Abstract
Irritable bowel syndrome (IBS) is a prevalent functional gastrointestinal disorder worldwide. Extensive research has identified multiple factors contributing to its development, including genetic predisposition, chronic infection, gut dysbiosis, aberrant serotonin metabolism, and brain dysfunction. Recent studies have emphasized the critical role of the early life stage as a susceptibility window for IBS. Current evidence suggests that diet can heighten the risk of IBS in offspring by influencing the microbiota composition, intestinal epithelium structure, gene expression, and brain-gut axis. The use of antibiotics during pregnancy and the neonatal period disrupts the normal gut microbiota structure, aligning it with the characteristics observed in IBS patients. Additionally, early life stress impacts susceptibility to IBS by modulating TLR4, NK1, and the hypothalamic-pituitary-adrenal (HPA) axis while compromising the offspring's immune system. Formula feeding facilitates the colonization of pathogenic bacteria in the intestines, concurrently reducing the presence of probiotics. This disruption of the Th1 and Th2 cell balance in the immune system weakens the intestinal epithelial barrier. Furthermore, studies suggest that delivery mode influences the occurrence of IBS by altering the composition of gut microbes. This review aims to provide a comprehensive summary of the existing evidence regarding the impact of adverse early life exposures on IBS during pregnancy, intrapartum, and neonatal period. By consolidating this knowledge, the review enhances our understanding of the direct and indirect mechanisms underlying early life-related IBS and offers new insights and research directions from childhood to adulthood.
Keywords: microbiota, early life, maternal, children, intrapartum, irritable bowel syndrome
1. Introduction
Irritable bowel syndrome (IBS) is a prevalent and chronic gastrointestinal disease that affects individuals of various genders and age groups, characterized by recurring symptoms (1). The pathogenesis of IBS involves several factors, including visceral hypersensitivity, small intestinal bacterial overgrowth, intestinal dysbiosis, gut immune dysregulation, dietary intolerance, alterations in the gut-brain axis, and stress, among others. However, the precise mechanisms underlying IBS remain incompletely understood.
In the 1980s, D. Barker introduced the “Developmental Origins of Health and Disease” hypothesis (2), revolutionizing chronic disease studies by highlighting the importance of early life stages. Subsequently, numerous studies have demonstrated that early life exposures can increase the risk of metabolic, mental, cardiac, and chronic intestinal diseases in offspring (3–5).
Recent evidence has also revealed that the uterus is not a sterile environment (6), and gut microbiota composition in early life is less stable than in adults. Factors such as antibiotic use and dietary intake can disrupt the gut microbiota composition. Considering the crucial role of microbiota in intestinal growth and the development of IBS, it is evident that early life exposures can influence IBS by altering the initial gut microbiota composition.
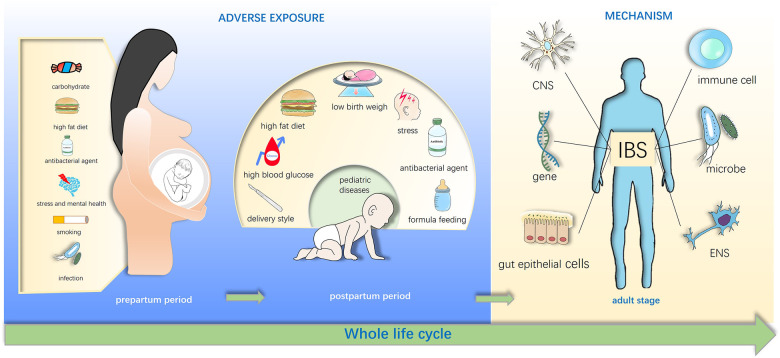
Furthermore, animal studies have shown that early-life exposure to maternal over-nutrition and antibiotics can cause dysbiosis, affecting offspring cognition and behavior (7, 8).While many studies indicate that early-life factors can affect offspring temporarily or long-term (9, 10) (Figure 1), the ability to prevent IBS is impeded by a lack of understanding of the intricate interplay between environmental factors and the disease.
Figure 1.
Early life adverse exposures might contribute to irritable bowel syndrome in adulthood. Adverse exposures in early life disturb the gut microbiota of infants and influence the brain-gut-microbiota axis, and these exposures include maternal diet exposure such as high fat diet, stress, drug use in pregnancy, cesarean section (C-section) and low birth weight, formula feeding of infants. These effects may directly or indirectly increase susceptibility of irritable bowel syndrome (IBS) in the childhood and even adulthood.
Therefore, this review aims to summarize the maternal, intrapartum, and neonatal adverse exposures directly or indirectly related to the development of IBS (Table 1) and explore possible mechanisms. By doing so, this review will offer new insights into the prevention and treatment of IBS.
Table 1.
Summary of early life adverse exposures affected directly in irritable bowel syndrome.
| Adverse exposures | Authors | Publication year | Study type | Study populution | Outcome | |
|---|---|---|---|---|---|---|
| High fat diet in offspring | Zhu et al. (11) | 2014 | Cross-sectional study | Chinese children in grades 1 through 6 | Eating fried food is a risk factor for IBS | |
| Stress exposure | NMS | Riba et al. (12) | 2018 | Experimental study | / | / |
| Yi et al. (13) | 2017 | Experimental study | / | / | ||
| Tang et al. (14) | 2017 | Experimental study | / | / | ||
| O'Mahony et al. (15) | 2009 | Experimental study | / | / | ||
| Early life adverse experiences | Park et al. (16) | 2016 | Case-control study | IBS patients and health controls | Various types of EALs are significant predictor of IBS | |
| Bradford et al. (17) | 2012 | Case-control study | IBS patients and health controls | Various types of EALs are associated with the development of IBS | ||
| Ju et al. (18) | 2020 | Case-control study | IBS Patients and health controls | A greater number of EALs and higher perceived trauma severity were associated with increased odds of IBS | ||
| Rahal et al. (19) | 2020 | Case-control study | IBS Patients and health controls | Fear improved prediction of IBS over the total number of EALs | ||
| Adverse perinatal period factors and formula feeding | C-section | Waehrens et al. (20) | 2018 | Cohort study | A national cohort of persons who were born in Sweden | Significant risk factors for IBS (caesarean, low birth weight, being second in birth orderfoetal growth ≥1 SD, young maternal age (<20 years), maternal marital status (divorced/widowed), maternal education of 10–11 years, maternal education of 12–14 years, parental history of IBS, parental history of anxiety, parental history of depression |
| Lower birth weight | Waehrens et al. (20) | 2018 | Cohort study | As above | As above | |
| Raslau et al. (21) | 2016 | Case-control study | IBS Patients and health controls | Lower birth weight increased the odds for IBS | ||
| Formula feeding | Koloski et al. (22) | 2005 | Case-control study | A random population sample from Sydney, Australia | Development of IBS was associated with childhood factors-a shorter duration of breastfeeding | |
| Pediatric diseases related to IBS | Acute gastroenteritis | Cremon et al. (23) | 2014 | Cohort study | 1,811 patients (primarily children) who were infected with foodborne Salmonella enteritidis in Bologna, Italy in 1994 | The prevalence of IBS was higher in individuals exposed Salmonella as children than in controls |
| Thabane et al. (24) | 2010 | Cohort study | Children exposed to domestic water contaminated with E. coli 0157:H7 and Campylobacter in Walkerton, Ontario, May 2000 | Acute bacterial gastroenteritis is associated with subsequent IBS in children as in adults | ||
| Functional constipation | Khan et al. (25) | 2007 | Case-control study | Pediatric FCC patients and health control | Childhood constipation appears to be a predictor of IBS in adulthood | |
| Urinary tract infection | Tan et al. (26) | 2018 | Cohort study | UTI infants and health controls | Infants with UTI had higher risks of childhood IBS | |
| Atopic dermatitis | Tsai et al. (27) | 2018 | Cohort study | AD children and health controls | AD children had a greater risk of developing IBS | |
| Food allergy/intolerance | Mansueto et al. (28) | 2015 | Experimental study | / | / | |
| Childhood physical/emotional trauma | Bradford et al. (17) | 2012 | Case-control study | 294 IBS patients and 435 controls | EALs had an independent association with IBS | |
| Childhood abdominal pain | Howell et al. (29) | 2005 | Cohort study | 1972 birth cohort (Dunedin, NZ) | CAP can progress to adult IBS in some children | |
| Asthma | Sjolund et al. (30) | 2021 | Cohort study | Children born in Sweden from 1994 through 1996 | Asthma is positively associated with IBS | |
NMS, Neonatal maternal separation; C-section, cesarean-section; EALs, early adverse life events; FCC, functional childhood constipation; UTI, urinary tract infection; AD, atopic dermatitis; CAP, childhood abdominal pain.
2. Adverse exposures in early life and IBS
2.1. Maternal adverse factors
2.1.1. Maternal inappropriate carbohydrate intake
Carbohydrates, found in diverse foods, are humans’ primary energy source. They can be classified into monosaccharides, disaccharides, and polysaccharides. Different dietary patterns can lead to variations in the composition of gut microbiota in offspring, and early-life microbiota alterations have been shown to influence susceptibility to diseases later in life (31). A cohort study conducted in Norway investigated 60 pregnant women and examined their dietary habits during pregnancy. Four days after delivery, the intestinal microbiota of these women was analyzed. The study found that increased sugar consumption was associated with a higher Actinobacteria/Firmicutes ratio (32). Another Australian pregnancy cohort study published in 2023 indicated a relationship between sugar consumption and decreased maternal microbial Shannon diversity (33). Conversely, reducing carbohydrate intake has been associated with an increased abundance of beneficial bacteria in the intestines, such as Roseburia spp., Bifidobacterium spp., and Eubacterium rectale (34). It has been established that there is an increase in pro-inflammatory bacteria in the gut of patients with irritable bowel syndrome (35). In 2021, Laura A. Bolte published high-quality evidence pointing out that the diet pattern is closely related to the composition of the microbiota and inflammation markers in human. Specifically, a high intake of carbohydrates has been shown to dramatically increase the presence of mucolytic bacteria and bacteria associated with energy harvesting, thereby promoting inflammation and increased gut permeability. Furthermore, long-term carbohydrate intake has been associated with higher quinone synthesis, which can also trigger intestinal inflammation (36). These studies have revealed the relationship between IBS, pro-inflammatory microbiota, and high sugar intake in adults. However, further evidence is needed to verify similar results in the early stages of life.
Carbohydrate intake has been closely linked to conditions like diabetes and obesity, as excessive consumption can lead to elevated blood glucose levels. The incidence of diabetes has been increasing alongside economic development. Interestingly, the incidence of IBS has also been on the rise in recent years. Specifically, a diet high in glucose and fructose was found to decrease the abundance of Bacteroidetes while increasing the levels of Proteobacteria and Akkermansia muciniphila in mice (37). These findings align with alterations observed in IBS patients and animal models (38, 39).
Interestingly, the offspring of the female rats in the diabetic model group had worse intestinal maturation when compared with those in the healthy control group. Specifically, the offspring from the diabetic model displayed thin intestinal mucosa and irregular arrangement of intestinal cells by the 10th day. As they reached the 45th day, the intestinal glands showed degeneration, and there was a further reduction in goblet cells (40). Furthermore, reports suggest that damage to the enteric nervous system (ENS) is involved in gastrointestinal motility changes in individuals with diabetes. This damage is believed to be linked to various factors, including enhanced apoptosis, oxidative stress, advanced glycation end products, alterations in intestinal muscle contractility, and brain-gut interactions. However, it is worth noting that these mechanisms are rarely discussed in the context of early life research (41).
Currently, there is no direct evidence demonstrating that a high-glycemic diet early in life promotes the occurrence of IBS (42). It is worth noting that the influence of maternal high sugar diet and maternal high blood glucose on IBS in offspring has not received adequate attention. Further research is needed to explore the potential effects of maternal high sugar diet and maternal high blood glucose levels on the development of IBS in offspring.
2.1.2. Maternal high-fat diet
Increasing evidence links a high-fat diet to a heightened risk of inflammatory bowel disease. Shared pathogenesis between IBD and IBS indicates overlapping risk factors. Some researchers even propose that IBS represents the pre-IBD period (43, 44). In animal study, long-term consumption of a high-fat and high-sugar diet has already been established as a risk factor for pre-IBD (45). Experiments of mice have shown that interventions with a high-fat diet before, throughout, and after gestation can significantly alter the composition of the microbiota. Offspring from mice subjected to high-fat diet interventions exhibited increased levels of Lachnospiraceae and Bacteroides, and decreased levels of Lactobacillus, Allobaculum, and Prevotella. Importantly, these changes were not entirely consistent with alterations in the maternal microbiota. Furthermore, the effects of a high-fat diet could persist until adulthood in mice study (46). These changes in the microbiota caused by a high-fat diet create an opportunity for IBS. This hypothesis was supported by a previous cross-sectional study, which found a direct link between a high-fat diet in early life and the presence of IBS in children aged 8–13 years. The authors of the study suggested that dietary changes should be considered to prevent IBS in early life (11). Latest findings suggest adults with IBS consume more high-fat diets than those without the condition (47). Unfortunately, this study did not explore whether high-fat dietary habits were formed in the early life stage. Therefore, more research is needed to investigate dietary habits in early life.
In-depth studies have shown that a high-fat diet can alter the expression of genes associated with colon structure and function during the gut development of two-week-old transgenic mice. Specifically, genes such as Abca1, Mgat4b, Id1, and Tpp1 were found to be affected by the high-fat diet. Further analysis using a clustered image map revealed that the high-fat diet induced changes in the function of the colon by regulating these genes in these mice, which the microbiota may influence in some way (48). Moreover, it has been reported that a maternal high-fat diet could increase anxiety-like behavior in female macaque offspring. This finding is significant given that psychological factors have been established as important pathogenesis mechanisms in IBS (49).
2.1.3. Maternal antibacterial agent exposure
Antibiotic exposure has been shown to be a risk factor for IBS in adulthood in human (50). However, there is limited direct evidence indicating that maternal antibiotic exposure can trigger the occurrence of IBS in adulthood.
The gut microbiota of infants is highly susceptible to the effects of antibiotics. Disruptions in microbiota composition and microbial colonization can significantly affect human health (51). One of the most notable changes in the gut microbiota following antibiotic exposure is the enrichment of three genera: Bacteroides, Peptostreptococci, and Enterobacteria, while the abundance of the Bacteroidetes phylum is decreased (52). Additionally, in a rat study, beneficial bacteria like Lactobacillus are reduced, and there is an increase in the relative abundance of pathogenic bacteria such as Enterobacter, Shigella sonnei, Enterococcus hormaechei, and Acinetobacter sp. (53). A European infants study observed that maternal antibiotic use during the perinatal and/or breastfeeding periods could decrease the number of Bacteroides, and antibiotic use in newborns could shape the microbiota composition by increasing the proportion of Enterobacteria (54), which is shared by patients with IBS. These changes in gut flora mentioned above can increase the risk of intestinal diseases, including IBS, by modulating the intestinal microbiota. However, a case-control study published in 2016 reported that early-life antibiotic use is not a risk factor for IBS (21). Long-term assessments of antibiotic effects on gut flora and large-scale retrospective studies are still needed to investigate the relationship between early-life antibiotic exposure and the incidence of IBS.
2.1.4. Maternal stress
Prenatal maternal stress treatment can inhibit intestinal development in offspring mice at 3 weeks of age, impair their intestinal barrier function, and induce low-grade inflammation in the gut (55) Beyond directly influencing the gastrointestinal development of the offspring, maternal stress can also disrupt the neuroimmune network in the offspring. The fetal immune system and central nervous system are very sensitive to external disturbances during pregnancy, so stress in early life can enhance the function of HPA axis, increase systemic immune response and disturb intestinal microbiota in offspring (15). The early immune system change can persist throughout the whole life, and the maternal immune functional disturbances caused by stress can be passed to the next generation, which means that the ability of offspring to absorb maternal immunoglobulin is impaired and immune system is depressed (56, 57). Similarly, research has begun to uncover how these disturbances may affect the gut environment of the offspring. In 2023, a study revealed the impact of maternal stress on the offspring's gut microbiota by examining the fecal microbiota of infants. The study found that maternal psychological stress led to a decrease in offspring's gut microbial alpha diversity and a reduction in the number of probiotic like Bifidobacterium. Therefore, maternal stress might cause the occurrence of IBS by triggering gut microbiota disorder in the offspring (58). Animal experiments have also found that prenatal stress leads to a reduction in the number of Bifidobacteria and Lactobacilli in the feces of young monkeys (59). Furthermore, a rat study has shown that prenatal maternal stress can lead to visceral hypersensitivity in the offspring, and this change may be mediated by the upregulation of cystathionine-β-synthase and Nav1.7 expression. Animal studies have also reported that miR-485/ASIC1 signaling and BDNF expression are associated with the occurrence of visceral hypersensitivity in offspring following prenatal stress (60, 61). Therefore, stress during pregnancy could also increase the risk of offspring developing IBS through a mechanism of visceral hypersensitivity (62).
2.1.5. Maternal smoking
Currently, there is no direct evidence that maternal smoking leads to an increased risk of IBS in offspring. However, human studies have found that maternal smoking exposure during pregnancy is associated with DNA methylation in offspring peripheral blood, unfortunately, these methylation sites were not found to be associated with IBS (63). Further research emphasizes the multifaceted impact of maternal smoking on offspring. Smoking during pregnancy leads to a decrease in fecal microbiota diversity in newborns, and an increase in colonization by Enterobacteriaceae. At 6 months, these infants have a higher abundance of Bacteroides and Staphylococcus in their feces (64).Studies on autism have shown that maternal smoking can cause the onset of autism by altering the gut microbiota and acting through the gut-brain axis, as well as shaping early brain development (65, 66). However, no studies have proven that maternal smoking promotes the occurrence of IBS through these mechanisms.
2.1.6. Maternal mental health
Maternal mental health may have an impact on the onset of IBS in offspring. Familial clustering of IBS has been observed, and in addition to genetic factors, sociological learning can also affect the prevalence of IBS in offspring. Early-life sociological learning primarily comes from parents. Studies have pointed out that the offspring of mothers with IBS are more likely to be troubled by gastrointestinal symptoms, and both mothers and children are more likely to experience anxiety and depression. This could be related to the intergenerational transmission of psychological disorders such as anxiety and depression (67). Therefore, maternal mental health is extremely important. Moreover, research on infant gut microbiota suggests that maternal anxiety and depression can affect the composition of the offspring's gut microbiota, reducing the number of probiotics, changing the levels of cytokines in the body, and thereby increasing the likelihood of offspring suffering from IBS through brain-gut axis mechanisms (58). However, there have also been contradictory conclusions. Some studies have confirmed a connection between maternal anxiety and a decrease in pro-inflammatory bacteria like Proteobacteria, in infants, while other studies have proposed opposite conclusions. The authors believe this may be due to differences in fecal microbiota detection methods and ways of measuring maternal anxiety (59).
2.1.7. Maternal infection
Current evidence suggests a strong association between maternal infection and the onset of neuropsychiatric disease in offspring (68), but there isn't enough evidence to indicate that maternal infection during pregnancy increases the risk of IBS in offspring. More animal experiments and cohort studies are needed in the future to elucidate the relationship between infection during pregnancy and IBS.
3.1. Postpartum adverse factors
3.1.1. Neonatal high blood glucose
Type 1 diabetes (T1D) typically occurs in early life stages. Although the mechanisms of T1D are diverse, it is useful for understanding the connection between high blood glucose and IBS in early life. A Finnish cohort study analyzed fecal microbiota compositions from pediatric T1D patients. The study observed a decrease in alpha diversity and an increase in the presence of Ruminococcus gnavus and Streptococcus infantarius in T1D patients. These two strains are known to be pathogenic bacteria that can disrupt the intestinal barrier and promote gut inflammation. Additionally, further investigation found elevated levels of human β-defensin 2 (hBD2) in samples from T1D patients. hBD2 is an antimicrobial peptide produced by epithelial cells to defend against pathogens (69). It has been previously established that IBS is characterized by low-grade inflammation in the gut (70). Therefore, the evidence mentioned above reveals the relationship between increased inflammation in the gut and metabolic disturbances in early life.
Glucagon-like peptide 1 (GLP-1) is a molecule that plays a significant role in regulating blood glucose levels. It is produced by enteroendocrine L-cells in the intestine. It has been established that carbohydrates in the lumen of the intestine stimulate the secretion of GLP-1, suppressing gastrointestinal motility in human (71, 72). While studies have indicated that GLP-1 analogs can alleviate abdominal pain in IBS patients, the exact role of GLP-1 in IBS physiology is still not fully understood. An animal study suggested that increased levels of GLP-1 are involved in the mechanism of IBS-C (73). However, a different human study found decreased levels of GLP-1 in the blood of IBS-C patients (74). Furthermore, a human study published in 2015 demonstrated that maternal high blood glucose levels could lead to decreased GLP-1 levels in offspring (75). From this, we can infer that maternal dietary patterns that result in high blood glucose may represent a new risk factor for the development of IBS in offspring.
3.1.2. High-fat diet in offspring
It is now understood that high-fat diets contribute to obesity and have been established as risk factors for cardiovascular and metabolic diseases. In addition, a cross-sectional study conducted in China demonstrated that a high-fat diet is also a risk factor for IBS in children (11). Interestingly, obesity has been extensively linked to the development of IBS. Studies have shown that obese adults are more likely to be diagnosed with IBS, and similar findings have also been reported in children (76). Dysbiosis, or an imbalance in the gut microbiota, is one of the main mechanisms associated with IBS. Interestingly, similar alterations in gut microbiota have been observed in obese individuals (77). Moreover, further evidence suggests possible connections between the microbiota profiles associated with IBS and those induced by a high-fat diet in early life (78, 79).
Currently, there is no direct evidence indicating that a high-fat diet in early life leads to an increased incidence of IBS in adulthood. An animal study showed that a high-fat diet given to mice from weaning until 6 weeks of age significantly reduced the relative abundance of the Muribaculaceae family in the mouse gut microbiota by the age of 14 weeks. Muribaculaceae is associated with the production of propionate, a short-chain fatty acid (80). Other animal studies have pointed out that a high-fat diet in juvenile mice reduces the number of Bifidobacterium and Akkermansia in the gut, and promotes the increase of pro-inflammatory bacteria like Dorea (81). Importantly, a study involving juvenile high-fat diet intervention demonstrated increased plasma insulin levels and decreased insulin sensitivity (82). These metabolic changes may interact with GLP-1 secretion, as discussed earlier. Furthermore, disruption of the enteric epithelial barrier and increased intestinal permeability have been observed in patients with IBS (83). Early-life high-fat diet intake might, based on evidence, predispose to IBS by damaging the gut barrier and enhancing intestinal permeability. Toll-like receptor 4 (TLR4) is a member of the pattern recognition receptor family and plays a role in the inflammatory process in animal models of obesity. Recent studies have indicated that mice receiving a high-fat diet for 16 weeks starting from 3 weeks old exhibited visceral hypersensitivity. This outcome could be attributed to the increased expression of TLR4 protein in both the central and peripheral nervous systems (84).
3.1.3. Neonatal stress exposure
According to the Rome IV criteria, IBS is considered a disease related to the gut-brain axis, where stress and psychological factors play a significant role in its pathogenesis. Clinical studies have shown a strong correlation between early life adverse experiences and the development of IBS (18). A case-control study supported that early-life emotional abuse increases the risk of developing IBS in adulthood. Additionally, another study identified household mental illness as the strongest predictor for IBS in adulthood among various early-life adverse events (16, 17). Further research has suggested that a sense of fear and dissociation following early-life trauma can predict the occurrence of IBS later in life (19).
Neonatal maternal separation (NMS) has become a widely accepted experimental model for studying IBS, and it has provided insights into the mechanisms related to stress in IBS development (12). Animal experiments using this model have demonstrated that NMS can induce visceral hypersensitivity and abnormal behavior in rats from early life to adulthood (13). The underlying mechanism for these effects involves increased Toll-like receptor 4 (TLR4) expression in microglial cells (14). Regarding visceral hyperalgesia, nerve growth factor (NGF) is crucial in mediating neuronal plasticity (85). In an animal experiment, male Wistar rats were subjected to NMS for 3 h per day starting from the second to fourteenth days after birth. When sacrificed at the 12th week, these rats exhibited colon mast cell hyperplasia and increased neurokinin 1 (NK1) receptor expression in the spinal cord. It has been established that substance P, which binds to NK1 receptors, can mediate pain transmission and regulates pain responses. During maternal separation, anti-NGF antibody treatment in NMS rats reduced enteric nerve synapses formation and mast cell counts, implying NGF's role in early-life stress-induced IBS development (86).
Early immune system disturbance and dysfunction of the HPA axis have been closely associated with IBS and can persist throughout an individual's life. male NMS rats have been observed to exhibit increased corticosterone levels, enhanced HPA axis activity, and abnormal behaviors (15). In NMS rats, the activation of metabotropic glutamate receptor-7 has been shown to reduce visceral hypersensitivity by modulating immune responses (87).
The intestinal mucosa contains a large number of immune cells and is vital to the functioning of the immune system. Animal studies have shown that at the age of 14 days, the colonic mucosa of neonatal maternal separation (NMS) rats showed an increase in mast cells compared to the control group, a phenomenon that persisted at 12 weeks. Simultaneously, the experiment found that at 14 days and 12 weeks, the expression of NGF in the mucosal and muscular layers of the NMS rat colon was significantly increased compared to the control group, consistent with changes in intestinal hypersensitivity in both groups (88). Other study has also revealed persistent hypersensitivity function of secretomotor neurons and upregulation of acetylcholine activity in pig offspring on the 15th day after weaning. Additionally, more enteric neurons have been observed, suggesting a potential link to IBS (89). These findings provide a foundation for understanding the relationship between early life stress and the development of IBS.
Psychological therapy has emerged as an important approach to managing stress-related disorders, including IBS. Studies have shown that cognitive behavioral therapy and hypnotherapy have effectively treated children and adults with IBS (90). These therapeutic approaches offer promising directions for future research and highlight the significant role of psychological interventions in managing IBS symptoms.
3.1.4. Neonatal formula feeding
Neonatal feeding modalities encompass breastfeeding, formula feeding, and mixed feeding. Recent research has shed light on the benefits of breastfeeding in growth programming (91). Breastfeeding during the initial three months has been linked to reduced infant functional constipation and favorable modulation of gut microbiota, influencing overall health (92). Evidence suggests that breastfed infants exhibit increased levels of probiotic species, such as Bifidobacterium, in their feces, often more than double that of formula-fed infants. A follow-up study published in 2023 indicated that formula feeding at 12 months of age was associated with a decrease in Verrucomicrobiota compared to breastfeeding (93). Conversely, formula-fed infants showed a decrease in Bifidobacterium levels and increased Bacteroides abundance (94, 95). Furthermore, formula-fed infants exhibited an overrepresentation of C. difficile, and a recent experiment on neonatal piglets demonstrated that formula-feeding predisposed them to C. difficile gut infection (96). Additionally, the effects of combining breast milk with formula feeding on the microbiota were more similar to exclusive formula feeding rather than exclusive breastfeeding (97).
Interestingly, prolonged infant breastfeeding has enhanced their resilience against IBS in adulthood (22). This effect is believed to be associated with the modulation of Th1 and Th2 immune responses. In individuals with IBS, the immune response shifts towards Th2 cells (98). Breastfeeding has been found to promote the development of a Th1 response in human (22). Additionally, human milk oligosaccharides have been shown to benefit by modulating cell signaling, gut microbiota composition and reducing mucosal invasion (99). In contrast, exclusive formula feeding can alter gut microbiota and increase susceptibility to gastrointestinal diseases (100). Studies have indicated that formula feeding can lead to changes in gastrointestinal morphology, microbial abundance, intestinal barrier proteins (such as vascular endothelial cadherin), and interleukin-10 (IL-10) production (101, 102). These alterations may result in reduced immune education and increased inflammatory processes. Improving feeding patterns can enhance immunity, reduce inflammation, and help prevent allergic and infectious diseases. Importantly, evidence suggests that immune impairments can have long-term health consequences. A study involving five European centers found that feeding patterns have a sustained influence on intestinal microbiota, which persists even after weaning (103).
Therefore, the choice of feeding method can have a long-term impact on the gut microbiota, immune system, and intestinal barrier of offspring. Optimal feeding methods play a crucial role in preventing the development of IBS from childhood to adulthood.
3.1.5. Neonatal antibiotic use
Studies have consistently shown that antibiotic exposure in adults and children increases the risk of abdominal symptoms, particularly abdominal pain (104). The changes in gut microbiota caused by early-life antibiotic use have been linked to the development of abdominal pain. For example, a Swedish study involving 2,732 12-year-old children found that antibiotic use during the first 2 years of life was associated with an increased risk of abdominal pain, especially in girls (105). Additionally, long-term or broad-spectrum antibiotic use between the ages of 9 and 12 was found to contribute to the risk of abdominal pain (106). Since the diagnosis of IBS is based on abdominal symptoms, it is noteworthy that disorders of gut-brain interaction in early life have been identified as independent predictors of IBS in adulthood (25). This suggests that many individuals experience IBS symptoms (such as altered bowel habits, changes in frequency of bowel movements, abdominal cramps, decreased appetite, and/or early satiety, gas and bloating) from early life into adulthood (107). Further research is needed to explore the long-term implications of these findings and provide a more comprehensive understanding of the underlying mechanisms.
Current research suggests that early-life antibiotic use directly impacts intestinal epithelial cells. In mouse experiments, early-life antibiotic exposure accelerated intestinal epithelial maturation, reducing permeability and the count of vacuolated enterocytes (108). These changes may be attributed to the stage of intestinal epithelium development during early life. In contrast, antibiotic use in adults is often associated with increased intestinal permeability, which may reflect differences in the developmental stage of the intestinal epithelium. The decrease in vacuolated enterocytes resulting from early-life antibiotic use can affect the absorption of breast milk and potentially diminish the protective effects associated with breastfeeding.
Indeed, antibiotic use in early life, including the first two years of childhood, has been associated with an increased risk of various allergic diseases, including food allergies (109). Allergic diseases, in turn, have been linked to an increased risk of abdominal pain (110). Food allergy has also been considered one of the possible causes of IBS (28). Furthermore, besides clinical use, antimicrobial substances like triclosan and triclocarban, commonly found in household and personal care products, can also disrupt the gut microbiota composition. Studies have shown that increased levels of triclosan are associated with decreased abundance of Bacteroides fragilis and enrichment of the Proteobacteria phylum in infants (111). Dysbiosis of the gut microbiota has been implicated in the development of functional gastrointestinal disorders and can potentially affect cognitive function (112). Therefore, it can be inferred that early-life antibiotic use, including exposure to antimicrobials, may negatively influence the gut microbiota of offspring and contribute to the onset of IBS in adulthood. However, more extensive research is still needed to better understand the relationship between antibiotic use, gut microbiota, and the development of IBS, especially through large-scale studies and data analysis.
3.1.6. Low birth weight
Low birth weight has been identified as another risk factor for IBS (20, 21). It is unclear what are causes of low birth weight (113). It has been suggested that the increased risk of IBS associated with low birth weight may be attributed to the immaturity of intestinal motor function (21, 114). Moreover, very low birth weight infants have been found to exhibit abnormal patterns of intestinal microbiota colonization, characterized by reduced population diversity and an increase in bacterial and fungal pathogens such as Escherichia coli, Enterococcus sp., Klebsiella pneumoniae, Candida spp., and Clavispora spp. These findings support the role of altered microbiota in the pathogenesis of IBS, as discussed earlier (115, 116).
3.1.7. Pediatric diseases related to IBS
Indeed, several pediatric diseases have been discovered to have notable correlations with IBS, highlighting a complex and multi-faceted etiological landscape. Functional constipation and Salmonella gastroenteritis in early life, for instance, have been identified as risk factors for IBS in adulthood (23). Furthermore, there is emerging evidence of a cross-talk between the urinary and intestinal systems, with studies supporting the idea that genitourinary disorders can contribute to gastrointestinal disorders (117, 118). Infants with urinary infections have been found to have a higher risk of developing IBS in childhood (26), possibly due to their shared embryonic origin and peripheral nerve connections (119).
Childhood physical or emotional trauma is a risk factor for developing IBS in adulthood, with studies indicating that early general trauma, physical punishment, emotional abuse, and sexual events are strong predictors for the onset of IBS in adulthood (17). Additionally, post-traumatic stress disorder in adult has also been confirmed as an adverse exposure for IBS (120), but the research did not focus on the early life period. Evidence suggests that fear emotions generated by early physical or emotional trauma play a critical role in the pathophysiology of IBS (19). A prospective study published in 2005 pointed out that some childhood abdominal pain can develop into IBS in adults, though the specific mechanisms remain elusive (29). In contrast, other research has found that early life recurrent abdominal pain may not be connected with IBS in adolescence, leaving unresolved questions regarding the relationship between childhood abdominal pain and adult IBS (121).
From the perspective of IBS research, there's a complex interplay between the syndrome and associated allergic diseases. Sudies have found that children with a history of atopic dermatitis (AD) are more prone to IBS (27). Genetic variations and local mucosal immune function have been implicated in both atopy and IBS (122, 123). Children with AD often experience food allergies closely related to mast cell activation, and these children have a higher risk of gastrointestinal dysfunction. Although the exact mechanisms are not yet fully understood, immune responses in the small bowel have been proposed as a potential link between food allergy and IBS in adults (124). Exploring the immune changes in the small bowel in children and their relationship to the development of IBS in adulthood holds promise for future research in this area.
Diving deeper into allergy-related diseases, a recent study have also drawn connections between childhood asthma and the onset of IBS by age 16. The same research pointed out that children who had eczema at 1–2 years of age might have an increased trend to develop IBS at 16 years of age, but unfortunately, the data did not show a significant statistical difference. Food hypersensitivity is generally associated with IBS onset at age 16, but the data did not indicate whether such a link exists in early life (30).
4.1. Delivery style
Microbiota is shared between the gut and vagina, and the mode of delivery significantly affects the microbiota in newborns. Several studies have demonstrated that caesarean section (C-section) delivery increases the risk for IBS (125). The mechanism underlying this association may be linked to the composition of the microbiota. Vaginally delivered and exclusively breastfed infants exhibit the “best” gut microbiota composition, characterized by a higher abundance of beneficial bacteria such as Bifidobacteria and Bacteroides, and a lower abundance of potentially harmful bacteria like Escherichia coli (E. coli) and Clostridium difficile (125). In contrast, infants born via C-section seem to have a lower abundance and diversity of gut microbiota and decreased and delayed colonization of Bifidobacterium spp. and Bacteroides spp. Instead, they tend to have increased levels of C. difficile and typical skin bacteria like Staphylococcus, Corynebacterium, and Propionibacterium spp (126). Similar results have been found in a large-scale study from the Netherlands, where C-section delivery was associated with lower amounts of Bifidobacteria and Bacteroides fragilis (B. fragilis)-group species and increased amounts of E. coli and C. difficile (125). Interestingly, emerging research suggests that the influence of maternal microbiota on the infant's microbiota is only significant in vaginally-delivered infants, and this influence is disturbed by C-section delivery (127). In addition, C-section delivery has been associated with a higher rate of acute gastroenteritis admission in children (128). It has been reported that a history of acute gastroenteritis in children is linked to an increased risk of IBS (24).
5. Conclusions
Lately, there has been increasing evidence showing that adverse exposures in early life can impact the development of functional gastrointestinal diseases, including IBS, in adulthood. These factors during early life stages can influence the risk of developing IBS later. Some factors, such as diet and stress, are relatively well-established, while others, including the use of antibiotics, delivery mode, and feeding patterns, still require long-term follow-up observations to gather sufficient evidence.
Proper diets and interventions with probiotics in early life have shown potential therapeutic prospects for preventing IBS. Therefore, understanding the relationship between adverse exposures in early life and IBS can open up a new field for preventing IBS in adulthood. This knowledge can ultimately help reduce the disease's burden and contribute to improving population health and the rational use of medical resources.
Author contributions
Conceptualization, GZ, MH, and MZ; methodology, GZ, XY, and NZ; investigation, GZ and ST; writing—original draft preparation, GZ and MH; writing—review and editing, GZ and MZ; visualization, XY, NZ, and GZ, supervision, MZ. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Camilleri M. Diagnosis and treatment of irritable bowel syndrome: a review. JAMA. (2021) 325(9):865–77. 10.1001/jama.2020.22532 [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell KJ, Meaney MJ. Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am J Psychiatry. (2017) 174(4):319–28. 10.1176/appi.ajp.2016.16020138 [DOI] [PubMed] [Google Scholar]
- 3.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. (2009) 27(5):358–68. 10.1055/s-0029-1237424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez-Roig MD, Pascal R, Cahuana MJ, García-Algar O, Sebastiani G, Andreu-Fernández V, et al. Environmental exposure during pregnancy: influence on prenatal development and early life: a comprehensive review. Fetal Diagn Ther. (2021) 48(4):245–57. 10.1159/000514884 [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RA, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. (2018) 15(1):39–49. 10.1038/nrgastro.2017.136 [DOI] [PubMed] [Google Scholar]
- 6.Willyard C. Could baby’s first bacteria take root before birth? Nature. (2018) 553(7688):264–6. 10.1038/d41586-018-00664-8 [DOI] [PubMed] [Google Scholar]
- 7.Bruce-Keller AJ, Fernandez-Kim SO, Townsend RL, Kruger C, Carmouche R, Newman S, et al. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS One. (2017) 12(4):e0175577. 10.1371/journal.pone.0175577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul HA, Bomhof MR, Vogel HJ, Reimer RA. Diet-induced changes in maternal gut microbiota and metabolomic profiles influence programming of offspring obesity risk in rats. Sci Rep. (2016) 6:20683. 10.1038/srep20683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rautava S. Early microbial contact, the breast milk microbiome and child health. J Dev Orig Health Dis. (2016) 7(1):5–14. 10.1017/S2040174415001233 [DOI] [PubMed] [Google Scholar]
- 10.Low E, Mandhari M, Herndon CC, Loo E, Tham EH, Siah K. Parental, perinatal, and childhood risk factors for development of irritable bowel syndrome: a systematic review. J Neurogastroenterol Motil. (2020) 26(4):437–46. 10.5056/jnm20109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X, Chen W, Zhu X, Shen Y. A cross-sectional study of risk factors for irritable bowel syndrome in children 8–13 years of age in Suzhou, China. Gastroenterol Res Pract. (2014) 2014:198461. 10.1155/2014/198461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riba A, Olier M, Lacroix-Lamandé S, Lencina C, Bacquié V, Harkat C, et al. Early life stress in mice is a suitable model for irritable bowel syndrome but does not predispose to colitis nor increase susceptibility to enteric infections. Brain Behav Immun. (2018) 73:403–15. 10.1016/j.bbi.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 13.Yi L, Zhang H, Sun H, Zhou L, Chen Y, Xuan L, et al. Maternal separation induced visceral hypersensitivity from childhood to adulthood. J Neurogastroenterol Motil. (2017) 23(2):306–15. 10.5056/jnm16089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang HL, Zhang G, Ji NN, Du L, Chen BB, Hua R. Toll-like receptor 4 in paraventricular nucleus mediates visceral hypersensitivity induced by maternal separation. Front Pharmacol. (2017) 8:309. 10.3389/fphar.2017.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. (2009) 65(3):263–7. 10.1016/j.biopsych.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 16.Park SH, Videlock EJ, Shih W, Presson AP, Mayer EA, Chang L. Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil. (2016) 28(8):1252–60. 10.1111/nmo.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. (2012) 10(4):385–90.e1–3. 10.1016/j.cgh.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju T, Naliboff BD, Shih W, Presson AP, Liu C, Gupta A, et al. Risk and protective factors related to early adverse life events in irritable bowel syndrome. J Clin Gastroenterol. (2020) 54(1):63–9. 10.1097/MCG.0000000000001153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahal H, Videlock EJ, Icenhour A, Shih W, Naliboff B, Gupta A, et al. Importance of trauma-related fear in patients with irritable bowel syndrome and early adverse life events. Neurogastroenterol Motil. (2020) 32(9):e13896. 10.1111/nmo.13896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waehrens R, Li X, Sundquist J, Sundquist K, Zöller B. Perinatal and familial risk factors for irritable bowel syndrome in a Swedish national cohort. Scand J Gastroenterol. (2018) 53(5):559–66. 10.1080/00365521.2017.1398345 [DOI] [PubMed] [Google Scholar]
- 21.Raslau D, Herrick LM, Locke GR, Schleck CD, Zinsmeister AR, Almazar A, et al. Irritable bowel syndrome and the perinatal period: lower birth weight increases the risk. Neurogastroenterol Motil. (2016) 28(10):1518–24. 10.1111/nmo.12849 [DOI] [PubMed] [Google Scholar]
- 22.Koloski NA, Jones M, MWeltman M, Kalantar J, Bone C, Gowryshankar A, et al. Identification of early environmental risk factors for irritable bowel syndrome and dyspepsia. Neurogastroenterol Motil. (2015) 27(9):1317–25. 10.1111/nmo.12626 [DOI] [PubMed] [Google Scholar]
- 23.Cremon C, Stanghellini V, Pallotti F, Fogacci E, Bellacosa L, Morselli-Labate AM, et al. Salmonella gastroenteritis during childhood is a risk factor for irritable bowel syndrome in adulthood. Gastroenterology. (2014) 147(1):69–77. 10.1053/j.gastro.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 24.Thabane M, Simunovic M, Akhtar-Danesh N, Garg AX, Clark WF, Collins SM, et al. An outbreak of acute bacterial gastroenteritis is associated with an increased incidence of irritable bowel syndrome in children. Am J Gastroenterol. (2010) 105(4):933–9. 10.1038/ajg.2010.74 [DOI] [PubMed] [Google Scholar]
- 25.Khan S, Campo J, Bridge JA, Chiappetta LC, Wald A, di Lorenzo C. Long-term outcome of functional childhood constipation. Dig Dis Sci. (2007) 52(1):64–9. 10.1007/s10620-006-9308-9 [DOI] [PubMed] [Google Scholar]
- 26.Tan TK, Saps M, Lin CL, Wei CC. Risks of irritable bowel syndrome in children with infantile urinary tract infection: a 13-year nationwide cohort study. J Investig Med. (2018) 66(6):998–1003. 10.1136/jim-2017-000703 [DOI] [PubMed] [Google Scholar]
- 27.Tsai JD, Wang IC, Shen TC, Lin CL, Wei CC. A 8-year population-based cohort study of irritable bowel syndrome in childhood with history of atopic dermatitis. J Investig Med. (2018) 66(4):755–61. 10.1136/jim-2017-000631 [DOI] [PubMed] [Google Scholar]
- 28.Mansueto P, D'Alcamo A, Seidita A, Carroccio A. Food allergy in irritable bowel syndrome: the case of non-celiac wheat sensitivity. World J Gastroenterol. (2015) 21(23):7089–109. 10.3748/wjg.v21.i23.7089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howell S, Poulton R, Talley NJ. The natural history of childhood abdominal pain and its association with adult irritable bowel syndrome: birth-cohort study. Am J Gastroenterol. (2005) 100(9):2071–8. 10.1111/j.1572-0241.2005.41753.x [DOI] [PubMed] [Google Scholar]
- 30.Sjölund J, Kull I, Bergström A, Järås J, Ludvigsson JF, Törnblom H, et al. Allergy-related diseases in childhood and risk for abdominal pain-related functional gastrointestinal disorders at 16 years-a birth cohort study. BMC Med. (2021) 19(1):214. 10.1186/s12916-021-02069-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr CA, Grice DM, Tran CD, Bauer DC, Li D, Hendry P, et al. Early life events influence whole-of-life metabolic health via gut microflora and gut permeability. Crit Rev Microbiol. (2015) 41(3):326–40. 10.3109/1040841X.2013.837863 [DOI] [PubMed] [Google Scholar]
- 32.Mandal S, Godfrey KM, McDonald D, Treuren WV, Bjørnholt JV, Midtvedt T, et al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome. (2016) 4(1):55. 10.1186/s40168-016-0200-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gow ML, Chua XY, El-Omar E, Susic D, Henry A. Relationship between diet quality and maternal stool microbiota in the MUMS Australian pregnancy cohort. Nutrients. (2023) 15(3):689. 10.3390/nu15030689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. (2015) 42(2):158–79. 10.1111/apt.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sciavilla P, Strati F, Paola MD, Modesto M, Vitali F, Cavalieri D, et al. Gut microbiota profiles and characterization of cultivable fungal isolates in IBS patients. Appl Microbiol Biotechnol. (2021) 105(8):3277–88. 10.1007/s00253-021-11264-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolte LA, Vila AV, Imhann F, Collij V, Gacesa R, Peters V, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. (2021) 70(7):1287–98. 10.1136/gutjnl-2020-322670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Do MH, Lee E, Oh MJ, Kim Y, Park HY. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients. (2018) 10(6):761. 10.3390/nu10060761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gobert AP, Sagrestani G, Delmas E, Wilson KT, Verriere TG, Dapoigny M, et al. The human intestinal microbiota of constipated-predominant irritable bowel syndrome patients exhibits anti-inflammatory properties. Sci Rep. (2016) 6:39399. 10.1038/srep39399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, et al. Gut microbiota in patients with irritable bowel syndrome-a systematic review. Gastroenterology. (2019) 157(1):97–108. 10.1053/j.gastro.2019.03.049 [DOI] [PubMed] [Google Scholar]
- 40.Sharma R, Kaur J, Chauhan SS, Mahmood A. Gestational diabetes affects postnatal development of transport and enzyme functions in rat intestine. Mol Cell Biochem. (2012) 361(1–2):71–7. 10.1007/s11010-011-1090-0 [DOI] [PubMed] [Google Scholar]
- 41.Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. (2007) 19(12):951–60. 10.1111/j.1365-2982.2007.01023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gulcan E, Taser F, Toker A, Korkmaz U, Alcelik A. Increased frequency of prediabetes in patients with irritable bowel syndrome. Am J Med Sci. (2009) 338(2):116–9. 10.1097/MAJ.0b013e31819f7587 [DOI] [PubMed] [Google Scholar]
- 43.Abdul RR, Raja AR, Lee YY. Irritable bowel syndrome and inflammatory bowel disease overlap syndrome: pieces of the puzzle are falling into place. Intest Res. (2016) 14(4):297–304. 10.5217/ir.2016.14.4.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porter CK, Cash BD, Pimentel M, Akinseye A, Riddle MS. Risk of inflammatory bowel disease following a diagnosis of irritable bowel syndrome. BMC Gastroenterol. (2012) 12:55. 10.1186/1471-230X-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnone D, Vallier M, Hergalant S, Chabot C, Ndiaye NC, Moulin D, et al. Long-term overconsumption of fat and sugar causes a partially reversible pre-inflammatory bowel disease state. Front Nutr. (2021) 8:758518. 10.3389/fnut.2021.758518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo Y, Wang Z, Chen L, Tang L, Wen S, Liu Y, et al. Diet induced maternal obesity affects offspring gut microbiota and persists into young adulthood. Food Funct. (2018) 9(8):4317–27. 10.1039/C8FO00444G [DOI] [PubMed] [Google Scholar]
- 47.Na W, Lee Y, Kim H, Kim YS, Sohn C. High-fat foods and FODMAPs containing gluten foods primarily contribute to symptoms of irritable bowel syndrome in Korean adults. Nutrients. (2021) 13(4):1308. 10.3390/nu13041308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steegenga WT, Mischke M, Lute C, Boekschoten MV, Lendvai A, Pruis MG, et al. Maternal exposure to a western-style diet causes differences in intestinal microbiota composition and gene expression of suckling mouse pups. Mol Nutr Food Res. (2017) 61(1):1600141. 10.1002/mnfr.201600141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. (2010) 30(10):3826–30. 10.1523/JNEUROSCI.5560-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Törnblom H, Holmvall P, Svenungsson B, Lindberg G. Gastrointestinal symptoms after infectious diarrhea: a five-year follow-up in a Swedish cohort of adults. Clin Gastroenterol Hepatol. (2007) 5(4):461–4. 10.1016/j.cgh.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 51.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung LM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. (2014) 158(4):705–21. 10.1016/j.cell.2014.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasmin F, Tun HM, Konya TB, Guttman DS, Chari RS, Field CJ, et al. Cesarean section, formula feeding, and infant antibiotic exposure: separate and combined impacts on gut microbial changes in later infancy. Front Pediatr. (2017) 5:200. 10.3389/fped.2017.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan I, Azhar EI, Abbas AT, Kumosani T, Barbour EK, Raoult D, et al. Metagenomic analysis of antibiotic-induced changes in gut microbiota in a pregnant rat model. Front Pharmacol. (2016) 7:104. 10.3389/fphar.2016.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. (2010) 51(1):77–84. 10.1097/MPG.0b013e3181d1b11e [DOI] [PubMed] [Google Scholar]
- 55.Sun Y, Xie R, Li L, Jin G, Zhou B, Huang H, et al. Prenatal maternal stress exacerbates experimental colitis of offspring in adulthood. Front Immunol. (2021) 12:700995. 10.3389/fimmu.2021.700995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vandenplas Y, Alturaiki MA, Al-Qabandi W, AlRefaee F, Bassil Z, Eid B, et al. Middle East consensus statement on the diagnosis and management of functional gastrointestinal disorders in <12 months old infants. Pediatr Gastroenterol Hepatol Nutr. (2016) 19(3):153–61. 10.5223/pghn.2016.19.3.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merlot E, Quesnel H, Prunier A. Prenatal stress, immunity and neonatal health in farm animal species. Animal. (2013) 7(12):2016–25. 10.1017/S175173111300147X [DOI] [PubMed] [Google Scholar]
- 58.Galley JD, Mashburn-Warren L, Blalock LC, Lauber CL, Carroll JE, Kharah M, Ross KM, et al. Maternal anxiety, depression and stress affects offspring gut microbiome diversity and bifidobacterial abundances. Brain Behav Immun. (2023) 107:253–64. 10.1016/j.bbi.2022.10.005 [DOI] [PubMed] [Google Scholar]
- 59.Hu J, Ly J, Zhang W, Huang Y, Glover V, Peter I, et al. Microbiota of newborn meconium is associated with maternal anxiety experienced during pregnancy. Dev Psychobiol. (2019) 61(5):640–9. 10.1002/dev.21837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu X, Li YC, Wu YY, Xu YC, Weng RX, Wang CL, et al. Upregulation of spinal ASIC1 by miR-485 mediates enterodynia in adult offspring rats with prenatal maternal stress. CNS Neurosci Ther. (2021) 27(2):244–55. 10.1111/cns.13542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winston JH, Li Q, Sarna SK. Chronic prenatal stress epigenetically modifies spinal cord BDNF expression to induce sex-specific visceral hypersensitivity in offspring. Neurogastroenterol Motil. (2014) 26(5):715–30. 10.1111/nmo.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang HJ, Xu X, Xie RH, Rui YY, Zhang PA, Zhu XJ, et al. Prenatal maternal stress induces visceral hypersensitivity of adult rat offspring through activation of cystathionine-beta-synthase signaling in primary sensory neurons. Mol Pain. (2018) 14:1744806918777406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiklund P, Karhunen V, Richmond RC, Parmar P, Rodriguez A, Silva MD, et al. DNA methylation links prenatal smoking exposure to later life health outcomes in offspring. Clin Epigenetics. (2019) 11(1):97. 10.1186/s13148-019-0683-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McLean C, Jun S, Kozyrskyj A. Impact of maternal smoking on the infant gut microbiota and its association with child overweight: a scoping review. World J Pediatr. (2019) 15(4):341–9. 10.1007/s12519-019-00278-8 [DOI] [PubMed] [Google Scholar]
- 65.Dawson SL, O'Hely M, Jacka FN, Ponsonby AL, Symeonides C, Loughman A, et al. Maternal prenatal gut microbiota composition predicts child behaviour. EBioMedicine. (2021) 68:103400. 10.1016/j.ebiom.2021.103400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Streit F, Prandovszky E, Send T, Zillich L, Frank J, Sabunciyan S, et al. Microbiome profiles are associated with cognitive functioning in 45-month-old children. Brain Behav Immun. (2021) 98:151–60. 10.1016/j.bbi.2021.08.001 [DOI] [PubMed] [Google Scholar]
- 67.Tilburg MA, Levy RL, Walker LS, Korff MV, Feld LD, Garner M, et al. Psychosocial mechanisms for the transmission of somatic symptoms from parents to children. World J Gastroenterol. (2015) 21(18):5532–41. 10.3748/wjg.v21.i18.5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Haddad B, Jacobsson B, Chabra S, Modzelewska D, Olson EM, Bernier R, et al. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry. (2019) 76(6):594–602. 10.1001/jamapsychiatry.2019.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kostic AD, Gevers D, Siljander H, Vatanen T, Hyötyläinen T, Hämäläinen AM, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. (2015) 17(2):260–73. 10.1016/j.chom.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akiho H, Ihara E, Nakamura K. Low-grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J Gastrointest Pathophysiol. (2010) 1(3):97–105. 10.4291/wjgp.v1.i3.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sandoval DA, D'Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. (2015) 95(2):513–48. 10.1152/physrev.00013.2014 [DOI] [PubMed] [Google Scholar]
- 72.Hellström PM, Näslund E, Edholm T, Schmidt PT, Kristensen J, Theodorsson E, Holst JJ, et al. GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome. Neurogastroenterol Motil. (2008) 20(6):649–59. 10.1111/j.1365-2982.2007.01079.x [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, Li Z, Yang Y, Lin L, Zhang H. Role of glucagon-like peptide-1 in the pathogenesis of experimental irritable bowel syndrome rat models. Int J Mol Med. (2013) 31(3):607–13. 10.3892/ijmm.2013.1252 [DOI] [PubMed] [Google Scholar]
- 74.Li ZY, Zhang N, Wen S, Zhang J, Sun XL, Fan XM, et al. Decreased glucagon-like peptide-1 correlates with abdominal pain in patients with constipation-predominant irritable bowel syndrome. Clin Res Hepatol Gastroenterol. (2017) 41(4):459–65. 10.1016/j.clinre.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 75.Kelstrup L, Clausen TD, Mathiesen ER, Hansen T, Holst JJ, Damm P. Incretin and glucagon levels in adult offspring exposed to maternal diabetes in pregnancy. J Clin Endocrinol Metab. (2015) 100(5):1967–75. 10.1210/jc.2014-3978 [DOI] [PubMed] [Google Scholar]
- 76.Aro P, Ronkainen J, Talley NJ, Storskrubb T, Bolling-Sternevald E, Agréus L. Body mass index and chronic unexplained gastrointestinal symptoms: an adult endoscopic population based study. Gut. (2005) 54(10):1377–83. 10.1136/gut.2004.057497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maruvada P, Leone V, Kaplan LM, Chang EB. The human microbiome and obesity: moving beyond associations. Cell Host Microbe. (2017) 22(5):589–99. 10.1016/j.chom.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 78.Pugliese G, Muscogiuri G, Barrea L, Laudisio D, Savastano S, Colao A. Irritable bowel syndrome: a new therapeutic target when treating obesity? Hormones (Athens). (2019) 18(4):395–9. 10.1007/s42000-019-00113-9 [DOI] [PubMed] [Google Scholar]
- 79.Pickett-Blakely O. Obesity and irritable bowel syndrome: a comprehensive review. Gastroenterol Hepatol (N Y). (2014) 10(7):411–6. 10.1111/apt.12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McNamara MP, Singleton JM, Cadney MD, Ruegger PM, Borneman J, Garland T. Early-life effects of juvenile western diet and exercise on adult gut microbiome composition in mice. J Exp Biol. (2021) 224(Pt 4):jeb.239699. 10.1242/jeb.239699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Villamil SI, Huerlimann R, Morianos C, Sarnyai Z, Maes GE. Adverse effect of early-life high-fat/high-carbohydrate (“western”) diet on bacterial community in the distal bowel of mice. Nutr Res. (2018) 50:25–36. 10.1016/j.nutres.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 82.Maejima Y, Yokota S, Horita S, Shimomura K. Early life high-fat diet exposure evokes normal weight obesity. Nutr Metab (Lond). (2020) 17:48. 10.1186/s12986-020-00464-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Camilleri M, Madsen K, Spiller R, Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. (2012) 24(6):503–12. 10.1111/j.1365-2982.2012.01921.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tramullas M, Finger BC, Dinan TG, Cryan JF. Obesity takes its toll on visceral pain: high-fat diet induces toll-like receptor 4-dependent visceral hypersensitivity. PLoS One. (2016) 11(5):e0155367. 10.1371/journal.pone.0155367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chung EK, Zhang XJ, Xu HX, Sung JJ, Bian ZX. Visceral hyperalgesia induced by neonatal maternal separation is associated with nerve growth factor-mediated central neuronal plasticity in rat spinal cord. Neuroscience. (2007) 149(3):685–95. 10.1016/j.neuroscience.2007.07.055 [DOI] [PubMed] [Google Scholar]
- 86.Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut. (2008) 57(5):582–90. 10.1136/gut.2007.126680 [DOI] [PubMed] [Google Scholar]
- 87.Shao L, Liu Y, Xiao J, Wang Q, Liu F, Ding J. Activating metabotropic glutamate receptor7 attenuates visceral hypersensitivity in neonatal maternally separated rats. Int J Mol Med. (2019) 43(2):761–70. 10.3892/ijmm.2018.4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. (2004) 127(2):524–34. 10.1053/j.gastro.2004.05.019 [DOI] [PubMed] [Google Scholar]
- 89.Medland JE, Pohl CS, Edwards LL, Frandsen S, Bagley K, Li Y, et al. Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterol Motil. (2016) 28(9):1317–29. 10.1111/nmo.12828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giannetti E, Staiano A. Probiotics for irritable bowel syndrome: clinical data in children. J Pediatr Gastroenterol Nutr. (2016) 63(Suppl 1):S25–6. 10.1097/MPG.0000000000001220 [DOI] [PubMed] [Google Scholar]
- 91.Sepúlveda-Valbuena N, Nieto-Ruiz A, Diéguez E, Herrmann F, Escudero-Marín M, De-Castellar R, et al. Growth patterns and breast milk/infant formula energetic efficiency in healthy infants up to 18 months of life: the COGNIS study. Br J Nutr. (2021) 126:1–14. 10.1017/S000711452100057X [DOI] [PubMed] [Google Scholar]
- 92.Turco R, Miele E, Russo M, Mastroianni R, Lavorgna A, Paludetto R, et al. Early-life factors associated with pediatric functional constipation. J Pediatr Gastroenterol Nutr. (2014) 58(3):307–12. 10.1097/MPG.0000000000000209 [DOI] [PubMed] [Google Scholar]
- 93.Plaza-Diaz J, Ruiz-Ojeda FJ, Morales J, Martín-Masot R, Climent E, Silva A, et al. Innova 2020: a follow-up study of the fecal microbiota of infants using a novel infant formula between 6 months and 12 months of age. Int J Mol Sci. (2023) 24(8):7392. 10.3390/ijms24087392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe. (2011) 17(6):478–82. 10.1016/j.anaerobe.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 95.Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology (Reading). (2010) 156(Pt 11):3329–41. 10.1099/mic.0.043224-0 [DOI] [PubMed] [Google Scholar]
- 96.Grzeskowiak L, Martínez-Vallespín B, Dadi TH, Radloff J, Amasheh S, Heinsen FA, et al. Formula feeding predisposes neonatal piglets to Clostridium difficile gut infection. J Infect Dis. (2018) 217(9):1442–52. 10.1093/infdis/jix567 [DOI] [PubMed] [Google Scholar]
- 97.Madan JC, Hoen AG, Lundgren SN, Farzan SF, Cottingham KL, Morrison HG, et al. Association of cesarean delivery and formula supplementation with the intestinal microbiome of 6-week-old infants. JAMA Pediatr. (2016) 170(3):212–9. 10.1001/jamapediatrics.2015.3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kindt S, Oudenhove LV, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, et al. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. (2009) 21(4):389–98. 10.1111/j.1365-2982.2008.01220.x [DOI] [PubMed] [Google Scholar]
- 99.Plaza-Diaz J, Fontana L, Gil A. Human milk oligosaccharides and immune system development. Nutrients. (2018) 10(8):1038. 10.3390/nu10081038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Le Huerou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. (2010) 23(1):23–36. 10.1017/S0954422410000065 [DOI] [PubMed] [Google Scholar]
- 101.Groer MW, Davis MW, Smith K, Casey K, Kramer V, Bukovsky E. Immunity, inflammation and infection in post-partum breast and formula feeders. Am J Reprod Immunol. (2005) 54(4):222–31. 10.1111/j.1600-0897.2005.00301.x [DOI] [PubMed] [Google Scholar]
- 102.Groer MW, Davis MW. Cytokines, infections, stress, and dysphoric moods in breastfeeders and formula feeders. J Obstet Gynecol Neonatal Nurs. (2006) 35(5):599–607. 10.1111/j.1552-6909.2006.00083.x [DOI] [PubMed] [Google Scholar]
- 103.Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology (Reading). (2011) 157(Pt 5):1385–92. 10.1099/mic.0.042143-0 [DOI] [PubMed] [Google Scholar]
- 104.Maxwell PR, Rink E, Kumar D, Mendall MA. Antibiotics increase functional abdominal symptoms. Am J Gastroenterol. (2002) 97(1):104–8. 10.1111/j.1572-0241.2002.05428.x [DOI] [PubMed] [Google Scholar]
- 105.Kamphorst K, Daele EV, Vlieger AM, Daams JG, Knol J, Elburg RM. Early life antibiotics and childhood gastrointestinal disorders: a systematic review. BMJ Paediatr Open. (2021) 5(1):e001028. 10.1136/bmjpo-2021-001028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uusijärvi A, Bergström A, Simrén M, Ludvigsson JF, Kull I, Wickman M, et al. Use of antibiotics in infancy and childhood and risk of recurrent abdominal pain–a Swedish birth cohort study. Neurogastroenterol Motil. (2014) 26(6):841–50. 10.1111/nmo.12340 [DOI] [PubMed] [Google Scholar]
- 107.Zanchi C, Pintaldi S, Leo GD, Ronfani L, Zamagni G, Viel M, et al. Fifteen-years follow-up in a cohort of children with functional gastrointestinal disorders: prevalence and risk factors to develop neuropsychiatric disorders and other comorbidities. Children (Basel). (2021) 8(10):838. 10.3390/children8100838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garcia TM, Roest M, Vermeulen JL, Meisner S, Smit WL, et al. Early life antibiotics influence in vivo and in vitro mouse intestinal epithelium maturation and functioning. Cell Mol Gastroenterol Hepatol. (2021) 12(3):943–81. 10.1016/j.jcmgh.2021.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ahmadizar F, Vijverberg SJ, Arets HG, Boer AD, Lang JE, Garssen J, et al. Early-life antibiotic exposure increases the risk of developing allergic symptoms later in life: a meta-analysis. Allergy. (2018) 73(5):971–86. 10.1111/all.13332 [DOI] [PubMed] [Google Scholar]
- 110.Olén O, Neuman A, Koopmann B, Ludvigsson JF, Ballardini N, Westman M, et al. Allergy-related diseases and recurrent abdominal pain during childhood—a birth cohort study. Aliment Pharmacol Ther. (2014) 40(11–12):1349–58. 10.1111/apt.12965 [DOI] [PubMed] [Google Scholar]
- 111.Ribado JV, Ley C, Haggerty TD, Tkachenko E, Bhatt AS, Parsonnet J. Household triclosan and triclocarban effects on the infant and maternal microbiome. EMBO Mol Med. (2017) 9(12):1732–41. 10.15252/emmm.201707882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. (2014) 20(39):14105–25. 10.3748/wjg.v20.i39.14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martínez-Galiano JM, Amezcua-Prieto C, Salcedo-Bellido I, González-Mata G, Bueno-Cavanillas A, Delgado-Rodríguez M. Maternal dietary consumption of legumes, vegetables and fruit during pregnancy, does it protect against small for gestational age? BMC Pregnancy Childbirth. (2018) 18(1):486. 10.1186/s12884-018-2123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berseth CL. Gestational evolution of small intestine motility in preterm and term infants. J Pediatr. (1989) 115(4):646–51. 10.1016/S0022-3476(89)80302-6 [DOI] [PubMed] [Google Scholar]
- 115.Unger S, Stintzi A, Shah P, Mack D, O'Connor DL. Gut microbiota of the very-low-birth-weight infant. Pediatr Res. (2015) 77(1–2):205–13. 10.1038/pr.2014.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gu Y, Zhou G, Qin X, Huang S, Wang B, Cao H. The potential role of gut mycobiome in irritable bowel syndrome. Front Microbiol. (2019) 10:1894. 10.3389/fmicb.2019.01894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Francis CY, Duffy JN, Whorwell PJ, Morris J. High prevalence of irritable bowel syndrome in patients attending urological outpatient departments. Dig Dis Sci. (1997) 42(2):404–7. 10.1023/A:1018838507545 [DOI] [PubMed] [Google Scholar]
- 118.Brumovsky PR, Feng B, Xu L, McCarthy CJ, Gebhart GF. Cystitis increases colorectal afferent sensitivity in the mouse. Am J Physiol Gastrointest Liver Physiol. (2009) 297(6):G1250–8. 10.1152/ajpgi.00329.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malykhina AP, Wyndaele JJ, Andersson KE, Wachter SD, Dmochowski RR. Do the urinary bladder and large bowel interact, in sickness or in health? ICI-RS 2011. Neurourol Urodyn. (2012) 31(3):352–8. 10.1002/nau.21228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qin Xiang Ng QX, Soh AY, Loke W, Venkatanarayanan N, Lim DY, Yeo WS. Systematic review with meta-analysis: the association between post-traumatic stress disorder and irritable bowel syndrome. J Gastroenterol Hepatol. (2019) 34(1):68–73. 10.1111/jgh.14446 [DOI] [PubMed] [Google Scholar]
- 121.Sjölund J, Uusijärvi A, Tornkvist NT, Kull I, Bergström A, Alm J, et al. Prevalence and progression of recurrent abdominal pain, from early childhood to adolescence. Clin Gastroenterol Hepatol. (2021) 19(5):930–8.e8. 10.1016/j.cgh.2020.04.047 [DOI] [PubMed] [Google Scholar]
- 122.Walker MM, Talley NJ, Prabhakar M, Pennaneac'h CJ, Aro P, Ronkainen J, et al. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. (2009) 29(7):765–73. 10.1111/j.1365-2036.2009.03937.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. (2014) 133(6):1521–34. 10.1016/j.jaci.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burns GL, Talley NJ, Keely S. Immune responses in the irritable bowel syndromes: time to consider the small intestine. BMC Med. (2022) 20(1):115. 10.1186/s12916-022-02301-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. (2006) 118(2):511–21. 10.1542/peds.2005-2824 [DOI] [PubMed] [Google Scholar]
- 126.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. (2010) 107(26):11971–5. 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Singh SB, Madan J, Coker M, Hoen A, Baker ER, Karagas MR, et al. Does birth mode modify associations of maternal pre-pregnancy BMI and gestational weight gain with the infant gut microbiome? Int J Obes (Lond). (2020) 44(1):23–32. 10.1038/s41366-018-0273-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bentley JP, Simpson JM, Bowen JR, Morris JM, Roberts CL, Nassar N. Gestational age, mode of birth and breastmilk feeding all influence acute early childhood gastroenteritis: a record-linkage cohort study. BMC Pediatr. (2016) 16:55. 10.1186/s12887-016-0591-0 [DOI] [PMC free article] [PubMed] [Google Scholar]