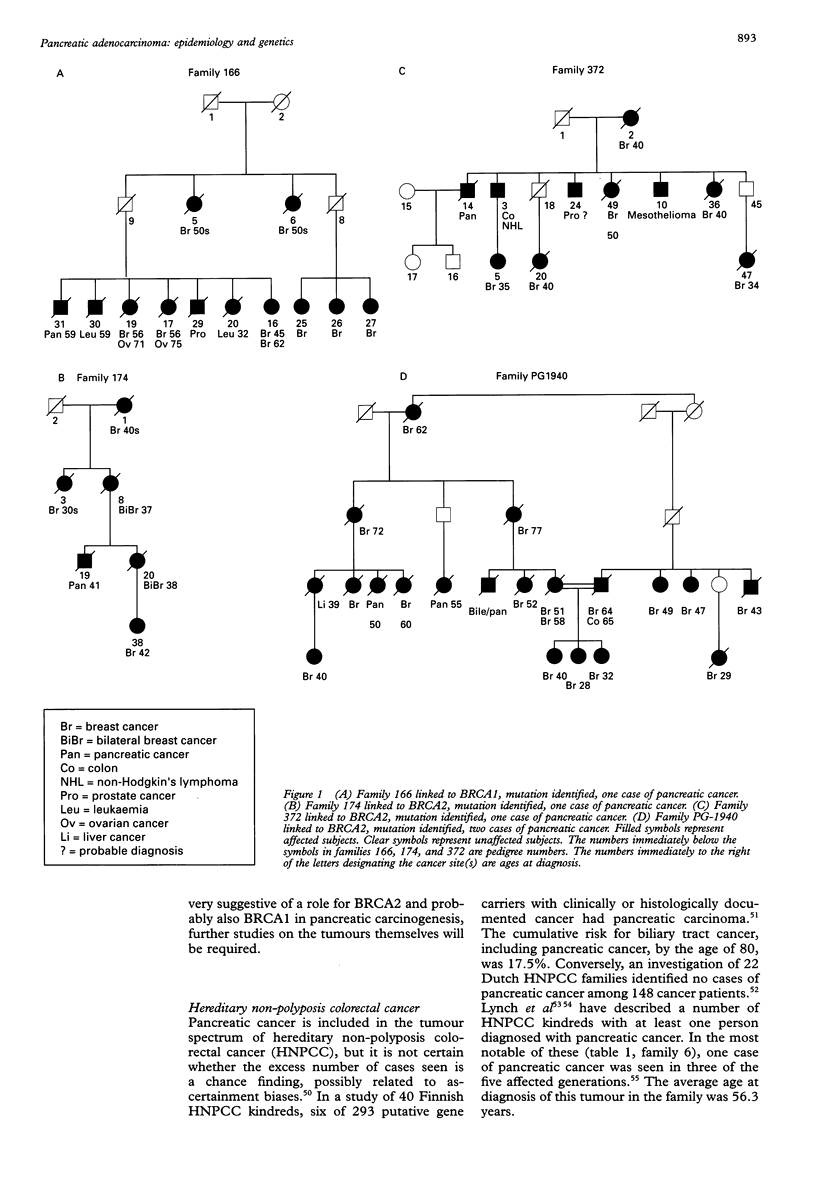
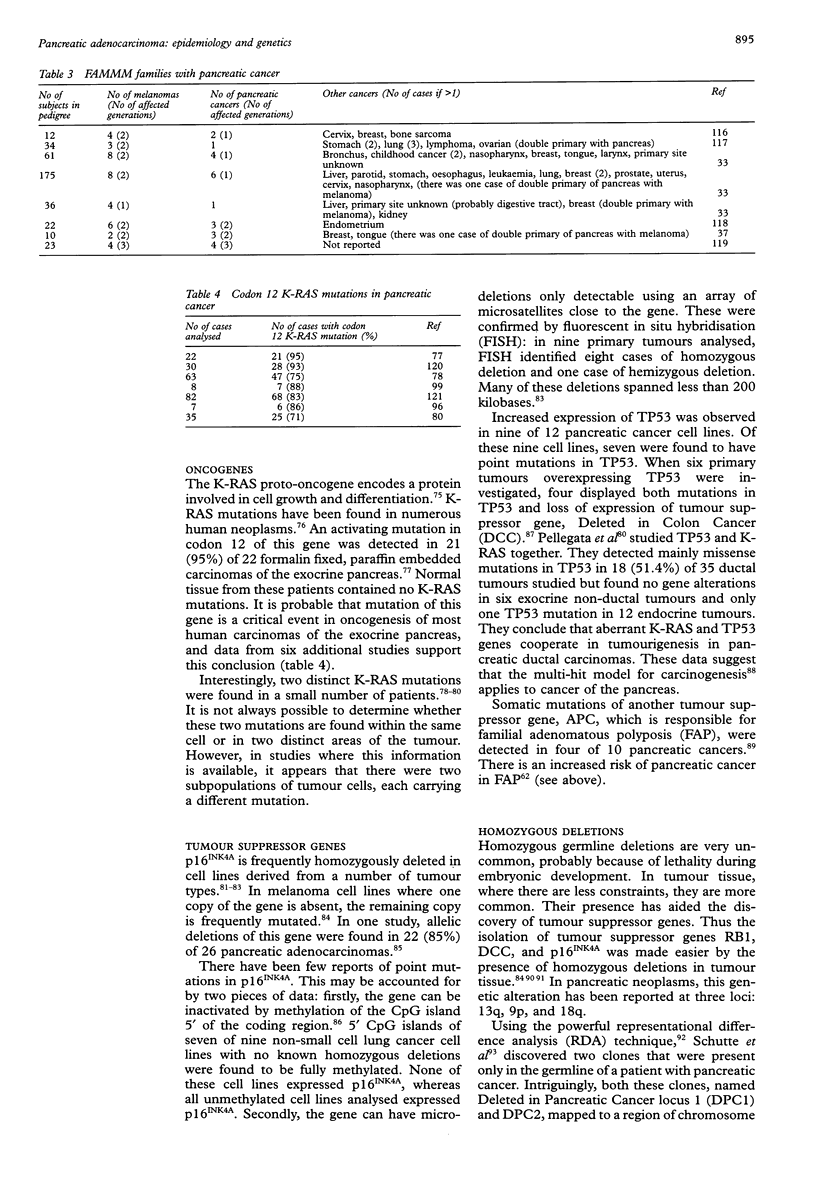
Abstract
Pancreatic adenocarcinoma is an important cause of death from cancer throughout the developed world. There are few established environmental risk factors, but a previous history of pancreatitis and exposure to tobacco and salted food appear to be the most important. A family history of pancreatic adenocarcinoma is not common in patients with this disease, but recent research has shown that pancreatic adenocarcinoma can be a feature of cancer susceptibility syndromes associated with germline mutations in p16, BRCA1, BRCA2, and APC. This highlights the need for a full family history in apparently sporadic cases. Somatic mutations in p16, BRCA2, and APC have also been reported in pancreatic cancer; however, K-RAS mutations appear to be the commonest oncogenic alteration. Recent advances in our understanding of the basis of hereditary cancer syndromes may be applicable to the diagnosis, treatment, and possibly prevention of pancreatic adenocarcinoma in the future.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarnio M., Mecklin J. P., Aaltonen L. A., Nyström-Lahti M., Järvinen H. J. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer. 1995 Dec 20;64(6):430–433. doi: 10.1002/ijc.2910640613. [DOI] [PubMed] [Google Scholar]
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Arason A., Barkardóttir R. B., Egilsson V. Linkage analysis of chromosome 17q markers and breast-ovarian cancer in Icelandic families, and possible relationship to prostatic cancer. Am J Hum Genet. 1993 Apr;52(4):711–717. [PMC free article] [PubMed] [Google Scholar]
- Arch R., Wirth K., Hofmann M., Ponta H., Matzku S., Herrlich P., Zöller M. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992 Jul 31;257(5070):682–685. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- Bansal P., Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology. 1995 Jul;109(1):247–251. doi: 10.1016/0016-5085(95)90291-0. [DOI] [PubMed] [Google Scholar]
- Bergman W., Watson P., de Jong J., Lynch H. T., Fusaro R. M. Systemic cancer and the FAMMM syndrome. Br J Cancer. 1990 Jun;61(6):932–936. doi: 10.1038/bjc.1990.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L. The ras gene family and human carcinogenesis. Mutat Res. 1988 May;195(3):255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Bowlby L. S. Pancreatic adenocarcinoma in an adolescent male with Peutz-Jeghers syndrome. Hum Pathol. 1986 Jan;17(1):97–99. doi: 10.1016/s0046-8177(86)80163-0. [DOI] [PubMed] [Google Scholar]
- Cairns P., Polascik T. J., Eby Y., Tokino K., Califano J., Merlo A., Mao L., Herath J., Jenkins R., Westra W. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet. 1995 Oct;11(2):210–212. doi: 10.1038/ng1095-210. [DOI] [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., Hruban R. H., Redston M. S., Yeo C. J., Kern S. E. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res. 1994 Jul 1;54(13):3568–3573. [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Ciotti P., Strigini P., Bianchi-Scarrà G. Familial melanoma and pancreatic cancer. Ligurian Skin Tumor Study Group. N Engl J Med. 1996 Feb 15;334(7):469–472. doi: 10.1056/NEJM199602153340714. [DOI] [PubMed] [Google Scholar]
- Dalager N. A., Kang H. K., Thomas T. L. Cancer mortality patterns among women who served in the military: the Vietnam experience. J Occup Environ Med. 1995 Mar;37(3):298–305. doi: 10.1097/00043764-199503000-00005. [DOI] [PubMed] [Google Scholar]
- Danes B. S., Lynch H. T. A familial aggregation of pancreatic cancer. An in vitro study. JAMA. 1982 May 28;247(20):2798–2802. [PubMed] [Google Scholar]
- Davidson P., Costanza D., Swieconek J. A., Harris J. B. Hereditary pancreatitis. A kindred without gross aminoaciduria. Ann Intern Med. 1968 Jan;68(1):88–96. doi: 10.7326/0003-4819-68-1-88. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Morphoregulatory molecules. Biochemistry. 1988 May 17;27(10):3533–3543. doi: 10.1021/bi00410a001. [DOI] [PubMed] [Google Scholar]
- Ehrenthal D., Haeger L., Griffin T., Compton C. Familial pancreatic adenocarcinoma in three generations. A case report and a review of the literature. Cancer. 1987 May 1;59(9):1661–1664. doi: 10.1002/1097-0142(19870501)59:9<1661::aid-cncr2820590923>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Evans J. P., Burke W., Chen R., Bennett R. L., Schmidt R. A., Dellinger E. P., Kimmey M., Crispin D., Brentnall T. A., Byrd D. R. Familial pancreatic adenocarcinoma: association with diabetes and early molecular diagnosis. J Med Genet. 1995 May;32(5):330–335. doi: 10.1136/jmg.32.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everhart J., Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995 May 24;273(20):1605–1609. [PubMed] [Google Scholar]
- Falk R. T., Pickle L. W., Fontham E. T., Correa P., Fraumeni J. F., Jr Life-style risk factors for pancreatic cancer in Louisiana: a case-control study. Am J Epidemiol. 1988 Aug;128(2):324–336. doi: 10.1093/oxfordjournals.aje.a114972. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Foley T. R., McGarrity T. J., Abt A. B. Peutz-Jeghers syndrome: a clinicopathologic survey of the "Harrisburg family" with a 49-year follow-up. Gastroenterology. 1988 Dec;95(6):1535–1540. doi: 10.1016/s0016-5085(88)80074-x. [DOI] [PubMed] [Google Scholar]
- Friedman G. D. Cholecystectomy not confirmed as a risk factor for pancreatic cancer. Int J Cancer. 1995 May 29;61(5):745–746. doi: 10.1002/ijc.2910610526. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Fialkow P. J. Familial carcinoma of the pancreas. Clin Genet. 1976 May;9(5):463–469. doi: 10.1111/j.1399-0004.1976.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Ghadirian P., Baillargeon J., Simard A., Perret C. Food habits and pancreatic cancer: a case-control study of the Francophone community in Montreal, Canada. Cancer Epidemiol Biomarkers Prev. 1995 Dec;4(8):895–899. [PubMed] [Google Scholar]
- Ghadirian P., Boyle P., Simard A., Baillargeon J., Maisonneuve P., Perret C. Reported family aggregation of pancreatic cancer within a population-based case-control study in the Francophone community in Montreal, Canada. Int J Pancreatol. 1991 Nov-Dec;10(3-4):183–196. doi: 10.1007/BF02924156. [DOI] [PubMed] [Google Scholar]
- Ghadirian P., Simard A., Baillargeon J. Cancer of the pancreas in two brothers and one sister. Int J Pancreatol. 1987 Oct-Dec;2(5-6):383–391. doi: 10.1007/BF02788437. [DOI] [PubMed] [Google Scholar]
- Ghadirian P., Simard A., Baillargeon J. Tobacco, alcohol, and coffee and cancer of the pancreas. A population-based, case-control study in Quebec, Canada. Cancer. 1991 May 15;67(10):2664–2670. doi: 10.1002/1097-0142(19910515)67:10<2664::aid-cncr2820671043>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Giardiello F. M., Offerhaus G. J., Lee D. H., Krush A. J., Tersmette A. C., Booker S. V., Kelley N. C., Hamilton S. R. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993 Oct;34(10):1394–1396. doi: 10.1136/gut.34.10.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardiello F. M., Welsh S. B., Hamilton S. R., Offerhaus G. J., Gittelsohn A. M., Booker S. V., Krush A. J., Yardley J. H., Luk G. D. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987 Jun 11;316(24):1511–1514. doi: 10.1056/NEJM198706113162404. [DOI] [PubMed] [Google Scholar]
- Gold E. B., Cameron J. L. Chronic pancreatitis and pancreatic cancer. N Engl J Med. 1993 May 20;328(20):1485–1486. doi: 10.1056/NEJM199305203282010. [DOI] [PubMed] [Google Scholar]
- Gold E. B. Epidemiology of and risk factors for pancreatic cancer. Surg Clin North Am. 1995 Oct;75(5):819–843. doi: 10.1016/s0039-6109(16)46730-7. [DOI] [PubMed] [Google Scholar]
- Goldgar D. E., Easton D. F., Cannon-Albright L. A., Skolnick M. H. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994 Nov 2;86(21):1600–1608. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- Goldstein A. M., Fraser M. C., Struewing J. P., Hussussian C. J., Ranade K., Zametkin D. P., Fontaine L. S., Organic S. M., Dracopoli N. C., Clark W. H., Jr Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995 Oct 12;333(15):970–974. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- Grajower M. M. Familial pancreatic cancer. Ann Intern Med. 1983 Jan;98(1):111–111. doi: 10.7326/0003-4819-98-1-111_2. [DOI] [PubMed] [Google Scholar]
- Gruis N. A., van der Velden P. A., Sandkuijl L. A., Prins D. E., Weaver-Feldhaus J., Kamb A., Bergman W., Frants R. R. Homozygotes for CDKN2 (p16) germline mutation in Dutch familial melanoma kindreds. Nat Genet. 1995 Jul;10(3):351–353. doi: 10.1038/ng0795-351. [DOI] [PubMed] [Google Scholar]
- Grünewald K., Lyons J., Fröhlich A., Feichtinger H., Weger R. A., Schwab G., Janssen J. W., Bartram C. R. High frequency of Ki-ras codon 12 mutations in pancreatic adenocarcinomas. Int J Cancer. 1989 Jun 15;43(6):1037–1041. doi: 10.1002/ijc.2910430614. [DOI] [PubMed] [Google Scholar]
- Gutman M., Cnaan A., Inbar M., Shafir R., Chaitchik S., Rozin R. R., Klausner J. M. Are malignant melanoma patients at higher risk for a second cancer? Cancer. 1991 Aug 1;68(3):660–665. doi: 10.1002/1097-0142(19910801)68:3<660::aid-cncr2820680337>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Hahn S. A., Hoque A. T., Moskaluk C. A., da Costa L. T., Schutte M., Rozenblum E., Seymour A. B., Weinstein C. L., Yeo C. J., Hruban R. H. Homozygous deletion map at 18q21.1 in pancreatic cancer. Cancer Res. 1996 Feb 1;56(3):490–494. [PubMed] [Google Scholar]
- Hahn S. A., Schutte M., Hoque A. T., Moskaluk C. A., da Costa L. T., Rozenblum E., Weinstein C. L., Fischer A., Yeo C. J., Hruban R. H. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996 Jan 19;271(5247):350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- Hahn S. A., Seymour A. B., Hoque A. T., Schutte M., da Costa L. T., Redston M. S., Caldas C., Weinstein C. L., Fischer A., Yeo C. J. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995 Oct 15;55(20):4670–4675. [PubMed] [Google Scholar]
- Hedrick L., Cho K. R., Fearon E. R., Wu T. C., Kinzler K. W., Vogelstein B. The DCC gene product in cellular differentiation and colorectal tumorigenesis. Genes Dev. 1994 May 15;8(10):1174–1183. doi: 10.1101/gad.8.10.1174. [DOI] [PubMed] [Google Scholar]
- Horii A., Nakatsuru S., Miyoshi Y., Ichii S., Nagase H., Ando H., Yanagisawa A., Tsuchiya E., Kato Y., Nakamura Y. Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res. 1992 Dec 1;52(23):6696–6698. [PubMed] [Google Scholar]
- Hruban R. H., van Mansfeld A. D., Offerhaus G. J., van Weering D. H., Allison D. C., Goodman S. N., Kensler T. W., Bose K. K., Cameron J. L., Bos J. L. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993 Aug;143(2):545–554. [PMC free article] [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Höhne M. W., Halatsch M. E., Kahl G. F., Weinel R. J. Frequent loss of expression of the potential tumor suppressor gene DCC in ductal pancreatic adenocarcinoma. Cancer Res. 1992 May 1;52(9):2616–2619. [PubMed] [Google Scholar]
- Jensen O. A., Warburg M., Dupont A. Ocular pathology in the elfin face syndrome (the Fanconi-Schlesinger type of idiopathic hypercalcaemia of infancy). Histochemical and ultrastructural study of a case. Ophthalmologica. 1976;172(6):434–444. doi: 10.1159/000307744. [DOI] [PubMed] [Google Scholar]
- Johannsson O., Ostermeyer E. A., Håkansson S., Friedman L. S., Johansson U., Sellberg G., Brøndum-Nielsen K., Sele V., Olsson H., King M. C. Founding BRCA1 mutations in hereditary breast and ovarian cancer in southern Sweden. Am J Hum Genet. 1996 Mar;58(3):441–450. [PMC free article] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kattwinkel J., Lapey A., Di Sant'Agnese P. A., Edwards W. A. Hereditary pancreatitis: three new kindreds and a critical review of the literature. Pediatrics. 1973 Jan;51(1):55–69. [PubMed] [Google Scholar]
- Kerber R. A., Slattery M. L. The impact of family history on ovarian cancer risk. The Utah Population Database. Arch Intern Med. 1995 May 8;155(9):905–912. [PubMed] [Google Scholar]
- Le Bodic L., Bignon J. D., Raguénès O., Mercier B., Georgelin T., Schnee M., Soulard F., Gagne K., Bonneville F., Muller J. Y. The hereditary pancreatitis gene maps to long arm of chromosome 7. Hum Mol Genet. 1996 Apr;5(4):549–554. doi: 10.1093/hmg/5.4.549. [DOI] [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr, Mulvihill J. J., Blattner W. A., Dreyfus M. G., Tucker M. A., Miller R. W. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988 Sep 15;48(18):5358–5362. [PubMed] [Google Scholar]
- Lisitsyn N. A., Lisitsina N. M., Dalbagni G., Barker P., Sanchez C. A., Gnarra J., Linehan W. M., Reid B. J., Wigler M. H. Comparative genomic analysis of tumors: detection of DNA losses and amplification. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):151–155. doi: 10.1073/pnas.92.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch H. T., Fitzsimmons M. L., Smyrk T. C., Lanspa S. J., Watson P., McClellan J., Lynch J. F. Familial pancreatic cancer: clinicopathologic study of 18 nuclear families. Am J Gastroenterol. 1990 Jan;85(1):54–60. [PubMed] [Google Scholar]
- Lynch H. T., Frichot B. C., Lynch P., Lynch J., Gurigis H. A. Family studies of malignant melanoma and associated cancer. Surg Gynecol Obstet. 1975 Oct;141(4):517–522. [PubMed] [Google Scholar]
- Lynch H. T., Fusaro L., Lynch J. F. Familial pancreatic cancer: a family study. Pancreas. 1992;7(5):511–515. doi: 10.1097/00006676-199209000-00001. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Fusaro L., Smyrk T. C., Watson P., Lanspa S., Lynch J. F. Medical genetic study of eight pancreatic cancer-prone families. Cancer Invest. 1995;13(2):141–149. doi: 10.3109/07357909509011683. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Fusaro R. M. Pancreatic cancer and the familial atypical multiple mole melanoma (FAMMM) syndrome. Pancreas. 1991 Mar;6(2):127–131. doi: 10.1097/00006676-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Krush A. J. Heredity and malignant melanoma: implications for early cancer detection. Can Med Assoc J. 1968 Jul 6;99(1):17–21. [PMC free article] [PubMed] [Google Scholar]
- Lynch H. T., Richardson J. D., Amin M., Lynch J. F., Cavalieri R. J., Bronson E., Fusaro R. M. Variable gastrointestinal and urologic cancers in a Lynch syndrome II kindred. Dis Colon Rectum. 1991 Oct;34(10):891–895. doi: 10.1007/BF02049703. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T., Watson P., Lanspa S. J., Boman B. M., Lynch P. M., Lynch J. F., Cavalieri J. Hereditary colorectal cancer. Semin Oncol. 1991 Aug;18(4):337–366. [PubMed] [Google Scholar]
- Lynch H. T., Voorhees G. J., Lanspa S. J., McGreevy P. S., Lynch J. F. Pancreatic carcinoma and hereditary nonpolyposis colorectal cancer: a family study. Br J Cancer. 1985 Aug;52(2):271–273. doi: 10.1038/bjc.1985.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Kramer P. Adenocarcinoma of the pancreas in four siblings. Gastroenterology. 1973 Jul;65(1):137–139. [PubMed] [Google Scholar]
- Mack T. M., Yu M. C., Hanisch R., Henderson B. E. Pancreas cancer and smoking, beverage consumption, and past medical history. J Natl Cancer Inst. 1986 Jan;76(1):49–60. [PubMed] [Google Scholar]
- Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995 Jul;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Morgan R. G., Wormsley K. G. Progress report. Cancer of the pancreas. Gut. 1977 Jul;18(7):580–596. doi: 10.1136/gut.18.7.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. A., Thomas I. T., Greenberg F. Williams syndrome: autosomal dominant inheritance. Am J Med Genet. 1993 Sep 15;47(4):478–481. doi: 10.1002/ajmg.1320470409. [DOI] [PubMed] [Google Scholar]
- Narita T., Takagi K. Ataxia-telangiectasia with dysgerminoma of right ovary, papillary carcinoma of thyroid, and adenocarcinoma of pancreas. Cancer. 1984 Sep 15;54(6):1113–1116. doi: 10.1002/1097-0142(19840915)54:6<1113::aid-cncr2820540632>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Neglia J. P., Wielinski C. L., Warwick W. J. Cancer risk among patients with cystic fibrosis. J Pediatr. 1991 Nov;119(5):764–766. doi: 10.1016/s0022-3476(05)80296-3. [DOI] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Offerhaus G. J., Giardiello F. M., Moore G. W., Tersmette A. C. Partial gastrectomy: a risk factor for carcinoma of the pancreas? Hum Pathol. 1987 Mar;18(3):285–288. doi: 10.1016/s0046-8177(87)80011-4. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Demetrick D. J., Spillare E. A., Hagiwara K., Hussain S. P., Bennett W. P., Forrester K., Gerwin B., Serrano M., Beach D. H. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin D. M., Lärä E., Muir C. S. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer. 1988 Feb 15;41(2):184–197. doi: 10.1002/ijc.2910410205. [DOI] [PubMed] [Google Scholar]
- Parkin D. M., Pisani P., Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993 Jun 19;54(4):594–606. doi: 10.1002/ijc.2910540413. [DOI] [PubMed] [Google Scholar]
- Partanen T., Kauppinen T., Degerth R., Moneta G., Mearelli I., Ojajärvi A., Hernberg S., Koskinen H., Pukkala E. Pancreatic cancer in industrial branches and occupations in Finland. Am J Ind Med. 1994 Jun;25(6):851–866. doi: 10.1002/ajim.4700250609. [DOI] [PubMed] [Google Scholar]
- Pellegata N. S., Sessa F., Renault B., Bonato M., Leone B. E., Solcia E., Ranzani G. N. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994 Mar 15;54(6):1556–1560. [PubMed] [Google Scholar]
- Phelan C. M., Lancaster J. M., Tonin P., Gumbs C., Cochran C., Carter R., Ghadirian P., Perret C., Moslehi R., Dion F. Mutation analysis of the BRCA2 gene in 49 site-specific breast cancer families. Nat Genet. 1996 May;13(1):120–122. doi: 10.1038/ng0596-120. [DOI] [PubMed] [Google Scholar]
- Pippard E. C., Hall A. J., Barker D. J., Bridges B. A. Cancer in homozygotes and heterozygotes of ataxia-telangiectasia and xeroderma pigmentosum in Britain. Cancer Res. 1988 May 15;48(10):2929–2932. [PubMed] [Google Scholar]
- Ranade K., Hussussian C. J., Sikorski R. S., Varmus H. E., Goldstein A. M., Tucker M. A., Serrano M., Hannon G. J., Beach D., Dracopoli N. C. Mutations associated with familial melanoma impair p16INK4 function. Nat Genet. 1995 May;10(1):114–116. doi: 10.1038/ng0595-114. [DOI] [PubMed] [Google Scholar]
- Reale M. A., Hu G., Zafar A. I., Getzenberg R. H., Levine S. M., Fearon E. R. Expression and alternative splicing of the deleted in colorectal cancer (DCC) gene in normal and malignant tissues. Cancer Res. 1994 Aug 15;54(16):4493–4501. [PubMed] [Google Scholar]
- Ross A. H., Smith M. A., Anderson J. R., Small W. P. Late mortality after surgery for peptic ulcer. N Engl J Med. 1982 Aug 26;307(9):519–522. doi: 10.1056/NEJM198208263070902. [DOI] [PubMed] [Google Scholar]
- Sadler L. S., Robinson L. K., Verdaasdonk K. R., Gingell R. The Williams syndrome: evidence for possible autosomal dominant inheritance. Am J Med Genet. 1993 Sep 15;47(4):468–470. doi: 10.1002/ajmg.1320470406. [DOI] [PubMed] [Google Scholar]
- Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D. A., Smith S., Uziel T., Sfez S. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995 Jun 23;268(5218):1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Schutte M., da Costa L. T., Hahn S. A., Moskaluk C., Hoque A. T., Rozenblum E., Weinstein C. L., Bittner M., Meltzer P. S., Trent J. M. Identification by representational difference analysis of a homozygous deletion in pancreatic carcinoma that lies within the BRCA2 region. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5950–5954. doi: 10.1073/pnas.92.13.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour A. B., Hruban R. H., Redston M., Caldas C., Powell S. M., Kinzler K. W., Yeo C. J., Kern S. E. Allelotype of pancreatic adenocarcinoma. Cancer Res. 1994 May 15;54(10):2761–2764. [PubMed] [Google Scholar]
- Shibata A., Mack T. M., Paganini-Hill A., Ross R. K., Henderson B. E. A prospective study of pancreatic cancer in the elderly. Int J Cancer. 1994 Jul 1;58(1):46–49. doi: 10.1002/ijc.2910580109. [DOI] [PubMed] [Google Scholar]
- Simard J., Tonin P., Durocher F., Morgan K., Rommens J., Gingras S., Samson C., Leblanc J. F., Bélanger C., Dion F. Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat Genet. 1994 Dec;8(4):392–398. doi: 10.1038/ng1294-392. [DOI] [PubMed] [Google Scholar]
- Simon B., Weinel R., Höhne M., Watz J., Schmidt J., Körtner G., Arnold R. Frequent alterations of the tumor suppressor genes p53 and DCC in human pancreatic carcinoma. Gastroenterology. 1994 Jun;106(6):1645–1651. doi: 10.1016/0016-5085(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Smit V. T., Boot A. J., Smits A. M., Fleuren G. J., Cornelisse C. J., Bos J. L. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988 Aug 25;16(16):7773–7782. doi: 10.1093/nar/16.16.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman A. D., Murday V., Phillips R. K. Cancer and the Peutz-Jeghers syndrome. Gut. 1989 Nov;30(11):1588–1590. doi: 10.1136/gut.30.11.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong L. C., Stine M., Norsted T. L. Cancer in survivors of childhood soft tissue sarcoma and their relatives. J Natl Cancer Inst. 1987 Dec;79(6):1213–1220. [PubMed] [Google Scholar]
- Swift M., Chase C. L., Morrell D. Cancer predisposition of ataxia-telangiectasia heterozygotes. Cancer Genet Cytogenet. 1990 May;46(1):21–27. doi: 10.1016/0165-4608(90)90004-t. [DOI] [PubMed] [Google Scholar]
- Swift M., Reitnauer P. J., Morrell D., Chase C. L. Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med. 1987 May 21;316(21):1289–1294. doi: 10.1056/NEJM198705213162101. [DOI] [PubMed] [Google Scholar]
- Tada M., Omata M., Kawai S., Saisho H., Ohto M., Saiki R. K., Sninsky J. J. Detection of ras gene mutations in pancreatic juice and peripheral blood of patients with pancreatic adenocarcinoma. Cancer Res. 1993 Jun 1;53(11):2472–2474. [PubMed] [Google Scholar]
- Takada M., Yamamoto M., Saitoh Y. The significance of CD44 in human pancreatic cancer: I. High expression of CD44 in human pancreatic adenocarcinoma. Pancreas. 1994 Nov;9(6):748–752. doi: 10.1097/00006676-199411000-00013. [DOI] [PubMed] [Google Scholar]
- Takada M., Yamamoto M., Saitoh Y. The significance of CD44 in human pancreatic cancer: II. The role of CD44 in human pancreatic adenocarcinoma invasion. Pancreas. 1994 Nov;9(6):753–757. doi: 10.1097/00006676-199411000-00014. [DOI] [PubMed] [Google Scholar]
- Tavtigian S. V., Simard J., Rommens J., Couch F., Shattuck-Eidens D., Neuhausen S., Merajver S., Thorlacius S., Offit K., Stoppa-Lyonnet D. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996 Mar;12(3):333–337. doi: 10.1038/ng0396-333. [DOI] [PubMed] [Google Scholar]
- Thorlacius S., Olafsdottir G., Tryggvadottir L., Neuhausen S., Jonasson J. G., Tavtigian S. V., Tulinius H., Ogmundsdottir H. M., Eyfjörd J. E. A single BRCA2 mutation in male and female breast cancer families from Iceland with varied cancer phenotypes. Nat Genet. 1996 May;13(1):117–119. doi: 10.1038/ng0596-117. [DOI] [PubMed] [Google Scholar]
- Tonin P., Ghadirian P., Phelan C., Lenoir G. M., Lynch H. T., Letendre F., Belanger D., Monté M., Narod S. A. A large multisite cancer family is linked to BRCA2. J Med Genet. 1995 Dec;32(12):982–984. doi: 10.1136/jmg.32.12.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulinius H., Olafsdottir G. H., Sigvaldason H., Tryggvadottir L., Bjarnadottir K. Neoplastic diseases in families of breast cancer patients. J Med Genet. 1994 Aug;31(8):618–621. doi: 10.1136/jmg.31.8.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasen H. F., den Hartog Jager F. C., Menko F. H., Nagengast F. M. Screening for hereditary non-polyposis colorectal cancer: a study of 22 kindreds in The Netherlands. Am J Med. 1989 Mar;86(3):278–281. doi: 10.1016/0002-9343(89)90296-9. [DOI] [PubMed] [Google Scholar]
- Visintainer P. F., Barone M., McGee H., Peterson E. L. Proportionate mortality study of Vietnam-era veterans of Michigan. J Occup Environ Med. 1995 Apr;37(4):423–428. doi: 10.1097/00043764-199504000-00013. [DOI] [PubMed] [Google Scholar]
- Watson P., Lynch H. T. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993 Feb 1;71(3):677–685. doi: 10.1002/1097-0142(19930201)71:3<677::aid-cncr2820710305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Whelan A. J., Bartsch D., Goodfellow P. J. Brief report: a familial syndrome of pancreatic cancer and melanoma with a mutation in the CDKN2 tumor-suppressor gene. N Engl J Med. 1995 Oct 12;333(15):975–977. doi: 10.1056/NEJM199510123331505. [DOI] [PubMed] [Google Scholar]


