Abstract
Interoceptive signals give rise to subjective feeling states that can drive motivational and behavioural responses. In the context of alcohol use behaviours, interoceptive signals may shape subjective alcohol experiences and thereby support biobehavioural mechanisms of drinking behaviour change. This study examined the acute effects of alcohol on participants’ interoceptive abilities and determined whether pharmacologically induced changes in heart beat detection correlate with subjective alcohol effects, craving and expectancies. Participants completed a two-session, double-blind placebo controlled experiment (n = 27). Participants consumed a beverage containing 0.4 g/kg of alcohol or a placebo. They also completed measurements of alcohol expectancies at baseline, and alcohol-induced changes in mood, craving and light-headedness. Interoceptive ability was measured using the heartbeat discrimination task prior to and following beverage administration, yielding indices of interoceptive accuracy, confidence and meta-cognition. Alcohol administration increased interoceptive accuracy compared with baseline and placebo; and those changes in interoception negatively correlated with negative alcohol expectancies. Further, changes in interoception positively correlated with perceived light-headedness and positive mood after alcohol administration, whereas null effects were found for craving. In the placebo condition, null results were obtained. Alcohol is well established to change bodily states, and here, we find that the extent to which alcohol increases participants’ sensitivity to bodily states correlates with their subjective drinking experiences. This was observed in relation to mood, light-headedness and prospective alcohol expectancies. We posit that over successive alcohol experiences, changes in bodily states may feed into the development of alcohol expectancies that could in turn predict future drinking behaviours.
Keywords: alcohol administration, alcohol expectancies, interoception
1. INTRODUCTION
Interoception is the sensing and integration of internal bodily states in the brain.1,2 Interoceptive signals are most commonly studied across the cardiac axis,3 and heart signals feed into neural pathways to inform cognitive, emotional and behavioural adaptations to environmental and internal demands.4,5 Cognitive and emotional experiences are key in addictive processes, as feelings of ‘high’ and negative withdrawal states drive aberrant decision making and craving6,7; and these can in turn trigger drug consumption behaviours. Given that physiological signals are integrated through interoceptive pathways to generate behavioural and emotional adaptations in healthy populations,1 it is likely that interoceptive processes also can support cognitive-affective processes in addictive behaviours.8–10 Alcohol administration modulates interoceptive responses towards alcohol cues, giving rise to alcohol cognitive biases in persons at high risk for alcohol use disorder.11 Alcohol administration can also affect participants’ internal bodily states, changing the value of alcohol-related cues and incentive salience processes as a function of cardiovascular reactivity to alcohol effects.12 There is however limited research on the effects of alcohol on cardiac interoceptive abilities, the perception of one’s own heartbeat. This could be an avenue to study how interoception supports addictive processes, determining if perceived drug-induced changes in bodily states contribute to cognitive and emotional components of alcohol use.
In this report, we measure interoceptive abilities using the heartbeat discrimination task13,14 in which participants are asked to indicate if an auditory stimulus is synchronized with their heartbeat or not. From this task, three indices can be extracted15: interoceptive accuracy (IAcc), participants’ ability to determine if a stimulus is presented in synchrony with their heartbeat; interoceptive confidence, their subjective confidence (ICon) in their responses; and interoceptive awareness (IAw), the relationship between accuracy and confidence, reflecting participants’ meta-cognitive knowledge about their performance on the task. IAw constitutes a novel examination of higher order interoceptive processing, indexing meta-cognitive representation of interoceptive abilities across organ-specific axes.3
To our knowledge, only two papers have examined the effects of alcohol on interoceptive ability indices. Abrams et al.16 found that alcohol impairs IAcc on a heartbeat counting task; however, these results were restricted to men, and this task has been subject to methodological criticisms17,18 as it primarily relies on heart rate estimation rather than actual heartbeat perception. Using the heartbeat discrimination task, Leganes-Fonteneau et al.19 found that IAw, but not IAcc, was lower in participants receiving a 0.4-g/kg dose of alcohol. Further, they found that higher levels of IAw positively correlated with subjective feelings of light-headedness after alcohol administration. This implies that participants’ awareness of their own ability to perceive internal bodily sensations after alcohol administration correlates with the perception of alcohol effects. However, in that study, participants only completed one experimental session, and no measure of baseline interoception was obtained, limiting the interpretation of the results. The first aim of the present experiment addresses these limitations using a within-subjects design.
The second aim of this experiment is to extend experimental evidence on the involvement of interoceptive mechanisms in the cognitive and emotional processes that support alcohol use.9 Two distinct processes may contribute to the role of interoception in the generation of craving and subjective responses to alcohol. On one hand, it has been posited that interoceptive signals provide a dynamic representation of bodily states associated with anxiety and tension20 and would therefore act through a negative reinforcement pathway to generate craving. On the other hand, interoceptive signals could support hedonic and incentive processes21 reflecting positive reinforcement components. Currently, this latter hypothesis is grounded mainly on a single lesion study showing that smokers with damage to the insular cortex, which is responsible for the processing of viscero-afferent signals, experienced a decrease in craving and quit smoking.22 Given that alcohol administration modulates bodily states in part through its effects on the cardiovascular system,11,12 we explore whether changes in interoceptive ability after alcohol administration correlate with alcohol-induced effects on craving (i.e., Rose and Duka23 and Schoenmakers et al.24). Further, based on previous findings that interoceptive signals provide broad mechanistic support for subjective feeling states,4 we examine whether interoceptive changes positively correlate with the acute subjective effects of alcohol on light-headedness25 and mood.26,27
Finally, alcohol expectancies are also a key factor in addiction as they have the ability to predict drinking patterns,28 clinical relapses29 and both subjective30 and autonomic31 reactivity towards alcohol cues. Alcohol expectancies are shaped in part by the previous experience of substance effects,32 as positive substance effects shape future positive expectancies.33 Interoceptive signals are posited to participate in associative learning processes through their support of a hedonic and incentive signature of alcohol effects.21,34 Within this learning framework,35 it is possible that alcohol expectancies are shaped not only by previous social or cognitive experiences but also by interoceptive experiences after alcohol consumption. Thus, in this report, we examine whether alcohol expectancies correlate with changes in interoceptive ability following alcohol consumption.
In a double-blind placebo controlled experiment, participants completed a baseline assessment of interoceptive ability and alcohol expectancies. Changes in interoception were measured after placebo and alcohol (0.4 g/kg) administration, together with changes in mood, craving and light-headedness. We expect to find changes in interoceptive ability after alcohol administration, and that these changes will correlate with subjective responses to alcohol.
2. METHODS
2.1. Participants
Thirty-one participants (mean age = 23.52, SD = 4.05, 16 males) were recruited using fliers posted on social media or near Rutgers University–New Brunswick, NJ, USA. Eligibility criteria were drinking more than three alcohol units per week in the past 3 months, being at least 21 years old, and not reporting a history of learning disability, psychiatric disorders, treatment for substance use disorder, current AUD or regular (weekly) illicit or prescription drug use. Pregnant women and participants with a body mass index (BMI) 20% overweight or underweight were excluded. Upon arrival to the laboratory, pregnancy status was confirmed using a pregnancy test, and participants were weighted in order to prepare the alcoholic beverage. Participants were compensated for their participation in the study (USD 35–45).
Due to premature study termination related to COVID-19, three participants completed only one session (alcohol session only, n = 2; placebo session only, n = 1). An a priori power analysis was performed for sample size estimation. Although effect size estimations were limited by a lack of previous research, we used the previously reported correlation between meta-cognitive interoception and light-headedness (R2 = 0.463),19 but acknowledge that this effect size was derived from a model that included multiple covariates and focused on only one of the three interoception variables of interest (the other variables were non-significant in this previous, small study limiting the utility of these effect sizes for a priori power analyses). With an α = 0.05, n = 25 participants would generate a power = 0.95. Regarding the effects of alcohol administration on interoception (η2 = 0.154; Leganes-Fonteneau et al.19), again, n = 20 would yield a power > 0.95. We recruited a larger sample in the context of a more extensive experiment and the aforementioned limitations of previous studies.
2.2. Questionnaires
The Anticipated Effects of Alcohol Scale (AEAS33) evaluates expectancies across two axes (high/low arousal crossed with positive/negative valence). Participants respond to two sets of 22 items indicating how they expect to feel immediately after having four drinks (five for males) within a 2-h period and how they expect to feel an hour and a half after finishing their fourth drink. This way, four different scores (high+, high−, low+ and low−) are obtained for the ascending and descending limbs separately. Because the AEAS yields eight different variables, a principal components factor analysis was conducted to further reduce the results of this questionnaire. Upon initial examination of eigenvalues > 1 and scree plot, a two-factor solution with orthogonal varimax rotation was forced. Unsurprisingly, the four positive and the four negative scores loaded on two different factors (positive expectancies and negative expectancies) with 69.62% of cumulative variance were explained, and factor scores were computed using the Anderson–Rubin method. This allows reducing multiple comparisons when examining the correlates of alcohol expectancies.
A series of questionnaires were used to characterize the sample. The alcohol use disorders identification test (AUDIT36) and the alcohol use questionnaire (AUQ37) assessed participants’ alcohol consumption. The AUQ was modified to reflect current, prevalent college drinking preferences (e.g., hard seltzers). Alcohol consumption was quantified in alcohol units per week. A binge drinking score was extracted38 accounting for number of drinks consumed per hour, percentage of times participants reach intoxication after consuming alcohol and number of times intoxicated in the last 6 months.
Alcohol effects on subjective states were measured using the alcohol-induced effects Visual Analogue Scale (Alcohol VAS25). Participants had to indicate the extent to which they experience a variety of states (e.g., ‘light-headed’, ‘stimulated’, ‘alert’, ‘relaxed’, ‘irritated’ and ‘contented’) on a VAS from 0 (not at all) to 100 (extremely). In this report, we focus on light-headedness as this subjective effect has been shown to correlate with IAw19 and to serve as an interoceptive cue in alcohol discrimination procedures.39 The Positive and Negative Affect Scale (PANAS40) measured changes in mood, and the abbreviated version of the Desires for Alcohol Questionnaire (DAQ41) measured changes in craving across three factors (intention to drink, positive reinforcement and negative reinforcement).
2.3. Alcohol administration
According to a double-blind protocol, participants completed two sessions and were administered either an alcohol or placebo drink in each session. The order of the sessions was counterbalanced accounting for gender. In the alcohol session, participants received a dose of 0.4 g/kg of alcohol (190-proof Everclear) mixed with sugar-free Schweppes tonic water and five drops of angostura bitters to make up a 200-ml solution. The placebo beverage consisted of 200 ml of Schweppes with five drops angostura bitters. The drink was poured in five plastic glasses (40 ml each), and alcohol was sprinkled over the serving tray for olfactory cues. Participants were instructed to drink each glass at their own pace within a 2-min window for each serving. We measured blood–alcohol content (BAC) at two different time points starting 10 min after the beverage consumption was completed (Alco-Pro Alco Sensor FST).
2.4. Cardiac interoception
Participants’ interoceptive ability was measured at different time points using the cardiac discrimination task.13 For 20 trials, participants heard 10 tones (100 ms, 440 Hz) that were either synchronized (50% of the trials) or nonsynchronized with the participant’s own heartbeat. Trials were presented in random order. On nonsynchronized trials, a 300-ms delay was introduced between each heartbeat and the tone. Participants’ task was to indicate verbally at the end of each trial whether the tones were synchronized or not with their heartbeat and to indicate how confident they were in their response on a VAS using Qualtrics. A finger pulse oximeter (8000SM, Nonin Medical, Inc., Minnesota, USA) was used to track participants’ heartbeat.
Three indexes of interoceptive ability were extracted from the discrimination task. IAcc was computed as the rate of correct responses (i.e., replying ‘Yes’ on a synchronized trial or ‘No’ on a nonsynchronized trial). ICon scores were computed as the mean response on the VAS. IAw was computed using an area under the receiver operating characteristic curve (AUROC) by which it is possible to determine the extent to which confidence scores predict accuracy on each trial (see Garfinkel et al.15 for a detailed explanation of the different measures and scorings).
Prior to the discrimination task, participants also completed the heartbeat counting task,42 but given the serious concerns raised about this task and published since the conception of the experiment,17,18 we did not examine these data.
During the cardiac discrimination task, mean heart rate was obtained, and measures of systolic and diastolic blood pressure were also taken before each task.
2.5. Protocol
This study was approved by the Rutgers University Arts and Sciences Institutional Review Board for the Protection of Human Subjects Involved in Research, and all participants provided written informed consent. All tasks were programmed using Matlab Psychtoolbox.
Participants were invited to complete the experimental sessions a minimum of one and a maximum of two weeks apart. Before the experiment, they were instructed to have a low-fat breakfast, not eat for the 2-h period prior to the experiment, and avoid alcohol for 24 h before the sessions. Participants were told they would receive beverage containing different amounts of alcohol in each of the sessions.
In the first session, participants signed the consent form and completed the AEAS questionnaires and the PANAS, DAQ and Alcohol VAS at t0, followed by the baseline heartbeat discrimination task at t0 (administered in the first session only). Participants were administered a beverage according to the procedure described above. After a 10-min rest, BAC was measured (mean BAC t0 = 58 mg/dl, SD = 18), and participants completed a modified flanker task and a cue reactivity task (~25 min, data not presented here). BAC was collected again (mean BAC t1 = 57 mg/dl, SD = 13), and participants completed PANAS, Alcohol VAS and DAQ questionnaires and the heartbeat discrimination task at t1. At the end, participants indicated how many standard drinks they thought they had consumed and remained in the facilities until they reached a BAC < 0.03. If participants were in the placebo condition, their BAC was measured twice, and they were dismissed after 15 min. See Figure 1 for a description of the protocol.
Figure 1.
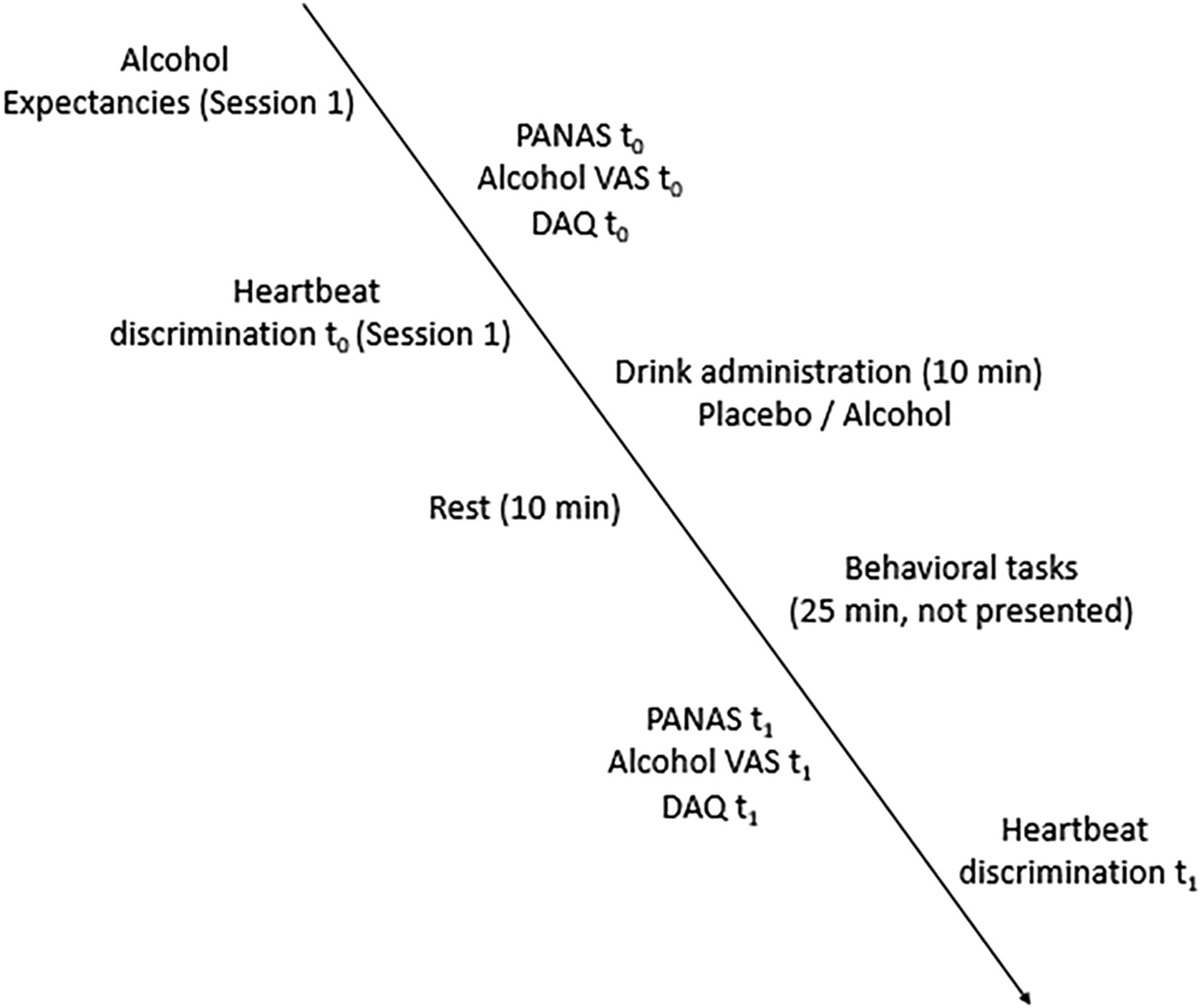
Experimental protocol
3. RESULTS
In this sample, the mean AUQ Binge score was 17.35, SD = 13.01, mean AUQ Units per week was 12.60, SD = 7.68, and mean AUDIT score was 7.96, SD = 3.50. Bayes factors (BFs) were used to examine evidence in favour of the null or alternative hypotheses. A BF > 3 implies evidence for the alternative hypotheses, whereas a BF < 1/3 provides evidence for the null hypothesis. For the post hoc analyses of the effects of alcohol administration on interoception, we modelled H1 as a uniform from [0 to max]43 using previous findings of the effects of alcohol on IAw,19 whereas for regression analyses, we used default priors in SPSS 26.
Data from participants who had only completed one experimental session were excluded. An outlier with extremely low IAcc at t1 (0.10, chance level = 0.50) was also excluded (n = 27); for analyses on light-headedness, one outlier who reported a large decrease in LH after alcohol administration was excluded (n = 26), and all other participants showed either no change or an increase in LH after alcohol.
We examined the effects of alcohol administration on interoceptive ability. A series of two-way mixed ANOVAs, with time (baseline vs. alcohol vs. placebo) as within-subjects factor and condition (controlling for session order effects) as between-subjects factor, was conducted for all three measures of interoception. For IAcc, there was a significant main effect of time, F(2,46) = 4.373, p = 0.018, partial η2 = 0.160, and there was no significant interaction with condition, p = 0.661. Pairwise post hoc analyses adjusted for multiple comparisons using LSD show that IAcc after alcohol administration was higher than at baseline, p = 0.018, BFU[0,0.11] = 14.58, and placebo, p = 0.009, BFU[0,0.11] = 34.52, whereas there was no significant difference between placebo and baseline, p = 0.859, BFU[0,0.11] = 0.44 (see Figure 2).
Figure 2.
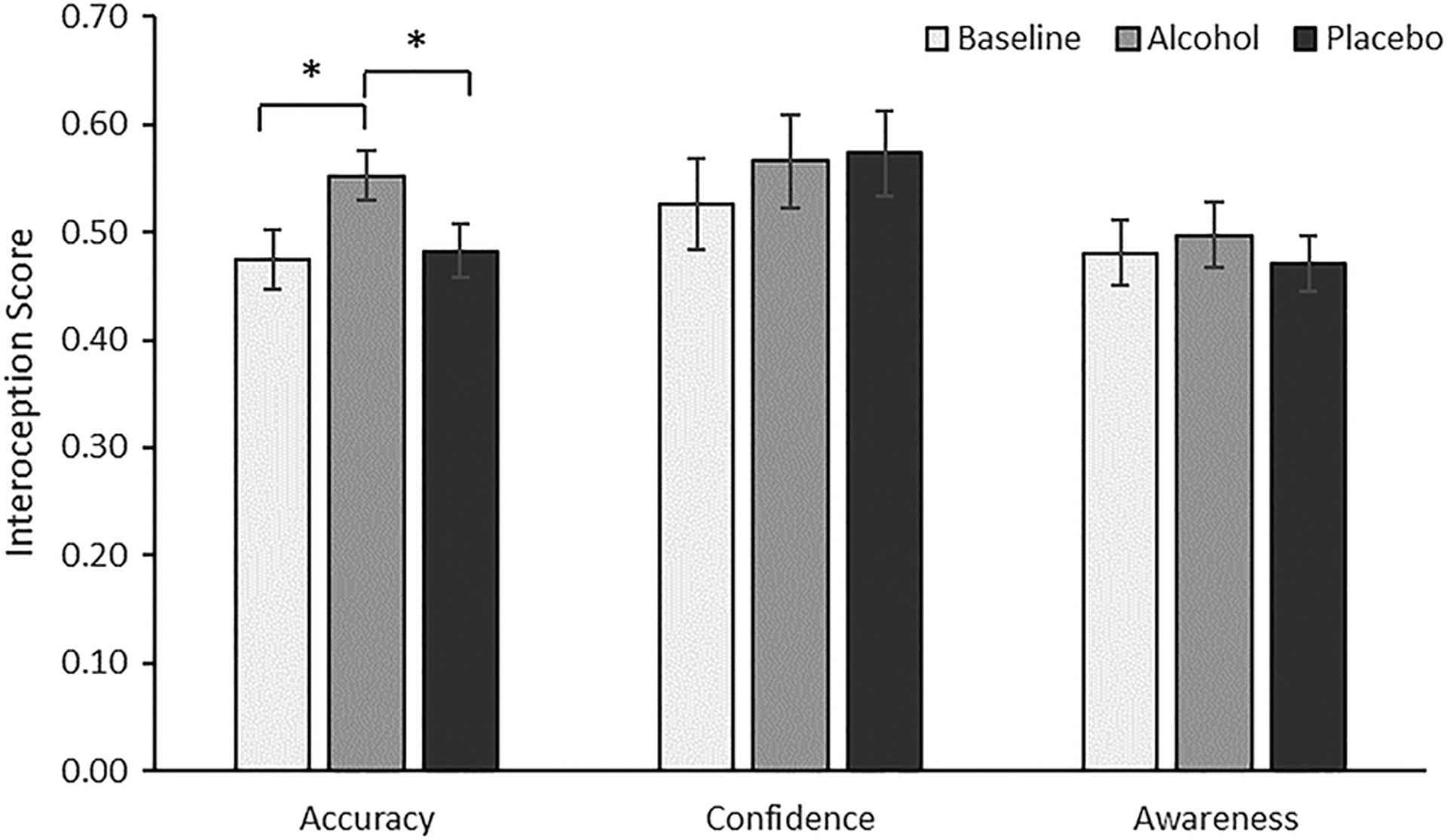
Interoceptive ability scores as a function of drink administered: alcohol administration increased interoceptive accuracy compared with baseline and placebo
An exploratory analysis of IAcc included changes in systolic and diastolic blood pressure and heart rate after alcohol administration as covariates. The effect of time remained significant, F(2,38) = 4.532, p = 0.017.
Alcohol administration had no effect for IAw, F(2,46) = 0.192, p = 0.826, partial η2 = 0.008, BF01 = 0.022, or ICon, F(2,46) = 1.141, p = 0.328, partial η2 = 0.047, BF01 = 0.117.
A series of multiple linear regressions examined changes in light-headedness, mood and craving as a function of changes in interoceptive ability. Difference scores (post drink minus baseline/t1 − t0) were computed for each interoceptive ability measure and for each dependent variable. Additionally, we also examined if changes in interoceptive ability predicted alcohol expectancies. Analyses were conducted separately for the alcohol and placebo sessions. False discovery rate was used to account for multiple comparisons in the six regression models computed in the alcohol condition as those were the primary models of interest (https://tools.carbocation.com/FDR). Corrected p values are reported for significant results. All regression models showed high degrees of tolerance (>0.70) revealing no issues with multicolinearity.
For light-headedness scores, the regression for the alcohol condition was significant, R2 = 0.305, F(3,25) = 3.759, p = 0.026, BF01 = 1.88, power = 0.74, corrected p = 0.052. Changes in IAw and ICon were significant predictors, ps < 0.05 (see Table 1 and Figure 3). In the placebo condition, the regression showed null results, p = 0.441, BF01 = 0.041.
Table 1.
Results of the regression analyses examining changes in light-headedness and PANAS positive and negative expectancies as a function of interoceptive accuracy, confidence and awareness
| β | t | p | |
|---|---|---|---|
| Light-headedness (t1 − t0) | |||
| Accuracy | 0.116 | 0.567 | 0.576 |
| Confidence | 0.364 | 2.091 | 0.048* |
| Awareness | 0.506 | 2.482 | 0.021* |
| PANAS positive (t1 − t0) | |||
| Accuracy | 0.706 | 4.039 | 0.001* |
| Confidence | −0.029 | −0.187 | 0.854 |
| Awareness | 0.542 | 3.129 | 0.005* |
| Negative expectancies | |||
| Accuracy | −0.558 | −2.893 | 0.008* |
| Confidence | 0.142 | 0.822 | 0.420 |
| Awareness | −0.039 | −0.204 | 0.840 |
Significant predictor.
Figure 3.
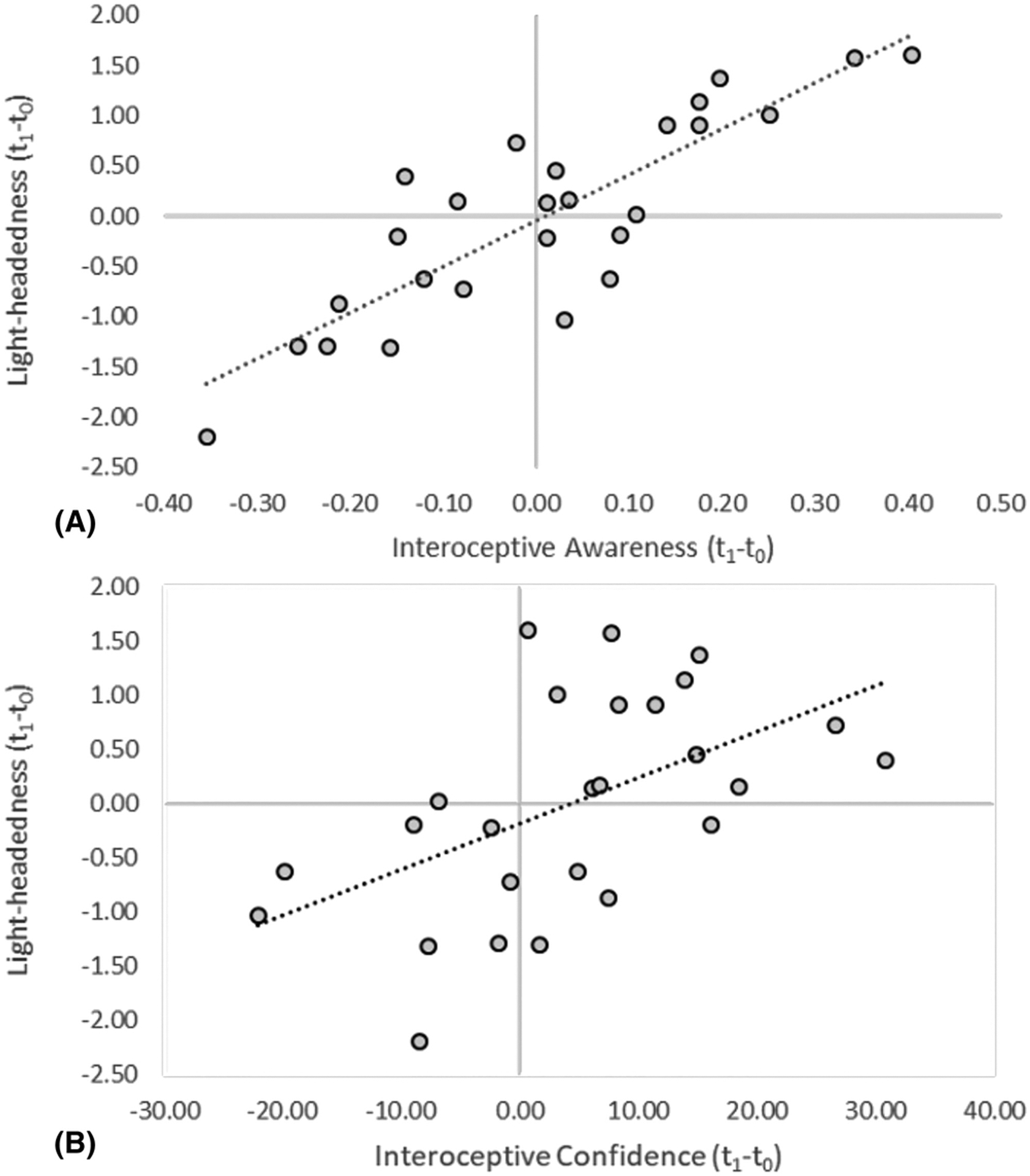
Relationship between changes in light-headedness (standardized predicted values) and (A) interoceptive awareness and (B) interoceptive confidence after alcohol administration
Regarding alcohol-induced mood changes, the regression examining PANAS positive scores was significant, R2 = 0.455, F(3,26) = 6.394, p = 0.003, BF01 = 9.447, power = 0.97, corrected p = 0.018. IAcc and IAw were significant positive predictors, ps < 0.005. In the placebo condition, results were null, p = 0.632, BF01 = 0.026. For PANAS negative scores, results were null in the alcohol, p = 0.214, BF01 = 0.084, and in the placebo, p = 0.173, BF01 = 0.118, conditions (see Table 1 and Figure 4).
Figure 4.
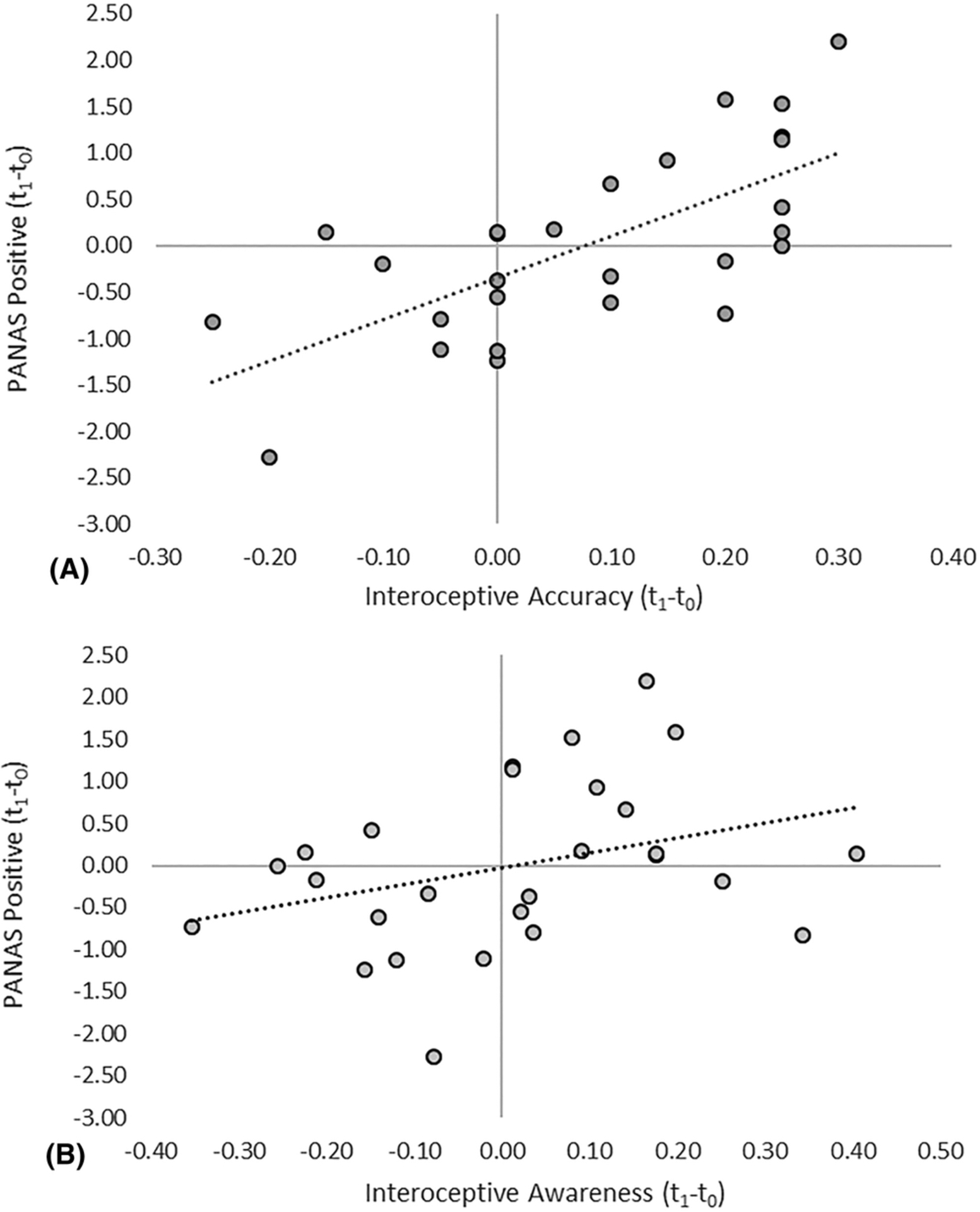
Relationship between changes in PANAS positive (standardized predicted values) and (A) interoceptive accuracy and (B) interoceptive awareness after alcohol administration
For total craving scores, the regression for the alcohol condition showed null results, F(3,26) = 0.110, p = 0.953, BF01 = 0.011. Results in the placebo condition were also null, p = 0.494, BF01 = 0.034.
Equivalent analyses were run for alcohol expectancies. For negative expectancies, the regression was significant in the alcohol condition, R2 = 0.336, F(3,26) = 3.228, p = 0.022, BF01 = 1.017, power = 0.82, corrected p = 0.066. IAcc was a significant negative predictor, p < 0.01, whereas in the placebo condition, the regression showed null results, p = 0.349, BF01 = 0.052. For positive expectancies, the regression was null in the alcohol, p = 0.993, BF01 = 0.010, and placebo, ps > 0.978, BF01 = 0.011, conditions (see Table 1 and Figure 5).
Figure 5.
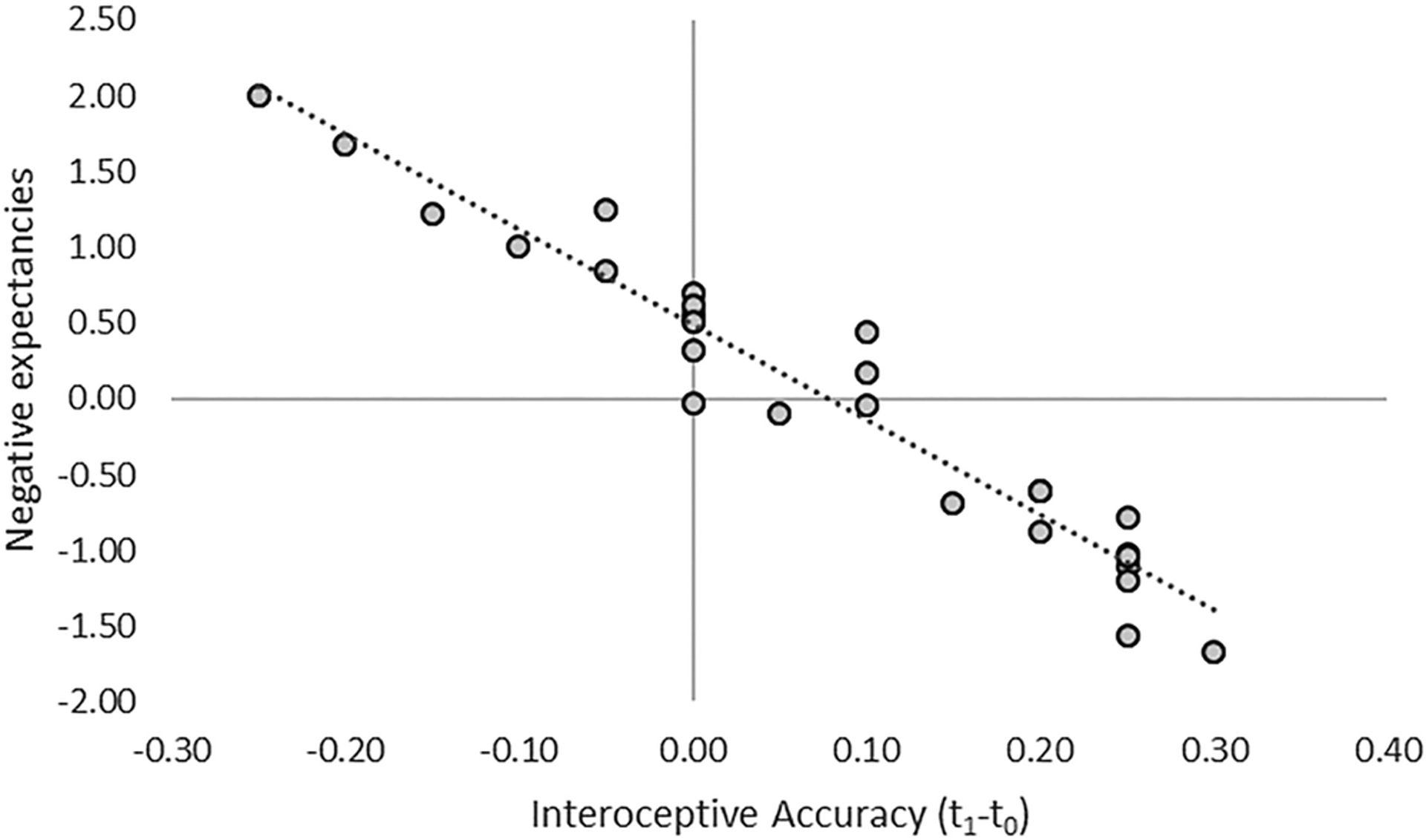
Relationship between changes in negative expectancies (standardized predicted values) and interoceptive accuracy after alcohol administration
Results for the analyses of the interaction between time and condition on DAQ, PANAS and light-headedness are available as Supporting Information. We ran a set of exploratory regressions including AUDIT and AUQ scores as a covariate and found no significant interactions, ps > 0.1.
In the alcohol session, participants thought they had more alcohol (mean = 3.31, SD = 1.32) than in the placebo condition (mean = 1.77, SD = 1.11), t(25) = 6.884, p < 0.001, although all participants except one in the placebo condition reported they had at least one drink.
4. DISCUSSION
In this report, we examined within-subjects effects of acute alcohol on participants’ ability to feel their heart and determined whether pharmacologically induced changes in interoceptive ability correlate with subjective alcohol effects, craving and expectancies. We found significant differences in IAcc after alcohol administration; BFs showed no effects of placebo administration and no effects of alcohol on IAw or ICon. We did not replicate previous between-subjects findings that alcohol would impair IAw,19 but the use of a within-subjects design with baseline measurements strengthens the evidence obtained herein. The increase in IAcc after alcohol administration is intriguing considering the sedative effects of alcohol and suggests that further research is needed to better understand alcohol effects on interoceptive abilities across ascending and descending alcohol limbs.
We found that individual differences in the modulation of IAw correlate with subjective alcohol effects. This replicates previous findings that IAw correlates with perceived light-headedness after alcohol administration.19 That is, changes in the awareness of one’s accuracy in the discrimination task positively correlated with the perception of light-headedness. Light-headedness is considered to be an index of bodily response to alcohol administration and has been used as an interoceptive cue in the discrimination of alcohol dose.39 ICon also positively correlated with light-headedness changes, although to a lesser extent. Meta-cognitive scores obtained using AUROC, however, are independent of confidence biases,44 showing that two distinct components of subjective cardiac interoception correlate with the development of light-headedness. Changes in positive mood on the other hand positively correlated with IAcc and IAw. This suggests that there is a link between the perception of alcohol effects and the changes elicited by a pharmacological challenge across interoceptive dimensions.15
Negative expectancies about alcohol effects, measured at the beginning of the first session, negatively correlated with alcohol-induced changes in IAcc. We are cautious about extrapolating causality from this correlation, but directionality could be relevant in understanding these results. One interpretation could be that negative expectancies somehow bias the ability of participants to feel their heart and therefore better perform the task. However, the session order was randomized between participants, and no effects were observed in the placebo condition, even though virtually all participants thought they had consumed some alcohol regardless of condition. Further, IAcc is an index that is less likely to be affected by conscious expectancies than ICon or IAw, which are akin to subjective interoceptive experiences. It is therefore unlikely that beliefs about negative alcohol expectancies could cause a decrease in participants’ ability to complete the heartbeat discrimination task.
Alternatively, these results may constitute an experimental perspective on how changes in interoception after alcohol administration result in future expectancies. It is possible that the large individual differences observed in IAcc change reflect the effects on interoception participants usually experience after drinking. If across their history of repeated alcohol consumption, participants experience a similar interoceptive effect, those interoceptive experiences could act as unconditioned stimuli shaping future expectancies through classical conditioning mechanisms.34,35 This latter hypothesis reconciles our findings that IAcc positively correlates with positive mood changes after alcohol administration, implying that changes in mood could participate in learning mechanisms shaping future expectancies. Likewise, we previously found that impaired body–brain connectivity, measured as decreases in 0.1-Hz HRV, correlated with a decrease in cognitive biases towards alcohol cues,12 the implication being that changes in bodily states after alcohol administration shape the value of alcohol-related stimuli. We posit therefore that interoceptive experiences after alcohol administration shape the perceived effects of the substance on the body and the incentive value of reinforcers through alliesthetic mechanisms,21,45 in turn determining future expectancies. This is also consistent with predictive coding perspectives, by which interoceptive signals shape predictions about external challenges,46,47 contributing to maladaptive learnt responses that derive into substance use disorders.48 In other words, people’s expectation of intoxication may be influenced by how alcohol usually disrupts their interoceptive processes.
4.1. Limitations and future directions
Regression analyses for light-headedness and negative expectancies need to be considered with caution as correction for multiple comparisons rendered results marginally significant; despite not being preregistered, all analyses conducted were reported in the manuscript. However, the inclusion of BFs and the finding of sensitively null results in the placebo condition indicate a specificity of effects after alcohol administration. Considering the impact COVID-19 had on participant recruitment, we consider our results constitute an adequate basis for future studies.
The cardiac discrimination task has also been subject to criticism and validity49 and reliability issues, particularly due to the small number of trials,50 and results should be interpreted with caution. Ideally, a method of constant stimuli should be used in the cardiac discrimination task, although the lengthy procedure of this task (>60 min) does not fit well with pharmacological paradigms such as the one used herein because blood–alcohol levels vary over time after beverage administration. More recently, novel tasks to measure interoceptive ability51 have been developed and will be examined as part of a preregistered pharmacological administration of alcohol.52
Contrary to our hypothesis, we found that interoceptive ability did not correlate with craving. However, as shown in the Supporting Information, our alcohol administration procedure did not generate subjective craving effects, as opposed to previous research conducted with similar doses.23,24 We tested participants during the day and in a laboratory setting, and these experimental constraints may have dampened the generation of craving. We cannot therefore rule out that interoception mediates the generation of subjective craving, and this study does not seem suitable to characterize such phenomenon. In any case, an interoceptive basis for craving is not necessarily observable through subjective ratings, and our previous findings of increased cognitive cue reactivity associated with alcohol-induced bodily states11,12 would provide support for the role of interoception in alcohol urges. Further, here, we characterize the role of interoception in the reinforcing and incentive components of alcohol, fitting the predictions of Paulus et al.21 Conversely, Gray and Critchley20 conceptualized the role of interoception in craving through the development of anxiety and tension, and our experiment does not address the negative reinforcement framework. Future clinical research should clarify the interoceptive basis of craving as a by-product of substance withdrawal.
Future studies are needed to replicate these findings and to extend the results with the use of other tools measuring alcohol effects. Assessing expectancies days before the experiment as a trait construct would further establish expectancies as a stable learning effect shaped by previous interoceptive experiences, disconnected from the possible influence of a laboratory setting. More advanced cardiovascular assessments, such as baroreflex sensitivity, are also necessary to understand the physiological mechanisms that support interoception53 and the bodily adaptations to alcohol effects.48 Further, different measures of interoceptive ability correlated with alcohol-related variables, and although most participants thought they had consumed alcohol in the placebo condition, they clearly perceived stronger intoxication effects in the alcohol condition, which could engage demand characteristics. Future research needs to disentangle the physiological basis for each of the interoceptive measures and further delineate the relationship between these measures.15
5. CONCLUSION
We propose that changes in the perception of bodily states construct an interoceptive experience of reinforcing alcohol effects,21,35 which in turn informs future alcohol expectancies to mediate the development and prognosis of alcohol use behaviours. Together with our previous findings that alcohol bodily states shape alcohol cue reactivity,11,12 this report provides novel experimental perspectives on interoception and drinking behaviour that were lacking.9
Supplementary Material
ACKNOWLEDGEMENT
This research was supported in part by Grants R01AA023667 and K02AA025123 from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
CONFLICT OF INTEREST
We disclose no conflicts of interest.
DATA AVAILABILITY STATEMENT
Data will be made available on OSF upon manuscript acceptance.
References
- 1.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. [DOI] [PubMed] [Google Scholar]
- 2.Sherrington CS. The Integrative Action of the Nervous System. CUP Archive; 1948. [Google Scholar]
- 3.Garfinkel SN, Manassei MF, Hamilton-Fletcher G, In den Bosch Y, Critchley HD, Engels M. Interoceptive dimensions across cardiac and respiratory axes. Philos Trans R Soc B Biol Sci. 2016;371(1708):20160014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77(4):624–638. [DOI] [PubMed] [Google Scholar]
- 5.Leganes-Fonteneau M, Buckman JF, Suzuki K, Pawlak A, Bates ME. More than meets the heart: systolic amplification of different emotional faces is task dependent. Cogn Emot. 2020;35(2):400–408. 10.1080/02699931.2020.1832050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clore GL, Huntsinger JR. How emotions inform judgment and regulate thought. Trends Cogn Sci. 2007;11(9):393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32(4):777–810. [DOI] [PubMed] [Google Scholar]
- 8.Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76(Pt B):342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36(8):1857–1869. [DOI] [PubMed] [Google Scholar]
- 10.Bonaz B, Lane RD, Oshinsky ML, et al. Diseases, disorders, and comorbidities of interoception. Trends Neurosci. 2021;44(1):39–51. [DOI] [PubMed] [Google Scholar]
- 11.Leganes-Fonteneau M, Buckman J, Pawlak A, Vaschillo B, Vaschillo E, Bates M. Interoceptive signaling in alcohol cognitive biases: role of family history and alliesthetic components. Addict Biol. 2020;26(3):e12952. 10.1111/adb.12952 [DOI] [PubMed] [Google Scholar]
- 12.Leganes-Fonteneau M, Bates ME, Vaschillo EG, Buckman JF. An interoceptive basis for alcohol priming effects. Psychopharmacology (Berl). 2021;1–11. 10.1007/s00213-021-05796-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katkin E, Reed S & Deroo C A methodological analysis of 3 techniques for the assessment of individual-differences in heartbeat detection. (1983).
- 14.Whitehead WE, Drescher VM, Heiman P, Blackwell B. Relation of heart rate control to heartbeat perception. Biofeedback Self Regul. 1977;2(4):371–392. [PubMed] [Google Scholar]
- 15.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol. 2015;104:65–74. [DOI] [PubMed] [Google Scholar]
- 16.Abrams K, Cieslowski K, Johnson S, et al. The effects of alcohol on heartbeat perception: Implications for anxiety. Addict Behav. 2018;79:151–158. [DOI] [PubMed] [Google Scholar]
- 17.Zamariola G, Maurage P, Luminet O, Corneille O. Interoceptive accuracy scores from the heartbeat counting task are problematic: evidence from simple bivariate correlations. Biol Psychol. 2018;137:12–17. [DOI] [PubMed] [Google Scholar]
- 18.Desmedt O, Luminet O, Corneille O. The heartbeat counting task largely involves non-interoceptive processes: evidence from both the original and an adapted counting task. Biol Psychol. 2018;138:185–188. [DOI] [PubMed] [Google Scholar]
- 19.Leganes-Fonteneau M, Cheang Y, Lam Y, Garfinkel S, Duka T. Interoceptive awareness is associated with acute alcohol-induced changes in subjective effects. Pharmacol Biochem Behav. 2019;181:69–76. [DOI] [PubMed] [Google Scholar]
- 20.Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54(2):183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav. 2009;94(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose AK, Duka T. Effects of dose and time on the ability of alcohol to prime social drinkers. Behav Pharmacol. 2006;17(1):61–70. [DOI] [PubMed] [Google Scholar]
- 24.Schoenmakers T, Wiers RW, Field M. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology (Berl). 2008;197(169–178):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duka T, Stephens DN, Russell C, Tasker R. Discriminative stimulus properties of low doses of ethanol in humans. Psychopharmacology (Berl). 1998;136(379–389):379–389. [DOI] [PubMed] [Google Scholar]
- 26.Smith AP. Effects of caffeine and alcohol on mood and performance changes following consumption of lager. Psychopharmacology (Berl). 2013;227(595–604):595–604. [DOI] [PubMed] [Google Scholar]
- 27.Wilkie H, Stewart SH. Reinforcing mood effects of alcohol in coping and enhancement motivated drinkers. Alcohol Clin Exp Res. 2005;29(5):829–836. [DOI] [PubMed] [Google Scholar]
- 28.Goldman MS, Del Boca FK, Darkes J. Alcohol expectancy theory: the application of cognitive neuroscience. In: Psychological Theories of Drinking and Alcoholism. The Guilford Press; 1999:203–246. [Google Scholar]
- 29.Sebold M, Nebe S, Garbusow M, et al. When habits are dangerous: alcohol expectancies and habitual decision making predict relapse in alcohol dependence. Biol Psychiatry. 2017;82(11):847–856. [DOI] [PubMed] [Google Scholar]
- 30.Drobes DJ, Carter AC, Goldman MS. Alcohol expectancies and reactivity to alcohol-related and affective cues. Exp Clin Psychopharmacol. 2009;17(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eddie D, Buckman JF, Mun EY, et al. Different associations of alcohol cue reactivity with negative alcohol expectancies in mandated and inpatient samples of young adults. Addict Behav. 2013;38(4):2040–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maisto SA, Carey KB, Bradizza CM. Social learning theory. In: Psychological Theories of Drinking and Alcoholism. 2nd ed. The Guilford Press; 1999:106–163. [Google Scholar]
- 33.Morean ME, Corbin WR, Treat TA. The Anticipated Effects of Alcohol Scale: development and psychometric evaluation of a novel assessment tool for measuring alcohol expectancies. Psychol Assess. 2012;24(4):1008–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson BM, Bevins RA, Murray JE. Interoceptive Stimulus Effects of Drugs of Abuse. Neural Mech Addict. 2019;89–101. 10.1016/B978-0-12-812202-0.00007-5 [DOI] [Google Scholar]
- 35.Bevins RA, Besheer J. Interoception and learning: import to understanding and treating diseases and psychopathologies. ACS Chem Nerosci. 2014;5(8):624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 37.Mehrabian A, Russell JA. A questionnaire measure of habitual alcohol use. Psychol Rep. 1978;43(3):803–806. [DOI] [PubMed] [Google Scholar]
- 38.Townshend J, Duka T. Patterns of alcohol drinking in a population of young social drinkers: a comparison of questionnaire and diary measures. Alcohol Alcohol. 2002;37(2):187–192. [DOI] [PubMed] [Google Scholar]
- 39.Duka T, Jackson A, Smith DC, Stephens DN. Relationship of components of an alcohol interoceptive stimulus to induction of desire for alcohol in social drinkers. Pharmacol Biochem Behav. 1999;64(2):301–309. [DOI] [PubMed] [Google Scholar]
- 40.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 41.Kramer JR, Chan G, Hesselbrock VM, et al. A principal components analysis of the abbreviated Desires for Alcohol Questionnaire (DAQ). J Stud Alcohol Drugs. 2010;71(1):150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schandry R Heart beat perception and emotional experience. Psychophysiology. 1981;18(4):483–488. [DOI] [PubMed] [Google Scholar]
- 43.Dienes Z Using Bayes to get the most out of non-significant results. Front Psychol. 2014;5:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleming SM, Lau HC. How to measure metacognition. Front Hum Neurosci. 2014;8:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avery JA, Burrows K, Kerr KL, et al. How the brain wants what the body needs: the neural basis of positive alliesthesia. Neuropsychopharmacology. 2017;42(4):822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seth AK, Friston KJ. Active interoceptive inference and the emotional brain. Philos Trans R Soc B Biol Sci. 2016;371(1708):20160007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Critchley HD, Garfinkel SN. The influence of physiological signals on cognition. Curr Opin Behav Sci. 2018;19:13–18. [Google Scholar]
- 48.Eddie D, Bates ME, Buckman JF. Closing the brain–heart loop: towards more holistic models of addiction and addiction recovery. Addict Biol. 2020;e12958. 10.1111/adb.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brener J, Ring C. Towards a psychophysics of interoceptive processes: the measurement of heartbeat detection. Philos Trans R Soc B Biol Sci. 2016;371(1708):20160015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleckner IR, Wormwood JB, Simmons WK, Barrett LF, Quigley KS. Methodological recommendations for a heartbeat detection-based measure of interoceptive sensitivity. Psychophysiology. 2015;52(11):1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Legrand N & Allen M The heart rate discrimination task: a psychophysical method to estimate the accuracy and precision of interoceptive beliefs. bioRxiv 1, 2 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Leganes M, Bates M, Pawlak A, Buckman J. Does alcohol affect emotional face processing via interoceptive pathways? Drug Alcohol Depend. 2021;226:108845. 10.31234/OSF.IO/43UMW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leganes-Fonteneau M, Buckman J, Islam S, et al. The cardiovascular mechanisms of interoceptive awareness: effects of resonance breathing. Int J Psychophysiol Stage. 2021;1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on OSF upon manuscript acceptance.


