Abstract
Leaf epidermal cells make ideal specimens for the investigation of the plant secretory pathway in that it is relatively easy to tag with fluorescent proteins and visualize in vivo the various organelles of the pathway. A number of techniques can be employed to identify and study proteins within the endomembrane organelles and to study their dynamics and interactions. Here, we discuss the most commonly used approaches to express proteins within arabidopsis and tobacco leaves, the use of mutant screens to identify trafficking proteins, and the use of two in vivo techniques, Fluorescence recovery after photobleaching and Förster resonance energy transfer, to study protein dynamics in plant cells.
INTRODUCTION
At the heart of the cell lies the secretory pathway: an intricate system of organelles that work in concert to produce and modify one-third of all cellular proteins, as well as lipids, which make up the majority of the cell membranes, and carbohydrates, which are attached to most secretory proteins. In plant cells, these organelles also make important components of the cell wall. The first organelle of the secretory pathway is the ER. The synthesis of most of the plant secretory products is initiated in the reticulated network of the ER, from which they traffic to the Golgi to be distributed to other secretory compartments and, as recently discovered, also to chloroplasts (Matheson, Hanton, & Brandizzi, 2006). The Golgi apparatus, which in plants is made of dispersed, mobile, and polarized stacks in close proximity to the ER (Brandizzi, Snapp, Roberts, Lippincott-Schwartz, & Hawes, 2002), collects and processes secretory materials before sorting them to their destinations. The Golgi also receives materials from the vacuole and the plasma membrane (Foresti & Denecke, 2008; Matheson et al., 2006). In plants, the Golgi synthesizes materials of a cellulosic cell wall that is not generally found in nonplant organisms and sorts the machinery involved in wall deposition to the plasma membrane (Keegstra, 2010). The secretory pathway is upregulated in response to biotic stresses and serves as a reservoir of membrane-tethered transcription factors that help cells to cope with abiotic stress and developmental clues (Chen, Slabaugh, & Brandizzi, 2008). In addition, the secretory pathway has important roles in synthesizing and retaining important receptors for plant hormones (e.g., the ethylene receptor; Chen, Randlett, Findell, & Schaller, 2002) and mediating their subcellular traffic (e.g., BRI; Geldner, Hyman, Wang, Schumacher, & Chory, 2007). Implicit in the vital roles of the secretory pathway for the inner workings of the cell as well as plant growth and development is the existence of a tight regulatory network to ensure membrane homeostasis during the processes of membrane traffic which entails movement of lipids, membrane, and sugars among distinct cellular subcompartments.
Compared to other systems that are less amenable than plants to visual inspection of endomembranes, such as yeast, or that do not benefit from powerful genetic tools, such as mammalian culture cells, the use of model plant species enables modern scientists to address specific questions that are fundamental in plants and all other eukaryotes using a powerful system for live cell biology combined with genetics studies. In this chapter, we discuss a number of techniques and experimental setups that are enabling plant researchers to discover the identity of the players and their dynamics and interactions that influence endomembrane traffic in living cells. The applications of the approaches described in this chapter are instrumental in defining the mechanisms that ensure establishment and maintenance of the secretory organelles with relevance beyond the field of plant biology for morphological and functional characteristics of the secretory pathway that are conserved among eukaryotes.
5.1. LEAVES AS A MODEL SYSTEM FOR ANALYZING THE PLANT GOLGI AND SECRETORY PATHWAY
Leaf epidermal cells are perfect material for high-resolution fluorescence live cell imaging of the components of the plant secretory pathway and as such can be considered to represent self-contained miniature laboratories where besides observational imaging experiments can be carried out on protein transport and dynamics, protein interactions, and organelle interactions. They have the advantage over root cells of being relatively large with a massive central vacuole and thin layer of cortical cytoplasm with few chloroplasts, within which one can carry out imaging and other manipulations. Therefore, such cells constitute optimal material for microscopy analyses of the plant endomembranes because they allow capturing fine details of the subcellular structures.
Expression of fluorescent protein (FP) fusions targeted to various secretory organelles has for a number of years been successfully used to investigate protein localization and protein–protein interactions (Lippincott-Schwartz, Cole, & Presley, 1998; Sparkes & Brandizzi, 2012). Furthermore, thanks to advanced confocal techniques developed over the past few years, more detailed information about protein movement both in aqueous and membrane environments have become possible. A protein of interest tagged with a FP is usually introduced in the plant tissue either by transient or stable transformation. Transient transformation allows the observation of the protein tagged with FPs within 2–4 days from the transformation event, while it takes about a couple of months or longer to obtain transgenic plants through stable transformation (Sparkes, Runions, Kearns, & Hawes, 2006).
Both tobacco species and arabidopsis possess specific characteristics making them useful for the different forms of transformation. Transient transformation can be obtained through various means including infection with a virus carrier (Boevink et al., 1998) or biolistic bombardment of DNA into cells. However, one of the cheapest and safest methods that does not require specialized equipment is infiltration using Agrobacterium carrying a binary vector encoding the FP-fusion of interest (Sparkes et al., 2006). This technique is fast and reliable and especially suitable for the expression of multiple constructs tagged to different colored FPs. The reader is referred to a paper by Denecke et al. (2012) for a discussion on the merits of transient versus stable expression and native versus constitutive promoters for the study of the secretory pathway. To date, most work has been restricted to Arabidopsis thaliana, with the advantage of genetic manipulation, or various tobacco varieties (Nicotiana tabacum, N. benthamiana, N. clevelandii) which are easy to exploit for transient expression. Transient transformation in tobacco epidermal cells usually shows a higher frequency of transformed cells compared to Arabidopsis. With transient expression, the protein of interest is only expressed for few days before the fluorescent signal disappears. Because of this, however, transient expression gives the advantage to study proteins or combination thereof that are potentially lethal and would result in no stable transformants. Transient expression generally provides a useful window to monitor localization and dynamics of proteins of interest before cell death processes start. To control the level of protein expression, the ubiquitin promoter instead of the most commonly used CaMV35S promoter can be adopted. The latter can on occasion cause the production of very high level of proteins that could alter or disturb their subcellular distribution (Grefen et al., 2010). The ubiquitin promoter may provide more homogeneous tissue transformation in stable transformation.
In transformation of Arabidopsis or tobacco plants, the T-DNA carried within the transforming DNA vector is inserted randomly into the genome; as consequence, one or more genes could be interrupted in the same time. In this case, in stable transformation, backcrosses can be made to ensure that the integration of the T-DNA is limited to one insertion. Upon obtaining stable lines, the ideal situation is to perform tail-PCR and identify where and how many genes are disrupted by the T-DNA insertion. It is also important to analyze multiple independent lines, at least five, to ensure that the FP-fusion shows a similar pattern of distribution among the transformants. In transient expression, this issue is solved by analyzing multiple cells within the transformed areas in the tissue.
5.1.1. Methods
Full protocols for the production and expression of FP constructs have been detailed in a previous volume (Runions, Hawes, & Kurup, 2006) and by Sparkes et al. (2006).
5.1.1.1. Transformation
5.1.1.1.1. Transient transformation in tobacco
Tobacco transient transformation can be performed as described in a published detailed protocol (Sparkes et al., 2006). We have found that higher transformation efficiency using plants that are 3–4-weeks old, grown in a controlled chamber with a cycle of 23 °C for 18 h light and 18 °C for 6 h night or grown in a greenhouse and transferred to a chamber for a few days before infiltration. In case of very low efficiency transformation, we recommend infiltrating agrobacteria carrying the gene of interest in plants at different stages (ranging from 2–3 weeks to 5–6 weeks).
5.1.1.1.2. Transient and stable transformation in Arabidopsis
Different protocols have been published for transient transformation of Arabidopsis plants; unfortunately, the percentage rate of fluorescent cells obtained after transformation is generally lower compared to tobacco plants (Campanoni, Sutter, Davis, Littlejohn, & Blatt, 2007; Li, Park, von Arnim, & Nebenführ, 2009; Marion et al., 2008). One of the most important factors that could increase the efficiency is the age of seedlings used, which we recommend to be not older than 4–5 day after germination.
Stable transformation has been performed in different ways using different media, chemicals, or methods, such as dipping, vacuum infiltration, or a combination of the two. However, the most used and successful method remain the protocol described by Clough and Bent (1998) which uses floral dipping. In all the methods used, the most important factor, which affects the percentage of stable transformants, is the health/age of the plants used.
5.1.1.1.3. Imaging leaf material
Arabidopsis cotyledons (7–10-days old) or 0.5 cm2 square section of leaves (tobacco or Arabidopsis) are mounted on a microscope slide (1 in. × 3 in., 1.2 mm thick) in water with the abaxial side oriented toward the objective lens and enclosed with a coverslip. For confocal microscopy, the use of oil or water immersion lenses of the highest possible numerical aperture is recommended.
5.2. MUTANT SCREENS FOR TRAFFICKING PROTEINS
The introduction of genetically encoded FP reporters has permitted the adoption of new mutant screen opportunities, which are profoundly different from earlier screens set to identify plant phenotypic change (Sparkes & Brandizzi, 2012; Zwiewka & Friml, 2012). Microscopy-based screens allow the identification of mutants with misplaced subcellular FP-based reporters, which can be informative for specific defects in protein traffic routes and organelle integrity. This approach benefits from the availability of the sequenced model plant, A. thaliana. The screens also benefit from the availability of fluorescent markers targeted to all the endomembrane compartments that permit the analyses of specific trafficking pathways inside the cell and the morphology of specific organelles. Furthermore, FPs have been successfully engineered to improve maturation time as well as resistance to various degrees of environmental conditions including chloride, pH, and light irradiation. Extremely important for plant cells studies are the FP variants that are resistant to changes in pH and enable the monitoring of highly acidic microenvironments such as vacuole and apoplast where original FPs are not visible. On the other hand, however, FP quenching due to the acidic ambient can be exploited as system to identify mutants. Based on this approach, the secreted marker SEC-green fluorescent protein (GFP) in which the sporamin signal peptide is fused to the GFP allows the GFP to be secreted/transported to the apoplast. Seedlings mutagenized have been successfully screened for intracellular accumulation of SEC-GFP where the pH situation is favorable for fluorescence (Zheng, Kunst, Hawes, & Moore, 2004).
The possibility to visualize FP reporters in combination with the available strategies to produce mutagenized plants with ethyl methane sulfonate (EMS), gamma-ray, fast neutron, or T-DNA insertions can be used in forward genetic screens to identify proteins involved in protein trafficking and organelle integrity (Quesada, Ponce, & Micol, 2000; Stefano, Renna, & Brandizzi, 2012). EMS treatment produces point mutation, mainly G/C-to-A/T transitions with high frequency, while gamma-ray and fast neutron produce many types of mutations including base substitutions, deletions, and chromosomal alterations, but the mutation frequency is lower compared to EMS treatment. As EMS induces a high frequency of mutations, a relatively small number of plants is needed to reach saturation and the generation of a large number of gene knockouts as well as hypomorphic knockdowns is possible. Once the mutants have been identified, the mutation can be mapped using classical mapping or next generation sequencing which can be performed in a relatively short time (Stefano, Renna, & Brandizzi, 2012). With these approaches, it has proved possible to identify a number of proteins that are key to the functioning of the plant secretory pathway such as potential Golgi–ER tethers as well as proteins involved in Golgi trafficking and membrane integrity (Renna et al., 2013; Sparkes & Brandizzi, 2012).
5.2.1. Methods
5.2.1.1. EMS treatment, confocal screening, and mapping
Arabidopsis lines stably expressing a fluorescent marker are mutagenized using EMS (for mutagenesis procedures refer to detailed protocols (Stefano et al., 2012)). This provides the so-called M1 generation. Seeds are collected from individual M1 plants to generate M2 lines. M2 seeds from individual lines are plated and screened for aberrant localization of the fluorescent marker using either a laser scanning confocal microscope or a fluorescence microscope. The use of confocal versus conventional fluorescence microscopy has some advantages. Confocal gives us a good resolution in each focal plane eliminating out of focus light, and it is possible to perform 3D reconstruction of protein distribution along the -axis. Our screening method starts with plating 60 seeds from each M2 lines as well as 5–10 control seeds of the nonmutagenized background. Seedlings are left to grow for 7–10 days. It is important to note that in some case the seeds show aberrant phenotype early in the development and other later in development, so we suggest observing the same line both in early and late developmental stages. With seedlings, it is possible to mount them directly on slides. As observing material we use cotyledon cells from the abaxial region that are very easy to observe compared to other cell types. Of course, depending on the fluorescent marker used and the tissue of interest, any part of the seedlings can be used in the screening. Often, the CaMV 35S promoter provides a patchy expression pattern with some of the fluorescent marker expressing only in some tissues. An alternative could be to use protein tagged with fluorescent marker expressed under the ubiquitin promoter that gives a very even distribution of the fluorescence in the plants. At the microscope, the samples should be observed from the cortical to the medial region of the cell as any altered distribution of the fluorescent marker could be affecting just specific regions of the cytoplasm.
Identified mutants are transferred to soil and reobserved in the next generation (M3) to make sure that the subcellular phenotypes are maintained. Mutants are then allowed to flower for crossing with an alternate genotype or the same wild-type genotype. This permits assessment of whether the mutation is recessive or dominant through confocal analyses of the F2 progeny of the crosses. For confirmed recessive mutations, the mutation can be mapped using a combination of DNA hybridization and sequencing protocols recently described (Stefano et al., 2012), which is faster if compared to traditional mapping methods. This relatively new approach is based on the ability to identify in a single assay numerous single feature polymorphisms using high-density oligonucleotide arrays, like the Affymetrix Arabidopsis ATH1 Gene-Chip array that can analyze circa 24,000 genes. The advantages of this method compared to the classical approach is the fact that mapping can be performed using a relatively small number of F2 individuals to locate the mutation allowing the identification of the mutated gene in a shorter time.
5.3. PHOTOBLEACHING FOR TRANSPORT STUDIES
Fluorescence recovery after photobleaching (FRAP) is a powerful tool enabling the study of active protein movement or diffusion within or on and off membranes as well as interconnected soluble environments (Martinière et al., 2012), or for the study of transport of fluorescently tagged proteins between compartments of the secretory pathway in real time (Brandizzi et al., 2002; Da Silva et al., 2004; Matheson et al., 2007; Renna et al., 2013). As such we have used the technique to quantify membrane protein transport between the ER and the Golgi apparatus, showing that such movement is energy-dependent but independent of the cytoskeleton (Brandizzi et al., 2002), as well as reversible binding of small GTPases, such as ARFs and Sar1, on and off ER, and Golgi membranes. Essentially, with FRAP, a high-energy laser beam is used to irreversibly quench the fluorophore fluorescence in a predetermined area of the specimen and recovery of fluorescence is monitored through time-lapse imaging. As such, movement of fluorescent molecules into the bleached area can be followed and in most cases it must be assumed that this is balanced by an equal loss of quenched molecules out of the bleached area.
5.3.1. Methods
Most of the organelles of the plant secretory pathway demonstrate a high degree of motility which is almost exclusively actin-mediated (Boevink et al., 1998; Brandizzi et al., 2002). Therefore, although it is possible to bleach FPs targeted to motile organelles such as Golgi bodies if they show at least partial interruptions in movement (Da Silva et al., 2004), it is generally best to inhibit movement with an actin depolymerizing drug such as latrunculin B or cytochalasin D (Brandizzi et al., 2002). Depending on the confocal microscope being used, it is possible to bleach multiple regions of interest; however, it is advisable to make sure that there are unbleached organelles in the field of view that can serve as control. In other words, from such regions, a time series of fluorescence intensity can be established to monitor any photobleaching by the imaging laser during the recovery period being monitored. Such bleaching will have to be factored into calculations of fluorescence recovery on the bleached regions. Furthermore, a region in the field of view where the FP fusions do not localize is necessary to calculate the background fluorescence. This will be subsequently subtracted from the fluorescence values of the bleached areas as well as the selected unbleached areas that serve as control reference.
5.3.1.1. Bleach protocol
Below is a typical series of steps to be taken when photobleaching an region of interest (ROI). Most modern confocal microscopes come supplied with easily programmable bleach protocols.
To initiate the experiment it is necessary to treat specimens with actin depolymerizing agents to stop movement of organelles. We find that for leaf material, treatment with 25 μM latrunculin B from a 10 mM stock solution in DMSO for at least 30 min is suitable. Alternatively, treatment with 20 μg/mL cytochalasin D from a 1 mg/mL stock solution in DMSO for at least 30 min can also be used. Generally, we submerge the leaf tissue in a tube containing the working solution and the tissue is then mounted on the slide with the same solution. We recommend leaving the tissue in the actin-depolymerizing solution for no longer than 90 min in order to reduce excessive cellular stress that may confound the results. We also find that the thickness of the coverslip can influence the results; therefore, we recommend using coverslips with consistent thickness (No. 1). It is also important to use consistent settings of magnification (i.e., same objective and optical zoom).
The excitation and emission wavelengths for imaging will depend on the fluorochrome of choice and they should provide maximum signal yield with minimal laser intensity power. Avoid imaging oversaturation or undersaturation by monitoring pixel saturation levels with the microscope software. If the purpose of the experiment is quantitative (e.g., comparing recovery rates of FPs in the Golgi or in ER regions), the size (i.e., area and shape) of the ROIs should be identical.
To initiate the FRAP experiment at the microscope, select 2–3 bleach regions using ROI settings on the confocal. As control ROIs, select two additional ROIs outside the bleach region: (a) an ROI in an area where the FP signal is visible (reference ROI), and (b) an ROI where no fluorescent signal is visible (background ROI). As explained above, the reference ROI will allow the levels of nonspecific bleaching that can be achieved during scanning to be assessed. This region should be therefore placed relatively far away from the bleach ROIs within the imaging field. The background ROI will define the levels of fluorescence that should be achieved upon the bleach scan in the FRAP analysis and will be subtracted from the bleach ROI value in the computational phase of the FRAP experiment.
Record 10 full field scans with the lowest possible laser beam intensity (i.e., generally, 1–5% transmittance). Then bleach ROIs by scanning with full-power laser beam (i.e., 100% transmittance for a predetermined number of scans). Generally, the number of scans should be sufficient to lower the fluorescence of the bleached area to near background levels, although this is not an absolute necessity. This can be established by comparing the levels of fluorescence in the ROI set on the organelle with the background ROI intensity.
Record full-field scans with low laser settings during recovery period until a plateau of signal intensity values is reached. The number of scans during the recovery period is generally established empirically and will depend on the experiment in hand. As scanning causes intrinsic bleaching, it is generally advisable to keep the number of the fluorescence recovery scans to a minimum. On the other hand, it is also important to ensure that the number of scans is sufficient to record the fluorescence recovery in the bleached ROI over time. Therefore, as an initial step to get familiar with the FRAP protocol, it may be useful to set up imaging conditions for the FRAP experiments with cells expressing the FP targeted to the organelle of interest that have been fixed with paraformaldehyde. The material should be used to record ROI fluorescence with low laser intensity in order to define the conditions to obtain no significant bleaching during the time course of the experiment.
It is customary to express fluorescence recovery in terms of the half time which is the time required for fluorescence in the bleached region to recover 50% of its initial fluorescence. This can be computed by scaling the postbleach fluorescence recovery to a 0–100% scale with the immediate postbleach intensity being 0% and the asymptote of the recovery being 100%. The bleached ROI intensity values should be subtracted from the background ROI values as well as normalized to the reference ROI values at equivalent imaging time points. The number of ROIs to be analyzed will depend of the FRAP experiment but they should be set to achieve statistical significance.
5.4. FÖRSTER RESONANCE ENERGY TRANSFER FOR PROTEIN INTERACTIONS
Whilst coexpression of proteins tagged to different FPs is a powerful technique to assess colocalization of proteins within specific organelles or regions of organelles (French, Mills, Swarup, Bennett, & Pridmore, 2008), there is no information in such images on any interactions between the proteins. Several techniques have been developed that permit the in vivo assessment of protein interactions. FRET (Förster or fluorescence resonance energy transfer), being the most powerful, permits the in vivo analysis of protein–protein interaction through measurement of nonradiative energy transfer from a donor fluorophore to an acceptor fluorophore of high excitation wavelength (Majoul, Jia, & Duden, 2006). Thus, FP constructs, where donor and acceptor are fused to putative interaction partners, are particularly suitable for this form of analysis. Suitable pairings that display a sufficient spectral overlap between excitation and emission spectra may be used, such as CFP/YFP or GFP/mRFP. As FRET efficiency decreases with the universal sixth power of the separation distance between the fluorophores, FRET can only be measured when the fluorophores are very close to each other, between 1 and 10 nm (Sparkes et al., 2011). This proximity only occurs when the two fusion proteins interact, and not when they simply colocate to the same cell structure (Majoul et al., 2006). FRET can be easily measured on a confocal microscope by a decrease in donor emission (quenching of the fluorescence) and with a corresponding increase in acceptor fluorophore fluorescence. A popular method is to photobleach the acceptor fluorophore and measure the accompanying increase in fluorescence of the donor fluorophore (Fricker, Runions, & Moore, 2006; Harholt et al., 2012).
A considerable improvement over the classic FRET measurement techniques is to utilize changes in the fluorescence excited-state lifetime of the donor fluorophore as it loses energy to the acceptor (Gerritsen, Draaijer, van den Heuvel, & Agronskaia, 2006; Sun, Day, & Perisamy, 2011). This has several advantages over conventional FRET in that fluorophore lifetime is unaffected by fluorophore concentration of excitation intensity and spectral bleed-through is not a problem as only the donor lifetime is measured. The lifetime of a fluorescent molecule is the average time that it stays in its excited state before decaying to its ground state, although it can change under different microenvironmental and physiological conditions in the cell (Becker, 2012). For FP constructs lifetimes can vary depending on the specific microenvironment of a protein spliced to the FP (Osterrieder et al., 2009). Lifetimes can be measured using photon counting detectors linked to time-correlated single-photon counting (SPC) modules available from specialist suppliers such as Becker and Hickl, and Picoquant, time gating modes as intensified charge coupled devices and frequency modulated methods. All of these techniques need pulsed light sources. These can be interfaced with confocal microscopes (Stahl et al., 2013) or two-photon systems, the latter giving better signal to noise ratio, resolution, and less background fluorescence in thick samples such as whole leaves, resulting in cleaner lifetime signals (Schoberer, Liebminger, Botchway, Strasser, & Hawes, 2013). Interactions between pairs of fluorophores can be measured through a reduction in the lifetime of the donor molecule (quenching) as a result of energy transfer to the acceptor molecule. This can simply be calculated by measuring the fluorescence lifetime of the donor alone or in combination with the acceptor fluorophore. This transfer or FRET percentage efficiency is calculated as follows:
and are the mean pixel-by-pixel excited-state lifetimes of the donor fluorophore in the presence and absence, respectively, of the acceptor fluorophore determined for each pixel. For GFP the decrease in average donor lifetimes by a minimum of 0.2 ns or 8% (where donor lifetime is determined to be 2.5 ns) in the presence of the acceptor can be considered relevant to indicate FRET and thus indicate potential protein–protein interaction (Schoberer et al., 2013; Sun et al., 2011).
There are not many reports of the use of FRET to measure interactions within the plant secretory pathway (Harholt et al., 2012). We have concentrated on using a two-photon FLIM system for assessing the interactions of Golgi matrix proteins with small GTPases, such as ARL1 (Osterrieder et al., 2009; Fig. 5.1) and interactions between -glycan processing enzymes in the Golgi stack (Schoberer et al., 2013). Likewise, homotypic interactions between the ER membrane proteins, the reticulons which are responsible for inducing tubule curvature, have been assessed by FRET-FLIM (Sparkes et al., 2010).
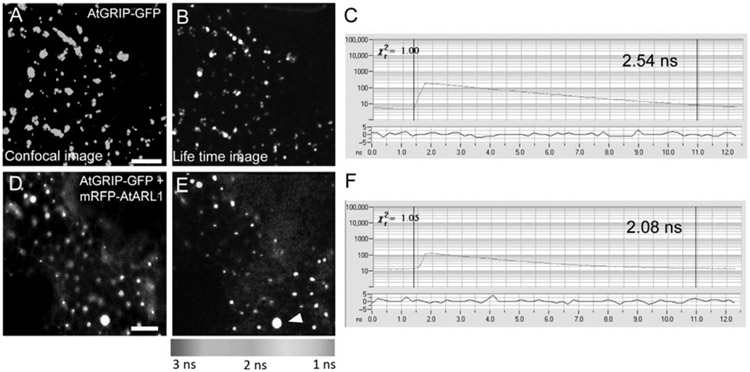
FIGURE 5.1.
Example of FLIM data—interactions between coiled-coil proteins and small regulatory GTPases. The subcellular localization of interactions between coiled-coil proteins fused to GFP and small regulatory GTPases fused to mRFP were visualized with pseudo-colored lifetime maps generated in SPCImage (Becker & Hickl, Germany). (A) Confocal image of a tobacco leaf epidermal cell expressing the trans-Golgi matrix protein AtGRIP-GFP as control. (B) Pseudo-colored lifetime map of the same cell. Golgi bodies appear in blue, indicating an unquenched lifetime around 2.5 ns. (C) Representative decay curve for a single point analysis of AtGRIP-GFP with a lifetime of 2.54 ns and a χ2 value of 1, indicating an optimal single exponential fit. (D) Confocal image of a cell expressing AtGRIP-GFP and the small regulatory GTPase mRFP-AtARL1. (E) Lifetime map showing quenching of AtGRIP-GFP in punctate structures colocating with mRFP–AtARL1. The lifetime reduction is reflected in the green color. Occasionally, large bright aggregates labeled by both proteins were observed, which did not show reduced lifetime values (arrowhead). (F) Representative decay curve for a single point analysis of AtGRIP-GFP + mRFP-ARL1 with a reduced lifetime of 2.08 ns. The χ2 of 1.05 indicates a single exponential optimal fit.
A typical two-photon microscope set-up connected to a confocal microscope would produce 700–1000 nm laser beam from a mode-locked titanium sapphire laser producing 180-fs pulses at 75 MHz pumped by a solid state, continuous wave, 532-nm laser. Emission signals are collected without descanning, filtered to remove near infra-red light, detected with a fast microchannel plate photomultiplier tube. Line, frame, and pixel clock signals generated by the scanning system are linked to a time-correlated SPC module to generate the raw FLIM data. FLIM images are analyzed using proprietary SPC image analysis software, although other analysis software such as TIMP are available.
5.4.1. Methods
Exact procedures for FRET measurements will depend on the microscope and systems being used. Our standard procedure for two-photon FRET-FLIM of leaf material expressing GFP and mRFP constructs is given below.
In order to achieve adequate photon counts for fluorescence lifetime acquisition (>100 in the peak channel of 256 bins), samples should be scanned several times to accumulate sufficient signal. This means that the area of interest needs to be immobilized in order to collect cumulative data. Depending on the cell structure of interest, this could mean drug-mediated cytoskeleton depolymerization (Osterrieder et al., 2009) or even fixation with paraformaldehyde, or by restricting analysis to an area of the structure that has less remodeling (e.g., measuring interaction of endoplasmic reticulum proteins near the nuclear envelope, Sparkes et al., 2010).
Mount 5 × 5 mm2 leaf samples or if using arabidopsis whole young leaves, pretreated as required on a glass slide with coverslip thickness no. 1. To prevent sample drift it is advisable to secure coverslip edges with sticky tape. Find a suitable region of the sample to measure using confocal microscopy and save a reference confocal image. Ideally, data should be collected from cells showing double expression of both constructs and also from cells expressing just the GFP construct for acquisition of control FLIM data.
Switch to two-photon laser to perform lifetime reading. A signal intensity of at least 100 photons is required in the peak channel. For Golgi bodies labeled with GFP we usually perform between 3–5 scans, each of 30 s. For brighter structures one scan with 30 s may be sufficient. The laser power should be adjusted for every construct depending on fluorochrome brightness, and care should be taken as exposing samples to too high laser powers can cause photodamage and dielectric breakdown of water in the cells.
Save images and import into FLIM analysis software. Refer to manufacturer instructions for specific procedures, for example, adjusting the decay model in the software or binning (the number of pixels the program takes into account for analysis). Becker & Hickl software translates lifetime image into a false-colored map in which different colors indicate different lifetimes. The first step is to perform curve-fitting analysis. Chi-square is used as an indicator for best exponential curve fit, where a χ2 = 1.0 in the structure is best fit of the data points to the lifetime value generated. Values of Chi-square that deviate from 1 indicates poor fitting to the data points when a single exponential condition is applied. Since we expect the lifetime value of GFP to be a single exponential decay, we discard data under χ2 = 0.8 and over χ2 = 1.5. (These indicate multiple decay components.)
Data can be extracted from lifetime images either on pixel basis and plotted into distribution histograms (see Osterrieder et al., 2009) or by drawing regions of interest and collecting average (Sparkes et al., 2010).
SUMMARY
Here, we have detailed just a few of the techniques that form just part a much larger toolbox available to the plant biologist for the study of the Golgi and the secretory pathway, but which can just as equally be applied to other cytoplasmic organelles. These include the use of photoactivatable and photoswitchable FPs, laser trapping and ablation techniques, and the whole range of recently developed high-resolution fluorescence microscope technologies. In conclusion, FP expression in leaf epidermal cells offers an extremely tractable experimental environment in which to apply such laser technologies to explore in vivo the dynamics and interactions of components and proteins of the secretory pathway.
Acknowledgments
Some of this work was supported by grants from the National Institutes of Health (R01 GM101038-01), Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. DOE (DE-FG02-91ER20021), NASA (NNX12AN71G), and the National Science Foundation (MCB 0948584 and MCB1243792) (FB) and the BBSRC (BB/F008147/1) (CH). We also thank Stan Botchway (STFC, Central Laser Facility, Harwell, UK) for reading the chapter.
References
- Becker W. (2012). Fluorescence lifetime imaging—Techniques and applications. Journal of Microscopy, 247, 119–136. [DOI] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, & Hawes CR (1998). Stacks on tracks: The plant Golgi apparatus traffics on an actin/ER network. Plant Journal, 15, 441–447. [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Snapp E, Roberts A, Lippincott-Schwartz J, & Hawes CR (2002). Membrane protein transport between the ER and Golgi in tobacco leaves is energy dependent but cytoskeleton independent: Evidence from selective photobleaching. Plant Cell, 14, 1293–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanoni P, Sutter JU, Davis CS, Littlejohn GR, & Blatt MR (2007). A generalized method for transfecting root epidermis uncovers endosomal dynamics in Arabidopsis root hairs. Plant Journal, 51, 322–330. [DOI] [PubMed] [Google Scholar]
- Chen YF, Randlett MD, Findell JL, & Schaller GE (2002). Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. Journal of Biological Chemistry, 277, 19861–19866. [DOI] [PubMed] [Google Scholar]
- Chen YN, Slabaugh E, & Brandizzi F (2008). Membrane-tethered transcription factors in Arabidopsis thaliana: Novel regulators in stress response and development. Current Opinion in Plant Biology, 11, 695–701. [DOI] [PubMed] [Google Scholar]
- Clough SJ, & Bent AF (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal, 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Da Silva LLP, Snapp EL, Denecke J, Lippincott-Schwartz L, Hawes C, & Brandizzi F (2004). Membrane cargo-mediated recruitment of GTPase Sar1p to specific export sites of the ER membrane. Plant Cell, 16, 1753–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J, Aniento F, Frigerio L, Hawes C, Hwang I, Mathur J, et al. (2012). Secretory pathway research: The more experimental systems the better. The Plant Cell, 24, 1316–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti O, & Denecke J (2008). Intermediate organelles of the plant secretory pathway: Identity and function. Traffic, 9, 1599–1612. [DOI] [PubMed] [Google Scholar]
- French AP, Mills S, Swarup R, Bennett MJ, & Pridmore TP (2008). Colocalization of fluorescent markers in confocal microscope images of plant cells. Nature Protocols, 3, 619–628. [DOI] [PubMed] [Google Scholar]
- Fricker M, Runions J, & Moore I (2006). Quantitative fluorescence microscopy: From art to science. Annual Review of Plant Biology, 57, 79–107. [DOI] [PubMed] [Google Scholar]
- Geldner N, Hyman DL, Wang X, Schumacher K, & Chory J (2007). Endosomal signaling of plant steroid receptor kinase BRI1. Genes & Development, 21, 1598–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen HC, Draaijer DA, van den Heuvel DJ, & Agronskaia AV (2006). Fluorescence lifetime imaging in scanning microscopy. In Pawley JB (Ed.), Handbook of biological confocal microscopy (pp. 516–534). New York: Springer. [Google Scholar]
- Grefen C, Donald N, Hashimoto K, Kudla J, Schumacher K, & Blatt MR (2010). A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. The Plant Journal, 64, 355–365. [DOI] [PubMed] [Google Scholar]
- Harholt J, Jensen JK, Verhertbruggen Y, Søgaard C, Bernard S, Nafisi M, et al. (2012). ARAD proteins associated with pectic arabinan biosynthesis form complexes when transiently overexpressed in planta. Planta, 236, 115–128. [DOI] [PubMed] [Google Scholar]
- Keegstra K. (2010). Plant cell walls. Plant Physiology, 154, 483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JF, Park E, von Arnim AG, & Nebenführ A (2009). The FAST technique: A simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods, 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Cole N, & Presley J (1998). Unravelling Golgi membrane traffic with green fluorescent protein chimeras. Trends in Cell Biology, 8, 16–20. [DOI] [PubMed] [Google Scholar]
- Majoul I, Jia Y, & Duden R (2006). Practical fluorescence resonance energy transfer or molecular nanobioscopy of living cells. In Pawley JB (Ed.), Handbook of biological confocal microscopy (pp. 788–807). New York: Springer. [Google Scholar]
- Marion J, Bach L, Bellec Y, Meyer C, Gissot L, & Faure JD (2008). Systematic analysis of protein subcellular localization and interaction using high-throughput transient transformation of Arabidopsis seedlings. The Plant Journal, 56, 169–179. [DOI] [PubMed] [Google Scholar]
- Martinière A, Lavagi I, Nageswaran G, Rolfe DL, Maneta-Peyret L, Luu D-T, et al. (2012). The cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proceedings of the National Academy of Sciences of the United States of America, 109, 12805–12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson LA, Hanton SL, & Brandizzi F (2006). Traffic between the plant endoplasmic reticulum and Golgi apparatus: To the Golgi and beyond. Current Opinion in Plant Biology, 9, 601–609. [DOI] [PubMed] [Google Scholar]
- Matheson LA, Hanton SL, Rossi M, Latijnhouwers M, Stefano G, Renna L, et al. (2007). Multiple roles of ADP-ribosylation factor 1 in plant cells include spatially regulated recruitment of coatomer and elements of the Golgi matrix. Plant Physiology, 143, 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieder A, Carvalho CM, Latijnhouwers M, Johansen JM, Stubbs C, Botchway S, et al. (2009). Fluorescence lifetime imaging of interactions between Golgi tethering factors and small GTPases in plants. Traffic, 10, 1–13. [DOI] [PubMed] [Google Scholar]
- Quesada V, Ponce MR, & Micol JL (2000). Genetic analysis of salt-tolerant mutants in Arabidopsis thaliana. Genetics, 154, 421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna L, Stefano G, Majeran W, Micalella C, Meinnel T, Giglione C, et al. (2013). Golgi traffic and integrity depend on N-myristoyl transferase-1 in Arabidopsis. Plant Cell, 25, 1756–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runions J, Hawes C, & Kurupp S (2006). Fluorescent protein fusions for protein localization in plants. In Methods in Cell Biology, 390, 239–255. [DOI] [PubMed] [Google Scholar]
- Schoberer J, Liebminger E, Botchway SW, Strasser R, & Hawes C (2013). Time-resolved fluorescence imaging reveals differential interactions of -glycan processing enzymes across the Golgi stack in planta. Plant Physiology, 161, 1737–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes I, & Brandizzi F (2012). Fluorescent protein-based technologies: Shedding new light on the plant endomembrane system. The Plant Journal, 70, 96–107. [DOI] [PubMed] [Google Scholar]
- Sparkes IA, Graumann K, Martiniére A, Schoberer J, Wang P, & Osterrieder A (2011). Bleach it, switch it, bounce it, pull it: Using lasers to reveal plant cell dynamics. Journal of Experimental Botany, 62, 1–7. [DOI] [PubMed] [Google Scholar]
- Sparkes I, Runions J, Kearns A, & Hawes C (2006). Rapid, transient expression of fluorescent protein fusions in tobacco plants and generation of stably transformed plants. Nature Protocols, 1, 2019–2025. [DOI] [PubMed] [Google Scholar]
- Sparkes I, Tolley N, Aller I, Svozil J, Osterrieder A, Botchway S, et al. (2010). Five plant reticulon isoforms share ER localisation, topology, ER membrane shaping properties. Plant Cell, 22, 1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl Y, Grabowski S, Bleckmann A, Kühnemuth R, Weidtkamp-Peters S, Pinto KG, et al. (2013). Moderation of Arabidopsis root stemless by CLAVATA1 and ARABIDOPSIS CRINKLY4 receptor kinase complexes. Current Biology, 23, 362–371. [DOI] [PubMed] [Google Scholar]
- Stefano G, Renna L, & Brandizzi F (2012). Fluorescence-microscopy screening and next-generation sequencing: Useful tools for the identification of genes involved in organelle integrity. Journal of Visualized Experiments, 62, e3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Day RN, & Perisamy A (2011). Investigating protein-protein interactions in living cells using fluorescence lifetime imaging microscopy. Nature Protocols, 6, 1324–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Kunst L, Hawes C, & Moore I (2004). A GFP-based assay reveals a role for RHD3 in transport between the endoplasmic reticulum and Golgi apparatus. Plant Journal, 37, 398–414. [DOI] [PubMed] [Google Scholar]
- Zwiewka M, & Friml J (2012). Fluorescence imaging-based forward genetic screens to identify trafficking regulators in plants. Frontiers in Plant Science, 3, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]