Abstract
Background
Ocular discomfort is the leading cause of permanent discontinuation of soft contact lens (SCL) wear. Silicone hydrogel and hydrogel materials are the two major categories of SCLs, with silicone hydrogel materials being newer and more breathable than hydrogel materials. Whether comfort is associated with SCL material is controversial despite numerous studies. Similarly, the difference between these materials in terms of safety outcomes (e.g. frequency of microbial keratitis) is unclear.
Objectives
To evaluate the comparative effectiveness and safety of silicone hydrogel compared with hydrogel SCLs on self‐reported comfort, dry eye test results, and adverse events in SCL‐wearing adults 18 years of age or older.
Search methods
The Cochrane Eyes and Vision Information Specialist searched the electronic databases for randomized controlled trials (RCTs). There were no restrictions on language or date of publication. We searched the Cochrane Central Register of Controlled Trials (CENTRAL, including the Cochrane Eyes and Vision Trials Register; 2022, Issue 6), MEDLINE Ovid, Embase.com, PubMed, LILACS (Latin American and Caribbean Health Science Information database), ClinicalTrials.gov, and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We also searched the reference lists of identified studies, review articles, and guidelines for information about relevant studies that may not have been identified by our search strategy. Furthermore, we contacted investigators regarding ongoing trials. The most recent database search was conducted on 24 June 2022.
Selection criteria
Our search selection criteria included RCTs, quasi‐RCTs, and cross‐over RCTs.
Data collection and analysis
We applied standard Cochrane methodology.
Main results
We included seven parallel‐group RCTs conducted in the USA, the UK, Australia, Germany, India, and Turkey. A total of 1371 participants were randomized. The duration of SCL wear ranged from one to 52 weeks.
Study characteristics and risk of bias
The median number of participants per trial was 120 (interquartile range: 51 to 314), and the average age ranged from 20.7 to 33.0 years. Women represented the majority of participants (range 55% to 74.9%; 5 RCTs). Collectively, the included trials compared eight different silicone hydrogel SCLs with three different hydrogel SCLs. Five trials compared daily disposable SCLs, and two compared extended wear SCLs (worn for seven days and six nights). New SCL wearers were enrolled in three trials. Two trials included both new and established SCL wearers, and two trials did not report participants' history of SCL use. Five trials were sponsored by industry. We judged the overall risk of bias to be 'high' or 'some concerns' for the safety and efficacy outcomes.
Findings
One trial reported Ocular Surface Disease Index (OSDI) results, with the evidence being very uncertain about the effects of SCL material on OSDI scores (mean difference −1.20, 95% confidence interval [CI] −10.49 to 8.09; 1 RCT, 47 participants; very low certainty evidence). Three trials reported visual analog scale comfort score results, with no clear difference in comfort between materials, but the evidence was of very low certainty; trial results could not be combined because the three trials reported results at different time points. The evidence is very uncertain about the effect of SCL material on discontinuation of contact lens wear (risk ratio [RR] 0.64, 95% CI 0.11 to 3.74; 1 RCT, 248 participants). None of the included trials reported Contact Lens Dry Eye Questionnaire (CLDEQ‐8) or Standard Patient Evaluation of Eye Dryness (SPEED) scores.
There was no evidence of a clinically meaningful difference (> 0.5 unit) between daily disposable silicone hydrogel and hydrogel SCLs in corneal staining, conjunctival staining, or conjunctival redness (very low certainty evidence).
Adverse events
Very low certainty evidence from two trials comparing daily disposable SCLs suggested no evidence of a difference between lens materials in the risk of vision‐threatening adverse events at one to four weeks (RR 0.68, 95% CI 0.08 to 5.51; 2 RCTs, 368 participants). Two trials comparing extended wear SCLs indicated that hydrogel SCLs may have a 2.03 times lower risk of adverse events at 52 weeks compared with silicone hydrogel SCLs (RR 2.03, 95% CI 1.38 to 2.99; 815 participants), but the certainty of evidence was very low.
Authors' conclusions
The overall evidence for a difference between all included silicone hydrogel and hydrogel SCLs was of very low certainty, with most trials at high overall risk of bias. The majority of studies did not assess comfort using a validated instrument. There was insufficient evidence to support recommending one SCL material over the other. For extended wear, hydrogel SCL may have a lower risk of adverse events at 52 weeks compared to silicon hydrogel. Future well‐designed trials are needed to generate high certainty evidence to further clarify differences in SCL material comfort and safety.
Keywords: Adolescent; Adult; Female; Humans; Young Adult; Contact Lenses, Hydrophilic; Contact Lenses, Hydrophilic/adverse effects; Face; Hydrogels; Patient Reported Outcome Measures; Randomized Controlled Trials as Topic; Silicones
Plain language summary
What are the benefits and harms of silicon hydrogel versus hydrogel soft contact lenses for eye discomfort?
Key messages
1) Silicone hydrogel and hydrogel soft contact lens (SCL) wearers may experience similar eye comfort, but we are very uncertain about the results.
2) In the long term, the risk of experiencing an adverse eye event may be more likely with extended wear silicone hydrogel SCLs than hydrogel SCLs. As above, the small number of studies and limitations in the evidence mean we are very uncertain about the results.
What are silicone hydrogel and hydrogel soft contact lenses?
Contact lenses are often used as an alternative to eyeglasses for vision correction. Unlike eyeglasses, contact lenses are placed directly onto the surface of the eye. SCLs are made from flexible plastics, which are typically made from either silicone hydrogel or hydrogel plastic materials. These materials are porous, with tiny spaces or holes that allow liquid and air to pass through to the surface of the eye. Silicone hydrogel materials are more breathable and have less water content compared with hydrogel SCLs.
How do the two types of SCLs differ in causing eye discomfort?
Contact lenses can block oxygen and change tears on the eye's surface. This can lead to eye irritation, discomfort, and even damage (such as scratches or infections) to the eye surface. The differences in lens materials may result in discomfort and harmful effects.
What did we want to find out?
We wanted to find out if silicone hydrogel SCLs can decrease eye discomfort and complications such as eye infections compared with hydrogel SCLs.
What did we do?
We searched for studies that compared silicone hydrogel SCLs versus hydrogel SCLs in adults 18 years of age or older. We compared and summarized the results of the studies and rated our confidence in the evidence based on study designs and methods.
What did we find?
We found seven studies including a total of 1371 participants who ranged in age between 21 and 33 years, with more women than men participating in the majority of the studies. Three studies enrolled only new SCL wearers; two studies enrolled both new and experienced SCL users; and two studies did not describe the experience level of the participants. Study periods ranged from one week to one year, with most studies lasting three months. Most studies were funded by companies that make SCLs or had authors who were employed by those companies, or both.
When comparing hydrogel and silicone hydrogel SCLs in terms of comfort, the evidence is of very low certainty that one provides better comfort than the other. While the evidence is of very low certainty, hydrogel SCLs may be safer than silicone hydrogel SCLs after one year of wear.
What are the limitations of the evidence?
We have low confidence in the evidence for the safety and comfort of silicone hydrogel versus hydrogel contact lenses. Our confidence was mainly influenced by flawed study design and conduct. It is also possible that people in the studies were aware of which treatment they received, such that the self‐reported comfort level might be biased.
How up‐to‐date is this evidence?
The evidence is current to June 2022.
Summary of findings
Summary of findings 1. Silicone hydrogel versus hydrogel soft contact lenses for differences in patient‐reported comfort and safety.
| Silicone hydrogel versus hydrogel soft contact lenses for differences in patient‐reported comfort and safety | ||||||
|
Patient or population: adults with refractive errors Settings: community private practices or university‐affiliated eye clinics Intervention: silicone hydrogel soft contact lenses (Balafilcon A, Comfilcon A, Delefilcon A, Galyfilcon A, Lotrafilcon A, Lotrafilcon B, Narafilcon A, Senofilcon A) Comparison: hydrogel soft contact lenses (Etafilcon A, Nelfilcon A, Ocufilcon B) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
No. of participants (studies) |
Certainty of evidence (GRADE) |
Comments | |
|
Assumed risk hydrogel |
Corresponding risk silicone hydrogel |
|||||
| Mean change in patient‐reported comfort score from baseline using CLDEQ‐8 | No studies assessed this outcome. | |||||
|
Mean change in patient‐reported comfort score from baseline using OSDI, at 1 month (MD < 0 favored) |
23.44 (SD 17) | 22.24 (12.95 to 31.53) | MD −1.20 (−10.49 to 8.09) |
47 (1 RCT) |
⊕⊝⊝⊝ Very low1,2 |
Results reported at 3 months for this RCT were similar (MD 1.43, 95% CI −8.05 to 10.91). |
|
Mean change in patient‐reported comfort score from baseline using VAS, at 1 week (MD > 0 favored) |
3.35 (SD 1.5) | 3.9 (3.51 to 4.29) | MD 0.55 (0.16 to 0.94) |
240 (1 RCT) |
⊕⊝⊝⊝ Very low3,4 |
VAS 0 to 5; 5 = excellent comfort |
|
Proportion of participants who discontinued contact lens wear, at 1 week (RR < 1 favored) |
25 per 1000 | 16 (3 to 94) per 1000 | RR 0.64 (0.11 to 3.74) |
248 (1 RCT) |
⊕⊝⊝⊝ Very low1,2 |
Pooled results by adding data from 4 other RCTs with follow‐up time up to 52 weeks (total n = 1273) were similar (RR 0.92, 95% CI 0.73 to 1.16). |
|
Corneal staining scores, at 1 week (MD < 0 favored) |
0.56 (SD 0.43) | 0.31 (0.20 to 0.42) | MD −0.25 (−0.36 to −0.14) |
243 (1 RCT) |
⊕⊝⊝⊝ Very low3,4 |
≥ 0.5 units clinically meaningful (Dundas 2001) NEI grading scale5 |
|
Conjunctival staining scores, at 1 month (MD < 0 favored) |
0.3 (SD 0.5) | 0.5 (0.28 to 0.72) | MD 0.20 (−0.02 to 0.42) |
80 (1 RCT) |
⊕⊝⊝⊝ Very low3,4 |
≥ 0.5 units clinically meaningful (Dundas 2001) 5‐point scale6 |
|
Proportion of participants with vision‐threatening adverse events (RR < 1 favored) |
4 weeks |
⊕⊝⊝⊝ Very low1,2 |
||||
| 5 per 1000 | 3 (0 to 28) per 1000 | RR 0.68 (0.08 to 5.51) |
368 (2 RCTs) |
|||
| 3 months | ||||||
| 50 per 1000 | 178 (10 to 1000) per 1000 | RR 3.56 (0.19 to 66.72) |
90 (1 RCT) |
|||
| 52 weeks | ||||||
| 271 per 1000 | 550 (374 to 810) per 1000 | RR 2.03 (1.38 to 2.99) |
815 (2 RCTs) |
|||
| *The basis for the assumed risk is the mean baseline risk from the studies in the meta‐analysis; the total number of events in the control group divided by the total number of participants in the control groups, scaled to 1000. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI, confidence interval; CLDEQ‐8, Contact Lens Dry Eye Questionnaire‐8; MD, mean difference; NEI, National Eye Institute; OSDI, Ocular Surface Disease Index; RCT, randomized controlled trial; RR, risk ratio; SD, standard deviation; VAS, visual analog scale | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded for risk of bias (−1). 2Downgraded for extreme imprecision (−2). 3Downgraded for high risk of bias (−2). 4Downgraded for imprecision (−1). 5NEI grading scale 0 to 3; 0 = normal, 1 = mild, 2 = moderate, 3 = severe; inferior region only. 65‐point scale 0 to 4; 0 = none, 4 = severe; scores were averages of quadrants.
Background
Description of the condition
Description of the contact lens materials
The first contact lenses were manufactured in the late 1800s from ground glass, a material impermeable to oxygen, with severe hypoxia drastically limiting wear times (Jacob 2013). Innovations over the past 100 years or more have resulted in the development of gas‐permeable contact lenses as well as reusable and daily disposable soft contact lens (SCL) materials that are primarily made of either hydrogel or silicone hydrogel polymers (Efron 2015; Jacob 2013). Hydrogel SCLs generally have higher water content and much lower oxygen permeability than silicone hydrogel SCLs. Low oxygen transmissibility is a major contributor to contact lens‐related complications, such as corneal inflammation or neovascularization (Dillehay 2007). Early studies suggested that contact lens materials required oxygen transmissibility of 24.1 × 10‐9 oxygen permeability (Dk)/L to avoid corneal swelling during daily wear, and 87.0 × 10‐9 Dk/L for overnight wear (Holden 1984). No hydrogel contact lenses meet the above oxygen transmissibility requirements for overnight wear, and some do not meet the oxygen requirements for daily wear. Prescribing trends show the frequency of silicone hydrogel SCL prescriptions have increased, while hydrogel SCL prescriptions have declined (Efron 2015). Distributions between SCL materials have remained steady since 2010, with silicone hydrogel and hydrogel SCLs accounting for approximately 70% and 30% of the market share, respectively (Efron 2015).
Epidemiology and wearing patterns of contact lenses
Globally, approximately 140 million people are contact lens wearers, with 90% using SCLs (Markoulli 2017). The USA is considered to be one of the largest markets with an estimated 38.5 million wearers (Efron 2015). Each year, the number of new contact lens users is nearly balanced out by a corresponding number of people who stop using contact lenses ('contact lens dropouts'), prompting numerous studies into potential reasons for discontinuation (Markoulli 2017; Pucker 2020). Despite innovations in contact lens materials over the past 50 years, the top reason for established contact lens wearers to discontinue use is ocular discomfort (Grant 2020; Pucker 2020). Other potential reasons for discontinued use include blurry vision, lack of motivation, and handling issues (Grant 2020; Pucker 2020). Ocular surface health is a concern of contact lens wear, and safety is primarily evaluated by findings such as corneal and conjunctival staining (Markoulli 2017). Meibomian gland health has also been recognized as an important factor associated with contact lens success (Pucker 2019). Compromised ocular surface integrity may promote ocular discomfort (Markoulli 2017). It is unclear whether ocular surface findings differ between hydrogel and silicone hydrogel SCL wearers.
Diagnosis of contact lens discomfort and safety concerns
Information about contact lens comfort is typically collected informally from the wearer during routine contact lens fitting. Clinical evaluation of contact lens fit and tests for dry eye are sometimes used to further elucidate the etiology of discomfort complaints and identify safety concerns related to contact lens wear (Pucker 2019; Young 2002). Anecdotally, standardized patient questionnaires are rarely used to assess contact lens comfort in clinical settings. In contrast, well‐designed research studies usually apply formal questionnaires administered via paper or electronic format to diagnose contact lens discomfort (Pucker 2018). The Tear Film and Ocular Surface Society (TFOS) International Workshop on Contact Lens Discomfort describes several potentially useful symptom‐based questionnaires for measuring patient‐reported ocular comfort (Nichols 2013).
McMonnies pioneered the first ocular surface specific symptom questionnaire, although it is not specific to contact lens use and lacks the ability to evaluate symptom severity (McMonnies 1986; McMonnies 1987). The 8‐item Contact Lens Dry Eye Questionnaire (CLDEQ‐8) has total scores ranging from 1 to 37; it was developed from the much longer CLDEQ, which contains 36 questions with nine subscale scores (Chalmers 2012; Nichols 2002). The CLDEQ‐8 is used primarily to compare baseline scores with change following contact lens refits (Chalmers 2012). A newer tool, the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire, was developed to improve assessment of lid wiper epitheliopathy‐related eye discomfort symptoms (Blackie 2009; Korb 2005; Ngo 2013). The SPEED score is calculated using two subscales of frequency and severity of symptoms; scores range from 0 to 28, with a score of 1 to 9 points being diagnostic of mild to moderate dry eye, and 10 points or more considered severe dry eye (Blackie 2009; Korb 2005; Ngo 2013). Pucker 2018 psychometrically validated the CLDEQ‐8 and SPEED questionnaire for tracking both the severity and frequency of symptoms in contact lens wearers. Another commonly used dry eye symptom questionnaire that is not validated for use in contact lens wearers is the Ocular Surface Disease Index (OSDI) (Schiffman 2000). This questionnaire has three subscales to classify people as having mild, moderate, or severe forms of dry eye, on a scale from 0 to 100 (Schiffman 2000). Visual analog scales (VAS) are also commonly used to assess eye discomfort with a capped continuous linear scale.
Description of the intervention
It is possible to manage refractive ametropias (i.e. myopia, hyperopia, astigmatism, and presbyopia) using non‐invasive or invasive options, or a combination of options. The most common ones include spectacles, contact lenses, and refractive surgery. Contact lens options include rigid, corneal, gas‐permeable, soft, hybrid (rigid, gas‐permeable optic zone with a soft skirt to support the optic zone), and scleral lens designs. Occasionally, a SCL will be used under a rigid, corneal, gas‐permeable contact lens to improve comfort or centration, or both (Jacobs 2021). Of the contact lens options, SCLs are by far the most commonly prescribed (Efron 2015).
SCLs can be broadly divided into either poly‐2‐hydroxyethyl methacrylate (poly‐HEMA; hydrogel) or siloxane‐based materials (silicone hydrogel). The combination of two hydrogel copolymers led to the development of the first spin cast hydrogel SCL (Key 2007; Wichterle 1960; Wichterle 1961), and US Food and Drug Administration (FDA) approval in 1971. Hydrogel SCLs improved comfort over rigid contact lens designs by significantly lowering the modulus of elasticity and providing whole corneal coverage, while reducing manufacturing costs (Jacob 2013). The demand for overnight wear began in England in the late 1970s (Carle 1972), and for continuous (30 nights) wear in the USA in 1981 (Nicolson 2001). Continuous wear was rescinded by the FDA in 1989 in response to findings of increased risks of ulcerative keratitis (Poggio 1989). After the introduction of siloxane‐based materials in 1999, continuous wear options became available again (Sankaridurg 2013). Further refinements were attempted to address comfort, vision, and safety issues, including adverse physiological events, oxygen permeability, deposit formation, and solution‐related issues (Cho 2013; Covey 2001). Siloxane‐based polymers released in the late 1990s were developed to address the comfort, lower modulus, and wettability issues encountered with dimethylsiloxane, while affording higher oxygen permeability than hydrogels (Morgan 2010). This led to a proliferation of research into bulk and surface properties across all wearing and replacement modalities without adequately addressing causes of physiological adverse events (Chalmers 2015). Instead, the novel material or design has introduced other complications due to mechanical interactions (e.g. silicone hydrogel tend to have a higher modulus) with the ocular surface (Sankaridurg 2013). However, the improvements in oxygen permeability did not alter rates of microbial keratitis, Alipour 2017; Diec 2018; Holden 2003; Lim 2018; Stapleton 2013; Sweeney 2013, or other adverse events, such as increased inflammatory events (Richdale 2016; Szczotka‐Flynn 2014), surface deposits (Millar 2003; Nichols 2013), or undesirable contact lens‐solution interactions (Diec 2013; Lazon de la Jara 2013). Multifactorial comfort issues remain (Guillon 2013; Lin 2013; Stapleton 2017; Varikooty 2013).
How the intervention might work
The tear film is a 2‐ to 5‐micrometer thick layer of fluid that covers the ocular surface, hydrates the eye, and covers the irregularly shaped corneal surface; this smooth interface with the external world allows for comfortable, clear, and crisp vision (Bai 2018; Holden 2016; Maurice 1990; Szczesna 2006; Wang 2006). As described above, SCLs are commonly used to correct refractive error. When a contact lens is applied to the eye, it splits the tears into two layers, a pre‐lens tear film and a post‐lens tear film (Nichols 2003). This destabilizes the tears and may result in evaporation, associated with characteristic symptoms associated with contact lens discomfort such as dryness and burning sensation (Begley 2000; Efron 1991). Contact lens discomfort may also stem from inherent individual factors such as age, contact lens care systems, or contact lens materials/designs (Nichols 2013). While the introduction of silicone hydrogel SCL materials was intended to solve many of the contact lens‐related issues, it is unclear whether silicone hydrogel SCLs result in better ocular health and comfort than hydrogel SCLs (Guillon 2013; Stapleton 2017).
Why it is important to do this review
Unanswered research questions regarding self‐reported SCL comfort and safety remain (Doughty 1997). Discontinuation of contact lens wear is most frequently attributed to discomfort. Many people would still prefer contact lens wear over other vision correction modalities if comfort issues were resolved (Dumbleton 2013; Pritchard 1999; Richdale 2007). Globally, it is estimated that up to 30% of established contact lens wearers permanently discontinue lens wear because of ocular discomfort (Pucker 2020; Rumpakis 2010; Young 2002). While the comfort and safety of current contact lens designs have improved, the full etiology of contact lens discomfort remains largely unresolved, and conflicting results regarding safety and efficacy of various SCLs remain (Nichols 2013). Understanding the differences between silicone hydrogel and hydrogel SCL materials will therefore help doctors and contact lens wearers make informed decisions about SCL selection.
Objectives
To evaluate the comparative effectiveness and safety of silicone hydrogel compared with hydrogel SCLs on self‐reported comfort, dry eye test results, and adverse events in SCL‐wearing adults 18 years of age or older.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐RCTs. We included cross‐over RCTs where the reported conduct and analysis accounted for the study design (see Differences between protocol and review).
Types of participants
We included trials that enrolled adults (age 18 years and over). We imposed no restrictions based on race, ethnicity, or gender.
Types of interventions
We included trials that compared hydrogel and silicone hydrogel SCLs, worn for vision correction as daily disposable, daily wear, extended wear, or continuous wear modalities.
Types of outcome measures
Primary outcomes
Critical outcome
Mean change from baseline in patient‐reported comfort score measured using the Contact Lens Dry Eye Questionnaire‐8 (CLDEQ‐8) at one to four weeks (Chalmers 2012).
Secondary outcomes
Important outcomes
We assessed the following patient‐reported comfort scores as important outcomes, measured as the mean change from baseline to follow‐up at one to four weeks:
Ocular Surface Disease Index (OSDI) scores (Schiffman 2000);
Standard Patient Evaluation of Eye Dryness (SPEED) scores (Blackie 2009);
visual analog scale (VAS) scores.
If the included trial did not report mean change from baseline, we used the patient‐reported comfort scores at one to four weeks. If a trial reported multiple measurements during the one‐to‐four‐week time period, we used data at the longest follow‐up. If the included trial assessed comfort in multiple ways (e.g. comfort on insertion, comfort at the end of the day, comfortable wearing time, overall comfort), we prioritized overall comfort.
Adverse events
We assessed adverse events at one to four weeks. Additionally, we included vision‐threatening adverse events at the furthest time point (see Differences between protocol and review):
proportion of participants who discontinued contact lens wear;
corneal staining scores, as assessed by any integer grading scale (e.g. Cornea and Contact Lens Research Unit (CCLRU), Terry 1993, or Oxford, Bron 2003, scales);
conjunctival staining scores, as assessed by any integer grading scale (e.g. CCLRU, Terry 1993, or National Eye Institute/Industry, Lemp 1995, scales);
conjunctival redness scores, as assessed by any integer grading scale (e.g. Efron, Efron 2001, or McMonnies and Chapman‐Davies, McMonnies 1987, scales);
proportion of participants who had vision‐threatening adverse events (e.g. microbial keratitis).
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases without restrictions on language or date of publication. The most recent database search was on 24 June 2022.
Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register; 2022, Issue 6) in the Cochrane Library (Appendix 1).
MEDLINE Ovid (1946 to 24 June 2022) (Appendix 2).
Embase.com (1947 to 24 June 2022) (Appendix 3).
PubMed (1948 to 24 June 2022) (Appendix 4).
LILACS (Latin American and Caribbean Health Science Information database) (1982 to 24 June 2022) (Appendix 5).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/clinical-trials-registry-platform) (Appendix 7).
Searching other resources
We searched the reference lists of included studies, review articles, and guidelines for information about relevant studies that may not have been identified by our search strategy. We imposed no restriction on language or date of publication. We also contacted experts in the field regarding information about ongoing trials on silicone hydrogel and hydrogel SCLs.
Data collection and analysis
Selection of studies
The Cochrane Eyes and Vision Information Specialist removed duplicate records prior to our uploading the unique records into Covidence, the internet‐based review management software we used for screening and data extraction (Covidence). Two review authors (two of KH, DT, LL, DF, ADP) independently screened the titles and abstracts for eligible studies, resolving any disagreements by discussion. We retrieved the full‐text reports for relevant or possibly relevant records. Two review authors (two of KH, DT, LL, DF, ADP) independently reviewed the full‐text reports for eligibility, resolving any disagreements by discussion. We contacted study investigators to obtain further information when necessary to determine study eligibility; if they did not respond within two weeks, we used the information available from the reports. We recorded the reasons for exclusion of all full‐text reports in the Characteristics of excluded studies section. We assessed eligible trials in progress as ongoing, and trials with missing results as awaiting classification.
Data extraction and management
Two review authors (two of KH, DT, LL, DF, ADP) independently extracted data using the data collection form in Appendix 8 and Covidence software (Covidence). One review author (LL) exported data from Covidence into Review Manager Web (RevMan Web 2022), and a second review author (ADP) then verified all data entries to ensure that data were consistent and error‐free. We extracted the following information: study setting, countries where participant recruitment took place, study design, sample size, study duration (planned and actual), participants, interventions, comparators, outcomes, sources of funding, and potential conflicts of interests. We collected and used the most detailed numerical data available from the included studies to facilitate analysis. We contacted study investigators and organizations to obtain missing or unclear information. If the investigators did not respond within two weeks, we proceeded with the existing information. Where data were only available in graphical displays, two review authors independently extracted the data electronically using browser‐based data extraction software (WebPlotDigitizer). In the case of discrepancies in data extraction, a consensus was reached through discussion or by consulting a third review author.
Assessment of risk of bias in included studies
We planned to assess risk of bias for two review outcomes: change from baseline in patient‐reported comfort score measured using the CLDEQ‐8 and proportion of participants who had vision‐threatening adverse events (Haworth 2021). Given that no included trial reported CLDEQ‐8 scores, we decided to include VAS scores for bias assessment. Two review authors (two of KH, DT, LL, DF, ADP) independently assessed risk of bias for the effect of assignment to interventions using the RoB 2 tool, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). We used the RoB 2 Excel tool and 2019 guidance (available at www.riskofbias.info/).We considered the following domains of bias:
bias arising from the randomization process;
bias from deviations from intended interventions;
bias from missing outcome data;
bias in measurement of the outcome, which will include a two‐day minimum wash‐out period for cross‐over studies;
bias in selection of the reported result.
We evaluated the risk of bias in every bias domain as well as an overall risk of bias as 'low risk,' 'high risk,' or 'some concerns'; the assessment of each domain was guided by signaling questions.
For an overall risk of bias judgment, we considered a study to have:
low risk if it is of low risk of bias for all domains for this result;
some concerns if the trial is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain;
high risk if the trial is judged to be at high risk of bias in at least one domain, or to have some concerns for multiple domains such that confidence in the result is substantially lowered.
In case of disagreement or discrepancy between review authors, a third review author adjudicated the risk of bias assessment.
Measures of treatment effect
For continuous outcomes measured using the same scales, we assessed the normality of distributions and calculated mean differences (MDs) with 95% confidence intervals (CIs) where outcomes were normally distributed. Where trials measured continuous outcomes using difference scales, we calculated standardized mean differences (SMDs), interpreting them as follows: 0.2 for small, 0.5 for moderate, and 0.8 for large effects (Cohen 1988). Continuous outcomes for this review included CLDEQ‐8, OSDI, SPEED, and VAS scores. We calculated risk ratios (RR) with 95% CIs for dichotomous outcomes. We considered the proportion of participants with an adverse event to be a dichotomous outcome. We checked data for skewness and analyzed skewed data using the guidance outlined in Chapter 9 of the Cochrane Handbook (Deeks 2022).
Unit of analysis issues
The participant was the primary unit of analysis. If a trial randomized both eyes of participants (to the same or different interventions), we extracted the results that accounted for the correlation between eyes and referred to Chapter 23 of the Cochrane Handbook for guidance regarding including variants of randomized trials (Higgins 2022b). In the protocol, we planned to perform sensitivity analysis by excluding studies that failed to consider the correlation between two eyes. Given that none of the paired‐eye studies reported usable data for qualitative or quantitative synthesis, we excluded those studies from data extraction. For included studies with more than two groups, we evaluated each relevant comparison separately and selected one pair‐wise comparison that was relevant to the review to avoid double counting the studies in the analysis (Higgins 2022b).
Dealing with missing data
We analyzed outcomes on an intention‐to‐treat basis. If we received no response from investigators within two weeks, we proceeded with the best information available for analysis. We did not impute missing data for the purposes of this review.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by examining differences in participants, interventions (silicone hydrogel versus hydrogel), and aspects of study design. We assessed statistical heterogeneity by examining the overlap in CIs of forest plots, and by using the Chi2 and I2 statistics to determine the proportion of total variation due to statistical heterogeneity, as described in Chapter 10 of the Cochrane Handbook (Deeks 2022). We considered the following thresholds for the interpretation of the I2 statistic:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We assessed selective outcome reporting for each trial by comparing the outcomes specified in a protocol or clinical trial registry with the final report. Where trial protocols or trial registry records were unavailable or inaccessible, we compared outcomes specified in the methods section of the study reports with outcomes reported in the study. We did not assess publication bias by funnel plots as there were too few trials for meaningful analysis.
Data synthesis
We synthesized and analyzed data following the guidelines in Chapter 9, McKenzie 2022, and Chapter 10, Deeks 2022, of the Cochrane Handbook. When more than two studies contributed data to a meta‐analysis or there was statistical or clinical heterogeneity, we used a random‐effects model to estimate intervention effects; otherwise we used a fixed‐effect model. If the direction of treatment effects was inconsistent across studies, or we detected the presence of substantial or considerable statistical heterogeneity, we did not combine results in a meta‐analysis and presented a narrative summary of results instead.
Subgroup analysis and investigation of heterogeneity
We planned to conduct a subgroup analysis based on SCL replacement frequency and material subtypes of SCLs. However, we did not perform either subgroup analysis due to the limited number of included trials.
Sensitivity analysis
To assess the robustness of the effect estimates, we planned sensitivity analysis by excluding studies with high risk of bias, industry‐funded studies, and studies that did not address the unit of analysis issue properly.
Summary of findings and assessment of the certainty of the evidence
We prepared a summary of findings table according to the methods described in Chapter 14 of the Cochrane Handbook (Schünemann 2022a), and presented the estimated effects of silicone hydrogel versus hydrogel SCLs at one to four weeks. We included the following outcomes:
comfort scores measured using CLDEQ‐8;
comfort scores measured using OSDI scores;
comfort scores measured using VAS scores;
proportion of participants who discontinued contact lens wear;
corneal staining scores;
conjunctival staining scores;
proportion of participants with vision‐threatening adverse events.
Two review authors (two of KH, DT, LL, DF, ADP) independently judged the certainty of the evidence for each outcome using the GRADE approach. We judged the certainty of evidence as 'high,' 'moderate,' 'low,' or 'very low' by considering (1) high risk of bias; (2) indirectness of evidence; (3) unexplained heterogeneity or inconsistency of results; (4) imprecision; (5) high probability of publication bias (Langendam 2013), and the contextualized certainty of evidence (Schünemann 2022b). Any disagreements between review authors were resolved by discussion or consultation with a third review author. We applied study‐level risk of bias assessments, based on responses to signaling questions in domains 1 to 3 of the RoB 2 tool, to judge the risk of bias when we graded outcomes not listed in the Assessment of risk of bias in included studies section.
Results
Description of studies
Results of the search
Our first search of the electronic databases was conducted in July 2021 and yielded 7096 records. We found two additional records by searching the reference lists of included studies. We performed an additional search in June 2022, which identified a further 331 records.
In total, we screened 5536 unique titles and abstracts and retrieved 111 full‐text reports from 86 studies. After full‐text screening, we included 7 studies (10 reports) (see Characteristics of included studies) (Figure 1); listed 18 studies (20 reports) as awaiting classification (see Characteristics of studies awaiting classification); and excluded 61 studies (81 reports) with reasons (see Characteristics of excluded studies).
1.
Study flow diagram.
Included studies
Types of studies
Study design
The seven included trials were parallel‐group RCTs that masked participants alone (Hall 2009), investigator alone (NCT00762788), or both (NCT00241280). Two trials applied open‐label assignment when dispensing the SCLs (Diec 2012; NCT01354223). Two trials did not report whether they masked the participants or the investigators for outcome assessment (Marx 2009; Muhafiz 2019). Four trials randomized individual participants to two SCL groups (Hall 2009; Marx 2009; NCT00241280; NCT01354223); two trials randomized participants to three SCL groups (Diec 2012; Muhafiz 2019); and one trial randomized participants to six SCL groups (NCT00762788). One arm of the Muhafiz 2019 trial used a different care system, therefore only two arms of the Muhafiz 2019 trial were included in the review.
Setting
Three of the seven trials were conducted in multiple countries, including Germany (Marx 2009), Turkey (Muhafiz 2019), and the UK (Hall 2009). Two trials were conducted in the USA (NCT00241280; NCT01354223), one in India (NCT00762788), and one in Australia (Diec 2012). The majority of the trials enrolled participants at multiple sites, ranging from two sites to 25 sites (NCT00241280).
Funding
Most included trials reported industry sponsorship. One was sponsored by CooperVision (NCT01354223), three by Johnson & Johnson Vision Care (Hall 2009; NCT00241280; NCT00762788), and one by Alcon (Diec 2012). One meeting abstract did not report funders, though co‐authors reported industry affiliations with CIBA Vision (Marx 2009). Two trials were sponsored by non‐industry sources, one by an academic institution (Muhafiz 2019), and one by the Brien Holden Vision Institute, a nonprofit organization (Diec 2012).
Types of participants
A total of 1371 participants were enrolled in the included trials, with a median number of 120 participants (interquartile range: 51 to 314) per trial. The average age of the trial populations ranged from 20.7 to 33.0 years, with women representing the largest proportion in five trials that reported a gender distribution (per cent women range: 55% to 75%). Only one trial reported the study participants were predominantly "White" or "Caucasian" (NCT00241280); the remaining trials did not provide this information.
Three trials specifically enrolled new SCL wearers (Marx 2009; Muhafiz 2019; NCT01354223), whereas another two trials included both new and experienced SCLs wearers (Diec 2012; Hall 2009). Two trials did not report the SCL‐wearing experiences of the study population (NCT00241280; NCT00762788).
Types of interventions
The included trials compared a total of eight different silicone hydrogel SCLs with three hydrogel SCLs. The most frequently used silicone hydrogel SCL was Senofilcon A, Diec 2012; NCT00762788; NCT01354223, and Narafilcon A, Diec 2012; Hall 2009; Marx 2009; other silicone hydrogel SCLs included Delefilcon A (Muhafiz 2019), Galyfilcon A (NCT00241280), Lotrafilcon A (NCT00762788), Lotrafilcon B (NCT00762788), Balafilcon A (NCT00762788), and Comfilcon A (NCT00762788). The most commonly used hydrogel SCL was Etafilcon A (Diec 2012; Muhafiz 2019; NCT00241280; NCT00762788), followed by Nelfilcon A (Hall 2009; Marx 2009), and Ocufilcon B (NCT01354223). In two trials, all treatment arms used continuous (or extended) wear SCLs, which were worn for seven days and six nights before replacement (NCT00241280; NCT00762788); the other five trials only compared daily disposable SCLs. The duration of the intervention ranged from one week to 52 weeks, with three months being the most common study duration (n = 3).
Types of outcomes
Critical outcomes
CLDEQ‐8 scores
No included trials reported this outcome.
Important outcomes
OSDI scores
Only Muhafiz 2019 measured OSDI scores. Applying repeated‐measure analysis, the authors correctly accounted for within‐person comparisons of OSDI scores over time within the same group, but they did not perform or report comparisons between participants wearing silicone hydrogel SCLs versus those wearing hydrogel SCLs. We extracted raw OSDI scores at each time point (baseline, one month, three months) for each group from Figure 1 of the publication (Muhafiz 2019).
SPEED scores
No included trials reported this outcome.
VAS scores
Three trials employed questionnaires to collect patient‐reported discomfort symptoms on different VAS systems (Diec 2012; Hall 2009; NCT01354223). In Diec 2012, 120 participants, 80 in two groups using silicone hydrogel SCLs and 40 in one group using hydrogel SCLs, were asked to rate their overall comfort level on a 1‐to‐100 numeric scale over the three‐month study period at two weeks, one month, and three months. Investigators of another trial asked 248 participants to rate their overall comfort on a 5‐point scale (1 to 5) at the end of the one‐week trial (Hall 2009).
In contrast, investigators in NCT01354223 employed a questionnaire of 0‐to‐4 scale to assess participants' symptoms of eye discomfort (0 = no discomfort, 4 = severe discomfort) and reported percentages of eyes with no symptoms of discomfort at each trial visit at week one and two and month one and two.
Discontinuation of contact lens wear
Five of the seven trials reported this outcome; two did not (Marx 2009; Muhafiz 2019). None of the included trials specified discontinuation of SCL wear as a trial outcome. We chose to treat trial participants who dropped out as having discontinued SCL wear, rather than imputing outcomes from the reasons that were provided for which they left the trial (see Differences between protocol and review).
Corneal staining
Only Hall 2009 examined study participants at baseline and after one week of wear, assessing the degree of corneal staining based on the National Eye Institute (NEI) 0‐to‐3 scale for the inferior region only. The staining procedure and dye used were not reported.
Conjunctival staining
Diec 2012 measured conjunctival staining, divided into quadrants, using the Cornea and Contact Lens Research Unit (CCLRU; now known as Brien Holden Vision Institute grading scale) 0‐to‐4 grading scale. The scale used 0.1 increments, a higher score reflecting a more severe condition (Terry 1993). The staining procedure and name of dye were not reported. Assessments were made at two weeks, one month, and three months; only the one‐month results were reported.
Conjunctival redness
Investigators of one trial presented only the mean results (overall postbaseline visits) of limbal redness by quadrants (inferior, superior, nasal, temporal) in a figure, from which we extracted data for the nasal and temporal quadrants for meta‐analysis (Diec 2012). The investigators did not report data for bulbar conjunctival or palpebral conjunctival redness, although the methods section of the published article stated that these outcomes had been assessed (Diec 2012). The authors of another trial reported limbal redness scores based on an unspecified 5‐point scale (0 to 4, half‐grade increments; higher is worse) after one week of wear (Hall 2009).
Vision‐threatening adverse events
The authors of one trial did not report adverse events (Marx 2009). Hall 2009 reported adverse events as an outcome on the trial registration site and separately as a reason for leaving the trial. We included those leaving the trial due to adverse events (see Differences between protocol and review).
Among five trials that reported serious or non‐serious ocular adverse events (Diec 2012; Hall 2009; NCT00241280; NCT00762788; NCT01354223), three specified a reporting threshold at 5% (Hall 2009; NCT00241280; NCT01354223); NCT00762788 reported anything that was not 0%; and Diec 2012 did not provide this information.
Two of the five trials reported this outcome at one week (Diec 2012; Hall 2009), while the other three trials reported at three months, NCT01354223, or 52 weeks, NCT00241280; NCT00762788. Of note, the two trials reporting outcomes at 52 weeks were of extended wear SCLs (NCT00241280; NCT00762788)
Excluded studies
Of the 61 excluded studies, 16 compared SCLs of the same material (26%); six tested prototype SCLs (10%); seven examined different SCL materials, SCL design, replacement schedules, varying duration of SCL wear, or other conditions at the same time (11%); six trials lasted shorter than a week (10%); six trials were non‐RCTs (10%); and five trials included children or adolescents younger than 18 years old (8%). One cross‐over trial (2%) used a paired‐eye design and exposed both comparison groups to both silicone hydrogel and hydrogel SCL in the two intervention periods (Arroyo‐Del Arroyo 2021). Additionally, we excluded 14 trials that did not apply appropriate analysis to account for the cross‐over of the same participants (N = 10) or pairing of eyes (N = 4) in the study design (23%).
Ongoing studies and studies awaiting classification
We categorized 18 studies of either cross‐over or paired‐eye design as awaiting classification. These studies did not analyze or report comparative results according to their trial design such that there was no usable data for the purpose of evidence synthesis (see Studies awaiting classification). We did not identify any eligible studies that were ongoing (Figure 1).
Risk of bias in included studies
We planned to assess risk of bias for the outcomes comfort scores measured by CLDEQ‐8 and proportion of participants with vision‐threatening adverse events (Haworth 2021). Given that none of the included trials measured participants' comfort using CLDEQ‐8, we chose to evaluate risk of bias for comfort scores measured by VAS instead (see Differences between protocol and review). Furthermore, the small number of included studies precluded sensitivity analysis (see Differences between protocol and review).
Of the seven included trials, three reported VAS comfort scores (Diec 2012; Hall 2009; NCT01354223), and five reported ocular adverse events (Diec 2012; Hall 2009; NCT00241280; NCT00762788; NCT01354223).
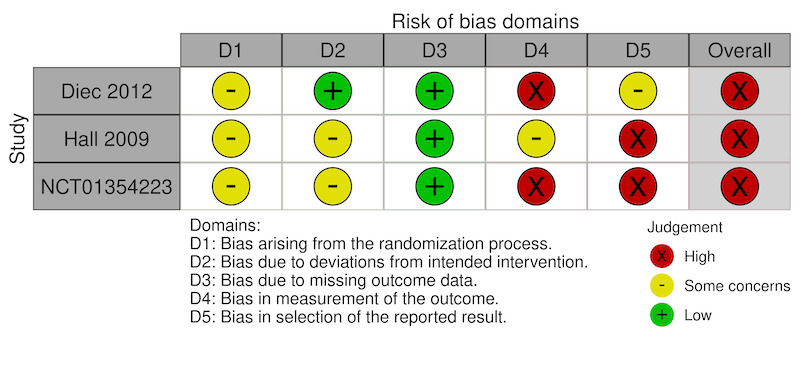
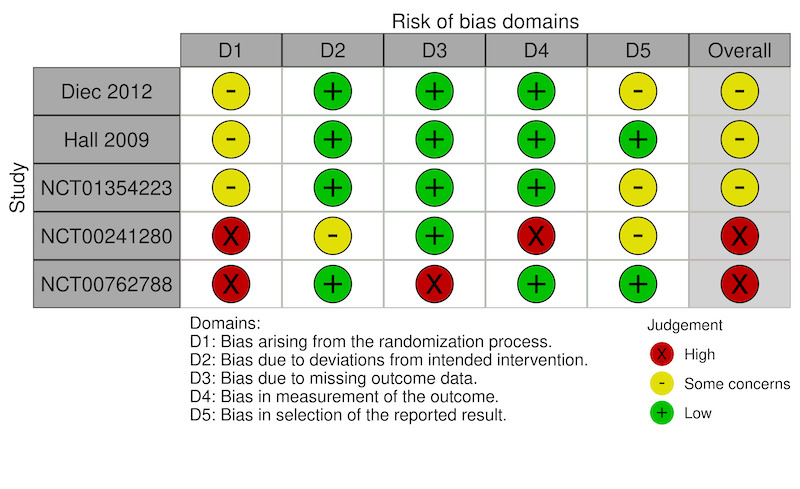
We judged the overall risk of bias as high for all three trials reporting VAS comfort scores (Figure 2). We judged the overall risk of bias as high for two trials and some concerns for three trials reporting adverse events (Figure 3).
2.
Risk of bias summary: comfort scores measured using a visual analog scale.
3.
Risk of bias summary: proportion of participants with vision‐threatening adverse events.
Domain 1: Bias arising from the randomization process
We judged the risk of bias for five of the seven trials as some concerns for this domain due to lack of information on the method of allocation concealment. We judged two trials, both of extended wear SCL, as having a high risk of bias due to substantial dropouts after randomization in one trial (NCT00762788), and large differences in the number of participants randomized to each group in the other trial (NCT00241280).
Domain 2: Bias arising from deviations from intended interventions
VAS comfort scores
We assessed two of the three trials that reported VAS comfort scores as having some concerns because of inadequate reporting of participant masking, although there was no evidence of potential deviations from the intended intervention (Hall 2009; NCT01354223). We judged the third trial as at low risk of bias for this domain (Diec 2012).
Vision‐threatening adverse events
We judged all five trials as at low risk of bias for this domain.
Domain 3: Bias due to missing outcome data
VAS comfort scores
We judged all three trials as at low risk of bias for this domain.
Vision‐threatening adverse events
We judged one trial as at high risk of bias due to substantial missing outcome data (NCT00762788). We judged the other trials as at low risk of bias for this domain.
Domain 4: Bias in outcome measurement
VAS comfort scores
We judged two open‐label (no masking) trials as at high risk of bias for this domain (Diec 2012; NCT01354223). We judged the risk of bias as some concerns for one trial that masked participants ('single‐masked')(Hall 2009).
Vision‐threatening adverse events
We judged all five trials as at low risk of bias for this domain. Although investigators in only two trials (both of extended wear SCL) were masked to the intervention received by participants (NCT00241280; NCT00762788), the severity of the outcome reduced the risk of biased assessments.
Domain 5: Bias in selective reporting of outcome data
VAS comfort scores
We judged two trials as at high risk of bias for this domain (Hall 2009; NCT01354223). One trial reported inconsistent data between the meeting abstract and the trial registry website (Hall 2009), whereas another presented the VAS scores in an unusual manner, as percentages of eyes "with no discomfort"(NCT01354223).
We judged the third trial as having some concerns for this domain (Diec 2012). Although the authors correctly accounted for repeated measurements using appropriate statistical models, they presented the postintervention scores as period averages (across all follow‐up visits), rather than as endpoint values or changes from baseline (Diec 2012).
Vision‐threatening adverse events
We judged three of the five trials as having some concerns because of a reporting threshold of 5% for adverse events. We judged two trials as at low risk of bias for this domain (Diec 2012; NCT00762788).
Effects of interventions
See: Table 1
Critical outcomes
CLDEQ‐8 scores
No included trials reported CLDEQ‐8 scores.
Important outcomes
OSDI scores
Only one trial reported this outcome (Muhafiz 2019). The single‐trial estimate for the mean difference (MD) in OSDI scores at one month was MD −1.20 (95% confidence interval (CI) −10.49 to 8.09; 47 participants; Figure 4), suggesting no evidence of differences in patient‐reported comfort between participants wearing silicone hydrogel and those wearing hydrogel SCLs. The trial also reported similar findings at postintervention three months (MD 1.43, 95% CI −8.05 to 10.91; 47 participants; Analysis 1.1); we did not combine data reported at the two time points as the protocol only specified up to week four for this important outcome (Haworth 2021). The certainty of the evidence was very low, downgraded for risk of bias (−1) and extreme imprecision (−2).
4.

Forest plot of silicon hydrogel SCL versus hydrogel SCL, outcome 1.1: OSDI scores.
1.1. Analysis.

Comparison 1: Silicone hydrogel versus hydrogel, Outcome 1: OSDI scores
SPEED scores
No included trials reported SPEED scores.
VAS scores
Hall 2009 reported different comparison results in VAS scores at one week between the meeting abstract (MD 0.43, 97.5% CI 0.10 to 0.76; Hall 2009) and the trial registration (MD 0.55, 97.5% CI 0.17 to 0.55; 240 participants; NCT00727558). Based on their original VAS scoring system (0 to 5; 5 = "excellent"), the single‐study estimate suggested a small improvement in overall comfort in silicone hydrogel SCL wearers compared to hydrogel SCL wearers after one week of wearing SCLs (MD 0.55, 95% CI 0.16 to 0.94; 240 participants; Figure 5).
5.

Forest plot of silicon hydrogel SCL versus hydrogel SCL, outcome 1.2: VAS scores.
The authors of Diec 2012 also reported overall comfort scores at three months using a different VAS scale (1 to 100; 100 = "extremely comfortable"). The study indicated no evidence of a difference in comfort when comparing silicone hydrogel to hydrogel SCLs (MD −1.00, 95% CI −5.19 to 3.19; 120 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: Silicone hydrogel versus hydrogel, Outcome 2: VAS scores
A third trial reported the percentage of eyes "with no symptoms of discomfort" at one month (NCT01354223). The authors reported that hydrogel SCLs were favored (55.4% in 114 unique eyes) over silicone hydrogel SCLs (60.3% in 58 unique eyes), although there was no evidence of a statistical difference (post hoc P = 0.539).
The certainty of the evidence was very low, downgraded for risk of bias (−2) and imprecision (−1).
Discontinuation of contact lens wear
A single trial reported participants lost to follow‐up at one week (Hall 2009). The single‐study estimate indicated little to no differences in discontinuation rates between the two groups (risk ratio (RR) 0.64, 95% CI 0.11 to 3.74; 248 participants; Figure 6). Another four trials also reported participants that were lost to follow‐up or withdrawal before the end of the trial at three months, Diec 2012; NCT01354223 or 52 weeks, NCT00241280; NCT00762788. There was no evidence of differential discontinuation rates by SCL material at either time point (Analysis 1.3).
6.

Forest plot of silicon hydrogel SCL versus hydrogel SCL, outcome 1.3: discontinuation of contact lens wear.
1.3. Analysis.

Comparison 1: Silicone hydrogel versus hydrogel, Outcome 3: Discontinuation of contact lens wear
The certainty of the evidence was very low, downgraded for risk of bias (−1) and extreme imprecision (−2).
Corneal staining
One trial reported corneal staining scores based on the NEI 0‐to‐3 scale in the trial registry online (MD −0.25, 97.5% CI −0.25 to −0.16; 243 participants; NCT00727558), but did not report results in the meeting abstract (Hall 2009). According to this single‐trial estimate, silicone hydrogel SCLs appeared to improve corneal staining scores when compared with hydrogel SCLs at one week (MD −0.25, 95% CI −0.36 to −0.14; 243 participants; Analysis 1.4).
1.4. Analysis.

Comparison 1: Silicone hydrogel versus hydrogel, Outcome 4: Corneal staining scores
The certainty of the evidence was very low, downgraded for risk of bias (−2) and imprecision (−1).
Conjunctival staining
One included trial reported conjunctival staining based on the CCLRU 0‐to‐4 scale at one month (Diec 2012). The authors compared two groups of participants (40 in each group, 80 total) wearing silicone hydrogel SCLs with those wearing hydrogel SCLs (n = 40), but only reported this outcome comparing one silicone hydrogel group versus the hydrogel group. The single‐study estimate suggested no differences in conjunctival staining scores (MD 0.20, 95% CI −0.02 to 0.42; 80 participants; Analysis 1.5).
1.5. Analysis.

Comparison 1: Silicone hydrogel versus hydrogel, Outcome 5: Conjunctival staining score
The certainty of the evidence was very low, downgraded for risk of bias (−2) and imprecision (−1).
Conjunctival redness scores
Two trials reported conjunctival redness scores at one week and up to three months, respectively (Diec 2012; Hall 2009).
Hall 2009 reported limbal conjunctival hyperemia at one week, measured in half‐grade increments on a 0‐to‐4 scale. Although the reported results were inconsistent between the meeting abstract (MD −0.17, 97.47% CI −0.24 to −0.10; Hall 2009) and the trial registration (MD −0.17, 97.47% CI −0.17 to −0.10; NCT00727558), the single‐study estimate and the calculated 95% CI showed a small reduction in limbal redness score comparing silicone hydrogel SCLs to hydrogel SCLs at one week (MD −0.18, 95% CI −0.33 to −0.03; 243 participants; Analysis 1.6).
1.6. Analysis.

Comparison 1: Silicone hydrogel versus hydrogel, Outcome 6: Conjunctival redness scores
Diec 2012 presented a figure of the three‐month average of all postbaseline assessments. Results reported for nasal and temporal conjunctival redness scores were similar between groups: nasal MD −0.10 (95% CI −0.16 to −0.04) and temporal MD −0.20 (95% CI −0.32 to −0.08; 120 participants; Analysis 1.6).
The certainty of the evidence was very low, downgraded for risk of bias (−2) and imprecision (−1).
Vision‐threatening adverse events
None of the included trials specifically reported adverse events that were vision threatening, therefore we chose to include potentially vision‐threatening ocular complications based on the reported data.
The investigators of one trial, Hall 2009, reported on the online trial registry that none of the 248 participants experienced "serious adverse events," although there was one participant in the hydrogel group who discontinued with the reason "adverse event" given (NCT00727558). Assuming the participant's complication was severe enough to drop out of the trial, we included this incident for the current analysis. In Diec 2012, the authors reported one case of infiltrative keratitis in the silicone hydrogel group at one month (n = 80). The combined estimate for the associated risk up to four weeks suggested no evidence of differences when comparing silicone hydrogel SCL with hydrogel SCL (RR 0.68, 95% CI 0.08 to 5.51; 2 RCTs, 368 participants; Figure 7).
7.

Forest plot of silicon hydrogel SCL versus hydrogel SCL, outcome 1.7: proportion of vision‐threatening adverse events.
One trial that followed participants over three months reported no "serious adverse events" in either comparison group (NCT01354223); there were three cases of papillary conjunctivitis observed in the silicone hydrogel group (RR 3.56, 95% CI 0.19 to 66.72; 90 participants; Analysis 1.7).
1.7. Analysis.

Comparison 1: Silicone hydrogel versus hydrogel, Outcome 7: Proportion of vision‐threatening adverse events
Two trials that followed 815 participants for up to 52 weeks compared extended wear hydrogel and silicon hydrogel SCLs. Both trials listed serious and non‐serious adverse events that could be considered as potentially vision threatening (NCT00241280; NCT00762788). The investigators of one trial reported cases of non‐serious ocular events (NCT00241280), including conjunctivitis and contact lens peripheral ulcers, in the silicone hydrogel (n = 43) and hydrogel groups (n = 23). The investigators of another trial noted two cases of microbial keratitis in the silicone hydrogel group along with an additional 105 cases of ocular adverse events (silicone hydrogel n = 95; hydrogel n = 8) (NCT00762788), including contact lens peripheral ulcer, significant infiltrative event, new corneal scar, slit lamp finding of Grade 1 or 2 corneal lesions requiring treatment, and other non‐specified significant adverse events. Over the 52 weeks during which participants were followed, the combined estimate RR was 2.03 (95% CI 1.38 to 2.99; 2 RCTs, 815 participants; I2 = 0; Figure 7), suggesting that silicone hydrogel SCLs may increase the risk of potentially vision‐threatening adverse events compared with hydrogel SCLs.
The certainty of the evidence was very low, downgraded for risk of bias (−1) and extreme imprecision (−2).
Subgroup analysis
There were too few studies to perform a meaningful subgroup analysis as planned.
Discussion
Summary of main results
This systematic review identified seven RCTs that compared the comfort or safety of at least one silicone hydrogel SCL and at least one hydrogel SCL at follow‐up ranging from one to 52 weeks. The evidence for all safety and efficacy outcomes was of very low certainty. Silicone hydrogel SCL may result in a slight reduction in corneal staining. These differences are not clinically meaningful when a difference of ≥ 0.5 units on an ocular surface Likert scale is considered to be clinically meaningful (Dundas 2001). We found very low certainty evidence that silicone hydrogel SCLs result in little to no difference in conjunctival staining at one month and that SCL material may result in little or no difference in vision‐threatening adverse events at one to four weeks. However, hydrogel SCLs may reduce the risk of adverse events compared to silicone hydrogel SCL when evaluated over 52 weeks.
Overall completeness and applicability of evidence
Ocular discomfort is consistently cited as the top reason for contact lens dropout in established wearers, despite innovations in contact lens materials over the past 50 years (Grant 2020; Pucker 2020). Most modern SCL materials are made of either hydrogel or silicone hydrogel materials (Efron 2015; Jacob 2013). Hydrogel SCLs generally have higher water content and much lower oxygen permeability than silicone hydrogel SCLs. Low oxygen transmissibility has the potential to lead to a higher frequency of contact lens‐related adverse events such as conjunctival redness, limbal redness, corneal neovascularization, and corneal edema (Dillehay 2007).
Population representativeness
While the median number of participants in the included studies likely suggested adequate sample sizes (n = 120), limited information was available about participant race or ethnicity, vision correction, or indication for SCLs, making the applicability of the review findings to the general population a legitimate concern. There were studies with only experienced SCL wearers or only neophytes, as well as trials with both neophyte and experienced SCL wearers. Loss to follow‐up was a particular concern in one trial that was of extended wear SCLs (NCT00762788).
Intervention lenses
The SCL materials included eight different material types for silicone hydrogel SCLs and three for hydrogel SCLs. There were too few studies to perform a meaningful subgroup analysis as planned. Only two of the included trials used extended wear modality (defined as seven days and six nights before replacement); both of these studies assessed adverse events in detail up to 52 weeks, yet neither assessed participant‐reported comfort. The applicability of the review findings to SCLs with different replacement schedules is limited.
Outcome measurement
The included trials did not have a consistent tool or timing of measuring ocular comfort. Although VAS or VAS‐like scales were the most commonly used instrument, not all scales had the same incremental unit. The scale was 1 to 5 in Hall 2009 and 1 to 100 in Diec 2012. Where multiple instruments were used (e.g. overall comfort, symptoms leading to discomfort), we selected those reflecting overall comfort. The large number of individual instruments used could suggest a risk of selective outcome reporting of favorable results by individual primary studies, although this was not directly conceivable without a publicly available study protocol for comparison and evaluation. The lack of reporting about the standardized assessment method and grading schemes used for corneal staining, conjunctival staining, and limbal hyperemia is similarly problematic.
There were several cross‐over and paired‐eye trials that met our inclusion criteria; however, these studies were excluded because their design or analysis methods were inadequate (see Differences between protocol and review). These excluded studies typically lacked a wash‐out period and did not use statistical methods to account for the study design and paired data.
Certainty of the evidence
The certainty of evidence was very low for the outcomes included in this review. Most of the included studies were trial registration records and meeting abstracts without published manuscripts. Additionally, the majority of included studies contained incomplete reporting of the method of randomization and allocation concealment. Several studies reported that participants were not masked, predisposing participant‐reported study outcome data to high risk of bias.
Potential biases in the review process
A Cochrane Information Specialist designed and conducted a comprehensive search of literature published in all relevant databases. The methods followed were aligned with our published protocol (Haworth 2021), with the primary exception of excluding some cross‐over studies for the reasons described above (Differences between protocol and review). Our search was not limited by language or publication date. Two review authors independently reviewed each study report to confirm eligibility based on predefined criteria. Two review authors independently completed data extraction, with a third review author adjudicating any differences. Risk of bias during the review process is considered minimal, especially given that none of the authors have topical conflicts of interest directly related to this work. The number of trial registration records and meeting abstracts without full publications could indicate selective non‐publication. Selective non‐reporting bias is also plausible. When necessary, we contacted investigators for information on study design and data, with mixed success. Obtaining complete data may have required searches not performed for this review as they were beyond what was outlined in the protocol (i.e. data repository requests, grey literature from manufacturers, and regulatory agency documents).
Agreements and disagreements with other studies or reviews
We identified 11 review manuscripts that compared the treatment of refractive error with hydrogel and silicone hydrogel contact lenses. However, only one of these manuscripts performed a meta‐analysis (Szczotka‐Flynn 2007). The investigators of this trial specifically performed a meta‐analysis that compared the safety of hydrogel and silicone hydrogel SCL materials and found that silicone hydrogel SCLs worn on a 30‐day continuous wear basis doubled the risk of developing a corneal inflammatory event compared to hydrogel SCLs worn on a 7‐day extended wear basis. The authors acknowledged that the increased risk of inflammatory events may have been linked to the silicone hydrogel SCLs being worn for more days than the hydrogel SCLs. This analysis was further confounded by the inclusion of non‐randomized trials and trials that did not directly compare material types.
There have been two international white paper efforts that have involved comparing hydrogel and silicone hydrogel SCL materials, the first being the Tear Film and Ocular Surface Society’s (TFOS) International Workshop on Contact Lens Discomfort (Jones 2013), and the second the British Contact Lens Association’s (BCLA) Contact Lens Evidence‐Based Academic Reports (CLEAR) (Morgan 2021; Stapleton 2021). TFOS concluded that overall there was limited evidence linking SCL materials to discomfort (Jones 2013). CLEAR described evidence suggesting that wearing silicone hydrogel SCL may result in less conjunctival redness and less corneal staining than hydrogel SCLs use (Morgan 2021). CLEAR likewise found reports that corneal infiltrative events were more common in silicone hydrogel SCL wearers than hydrogel SCL wearers; SCL‐induced dry eye was more common in hydrogel SCL wearers than silicone hydrogel SCL wearers; and that there was no clear difference in SCL comfort or the frequency of microbial keratitis between the two SCL material types (Stapleton 2021).
Multiple literature reviews have compared hydrogel and silicone hydrogel SCLs. One trial found no clear link between the two material types and SCL wear discontinuation (Pucker 2020). Another review described evidence from multiple studies that documented improvements in ocular surface signs and symptoms when patients were switched from hydrogel SCLs to silicone hydrogel SCLs (Dillehay 2007). A review by Guillon 2013 demonstrated no clear difference in comfort between hydrogel and silicone hydrogel SCL wearers based on data only from studies utilizing controls and masking (and not participants simply switching from their habitual SCL to another), which was the same finding of two other reviews (Efron 2022; Stapleton 2021). A review by Efron and colleagues concluded there were few reports of corneal vascularization with modern hydrogel or silicone hydrogel SCLs (Efron 2022).
The current meta‐analysis showed no evidence of clinically meaningful differences between the two material types with respect to comfort. However, silicone hydrogel SCLs may be more likely to cause an adverse event than hydrogel SCLs. It is important to recognize that this evidence was of very low certainty. One reason why hydrogel SCLs may have been found to be safer than silicone hydrogel SCLs was the limitation of studies to RCTs of modern hydrogel SCLs.
Authors' conclusions
Implications for practice.
We determined that there is insufficient evidence to show any clear differences in comfort between silicone hydrogel and hydrogel soft contact lenses (SCLs). However, in trials (both of extended wear SCLs) that had a duration of about one year, there is very low certainty evidence that wearing hydrogel SCLs may be less likely to result in an adverse event than wearing silicone hydrogel SCLs. The current review provides very low certainty evidence suggesting that both hydrogel and silicone hydrogel SCL materials could be expected to provide similar comfort and safety while correcting of ametropia. Factors other than categorical differences in SCL material exist (e.g. material may affect lens mobility on the eye) that may contribute more significantly to contact lens comfort (Nichols 2013; Stapleton 2017). There are a confounding number of variations in contact lens material properties across categories. Knowledge gaps exist due to the lack of quality primary studies that systematically vary contact lens material, design parameters, and fitting characteristics (Jones 2013). The findings of this review suggest that individual patient needs should be considered more important than categorical SCL material differences when selecting an SCL.
Implications for research.
This review identified no conclusive evidence of a difference in the comfort or safety of hydrogel versus silicone hydrogel SCLs. Areas of research identified in this review should be investigated in the future. Based on this review, future studies should consider ways to minimize bias and maximize pooling of study data, including the following.
Standardization of comfort assessment questionnaires.
Standardization of safety assessment.
Conduct of parallel randomized controlled trials rather than cross‐over trials.
If cross‐over studies are absolutely required for scientifically justified reasons, investigators should ensure an appropriate wash‐out period is included and data analyzed and reported in accordance with the study design.
Masking of participants and investigators.
Additional research should be done to increase our understanding of the effect of SCL materials on comfort.
Additional studies comparing extended wear SCLs, as these have a higher risk of microbial keratitis due to their longer wear times.
History
Protocol first published: Issue 5, 2021
Risk of bias
Risk of bias for analysis 1.2 VAS scores.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.2.1 At one week, VAS 0 to 5 (higher is better) | ||||||||||||
| Hall 2009 | Some concerns | The authors did not describe details about the process of allocation concealment was performed if any at all. | Some concerns | Trial registration listed the trial as 'single‐blind (participants)'. The analyses were per protocol and excluded those with incomplete data. The reasons for missing data were not described. No information available to determine whether there were potential deviations from the intervention. | Low risk of bias | Data of this outcome were available for nearly all participants (240/248 = 97%). | Some concerns | Single questionnaire of VAS of 0 to 5 scale was used for assessing overall comfort. Although not uncommon, the instrutment was not previously validated. Outcome assessment was based on participants' self‐reporting and the participants were masked (single‐blinded). | High risk of bias | There was no prespecified analysis plan provided. There were differences in the reported mean difference and its associated 97.5% confidence intervals between the conference abstract and the trial registry record online. We collected data from the trial registry website for the current meta‐analysis. | High risk of bias | The trial was judged as high risk in selective outcome reporting. Some concerns also existed in the randomization process, risks of potential deviations from the intervention, and biased outcome measurement. |
| Subgroup 1.2.2 At three months, VAS 0 to 100 (higher is better) | ||||||||||||
| Diec 2012 | Some concerns | "Randomisation list will be generated from simple randomisation using randomisation computer software." The method of allocation concealment is not detailed. The authors reported no statistical differences in baseline characteristics. Neophytes were split equally across arms. It is not clear how they accounted for this in randomization. | Low risk of bias | The study was an open‐label, prospective clinical trial that included experienced and neophyte myopic participants randomized to one of three lens types, worn bilaterally. There do not appear to be deviations. Linear mixed models included multiple measurements. Appears to be an intention‐to‐treat analysis. | Low risk of bias | 7/140 participants are missing. There are 6 discontinuations in Narafilcon A and 1 in Senofilcon A. The timepoint and impact on outcome are not described. | High risk of bias | A numeric scale of 0 to 100 was not inappropriate. The outcome was measured in the same manner across groups. The participant was not masked (open‐label) and it was possible that observers were influenced by the knowledge of the lens type. | Some concerns | There was no statistical analysis plan available for evaluation. Data of this outcome were analyzed based on repeated measure analysis (averaged over all follow‐up visits) to take advantage of the multiple measurements. It was unclear whether this analytic approach was defined apriori or based on resutls of signigicance testing. | High risk of bias | The trial was judged to be at high risk in biased outcome measurement as well as some concerns in allocation concealment, the randomization process, and selective outcome reporting. |
Risk of bias for analysis 1.7 Proportion of vision‐threatening adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.7.1 ≤ 4 weeks | ||||||||||||
| Diec 2012 | Some concerns | "Randomisation list will be generated from simple randomisation using randomisation computer software." The method of allocation concealment is not detailed. The authors reported no statistical differences in baseline characteristics. Neophytes were split equally across arms. It is not clear how they accounted for this in randomization. | Low risk of bias | The study was an open‐label, prospective clinical trial that included experienced and neophyte myopic participants randomized to one of three lens types, worn bilaterally. It does not seem like there were deviations due to the trial context. Proportions are reported as an intention‐to‐treat analysis. | Low risk of bias | 7/140 participants discontinued. The adverse events and time points are noted. There are 6 discontinuations in Narafilcon A and 1 in Senofilcon A. | Low risk of bias | Although the investigators were aware of the interventions received by the participants, the severity of this outcome should not be easily missed or ignored. | Low risk of bias | The was no analytic plan for evaluation but the data for the outcome was analyzed and reported at the event level, as usual. | Some concerns | The study was judged to have some concerns associated with bias in process of the allocation concealment. |
| Hall 2009 | Some concerns | The authors did not describe details about the process of allocation concealment was performed if any at all. | Low risk of bias | The trial registration record stated 'single‐blind (participants)'. There was no evidence of deviations from the intended intervention. | Low risk of bias | Data for this outcome were available for nearly all participants (240/248 = 97%). | Low risk of bias | Participants were followed at the end of the one‐week wear and assessed by trial investigators (or study physicians) who were aware of the assigned intervention status. The severity of the outcome could not be easily missed or ignored though. | Some concerns | Adverse events were reported at the person level (with no events recorded). It was unclear how the reporting threshold (5%) was determined a priori. | Some concerns | The trial was judged to have some concerns in the randomization process and selective outcome reporting. |
| Subgroup 1.7.2 At 3 months | ||||||||||||
| NCT01354223 | Some concerns | The authors only stated that it was randomized; details about allocation concealment were not described. | Low risk of bias | It was an open‐label (no masking) trial. There was no evidence for deviations from the intended intervention. | Low risk of bias | Data for this outcome was available for almost all eyes randomized (OcufilconB: 58/60 eyes, Stenfilcon A: 114/120 eyes). | Low risk of bias | It was unclear how vision‐threatening adverse events were actively monitored by the investigators or passively by participants' self reporting. However, the severity of the outcome should not be easily ignored or missed. | Some concerns | Minimal information was provided regarding the measurement or the analysis plan though the reporting threshold for nonserious ocular adverse events was reported as 5%, suggesting potential risks for under‐reporting or selective reporting of the outcome. | Some concerns | The trial was judged to have some concerns in the randomization process and selective outcome reporting. |
| Subgroup 1.7.3 At 52 weeks | ||||||||||||
| NCT00241280 | High risk of bias | Only states it was randomized. Randomization of lenses was very uneven. Two lenses only had 3 participants allocated and all 6 were lost to follow‐up. | Some concerns | No information on masking. Appears to be an intention‐to‐treat analysis with all participants included. | Low risk of bias | All participants appear to be included. | High risk of bias | There is insufficient information to determine whether there is bias in the measurement of the outcome. | Some concerns | No analysis plan provided. It is not clear how the frequency threshold (5%) was applied, whether there was a systematic and non systematic collected but only systematic above 5% reported. Not enough information to determine if this resulted in selection of the reported result. | High risk of bias | The trial was judged to be at high risk in the randomization process and in outcome measurement. There were also some concerns in selective outcome reporting. |
| NCT00762788 | High risk of bias | The authors did not provide details of randomization or allocation concealment but only stated the trial was 'randomized'. There appear to be post‐randomization exclusions labeled in the investigator's note: "Cautions for results: low completed enrollment numbers and number of non‐neophytes lower than required per protocol. Arms not gender matched; Proportion of neophytes/non‐neophytes not balanced; High rate of drop‐out among subjects enrolled." | Low risk of bias | The trial was described as single‐masked (investigator). There was no evidence for deviations from the intended interventions. | High risk of bias | Data for this outcome were reported for all participants. However, considering the large dropout rates, bias might have been introduced by imputing data for those who did not complete the study. | Low risk of bias | The trial was single‐masked (investigators). Measurement of this outcome was unlikely biased as the investigators had no knowledge about the intervention received by the participants. | Low risk of bias | No analysis plan was provided but the data were reported at both the person and event level. The data presented in ClinicalTrial.gov have significant and non‐signficant in different sections. | High risk of bias | The trial was judged to be at high risk in the randomization process followed by substantial lost to follow‐up after randomization. |
Acknowledgements
Acknowledgments from the authors
We would like to thank the following peer reviewers for their comments on the review protocol: Renee Bovelle (Howard University) and Lindsay Sicks (Illinois College of Optometry). We also thank Samuel A Abariga of Cochrane Eyes and Vision US project (CEV@US) for his assistance with preparing the review protocol.
Editorial and peer‐reviewer contributions
CEV@US supported the authors in the development of this update. The following people conducted the editorial process for this update:
Sign‐off Editors (final editorial decision): Dr Tianjing Li (University of Colorado Anschutz Medical Campus), Dr Gianni Virgilli (Queen's University Belfast, University of Florence);
Managing Editor and Assistant Managing Editors (selected peer reviewers, collated peer‐reviewer comments): Anupa Shah (Queen's University Belfast), Genie Han (Johns Hopkins University);
Methodologist (provided methodological and editorial guidance to authors, edited the article): Alison Su‐Hsun Liu (University of Colorado Anschutz Medical Campus);
Information Specialist: Lori Rosman (Johns Hopkins University);
Copy Editor: Lisa Winer (Cochrane Central Production Service);
Peer reviewers: Kelsy Steele (Ohio State University) and Lindsay Sicks (Illinois College of Optometry).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Contact Lenses] explode all trees #2 ((contact or contacts) NEXT/2 (lens or lenses)) #3 ((hydrogel* or hydrophilic* or silicone*) NEXT/2 (contact or contacts)) #4 ((hydrogel* or hydrophilic* or silicone*) NEXT/2 (lens or lenses)) #5 ((soft or disposable or disposables or daily or dailies or monthly or monthlies or weekly or weeklies or "extended wear" or "continuous wear" or hybrid* or biweek* or replacement*) NEXT/2 (contact or contacts)) #6 ((soft or disposable or disposables or daily or dailies or monthly or monthlies or weekly or weeklies or "extended wear" or "continuous wear" or hybrid* or biweek* or replacement*) NEXT/2 (lens or lenses)) #7 {OR #1‐#6} #8 MeSH descriptor: [Surveys and Questionnaires] this term only #9 MeSH descriptor: [Health Surveys] this term only #10 MeSH descriptor: [Health Status Indicators] this term only #11 MeSH descriptor: [Patient Reported Outcome Measures] explode all trees #12 MeSH descriptor: [Self Report] explode all trees #13 MeSH descriptor: [Patient Satisfaction] explode all trees #14 MeSH descriptor: [Personal Satisfaction] explode all trees #15 MeSH descriptor: [Patient Compliance] explode all trees #16 MeSH descriptor: [Patient Dropouts] explode all trees #17 MeSH descriptor: [Quality of Life] explode all trees #18 MeSH descriptor: [Qualitative Research] explode all trees #19 MeSH descriptor: [Focus Groups] explode all trees #20 MeSH descriptor: [Self Disclosure] explode all trees #21 (PROM or PROMS) #22 ((quality NEAR/2 life) or (QOL or HRQL or HRQOL)) #23 (dropout* or "drop out" or "drop outs") #24 (interview* or focus group* or qualitative* or survey or surveys or surveyed or questionnaire* or index or indices or scale or scales or rating or ratings) #25 ((patient* or self or client* or participant* or subject* or personal or consumer* or wearer*) NEXT/5 (report* or guided or relate* or view* or expectation* or perception* or perspective* or experience* or described or outcome* or measure* or assess* or monitor* or symptom* or domain* or burden* or impact* or effect* or satisf* or response* or opinion* or comfort* or discomfort* or complaint* or safety)) #26 (("contact lens" or "contact lenses") NEXT/5 (comfort* or discomfort*)) #27 MeSH descriptor: [Patient Comfort] explode all trees #28 MeSH descriptor: [Patient Safety] explode all trees #29 MeSH descriptor: [Long Term Adverse Effects] explode all trees #30 MeSH descriptor: [Dry Eye Syndromes] explode all trees #31 MeSH descriptor: [Meibomian Glands] explode all trees #32 MeSH descriptor: [Tears] explode all trees #33 ("dry eye" or "dry eyes" or "eye dryness" or "lens dehydration" or "lens lubricant" or "lens lubricants" or "lacrimal fluid" or "lacrimal fluids") #34 (tear* or meibomian* or schirmer* or ("phenol red" NEXT/1 thread*)) #35 (CLDEQ or "CLDEQ8" or SPEED or OSDI or VAS) #36 ((corneal or conjunctival or epithelial) NEAR/2 (staining or redness)) #37 ("ocular surface" NEAR/3 (gland* or alteration* or response* or sign* or physiology or comfort* or discomfort*)) #38 ((ocular or vision* or eye or eyes) NEAR/3 (safe* or health* or comfort* or discomfort*)) #39 ((adverse or dangerous or harmful or indirect or injurious or secondary or side or undesirable) NEAR/2 (complication* or consequence* or effect* or event* or impact* or outcome* or reaction*)) #40 (symptom or symptoms or symptomatic or asymptomatic) #41 {OR #8‐#40} #42 #7 AND #41
Appendix 2. MEDLINE (Ovid) search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Contact Lenses/ 13. ((contact or contacts) adj2 (lens or lenses)).tw. 14. ((hydrogel* or hydrophilic* or silicone*) adj2 (contact or contacts)).tw. 15. ((hydrogel* or hydrophilic* or silicone*) adj2 (lens or lenses)).tw. 16. ((soft or disposable or disposables or daily or dailies or monthly or monthlies or weekly or weeklies or "extended wear" or "continuous wear" or hybrid* or biweek* or replacement*) adj2 (contact or contacts)).tw. 17. ((soft or disposable or disposables or daily or dailies or monthly or monthlies or weekly or weeklies or "extended wear" or "continuous wear" or hybrid* or biweek* or replacement*) adj2 (lens or lenses)).tw. 18. or/12‐17 19. "Surveys and Questionnaires"/ 20. Health surveys/ 21. Health Status Indicators/ 22. exp Patient Reported Outcome Measures/ 23. exp Self Report/ 24. exp Patient Satisfaction/ 25. exp Personal Satisfaction/ 26. exp Patient Compliance/ 27. exp Patient Dropouts/ 28. exp "Quality of Life"/ 29. exp Qualitative Research/ 30. exp Focus Groups/ 31. exp Self Disclosure/ 32. (PROM or PROMS).tw. 33. ((quality adj2 life) or (QOL or HRQL or HRQOL)).tw. 34. (dropout* or drop out*).tw. 35. (interview* or focus group* or qualitative* or survey or surveys or surveyed or questionnaire* or index or indices or scale or scales or rating or ratings).tw. 36. ((patient* or self or client* or participant* or subject* or personal or consumer* or wearer*) adj5 (report* or guided or relate* or view* or expectation* or perception* or perspective* or experience* or described or outcome* or measure* or assess* or monitor* or symptom* or domain* or burden* or impact* or effect* or satisf* or response* or opinion* or comfort* or discomfort* or complaint* or safety)).tw. 37. (contact lens* adj5 (comfort* or discomfort*)).tw. 38. exp Patient Comfort/ 39. exp Patient Safety/ 40. exp Long Term Adverse Effects/ or adverse effects.fs. 41. exp Dry Eye Syndromes/ 42. exp Meibomian Glands/ 43. exp Tears/ 44. (dry eye or dry eyes or eye dryness or lens dehydration or lens lubricant* or lacrimal fluid*).tw. 45. (tear* or meibomian* or schirmer* or phenol red thread*).tw. 46. (CLDEQ or "CLDEQ8" or SPEED or OSDI or VAS).tw. 47. ((Corneal or conjunctival or epithelial) adj2 (staining or redness)).tw. 48. (ocular surface adj3 (gland* or alteration* or response* or sign* or physiology or comfort* or discomfort*)).tw. 49. ((ocular or vision* or eye or eyes) adj3 (safe* or health* or comfort* or discomfort*)).tw. 50. ((adverse or dangerous or harmful or indirect or injurious or secondary or side or undesirable) adj2 (complication* or consequence* or effect* or event* or impact* or outcome* or reaction*)).tw. 51. (symptom or symptoms or symptomatic or asymptomatic).tw. 52. or/19‐51 53. 11 and 18 and 52
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville and colleagues (Glanville 2006).
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'contact lens'/exp #34 ((contact OR contacts) NEXT/2 (lens OR lenses)):ab,ti,kw #35 ((hydrogel* OR hydrophilic* OR silicone*) NEXT/2 (contact OR contacts)):ab,ti,kw #36 ((hydrogel* OR hydrophilic* OR silicone*) NEXT/2 (lens OR lenses)):ab,ti,kw #37 ((soft OR disposable OR disposables OR daily OR dailies OR monthly OR monthlies OR weekly OR weeklies OR 'extended wear' OR 'continuous wear' OR hybrid* OR biweek* OR replacement*) NEXT/2 (contact OR contacts)):ab,ti,kw #38 ((soft OR disposable OR disposables OR daily OR dailies OR monthly OR monthlies OR weekly OR weeklies OR 'extended wear' OR 'continuous wear' OR hybrid* OR biweek* OR replacement*) NEXT/2 (lens OR lenses)):ab,ti,kw #39 #33 OR #34 OR #35 OR #36 OR #37 OR #38 #40 'questionnaire'/exp #41 'health survey'/exp #42 'patient‐reported outcome'/exp #43 'self report'/exp #44 'patient satisfaction'/exp #45 'satisfaction'/de #46 'patient compliance'/de #47 'patient dropout'/exp #48 'quality of life'/exp #49 'qualitative research'/exp #50 'focus group'/exp #51 'self disclosure'/exp #52 prom:ab,ti,kw OR proms:ab,ti,kw #53 ((quality NEAR/2 life):ab,ti,kw) OR qol:ab,ti,kw OR hrql:ab,ti,kw OR hrqol:ab,ti,kw #54 dropout*:ab,ti,kw OR 'drop out*':ab,ti,kw #55 interview*:ab,ti,kw OR 'focus group*':ab,ti,kw OR qualitative*:ab,ti,kw OR survey:ab,ti,kw OR surveys:ab,ti,kw OR surveyed:ab,ti,kw OR questionnaire*:ab,ti,kw OR index:ab,ti,kw OR indices:ab,ti,kw OR scale:ab,ti,kw OR scales:ab,ti,kw OR rating:ab,ti,kw OR ratings:ab,ti,kw #56 ((patient* OR self OR client* OR participant* OR subject* OR personal OR consumer* OR wearer*) NEXT/5 (report* OR guided OR relate* OR view* OR expectation* OR perception* OR perspective* OR experience* OR described OR outcome* OR measure* OR assess* OR monitor* OR symptom* OR domain* OR burden* OR impact* OR effect* OR satisf* OR response* OR opinion* OR comfort* OR discomfort* OR complaint* OR safety)):ab,ti,kw #57 ('contact lens' NEXT/5 (comfort* OR discomfort*)):ab,ti,kw #58 'patient comfort'/exp #59 'patient safety'/exp #60 'adverse event'/exp #61 'dry eye'/exp #62 'meibomian gland'/exp #63 'lacrimal fluid'/exp #64 'dry eye':abco, ti,kw OR 'dry eyes':ab,ti,kw OR 'eye dryness':ab,ti,kw OR 'lens dehydration':ab,ti,kw OR 'lens lubricant*':ab,ti,kw OR 'lacrimal fluid*':ab,ti,kw #65 tear*:ab,ti,kw OR meibomian*:ab,ti,kw OR schirmer*:ab,ti,kw OR 'phenol red thread*':ab,ti,kw #66 cldeq:ab,ti,kw OR 'cldeq8':ab,ti,kw OR speed:ab,ti,kw OR osdi:ab,ti,kw OR vas:ab,ti,kw #67 ((corneal OR conjunctival OR epithelial) NEAR/2 (staining OR redness)):ab,ti,kw #68 ('ocular surface' NEAR/3 (gland* OR alteration* OR response* OR sign* OR physiology OR comfort* OR discomfort*)):ab,ti,kw #69 ((ocular OR vision* OR eye OR eyes) NEAR/3 (safe* OR health* OR comfort* OR discomfort*)):ab,ti,kw #70 ((adverse OR dangerous OR harmful OR indirect OR injurious OR secondary OR side OR undesirable) NEAR/2 (complication* OR consequence* OR effect* OR event* OR impact* OR outcome* OR reaction*)):ab,ti,kw #71 symptom:ab,ti,kw OR symptoms:ab,ti,kw OR symptomatic:ab,ti,kw OR asymptomatic:ab,ti,kw #72 #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 #73 #39 AND #72 #74 #32 AND #73
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. "contact lens*"[tiab] 3. ((hydrogel*[tiab] OR hydrophilic*[tiab] OR silicone*[tiab]) AND (contact[tiab] OR contacts[tiab])) 4. ((hydrogel*[tiab] OR hydrophilic*[tiab] OR silicone*[tiab]) AND (lens[tiab] OR lenses[tiab])) 5. ((soft[tiab] OR disposable[tiab] OR disposables[tiab] OR daily[tiab] OR dailies[tiab] OR monthly[tiab] OR monthlies[tiab] OR weekly[tiab] OR weeklies[tiab] OR "extended wear"[tiab] OR "continuous wear"[tiab] OR hybrid*[tiab] OR biweek*[tiab] OR replacement*[tiab]) AND (contact[tiab] OR contacts[tiab])) 6. ((soft[tiab] OR disposable[tiab] OR disposables[tiab] OR daily[tiab] OR dailies[tiab] OR monthly[tiab] OR monthlies[tiab] OR weekly[tiab] OR weeklies[tiab] OR "extended wear"[tiab] OR "continuous wear"[tiab] OR hybrid*[tiab] OR biweek*[tiab] OR replacement*[tiab]) AND (lens[tiab] OR lenses[tiab])) 7. #2 OR #3 OR #4 OR #5 OR #6 8. (PROM[tiab] OR PROMS[tiab]) 9. ("quality of life"[tiab] OR "life quality"[tiab] OR QOL[tiab] OR HRQL[tiab] OR HRQOL[tiab]) 10. (dropout*[tiab] OR "drop out*"[tiab]) 11. (interview*[tiab] OR "focus group*"[tiab] OR qualitative*[tiab] OR survey[tiab] OR surveys[tiab] OR surveyed[tiab] OR questionnaire*[tiab] OR index[tiab] OR indices[tiab] OR scale[tiab] OR scales[tiab] OR rating[tiab] OR ratings[tiab]) 12. ((patient*[tiab] OR self[tiab] OR client*[tiab] OR participant*[tiab] OR subject*[tiab] OR personal[tiab] OR consumer*[tiab] OR wearer*[tiab]) AND (report*[tiab] OR guided[tiab] OR relate*[tiab] OR view*[tiab] OR expectation*[tiab] OR perception*[tiab] OR perspective*[tiab] OR experience*[tiab] OR described[tiab] OR outcome*[tiab] OR measure*[tiab] OR assess*[tiab] OR monitor* OR symptom*[tiab] OR domain*[tiab] OR burden*[tiab] OR impact*[tiab] OR effect*[tiab] OR satisf*[tiab] OR response*[tiab] OR opinion*[tiab] OR comfort*[tiab] OR discomfort*[tiab] OR complaint*[tiab] OR safety[tiab])) 13. ("contact lens*"[tiab]) AND (comfort*[tiab] OR discomfort*[tiab]) 14. ("dry eye*"[tiab] OR "eye dryness"[tiab] OR "lens dehydration"[tiab] OR "lens lubricant*" OR "lacrimal fluid*"[tiab]) 15. (tear*[tiab] OR meibomian*[tiab] OR schirmer*[tiab] OR "phenol red thread*"[tiab]) 16. (CLDEQ[tiab] OR "CLDEQ8"[tiab] OR SPEED[tiab] OR OSDI[tiab] OR VAS[tiab]) 17. ((Corneal[tiab] OR conjunctival[tiab] OR epithelial[tiab]) AND (staining[tiab] OR redness[tiab])) 18. ("ocular surface" AND (gland*[tiab] OR alteration*[tiab] OR response*[tiab] OR sign*[tiab] OR physiology[tiab] OR comfort*[tiab] OR discomfort*[tiab])) 19. ((ocular[tiab] OR vision*[tiab] OR eye[tiab] OR eyes[tiab]) AND (safe*[tiab] OR health*[tiab] OR comfort*[tiab] OR discomfort*[tiab])) 20. ((adverse[tiab] OR dangerous[tiab] OR harmful[tiab] OR indirect[tiab] OR injurious[tiab] OR secondary[tiab] OR side[tiab] OR undesirable[tiab]) AND (complication*[tiab] OR consequence*[tiab] OR effect*[tiab] OR event*[tiab] OR impact*[tiab] OR outcome*[tiab] OR reaction*[tiab])) 21. (symptom[tiab] OR symptoms[tiab] OR symptomatic[tiab] OR asymptomatic[tiab]) 22. #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 23. #1 AND #7 AND #22 24. Medline[sb] 25. #23 NOT #24
Appendix 5. LILACS search strategy
("Contact Lens" OR " Contact Lenses" OR "Lentes de Contacto" OR "Lentes de Contato" OR MH:E07.632.500.276$ OR MH: VS2.006.001.009.001$ OR ((hydrogel$ contact$) OR (hydrophilic$ contact$) OR (silicone$ contact$) OR "soft contact" OR "soft contacts" OR "disposable contact" OR "disposable contacts" OR disposables OR "daily contacts" OR dailies OR "monthly contacts" OR monthlies OR "weekly contacts" OR weeklies OR "extended wear" OR "continuous wear" OR "hybrid contacts" OR "biweekly contacts" OR (replacement$ contact$)) OR ((hydrogel$ OR hydrophilic$ OR silicone$ OR soft OR disposable OR disposables OR daily OR dailies OR monthly OR monthlies OR weekly OR weeklies OR "extended wear" OR "continuous wear" OR hybrid$ OR biweek$ OR replacement$) AND (lens OR lenses))) AND (MH:E05.318.308.980 OR MH:N05.715.360.300.800 OR MH:N06.850.520.308.980 OR MH;E05.318.308.980.438 OR MH:N05.715.360.300.800.438 OR MH:N06.850.520.308.980.438 OR MH:SP5.006.062.213 OR MH:E05.318.308.980.438.475 OR MH:N05.715.360.300.800.438.375 OR MH:N06.850.520.308.980.438.475 OR MH:SH1.030.050.030 OR MH:SP2.001.030 OR MH:SP4.127.413.629.890 OR MH:SP5.006.067 OR MH:E05.318.308.980.344.500$ OR MH:N03.349.380.210.750$ OR MH:N04.761.559.590.399.875$ OR MH:N05.425.210.500$ OR MH:N05.715.360.300.800.344.500$ OR MH:N05.715.360.575.575.399.875$ OR MH:N06.850.520.308.980.344.500$ OR MH:E05.318.308.980.500$ OR MH:N05.715.360.300.800.500$ OR MH:N06.850.520.308.980.500$ OR MH:F01.100.150.750.625$ OR MH:F01.145.488.887.625$ OR MH:N04.452.822.700$ OR MH:N05.300.150.800.625$ OR MH:N05.715.360.600$ OR MH:F01.145.677$ OR MH:F01.100.150.750.500.600$ OR MH:F01.145.488.887.500.600$ OR MH:N05.300.150.800.500.600$ OR MH:F01.100.150.750.500.610$ OR MH:F01.145.488.887.500.610$ OR MH:N05.300.150.800.500.610$ OR MH:I01.800$ OR MH:K01.752.400.750$ OR MH:N06.850.505.400.425.837$ OR MH:SP4.077.593$ OR MH:H01.770.644.241.850$ OR MH:E05.318.308.112$ OR MH:N05.715.360.300.269$ OR MH:N06.850.520.308.112$ OR MH:F01.752.747.792.662$ OR PROM OR PROMS OR "quality of life" OR "life quality" OR QOL OR HRQL OR HRQOL OR dropout$ OR "drop out" OR "drop outs" OR interview$ OR "focus group" OR "focus groups" OR qualitative$ OR survey OR surveys OR surveyed OR questionnaire$ OR index OR indices OR scale OR scales OR rating OR ratings OR ((patient$ OR self OR client$ OR participant$ OR subject$ OR personal OR consumer$ OR wearer$) AND (report$ OR guided OR relate$ OR view$ OR expectation$ OR perception$ OR perspective$ OR experience$ OR described OR outcome$ OR measure$ OR assess$ OR monitor$ OR symptom$ OR domain$ OR burden$ OR impact$ OR effect$ OR satisf$ OR response$ OR opinion$ OR comfort$ OR discomfort$ OR complaint$ OR safety)) OR ((contact lens$) AND (comfort$ OR discomfort$)) OR MH:N02.421.585.683$ OR MH:N06.850.135.060.075.399 OR MH:C23.550.543$ OR MH:C11.496.260 OR MH:A09.371.337.614$ OR MH:A10.336.827.600$ OR MH:A12.200.882 OR "dry eye" OR "dry eyes" OR "eye dryness" OR "lens dehydration" OR "lens lubricant" OR "lens lubricants" OR "lacrimal fluid" OR "lacrimal fluids" OR tear$ OR meibomian$ OR Schirmer$ OR (phenol red thread$) OR CLDEQ OR "CLDEQ8" OR SPEED OR OSDI OR VAS OR ((corneal OR conjunctival OR epithelial) AND (staining OR redness)) OR ("ocular surface" AND (gland$ OR alteration$ OR response$ OR sign$ OR physiology OR comfort$ OR discomfort$)) OR ((ocular OR vision$ OR eye OR eyes) AND (safe$ OR health$ OR comfort$ OR discomfort$)) OR ((adverse OR dangerous OR harmful OR indirect OR injurious OR secondary OR side OR undesirable) AND (complication$ OR consequence$ OR effect$ OR event$ OR impact$ OR outcome$ OR reaction$)) OR symptom$)
Appendix 6. ClinicalTrials.gov search strategy
(Contact lens OR ((hydrogel OR hydrophilic OR silicone OR soft OR disposable OR "extended wear" OR "continuous wear" OR replacement OR hybrid OR biweekly) AND (contact OR lens)))
Appendix 7. WHO ICTRP search strategy
Contact lens OR Hydrogel contacts OR hydrophilic contacts OR silicone contacts OR soft contacts OR disposable contacts OR extended wear contacts OR continuous wear contacts OR replacement contacts OR hybrid contacts OR biweekly contacts OR hydrogel lens OR hydrophilic lens OR silicone lens OR soft lens OR disposable lens OR extended wear lens OR continuous wear lens OR replacement lens or hybrid lens or biweekly lens
Appendix 8. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design |
|
Exclusions after randomization Losses to follow‐up Number randomized/analyzed How were missing data handled? e.g. available‐case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes or Unit of randomization/unit of analysis |
|
|
| Participants | ||
| Country | Setting Ethnic group Equivalence of baseline characteristics (Y/N) |
|
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are only reported for the people who were followed up, please indicate. | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | ||
| Intervention (n = ) Comparator (n = ) See MECIR 65 and 70 |
|
|
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports See MECIR R70 |
List outcomes Adverse events reported (Y/N) Length of follow‐up and intervals at which outcomes assessed |
Planned/actual length of follow‐up |
Data and analyses
Comparison 1. Silicone hydrogel versus hydrogel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 OSDI scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.2 At 3 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 VAS scores | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 At one week, VAS 0 to 5 (higher is better) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 At three months, VAS 0 to 100 (higher is better) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Discontinuation of contact lens wear | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 ≤ 4 weeks | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.74] |
| 1.3.2 At 3 months | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.53 [0.65, 19.08] |
| 1.3.3 At 52 weeks | 2 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.11] |
| 1.4 Corneal staining scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5 Conjunctival staining score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6 Conjunctival redness scores | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.1 Nasal conjunctival redness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.2 Temporal conjunctival redness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.3 Limbal conjunctival redness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7 Proportion of vision‐threatening adverse events | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 ≤ 4 weeks | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.08, 5.51] |
| 1.7.2 At 3 months | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [0.19, 66.72] |
| 1.7.3 At 52 weeks | 2 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.38, 2.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Diec 2012.
| Study characteristics | |
| Methods | Study design: RCT, parallel‐group design, 3 arms Numbers randomized (total and per group): total 126 participants enrolled, 120 were dispensed lenses, 40 in each group Unit of randomization: person Masking: not masked, "open‐label" Exclusions and losses to follow‐up (total and per group): 6 participants failed to meet the exclusion criteria overall, and there were no dropouts Number analyzed (total and per group): total 120 (40 in each group) Unit of analysis (person or eye): person Study duration (planned and actual): 3 months Intervention duration: 3 months How were missing data handled: not reported Sample size and power calculation: "A sample size of 40 participants for each lens type was required to have 80% power to detect a statistically significant difference of 0.5 ± 0.7 units in the 0 to 4 grading scale and a 10 ± 15 units in the subjective ratings between the lens types." |
| Participants |
Country: Australia
Setting: Brien Holden Vision Institute Inclusion criteria: age 18 years and up, willing to comply with contact lens schedule, normal ocular health, vision correctable to at least 6/12 (20/40) or better in each eye, may be experienced or inexperienced with contact lenses Exclusion criteria: pre‐existing injury, irritation, or conditions of the cornea, conjunctiva, or eyelids precluding wearing of contact lenses; systemic diseases that impact eye health (diabetes, Graves disease, autoimmune disorders); use of ocular medications (category S3 and above) within 12 weeks prior to trial; use of systemic or topical medications that may alter eye health; eye surgery within 12 weeks prior to trial; previous corneal refractive surgery; allergy to topical anesthesia; pregnant; currently enrolled in another trial; previously participated in a clinical trial within 2 weeks (or 48 hours for "short term" trials) prior to this trial Baseline characteristics Refractive condition: myopia Etafilcon A (N = 40)
Narafilcon A (N = 40)
Senofilcon A (N = 40)
Overall (N = 120)
|
| Interventions | Etafilcon A
Narafilcon A
Senofilcon A
Notes: participants were instructed to wear a minimum of 6 days per week and 8 hours per day |
| Outcomes |
Primary outcome:
Secondary outcomes:
Adverse outcomes (Y/N), if yes, please describe: Y, serious and non‐serious adverse events Measurement time points: baseline, 2 weeks, 1 month, and 3 months |
| Notes | Sponsorship source: Alcon Labs and Brien Holden Vision Institute Author's name: Jennie Diec Institution: Brien Holden Vision Institute Conflicts of interest : not reported Publication language: English Trial register: ACTRN12609000812291 |
Hall 2009.
| Study characteristics | |
| Methods | Study design: RCT, parallel‐group design Numbers randomized (total and per group): total 248 participants; 127 in Narafilcon A and 121 in Nelfilcon A group Unit of randomization: person Masking: "single (participant)" Exclusions and losses to follow‐up (total and per group): total 5; 2 in Narafilcon A and 3 in Nelfilcon A group (1 participant in Nelfilcon A group was withdrawn due to adverse event) Number analyzed (total and per group): total 243 participants; 125 in Narafilcon A and 118 in Nelfilcon A group Unit of analysis (person or eye): person Study duration (planned and actual): 1 week Intervention duration: 1 week How were missing data handled: excluded Sample size and power calculation: not reported |
| Participants |
Country: UK
Setting: 21 sites Inclusion criteria: aged 18 years or older, willing to follow protocol, maximum of 1.00 D of refractive astigmatism; VA of 6/9 (20/30) in each eye with contact lenses, able to wear contact lenses with a back vertex power of −1.00 to −6.00 DS; successfully worn contact lenses within 6 months prior to enrollment Exclusion criteria: systemic disorders or ocular disorders (including surface signs) preventing contact lens wear; aphakic; corneal distortions (due to hard contact lens wear or keratoconus); using any topical medication (ointment, eye drops); previous corneal refractive surgery; infectious or immunosuppressive disease (hepatitis, HIV); diabetes; taken part in any other clinical trial 2 weeks prior to the start of the study Baseline characteristics Refractive condition: a maximum of 1.00 D of refractive astigmatism Narafilcon A (N = 125)
Nelfilcon A (N = 118)
Overall (N = 243)
|
| Interventions | Narafilcon A
Nelfilcon A
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Measurement time points: 1 week Notes: initial trial outcomes changed as indicated on the trial registry website |
| Notes | Sponsorship source: supported by Johnson & Johnson Vision Care Inc. Author's name: Lee Hall Institution: Visioncare Research Ltd. Conflicts of interest: not reported Publication language: English Trial register:NCT00727558 |
Marx 2009.
| Study characteristics | |
| Methods | Study design: RCT, parallel‐group design Numbers randomized (total and per group): total 51 participants Unit of randomization: person Masking: not reported Exclusions and losses to follow‐up (total and per group): not reported Number analyzed (total and per group): not reported Unit of analysis (person or eye): person Study duration (planned and actual): 4 weeks Intervention duration: 4 weeks How were missing data handled: not reported Sample size and power calculation: not reported |
| Participants |
Country: Germany
Setting: university clinics Inclusion criteria: neophyte contact lens wearers Exclusion criteria: not reported Baseline characteristics Refractive condition: not reported Nelfilcon A
Narafilcon A
Overall (N = 51)
|
| Interventions | Nelfilcon A
Narafilcon A
|
| Outcomes |
Primary outcomes:
Secondary outcomes: not specified Adverse outcomes (Y/N), if yes, please describe: N Measurement time points: 1, 2, and 4 weeks |
| Notes | Sponsorship source: CIBA VISION Corporation Author's name: Sebastian Marx Institution: Ernst Abbe University of Applied Sciences Conflicts of interest: not reported Publication language: English Trial register: not reported |
Muhafiz 2019.
| Study characteristics | |
| Methods | Study design: RCT, parallel‐group design, 3 arms Numbers randomized (total and per group): total 71 participants; 22 in Etafilcon A, 25 in Delefilcon A, 24 in Balafilcon A (monthly reusable lenses, not included in this review) Unit of randomization: person (1 eye per person) Masking: not reported Exclusions and losses to follow‐up (total and per group): not reported Number analyzed (total and per group): 22 in Etafilcon A, 25 in Delefilcon A Unit of analysis (person or eye): person (1 eye per person) Study duration (planned and actual): 3 months Intervention duration: 3 months How were missing data handled: not reported Sample size and power calculation: "Power analysis was conducted using WSSPAS: Web‐Based Sample Size & Power Analysis Software," but details were not reported. |
| Participants |
Country: Turkey
Setting: university clinic Inclusion criteria: "Individuals who had not previously used CLs and requested the use of spherical CLs" Exclusion criteria: "Patients were excluded if they had astigmatism over ± 0.5 diopters, if they had a history of CL use, and if they used the CL irregularly" Baseline characteristics Refractive condition: not reported Etafilcon A (N = 22)
Delefilcon A (N = 25)
Total (N = 47)
Notes: characteristics of 24 participants in Balafilcon A group (monthly reusable lenses) were not included. "No significant difference was determined between the groups in respect to age and sex (P = 0.05). At the beginning of the study, no statistically significant difference was determined between the groups in respect to the OSDI score, tear osmolarity, TBUT, and Schirmer test (all P = 0.05)" |
| Interventions | Etafilcon A
Delefilcon A
Notes: participants were instructed to wear SCL for 10 hours every day |
| Outcomes |
Primary outcome:
Secondary outcomes: not specified Adverse outcomes (Y/N), if yes, please describe: N Measurement time points: baseline, 1 month, 3 months |
| Notes | Sponsorship source: "Supported by the Bozok University Scientific Research Projects Unit." Author's name: Ersin Muhafiz, MD Institution: Saglik Bilimleri University Conflicts of interest: "The authors have no conflicts of interest to disclose." Publication language: English Trial register: not reported |
NCT00241280.
| Study characteristics | |
| Methods |
Study design: RCT, parallel‐group design
Numbers randomized (total and per group): total 501 participants; 247 in Etafilcon A, 254 in Galyfilcon A group
Unit of randomization: person
Masking: double (participant, investigator)
Exclusions and losses to follow‐up (total and per group): total 118 participants; 48 in Etafilcon A, 68 in Galyfilcon A group
Number analyzed (total and per group):
Unit of analysis (person or eye): person and eye Study duration (planned and actual): 52 weeks Intervention duration: 52 weeks How were missing data handled: not reported Sample size and power calculation: not reported |
| Participants |
Country: USA
Setting: multicenter (25 sites) in university hospital and private practices Inclusion criteria: at least 18 years of age; minimum of 7 days successful lens wear; contact lens prescription requiring between −1.00 to −6.00 D spherical power; less than 1.00 D of astigmatism in either eye Exclusion criteria: not reported Baseline characteristics Refractive condition: myopia Etafilcon A (N = 247)
Galyfilcon A (N = 254)
Overall (N = 501)
|
| Interventions | Etafilcon A
Galyfilcon A
|
| Outcomes |
Primary outcome:
Secondary outcomes: not specified Adverse outcomes (Y/N), if yes, please describe: incidence of rates of contact lens‐related serious and significant adverse events Measurement time points: baseline, 24 hours, 1, 4, 12, 24, 36, and 52 weeks |
| Notes | Sponsorship source: Johnson & Johnson Vision Care Inc. Author's name: David C Turner, PhD Institution: Johnson & Johnson Vision Care Inc. Conflicts of interest: not reported Publication language: English Trial register:NCT00241280 |
NCT00762788.
| Study characteristics | |
| Methods | Study design: RCT, parallel‐group design Numbers randomized (total and per group): total 314 participants Unit of randomization: person Masking: single (investigator) Exclusions and losses to follow‐up (total and per group): total 144 participants (45.9%) lost to follow‐up Number analyzed (total and per group): total 314 participants analyzed for safety outcomes Unit of analysis (individual or eye): person Study duration (planned and actual): 52 weeks Intervention duration: 52 weeks How were missing data handled: assuming no adverse events incurred to those lost to follow‐up Sample size and power calculation: not reported |
| Participants |
Country: India
Setting: Vision Research Foundation in Chennai, Tamil Nadu, India Inclusion criteria: between 18 and 39 years of age; requires visual correction in both eyes; require a soft contact lens spherical correction between −0.50 and −9.00 D; have an astigmatic correction less than 1.50 D in both eyes; correctable to a VA of 20/30 or better in each eye; able to regularly wear the lenses on a 7‐day/6‐night extended wear basis (e.g. does not regularly swim more than once a week); no evidence of eye abnormality or disease (no amblyopia); no lid abnormality or infection; no clinically significant slit lamp findings; no previous ocular surgery Exclusion criteria: concurrent ocular medication; clinically significant slit lamp findings (grade 3 or 4); systemic illness or treatments that would contraindicate lens wear; diabetes; infectious disease (e.g. hepatitis, tuberculosis); immunosuppressive disease (e.g. HIV); hard contact lens wear in the previous 8 weeks; previous refractive surgery; injury/surgery within 8 weeks immediately prior to enrollment; keratoconus or other corneal irregularity; pregnancy, lactating or planning a pregnancy; participation in any concurrent clinical trial; currently wearing any of the study lenses on an extended wear basis; previous adverse events contraindicating extended wear (microbial keratitis, corneal scars) Baseline characteristics Refractive condition: myopia Senofilcon A (N = 55)
Lotrafilcon A (N = 58)
Lotrafilcon B (N = 42)
Balafilcon A (N = 64)
Comfilcon A (N = 43)
Etafilcon A (N = 52)
Overall (N = 314)
|
| Interventions | Senofilcon A
Lotrafilcon A
Lotrafilcon B
Balafilcon A
Comfilcon A
Etafilcon A
|
| Outcomes |
Primary outcome:
Secondary outcomes: not specified Adverse outcomes (Y/N), if yes, please describe: Y, "significant" events were defined as events that are usually symptomatic and warrant either temporary or permanent discontinuation of lens wear. "Non‐significant" events were defined as events that are usually asymptomatic and do not warrant discontinuation of lens wear. Measurement time points: baseline and 52 weeks; trial registration Version 1 assessments planned for baseline, 24 hours, 1 week, 1 month, 3 months, 6 months, 1 year Notes: incidence was calculated as the number of events divided by the total number of participants assigned to that lens type. High rates of lost to follow‐up across intervention groups; numbers of non‐neophytes were lower than required per protocol. |
| Notes | Sponsorship source: Johnson & Johnson Vision Care Author's name: Kathy Osborn, OD, MS, FAAO, FBCLA Institution: Vistakon Conflicts of interest: not reported Publication language: English Trial register:NCT00762788; other study ID: CR‐4472 |
NCT01354223.
| Study characteristics | |
| Methods | Study design: RCT, parallel‐group design Numbers randomized (total and per group): total 90 participants; 60 in Stenfilcon A group, 30 in Ocufilcon B Unit of randomization: person (1 eye per person) Masking: open‐label Exclusions and losses to follow‐up (total and per group): total 4 participants; 3 in Stenfilcon A, 1 in Ocufilcon B Number analyzed (total and per group): total 86 participants; 57 in Stenfilcon A, 29 in Ocufilcon B Unit of analysis (individual or eye): person Study duration (planned and actual): 3 months Intervention duration: 3 months How were missing data handled: not reported Sample size and power calculation: not reported |
| Participants |
Country: USA
Setting: 4 centers Inclusion criteria: at least 18 years of age; good general health; adapted current full‐time silicone hydrogel or soft contact lens wearer (at least 8 hours per day, worn daily for at least 1 month); requires spectacle lens powers between −1.00 and −6.00 DS; no more than 1.00 D of refractive astigmatism; willing to wear contact lenses in both eyes; manifest refraction Snellen VA equal to or better than 20/25 in each eye; contact lens correction to Snellen VA equal to or better than 20/30 in each eye Exclusion criteria: monovision modality; unwilling to be fit with distance lenses in both eyes; poor personal hygiene; active participation in another clinical trial within 30 days prior to this study; pregnant lactating or is planning a pregnancy within the next 3 months; a member, relative, or household member of the investigator or of the investigational office staff; sensitivity to study lubricants; previous refractive surgery; history of orthokeratology treatment; aphakic or pseudophakic; ocular diseases (glaucoma, uveitis, iritis, keratoconus); systemic diseases (diabetes, Sjögren's syndrome, lupus erythematosus); use of topical ocular medications or any medication that might interfere with contact lens wear or that would require the lenses to be removed during the day; clinically significant (grade 3 or 4) anterior segment abnormalities; history of corneal hypoesthesia, corneal ulcer, corneal infiltrates or fungal infections, papillary conjunctivitis. Slit lamp findings that would contraindicate contact lens wear (dry eye, punctate staining over grade 2, corneal scars, neovascularization, seborrheic conjunctivitis, seborrheic eczema, blepharitis) Baseline characteristics Refractive condition: myopia Stenfilcon A (N = 60)
Ocufilcon B (N = 30)
Overall (N = 90)
Notes: baseline characteristics were comparable |
| Interventions | Stenfilcon A
Ocufilcon B
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Adverse outcomes (Y/N), if yes, please describe: Y, serious and non‐serious adverse events (papillary conjunctivitis: 6 events in 3 participants (5.00%) in Stenfilcon A group) Measurement time points: baseline, weeks 1, 2, months 1, 2, and 3 |
| Notes | Sponsorship source: CooperVision Inc. Authors' names: Stephen Byrnes, Lee Rigel, Mary Jo Stiegemeier, Peter Van Hoven, Eric White Institution: Eric M. White, OD, Inc Conflicts of interest : not reported Publication language: English Trial register:NCT01354223 |
CCLRU: Cornea and Contact Lens Research Unit CL: contact lens(es) D: diopters DS: diopters sphere logMAR: Logarithm of the Minimum Angle of Resolution NEI: National Eye Institute OSDI: Ocular Surface Disease Index RCT: randomized controlled trial SCL: soft contact lens(es) SD: standard deviation TBUT: tear breakup time VAS: visual analog scale VA: visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12610000639022 | Ineligible study design: only silicone hydrogel |
| ACTRN12618000039280 | Ineligible intervention: prototype lens |
| Arroyo‐Del Arroyo 2021 | Ineligible study design: both comparison groups had exposure to hydrogel and silicone hydrogel |
| Bergenske 2005a | Ineligible study design: analysis did not account for the cross‐over design |
| Bishop 2022 | Ineligible study design: secondary data analysis |
| Brennan 2002 | Ineligible study design: same lens material, comparing replacement schedules |
| Bucci 1997 | Ineligible study design: only hydrogel |
| Carpena‐Torres 2021 | Ineligible study design: intervention duration less than 1 week |
| Cheung 2007 | Ineligible study design: analysis did not account for the paired‐eye design |
| Cho 2000 | Ineligible population: ages 17 to 35 years old |
| Comstock 2001 | Ineligible study design: analysis did not account for the paired‐eye design |
| CTRI/2015/04/005701 | Ineligible intervention: prototype lens |
| Dalcoll 2008 | Ineligible study design: only hydrogel |
| Davies 2006 | Ineligible study design: only silicone hydrogel |
| Dumbleton 1998 | Ineligible study design: comparing lens materials and lens replacement schedules |
| Dávila 2012 | Ineligible study design: no randomization |
| Fahmy 2017 | Ineligible population: ages 10 to 60 years old |
| Gan 2012 | Ineligible study design: only hydrogel |
| Garaszczuk 2020 | Ineligible study design: observational study |
| Ghorbani‐Mojarrad 2022 | Ineligible study design: only hydrogel |
| Guillon 2000 | Ineligible study design: intervention duration less than 1 week |
| Guthrie 2022 | Ineligible study population: under 18 years old |
| He 2013 | Ineligible study design: only hydrogel |
| Insua Pereira 2018 | Ineligible study design: analysis did not account for the paired‐eye design |
| ISRCTN14364515 | Ineligible intervention: prototype lens |
| ISRCTN62897847 | Ineligible intervention: prototype lens |
| Jones 2000 | Ineligible study design: only hydrogel |
| jRCT1032200350 | Ineligible study design: observational study |
| Labuz 2017 | Ineligible study design: intervention duration less than 1 week |
| Lira 2006 | Ineligible study design: comparing lens materials and lens replacement schedules |
| Maldonado‐Codina 2004 | Ineligible study design: observational study |
| Malet 2003 | Ineligible study design: cross‐over trial with varying durations between interventions and between periods |
| Martin 2007 | Ineligible study design: analysis did not account for the cross‐over design |
| Maïssa 2012 | Ineligible study design: only silicone hydrogel, comparing lens materials and edge design |
| McNally 2002 | Ineligible study design: comparing lens materials and durations of wear |
| Michaud 2016 | Ineligible study design: analysis did not account for the cross‐over design |
| Miller 2021 | Ineligible study design: analysis did not account for the cross‐over design |
| Mousavi 2021 | Ineligible study design: no randomization |
| Navascues‐Cornago 2022 | Ineligible study design: intervention duration less than 1 week |
| NCT00584220 | Ineligible study design: analysis did not account for the cross‐over design |
| NCT00597467 | Ineligible study design: only silicone hydrogel |
| NCT00912028 | Ineligible study design: analysis did not account for the non‐independence of the data from the same person |
| NCT00985231 | Ineligible study design: comparing same lens material but different design (single versus multi‐focal) |
| NCT01634659 | Ineligible study design: only silicone hydrogel |
| NCT02055404 | Ineligible study design: intervention duration less than 1 week |
| NCT02410824 | Ineligible study population: includes ages 17 years and up |
| NCT02801006 | Ineligible study design: analysis did not account for the cross‐over design |
| NCT03006458 | Ineligible study design: analysis did not account for the cross‐over design |
| NCT03969290 | Ineligible study design: intervention duration less than 1 week |
| NCT04067141 | Ineligible study design: analysis did not account for the cross‐over design |
| NCT04310566 | Ineligible study design: only silicone hydrogel |
| NCT04585646 | Ineligible study design: only silicone hydrogel |
| NCT04615507 | Ineligible study design: only silicone hydrogel |
| NCT04968925 | Ineligible study design: only silicone hydrogel |
| Ozkan 2018 | Ineligible study design: comparing lens materials and lens design (single vision vs multifocal) |
| Sha 2018 | Ineligible study design: only hydrogel |
| Spyridon 2012 | Ineligible study design: varied comparator (undefined habitual lens) |
| Subbaraman 2021a | Ineligible intervention: prototype lens |
| Subbaraman 2021b | Ineligible intervention: prototype lens |
| Sulley 2011 | Ineligible population: ages 16 to 60 years old |
| Szczotka‐Flynn 2018 | Ineligible study design: analysis did not account for the cross‐over design |
Characteristics of studies awaiting classification [ordered by study ID]
Bergenske 2005b.
| Methods | RCT, paired‐eye design |
| Participants | Country: not reported Setting: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Omafilcon A
Galyfilcon A
|
| Outcomes |
Primary outcomes:
Secondary outcomes: not specified Measurement time points: baseline and 4 weeks per sequence |
| Notes | Sponsorship source: "Supported by a grant from CopperVision." Author's name: Peter Bergenske Institution: Pacific University, College of Optometry Comments: whether analysis accounted for cross‐over design was unclear |
Eiden 2007.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: 18 years old or older; current SCL wearer with symptoms and/or reduced wearing time from contact lens dryness or discomfort; spherical refractive error ranged from –1.00 to –6.00 DS; refractive astigmatism between 0.75 DC and 1.50 DC Exclusion criteria: not reported |
| Interventions | Galyfilcon A
Balafilcon A
Lotrafilcon B
Omafilcon B
Ocufilcon D
|
| Outcomes |
Primary outcomes:
Secondary outcomes: not specified |
| Notes | Sponsorship source: "The study was conducted via a non‐restricted research grant from CooperVision to EyeVis Eye and Vision Research Institute" Author's name: Barry Eiden Institution: EyeVis Eye and Vision Research Institute and a private practice Comments: whether analysis accounted for cross‐over design was unclear |
ISRCTN50917346.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: at least 40 years old; BCVA of at least 20/25 in each eye; current multifocal SCL wearer; refraction distance sphere: −6.00 D to +4.00 D; astigmatism: 0.00 D to −0.75 D; near addition: +0.75 D to +2.50 D Exclusion criteria: currently wearing study contact lenses; ocular condition(s) preventing contact lens wear; monocular correction; pregnant or lactating |
| Interventions | Invigor
Etafilcon A
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
| Notes | Sponsorship source: CooperVision Inc. Author's name: Deborah Moore Institution: Ocular Technology Group ‐ International Comments: whether analysis accounted for cross‐over design was unclear |
ISRCTN54358864.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: at least 40 years old; BCVA of at least 20/25 in each eye; current multifocal SCL wearer; refraction distance sphere: −6.00 D to + 4.00 D; astigmatism: 0.00 D to −0.75 D; near addition: +0.75 D to +2.50 D Exclusion criteria: currently wearing study contact lenses; ocular condition(s) preventing contact lens wear; monocular correction; pregnant or lactating |
| Interventions | Hioxifilcon A
Etafilcon A
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Sponsorship source: CooperVision Author's name: Deborah Moore Institution: Ocular Technology Group and CooperVision Comments: whether the analysis accounted for cross‐over design was unclear |
jRCT2052210025.
| Methods | RCT, paired‐eye design |
| Participants |
Inclusion criteria: age 20 years or older; refractive error (equivalent spherical power: −1.00 D to −6.00 D); subjective symptoms with SCL wear: eye discomfort (questionnaire score of 3 to 4 in each eye), itching (over 2 points in each eye); positive allergy test within the last year; astigmatism (less than or equal to −1.50 D); BCVA equal or better than 1.0 in each eye; habitual SCL wearers Exclusion criteria: moderate or higher corneal, conjunctival, and eyelid findings; allergic symptoms to antiallergic agents; eye diseases except for refractive error and allergic conjunctivitis; dry eye and lacrimal disorders; concomitantly prohibited drugs; history of anaphylaxis to sodium cromoglycate; previous hard contact lens use; refractive surgery; dry, high‐dust, and chemical living environments; pregnant or lactating |
| Interventions | 2 types of daily disposable SCL (C‐BAC1423) worn at the same time (left or right eyes based on randomization) for 2 weeks Notes: lens material is unknown. No response to an email requesting clarification from investigators |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Sponsorship source: SEED Co. Ltd. Author's name: Kikue Nakamura Institution: SEED Co. Ltd. Comments: no trial results available |
Maldonado‐Codina 2021.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: 18 years of age and older; currently using SCL or previous 6 months; sphere between −1.00 and −6.00 DS; cylinder between −0.75 and −2.00 DC; attain at least 0.20 logMAR high contrast VA in each eye; able to use SCL at least 8 hours per day, 5 days per week Exclusion criteria: ocular or systemic disorder that would normally contradict SCL wear; use of topical eye medication; cataract or refractive surgery; pregnant or breastfeeding |
| Interventions | Somofilcon A
Etafilcon A
Nelfilcon A
Notes: back surface toric; dual thin zones superiorly and inferiorly |
| Outcomes |
Primary outcome:
Secondary outcomes: not specified |
| Notes | Sponsorship source: "This work was funded by CooperVision Incorporated." Author's name: Carole Maldonado‐Codina Institution: University of Manchester, UK Comments: whether the analysis accounted for cross‐over design was unclear |
NCT00639379.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: between 18 and 45 years of age; currently successful wearer for at least 3 months of Bausch + Lomb SofLens 66 Toric hydrogel lenses; agree to daily wear schedule for at least 8 hours per day every day during the study; spherical component between −1.00 D and −5.25 D; cylinder between 0.75 D and 2.25 D and cylinder axis within 10° of the vertical and within 30° of the horizontal in both eyes; BCVA of at least 20/25 or better in each eye Exclusion criteria: presbyopic; previous refractive surgery; current or previous orthokeratology treatment; presence of ocular or systemic disease or has need for medication (ocular or systemic) that might interfere with contact lens wear; clinical findings that would contraindicate contact lens wear; pregnant or lactating |
| Interventions | Senofilcon A
Alphafilcon A
|
| Outcomes |
Primary outcome:
Secondary outcomes: not specified |
| Notes | Sponsorship source: Johnson & Johnson Vision Care Inc. Author's name: Kurt Moody, OD Institution: Vistakon Comments: whether the analysis accounted for cross‐over design was unclear |
NCT00886119.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: at least 35 years of age; BCVA of at least 20/40 in each eye; spectacle add from +1.50 D to +2.50 D; can fit study sphere powers (Plano to −4.00 D); currently wearing SCL at least 5 days a week Exclusion criteria: requires concurrent ocular medication; eye injury or surgery within 12 weeks immediately prior to enrollment; systemic or ocular abnormality likely to affect successful wear of SCL; previous refractive surgery; astigmatism > 1.00 D |
| Interventions | Lotrafilcon B
Omafilcon A
|
| Outcomes |
Primary outcome:
Secondary outcomes: not specified |
| Notes | Sponsorship source: CIBA Vision (Alcon Research) Author's name: not reported Institution: CIBA Vision Comments: whether the analysis accounted for cross‐over design was unclear |
NCT01669629.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: between 18 and 45 years of age; willing to wear the study lenses for at least 8 hours per day, 7 days per week; current successful SCL wearer; spherical prescription −1.00 D to −6.00 D; astigmatism less than or equal to 0.75 D; BCVA of 20/30 (6/9) or better Exclusion criteria: ocular or systemic diseases or treatments that may interfere with contact lens wear; clinically significant biomicroscopy findings (Grade 3 or 4); previous hard contact lens use; pregnancy or lactation; no extended wear in the last 3 months |
| Interventions | Delefilcon A
Etafilcon A
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Sponsorship source: Johnson & Johnson Vision Care Inc. Author's name: Kathy Osborn Institution: Vistakon Comments: whether the analysis accounted for cross‐over design was unclear |
NCT01917162.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: adapted SCL wearer; spherical distance contact lens prescription between −1.00 and −6.00 D; spectacle cylinder ≤ 0.75 D in the least astigmatic eye, ≤ 1.00 D in the other; correctable to 6/9 (20/30) in both eyes Exclusion criteria: a systemic or ocular disease that might interfere with contact lens wear; corneal refractive surgery and any anterior segment surgery; systemic or topical medication contraindicating contact lens wear; use of gas‐permeable contact lenses within 1 month preceding the study |
| Interventions | Nelfilcon A
UltraFilcon B
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Sponsorship source: Alcon Research Author's name: Joachim Nick Institution: Alcon Research Comments: no trial results available |
NCT02920983.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: age 18 years or older; contact lens spherical prescription between −1.00 and −6.00 D; cylindrical correction of −0.75 D or less; can attain at least 0.10 logMAR; currently use SCL or have done so in the previous 6 months; willing to wear SCL at least 5 days per week and for at least 8 hours per day Exclusion criteria: ocular or systemic disorder or treatments that would normally contraindicate contact lens wear; previous cataract or refractive surgery; previous hard contact lens wear; pregnant or lactating |
| Interventions | Somofilcon A
Nelfilcon A II 2
Omafilcon A ll 2
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Sponsorship source: CooperVision Inc. Author's name: Carole Maldonado‐Codinal, PhD, FAAO Institution: Eurolens Research, University of Manchester Comments: whether the analysis accounted for cross‐over design was unclear |
NCT03341923.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: age 40 years or older; habitual multifocal SCL silicone hydrogel wearer; requiring a near spectacle addition of +0.50 to +2.50 D; power range +5.00 to −9.00 D; cylinder less than 1.00 D; VA correctable to 20/30 or 0.2 logMAR or better; willing to wear lenses every day (a minimum of 10 days) for 6 hours per day Exclusion criteria: currently wearing study lenses; systemic or ocular disease that contraindicate contact lens wear; use of systemic or ocular medications that contraindicate contact lens wear; monovision; moderate or severe slit lamp findings; refractive surgery; eye injury or surgery within 12 weeks |
| Interventions | Delefilcon A
Etafilcon A
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Sponsorship source: Alcon Japan Ltd. Author's name: Alcon Japan Ltd. Institution: Alcon Japan Ltd. Comments: whether the analysis accounted for cross‐over design was unclear |
NCT03349632.
| Methods | RCT, parallel‐group, cross‐over design |
| Participants |
Inclusion criteria: aged 18 years and older; successfully wears daily disposable spherical SCL in both eyes for a minimum of 5 days per week, 8 hours per day, at least the past 3 months; BCVA of 20/25 Snellen or better in each eye Exclusion criteria: any eye condition, surgery, disease, or use of medication that contraindicates contact lens wear; routinely sleeps in habitual contact lenses; currently wears any study SCL |
| Interventions | Verofilcon A
Senofilcon A
Stenfilcon A
Etafilcon A
|
| Outcomes |
Primary outcome:
Secondary outcomes: not specified |
| Notes | Sponsorship source: Alcon Research Author's name: not reported Institution: Alcon Research Comments: whether the analysis accounted for cross‐over design was unclear |
NCT04032457.
| Methods | RCT, parallel‐group, cross‐over design |
| Participants |
Inclusion criteria: 18 to 42 years of age; currently wearing spherical daily disposable contact lenses at least 5 days/week; vision correctable to 20/30 with spherical lenses in powers from +3.00 to +1.00 DS and from −1.00 to −7.00 DS; VA corrected to at least 20/30 with spherical contact lens Exclusion criteria: ocular or systemic disease that contraindicates SCL wear; sensitivity to diagnostic pharmaceuticals; pregnant, lactating, or planning pregnancy; aphakic; undergone refractive error surgery |
| Interventions |
Phase 1 Olifilcon B with tangible coatings
Etafilcon A
Senofilcon A
Delefilcon A
Phase 2 Etafilcon A with tangible coatings
Etafilcon A
Nesofilcon A
Nelfilcon A
Notes: 2 phases, only 6 parallel groups in phase A compared silicone hydrogel SCL to daily SCL in 12 cross‐over interventions (12 participants in each intervention period); the other 6 groups in phase B compared a new‐to‐market hydrogel SCL to daily SCL |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Sponsorship source: Vision Service Plan Authors' names: Robin L Chalmers, OD (investigator), Alex Dreu, Plexus Optix (results) Institution: Vision Service Plan, Foresight Regulatory Strategies Inc. Comments: quality control review of results not completed; whether the analysis accounted for cross‐over design was unclear |
NCT04527978.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: aged 18 years and older; successful wear of spherical SCL in both eyes for a minimum of 5 days per week and 10 hours per day during the past 3 months; willing to wear contact lenses for at least 16 hours per day Exclusion criteria: ocular condition that contraindicates contact lens wear; previous or current habitual wearer of study contact lenses |
| Interventions | Verofilcon A
Nesofilcon A
|
| Outcomes |
Primary outcomes:
Secondary outcomes: not specified |
| Notes | Sponsorship source: Alcon Research Author's name: CDMA Project Lead, Vision Care Institution: Alcon Research LLC Comments: whether the analysis accounted for cross‐over design was unclear |
NCT04908488.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: aged 18 years and older; successful wear of toric SCL in both eyes for a minimum of 5 days per week and 10 hours per day during the past 3 months; willing to wear study contact lenses for at least 16 hours per day Exclusion criteria: habitual wear of study contact lenses; spherical monovision or multifocal lens wearer; habitual contact lenses when sleeping for at least 1 night per week over 3 months prior to enrollment |
| Interventions | Verofilcon A
Etafilcon A
|
| Outcomes |
Primary outcome:
Secondary outcome: not specified |
| Notes | Sponsorship source: Alcon Research Author's name: Clinical Trial Lead, Vision Care, Alcon Research LLC Institution: Alcon Research LLC Comments: no trial results |
NCT05138783.
| Methods | RCT, cross‐over design |
| Participants |
Inclusion criteria: age 18 years and older; successful wear of spherical SCL for at least 3 months, with a minimum wearing time of 5 days per week and 10 hours per day; willing to wear contact lenses for at least 16 hours on the day prior to specified visit days Exclusion criteria: participation in a clinical trial within the previous 30 days or currently enrolled in any clinical trial; habitual use of study SCL; habitual monovision or multifocal lens wear |
| Interventions | Verofilcon A
Nesofilcon A
|
| Outcomes |
Primary outcome:
Secondary outcome: not specified |
| Notes | Sponsorship source: Alcon Research Author's name: Clinical Trial Lead, Vision Care, Alcon Research LLC Institution: Alcon Research LLC Comments: no trial results |
Wong 2008.
| Methods | RCT, cross‐over design |
| Participants | Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Balafilcon A
Omafilcon A
|
| Outcomes |
Primary outcomes:
Secondary outcomes: not specified |
| Notes | Sponsorship source: "Supported by a grant from CopperVision." Author's name: Jennifer Wong Institution: co‐author affiliations with Ohio State University, College of Optometry Comments: conference abstract; whether the analysis accounted for cross‐over design was unclear |
BCVA: best‐corrected visual acuity CLDEQ‐8: Contact Lens Dry Eye Questionnaire‐8 D: diopters DC: diopters cylinder DS: diopters sphere logMAR: Logarithm of the Minimum Angle of Resolution NEI: National Eye Institute OSDI: Ocular Surface Disease Index RCT: randomized controlled trial SCL: soft contact lens(es) VA: visual acuity VAS: visual analog scale
Differences between protocol and review
We used the investigator's definition when provided or the company's definition as described on their official website when there was a contact lens material that was not explicitly defined as silicon hydrogel or hydrogel.
-
We redefined the following important outcomes to reflect how the included primary studies had reported them:
corneal staining scores, as assessed by any integer grading scale;
conjunctival staining scores, as assessed by any integer grading scale;
conjunctival redness scores, as assessed by any integer grading scale.
The time frame for reporting vision‐threatening ocular adverse events was extended beyond the four weeks specified in the protocol, Haworth 2021, to allow for longer‐term adverse events as reported by the primary studies.
We opted to treat participants leaving a trial due to an adverse event as a possible vision‐threatening adverse event. We did not impute any outcome data.
We planned to assess risk of bias for 'patient comfort as measured by CLDEQ‐8.' Given that none of the included studies reported CLDEQ‐8 scores, we chose to assess risk of bias on 'comfort scores as measured by VAS.'
We planned to include 'comfort score as measured by SPEED' in Table 1; however, none of the included studies reported this outcome. We chose to include 'comfort score as measured by VAS' instead.
We planned sensitivity analysis by excluding trials at high risk of bias, industry‐funded studies, and studies that failed to address the unit of analysis. However, too few trials provided outcome data, rendering any sensitivity analysis uninformative or impossible.
We excluded cross‐over trials where the reported conduct and analysis did not account for the cross‐over design. We assessed cross‐over trials as awaiting classification.
We added Louis Leslie of CEV@US as an author and removed Samuel A Abariga of CEV@US, who was an author of the protocol (Haworth 2021).
Contributions of authors
Conception and design of protocol (ADP, KH, DF, Samuel A Abariga, DT)
Drafting the review or commenting on it critically for intellectual content (KH, DT, LL, DF, ADP)
Final approval of the document to be published (KH, DT, LL, DF, ADP)
Sources of support
Internal sources
-
None, Other
No internal source of support
External sources
-
National Eye Institute, National Institutes of Health, USA
Cochrane Eyes and Vision US Project, supported by grant UG1EY020522 (PI: Tianjing Li, MD, MHS, PhD)
-
Public Health Agency, UK
The HSC Research and Development (R&D) Division of the Public Health Agency funds the Cochrane Eyes and Vision editorial base at Queen's University Belfast (ended in April 2023).
-
Queen's University Belfast, UK
Gianni Virgili, Co‐ordinating Editor for Cochrane Eyes and Vision's work, is funded by the Centre for Public Health, Queen's University of Belfast, Northern Ireland (ended in April 2023).
Declarations of interest
KH: Integra LifeSciences (Research), Southern College of Optometry (Research, Honorarium), Association of Schools and Colleges of Optometry (Research). None of these activities are directly related to the proposed review.
DT: None
LL: Reports a grant from the National Eye Institute, National Institutes of Health, USA; payment to institution.
DF: Southern College of Optometry (Honorarium), American Academy of Optometry (Honorarium), PentaVision (Honorarium), Scleral Lens Education Society (Honorarium), Gas Permeable Lens Institute (Honorarium). None of these activities are directly related to the proposed review.
ADP: Alcon Research LLC (Consulting, Research), Bausch + Lomb (Research), Contamac (Research), CooperVision (Consulting), Euclid Systems (Research, Consulting), EyeGate Pharmaceuticals Inc. (Consulting), EpiTech (Consulting), Lexitas Pharma Services (Employee), Optikal Care Inc. (Consulting), Paragon Vision Sciences (Consulting), PentaVision (Honorarium), Southern College of Optometry (Honorarium), American Academy of Optometry (Honorarium), and American Optometric Association (Honorarium). None of these activities are directly related to the proposed review.
New
References
References to studies included in this review
Diec 2012 {published data only}
- ACTRN12609000812291. Prospective, open labelled, randomised daily wear trial comparing the ocular response and product performance between 1 Day Acuvue Moist, 1 Day Acuvue TruEye and Acuvue Oasys contact lenses in new and experienced wearers. anzctr.org.au/ACTRN12609000812291.aspx (first received 17 September 2009).
- Diec J, De La Jara P, Willcox M, Holden B. Effect of contact lenses on the ocular surface during daily disposable wear. Contact Lens and Anterior Eye 2011;34(Suppl 1):S23. [CENTRAL: CN-01129762] [Google Scholar]
- Diec J, Lazon de la Jara P, Willcox M, Holden BA. The clinical performance of lenses disposed of daily can vary considerably. Eye and Contact Lens 2012;38(5):313‐8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hall 2009 {published data only}
- Hall L, Topic C, Henderson T, Young G, Hunt C, Moody K. Multi-centre clinical evaluation of two daily disposable contact lenses. In: American Academy of Optometry Annual Meeting; 2009 Nov 11-14; Orlando (FL). American Academy of Optometry, 2009.
- NCT00727558. A comparison of daily disposable contact lenses. ClinicalTrials.gov/show/NCT00727558 (first received 4 August 2008).
Marx 2009 {published data only}
- Marx S, Sickenberger W, Michel M, Fahmy M, Giles T. Contact lens-wearing experience with daily disposable lenses in neophytes. In: American Academy of Optometry Annual Meeting; 2009 Nov 11-14; Orlando (FL). American Academy of Optometry, 2009.
Muhafiz 2019 {published data only}
- Muhafiz E, Bayhan HA, Sahin S, Gocmen AY, Aslan Bayhan S, Gurdal C. Evaluation of the ocular surface in different contact lens replacement schedules. Cornea 2019;38(5):587-94. [DOI] [PubMed] [Google Scholar]
NCT00241280 {published data only}
- NCT00241280. Study intended to evaluate the safety and effectiveness of a Johnson & Johnson Vision Care Inc, (JJVCI) contact lens for use on an extended-wear basis for up to 7 days (6 nights). ClinicalTrials.gov/show/NCT00241280 (first received 18 October 2005).
NCT00762788 {published data only}
- NCT00762788. Clinical trial of several contact lenses in extended wear. ClinicalTrials.gov/show/NCT00762788 (first received 30 September 2008).
NCT01354223 {published data only}
- NCT01354223. Clinical evaluation of stenfilcon a soft contact lens when compared to ocufilcon b soft contact lens [Evaluation of the CooperVision Stenfilcon A soft contact lens for daily wear when compared to the CooperVision Ocufilcon B soft contact lens]. ClinicalTrials.gov/show/NCT01354223 (first received 16 May 2011).
References to studies excluded from this review
ACTRN12610000639022 {published data only}
- ACTRN12610000639022. Contralateral, cross over clinical trial to assess the effect of novel and commercially available contact lenses on corneal topography [A prospective, open-label, contralateral daily wear, cross over, randomised clinical trial assessing the effect of wearing a novel and five commercially available contact lenses for 1 week on corneal topography worn in experienced myopic contact lens wearers]. anzctr.org.au/ACTRN12610000639022.aspx (first received 5 August 2010).
ACTRN12618000039280 {published data only}
- ACTRN12618000039280. A clinical trial where 120 participants will wear one of three contact lenses (SEED Pure, CooperVision Proclear or Johnson & Johnson Vita), for three months with monthly replacement to determine clinical performance [A prospective, randomised, open-label, bilateral wear, parallel-group, dispensing clinical trial to compare the clinical performance of SEED ionic bond (SIB) contact lens material against two commercially-available lens materials when worn for three months daily-wear with monthly replacement, and compare the clinical performance of SIB contact lens material after two weeks and one month of wear.]. anzctr.org.au/ACTRN12618000039280.aspx (first received 12 January 2018).
Arroyo‐Del Arroyo 2021 {published data only}
- Arroyo-Del Arroyo C, Novo-Diez A, Blanco-Vazquez M, Fernandez I, Lopez-Miguel A, Gonzalez-Garcia MJ. Does placebo effect exist in contact lens discomfort management? Contact Lens and Anterior Eye 2021;44(4):101370. [DOI: 10.1016/j.clae.2020.09.003] [DOI] [PubMed] [Google Scholar]
Bergenske 2005a {published data only}
- Bergenske P, Morgan D, Jensen B, Peine R, Schaefer S, Irravani N. Comfort and clinical performance comparison between a silicone hydrogel and a biomimetic hydrogel for daily wear. In: American Academy of Optometry Annual Meeting; 2005 Dec 8-11; San Diego (CA). American Academy of Optometry, 2005.
Bishop 2022 {published data only}
- Bishop MJ, Sun CK, Coles-Brennan C, Gallois-Bernos A. Evaluation of daily disposable senofilcon A contact lenses in a symptomatic population. Contact Lens and Anterior Eye 2022;45(5):101574. [DOI: 10.1016/j.clae.2022.101574] [DOI] [PubMed] [Google Scholar]
Brennan 2002 {published data only}
- Brennan N, Coles M, Comstock T, Levy B. A 1-year prospective clinical trial of balafilcon a (PureVision) silicone-hydrogel contact lenses used on a 30-day continuous wear schedule. Ophthalmology 2002;109(6):1172‐7. [DOI] [PubMed] [Google Scholar]
Bucci 1997 {published data only}
- Bucci F, Tanner J, Moody K, Myers P. Clinical performance of the Optima toric contact lens versus the CSI toric contact lens. CLAO Journal: official publication of the Contact Lens Association of Ophthalmologists 1997;23(1):43‐8. [PubMed] [Google Scholar]
Carpena‐Torres 2021 {published data only}
- Carpena-Torres C, Pastrana C, Rodríguez-Pomar C, Serramito M, Carracedo G. Stabilization of comfort and visual quality after the insertion of soft contact lenses. Contact Lens and Anterior Eye 2021;45(4):101498. [DOI: 10.1016/j.clae.2021.101498] [DOI] [PubMed] [Google Scholar]
Cheung 2007 {published data only}
- Cheung SW, Cho P, Chan B, Choy C, Ng V. A comparative study of biweekly disposable contact lenses: silicone hydrogel versus hydrogel. Clinical and Experimental Optometry 2007;90(2):124‐31. [DOI] [PubMed] [Google Scholar]
Cho 2000 {published data only}
- Cho P, Ng V. Clinical performances of two disposable soft contact lenses of different materials on Hong Kong-Chinese. Contact Lens Anterior Eye 2000;23(2):53-60. [DOI] [PubMed] [Google Scholar]
Comstock 2001 {published data only}
- Comstock T, Reindel W, Dey D. The evaluation of silicone hydrogel and traditional hydrogel materials for 7 day continuous wear among new contact lens wearers. Optometry and Vision Science 2001;78(12):201. [Google Scholar]
CTRI/2015/04/005701 {published data only}
- CTRI/2015/04/005701. A clinical trial to evaluate investigational silicone hydrogel contact lenses worn continuously for one week [Echo ionic silicone hydrogel 6-month extended wear investigation - NIL]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=11407 (first received 16 April 2015).
- NCT02543528. Echo ionic silicone hydrogel 6-month extended wear investigation [A clinical trial to evaluate investigational silicone hydrogel contact lenses worn continuously for one week]. clinicaltrials.gov/show/NCT02543528 (first received 7 September 2015).
Dalcoll 2008 {published data only}
- Dalcoll MW, Alves MR, Barreto J Jr, Yamane Ide S, Bechara S, Mukai A. Evaluation of optical performance of soft contact lenses in myopic correction [Avaliação da qualidade óptica de lentes de contato gelatinosas na correção de miopia]. Arquivos Brasileiros de Oftalmologia 2008;71(6 Suppl):37‐41. [DOI] [PubMed] [Google Scholar]
Davies 2006 {published data only}
- Davies I, Veys J. Subjective assessment of results obtained with two silicon-hydrogel contact lenses. Oftalmologia (Bucharest, Romania: 1990) 2006;50(2):126-8. [PubMed] [Google Scholar]
Dávila 2012 {published data only}
- Dávila J, Romero Y, Rodríguez M. Changes in the ocular surface and tear film during 30 days of daily use of hydrogel and silicone hydrogel soft contact lens [Cambios en la superficie ocular y en la película lagrimal durante 30 días de uso diario de lentes de contacto blandos de hidrogel e hidrogel de silicona]. Ciencia y Tecnología para la Salud Visual y Ocular 2012;10(2):47-56. [Google Scholar]
Dumbleton 1998 {published data only}
- Dumbleton K, Richter D, Simpson T, Fonn D, Chalmers R. A comparison of the vascular response to extended wear of conventional lower dk and experimental high dk hydrogel contact lenses. In: American Academy of Optometry Annual Meeting; 1998 Dec 10-14; San Francisco (CA). American Academy of Optometry, 1998:170.
- Dumbleton KA, Robin CL, Richter DB, Fonn D. Vascular response to extended wear of hydrogel lenses with high and low oxygen permeability. Optometry and Vision Science 2001;78(3):147-51. [DOI] [PubMed] [Google Scholar]
Fahmy 2017 {published data only}
- Fahmy M, Wang CH, Sickenberger W, Marx S. Clinical evaluation of two daily disposable contact lenses in neophytes. Investigative Ophthalmology and Visual Science 2017;58(8):3050. [Google Scholar]
Gan 2012 {published data only}
- Gan J-h, Zhang J, Lu H-b. Clinical application and follow-up of new-type HD soft hydrophilic contact lenses. Chinese Journal of Tissue Engineering Research 2012;16(29):5506‐10. [CENTRAL: CN-01019855] [Google Scholar]
Garaszczuk 2020 {published data only}
- Garaszczuk IK, Mousavi M, Szczesna-Iskander DH, Cerviño A, Iskander DR. A 12-month prospective study of tear osmolarity in contact lens wearers refitted with daily disposable soft contact lenses. Optometry and Vision Science 2020;97(3):178-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT03531346. Tear osmolarity changes in habitual contact lens wearers [A 12-month prospective study of tear osmolarity in contact lens wearers refitted with daily disposable soft contact lenses]. clinicaltrials.gov/ct2/show/NCT03531346 (first received 6 October 2016).
Ghorbani‐Mojarrad 2022 {published data only}
- Ghorbani-Mojarrad N, Cargill C, Collard S, Terry L. Patient experience and physiological response to two commercially available daily disposable myopia control contact lenses. Contact Lens and Anterior Eye 2022;45(2):101426. [DOI] [PubMed] [Google Scholar]
Guillon 2000 {published data only}
- Guillon M, Girard-Claudon K, Cooper P, Maissa C, Poling T. Comparative visual performance of acuvue brand bifocal contact lenses and focus progressives contact lenses. Optometry and Vision Science 2000;77(Suppl 12):162. [Google Scholar]
Guthrie 2022 {published data only}
- Guthrie S, Ng A, Woods J, Vega J, Orsborn G, Jones L. Exploring the factors which impact overall satisfaction with single vision contact lenses. Contact Lens and Anterior Eye 2022;45(5):101579. [DOI: 10.1016/j.clae.2022.101579] [DOI] [PubMed] [Google Scholar]
He 2013 {published data only}
- He Y, Lü HB, Ouyang K, Gan JH, Xu M, Yu L, et al. Extra-care soft contact lenses: clinical efficacy and safety. Chinese Journal of Tissue Engineering Research 2013;17(8):1423‐9. [DOI: 10.3969/j.issn.2095-4344.2013.08.016] [DOI] [Google Scholar]
Insua Pereira 2018 {published data only}
- Insua Pereira E, Lira M. Comfort, ocular dryness, and equilibrium water content changes of daily disposable contact lenses. Eye and Contact Lens 2018;44(Suppl 2):S233‐40. [DOI] [PubMed] [Google Scholar]
ISRCTN14364515 {published data only}
- ISRCTN14364515. Comparative performance and acceptance validation study of CVI new multifocal contact lenses [Comparative performance and acceptance validation study of CooperVision new multifocal contact lenses vs. 1-Day Acuvue® moist contact lenses]. isrctn.com/ISRCTN14364515 (first received 12 February 2020). [DOI: 10.1186/ISRCTN14364515] [DOI]
ISRCTN62897847 {published data only}
- ISRCTN62897847. Validation of CV multifocal daily disposable contact lenses performance in a multisite study [Clinical validation multisite study of CV multifocal contact lenses]. isrctn.com/ISRCTN62897847 (first received 24 January 2019). [DOI: 10.1186/ISRCTN62897847] [DOI]
Jones 2000 {published data only}
- Jones L, Mann A, Evans K, Franklin V, Tighe B. An in vivo comparison of the kinetics of protein and lipid deposition on group II and group IV frequent-replacement contact lenses. Optometry and Vision Science 2000;77(10):503‐10. [DOI] [PubMed] [Google Scholar]
jRCT1032200350 {published data only}
- jRCT1032200350. Wear study of "clariti 1 day" [Wear study of daily disposable silicone hydrogel lens "clariti 1 day"]. jrct.niph.go.jp/en-latest-detail/jRCT1032200350 (first received 5 February 2021).
Labuz 2017 {published data only}
- Łabuz G, López-Gil N, den Berg TJ, Vargas-Martín F. Ocular straylight with different multifocal contact lenses. Optometry and Vision Science 2017;94(4):496‐504. [DOI] [PubMed] [Google Scholar]
Lira 2006 {published data only}
- Lira MM, Oliveira MECDR, Vilar EY, Azeredo J, Santos L. The effect of conventional and silicone hydrogel contact lenses wear on the tear film. Investigative Ophthalmology and Visual Science 2006;47(13):116. [Google Scholar]
Maïssa 2012 {published data only}
- Maïssa C, Guillon M, Garofalo RJ. Contact lens-induced circumlimbal staining in silicone hydrogel contact lenses worn on a daily wear basis. Eye and Contact Lens 2012;38(1):16-26. [DOI] [PubMed] [Google Scholar]
- NCT00940459. Subjective and conjunctival response to edge design of different silicone hydrogels. ClinicalTrials.gov/ct2/show/NCT00940459 (first received 16 July 2009).
Maldonado‐Codina 2004 {published data only}
- Maldonado-Codina C, Morgan P, Schnider C, Efron N. Short-term physiologic response in neophyte subjects fitted with hydrogel and silicone hydrogel contact lenses. Optometry and Vision Science 2004;81(12):911‐21. [PubMed] [Google Scholar]
- Maldonado-Codina C, Morgan P, Schnider C, Efron N. Short-term physiological response in neophyte subjects fitted with hydrogel and silicone hydrogel contact lenses. In: American Academy of Optometry Annual Meeting; 2004 Dec 9-12; Tampa (FL). American Academy of Optometry, 2004. [PubMed]
Malet 2003 {published data only}
- Malet F, Pagot R, Peyre C, Subirana X, Lejeune S, George-Vicariot MN, et al. Clinical results comparing high-oxygen and low-oxygen permeable soft contact lenses in France. Eye and Contact Lens 2003;29(1):50‐4. [DOI] [PubMed] [Google Scholar]
- Malet F, Pagot R, Peyre C, Subirana X, Lejeune S, George-Vicariot MN, et al. Subjective experience with high-oxygen and low-oxygen permeable soft contact lenses in France. Eye and Contact Lens 2003;29(1):55‐9. [DOI] [PubMed] [Google Scholar]
Martin 2007 {published data only}
- Martin R, Izquierdo M, Saber A. Investigation of posterior corneal curvature in CL-induced corneal swelling. Contact Lens and Anterior Eye 2009;32(6):288‐93. [DOI] [PubMed] [Google Scholar]
- Martin R, Nuñez L, Sastre J, Juan V, Rodriguez G. Constancy of the orbscan acoustic factor to detect contact lens-induced corneal swelling. Clinical and Experimental Optometry 2011;94(4):352‐60. [DOI] [PubMed] [Google Scholar]
- Martin R, Juan V, Rodriguez G, Cuadrado R, Fernandez I. Measurement of corneal swelling variations without removal of the contact lens during extended wear. Investigative Ophthalmology and Visual Science 2007;48(7):3043-50. [DOI] [PubMed] [Google Scholar]
- Martin R, Juan V, Rodriguez G, Fonseca S, Martin S. Contact lens-induced corneal peripheral swelling differences with extended wear. Cornea 2008;27(9):976‐9. [DOI] [PubMed] [Google Scholar]
- Martin R, Juan V, Rodriguez G, Fonseca S, Martin S. Contact lens-induced corneal peripheral swelling: orbscan repeatability. Optometry and Vision Science 2009;86(4):340‐9. [DOI] [PubMed] [Google Scholar]
- Martin R, Juan V, Rodriguez G, Martin S, Fonseca S. Initial comfort of lotrafilcon A silicone hydrogel contact lenses versus etafilcon A contact lenses for extended wear. Contact Lens and Anterior Eye 2007;30(1):23‐8. [DOI] [PubMed] [Google Scholar]
McNally 2002 {published data only}
- McNally JJ, Chalmers RL, McKenney CD. Factors associated with change in refractive error during a year of 6 night or 30 night extended wear. Investigative Ophthalmology and Visual Science 2002;43(13):3110. [Google Scholar]
Michaud 2016 {published data only}
- Michaud L, Forcier P. Comparing two different daily disposable lenses for improving discomfort related to contact lens wear. Contact Lens and Anterior Eye 2016;39(3):203‐9. [DOI] [PubMed] [Google Scholar]
Miller 2021 {published data only}
- Miller J, Giedd B, Subbaraman LN. Clinical comparison of a silicone hydrogel and a conventional hydrogel daily disposable contact lens. Clinical Ophthalmology 2021;15:4339-45. [DOI: 10.2147/OPTH.S332651] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03888469. Clinical comparison of DDT2 contact lens and a daily disposable contact lens - study 1. clinicaltrials.gov/ct2/show/NCT03888469 (first received 25 March 2019).
- Subbaraman LN, Miller J, Giedd B. Clinical subjective performance of two daily disposable soft contact lenses. Investigative Ophthalmology and Visual Science 2021;62(8):670. [CENTRAL: CN-02324854]
Mousavi 2021 {published data only}
- Mousavi M, Garaszczuk IK, De Jesus DA, Szczesna-Iskander DH, Armstrong RA, Nichols KK, et al. Tear film surface quality in modern daily disposable contact lens wear. Eye and Contact Lens 2021;47(12):631-7. [DOI] [PubMed] [Google Scholar]
Navascues‐Cornago 2022 {published data only}
- Navascues-Cornago M, Sun T, Read ML, Morgan PB. The short-term effect of contact lens wear on blink characteristics. Contact Lens and Anterior Eye 2022;45(5):101596. [DOI: 10.1016/j.clae.2022.101596] [DOI] [PubMed] [Google Scholar]
NCT00584220 {published data only}
- NCT00584220. Evaluation of two toric soft contact lenses after two weeks of wear [Evaluation of fit and visual acuity of the investigational toric lens in two phases: part b: a dispensing cross-over comparison to SofLens66 toric]. ClinicalTrials.gov/show/NCT00584220 (first received 2 January 2008).
NCT00597467 {published data only}
- NCT00597467. Study of soft contact lens use with 7 day extended wear [An open-labeled, multi-center, randomized investigation of the VISA (comfilcon A) silicone hydrogel soft (hydrophilic) contact lens for use in a 7 day extended wear regimen]. ClinicalTrials.gov/show/NCT00597467 (first received 18 January 2008).
NCT00912028 {published data only}
- NCT00912028. Clinical performance comparison of several different contact lenses [Multicenter, single-masked, randomized, parallel, controlled study to compare senofilcon A contact lenses to currently used contact lenses]. ClinicalTrials.gov/show/NCT00912028 (first received 3 June 2009).
NCT00985231 {published data only}
- NCT00985231. Performance evaluation of contact lenses among a population of adapted contact lens wearers. ClinicalTrials.gov/ct2/show/NCT00985231 (first received 28 September 2009).
NCT01634659 {published data only}
- NCT01634659. Clinical evaluation of two daily disposable silicone hydrogel contact lenses. ClinicalTrials.gov/ct2/show/NCT01634659 (first received 6 July 2012).
NCT02055404 {published data only}
- NCT02055404. On-eye evaluation of contact lens rotation marks. ClinicalTrials.gov/ct2/show/NCT02055404 (first received 5 February 2014).
NCT02410824 {published data only}
- NCT02410824. Bilateral dispensing clinical trial of stenfilcon A against etafilcon A for astigmatism. ClinicalTrials.gov/ct2/show/NCT02410824 (first received 8 April 2015).
NCT02801006 {published data only}
- NCT02801006. The clinical study of silicone hydrogel daily disposable stenfilcon A Toric Lens. ClinicalTrials.gov/show/NCT02801006 (first received 15 June 2016).
NCT03006458 {published data only}
- NCT03006458. A clinical comparison of 2 multifocal toric XR (extended range) contact lenses [A clinical comparison of the comfilcon A (extended range) XR toric multifocal and omafilcon B toric XR multifocal contact lenses]. ClinicalTrials.gov/show/NCT03006458 (first received 30 December 2016).
NCT03969290 {published data only}
- NCT03969290. Clinical performance of two daily disposable contact lenses - study 1. ClinicalTrials.gov/ct2/show/NCT03969290 (first received 31 May 2019).
NCT04067141 {published data only}
- NCT04067141. The clinical comparison of somofilcon a 1 day and nelfilcon a daily disposable contact lenses. ClinicalTrials.gov/show/NCT04067141 (first received 26 August 2019).
NCT04310566 {published data only}
- NCT04310566. Evaluation of a daily disposable novel multifocal contact lens in a myopic population. ClinicalTrials.gov/show/NCT04310566 (first received 17 March 2020).
NCT04585646 {published data only}
- NCT04585646. A two-week crossover dispensing evaluation of orion daily wear soft contact lens. ClinicalTrials.gov/show/NCT04585646 (first received 14 October 2020).
NCT04615507 {published data only}
- NCT04615507. Evaluation of a daily disposable novel multifocal contact lens in a myopic population; part 2. ClinicalTrials.gov/show/NCT04615507 (first received 4 November 2020).
NCT04968925 {published data only}
- NCT04968925. Evaluation of comfort for two marketed daily disposable contact lenses. ClinicalTrials.gov/show/NCT04968925 (first received 20 July 2021).
Ozkan 2018 {published data only}
- Ozkan J, Fedtke C, Chung J, Thomas V, Bakaraju Rc. Short-term adaptation of accommodative responses in myopes fitted with multifocal contact lenses. Eye and Contact Lens 2018;44(Suppl 1):S30‐7. [DOI] [PubMed] [Google Scholar]
Sha 2018 {published data only}
- ACTRN12615000652572. Dispensing study to assess the visual performance of optimised prototype contact lenses [Prospective, double-masked, bilateral wear, crossover, dispensing clinical trial to assess visual performance of multiple optimised prototype contact lens designs compared to commercial contact lenses]. anzctr.org.au/ACTRN12615000652572.aspx (first received 5 June 2015).
- ACTRN12617000247370. Dispensing study to assess the visual performance of optimised prototype contact lenses [Prospective, double-masked, bilateral wear, crossover, dispensing clinical trial to assess visual performance of multiple optimised prototype contact lens designs compared to commercial contact lenses]. anzctr.org.au/ACTRN12617000247370.aspx (first received 24 April 2015).
- Jong M, Tilia D, Sha J, Diec J, Thomas V, Bakaraju R. The relationship between visual acuity, subjective vision and willingness to purchase simultaneous-image contact lenses. Investigative Ophthalmology and Visual Science 2019;96(4):283-90. [DOI] [PubMed] [Google Scholar]
- NCT02484586. Dispensing study to assess the visual performance of optimised prototype contact lenses. ClinicalTrials.gov/ct2/show/NCT02484586 (first received 29 June 2015).
- Sha J, Tilia D, Kho D, Amrizal H, Diec J, Yeotikar N, et al. Visual performance of daily-disposable multifocal soft contact lenses: a randomized, double-blind clinical trial. Optometry and Vision Science 2018;95(12):1096‐1104. [DOI] [PubMed] [Google Scholar]
- Sha J, Tilia D, Kho D, Diec J, Yeotikar N, Jong M, et al. Visual performance of daily disposable multifocal soft contact lenses. Investigative Ophthalmology and Visual Science 2018;59(9):1793. [DOI] [PubMed] [Google Scholar]
Spyridon 2012 {published data only}
- Spyridon M, Hickson-Curran S, Hunt C, Young G. Eye sensitivity in soft contact lens wearers. Optometry and Vision Science 2012;89(12):1682‐90. [DOI] [PubMed] [Google Scholar]
Subbaraman 2021a {published data only}
- Subbaraman L, Cummings S, Brobst A. Comparison of the clinical performance of a novel silicone hydrogel and a HEMA-based daily disposable contact lens. Contact Lens and Anterior Eye 2021;44(1):7. [Google Scholar]
Subbaraman 2021b {published data only}
- Subbaraman L, Spear KG, Brobst A, Cummings S. Clinical lens fit characteristics of a new silicone hydrogel daily disposable and two commercially available daily disposable contact lenses. Contact Lens and Anterior Eye 2021;44(1):5. [Google Scholar]
Sulley 2011 {published data only}
- Sulley A, Young G, Lorenz KO, Hunt C. Clinical evaluation of fitting toric soft contact lenses to current non-users. Ophthalmic and Physiological Optics 2013;33(2):94‐103. [DOI] [PubMed] [Google Scholar]
- Sulley AL, Young G, Osborn KE, Hunt C. A multi-centre study of astigmatic non-users of toric soft contact lenses. Contact Lens and Anterior Eye 2011;34:S20. [Google Scholar]
Szczotka‐Flynn 2018 {published data only}
- NCT02328937. Central corneal swelling with silicone hydrogel materials. ClinicalTrials.gov/show/NCT02328937 (first received 31 December 2014).
- Szczotka-Flynn LB, Debanne S, Benetz BA, Wilson T, Brennan N. Daily wear contact lenses manufactured in etafilcon a are noninferior to two silicone hydrogel lens types with respect to hypoxic stress. Eye and Contact Lens 2018;44(3):190‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bergenske 2005b {published data only}
- Bergenske P, Borstad J, Gorger R, Romfh D, Smythe J, Iravani N. Comparative clinical evaluation of biomimetic and silicone hydrogel lenses in daily wear. In: American Academy of Optometry Annual Meeting; 2005 Dec 8-11; San Diego (CA). American Academy of Optometry, 2005.
Eiden 2007 {published data only}
- Eiden BS, Davis R. Clinical comparative evaluation of silicon hydrogel vs. non-silicon hydrogel toric contact lens designs for monthly replacement. In: American Academy of Optometry Annual Meeting; 2007 Oct 24-27; Tampa (FL). American Academy of Optometry, 2007.
ISRCTN50917346 {published data only}
- ISRCTN50917346. Vision comparison of two multifocal contact lenses [Clinical performance and acceptance of Invigor multifocal vs 1-day Acuvue® moist multifocal contact lenses]. isrctn.com/ISRCTN50917346 (first received 12 June 2020). [DOI: 10.1186/ISRCTN50917346] [DOI]
ISRCTN54358864 {published data only}
- ISRCTN54358864. Multifocal contact lens performance and acceptance [Miru multifocal contact lens performance and acceptance assessment]. isrctn.com/ISRCTN54358864 (first received 10 December 2020). [DOI: 10.1186/ISRCTN54358864] [DOI]
jRCT2052210025 {published data only}
- jRCT2052210025. Exploratory clinical trial to evaluate the safety of C-BAC1423 soft contact lens. jrct.niph.go.jp/en-latest-detail/jRCT2052210025 (first received 13 May 2021).
Maldonado‐Codina 2021 {published data only}
- Maldonado-Codina C, Navascues Cornago M, Read ML, Plowright AJ, Vega J, Orsborn GN, et al. The association of comfort and vision in soft toric contact lens wear. Contact Lens and Anterior Eye 2021;44(4):101387. [DOI] [PubMed] [Google Scholar]
- Orsborn G, Vega J. Correlation between ocular comfort and vision quality of three daily disposable soft toric contact lenses with different moduli of elasticity. Contact Lens and Anterior Eye 2019;42(6):e12. [Google Scholar]
NCT00639379 {published data only}
- NCT00639379. Comparison of two toric contact lenses on current toric wearers [A multi-center, subject masked, randomized, two week crossover design, investigation of the Acuvue Cypress toric silicone hydrogel lens compared to the Bausch & Lomb SofLens66® toric hydrophilic lens]. ClinicalTrials.gov/show/NCT00639379 (first received 20 March 2008).
NCT00886119 {published data only}
- NCT00886119. Comparison of a multifocal contact lens to a traditional multifocal contact lens [Lotrafilcon B multifocal evaluations—comparison to a traditional multifocal in higher spectacle adds]. ClinicalTrials.gov/show/NCT00886119 (first received 22 April 2009).
NCT01669629 {published data only}
- NCT01669629. Comparison of two daily disposable contact lenses over 1-week of wear. clinicaltrials.gov/show/NCT01669629 (first received 21 August 2012).
NCT01917162 {published data only}
- NCT01917162. Multi-center clinical evaluation of two daily disposable contact lenses (study 2). ClinicalTrials.gov/show/NCT01917162 (first received 6 August 2013).
NCT02920983 {published data only}
- NCT02920983. A clinical comparison of three soft daily disposable contact lenses. ClinicalTrials.gov/show/NCT02920983 (first received 30 September 2016).
NCT03341923 {published data only}
- JPRN-UMIN000030247. Clinical evaluation of DAILIES TOTAL 1 multifocal compared to 1-Day Acuvue Moist multifocal in a Japanese population. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000030247 (first received 4 December 2017).
- NCT03341923. Clinical evaluation of DAILIES TOTAL 1® multifocal compared to 1-Day Acuvue® Moist® multifocal in a Japanese population. ClinicalTrials.gov/show/NCT03341923 (first received 14 November 2017).
NCT03349632 {published data only}
- NCT03349632. Clinical comparison of 4 daily disposable soft contact lenses. ClinicalTrials.gov/show/NCT03349632 (first received 21 November 2017).
NCT04032457 {published data only}
- NCT04032457. PAVES benchmarking pilot study of daily disposable contact lenses. ClinicalTrials.gov/show/NCT04032457 (first received 15 March 2019).
NCT04527978 {published data only}
- NCT04527978. Clinical comparison of 2 daily disposable contact lenses—pilot study 1. ClinicalTrials.gov/show/NCT04527978 (first received 27 August 2020).
NCT04908488 {published data only}
- NCT04908488. Clinical performance of two daily disposable toric soft contact lenses. ClinicalTrials.gov/show/NCT04908488 (first received 1 June 2021).
NCT05138783 {published data only}
- NCT05138783. Clinical performance of two daily disposable soft contact lenses. ClinicalTrials.gov/show/NCT05138783 (first received 1 December 2021).
Wong 2008 {published data only}
- Wong J, Reuter KS, Kenrick C, Jansen M, Nichols JJ, Kollbaum PS. Comparison of two aspheric multifocal contact lens designs in the correction of early presbyopia. In: American Academy of Optometry Annual Meeting; 2008 Oct 22-25; Anaheim (CA). American Academy of Optometry, 2008.
Additional references
Alipour 2017
- Alipour F, Khaheshi S, Soleimanzadeh M, Heidarzadeh S, Heydarzadeh S. Contact lens-related complications: a review. Journal of Ophthalmic and Vision Research 2017;12(2):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bai 2018
- Bai Y, Ngo W, Gu B, Zhang Y, Nichols JJ. An imaging system integrating optical coherence tomography and interferometry for in vivo measurement of the thickness and dynamics of the tear film. Biomedical Engineering Online 2018;17(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begley 2000
- Begley CG, Caffery B, Nichols KK, Chalmers R. Responses of contact lens wearers to a dry eye survey. Optometry and Vision Science 2000;77(1):40-6. [DOI] [PubMed] [Google Scholar]
Blackie 2009
- Blackie CA, Solomon JD, Scaffidi RC, Greiner JV, Lemp MA, Korb DR. The relationship between dry eye symptoms and lipid layer thickness. Cornea 2009;28(7):789-94. [DOI] [PubMed] [Google Scholar]
Bron 2003
- Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22(7):640-50. [DOI] [PubMed] [Google Scholar]
Carle 1972
- Carle J. Developing hydrophilic lenses for continuous wearing. Australian Journal of Optometry 1972;55(9):343-6. [Google Scholar]
Chalmers 2012
- Chalmers RL, Begley CG, Moody K, Hickson-Curran SB. Contact Lens Dry Eye Questionnaire-8 (CLDEQ-8) and opinion of contact lens performance. Optometry and Vision Science 2012;89(10):1435-42. [DOI] [PubMed] [Google Scholar]
Chalmers 2015
- Chalmers RL, Hickson-Curran SB, Keay L, Gleason WJ, Albright R. Rates of adverse events with hydrogel and silicone hydrogel daily disposable lenses in a large postmarket surveillance registry: the TEMPO Registry. Investigative Ophthalmology and Visual Science 2015;56(1):654-63. [DOI] [PubMed] [Google Scholar]
Cho 2013
- Cho P, Boost MV. Daily disposable lenses: the better alternative. Contact Lens and Anterior Eye 2013;36(1):4-12. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale, NJ: Lawrence Erlbaum Associates Inc., 1988. [Google Scholar]
Covey 2001
- Covey M, Sweeney DF, Terry R, Sankaridurg PR, Holden BA. Hypoxic effects on the anterior eye of high-Dk soft contact lens wearers are negligible. Optometry and Vision Science 2001;78(2):95-9. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 15 May 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Diec 2013
- Diec J, Papas E, Naduvilath T, Xu P, Holden BA, la Jara PL. Combined effect of comfort and adverse events on contact lens performance. Optometry and Vision Science 2013;90(7):674-81. [DOI] [PubMed] [Google Scholar]
Diec 2018
- Diec J, Tilia D, Thomas V. Comparison of silicone hydrogel and hydrogel daily disposable contact lenses. Eye and Contact Lens 2018;44:S167-72. [DOI] [PubMed] [Google Scholar]
Dillehay 2007
- Dillehay SM. Does the level of available oxygen impact comfort in contact lens wear?: a review of the literature. Eye and Contact Lens 2007;33(3):148-55. [DOI] [PubMed] [Google Scholar]
Doughty 1997
- Doughty MJ, Fonn D, Richter D, Simpson T, Caffery B, Gordon K. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optometry and Vision Science 1997;74(8):624-31. [DOI] [PubMed] [Google Scholar]
Dumbleton 2013
- Dumbleton K, Woods CA, Jones LW, Fonn D. The impact of contemporary contact lenses on contact lens discontinuation. Eye and Contact Lens 2013;39(1):93-9. [DOI] [PubMed] [Google Scholar]
Dundas 2001
- Dundas M, Walker A, Woods RL. Clinical grading of corneal staining of non-contact lens wearers. Ophthalmic and Physiological Optics 2001;21(1):30-5. [PMID: ] [PubMed] [Google Scholar]
Efron 1991
- Efron N, Golding T, Brennan NA. The effect of soft lens lubricants on symptoms and lens dehydration. Eye and Contact Lens 1991;17(2):114-9. [PubMed] [Google Scholar]
Efron 2001
- Efron N, Morgan PB, Katsara SS. Validation of grading scales for contact lens complications. Ophthalmic and Physiological Optics 2001;21(1):17-29. [PubMed] [Google Scholar]
Efron 2015
- Efron N, Nichols JJ, Woods CA, Morgan PB. Trends in US contact lens prescribing 2002 to 2014. Optometry and Vision Science 2015;92(7):758-67. [DOI] [PubMed] [Google Scholar]
Efron 2022
- Efron N, Morgan PB, Nichols JJ, Walsh K, Willcox MD, Wolffsohn JS, et al. All soft contact lenses are not created equal. Contact Lens and Anterior Eye 2022;45(2):101515. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Grant 2020
- Grant T, Tang A. A survey of contact lens wearers and eye care professionals on satisfaction with a new smart-surface silicone hydrogel daily disposable contact lens. Clinical Optometry 2020;12:9-15. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guillon 2013
- Guillon M. Are silicone hydrogel contact lenses more comfortable than hydrogel contact lenses? Eye and Contact Lens 2013;39(1):86-92. [DOI] [PubMed] [Google Scholar]
Higgins 2022a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Higgins 2022b
- Higgins JPT, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Holden 1984
- Holden BA, Mertz GW. Critical oxygen levels to avoid corneal edema for daily and extended wear contact lenses. Investigative Ophthalmology and Visual Science 1984;25(10):1161-7. [PubMed] [Google Scholar]
Holden 2003
- Holden BA, Sweeney DF, Sankaridurg PR, Carnt N, Edwards K, Stretton S, et al. Microbial keratitis and vision loss with contact lenses. Eye and Contact Lens 2003;29(1):S131-4. [DOI] [PubMed] [Google Scholar]
Holden 2016
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016;123(5):1036-42. [DOI] [PubMed] [Google Scholar]
Jacob 2013
- Jacob JT. Biocompatibility in the development of silicone-hydrogel lenses. Eye and Contact Lens 2013;39(1):13-9. [DOI] [PubMed] [Google Scholar]
Jacobs 2021
- Jacobs DS, Carrasquillo KG, Cottrell PD, Fernández-Velázquez FJ, Gil-Cazorla R, Jalbert I, et al. CLEAR—Medical use of contact lenses. Contact Lens and Anterior Eye 2021;44(2):289-329. [DOI] [PubMed] [Google Scholar]
Jones 2013
- Jones L, Brennan NA, González-Méijome J, Lally J, Maldonado-Codina C, members of the TFOS International Workshop on Contact Lens Discomfort. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design, and care subcommittee. Investigative Ophthalmology and Visual Science 2013;54(11):TFOS37-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Key 2007
- Key JE. Development of contact lenses and their worldwide use. Eye and Contact Lens 2007;33(6):343-5. [DOI] [PubMed] [Google Scholar]
Korb 2005
- Korb DR, Herman JP, Greiner JV, Scaffidi RC, Finnemore VM, Exford JM, et al. Lid wiper epitheliopathy and dry eye symptoms. Eye and Contact Lens 2005;31(1):2-8. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [DOI: 10.1186/2046-4053-2-81] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lazon de la Jara 2013
- Lazon de la Jara P, Papas E, Diec J, Naduvilath T, Willcox MD, Holden BA. Effect of lens care systems on the clinical performance of a contact lens. Optometry and Vision Science 2013;90(4):344-50. [DOI] [PubMed] [Google Scholar]
Lemp 1995
- Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. The CLAO Journal: official publication of the Contact Lens Association of Ophthalmologists 1995;21(4):221-32. [PubMed] [Google Scholar]
Lim 2018
- Lim CH, Stapleton F, Mehta JS. Review of contact lens-related complications. Eye 2018;44:S1-S10. [DOI] [PubMed] [Google Scholar]
Lin 2013
- Lin MC, Yeh TN. Mechanical complications induced by silicone hydrogel contact lenses. Eye and Contact Lens 2013;39(1):115-24. [DOI] [PubMed] [Google Scholar]
Markoulli 2017
- Markoulli M, Kolanu S. Contact lens wear and dry eyes: challenges and solutions. Clinical Optometry 2017;9:41-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maurice 1990
- Maurice D. The Charles Prentice award lecture 1989: the physiology of tears. Optometry and Vision Science 1990;67(6):391-9. [DOI] [PubMed] [Google Scholar]
McKenzie 2022
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Chapter 9: Summarizing study characteristics and preparing for synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
McMonnies 1986
- McMonnies CW. Key questions in a dry eye history. American Optometric Association 1986;57(7):512-7. [PubMed] [Google Scholar]
McMonnies 1987
- McMonnies CW, Ho A. Patient history in screening for dry eye conditions. American Optometric Association 1987;58(4):296-301. [PubMed] [Google Scholar]
Millar 2003
- Millar TJ, Papas EB, Ozkan J, Jalbert I, Ball M. Clinical appearance and microscopic analysis of mucin balls associated with contact lens wear. Cornea 2003;22(8):740-5. [DOI] [PubMed] [Google Scholar]
Morgan 2010
- Morgan PB, Efron N, Helland M, Itoi M, Jones D, Nichols JJ, et al. Twenty first century trends in silicone hydrogel contact lens fitting: an international perspective. Contact Lens and Anterior Eye 2010;33(4):196-8. [DOI] [PubMed] [Google Scholar]
Morgan 2021
- Morgan PB, Murphy PJ, Gifford KL, Gifford P, Golebiowski B, Johnson L, et al. CLEAR—Effect of contact lens materials and designs on the anatomy and physiology of the eye. Contact Lens and Anterior Eye 2021;44(2):192-219. [DOI] [PubMed] [Google Scholar]
NCT00727558
- NCT00727558. A comparison of daily disposable contact lenses. ClinicalTrials.gov/show/NCT00727558 (first received 4 August 2008).
Ngo 2013
- Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea 2013;32(9):1204-10. [DOI] [PubMed] [Google Scholar]
Nichols 2002
- Nichols JJ, Mitchell GL, Nichols KK, Chalmers R, Begley C. The performance of the contact lens dry eye questionnaire as a screening survey for contact lens-related dry eye. Cornea 2002;21(5):469-75. [DOI] [PubMed] [Google Scholar]
Nichols 2003
- Nichols JJ, King-Smith PE. Thickness of the pre-and post–contact lens tear film measured in vivo by interferometry. Investigative Ophthalmology and Visual Science 2003;44(1):68-77. [DOI] [PubMed] [Google Scholar]
Nichols 2013
- Nichols JJ. Deposition on silicone hydrogel lenses. Eye and Contact Lens 2013;39(1):20-3. [DOI] [PubMed] [Google Scholar]
Nicolson 2001
- Nicolson PC, Vogt J. Soft contact lens polymers: an evolution. Biomaterials 2001;22(24):3273-83. [DOI] [PubMed] [Google Scholar]
Poggio 1989
- Poggio EC, Glynn RJ, Schein OD, Seddon JM, Shannon MJ, Scardino VA, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. New England Journal of Medicine 1989;321(12):779-83. [DOI] [PubMed] [Google Scholar]
Pritchard 1999
- Pritchard N, Fonn D, Brazeau D. Discontinuation of contact lens wear: a survey. International Contact Lens Clinic 1999;26(6):157-62. [DOI] [PubMed] [Google Scholar]
Pucker 2018
- Pucker AD, Dougherty BE, Jones-Jordan LA, Kwan JT, Kunnen CM, Srinivasan S. Psychometric analysis of the SPEED questionnaire and CLDEQ-8. Investigative Ophthalmology and Visual Science 2018;59(8):3307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pucker 2019
- Pucker AD, Jones-Jordan LA, Marx S, Powell DR, Kwan JT. Clinical factors associated with contact lens dropout. Contact Lens and Anterior Eye 2019;42(3):318-24. [DOI] [PubMed] [Google Scholar]
Pucker 2020
- Pucker AD, Tichenor AA. A review of contact lens dropout. Clinical Optometry 2020;12:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Richdale 2007
- Richdale K, Sinnott LT, Skadahl E, Nichols JJ. Frequency of and factors associated with contact lens dissatisfaction and discontinuation. Cornea 2007;26(2):168-74. [DOI] [PubMed] [Google Scholar]
Richdale 2016
- Richdale K, Lam DY, Wagner H, Zimmerman AB, Kinoshita BT, Chalmers R, et al. Case-control pilot study of soft contact lens wearers with corneal infiltrative events and healthy controls. Investigative Ophthalmology and Visual Science 2016;57(1):47-55. [DOI] [PubMed] [Google Scholar]
Rumpakis 2010
- Rumpakis JM. New data on contact lens dropouts: an international perspective: more than one in six of your contact lens patients will discontinue lens wear, a new study finds. That's a big chunk of your bottom line. Review of Optometry 2010;147(1):37-42. [Google Scholar]
Sankaridurg 2013
- Sankaridurg P, la Jara PL, Holden B. The future of silicone hydrogels. Eye and Contact Lens 2013;39(1):125-9. [DOI] [PubMed] [Google Scholar]
Schiffman 2000
- Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Archives of Ophthalmology 2000;118(5):615-21. [DOI] [PubMed] [Google Scholar]
Schünemann 2022a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from handbook.cochrane.org.
Schünemann 2022b
- Schünemann HJ, Neumann I, Hultcrantz M, Brignardello-Petersen R, Zeng L, GRADE Working Group. GRADE guidance article 35: update on rating imprecision for assessing contextualized certainty of evidence and making decisions. Journal of Clinical Epidemiology 2022 Aug 5 [Epub ahead of print]. [DOI: ] [DOI] [PubMed]
Stapleton 2013
- Stapleton F, Keay L, Edwards K, Holden B. The epidemiology of microbial keratitis with silicone hydrogel contact lenses. Eye and Contact Lens 2013;39(1):79-85. [DOI] [PubMed] [Google Scholar]
Stapleton 2017
- Stapleton F, Tan J. Impact of contact lens material, design, and fitting on discomfort. Science and Clinical Practice 2017;43(1):32-9. [DOI] [PubMed] [Google Scholar]
Stapleton 2021
- Stapleton F, Bakkar M, Carnt N, Chalmers R, Vijay AK, Marasini S, et al. CLEAR—Contact lens complications. Contact Lens and Anterior Eye 2021;44(2):330-67. [DOI] [PubMed] [Google Scholar]
Sweeney 2013
- Sweeney DF. Have silicone hydrogel lenses eliminated hypoxia? Eye and Contact Lens 2013;39(1):53-60. [DOI] [PubMed] [Google Scholar]
Szczesna 2006
- Szczesna DH, Jaroński J, Kasprzak HT, Stenevi U. Interferometric measurements of dynamic changes of tear film. Journal of Biomedical Optics 2006;11(3):034028. [DOI] [PubMed] [Google Scholar]
Szczotka‐Flynn 2007
- Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear: a meta-analysis. Optometry and Vision Science 2007;84(4):247-56. [DOI] [PubMed] [Google Scholar]
Szczotka‐Flynn 2014
- Szczotka-Flynn L, Jiang Y, Raghupathy S, Bielefeld RA, Garvey MT, Jacobs MR, et al. Corneal inflammatory events with daily silicone hydrogel lens wear. Optometry and Vision Science 2014;91(1):3-12. [DOI] [PubMed] [Google Scholar]
Terry 1993
- Terry RL, Schnider CM, Holden BA, Cornish R, Grant T, Sweeney D, et al. CCLRU standards for success of daily and extended wear contact lenses. Optometry and Vision Science: official publication of the American Academy of Optometry 1993;70(3):234-43. [DOI] [PubMed] [Google Scholar]
Varikooty 2013
- Varikooty J, Keir N, Richter D, Jones LW, Woods C, Fonn D. Comfort response of three silicone hydrogel daily disposable contact lenses. Optometry and Vision Science 2013;90(9):945-53. [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang J, Aquavella J, Palakuru J, Chung S. Repeated measurements of dynamic tear distribution on the ocular surface after instillation of artificial tears. Investigative Ophthalmology and Visual Science 2006;47(8):3325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
WebPlotDigitizer [Computer program]
- WebPlotDigitizer. Version 4.5. Rohatgi A, accessed 10 October 2022. Available from automeris.io/WebPlotDigitizer.
Wichterle 1960
- Wichterle O, Lim D. Hydrophilic gels for biological use. Nature 1960;185(4706):117-8. [Google Scholar]
Wichterle 1961
- Wichterle O, Lim D, Dreifus M. The problem of contact lenses. Ceskoslovenska Oftalmologie 1961;17:70-5. [PubMed] [Google Scholar]
Young 2002
- Young G, Veys J, Pritchard N, Coleman S. A multi‐centre study of lapsed contact lens wearers. Ophthalmic and Physiological Optics 2002;22(6):516-27. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Haworth 2021
- Haworth K, Travis D, Abariga SA, Fuller D, Pucker AD. Silicone hydrogel versus hydrogel soft contact lenses for differences in patient-reported eye comfort and safety. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD014791. [DOI: 10.1002/14651858.CD014791] [DOI] [PMC free article] [PubMed] [Google Scholar]


