Abstract
Background
Diaphragm dysfunction is common in critically ill patients and associated with poorer outcomes. The function of the diaphragm can be evaluated at the bedside by measuring diaphragmatic excursion using ultrasonography. In this study, we investigated the ability of right-sided diaphragmatic excursion (RDE) to predict the need for invasive mechanical ventilation (IMV).
Methods
Critically ill patients aged 18 years and older who presented to our emergency department between May 20, 2021 and May 19, 2022 and underwent measurement of RDE within 10 min of arrival were enrolled in this prospective study. The ability of RDE to predict the need for IMV was assessed by multivariable logistic regression and analysis of the area under the receiver-operating characteristic curve (AUROC).
Results
A total of 314 patients were enrolled in the study; 113 (35.9%) of these patients required IMV. An increase of RDE value per each 0.1 cm was identified to be an independent predictor of IMV (adjusted odds ratio 0.08, 95% confidence interval [CI] 0.04–0.17, p < 0.001; AUROC 0.850, 95% CI 0.807–0.894). The RDE cutoff value was 1.2 cm (sensitivity 82.3%, 95% CI 74.0–88.8; specificity 78.1%, 95% CI 71.7–83.6). Time on a ventilator was significantly longer when the RDE was ≤ 1.2 cm (13 days [interquartile range 5, 27] versus 5 days [interquartile range 3, 8], p = 0.006).
Conclusions
In this study, RDE had a good ability to predict the need for IMV in critically ill patients. The optimal RDE cutoff value was 1.2 cm. Its benefit in patient management requires further investigation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40560-023-00690-3.
Keywords: Diaphragmatic excursion, Point-of-care ultrasound, Diaphragmatic dysfunction, Critical illness
Introduction
Critically ill patients who present in the emergency department (ED) often require respiratory support. Most of the evidence shows that non-invasive ventilation (NIV) and high-flow nasal cannula (HFNC) therapy reduce the likelihood of intubation in these patients [1–3]. However, delayed intubation is associated with high mortality. Therefore, close monitoring of vital signs and respiratory parameters is essential [1, 4–7].
The diaphragm is the main muscle involved in respiration. Diaphragm dysfunction is common in critically ill patients and is associated with worse outcomes, including requirement for invasive mechanical ventilation (IMV) and an increased mortality risk [8–11]. The function of the diaphragm can be evaluated by ultrasonography at the bedside in the intensive care unit (ICU) or ED to predict risks of intubation and re-intubation [11–13]. Measurement of diaphragmatic excursion by ultrasound is one of the methods that can be used to detect diaphragm dysfunction. In the ED, a few studies had utility diaphragmatic excursion measurement to predicting require IMV [14, 15]. A previous study showed that the decreased diaphragmatic excursion value in the ED has a good ability to detect paralysis of the diaphragm in patients with acute dyspnea and to predict failure of NIV in patients with acute exacerbation of chronic obstructive pulmonary disease [14]. However, the accuracy of measurement of diaphragmatic excursion in the ED and the cutoff value that predicts the need for IMV in critically ill patients remains to be determined [14, 15].
The aim of this study was to evaluate the ability of right-sided diaphragmatic excursion (RDE) to predict the need for IMV and to determine its optimal cutoff value.
Methods
Study design
This prospective single-center cohort study was performed at Ramathibodi Hospital, which is a tertiary teaching healthcare facility in Bangkok, Thailand. The study was approved by the Ethics Committee of Ramathibodi Hospital, Mahidol University (approval number COA MURA2021/403). Each study participant or a family member provided written informed consent before enrollment in the study.
Patient selection process and eligibility criteria
Critically ill patients aged 18 years or older who presented to the ED between May 20, 2021 and May 19, 2022 were enrolled. In terms of critically ill patients, any condition of vital organ dysfunction who high risk of life threatening [16]. Critical illness in this study was defined as any of the following: a respiratory rate > 24 breaths per minute, use of accessory respiratory muscles, hypoxemia (pulse oxygen saturation < 90% or ratio of arterial oxygen partial pressure to a fraction of inspired oxygen (PF ratio) < 300), hypotension (systolic blood pressure (SBP) < 90 mmHg or mean arterial pressure (MAP) < 65 mmHg), and a Glasgow Coma Scale (GCS) score of < 15 [17, 18]. Patients with a neuromuscular disorder, those who underwent endotracheal intubation within 60 min of arrival, those with traumatic injury, and those with a do-not-attempt intubation order were excluded.
Data measurements
Data were collected on patient background characteristics (sex, age, and body mass index), comorbidities, Charlson Comorbidity Index, heart rate, blood pressure, respiratory rate, blood oxygen saturation, PF ratio, GSC score, blood pH, partial pressure of arterial oxygen (PaO2), partial pressure of arterial carbon dioxide (PaCO2), bicarbonate level, blood lactate, serum creatinine, Sequential Organ Failure Assessment (SOFA) score, final diagnosis, and diaphragmatic excursion at the time of enrollment in the ED.
The fraction of inspired oxygen (FiO2) to use in the calculation PF ratio in conventional oxygen therapy (COT) such as nasal cannula and non-rebreathing mask with reservoir bag was estimated by 3% formula (21% + oxygen flow rate in L/min × 3) [19, 20]. In a previous study in HFNC with flow rate > 30 LPM in closed, delivered FiO2 was closed to measured FiO2 [21]. Hence, the delivered FiO2 in HFNC and NIV was collected from the setting to calculate the PF ratio. The decision to use COT, HFNC and NIV was dependent on the physician’s judgment.
Diaphragmatic excursion was measured by ultrasonography within 10 min of arrival in the ED by experienced emergency physicians, each of whom had performed more than 50 such measurements. All measurements were obtained on the right side during spontaneous tidal breathing (quiet breathing) without non-invasive respiratory support (NIV or HFNC) in a semi-recumbent position. The transducer was placed in the right subcostal area between the midclavicular and anterior axillary lines. The angle of ultrasound tracing is possible cranially and perpendicular to the dome of the diaphragm. Diaphragmatic excursion was measured in M-mode. The RDE was defined as the distance between the value of the diaphragm dome in end-inspiratory and end-expiratory (Additional file 1: S1) [8], which was measured using a Xario 100G ultrasound machine (Canon Medical Systems USA Inc., Tustin, CA, USA) with a 1.8–4.8-MHz sector probe. The average of three measurements was recorded. The emergency physician who performed the ultrasound was not involved in decision-making regarding IMV.
The requirement for IMV was based on the following: hypoxic respiratory failure defined by hypoxemia with PF ratio < 150, respiratory rate > 35 breaths per minute, significant accessory respiratory used after management with non-invasive respiratory support, respiratory acidosis defined by pH < 7.35 and PaCO2 > 45 mmHg while receiving non-invasive respiratory support, persistent hemodynamic instability after optimization of fluids and vasoactive agents, and need for intubation to protect the airway [22].
The primary outcome was the ability of RDE to predict the requirement for IMV within 48 h and its optimal cutoff value. Secondary outcomes were the associations of RDE with durations of IMV and mortality.
Calculation of sample size
The minimum total sample size required to detect an area under the receiver-operating characteristic curve (AUROC) value of 0.80 with an effect size of 0.10, the 95% confidence interval (CI), and a power of 80% was calculated to be 184 [23].
Statistical analysis
Patients were compared according to whether or not they required IMV. Categorical variables are expressed as the number (percentage) and continuous variables as the mean ± standard deviation if normally distributed. Variables with a skewed distribution are shown as the median (interquartile range [IQR]). Categorical variables were compared using the chi-squared test, and continuous variables using the unpaired t test or Mann–Whitney U test as appropriate.
Multivariable logistic regression was used to identify whether RDE was associated with the requirement for IMV. Important factors at risk in intubation, such as blood pH < 7.30, GCS score < 15, hypotension, respiratory rate > 30 per minute, and PF ratio < 200, and factors with p value < 0.100 in univariable logistic regression, were selected for regression. In multivariable logistic regression, multicollinearity was checked by a variance inflation factor (VIF), factors of which VIF > 2.5 will be removed. The AUROC method was used to assess the predictive performance of RDE. The algorithm of DeLong was used to compare AUROC in each variable. Sensitivity and specificity values were used to determine the optimal cutoff value for RDE. The probability of requiring IMV within 48 h according to the cutoff value was determined using Kaplan–Meier survival curves. The statistical analysis was performed using STATA version 16.1 (StataCorp LLC, College Station, TX, USA). A p value of < 0.05 was considered statistically significant.
Results
RDE was measured by ultrasound in 340 patients during the study period. Twenty-six patients were excluded (do-not-attempt intubation order, n = 14; required IMV within 60 min, n = 10); neuromuscular disorder, n = 2), leaving 314 patients for inclusion in the final analysis.
Two hundred and nineteen of the 314 patients received non-invasive respiratory support, 163 received NIV, 49 received HFNC, and seven received both NIV and HFNC (Table 1). One hundred and thirteen patients (35.9%) required IMV; 67 (59.3%) required IMV within 6 h, and 88 (77.9%) within 24 h of ultrasound evaluation. The median RDE value was 0.90 cm (IQR 0.76, 1.10) in patients who required IMV within 48 h and 1.80 cm (IQR 1.30, 2.40) in those who did not. In sex, the median RDE values were 0.9 cm (IQR 0.70, 1.10) and 1.0 cm (IQR 0.80, 1.20) in female and male patients who required IMV within 48 h, respectively, significantly different (p = 0.039). Moreover, the median RDE in patients who received non-invasive respiratory support was 0.90 cm (IQR 0.70, 1.10) in intubated patients and 1.70 cm (IQR 1.20, 2.40) in those who did not intubate (Additional file 2: S2).
Table 1.
Patient demographic and clinical characteristics
| Variable | Intubated patients (n = 113) | Non-intubated patients (n = 201) | p value |
|---|---|---|---|
| Sex, male, n (%) | 68 (60.2) | 101 (50.2) | 0.091 |
| Age (years), median [IQR] | 77 [67, 83] | 73 [65, 85] | 0.603 |
| BMI (kg/m2), mean ± SD | 22.7 ± 5.5 | 22.9 ± 5.8 | 0.678 |
| Charlson Comorbidity Index, median [IQR] | 3 [1, 5] | 3 [1, 5] | 0.968 |
| Comorbidities, n (%) | |||
| COPD | 22 (19.5) | 42 (20.9) | 0.764 |
| Chronic renal disease stage 4 or 5 | 13 (11.5) | 37 (18.4) | 0.127 |
| History of myocardial infarction | 31 (27.4) | 47 (23.4) | 0.426 |
| Heart failure | 32 (28.3) | 71 (35.3) | 0.205 |
| History of a cerebrovascular event | 32 (28.3) | 54 (26.9) | 0.782 |
| Dementia | 18 (15.9) | 24 (11.9) | 0.320 |
| Post-COVID-19 status | 18 (15.9) | 25 (12.4) | 0.388 |
| Vital signs | |||
| SBP, mmHg, mean ± SD | 126 ± 35 | 132 ± 34 | 0.137 |
| MAP, mmHg, mean ± SD | 88 ± 23 | 92 ± 22 | 0.072 |
| Hypotension (SBP ≤ 90 or MAP ≤ 65 mmHg), n (%) | 15 (13.3) | 24 (11.9) | 0.731 |
| Heart rate, bpm, mean ± SD | 102 ± 23 | 98 ± 23 | 0.216 |
| Respiratory rate, per minute, median [IQR] | 28 [24, 32] | 26 [24, 30] | 0.014 |
| Respiratory rate > 30 per minute, n (%) | 33 (29.2) | 42 (20.1) | 0.098 |
| SpO2, (%), median [IQR] | 94 [90, 98] | 96 [92, 99] | 0.006 |
| Glasgow Coma Scale score, median [IQR] | 15 [13, 15] | 15 [15] | < 0.001 |
| Glasgow Coma Scale score < 15, n (%) | 41 (36.3) | 33 (16.4) | 0.002 |
| Blood gas analysis | |||
| pH, mean ± SD | 7.37 ± 0.11 | 7.41 ± 0.08 | 0.001 |
| PaO2, mmHg, median [IQR] | 125 [84, 18] | 130 [82, 172] | 0.362 |
| PaCO2, mmHg, median [IQR] | 33 [27, 42] | 33 [29, 40] | 0.910 |
| PF ratio, mean ± SD | 377.77 ± 137.47 | 391.87 ± 135.20 | 0.379 |
| Bicarbonate, mEq/L, mean ± SD | 19.9 ± 6.1 | 21.6 ± 4.8 | 0.007 |
| Blood lactate, mmol/L, median [IQR] | 1.7 [0.8, 3.3] | 1.8 [1.0, 2.8] | 0.717 |
| Serum creatinine, mg/dL, median [IQR] | 1.18 [0.79, 1.72] | 1.14 [0.82, 1.82] | 0.848 |
| SOFA score, median [IQR] | 3 [2, 5] | 2 [1, 4] | 0.001 |
| Non-invasive respiratory support, n (%) | 88 (77.9) | 131 (65.2) | 0.019 |
| Final diagnosis, n (%) | |||
| Respiratory infection | 63 (55.8) | 73 (36.3) | 0.001 |
| Sepsis | 76 (67.3) | 73 (36.3) | < 0.001 |
| Acute exacerbation of COPD | 20 (17.7) | 37 (18.4) | 0.876 |
| Asthma attack | 4 (3.5) | 10 (5.0) | 0.555 |
| Cardiogenic pulmonary edema | 22 (19.5) | 61 (30.3) | 0.036 |
| COVID-19 pneumonia | 17 (15.0) | 7 (3.5) | 0.050 |
| Diaphragmatic excursion, cm, median [IQR] | 0.9 [0.76, 1.10] | 1.8 [1.30, 2.40] | < 0.001 |
BMI body mass index, COPD chronic obstructive pulmonary disease, COVID-19 coronavirus disease 2019, IQR interquartile range, PaO2 partial pressure of arterial oxygen, PaCO2 partial pressure of arterial carbon dioxide, PF ratio ratio of the partial pressure of arterial oxygen to inspired oxygen fraction, SD standard deviation, SOFA Sequential Organ Failure Assessment, SpO2 blood oxygen saturation level
Multiple logistic regression showed that the following five variables were significant independent predictors of the probability of requiring IMV within 48 h: increase RDE per 0.1 cm (adjusted odds ratio [aOR] 0.08, 95% CI 0.04–0.17, p < 0.001), PaO2 < 200 (aOR 0.26, 95% CI 0.07–0.96, p = 0.044), blood pH < 7.30 (aOR 3.79, 95% CI 1.36–10.57, p = 0.011), respiratory tract infection (aOR 2.31, 95% CI 1.12–4.77, p = 0.024), and sepsis (aOR 2.37, 95% CI 1.07–5.25, p = 0.033) (Table 2).
Table 2.
Univariable and multivariable logistic regression of factors associated with intubation within 48 h of critically ill patients in the emergency department
| Variable | Odds ratio (95% CI) | p value | Adjusted odds ratio (95% CI) | p value |
|---|---|---|---|---|
| Sex, male | 1.50 (0.94–2.38) | 0.091 | 1.50 (0.77–2.94) | 0.237 |
| Hypotension (SBP ≤ 90 or MAP ≤ 65 mmHg) | 1.13 (0.57–2.25) | 0.731 | 0.61 (0.21–1.75) | 0.362 |
| Respiratory rate > 30 per minute, n (%) | 1.56 (0.920–2.65) | 0.099 | 1.05 (0.56–2.26) | 0.892 |
| Glasgow Coma Scale score < 15 | 2.90 (1.70–4.95) | < 0.001 | 1.63 (0.72–3.66) | 0.236 |
| pH < 7.30 | 4.91 (2.31–10.42) | < 0.001 | 3.79 (1.36–10.57) | 0.011 |
| PF ratio < 200 | 0.76 (0.30–1.92) | 0.565 | 0.26 (0.07–0.96) | 0.044 |
| Bicarbonate | 0.94 (0.89–0.98) | 0.007 | 0.99 (0.92–1.06) | 0.678 |
| Blood lactate ≥ 4 mmol/L | 2.32 (1.19–4.50) | 0.013 | 2.01 (0.72–5.61) | 0.182 |
| SOFA score | 1.17 (1.06–1.29) | 0.002 | 1.06 (0.88–1.29) | 0.528 |
| Non-invasive respiratory support | 1.88 (1.11–3.20) | 0.020 | 2.02 (0.93–4.33) | 0.073 |
| Final diagnosis | ||||
| Respiratory infection | 2.21 (1.38–3.53) | 0.001 | 2.31 (1.12–4.77) | 0.024 |
| Sepsis | 3.60 (2.21–5.86) | < 0.001 | 2.37 (1.07–5.25) | 0.033 |
| Cardiogenic pulmonary edema | 0.55 (0.32–0.97) | 0.036 | 1.02 (0.46–2.26) | 0.963 |
| COVID-19 pneumonia | 2.05 (0.99–4.23) | 0.053 | 1.70 (0.59–4.91) | 0.324 |
| Diaphragmatic excursion (increase per 0.1 cm) | 0.08 (0.05–0.16) | < 0.001 | 0.08 (0.04–0.17) | < 0.001 |
BMI body mass index, CI confidence interval, COPD chronic obstructive pulmonary disease, COVID-19 coronavirus disease 2019, MAP mean arterial pressure, PaCO2 partial pressure of arterial carbon dioxide, PaO2 partial pressure of arterial oxygen, PF ratio ratio of the partial pressure of arterial oxygen to inspired oxygen fraction, SBP systolic blood pressure, SOFA Sequential Organ Failure Assessment, SpO2 blood oxygen saturation level
The ability of RDE to predict required for IMV
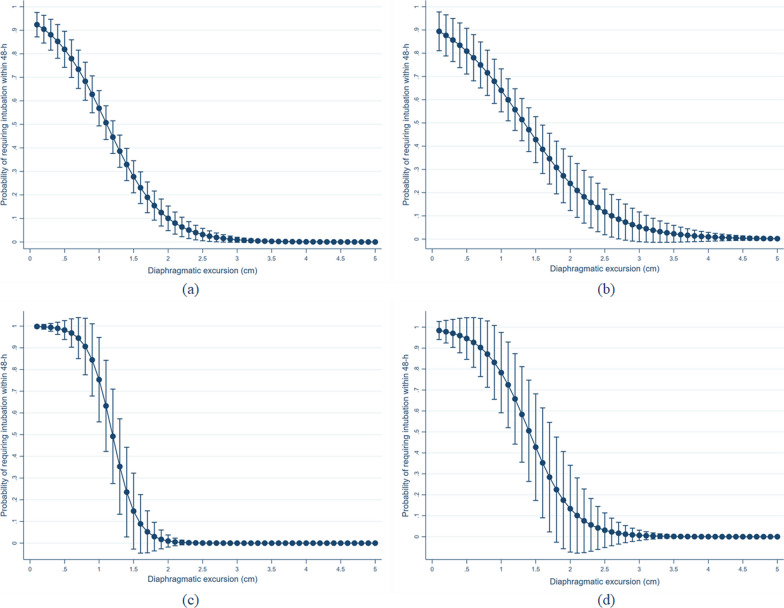
The AUROC for the ability of RDE to predict the requirement for IMV within 48 h was 0.850 (95% CI 0.807–0.894) and had better accuracy than blood pH (AUROC 0.601, 95% CI 0.533–0.669), and the SOFA score (AUROC 0.613, 95% CI 0.549–0.676). This finding was statistically significant between RDE and blood pH (p < 0.001), and between RDE and SOFA score (p < 001). In addition, the probability of requiring intubation within 48 h was increased in decreased RDE values (Fig. 1). Moreover, the association between RDE and non-invasive respiratory support was OR 0.76 (95% CI 0.60–0.96, p = 0.022), and AUROC for predicting requiring non-respiratory support was 0.589 (95% CI 0.521–0.657).
Fig. 1.
HYPERLINK "sps:id::fig1||locator::gr1||MediaObject::0"Probability of requiring intubation within 48 h in each value of right-sided diaphragmatic excursion a total populations, b sepsis, c acute exacerbation of chronic obstructive pulmonary disease and d coronavirus disease 2019
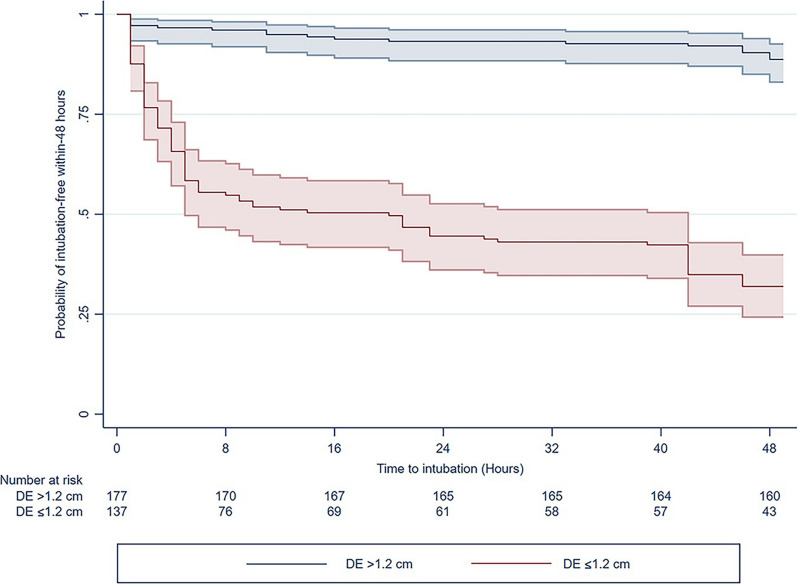
The RDE cutoff value was 1.20 cm with a sensitivity of 82.3% (95% CI 74.0–88.8) and a specificity of 78.1% (95% CI 71.7–83.6) (Table 3). The Kaplan–Meier curves for the probability of intubation-free within 48 h are shown according to the cutoff value in Fig. 2. In addition, 110 (80.3%) of 137 patients with RDE ≤ 1.20 cm required non-invasive respiratory support, and 75 (68.1%) patients required IMV within 48 h.
Table 3.
Predictive performance of diaphragmatic excursion in intubation within 48 h
| Diaphragmatic excursion (cm) | Sensitivity | Specificity | PPV | NPV | Predicted probability |
|---|---|---|---|---|---|
| Cohort (N = 314), AUROC 0.850 (95% CI 0.807–0.894) | |||||
| ≥ 2.00 | – | – | – | – | 3.4% (− 0.4, 7.3) |
| 1.60–1.99 | 97.3% (92.4–99.4) | 41.8% (34.9–48.9) | 48.5% (41.8–55.2) | 96.6% (90.3–99.3) | 12.8% (3.2–22.3) |
| 1.21–1.59 | 92.2% (85.4–96.3) | 62.2% (55.1–68.9) | 57.8% (50.2–65.1) | 93.3% (87.6–96.9) | 25.6% (12.5–38.6) |
| 0.91–1.20 | 82.3% (74.0–88.8) | 78.1% (71.7–83.6) | 67.9% (59.4–75.6) | 88.7% (83.1–93.0) | 58.5% (45.2–71.8) |
| ≤ 0.90 | 54.9% (45.2–64.2) | 89.1% (83.9–93.0) | 73.8% (63.1–82.8) | 77.8% (71.9–83.0) | 73.8% (64.4–83.2) |
AUROC area under the receiver-operating characteristic curve, NPV negative predictive value, PPV positive predictive value
Fig. 2.
HYPERLINK "sps:id::fig2||locator::gr2||MediaObject::0"Kaplan–Meier survival curves for the probability of intubation-free within-48 h according to the cutoff value of the right-sided diaphragmatic excursion
In addition, the AUROCs for the ability of the RDE to predict the requirement for IMV within 6 h and 24 h were 0.841 (95% CI 0.794–0.889) and 0.854 (95% CI 0.811–0.899), respectively. The median RDE value was 0.80 cm (IQR 0.70, 1.10) in patients who required IMV within 6 h and 0.90 cm (IQR 0.70, 1.10) in patients who required IMV within 24 h.
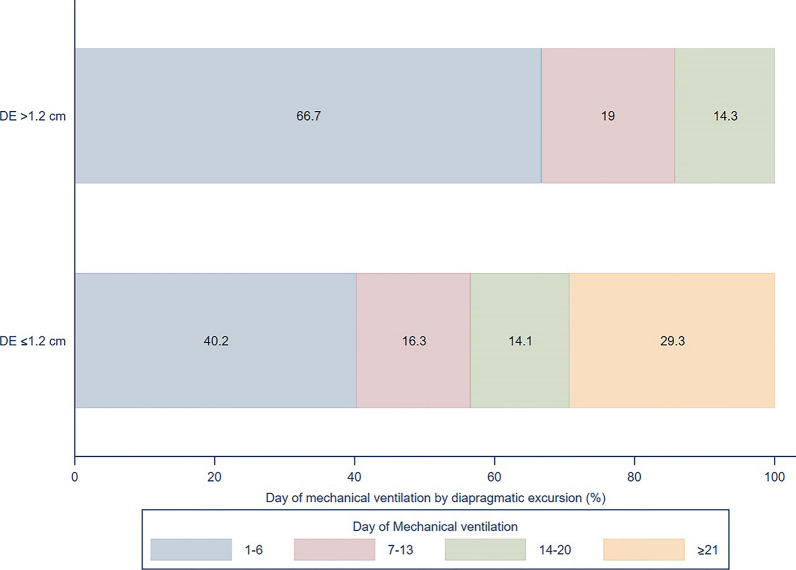
Ventilation time was significantly longer when the RDE was ≤ 1.2 cm than when it was > 1.2 cm (13 days [IQR 5, 27] versus 5 days [IQR 3, 8], p = 0.006). The probability of mechanical ventilation according to the RDE threshold is shown in Fig. 3. Moreover, RDE ≤ 1.2 cm was an independent risk factor for mortality (aOR 2.99, 95% CI 1.32–6.79, p = 0.009), including when adjusted by SOFA score (aOR 1.17, 95% CI 1.02–1.34, p = 0.020), blood lactate ≥ 4 mmol/L (aOR 1.13, 95% CI 0.46–2.81, p value = 0.788), blood pH < 7.30 (aOR 0.45, 95% CI 0.15–1.34, p = 0.152), and receiving IMV (aOR 2.94, 95% CI 1.34–6.42, p = 0.007).
Fig. 3.
HYPERLINK "sps:id::fig3||locator::gr3||MediaObject::0"Probability of number of days on mechanical ventilation according to the right-sided diaphragmatic excursion cutoff value
On subgroup analysis according to the final diagnosis, the AUROC was 0.893 (95% CI 0.782–0.999) for presence of acute exacerbation of chronic obstructive pulmonary disease (AECOPD), 0.830 (95% CI 0.779–0.882) for non-AECOPD, 0.792 (95% CI 0.714–0.869) for sepsis, and 0.893 (95% CI 0.782–0.999) for coronavirus disease 2019 (COVID-19) pneumonia (Additional file 3: S3).
Discussion
In this study, the RDE had a good ability to predict the need for IMV within 48 h in critically ill patients in ED and was better than the ratio of oxygen saturation to fraction of inspired oxygen and the SOFA score. Furthermore, a low RDE value was associated with longer IMV and increased mortality risk.
Point-of-care ultrasound (POCUS) has been found to be useful in the ICU and ED for guiding resuscitation of critically ill patients, such as fluid responsiveness, and the bedside lung ultrasound in emergency (BLUE) protocol for immediate diagnosis of respiratory failure [24–26]. Critical illness has been associated with diaphragm dysfunction that causes respiratory failure and difficulty in weaning from mechanical ventilation and with mortality [8, 9, 27]. Transdiaphragmatic pressure (Pdi) is used to measure diaphragmatic force; however, the gold standard of Pdi measurement is magnetic stimulation of phrenic nerves, which was unsuitable in ED [9]. Therefore, there is a need for evaluation of the ability of examination of the diaphragm by ultrasound to detect diaphragm dysfunction and predict the need for IMV.
The diaphragm ultrasound can assess diaphragm function by diaphragmatic excursion and diaphragmatic thickness fraction. In this study, we used the RDE, which is simpler to measure and more rapidly obtained than the diaphragmatic thickness fraction when assessing the function of the diaphragm and has demonstrated good reliability [27–29]. The diaphragm action is qualified for shortening and force generation, which are measured by volume change and inspiratory pressure, respectively. The relation between diaphragmatic excursion and tidal volume and inspiratory capacity (IC) in spontaneous breathing at rest and exercise in healthy was a linear correlation [30, 31]. Moreover, in COPD, inspiratory capacity was reduced compared to volunteers who had no chronic disease, and decreasing of the diaphragmatic excursion was associated with a reduced inspiratory capacity [32]. In a previous study, diaphragmatic excursion has been a reliable tool as fluoroscopy for measuring diaphragm contractile activity [33]. In spontaneous breathing, diaphragmatic excursion correlated well with Pdi and esophageal pressure in intubated patients with zero pressure [34].
Previous studies have shown that diaphragmatic excursion has poor accuracy when used to predict the need for use of a mechanical ventilator. Clément et al. showed that diaphragmatic excursion could not predict the need for NIV or IMV in patients with acute dyspnea in the ED [15]. A similar study by Barbariol et al. found that diaphragmatic excursion had a poor ability to predict failure of NIV in patients with acute hypoxic respiratory failure in the ICU [35]. These findings are in contrast with those of our present study, in which the RDE showed a good ability to predict IMV. Possible reasons for this inconsistency are that our study population included patients with shock or a change in mental status and different criteria for intubation. Furthermore, we found that the RDE could predict IMV regardless of whether or not the patient had AECOPD, especially when associated with COVID-19 pneumonia.
The diaphragmatic dysfunction in critical conditions was related to worsening outcomes. Previous studies have identified mechanical ventilation use, malnutrition, use of corticosteroids, inflammation, releases of cytokines, and mitochondrial impairment as causes of diaphragm dysfunction in critical illness due to the catabolic process occurring in the diaphragm and other respiratory muscles [8, 36, 37]. Many studies show that sepsis is one of the risks of diaphragmatic dysfunction. Increasing metabolic demands and inflammation in sepsis increased respiratory drive and effort. However, in animal studies, group B streptococcal sepsis in young piglets was associated decline in diaphragmatic contractility and tidal volume within 2 h [38], and endotoxin administration produced a reduction in diaphragmatic force generation in hamsters [39]. Similarly, other experimental studies in animals exposed to endotoxin showed increased ventilation in an early, then fall and decreased diaphragmatic function [40–42]. Chen et al. found that diaphragmatic thickening fraction and diaphragmatic excursion were significantly lower in sepsis with SOFA > 5 than in controls [43]. In ICU, diaphragm volume and strength were lower in sepsis when compared with non-septic. Moreover, diaphragm strength was correlated with diaphragm volume [44]. Systemic inflammation in sepsis increased diaphragm weakness and susceptibility to injury. In addition, diaphragm dysfunction may develop within 4 h of sepsis. Therefore, diaphragmatic evaluation may consider monitoring to choose ventilatory support to prevent worsening outcomes in delayed intubation and diaphragmatic weakness in mechanical ventilation [8, 22]. A previous study evaluated the performance of diaphragmatic function was found low diaphragmatic excursion values associated need for IMV, prolonged IMV, and mortality in sepsis [45]. Similarly, this study found RDE was a good performance to predict IMV within 48 h and mortality. In our population, respiratory rate was high, and accessory muscle used that indicated high respiratory effort was included in the inclusion criteria. However, low RDE was associated with requiring IMV, which results similar results to previous study [40–42, 45]. Therefore, the evidence of diaphragmatic ultrasound needs evaluation and validation in requiring intubation in sepsis.
In COPD, expiratory flow is limited by airway narrowing resulting from chronic inflammation and mucus plugging, causing to required prolonged time to exhale volume in the lung. In critically ill, almost increased minute ventilation from increased tidal volume and respiratory rate that were increasing in end-expiratory lung volume and reduced IC. In COPD with limited IC, increasing the minute ventilation by increased respiratory rate cause of dynamic hyperinflation by insufficient expiratory time and causes hypercapnic respiratory failure, especially in AECOPD. In addition, the work of breathing increased during hyperinflation and resulting in diaphragmatic dysfunction [46]. Previous studies show diaphragmatic dysfunction was associated with required IMV [10, 12, 14]. Diaphragmatic thickening fraction was shown to correlate with Pdi during the sniff maneuver and accurately identified risks of NIV failure in AECOPD [12]. However, this study did not measure the diaphragmatic thickening fraction. Diaphragmatic excursion in COPD with acute hypercapnic respiratory failure could predict NIV failure more accurately than arterial pH and PaCO2 [14], similar to our study. This result was in line with previous findings that diaphragmatic excursion was correlated with inspiratory capacity and tidal volume [30–32]; in spontaneous breathing that result presumed the low value of diaphragmatic excursion was associated with decreased IC due to dynamic hyperinflation and caused requiring IMV in AECOPD.
In COVID-19, the theory of diaphragmatic dysfunction was related to critical illness myopathy, cytokine storm, ventilator-induced diaphragmatic dysfunction, and directly viral infiltration via expression of the angiotensin-converting enzyme 2 receptor [36, 37, 47]. Other studies have also found associations between diaphragm dysfunction with the requirement for IMV and adverse outcomes in patients with severe COVID-19 pneumonia [48, 49] that support the results of our study. However, the sample size in our study on COVID-19 pneumonia was small. Therefore, clinical application in diaphragmatic ultrasound should be assessed in further study.
The normal diaphragmatic excursion value in quiet breathing is about 2 cm in the general population and 1.0–1.4 cm in critically ill patients in a semi-recumbent position [27, 29, 50]. In our study, the median RDE value was 0.9 cm in critically ill patients requiring IMV and 1.8 cm in their counterparts who did not; both these values are lower than those in the general population. Moreover, our study showed different diaphragmatic excursion between gender, since the excursion in males was displace greater than in females, that result supports previous studies [29, 50]. Several other studies have shown an association between a low value of diaphragmatic excursion value and requirements for IMV [15, 35, 42]. Many studies have shown various cutoff values of diaphragmatic excursion to be associated with adverse events [27, 29]. Therefore, our study showed sensitivity, specificity, and probability in predicting requiring IMV in each range of RDE for physician decisions. This study identified an RDE of 1.2 cm as the cutoff value below which there was a high probability of requiring IMV and an RDE of ≥ 2.0 cm to be the value above which the probability was low.
Motion of the diaphragm, indicated by the diaphragmatic thickness fraction and excursion, has been assessed as a prognostic factor in weaning from mechanical ventilation in the ICU. Patients with a diaphragmatic excursion or diaphragmatic thickness fraction lower than the threshold were found to have a prolonged period of IMV, which was a predictor of failure to extubate [8, 9]. In our study, an RDE ≤ 1.2 cm was similarly associated with a significantly prolonged period of IMV.
In view of our results, we believe that the RDE could be useful to implement in POCUS for assessment of the risk of failure of non-invasive respiratory support and identifying patients at risk who require close monitoring. For example, patients who present in the ED with acute respiratory distress and an RDE ≤ 1.2 cm could be admitted to ICU or a respiratory care unit for close monitoring and early intubation for IMV to prevent adverse conditions, such as mortality. In contrast, patients with an RDE > 2 cm could safely receive non-invasive respiratory support and routine care outside the ICU. Furthermore, monitoring the RDE during the weaning process could help to predict the outcomes of weaning from IMV. Using diaphragmatic excursion with clinical parameters that indicated intubation criteria could benefit the decision for early intubation to prevent adverse outcomes. However, integrating diaphragmatic excursion into POCUS to guide the resuscitation setting requires further studies to confirm the cutoff value and the benefit of implementing care.
This study has several limitations. First, it was performed in the ED of a single tertiary care center, which limits the generalizability of its findings. However, the sample size was adequate for assessments of the primary outcome. Second, we used the average of the RDE values obtained on ultrasound by a single emergency physician for each patient; therefore, the intra-rater and inter-rater correlations were not analyzed. However, all the emergency physicians who measured the diaphragmatic excursion had already performed the procedure more than 50 times, and previous research has shown that measurement of diaphragmatic excursion is an easy skill to master with a steep learning curve and good reliability [27, 28]. Third, we measured only RDE and did not include the diaphragmatic thickness fraction, which has been shown to be associated with respiratory effort in patients receiving positive pressure ventilation [27, 51]. Nevertheless, RDE was measured during spontaneous breathing, which has been reported to produce reliable results [27, 29]. Fourth, ultrasound was performed only once and not repeated after resuscitation. Finally, confounding factors, such as pharmacologic treatment, setting, and parameters of non-invasive respiratory support, were not collected in this study. Finally, the RDE value was unblinded to physicians who decided on intubation; however, the emergency physician who performed the ultrasound was not involved in deciding on intubation. Further studies are needed to validate our cutoff value in multi-center studies and determine the diaphragmatic excursion and the thickness fraction or a variation of diaphragmatic parameters should be implemented in POCUS to predict worsening outcomes.
Conclusions
In this study, the RDE had a good ability to predict the need for IMV in critically ill patients. A low diaphragmatic excursion value was associated with a need for IMV; the cutoff value was 1.2 cm. Patients with an RDE below the cutoff value required a longer period of IMV. The benefit of inclusion of the RDE in POCUS should be assessed further.
Supplementary Information
Additional file 1: S1. (a) The transducer (sector probe) was placed in the right subcostal area between the midclavicular and anterior axillary lines. (b) The angle of ultrasound tracing is possible to the diaphragmatic dome. The diaphragmatic excursion was measured in M-mode. The right diaphragmatic excursion (red dash) was measured as the distance between the value of the diaphragm dome in end-inspiration and end-expiration (green dash).
Additional file 2: S2. Comparison of right-sided diaphragmatic excursion between the non-intubated group and the intubated group in received and not received non-invasive respiratory support.
Additional file 3: S3. Predictive performance of diaphragmatic excursion in the emergency department according to the final diagnosis.
Acknowledgements
The authors are grateful to the emergency physicians and residents at our institution who cared for the patients who participated in this study.
Abbreviations
- AECOPD
Acute exacerbation of chronic obstructive pulmonary disease
- aOR
Adjusted odds ratio
- AUROC
Area under the receiver-operating characteristic curve
- BLUE
Bedside lung ultrasound in emergency
- CI
Confidence interval
- COT
Conventional oxygen therapy
- ED
Emergency department
- FiO2
Fraction of inspired oxygen
- GCS
Glasgow Coma Scale
- HFNC
High-flow nasal cannula
- IC
Inspiratory capacity
- ICU
Intensive care unit
- IMV
Invasive mechanical ventilation
- IQR
Interquartile range
- MAP
Mean arterial pressure
- NIV
Non-invasive ventilation
- PaCO2
Partial pressure of arterial carbon dioxide
- PaO2
Partial pressure of arterial oxygen
- Pdi
Transdiaphragmatic pressure
- PFratio
Ratio of arterial oxygen partial pressure to a fraction of inspired oxygen
- POCUS
Point-of-care ultrasound
- RDE
Right-sided diaphragmatic excursion
- SBP
Systolic blood pressure
- SOFA
Sequential Organ Failure Assessment
- VIF
Variance inflation factor
Author contributions
KS, SW, and PS designed the study and developed the protocol. SW and SS were responsible for collection of the study. KS and PS were responsible for the data analysis. KS wrote the first draft of the manuscript and all authors commented on this draft. All authors read and approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional files 1, 2, and 3.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Ramathibodi Hospital, Mahidol University (approval number COA MURA2021/403). All participants or a family member provided written informed consent before enrollment in the study.
Consent for publication
Informed consent was obtained from all patients or their relatives.
Competing interests
There is no competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rose L. Management of critically ill patients receiving noninvasive and invasive mechanical ventilation in the emergency department. Open Access Emerg Med. 2012;4:5–15. doi: 10.2147/OAEM.S25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao H, Wang H, Sun F, Lyu S, An Y. High-flow nasal cannula oxygen therapy is superior to conventional oxygen therapy but not to noninvasive mechanical ventilation on intubation rate: a systematic review and meta-analysis. Crit Care. 2017;21(1):184. doi: 10.1186/s13054-017-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreyro BL, Angriman F, Munshi L, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324(1):57–67. doi: 10.1001/jama.2020.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricard JD, Roca O, Lemiale V, et al. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Med. 2020;46(12):2238–2247. doi: 10.1007/s00134-020-06228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oczkowski S, Ergan B, Bos L, et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J. 2022;59(4):2101574. doi: 10.1183/13993003.01574-2021. [DOI] [PubMed] [Google Scholar]
- 6.Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41(4):623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 7.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 8.Supinski GS, Morris PE, Dhar S, Callahan LA. Diaphragm dysfunction in critical illness. Chest. 2018;153(4):1040–1051. doi: 10.1016/j.chest.2017.08.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med. 2017;43(10):1441–1452. doi: 10.1007/s00134-017-4928-4. [DOI] [PubMed] [Google Scholar]
- 10.Marchioni A, Tonelli R, Fantini R, et al. Respiratory mechanics and diaphragmatic dysfunction in COPD patients who failed non-invasive mechanical ventilation. Int J Chronic Obstr Pulm Dis. 2019;14:2575–2585. doi: 10.2147/COPD.S219125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fantini R, Tonelli R, Castaniere I, et al. Serial ultrasound assessment of diaphragmatic function and clinical outcome in patients with amyotrophic lateral sclerosis. BMC Pulm Med. 2019;19(1):160. doi: 10.1186/s12890-019-0924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchioni A, Castaniere I, Tonelli R, et al. Ultrasound-assessed diaphragmatic impairment is a predictor of outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease undergoing noninvasive ventilation. Crit Care. 2018;22(1):109. doi: 10.1186/s13054-018-2033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dres M, Similowski T, Goligher EC, et al. Dyspnoea and respiratory muscle ultrasound to predict extubation failure. Eur Respir J. 2021;58(5):2100002. doi: 10.1183/13993003.00002-2021. [DOI] [PubMed] [Google Scholar]
- 14.Cammarota G, Sguazzotti I, Zanoni M, et al. Diaphragmatic ultrasound assessment in subjects with acute hypercapnic respiratory failure admitted to the emergency department. Respir Care. 2019;64(12):1469–1477. doi: 10.4187/respcare.06803. [DOI] [PubMed] [Google Scholar]
- 15.Clément A, Zieleskiewicz L, Bonnec JM, et al. Diaphragmatic excursion measurement in emergency department patients with acute dyspnea to predict mechanical ventilation use. Am J Emerg Med. 2020;38(10):2081–2087. doi: 10.1016/j.ajem.2020.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Kayambankadzanja RK, Schell CO, Gerdin Wärnberg M, et al. Towards definitions of critical illness and critical care using concept analysis. BMJ Open. 2022;12(9):e060972. doi: 10.1136/bmjopen-2022-060972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Wijdicks EF, Kramer AA, Rohs T, Jr, et al. Comparison of the Full Outline of UnResponsiveness score and the Glasgow Coma Scale in predicting mortality in critically ill patients*. Crit Care Med. 2015;43(2):439–444. doi: 10.1097/CCM.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 19.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 20.Coudroy R, Frat JP, Girault C, Thille AW. Reliability of methods to estimate the fraction of inspired oxygen in patients with acute respiratory failure breathing through non-rebreather reservoir bag oxygen mask. Thorax. 2020;75(9):805–807. doi: 10.1136/thoraxjnl-2020-214863. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care. 2011;39(6):1103–1110. doi: 10.1177/0310057X1103900620. [DOI] [PubMed] [Google Scholar]
- 22.Darreau C, Martino F, Saint-Martin M, et al. Use, timing and factors associated with tracheal intubation in septic shock: a prospective multicentric observational study. Ann Intensive Care. 2020;10(1):62. doi: 10.1186/s13613-020-00668-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform. 2014;48:193–204. doi: 10.1016/j.jbi.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Messina A, Calabrò L, Pugliese L, et al. Fluid challenge in critically ill patients receiving haemodynamic monitoring: a systematic review and comparison of two decades. Crit Care. 2022;26(1):186. doi: 10.1186/s13054-022-04056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monnet X, Shi R, Teboul JL. Prediction of fluid responsiveness. What’s new? Ann Intensive Care. 2022;12(1):46. doi: 10.1186/s13613-022-01022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 2015;147(6):1659–1670. doi: 10.1378/chest.14-1313. [DOI] [PubMed] [Google Scholar]
- 27.Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF, Zangrillo A. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med. 2017;43(1):29–38. doi: 10.1007/s00134-016-4524-z. [DOI] [PubMed] [Google Scholar]
- 28.Haaksma ME, Smit JM, Boussuges A, et al. EXpert consensus On Diaphragm UltraSonography in the critically ill (EXODUS): a Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Crit Care. 2022;26(1):99. doi: 10.1186/s13054-022-03975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by M-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135(2):391–400. doi: 10.1378/chest.08-1541. [DOI] [PubMed] [Google Scholar]
- 30.Cohen E, Mier A, Heywood P, Murphy K, Boultbee J, Guz A. Excursion-volume relation of the right hemidiaphragm measured by ultrasonography and respiratory airflow measurements. Thorax. 1994;49(9):885–889. doi: 10.1136/thx.49.9.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones AYM, Ngai SPC, Ying MTC, et al. Sonographic evaluation of diaphragmatic function during breathing control. Physiother Theory Pract. 2017;33(7):560–567. doi: 10.1080/09593985.2017.1323363. [DOI] [PubMed] [Google Scholar]
- 32.Shiraishi M, Higashimoto Y, Sugiya R, et al. Diaphragmatic excursion correlates with exercise capacity and dynamic hyperinflation in COPD patients. ERJ Open Res. 2020;6(4):00589-2020. doi: 10.1183/23120541.00589-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayoub J, Metge L, Dauzat M, et al. Diaphragm kinetics coupled with spirometry. M-mode ultrasonographic and fluoroscopic study; preliminary results. J Radiol. 1997;78:563–568. [PubMed] [Google Scholar]
- 34.Koco E, Soilemezi E, Sotiriou P, et al. Ultrasonographic assessment of diaphragmatic contraction and relaxation properties: correlations of diaphragmatic displacement with oesophageal and transdiaphragmatic pressure. BMJ Open Respir Res. 2021;8(1):e001006. doi: 10.1136/bmjresp-2021-001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbariol F, Deana C, Guadagnin GM, Cammarota G, Vetrugno L, Bassi F. Ultrasound diaphragmatic excursion during non-invasive ventilation in ICU: a prospective observational study. Acta Biomed. 2021;92(3):e2021269. doi: 10.23750/abm.v92i3.11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan H, Bai H. Is mitochondrial oxidative stress the key contributor to diaphragm atrophy and dysfunction in critically ill patients? Crit Care Res Pract. 2020;2020:8672939. doi: 10.1155/2020/8672939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demoule A, Jung B, Prodanovic H, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact—a prospective study. Am J Respir Crit Care Med. 2013;188(2):213–219. doi: 10.1164/rccm.201209-1668OC. [DOI] [PubMed] [Google Scholar]
- 38.Murphy TD, Gibson RL, Standaert TA, Mayock DE, Woodrum DE. Effect of group B streptococcal sepsis on diaphragmatic function in young piglets. Pediatr Res. 1993;33(1):10–14. doi: 10.1203/00006450-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Supinski G, Nethery D, Stofan D, DiMarco A. Comparison of the effects of endotoxin on limb, respiratory, and cardiac muscles. J Appl Physiol. 1996;81(3):1370–1378. doi: 10.1152/jappl.1996.81.3.1370. [DOI] [PubMed] [Google Scholar]
- 40.Hussain SN, Simkus G, Roussos C. Respiratory muscle fatigue: a cause of ventilatory failure in septic shock. J Appl Physiol. 1985;58(6):2033–2040. doi: 10.1152/jappl.1985.58.6.2033. [DOI] [PubMed] [Google Scholar]
- 41.Hussain SN, Graham R, Rutledge F, Roussos C. Respiratory muscle energetics during endotoxic shock in dogs. J Appl Physiol. 1986;60(2):486–493. doi: 10.1152/jappl.1986.60.2.486. [DOI] [PubMed] [Google Scholar]
- 42.Boczkowski J, Dureuil B, Branger C, et al. Effects of sepsis on diaphragmatic function in rats. Am Rev Respir Dis. 1988;138(2):260–265. doi: 10.1164/ajrccm/138.2.260. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Liu Y, Han M, et al. Quantification of diaphragmatic dynamic dysfunction in septic patients by bedside ultrasound. Sci Rep. 2022;12(1):17336. doi: 10.1038/s41598-022-21702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung B, Nougaret S, Conseil M, et al. Sepsis is associated with a preferential diaphragmatic atrophy: a critically ill patient study using tridimensional computed tomography. Anesthesiology. 2014;120(5):1182–1191. doi: 10.1097/ALN.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 45.Zaki A, El Ghawaby HH, Gad MMM, Ismail AM, Nawar AS. Diaphragmatic function assessed by bed side ultrasonography in patients with sepsis or septic shock admitted to intensive care unit. Open Access Maced J Med Sci. 2021;9(B):1417–1423. doi: 10.3889/oamjms.2021.7060. [DOI] [Google Scholar]
- 46.Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3(2):176–179. doi: 10.1513/pats.200508-094DO. [DOI] [PubMed] [Google Scholar]
- 47.Shi Z, de Vries HJ, Vlaar APJ, et al. Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern Med. 2021;181(1):122–124. doi: 10.1001/jamainternmed.2020.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adolf Helmy M, Magdy Milad L, Hasanin A, Mostafa M. The novel use of diaphragmatic excursion on hospital admission to predict the need for ventilatory support in patients with coronavirus disease 2019. Anaesth Crit Care Pain Med. 2021;40(6):100976. doi: 10.1016/j.accpm.2021.100976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corradi F, Vetrugno L, Orso D, et al. Diaphragmatic thickening fraction as a potential predictor of response to continuous positive airway pressure ventilation in Covid-19 pneumonia: a single-center pilot study. Respir Physiol Neurobiol. 2021;284:103585. doi: 10.1016/j.resp.2020.103585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boussuges A, Finance J, Chaumet G, Brégeon F. Diaphragmatic motion recorded by M-mode ultrasonography: limits of normality. ERJ Open Res. 2021;7(1):00714–2020. doi: 10.1183/23120541.00714-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Umbrello M, Formenti P, Longhi D, et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care. 2015;19(1):161. doi: 10.1186/s13054-015-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: S1. (a) The transducer (sector probe) was placed in the right subcostal area between the midclavicular and anterior axillary lines. (b) The angle of ultrasound tracing is possible to the diaphragmatic dome. The diaphragmatic excursion was measured in M-mode. The right diaphragmatic excursion (red dash) was measured as the distance between the value of the diaphragm dome in end-inspiration and end-expiration (green dash).
Additional file 2: S2. Comparison of right-sided diaphragmatic excursion between the non-intubated group and the intubated group in received and not received non-invasive respiratory support.
Additional file 3: S3. Predictive performance of diaphragmatic excursion in the emergency department according to the final diagnosis.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional files 1, 2, and 3.