1146 (m) (R-COOH), 1058 (m) (CH3–Ph), 816 (s) (2 adjacent H (R-Ph-p-Me)), 703 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF) m/z: [3-H]− calculated for C20H17O3 305.1172; found 305.1170.
4-Phenyl-5-(3-methylphenyl)-cyclohex-2-en-1-one-3-carboxylic acid (4) (Enantiomeric Pair of RR and SS)
The product was synthesized according to the general procedure for in situ synthesized enone using m-tolualdehyde (2 mmol, 235 μL) instead of benzaldehyde. Product was isolated as 172 mg (28%) of a white powder. 1H NMR (500 MHz, acetone-d6): δ 7.29–7.23 (m, 2H), 7.22–7.17 (m, 3H), 7.16–7.12 (m, 1H), 7.07 (s, 1H), 7.07–6.98 (m, 2H), 6.80 (d, J = 1.8 Hz, 1H), 4.40 (dd, J = 6.6, 1.7 Hz, 1H), 3.48 (ddd, J = 9.0, 6.6, 4.7 Hz, 1H), 2.88 (dd, J = 16.4, 9.0 Hz, 1H), 2.65 (dd, J = 16.5, 4.6 Hz, 1H), 2.26 (s, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 198.9, 167.7, 150.9, 143.1, 141.8, 138.7, 133.4, 129.24, 129.23, 129.16, 129.0, 128.3, 127.7, 125.4, 49.2, 48.9, 42.4, 21.4. IR (atr) cm–1: 2800–3200 (broad) (R-COOH), 1711 (s) (C=C-ROOH), 1651 (s) (C=C-CO-R), 1454 (m), 1058 (m) (Ph–CH3), 1415 (m) (R-CO-CH2-R), 1228 (s) (R-CO-R), 880 (m) (isolated H (Ph)), 780 (m) (3 adjacent H (Ph)), 699 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF) m/z: [4-H]− calculated for C20H17O3 305.1172; found 305.1173.
4-Phenyl-5-(2-methylphenyl)-cyclohex-2-en-1-one-3-carboxylic acid (5) (Enantiomeric Pair of RR and SS)
The product was synthesized according to the general procedure for in situ synthesized enone by using o-tolualdehyde (2 mmol, 230 μL) instead of benzaldehyde. Product was isolated as 224 mg (37%) of a white powder. 1H NMR (500 MHz, acetone-d6): δ 7.53 (d, J = 7.8 Hz, 1H), 7.25–7.13 (m, 4H), 7.10–7.05 (m, 3H), 7.01 (d, J = 7.5 Hz, 1H), 6.80 (dd, J = 2.2, 0.6 Hz, 1H), 4.32 (dd, J = 8.2, 2.2 Hz, 1H), 3.76 (ddd, J = 11.2, 8.2, 4.5 Hz, 1H), 2.90 (dd, J = 16.3, 11.2 Hz, 1H), 2.57 (dd, J = 16.2, 4.4 Hz, 1H), 1.92 (s, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 198.9, 167.9, 152.1, 142.2, 141.4, 136.9, 133.0, 131.2, 129.1, 129.0, 127.6, 127.5, 127.4, 127.1, 49.5, 44.2, 43.3, 19.5. IR (atr) cm–1: 2800–3200 (broad) (R-COOH), 1704 (m) (C=C-ROOH), 1669 (s) (C=C-CO-R), 1454 (w), 1052 (m) (Ph–CH3), 1412 (w) (R-CO-CH2-R), 1254 (s) (R-CO-R), 1152 (m) (R-COOH), 762 (s) (4 adjacent H (Ph)), 701 (s), 726 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF) m/z: [5-H]− calculated for C20H17O3 305.1172; found 305.1174.
4-Phenyl-5-(4-methoxyphenyl)-cyclohex-2-en-1-one-3-carboxylic acid (6) (Enantiomeric Pair of RR and SS)
The product was synthesized according to the general procedure for in situ synthesized enone using 4-methoxybenzaldehyde (2 mmol, 245 μL) instead of benzaldehyde. Product was isolated as 175 mg (27%) of a white powder. 1H NMR (500 MHz, acetone-d6): δ 7.28–7.23 (m, 2H), 7.21–7.12 (m, 5H), 6.84–6.80 (m, 2H), 6.78 (d, J = 1.8 Hz, 1H), 4.36 (dd, J = 6.7, 1.6 Hz, 1H), 3.74 (s, 3H), 3.46 (ddd, J = 9.2, 6.7, 4.6 Hz, 1H), 2.86 (dd, J = 16.4, 9.2 Hz, 1H), 2.63 (dd, J = 16.4, 4.5 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 199.1, 167.8, 159.4, 151.2, 141.9, 135.0, 133.3, 129.4, 129.2, 129.0, 127.6, 114.6, 55.4, 49.5, 48.3, 42.7. IR (atr) cm–1: 2800–3200 (broad), 1182 (s), 1146 (s) (R-COOH), 1679 (s) (C=C-CO-R), 1610 (m) (R2-C=CH-R), 1238 (s) (R-CO-R), 1030 (m) (R-O–CH3), 824 (s) (2 adjacent H (Ph)), 773 (s), 703 (s) (5 adjacent H (Ph)), 755 (m) (Ph-O–CH3). HRMS (ESI-TOF) m/z: [6-H]− calculated for C20H17O4 321.1121; found 321.1120.
4-Phenyl-5-(3-methoxyphenyl)-cyclohex-2-en-1-one-3-carboxylic acid (7) (Enantiomeric Pair of RR and SS)
The product was synthesized according to the general procedure for in situ synthesized enone using 3-methoxybenzaldehyde (2 mmol, 245 μL) instead of benzaldehyde. Product was isolated as 374 mg (58% yield) of a white powder. 1H NMR (500 MHz, acetone-d6): δ 7.28–7.23 (m, 2H), 7.22–7.15 (m, 4H), 6.81–6.78 (m, 3H), 6.77–6.74 (m, 1H), 4.41 (dd, J = 6.8, 1.5 Hz, 1H), 3.73 (s, 3H), 3.50 (ddd, J = 9.4, 6.8, 4.6 Hz, 1H) 2.91 (dd, J = 16.4, 9.4 Hz, 1H), 2.65 (dd, J = 16.4, 4.5 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 198.9, 167.8, 160.7, 151.2, 144.7, 141.8, 133.2, 130.3, 129.2, 129.1, 127.7, 120.6, 114.4, 112.9, 55.4, 49.2, 49.1, 42.6. IR (atr) cm–1: 2800–3200 (broad) (R-COOH), 1720 (s) (C=C-ROOH), 1644 (s) (C=C-CO-R), 1454 (m) (R-O–CH3), 1439 (m) (R-CO-CH2-R), 1234 (s) (R-CO-R), 1051 (m), 754 (s) (Ph-O–CH3), 903 (m) (isolated H (Ph)), 771 (s), 706 (s) (3 adjacent H (Ph)), 695 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF): m/z: [7-H]− calculated for C20H17O4 321.1121; found 321.1118.
4-Phenyl-5-(2-methoxyphenyl)-cyclohex-2-en-1-one-3-carboxylic acid (8) (Enantiomeric Pair of RR and SS)
The product was synthesized according to the general procedure for in situ synthesized enone using 2-methoxybenzaldehyde (2 mmol, 240 μL) instead of benzaldehyde. Product was isolated as 111 mg (17%) of a white powder. 1H NMR (500 MHz, acetone-d6): δ 7.33–7.26 (m, 4H), 7.25–7.18 (m, 2H), 7.01 (m, 2H), 6.88 (m, 2H), 4.52 (d, J = 4.8 Hz, 1H), 3.89 (s, 3H), 3.83 (q, J = 5.0 Hz, 1H), 2.78 (dd, J = 17.0, 6.4 Hz, 1H), 2.65 (dd, J = 17.0, 5.3 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 199.6, 167.7, 158.1, 150.2, 142.1, 133.6, 131.5, 129.3, 129.0, 128.8, 128.5, 127.7, 121.3, 111.9, 55.8, 46.9, 43.1, 39.7. IR (atr) cm–1: 2800–3200 (broad) (R-COOH), 1716 (s) (C=C-ROOH), 1643 (s) (C=C-CO-R), 1463 (m), 1029 (m) (Ph-O–CH3), 1438 (w) (R-CO-CH2-R), 1221 (s) (R-CO-R), 1165 (m), 1115 (m) (R-COOH), 753 (s) (4 adjacent H (Ph)), 697 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF): m/z: [8-H]− calculated for C20H17O4 321.1121; found 321.1119.
4-Phenyl-5-(4-fluorophenyl)-cyclohex-2-en-1-one-3-carboxylic acid (9) (Enantiomeric Pair of RR and SS)
The product was synthesized according to the general procedure for in situ synthesized enone using 4-fluorobenzaldehyde (2 mmol, 215 μL) instead of benzaldehyde. Product was isolated as 61 mg (10%) of a white powder. 1H NMR (500 MHz, acetone-d6): δ 7.28–7.22 (m, 4H), 7.21–7.17 (m, 1H), 7.16–7.12 (m, 2H), 7.04–6.99 (m, 2H), 6.78 (d, J = 2.0 Hz, 1H), 4.36 (dd, J = 7.4, 1.9 Hz, 1H), 3.53 (ddd, J = 10.1, 7.4, 4.4 Hz, 1H), 2.93 (dd, J = 16.3, 10.1 Hz, 1H), 2.65 (dd, J = 16.3, 4.4 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 198.7, 167.7, 162.5 (d, JC–F = 243.6 Hz), 151.4, 141.7, 139.1 (d, JC–F = 3.2 Hz), 133,1, 130.3 (d, JC–F = 7.8 Hz), 129.2, 129.1, 127.7, 115.9 (d, JC–F = 21.1 Hz), 49.6, 48.5, 42.9. 19F NMR (470 MHz, acetone-d6): δ −117.63. IR (atr) cm–1: 2800–3200 (broad) (R-COOH), 1732 (m) (C=C-ROOH), 1671 (s) (C=C-CO-R), 1454 (m) (R-CO-CH2-R), 1268 (m), 533 (s) (Ph-F), 1217(s) (R-CO-R), 1154 (s), 1079 (m) (R-COOH), 823 (s), 780 (s) (2 adjacent H (R-Ph-p-F)), 757 (s) (C–F), 701 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF): m/z: [9-H]− calculated for C19H14O3F 309.0921; found 309.0919.
4-Phenyl-5-(4-chlorophenyl)-cyclohex-2-en-1-one-3-carboxylic acid (10) (Enantiomeric Pair of RR and SS)
The product was synthesized according to the general procedure for in situ synthesized enone using 4-clorobenzaldehyde (2 mmol, 281.1 mg) instead of benzaldehyde. Product was isolated as 131 mg (20%) of a white powder. 1H NMR (500 MHz, acetone-d6): δ 7.30–7.14 (m, 9H), 6.77 (d, J = 2.0 Hz, 1H), 4.36 (dd, J = 7.4, 2.0 Hz, 1H), 3.55 (ddd, J = 10.1, 7.5, 4.4 Hz, 1H), 2.93 (dd, J = 16.3, 10.1 Hz, 1H) 2.65 (dd, J = 16.3, 4.4 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 198.5, 167.7, 151.4, 142.0, 141.6, 133.1, 132.9, 130.3, 129.3, 129.1, 127.8, 49.4, 48.6, 42.8. IR (atr) cm–1: 2800–3200 (broad), 1149 (m) (R-COOH), 1732 (s) (C=C-ROOH), 1676 (s) (C=C-CO-R), 1427 (m) (R-CO-CH2-R), 1255(s) (R-CO-R), 1090 (m), 533 (s) (Ph–Cl), 821 (s), 780 (s) (2 adjacent H (R-Ph-p-Cl)), 703 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF): m/z: [10-H]− calculated for C19H14O3Cl 325.0626; found 325.0625.
4-Phenyl-5-(4-(trifluoromethyl)phenyl)-cyclohex-2-en-1-one-3-carboxylic acid (11) (Enantiomeric Pair of RR and SS)
The product was synthesized according to the general procedure for in situ synthesized enone using 4-(trifluoromethyl)benzaldehyde (2 mmol, 275 μL) instead of benzaldehyde. Product was isolated as 28 mg (4%) of a white powder. 1H NMR (500 MHz, acetone-d6): δ 7.61 (d, J = 8.1 Hz, 2H), 7.47 (d, J = 8.1 Hz, 2H), 7.27–7.16 (m, 5H), 6.79 (d, J = 2.0 Hz, 1H), 4.44 (dd, J = 7.5, 1.9 Hz, 1H), 3.67 (ddd, J = 10.3, 7.5, 4.4 Hz, 1H), 2.99 (dd, 16.4, 10.1 Hz, 1H), 2.69 (dd, J = 16.3, 4.4 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 198.3, 167.6, 151.3, 147.7 (d, JC–F = 1.4 Hz), 141.4, 133.2, 129.4, 129.3, 129.1, 127.9, 126.4, 126.1 (q, JC–F = 3.8 Hz), 124.3, 49.1, 49.0, 42.6. 19F NMR (470 MHz, acetone-d6): δ −62.93. IR (atr) cm–1: 2800–3200 (broad), 1111 (s) (R-COOH), 1723 (s) (C=C-ROOH), 1645 (s) (C=C-CO-R), 1421 (m) (R-CO-CH2-R), 1330 (s), 1160 (s), 512 (s) (R-CF3), 1240 (s) (R-CO-R), 836 (s) (2 adjacent H (R-Ph-p-CF3)), 703 (s) (5 adjacent H (Ph)) HRMS (ESI-TOF): m/z: [11-H]− calculated for C20H14O3F3 359.0890; found 359.0893.
4-Phenyl-5-(4-(benzyloxy)phenyl)-cyclohex-2-en-1-one-3-carboxylic acid (12a and 12b) (Enantiomeric Pair of RR/SS, 12a, and RS/SR, 12b)
Synthesis and separation of: 12a and 12b. The product was synthesized according to the general procedure for in situ synthesized enone using 4-(benzyloxy)benzaldehyde (2 mmol, 424.5 mg) instead of benzaldehyde. The products are obtained as a mixture of 12a and 12b (337 mg, 42%) as a white powder. According to the 1H NMR measurement, 62% of this mixture is 12a (RR/SS) and the remaining 38% is 12b (RS/SR).
Separation of 12a and 12b
Prior protonation of the product. The salt crystals formed of 12a and 12b are collected and washed with acetone and dried overnight under air. Then the crystals are heated with water to 90 °C for 15 min and cooled back down to room temperate. During the cooling crystals are forming and these are primarily the salts of 12b crystals. These 12b crystals are purified by dissolving them again with hot water (90 °C) and recrystallization to form pure 12b salt crystals. The water solutions from the filtrations contain mainly 12a crystals. The corresponding crystals are collected, dissolved into H2O (hot one for 12b), and protonated with HCl, followed by filtration, washing with water, and drying under vacuum. Utilization of this separation method provided us pure 12a and 12b as white powders.
Synthesis of 12a and 12b was also done in water. The general procedure for cyclohexenone acid synthesis was utilized in water using phenylpyruvic acid (2 mmol, 328.3 mg) and in situ synthesized enone from acetone (2 mmol, 150 μL) and 4-(benzyloxy)benzaldehyde (2 mmol, 424.5 mg). Product was obtained as a mixture of 12a and 12b as 661 mg (83%) of a white powder. According to 1H NMR measurement, the ratio of 12a (RR/SS, anti):12b (RS/SR, syn) was 62:38.
12a: 1H NMR: (500 MHz, acetone-d6): δ 7.47 (m, 2H), 7.39 (m, 2H), 7.32 (m, 1H), 7.26 (m, 2H), 7.22–7.11 (m, 5H), 6.91 (m, 2H), 6.79 (d, J = 1.9 Hz, 1H), 5.07 (s, 2H), 4.37 (dd, J = 6.7, 2.0 Hz, 1H), 3.47 (ddd, J = 9.3, 6.7, 4.5 Hz, 1H), 2.87 (dd, J = 16.4, 9.2 Hz, 1H), 2.64 (dd, J = 16.4, 4.5 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 199.0, 167.8, 158.6, 151.1, 141.9, 138.4, 135.4, 133.3, 129.5, 129.3, 129.1, 128.6, 128.5, 127.7, 115.6, 70.4, 49.5, 48.3, 42.7. Note: One carbon signal is missing, probably due to overlapping at the aromatic region. IR (atr) cm–1: 2800–3200 (broad) (R-COOH), 1723 (s) (C=C-ROOH), 1460 (m) (R-CH2–R), 1246 (s) (R-CO-R), 1025 (m) (Ph-O-R), 830 (m) (2 adjacent H (R-Ph-R)), 690 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF): m/z: [12a-H]− calculated for C26H21O4 397.1434; found 397.1430.
12b: 1H NMR (500 MHz, acetone-d6): δ 7.49–7.43 (m, 2H), 7.42–7.36 (m, 2H), 7.35–7.29 (m, 1H), 7.19–7.09 (m, 3H), 6.96 (s, 1H), 6.87–6.78 (m, 4H), 6.82 (d, J = 6.8 Hz, 2H), 5.08 (s, 2H), 4.36 (d, J = 4.9 Hz, 1H), 3.88 (dt, J = 15.2, 4.1 Hz, 1H), 2.88 (dd, J = 16.9, 15.1 Hz, 1H), 2.38 (dd, J = 16.8, 3.6 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 200.3, 167.1, 158.7, 151.0, 138.5, 135.9, 134.2, 134.0, 130.4, 129.8, 129.3, 128.7, 128.6, 128.4, 127.9, 115.2, 70.4, 48.6, 44.4, 38.5. IR (atr) cm–1: 2800–3200 (broad) (R-COOH), 1692 (s), 1613 (m) (C=C-ROOH), 1676 (s), 1254 (s) (R-CO-R), 1493 (w), 1453 (m), 1030 (m) (R-O-CH2-R), 1435 (m) (R-CH2-R), 828 (m) (2 adjacent H (R-Ph-R)), 700 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF): m/z: [12b-H]− calculated for C26H21O4 397.1434; found 397.1435.
4,5-Diphenyl-2-methyl-cyclohex-2-en-1-one-3-carboxylic acid (13a and 13b) (Enantiomeric pair of RR/SS (anti) and RS/SR (syn))
The synthesis of the mixture of anti and syn products was carried out according to the general procedure for cyclohexenone acid synthesis in water using phenylpyruvic acid (2 mmol, 328.3 mg) and in situ synthesized enone from 2-butanone (2 mmol, 180 μL) and benzaldehyde (2 mmol, 205 μL). Product was obtained as a mixture of 13a and 13b as 600 mg (98%) of a white powder. According to 1H NMR measurement, the ratio of 13a (RR/SS, anti):13b (RS/SR, syn) was 72:28.
Enrichment of 13b
The white powder mixture (600 mg) of 13a and 13b (72:28 ratio) was dissolved in ethanol (ca. 2 mL). Water (ca. 6 mL) was added into the solution. After a week crystals form in the solution, and the crystals were filtered and washed with water. This set of crystals (180 mg) was identified as 13a. The next set of crystals (300 mg) precipitated from the solution after another week. The second set of crystals were identified as a mixture of 13a and 13b (1:1 ratio). This enriched 13b powder mixture was dissolved into chloroform (ca. 2 mL), and hexane (ca. 6 mL) was added as an anti solvent. The following day, precipitation formed from the chloroform/hexane solution, which was identified as pure 13a, and the precipitate was put aside. The remaining solution was evaporated in vacuum, and the formed crude solid was collected by filtration and washed with hexane. The crude solid had a slightly higher 13b ratio than earlier. The slightly more enriched 13b is subjected to the same chloroform (solvent)/hexane (anti solvent) recrystallization treatment repeatedly until 85% enrichment was achieved.
Synthesis of 13a was carried out by the general procedure for in situ synthesized enone using 2-butanone (2 mmol, 180 μL) instead of acetone. Product was isolated as 233 mg (38%) of a white powder.
13a: 1H NMR (500 MHz, acetone-d6): δ 7.22–7.09 (m, 10H), 4.32 (dq, J = 9.3, 2.3 Hz, 1H), 3.48 (ddd, J = 12.4, 9.3, 4.0 Hz, 1H), 3.00 (dd, J = 15.9, 12.5 Hz, 1H), 2.64 (dd, J = 15.9, 4.0 Hz, 1H), 1.98 (d, J = 2.3 Hz, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 198.5, 168.9, 148.2, 142.9, 141.4, 135.4, 129.9, 129.2, 128.9, 128.5, 127.7, 127.6, 51.7, 49.2, 44.3, 12.9. IR (atr) cm–1: 2800–3200 (broad), 1347 (m), 1122 (m), 926 (m) (R-COOH), 1722 (s) (C=C-ROOH), 1638 (s) (C=C-CO-R), 1448 (m) (R-CH3), 1219 (s) (R-CO-R), 1034(w), 755 (s), 699 (s) (5 adjacent H (Ph)) HRMS (ESI-TOF): m/z: [13a-H]− calculated for C20H17O3 305.1172; found 305.1170.
13b: 1H NMR (500 MHz, acetone-d6): δ 7.21–7.17 (m, 3H), 7.15–7.12 (m, 1H), 7.11–7.07 (m, 2H), 6.90–6.86 (m, 2H), 6.74–6.69 (m, 2H), 4.39 (d, J = 5.1 Hz, 1H), 3.93 (ddd, J = 15.1, 5.0, 3.6 Hz, 1H), 2.96 (dd, J = 16.7, 15.1 Hz, 1H), 2.44 (dd, J = 16.7, 3.6 Hz, 1H), 2.12 (d, J = 1.3 Hz, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 199.8, 169.1, 146.9, 142.0, 137.9, 135.8, 130.7, 128.80, 128.79, 128.5, 127.9, 127.6, 50.6, 44.6, 37.8, 13.2. IR (atr) cm–1: 2800–3200 (broad) (R-COOH), 1695 (s) (C=C-ROOH), 1675 (s) (C=C-CO-R), 1452 (w) (R-CH3), 1245 (s) (R-CO-R), 763 (s), 696 (s) (5 adjacent H (Ph)) HRMS (ESI-TOF) m/z: [13b-H]− calculated for C20H17O3 305.1172; found 305.1173.
4,5-Diphenyl-2-ethyl-cyclohex-2-en-1-one-3-carboxylic acid (14a and 14b) (Enantiomeric Pair of RR/SS (anti) and RS/SR (syn))
The synthesis of the mixture of anti and syn products was carried out according to the general procedure for cyclohexenone acid synthesis in water using phenylpyruvic acid (2 mmol, 328.3 mg) and in situ synthesized enone from 2-pentanone (2 mmol, 215 μL) and benzaldehyde (2 mmol, 205 μL). Product was obtained as a mixture of 14a and 14b as 628 mg (98%) of a white powder. According to 1H NMR measurement, the ratio of 14a (RR/SS, anti):14b (RS/SR, syn) was 75:25.
Separation of 14a and 14b
The mixture of 14a and 14b was dissolved into acetone (ca. 4 mL), and water was added into it (ca. 4 mL). After 2 days the formed crystals were collected, and 180 mg of 14a were obtained as a white powder.
Enrichment of 14b
The enrichment process is the same as that for 13b, except acetone was used instead of EtOH at the initial crystallization. We managed to enrich the product to a 55% ratio.
14a: 1H NMR (500 MHz, acetone-d6): δ 7.23–7.19 (m, 4H), 7.19–7.09 (m, 6H), 4.32 (d, J = 9.2 Hz, 1H), 3.48 (ddd, J = 12.2, 9.3, 4.0 Hz,1H), 2.98 (dd, J = 15.9, 12.3 Hz), 2.64 (dd, J = 15.9, 4.0 Hz, 1H), 2.56 (m, 1H), 2.43 (dq, J = 12.5, 7.5 Hz, 1H), 1.08 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 198.1, 168.9, 148.1, 142.9, 141.2, 140.9, 129.9, 129.1, 128.9, 128.5, 127.7, 127.5, 51.6, 49.1, 44.6, 21.1, 14.6. IR (atr) cm–1: 2800–3200 (broad), 1174 (s), 918 (w) (R-COOH), 1725 (C=C-ROOH), 1641 (s) (C=C-CO-R), 1454 (m) (R-CO-CH2-R), 1216 (s) (R-CO-R), 762 (s), 697 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF) m/z: [14a-H]− calculated for C21H19O3 319.1329; found 319.1331.
14b: 1H NMR (500 MHz, acetone-d6): δ 7.22–7.07 (m, 6H), 6.91–6.85 (m, 2H), 6.74–6.69 (m, 2H), 4.36 (d, J = 5.0 Hz, 1H), 3.92 (ddd, J = 15.1, 5.0, 3.6 Hz, 1H), 2.96 (m, 1H), 2.69 (m, 1H), 2.56 (m, 1H), 2.43 (m, 1H), 1.14 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 199.4, 169.2, 146.9, 142.9, 141.9, 135.6, 130.7, 128.8, 128.8, 128.5, 127.9, 127.6, 50.5, 44.8, 38.1, 21.2, 14.5. Note: Aromatic area has heavy overlap with 14a; thus, proton integrals are not accurate. Carbon signal 146.9 is not observed in regular 13C{1H} NMR, but it is observed in the HMBC spectrum. IR (atr) cm–1: 2800–3200 (broad), 1275 (m) (R-COOH), 1674 (s) (C=C-CO-R), 1453 (m) (R-CH3), 1417 (w) (R-CO-CH2-R), 1241 (s) (R-CO-R), 764 (s), 697 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF) m/z: [14b-H]− calculated for C21H19O3 319.1329; found 319.1329.
2-Ethyl-1-hydroxy-3-oxo-5,6-diphenylcyclohexane-1-carboxylic acid (14A) (Enantiomeric Pair 1S, 2S, 5R, 6R and 1R, 2R, 5S, 6S)
The product was synthesized according to the general procedure for in situ synthesized enone using 2-pentanone (2 mmol, 215 μL) instead of acetone. Product was isolated as 88 mg (13%) of a white powder. 1H NMR (500 MHz, acetone-d6): δ 7.32–7.16 (m, 4H), 7.12 (t, J = 7.7 Hz, 2H), 7.06–6.94 (m, 4H), 4.01 (d, J = 12.2 Hz, 1H), 3.80 (td, J = 12.6, 4.4 Hz, 1H), 3.16 (dd, J = 9.3, 1.5 Hz, 1H), 3.03 (t, J = 13.2 Hz, 1H), 2.53 (dd, J = 13.5, 4.4 Hz, 1H), 2.04 (m, 1H), 1.04 (m, 1H), 0.94 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 206.6, 174.6, 143.9, 138.9, 129.0, 128.7, 128.4, 127.5, 127.0, 83.8, 58.8, 55.8, 50.8, 46.3, 18.5, 13.5. IR (atr) cm–1: 3467 (m, sharp) (Axial −OH), 2800–3200 (broad), 1724 (s), 1123 (R-COOH) 1682 (s), 1224 (s) (R-CO-R) 1454 (m) (R-CH2–R) 1373(m), 926 (m), (R-CH3), 1092 (s) (R–OH), 696 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF): m/z: [14A-H]− calculated for C21H21O4 337.1434; found 337.1438. See the X-ray diffraction section for the solved crystal structure of 14A (CCDC: 2193908). Notes: proton 2.04 overlapped by acetone-d6 signal. One aromatic carbon missing at the carbon spectrum. 128.74 is wider than usual; thus, it might be a double peak. Compound has sharp axial −OH signal in IR spectrum. Similar signals can be observed in 15b.
1-Hydroxy-2,2-dimethyl-3-oxo-5,6-diphenylcyclohexane-1-carboxylic acid (15a) (Enantiomeric Pair RRR and SSS) and (15b) Enantiomeric Pair (1S, 5R, 6R and 1R, 5S, 6S)
The product was synthesized according to the general procedure for in situ synthesized enone using 3-methyl-2-butanone (2 mmol, 215 μL) instead of acetone. Isolation: When acetone (5 mL) is added into the microwave tube, the product 15a salt is formed within a few hours in the tube. The salt is filtered and washed with acetone. The obtained solid is dissolved with water and protonated with HCl. 15a was isolated as 103 mg (15%) of a white powder. The remaining filtrated reaction solution contains the corresponding 15b salt. The 15b salt precipitated out from the filtrated reaction and washing solution overnight. The salt was filtered and washed with acetone. The salt is dissolved with water and protonated with HCl and dried under vacuum. 15b was isolated as 88 mg (13%) of a white powder.
Synthesis of 15a and 15b was also done in water
By utilizing the general procedure for cyclohexenone acid synthesis in water using phenylpyruvic acid (2 mmol, 328.3 mg) and in situ synthesized enone from 3-methyl-2-butanone (2 mmol, 215 μL) and benzaldehyde (2 mmol, 205 μL). Product was obtained as a mixture of 15a and 15b as 610 mg (90%) of a white powder. According to 1H NMR measurement, the ratio of 15a (RRR/SSS):15b (1S, 5R, 6R/1R, 5S, 6S) was 67:33.
15a: 1H NMR (500 MHz, acetone-d6): δ 7.33–7.26 (m, 2H), 7.24–7.18 (m, 2H), 7.16–7.06 (m, 4H), 7.05–6.96 (m, 2H), 4.06 (td, J = 12.6, 5.2 Hz, 1H), 3.90 (d, J = 12.5 Hz, 1H), 3.02 (m, 1H), 2.53 (dd, J = 15.4, 5.4 Hz), 1.52 (s, 3H), 1.07 (s, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 209.6, 175.1, 144.3, 138.8, 130.8, 129.2, 128.7, 128.4, 127.6, 127.1, 83.8, 53.1, 52.9, 46.1, 42.9, 23.1, 19.1. IR (atr) cm–1: 3633 (m, sharp), 1189 (s) (R–OH), 3250–3450 (m, broad) (intermolecular hydrogen bonds), 2800–3200 (broad), 1721 (s), 1102 (s) (R-COOH), 1703 (s), 1280 (s) (R-CO-R), 1454 (m), 1385 (m) (R-CH3), 751 (s), 695 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF): m/z: [15a-H]− calculated for C21H21O4 337.1434; found 337.1430. See the X-ray diffraction section for the solved crystal structure of 15a (CCDC: 2193909).
15b: 1H NMR (500 MHz, acetone-d6): δ 7.48–7.25 (m, 4H), 7.13 (t, J = 7.8 Hz, 2H), 7.07–6.94 (m, 4H), 4.19 (d, J = 12.3 Hz, 1H), 3.83 (td, J = 12.6, 5.3 Hz, 1H), 3.11 (dd, J = 14.9, 12.8 Hz, 1H), 2.46 (dd, J = 14.9, 5.3 Hz, 1H), 1.67 (s, 3H), 1.09 (s, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 210.1, 173.7, 144.0, 139.4, 131.1, 129.1, 128.8, 128.2, 127.4, 127.0, 84.3, 53.1, 50.3, 46.1, 45.6, 24.3, 19.1. IR (atr) cm–1: 3539 (m, sharp) (Axial −OH), 2800–3200 (broad), 1725 (s) (R-COOH), 1677 (s), 1189 (s) (R-CO-R), 1456 (w), 1387 (w), 965 (m) (R-CH3), 1121 (m) (tert–OH), 750 (m), 698 (s) (5 adjacent H (Ph)). HRMS (ESI-TOF): m/z: [15b-H]− calculated for C21H21O4 337.1434; found 337.1431. Note: IR spectrum has a similar sharp axial −OH signal as 14; this further suggests the axial nature of the alcohol group here.
5-(4-(Benzyloxy(phenyl)-1-hydroxy-2,2-dimethyl-3-oxo-6-phenylcyclohexane-1-carboxylic acid (16a) (Enantiomeric Pair RRR and SSS) and (16b) Enantiomeric Pair (1S, 5R, 6R and 1R, 5S, 6S)
The product was synthesized according to the general procedure for in situ synthesized enone using 3-methyl-2-butanone (2 mmol, 215 μL) instead of acetone and 4-(benzyloxy)benzaldehyde (2 mmol, 424.5 mg) instead of benzaldehyde. Isolation: When acetone (5 mL) is added into the microwave tube to initiate the crystallization, the product 16a salt is crystallized within a few hours. The salt is filtered and washed with acetone. The obtained solid is dissolved with water and protonated with HCl (1 M). 16a was isolated as 104 mg (12%) of a white powder. In order to precipitate 16b salt, acetone (ca. 20 mL) was added into the solution where the 16a salt precipitated out. After waiting overnight, 16b salt precipitated out from the remaining solution. It is then filtered and washed with acetone. Then the crystals are dissolved with water and protonated with HCl (1 M), washed with water, and dried under vacuum. 16b is obtained as a beige powder (72 mg, 8%).
16a: 1H NMR (500 MHz, acetone-d6): δ 7.45–7.38 (m, 2H), 7.38–7.32 (m, 2H), 7.32–7.27 (m, 1H), 7.27–7.16 (m, 4H), 7.12–7.05 (m, 2H), 7.05–7.00 (m, 1H), 6.77 (d, J = 8.7 Hz, 2H), 4.95 (s, 2H), 4.04 (td, J = 12.3, 5.4 Hz, 1H), 3.80 (d, J = 12.8 Hz, 1H), 2.92 (dd, J = 15.1, 12.4 Hz, 1H), 2.50 (dd, J = 15.2, 5.5 Hz, 1H), 1.49 (s, 3H), 1.06 (s, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 176.0, 158.1, 139.4, 138.5, 136.9, 130.9, 129.7, 129.2, 128.6, 128.5, 128.3, 127.3, 115.4, 83.5, 70.3, 53.4, 52.7, 46.1, 42.2, 22.8, 19.2. IR (atr) cm–1: 2800–3200 (broad), 1738 (s) (R-COOH), 1710 (s), 1249 (s) (R-CO-R), 1463 (m) (R-CH2–R), 1384 (m) (R-CH3), 1306 (m), 1075 (s), 1024 (w), (Ph-O-R), 1174 (m) ((CH3)2-C-R2), 900 (m), 735 (s), 688 (s) (5 adjacent H (Ph)), 830 (m) (2 adjacent H (R-Ph-R)). HRMS (ESI-TOF) m/z: [16a-H]− calculated for C28H27O5 443.1853; found 443.1849. Note: Ketone carbon ketone signal is missing. It is not obtained even with a wider spectral window or increased relaxation time.
16b: 1H NMR (500 MHz, acetone-d6): δ 7.43–7.26 (m, 7H), 7.21 (m, 2H), 7.08–6.95 (m, 3H), 6.77 (m, 2H), 4.96 (s, 2H), 4.14 (d, J = 12.2 Hz, 1H), 3.78 (td, J = 12.5, 5.2 Hz), 3.07 (dd, J = 14.9, 12.8 Hz, 1H), 2.43 (dd, J = 14.9, 5.2 Hz, 1H), 1.66 (s, 3H), 1.07 (s, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 210.2, 173.8, 158.2, 139.6, 138.5, 136.4, 129.8, 129.2, 128.6, 128.5, 128.2, 127.4, 115.3, 84.2, 70.3, 53.0, 50.6, 46.3, 44.8, 24.4, 19.1. IR (atr) cm–1: 2800–3200 (broad), 1712 (s) (R-COOH), 1678 (s), 1250 (s) (R-CO-R), 1467 (m) (R-CH2–R), 1426 (m) (R-CH2-CO-R), 1379 (m) (R-CH3), 1176 (s) ((CH3)2-C-R2), 1081 (m) (Ph-O-R), 1028 (m), 748 (s) (Ph–CH2–O-R), 880 (m), 694 (s) (5 adjacent H (Ph)), 827 (m) (2 adjacent H (R-Ph-R)). HRMS (ESI-TOF) m/z: [16b-H]− calculated for C28H27O5 443.1853; found 443.1853. Note: one carbon signal is missing, probably due to overlapping at the aromatic region.
Acknowledgments
A.E. acknowledges Magnus Ehrnrooth Foundation for financial support. J. Install and Dr. E. Lagerspets are acknowledged for language review and insightful comments on the manuscript.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c00757.
Author Contributions
A.E.: Experimental work, analytics and writing the manuscript. M.N. and T.A.K.: Crystallography. T.R.: Supervising. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Ledoux A.; St-Gelais A.; Cieckiewicz E.; Jansen O.; Bordignon A.; Illien B.; Di Giovanni N.; Marvilliers A.; Hoareau F.; Pendeville H.; Quetin-Leclercq J.; Frédérich M. Antimalarial Activities of Alkyl Cyclohexenone Derivatives Isolated from the Leaves of Poupartia borbonica. J. Nat. Prod. 2017, 80, 1750–1757. 10.1021/acs.jnatprod.6b01019. [DOI] [PubMed] [Google Scholar]
- Ledoux A.; Bériot D.; Mamede L.; Desdemoustier P.; Detroz F.; Jansen O.; Frédérich M.; Maquoi E. Cytotoxicity of Poupartone B, an Alkyl Cyclohexenone Derivative from Poupartia borbonica, against Human Cancer Cell Lines. Planta Med. 2021, 87, 1008–1017. 10.1055/a-1532-2384. [DOI] [PubMed] [Google Scholar]
- Shakil N. A.; Singh M. K.; Kumar J.; Sathiyendiran M.; Kumar G.; Singh M. K.; Pandey R. P.; Pandey A.; Parmar V. S. Microwave synthesis and antifungal evaluations of some chalcones and their derived diaryl-cyclohexenones. J. Environ. Sci. Health B 2010, 45, 524–530. 10.1080/03601234.2010.493482. [DOI] [PubMed] [Google Scholar]
- Ghavre M.; Froese J.; Murphy B.; Simionescu R.; Hudlicky T. A Formal Approach to Xylosmin and Flacourtosides E and F: Chemoenzymatic Total Synthesis of the Hydroxylated Cyclohexenone Carboxylic Acid Moiety of Xylosmin. Org. Lett. 2017, 19, 1156–1159. 10.1021/acs.orglett.7b00194. [DOI] [PubMed] [Google Scholar]
- Miyashita M.; Sasaki M.; Hattori I.; Sakai M.; Tanino K. Total Synthesis of Norzoanthamine. Science 2004, 305, 495–499. 10.1126/science.1098851. [DOI] [PubMed] [Google Scholar]
- Kuroda Y.; Nicacio K. J.; da Silva I. A. Jr.; Leger P. R.; Chang S.; Gubiani J. R.; Deflon V. M.; Nagashima N.; Rode A.; Blackford K.; Ferreira A. G.; Sette L. D.; Williams D. E.; Andersen R. J.; Jancar S.; Berlinck R. G. S; Sarpong R. Isolation, synthesis and bioactivity studies of phomactin terpenoids. Nat. Chem. 2018, 10, 938–945. 10.1038/s41557-018-0084-x. [DOI] [PubMed] [Google Scholar]
- Yang X.; Wang J.; Li P. Recent progress on asymmetric organocatalytic construction of chiral cyclohexenone skeletons. Org. Biomol. Chem. 2014, 12, 2499–2513. 10.1039/C3OB42293C. [DOI] [PubMed] [Google Scholar]
- Mohr P. J.; Halcomb R. L. Total Synthesis of (+)-Phomactin A Using a B-Alkyl Suzuki Macrocyclization. J. Am. Chem. Soc. 2003, 125, 1712–1713. 10.1021/ja0296531. [DOI] [PubMed] [Google Scholar]
- Bradshaw B.; Bonjoch J. The Wieland-Miescher Ketone: A Journey from Organocatalysis to Natural Product Synthesis. Synlett. 2012, 23, 337–356. 10.1055/s-0031-1290107. [DOI] [Google Scholar]
- Liu Z.-Q. How to Start a Total Synthesis from the Wieland-Miescher Ketone?. Current Organic Synthesis 2019, 16, 328–341. 10.2174/1570179416666190328233710. [DOI] [PubMed] [Google Scholar]
- Poplata S.; Bach T. Enantioselective Intermolecular [2 + 2] Photocycloaddition Reaction of Cyclic Enones and Its Application in a Synthesis of (−)-Grandisol. J. Am. Chem. Soc. 2018, 140, 3228–3231. 10.1021/jacs.8b01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-m; Zheng C.-w; Zhao G. Asymmetric Robinson-Type Annulation Reaction between β-Ketoamides and α,β-Unsaturated Ketones. J. Org. Chem. 2015, 80, 3798–3805. 10.1021/jo502904n. [DOI] [PubMed] [Google Scholar]
- Liu Z.-Q. An Overview on the Robinson Annulation. Curr. Org. Chem. 2018, 22, 1347–1372. 10.2174/1385272822666180511122631. [DOI] [Google Scholar]
- Wen Z.-K; Wu X.-X; Bao W.-K; Xiao J.-J; Chao J.-B. Palladium-Catalyzed Regioselective Coupling Cyclohexenone into Indoles: Atom-Economic Synthesis of β-Indolyl Cyclohexenones and Derivatization Applications. Org. Lett. 2020, 22, 4898–4902. 10.1021/acs.orglett.0c01763. [DOI] [PubMed] [Google Scholar]
- Tang L.; Luo Y.; Xue J.-W; He Y.-H; Guan Z. Highly enantioselective Michael-aldol-dehydration reaction for the synthesis of chiral 3,5-diaryl-cyclohexenones catalysed by primary amine. Tetrahedron 2017, 73, 1114–1119. 10.1016/j.tet.2017.01.004. [DOI] [Google Scholar]
- Lee J.; Wang S.; Callahan M.; Nagorny P. Copper(II)-Catalyzed Tandem Decarboxylative Michael/Aldol Reactions Leading to the Formation of Functionalized Cyclohexenones. Org. Lett. 2018, 20, 2067–2070. 10.1021/acs.orglett.8b00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Luo S.; Li J.; Li X.; Cheng J.-P. Organocatalytic kinetic resolution via intramolecular aldol reactions: Enantioselective synthesis of both enantiomers of chiral cyclohexenones. Org. Biomol. Chem. 2010, 8, 2627–2632. 10.1039/b927343c. [DOI] [PubMed] [Google Scholar]
- Kimaru N.; Komatsuki K.; Saito K.; Yamada T. Decarboxylation-triggered homo-Nazarov cyclization of cyclic enol carbonates catalyzed by rhenium complex. Chem. Commun. 2021, 57, 6133–6136. 10.1039/D1CC01144H. [DOI] [PubMed] [Google Scholar]
- Golec J. C.; Carter E. M.; Ward J. W.; Whittingham W. G.; Simón L.; Paton R. S.; Dixon D. J. BIMB-Catalyzed 1,3-Prototropic Shift for the Highly Enantioselective Synthesis of Conjugated Cyclohexenones. Angew. Chem., Int. Ed. 2020, 59, 17417–17422. 10.1002/anie.202006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobzev M. S.; Titov A. A.; Varlamov A. V. Synthesis of heterocyclic systems involving [3,3]-sigmatropic rearrangements. Russ. Chem. Bull., Int. Ed. 2021, 70, 1213–1259. 10.1007/s11172-021-3208-1. [DOI] [Google Scholar]
- Huang X.; Klimczyk S.; Maulide N. Charge-Accelerated Sulfonium [3,3]-Sigmatropic Rearrangements. Synthesis 2012, 2012, 175–183. 10.1055/s-0031-1289632. [DOI] [Google Scholar]
- Ilardi E. A.; Stivala C. E.; Zakarian A. [3,3]-Sigmatropic rearrangements: recent applications in the total synthesis of natural products. Chem. Soc. Rev. 2009, 38, 3133–3148. 10.1039/b901177n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. C.; May J. A.; Sarpong R.; Stoltz B. M. Toward a Symphony of Reactivity: Cascades Involving Catalysis and Sigmatropic Rearrangements. Angew. Chem., Int. Ed. 2014, 53, 2556–2591. 10.1002/anie.201302572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Pradilla R. F.; Tortosa M.; Viso A. Sulfur Participation in [3,3]-Sigmatropic Rearrangements. Top. Curr. Chem. 2006, 275, 103–129. 10.1007/128_059. [DOI] [PubMed] [Google Scholar]
- Eronen A. E. K; Mannisto J. K.; Moslova K.; Nieger M.; Heliövaara E.; Repo T. Synthesis of Diaryl Hydrocyl Dicarboxylic Acids from Amino Acids. J. Org. Chem. 2020, 85, 5799–5806. 10.1021/acs.joc.9b03320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen-Reh M. Reaction of phenylpyruvic acid with benzylidene- and anisylidene-acetone and corresponding saturated ketones. Bull. Soc. Chim. Fr. 1956, 882–887. [Google Scholar]
- Katsumura S.; Isoe S. An Efficient Synthesis of Jolkinolide E Involving the Butenolide Ring Formation by Intramolecular Wittig Reaction. Chem. Lett. 1982, 11, 1689–1692. 10.1246/cl.1982.1689. [DOI] [Google Scholar]
- Kimura A.; Katsumura S.; Isoe S. Total Synthesis of 6-Oxo-Grindelic Acid Methyl Ester. Chem. Lett. 1983, 12, 15–16. 10.1246/cl.1983.15. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.