Abstract
Background
Esophageal cancer survival is poor worldwide, though there is some variation. Differences in the distribution of anatomical sub‐site and morphological sub‐type may help explain international differences in survival for all esophageal cancers combined. We estimated survival by anatomic sub‐site and morphological sub‐type to understand further the impact of topography and morphology on international comparisons of esophageal cancer survival.
Methods
We estimated age‐standardized one‐year and five‐year net survival among adults (15‐99 years) diagnosed with esophageal cancer in each of 60 participating countries to monitor survival trends by calendar period of diagnosis (2000‐2004, 2005‐2009, 2010‐2014), sub‐site, morphology, and sex.
Results
For adults diagnosed during 2010‐2014, tumors in the lower third of the esophagus were the most common, followed by tumors of overlapping sub‐site and sub‐site not otherwise specified. The proportion of squamous cell carcinomas diagnosed during 2010‐2014 was generally higher in Asian countries (50%‐90%), while adenocarcinomas were more common in Europe, North America and Oceania (50%‐60%). From 2000‐2004 to 2010‐2014, the proportion of squamous cell carcinoma generally decreased, and the proportion of adenocarcinoma increased. Over time, there were few improvements in age‐standardized five‐year survival for each sub‐site. Age‐standardized one‐year survival was highest in Japan for both squamous cell carcinoma (67.7%) and adenocarcinoma (69.0%), ranging between 20%‐60% in most other countries. Age‐standardized five‐year survival from squamous cell carcinoma and adenocarcinoma was similar for most countries included, around 15%‐20% for adults diagnosed during 2010‐2014, though international variation was wider for squamous cell carcinoma. In most countries, survival for both squamous cell carcinoma and adenocarcinoma increased by less than 5% between 2000‐2004 and 2010‐2014.
Conclusions
Esophageal cancer survival remains poor in many countries. The distributions of sub‐site and morphological sub‐type vary between countries, but these differences do not fully explain international variation in esophageal cancer survival.
Keywords: Cancer, esophagus, morphology, survival, topography, trends
Abbreviations
- CI
confidence interval
- ICSS
International Cancer Survival Standard
- ICD‐O‐3
International Classification of Diseases for Oncology
- NOS
not otherwise specified
1. BACKGROUND
Esophageal cancer survival is relatively poor worldwide, with only limited improvement over the past few decades [1].
The second cycle of the CONCORD program established global surveillance of trends in cancer survival in 2015 [2]. CONCORD‐3 updated global survival trends in 2018 by analyzing data on over 37.5 million cancer patients diagnosed with one of 18 common cancers during 2000‐2014, contributed by 322 population‐based cancer registries in 71 countries [1]. CONCORD‐3 is the largest research program to date on population‐based cancer survival, including information on anatomic sub‐site and morphological sub‐type of the tumors included in analyses. Survival estimates are made as comparable as possible with centralized data quality control procedures and analysis and correction for background mortality in each region or country by age, sex, and calendar year.
CONCORD‐3 reported wide international variation in five‐year survival from esophageal cancer for all topographies and morphologies combined, ranging from 10% to 30% for adults diagnosed from 2010 to 2014[1]. Survival was highest in several East Asian countries. In addition, although survival increased in a few countries (e.g., China, Korea, and Japan), the improvements have been minimal.
Esophageal cancer is conventionally classified as upper third (cervical), middle third (thoracic) or lower third (abdominal). Squamous cell carcinoma and adenocarcinoma are the two most common morphological sub‐types. Squamous cell carcinoma has historically been the most common sub‐type, especially in low‐income and middle‐income countries in Asia where smoking, a known risk factor, is common[3, 4]. In North America and Western Europe, adenocarcinoma has more recently become the most common sub‐type, possibly due to the link with Barrett's esophagus and the increasing prevalence of obesity [3, 4].
Previous studies of esophageal cancer survival by sub‐site or morphology have been limited to one country or high‐income countries in Europe, North America, and Oceania [5, 6, 7, 8, 9, 10, 11]. A more global picture of the distribution of and survival from esophageal cancer by sub‐site and morphology is needed.
We have used data from CONCORD‐3 for a more detailed study of whether international differences in the distribution of sub‐site, morphology, and sex can help explain any of the international variation in esophageal cancer survival. We also provide estimates of time trends in esophageal cancer survival by sub‐site, morphology, sex, and country, to identify groups for which survival is lowest, in order to help drive cancer control policies to improve esophageal cancer survival.
2. METHODS
2.1. Data
Data from 288 population‐based cancer registries were available for 743,314 adults (15‐99 years) diagnosed with esophageal cancer during 2000‐2014 in 60 countries.
The CONCORD‐3 protocol, the ethical approvals and the data quality control procedures have been described [1]. We included only primary, invasive malignant tumors (International Classification Diseases of Oncology, 3rd edition [12] (ICD‐O‐3) behavior code 3) in survival analyses. If a patient was diagnosed with two or more primary, invasive tumors of the esophagus, only the first record was included. Patients whose cancer registration was from a death certificate or autopsy only were excluded from analysis because their true survival time was unknown (Supplementary Table S1). Follow‐up data on vital status (dead, alive, or lost to follow‐up) until 31 December 2014 were available.
We categorized topography into four sub‐sites based on the ICD‐O‐3 topographical code: cervical or upper third (C15.0 or C15.3), thoracic or middle third (C15.1 or C15.4) and abdominal or lower third (C15.2 or C15.5), with an additional category for cancers that overlapped sub‐sites or for which the sub‐site was not otherwise specified (NOS, C15.8 or C15.9).
We defined six morphological groups based on the literature and ICD‐O‐3 morphology codes [12]: squamous and transitional cell carcinomas, adenocarcinomas, other specified carcinomas, unspecified carcinomas, sarcomas and other soft tissue tumors, other specified cancers, and a separate category for tumors of non‐specific morphology (Table 1).
TABLE 1.
Morphological sub‐types.
| Morphological sub‐type | ICD‐O‐3 morphology code a |
|---|---|
| Squamous and transitional cell carcinomas | 8051‐8139 |
| Adenocarcinomas | 8140‐8149, 8160‐8169, 8180‐8229, 8250‐8509, 8520‐8559, 8570‐8579, 8940‐8949 |
| Other specified carcinomas | 8030‐8049, 8150‐8159, 8170‐8179, 8230‐8239, 8240‐8249, 8510‐8519, 8560‐8569, 8580‐8679 |
| Unspecified carcinomas | 8010‐8029, 8050 |
| Sarcomas and other soft tissue tumors | 8680‐8719, 8800‐8929, 8990‐8999, 9040‐9049, 9120‐9349, 9370‐9379, 9540‐9589 |
| Other specified tumors | 8720‐8799, 8930‐8939, 8950‐8989, 9000‐9039, 9050‐9119, 9360‐9369, 9380‐9539 |
| Non‐specific tumors | 8000‐8005 |
Fritz AG, Percy C, Jack A, Shanmugaratnam K, Sobin LH, Parkin DM, Whelan SL, editors. International Classification of Diseases for Oncology (ICD‐O). First revision of 3rd ed. Geneva: World Health Organisation; 2013.
2.2. Statistical analyses
We estimated age‐standardized one‐year and five‐year net survival by country, calendar period of diagnosis (2000‐2004, 2005‐2009, 2010‐2014), anatomic sub‐site, morphological sub‐type, and sex.
We used the cohort approach [13, 14] to estimate net survival for patients diagnosed during 2000‐2004 and 2005‐2009 because at least five years of follow‐up data were available for all patients by the end of 2014. We used the period approach [15] to estimate survival for patients diagnosed during 2010‐2014 because five years of follow‐up data were not available for all patients by 31 December 2014. Period estimates were obtained by multiplying the conditional probabilities of survival in each successive year up to five years after diagnosis that had been observed during the most recent period for which adequate follow‐up data were available.
We estimated net survival using the Pohar Perme estimator [16]. Net survival is the probability of a cancer patient surviving their cancer up to a given time since diagnosis, e.g., five years, after controlling for competing risks of death (background mortality), which are higher in the elderly. To account for the differences in background mortality between regions and over time, we constructed life tables of all‐cause mortality specific to each country or region, single year of age, sex, calendar year, and, where possible, race or ethnic group. The Pohar Perme estimator was implemented using stns [17] in Stata version 15 (StataCorp, College Station, Texas, USA).
We produced survival estimates for five age groups at diagnosis (15‐44, 45‐54, 55‐64, 65‐74, and 75‐99 years) and obtained age‐standardized estimates for all ages combined, using the International Cancer Survival Standard (ICSS) weights [18]. We did not estimate survival if fewer than ten patients were available for analysis. If 10‐49 patients were available in a given calendar period, we estimated survival for all ages combined. If 50 or more patients were available, we attempted survival estimation for each age group. If an age‐specific estimate could not be produced, data for adjacent age groups were pooled, and the re‐estimated survival was used for both age groups. If two or more age‐specific estimates could not be produced, we reported only the unstandardized estimates for all ages combined. We did not merge data between consecutive calendar periods.
The pooled estimates for countries with more than one registry do not include data from registries for which the estimates were considered less reliable. Less reliable estimates for a given country are shown with a flag in figures and tables when they are the only available information from a given country or territory. A survival estimate is considered less reliable if 15% or more patients were either lost to follow‐up or excluded because they were registered only from a death certificate or autopsy or registered with unknown vital status or incomplete dates. Detailed quality control indicators can be found for each registry that participated in CONCORD‐3 in the web appendix available online (https://doi.org/10.1016/S0140‐6736(17)33326‐3).
When examining trends in the distribution of sub‐site or sub‐type, we refer to increases or decreases in the proportion. Increases or decreases in the survival probabilities (%) are described in absolute terms.
We excluded 10,619 (1%) patients for whom the tumor morphology was unknown. Of the remaining 732,695 patients whose tumor morphology was known, we included all tumors reported by the registry as morphologically verified (684,821; 93%). Of the 40,465 (6%) tumors reported as not morphologically verified, we included 8,169 tumors with a specific ICD‐O‐3 morphology code (i.e., any code except 8000‐8005) as a specific morphology code implied morphological verification had been completed. Of the 7,409 (1%) tumors coded as unknown whether morphological verification had been completed, we included 3,984 tumors for which a specific morphological code was available.
2.3. Patient and public involvement
The CONCORD Steering Committee has included cancer patients since 2000. However, patients were not involved directly in the study design of this manuscript.
3. RESULTS
We analyzed survival with data for 696,974 adults from 288 population‐based cancer registries in 60 countries.
3.1. Distribution of anatomical sub‐sites
Patients with tumors in the lower third of the esophagus comprised 38% (n = 265,159) of those diagnosed during 2000‐2014, with the middle third accounting for 22% (n = 156,185) of the patients and the upper third for 8% (n = 52,927). For a further one‐third of patients (n = 222,703; 32%), the tumors were in overlapping sub‐sites or the sub‐site was not specified (NOS).
The sub‐site distribution varied between countries and by sex (Figure 1, Supplementary Table S2). For adults diagnosed during 2010‐2014, overlapping sub‐site and NOS tumors were the most frequent in 30 countries (Algeria, Mauritius, South Africa, 7 countries in Central and South America, 9 in Asia, and 11 in Europe). Tumors of the lower third were the most common in 21 countries (Puerto Rico, Canada and the US, Turkey, 15 countries in Europe, and Australia and New Zealand), while the middle third was the most common sub‐site in Guadeloupe, Japan, Korea, Singapore, Taiwan, and Russia.
FIGURE 1.
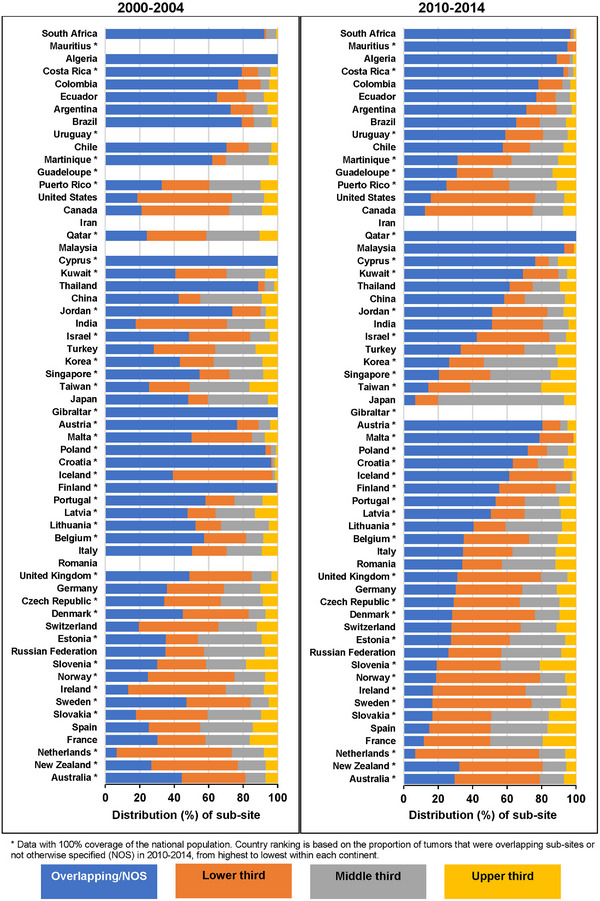
Distribution of anatomic sub‐site by country and calendar period of diagnosis: adults (15‐99 years) diagnosed with esophageal cancer.
* Data with 100% coverage of the national population. Country ranking is based on the proportion of tumors overlapping sub‐sites or not otherwise specified (NOS) in 2010‐2014, from highest to lowest within each continent.
The proportion of patients diagnosed with a tumor assigned to overlapping sub‐sites or NOS in 2010‐2014 was lower than the proportion for 2000‐2004. Correspondingly, the proportion of patients diagnosed with tumors in the middle or lower third of the esophagus increased over time (Figure 1, Supplementary Table S2). Given the high proportion of tumors assigned to an overlapping anatomic sub‐site or NOS, we focused on survival by morphological sub‐type.
3.2. Distribution of morphological sub‐type
Almost all esophageal tumors included in analyses (n = 680,709, 98%) had been coded to a specific morphology. Squamous cell carcinoma was the commonest morphological sub‐type worldwide, representing 53% of all esophageal tumors. Adenocarcinoma was the second most common (38%), while unspecified carcinoma (5%), other specified carcinomas (2%) and non‐specific tumors (2%) were rare. Other specified non‐carcinomas (0.2%) and sarcomas/other soft tissue tumors (0.1%) were extremely rare (Supplementary Table S3).
The distribution of morphological sub‐types differed between countries and by sex (Figure 2). In all participating countries in Africa, Central and South America and Asia, and in 20 European countries, squamous cell carcinoma was the commonest sub‐type. Among these 48 countries, the proportion of squamous cell carcinomas increased over time in 14 countries, with the largest increase in China (11%; from 66.6% of all tumors in 2000‐2004 to 77.3% in 2010‐2014) (Supplementary Table S3). Adenocarcinoma was the commonest sub‐type in Canada and the United States, eight European countries, Australia and New Zealand. In all of these 12 countries, the proportion of adenocarcinoma increased over time. Additionally, in 37 of 48 countries where squamous cell carcinoma was the most common subtype, the proportion of adenocarcinoma also increased over time, with the largest increase in Kuwait (17%; from 18.5% in 2000‐2004 to 35.9% in 2010‐2014).
FIGURE 2.
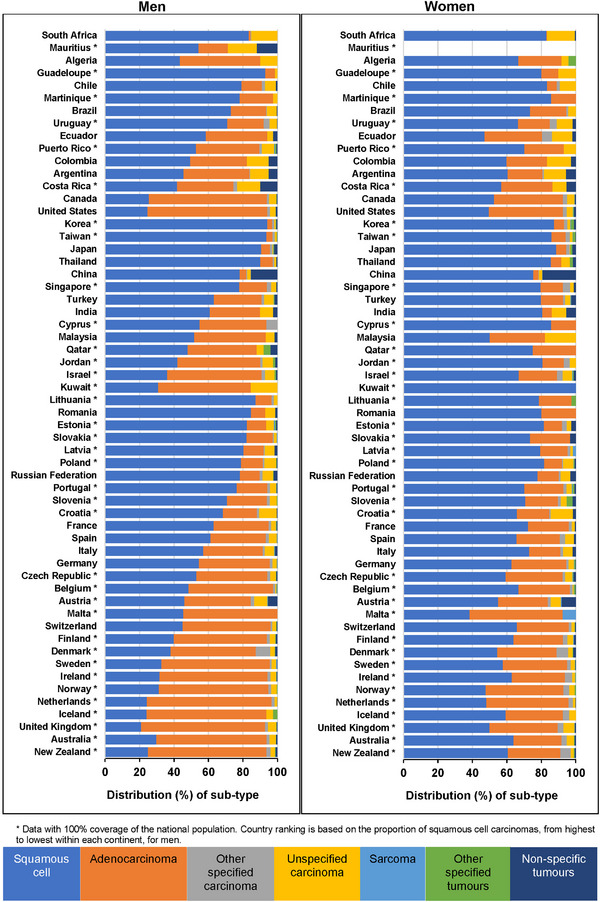
Distribution of morphological sub‐types by sex and country: adults (15‐99 years) diagnosed with esophageal cancer during 2010‐2014.
* Data with 100% coverage of the national population. Country ranking is based on the proportion of squamous cell carcinomas, from highest to lowest within each continent, for men.
In 39 of the 58 countries providing data for adults diagnosed during 2010‐2014, squamous cell carcinoma was the commonest morphological sub‐type for both men and women (Figure 2, Supplementary Table S4 [men], and Supplementary Table S5 [women]). In a further 18 countries, the distribution differed between men and women, with adenocarcinoma the most common sub‐type for men and squamous cell carcinoma the most common sub‐type for women. Malta was the only country where adenocarcinoma was the commonest sub‐type for both men and women (Figure 2, Supplementary Table S4 [men], and Supplementary Table S5 [women]).
3.3. Sex‐specific survival
Age‐standardized five‐year survival from esophageal cancer for all tumors combined was around 5% higher in women than in men, though there was wide global variation in survival by sex (Supplementary Table S6). For men diagnosed during 2010‐2014, the five‐year survival ranged from 3.5% (95% confidence interval (CI): 1.8%‐5.2%) in Lithuania to 34.8% (95% CI: 33.4%‐36.1%) in Japan. For women, the five‐year survival ranged from 7.1% (95% CI: 2.4%‐11.9%) in Latvia to 42.6% (95% CI: 39.7%‐45.5%) in Japan.
Age‐standardized five‐year survival from all esophageal tumors combined generally increased over time for both women and men (Supplementary Table S6). For men, survival increased by less than 5% in most countries, although in South Korea the increase reached 13.3%. In Russia, survival decreased slightly over time, from 11.1% (95% CI: 8.0%‐14.2%) in 2000‐2004 to 7.5% (95% CI: 5.6%‐9.3%) in 2010‐2014. For women, survival increased slightly in most countries (around 5%), with the largest increase in Israel (20.2%). A slight decrease in survival was seen in Finland: five‐year survival was 20.0% (95% CI: 15.3%‐24.7%) in 2000‐2004, which then decreased to 14.3% (95% CI: 10.6%‐18.1%) in 2010‐2014.
3.4. Survival by anatomical sub‐site
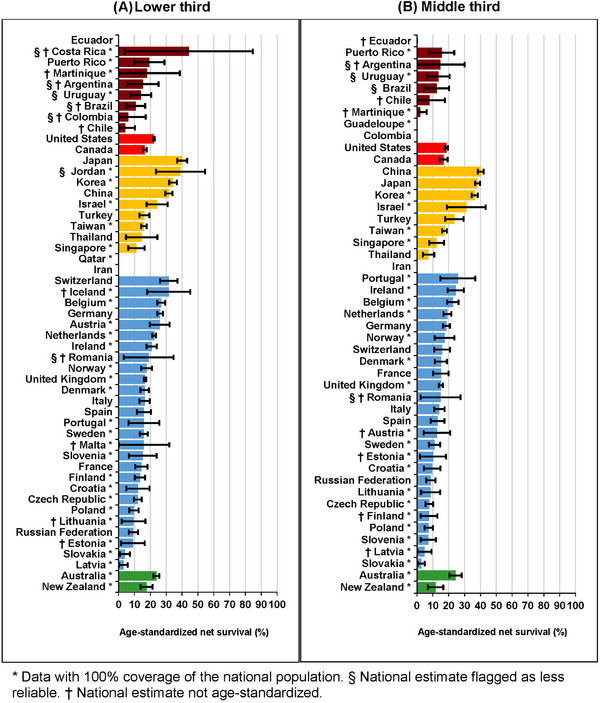
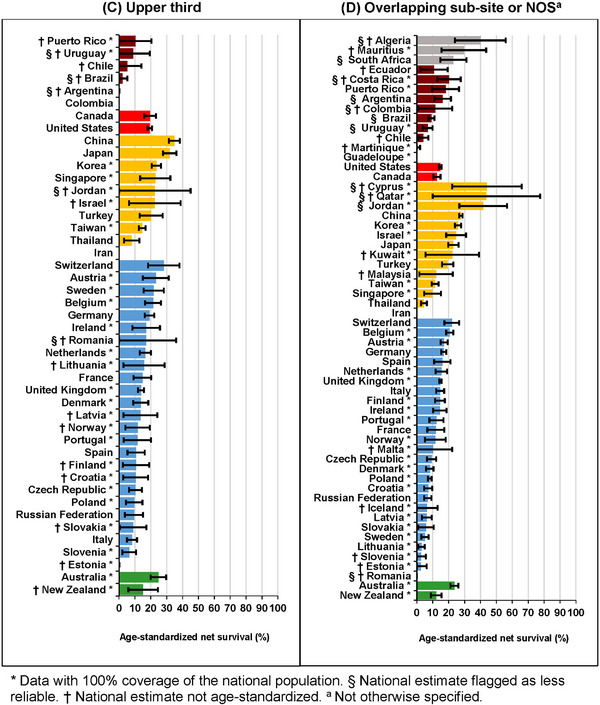
Five‐year survival for adults diagnosed with a tumor of the lower third was highest in Japan (40.1%, 36.9%‐43.3%) and lowest in Latvia (3.0%, 0.3%‐5.8%) (Figure 3a, Supplementary Table S7). Survival from tumors in the middle third during 2010‐2014 was highest in China (40.2%, 95% CI: 38.4%‐42.1%) and lowest in Slovakia (2.8%, 0.6%‐5.0%) (Figure 3b, Supplementary Table S6). For tumors of the upper third, the five‐year survival was highest in China (34.8%, 31.3%‐38.3%) and lowest in Slovenia (6.4%, 2.1%‐10.7%) (Figure 3c, Supplementary Table S6), while for tumors in overlapping sub‐sites or NOS, the five‐year survival was highest in China (27.7%, 26.6%‐28.8%), and lowest in Lithuania (2.9%, 0.9%‐4.9%) (Figure 3d, Supplementary Table S7).
FIGURE 3.
Age‐standardized five‐year net survival (%) by anatomic sub‐site and country: adults (15‐99 years) diagnosed with esophageal cancer during 2010‐2014. (A) Age‐standardized five‐year net survival (%) by country: adults (15‐99 years) diagnosed with tumors of the lower third of the esophagus during 2010‐2014. (B) Age‐standardized five‐year net survival (%) by country: adults (15‐99 years) diagnosed with tumors of the middle third of the esophagus during 2010‐2014. (C) Age‐standardized five‐year net survival (%) by country: adults (15‐99 years) diagnosed with tumors of the upper third of the esophagus during 2010‐2014. (D) Age‐standardized five‐year net survival (%) by country: adults (15‐99 years) diagnosed with tumors overlapping sub‐sites or not otherwise specified (NOS) during 2010‐2014.
* Data with 100% coverage of the national population. § National estimate flagged as less reliable. † National estimate not age‐standardized. a Not otherwise specified. The different colors represent the types of countries in terms of continental/geographical location, e.g., Africa, South America, North America, Asia, Middle East, Europe, etc.
Age‐standardized five‐year survival by sub‐site generally followed the same patterns in men and women: highest for tumors of the lower and middle thirds and lowest for tumors of the upper third or for tumors of overlapping sub‐site or NOS. The highest levels of survival in both men and women were generally seen in Asia (Supplementary Table S6 [all sub‐sites combined, upper third, middle third] and Supplementary Table S7 [lower third and overlapping or esophagus, NOS]).
3.5. Survival by morphological sub‐type
Age‐standardized one‐year survival from squamous cell carcinoma varied widely worldwide. For adults diagnosed in 2010‐2014, the one‐year survival was highest in Japan (67.7%, 95% CI: 66.3%‐69.1%) and lowest in Lithuania (21.8%, 17.0%‐26.6%) (Figure 4, Supplementary Table S8). One‐year survival from adenocarcinoma was similar, also with a wide international variation. For adults diagnosed in 2010‐2014, the one‐year survival was highest in Japan (69.0%, 64.7%‐73.3%) and lowest in Slovakia (25.0%, 13.3%‐36.7%). One‐year survival estimates for the less common morphological sub‐types can be found in Supplementary Table S9 and Supplementary Table S10.
FIGURE 4.
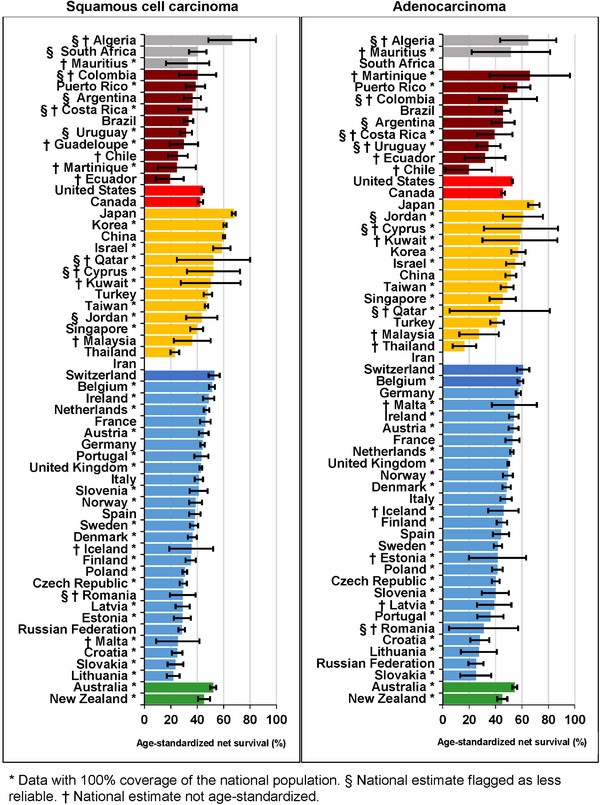
Age‐standardized one‐year net survival (%) by morphological sub‐type and country: adults (15‐99 years) diagnosed with esophageal cancer during 2010‐2014.
* Data with 100% coverage of the national population. § National estimate flagged as less reliable. † National estimate not age‐standardized. The different colors represent the types of countries in terms of continental/geographical location, e.g., Africa, South America, North America, Asia, Middle East, Europe, etc.
Age‐standardized five‐year survival from squamous cell carcinoma was generally around 15%‐20% (Supplementary Table S11). For adults diagnosed in 2010‐2014, the five‐year survival was highest in Japan (37.6%, 95% CI: 36.2%‐38.9%) and lowest in India (2.0%, 95% CI: 0.0%‐4.6%). For adenocarcinomas, age‐standardized five‐year survival was similar but with less worldwide variation (Figure 5, Supplementary Table S6). The highest survival for patients diagnosed during 2010‐2014 was seen in Japan (37.5%, 95% CI: 32.7%‐42.3%) and the lowest in Russia (9.0%, 95% CI: 5.1%‐12.9%). Five‐year survival estimates for the other morphological sub‐types can be found in Supplementary Table S12 and Supplementary Table S13.
FIGURE 5.
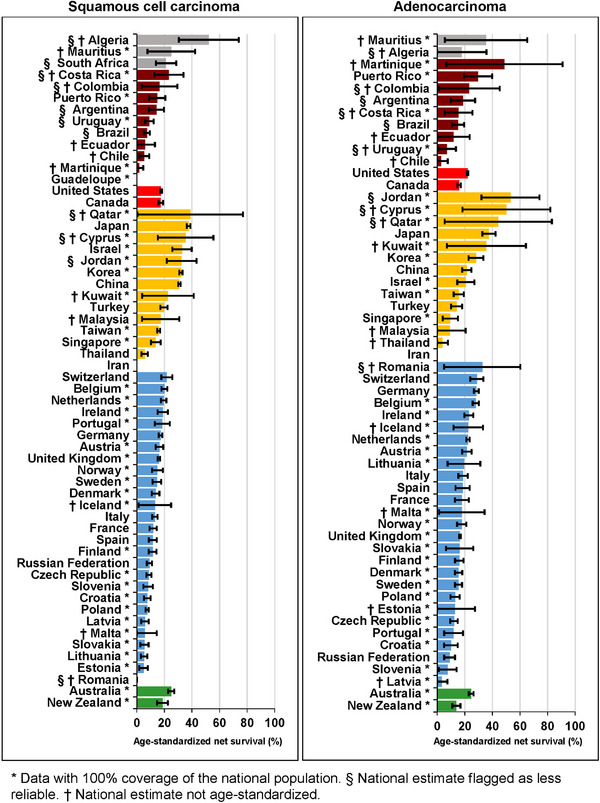
Five‐year net survival (%) by morphological sub‐type and country: adults (15‐99 years) diagnosed with esophageal cancer during 2010‐2014.
* Data with 100% coverage of the national population. § National estimate flagged as less reliable. † National estimate not age‐standardized. The different colors represent the types of countries in terms of continental/geographical location, e.g., Africa, South America, North America, Asia, Middle East, Europe, etc.
One‐ and five‐year survival from both squamous cell carcinoma and adenocarcinoma were generally higher for women than men (Supplementary Table S8 [one‐year survival] and Supplementary Table S11 [five‐year survival]). During 2010‐2014, one‐year survival from squamous cell carcinoma reached 67.1% (95%CI: 65.5%‐68.8%) for men and 70.0% (95% CI: 67.1%‐72.9%) for women in Japan. There was a wider gap in the five‐year survival from squamous cell carcinoma: 36.2% (95% CI: 34.7%‐37.7%) for men in Japan and 46.4% (95% CI: 37.7‐55.0%) for women in Israel. Survival was lowest for men in Lithuania (one‐year: 20.6%, 95% CI: 15.7%‐25.5%; five‐year: 4.0%, 95% CI: 1.8%‐6.2%) and for women in Thailand (one‐year: 18.0%, 95% CI: 11.3%‐24.7%; five‐year: 6.1%, 95% CI: 2.4%‐9.8%).
For adenocarcinoma, one‐year and five‐year survival estimates were highest for men in Japan (one‐year: 70.5%, 95% CI: 65.8%‐75.1%; five‐year: 37.0%, 95% CI: 31.8%‐42.3%) (Supplementary Table S8 [one‐year survival] and Supplementary Table S11 [five‐year survival]). For women, one‐ and five‐year survival were highest in Belgium (one‐year: 58.2%, 95% CI: 52.6%‐63.8%; five‐year: 28.6%, 95% CI: 23.1%‐34.0%). Survival was lowest for men in Russia (one‐year: 23.5%, 95% CI: 17.0%‐30.0%; five‐year: 7.1%, 95% CI: 4.0%‐10.1%). For women with an adenocarcinoma, one‐year survival was lowest in the Czech Republic (30.2%, 95% CI: 23.3%‐37.1%), and five‐year survival was lowest in France (7.3%, 95% CI: 0.0%‐15.3%).
One‐year survival increased by around 5%‐10% for both squamous cell carcinoma and adenocarcinoma, while five‐year survival increased by less than 5% (Supplementary Table S8 [one‐year survival] and Supplementary Table S11 [five‐year survival]). For squamous cell carcinoma, the greatest improvement in one‐year survival was in Slovenia (15.4% increase), while for five‐year survival, the largest improvement was in Israel (13.8%). One‐ and five‐year survival improved the most in Puerto Rico (one‐year: 23.0%; five‐year: 17.7%) for adenocarcinoma.
One‐year survival from squamous cell carcinoma improved the most for men in Slovenia (18.3%), while, for women, improvements were greatest in Norway (17.2%). There were large increases in five‐year survival from squamous cell carcinoma in Korea (12.9%) for men and in Israel (25.7%) for women. For adenocarcinoma, one‐year survival improved the most for men in Puerto Rico (21.2%) and for women in Norway (13.6%), while five‐year survival improved the most for men in Switzerland (16.1%) and women in Italy (11.5%).
4. DISCUSSION
This study included high‐quality individual records for 696,974 patients diagnosed with esophageal cancer from 288 population‐based cancer registries in 60 countries. It is the largest study to date of trends in esophageal cancer survival by sub‐site, morphology, and sex. We used the same standardized data quality controls and the same robust methods to produce net survival estimates for all countries included in the analyses.
Survival from esophageal cancer remains poor in many countries, regardless of the anatomic sub‐site or morphological sub‐type, despite some improvements during the 15‐year period from 2000 to 2014. The distribution of anatomic sub‐site has changed slightly, with an increase in the proportion of tumors arising in the lower and middle thirds of the esophagus and a decline in the proportion assigned to overlapping sub‐sites or sub‐sites not otherwise specified (NOS). Esophageal cancer is generally diagnosed at endoscopy, with biopsies taken for pathological confirmation [3]. As diagnostic techniques improve, fewer patients should be diagnosed with a non‐specific sub‐site. However, 37 of the 58 countries providing data for 2010‐2014 still coded 30% or more of tumors to overlapping or unspecified sub‐sites. This indicates that adequate diagnostic techniques are either not routinely available or are not routinely used in pathological reports of the anatomic sub‐site.
The distribution of morphological sub‐types has also changed. The proportion of squamous cell carcinomas has fallen in most countries, but there were increases in some African, Asian, and Eastern European countries, with the largest increases in China (11%) and South Africa (10%). Conversely, the proportion of adenocarcinomas has increased in most countries, with the largest increases in Kuwait (17%) and Finland (14%). These results confirm that while squamous cell carcinoma has historically been the most common morphological sub‐type worldwide, especially in low‐ and middle‐income countries, adenocarcinoma is becoming more common in most high‐income countries [3, 8].
If we disregard tumors that were coded as not morphologically verified or unknown whether or not they were morphologically verified, the proportion coded to a non‐specific morphology was less than 5% in 53 of 58 countries with data for 2010‐2014. The proportion of tumors of non‐specific morphology (ICD‐O‐3 codes 8000‐8005) remained relatively stable in most countries, though there were large decreases in China, Japan, and Latvia. In these three countries, the proportions of squamous cell carcinoma and adenocarcinoma increased, suggesting improvement in diagnostic techniques. However, for 51 of the 60 countries, the proportions of squamous cell carcinoma and adenocarcinoma showed opposite trends, where an increase in one sub‐type corresponded with a decrease in the other. In most of these countries, the proportion of tumors with a non‐specific morphology code was less than 2% and remained stable over time. Thus, the change in the morphology distributions over time for most countries is less likely to be attributable to improvement in the quality of pathological reporting or changes in the definition of the morphological sub‐types and more likely to a true shift in morphological types, in turn presumably attributable to a change in the prevalence of the different risk factors for each sub‐type of esophageal cancer [4, 19]. Given the availability of more detailed information on morphology, it may be more beneficial to examine trends in survival by morphological sub‐type than by anatomic sub‐site.
Smoking is a major risk factor for squamous cell carcinoma. In low‐ and middle‐income countries where smoking is still common, the proportion of squamous cell carcinoma continues to increase. However, smoking cessation can quickly reduce the risk of developing squamous cell carcinoma, and in countries where smoking cessation programs have been developed, the proportion of these tumors is decreasing [3, 4]. By contrast, risk factors for adenocarcinoma, such as obesity and gastro‐esophageal reflux, are increasing in high‐income countries; and this may explain the increase in adenocarcinomas in these regions [3]. Examining the trends in the incidence rates of the various sub‐types may help explain further the changing distribution over time.
Squamous cell carcinomas generally develop in the middle third of the esophagus, while adenocarcinomas tend to develop in the lower third [4]. Thus, the increasing proportion of tumors in the middle third in some Asian countries corresponds to the increase in squamous cell tumors. Similarly, the increase in tumors of the lower third in Central and South America, North America, Europe, and Oceania corresponds with the increase in adenocarcinoma in these regions. There may be some misclassification of adenocarcinomas in the gastro‐esophageal junction, with some esophageal tumors reported as arising in the stomach and vice versa. Though the impact of this misclassification is most likely to be small, it may have diminished the increasing trend in adenocarcinomas [20, 21].
While the distributions of anatomic sub‐site and morphological sub‐type vary worldwide, they do not appear to explain fully the international variations in survival for all esophageal cancers combined. Five‐year survival for adults with a middle or lower third tumor was higher (15%‐20%) than those with tumors of the upper third or overlapping sub‐sites (5%‐15%). While Asian countries had high proportions of tumors of the middle third, tumors of the lower third were more common in North America, Europe, and Oceania. Thus, the high survival for all sub‐sites combined in Asia is not explained by the high proportion of tumors in the middle third.
A similar conclusion can be drawn for the distribution of morphological sub‐type. One‐year survival from both squamous cell carcinoma and adenocarcinoma was generally around 30%‐50%, while five‐year survival ranged from 15%‐20%. One‐ and five‐year survival for each morphological sub‐type was highest in Asia. Thus, a higher proportion of squamous cell carcinomas does not appear to explain the higher levels of esophageal cancer survival in Asia.
Stage at diagnosis, as with many other cancers, is one of the most important prognostic factors of esophageal cancer, and stage‐specific survival has been shown to vary between high‐income countries [22]. Treatment for esophageal cancer will depend on the stage at diagnosis and is generally more effective for early‐stage disease, which can be curable [3]. Esophageal cancer usually presents at an advanced stage, primarily due to a lack of obvious symptoms for early‐stage disease [23]. The most common symptom is difficulty in swallowing, but this only occurs once the tumor is large enough to obstruct passage from the throat to the stomach and is, thus, no longer an early‐stage disease [24].
The high proportion of esophageal tumors diagnosed at an advanced stage could also occur because very few countries conduct population‐based screening programs for esophageal cancer. Despite precursor lesions existing for both squamous cell carcinoma and adenocarcinoma, many countries do not recommend screening at the population level, focusing instead only on high‐risk individuals, including those with Barrett's esophagus [19, 24]. Endoscopic screening at the population level can be costly, while cheaper non‐endoscopic techniques may not be accurate [25, 26].
However, higher survival in some Eastern Asian countries may be partially explained by comprehensive screening programs for esophageal and gastric cancers. Since 1983, gastric cancer screening has been offered to all adults aged 40 years or older in Japan [27, 28]. In 2006, free endoscopic screening was offered to the population in Yangzhong County, China. In this high‐risk population, screening effectively detected early‐stage tumors, which could then be treated with curative intent [29]. In South Korea, endoscopic screening for gastric cancer was incorporated in 1999 as part of the National Cancer Screening Programme [30]. Both countries, as well as other eastern Asian countries with population‐based gastric cancer screening programs, have experienced massive improvements in esophageal cancer survival over the past few decades and have achieved the highest levels of esophageal cancer survival worldwide.
In conclusion, the distributions of esophageal cancer by anatomic sub‐site and morphological sub‐type differ between continents and countries, but international variation in esophageal cancer survival does not appear to be explained by these differences. Further examination of survival by stage at diagnosis and trends in the incidence and mortality rates of the various sub‐site and sub‐types may help explain international variations in esophageal cancer survival.
DISCLOSURES
The interpretation of the findings in this report and the opinions, conclusions, and recommendations are those of the authors and do not necessarily reflect the views or official position of the British Columbia Cancer Agency or Cancer Care Ontario (Canada); the Centers for Disease Control and Prevention, the National Cancer Institute, Maryland Cancer Registry, New Hampshire Department of Health and Human Services, New York City Department of Health and Mental Hygiene, Ohio Department of Health, Pennsylvania Department of Health or West Virginia Cancer Registry (USA); the Health Directorate of the Australian Capital Territory, or the Institut National Du Cancer (France).
AUTHOR CONTRIBUTIONS
Study design: Melissa Matz, Claudia Allemani and Michel P Coleman. Acquisition of statutory and ethical approvals: Michel P Coleman and Claudia Allemani. Funding acquisition: Claudia Allemani and Michel P Coleman. Data quality control: Melissa Matz, Claudia Allemani, Michel P Coleman. Formal analyses: Melissa Matz. Writing original draft: Melissa Matz, Claudia Allemani, Michel P Coleman. Review and editing: All authors checked and contributed to writing the final report. All CONCORD Working Group members had access to the results of all steps of data preparation, quality control and analyses and contributed to the interpretation of the findings.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING INFORMATION
This project was supported by the Institut National du Cancer (2016‐101), La Ligue Contre le Cancer (EPDQJ18280), Centers for Disease Control and Prevention (200‐2017‐96189), Swiss Re, Swiss Cancer Research Foundation, Swiss Cancer League, Rossy Family Foundation, US National Cancer Institute (DAA3‐16‐62868‐1), and the American Cancer Society (35327). The funding sources played no part in the design, data collection, quality control, analysis, interpretation of the findings, writing of the manuscript or the decision to submit for publication. The corresponding author had full access to all the data and responsibility for submission for publication.
ETHICAL APPROVALS
The Cancer Survival Group maintains approval for processing sensitive personal data for the CONCORD programme from the UK's statutory Health Research Authority (reference ECC 3‐04(i)/2011; last update 17 October 2022), the UK National Health Service Research Ethics Service (11/LO/0331; 2 November 2022), and the Ethics Committee of the London School of Hygiene & Tropical Medicine (12171; 1 September 2022).
CONSENT FOR PUBLICATION
Consent for publication is not applicable because the data are anonymized by the source registries.
CONCORD Working Group
Africa—Algeria : S Bouzbid (Registre du Cancer d'Annaba); M Hamdi‐Chérif*, L Kara (Registre du Cancer de Sétif); K Meguenni, D Regagba (Registre du Cancer Tlemcen); Mali : S Bayo, T Cheick Bougadari (Kankou Moussa University); Mauritius : SS Manraj (Mauritius National Cancer Registry); Morocco : K Bendahhou (Registre du Cancer du Grand Casablanca); Nigeria : A Ladipo, OJ Ogunbiyi* (Ibadan Cancer Registry); South Africa : NIM Somdyala (Eastern Cape Province Cancer Registry)
America (Central and South)—Argentina : MA Chaplin, F Moreno (National Childhood Cancer Registry); GH Calabrano, SB Espinola (Chubut Cancer Registry); B Carballo Quintero, R Fita (Registro Provincial de Tumores de Córdoba); WD Laspada (Registro Provincial de Tumores de Mendoza); SG Ibañez (Population Registry of Cancer of the Province Tierra del Fuego); Brazil : CA Lima (Registro de Câncer de Base Populacional de Aracaju); A Mafra Da Costa (Registro de Câncer de Base Populacional da Região de Barretos); PCF De Souza (Registro de Câncer de Base Populacional de Cuiabá); J Chaves, CA Laporte (Registro de Curitiba); MP Curado, JC de Oliveira (Registro de Goiânia); CLA Veneziano, DB Veneziano (Registro de Câncer de Base Populacional de Jaú); ABM Almeida, MRDO Latorre (Registro de Câncer de São Paulo); MS Rebelo, MO Santos (Instituto Nacional de Câncer, Rio de Janeiro); G Azevedo e Silva* (University of Rio de Janeiro); Chile : JC Galaz (Registro Poblacional de Cáncer Region de Antofagasta); M Aparicio Aravena, J Sanhueza Monsalve (Registro Poblacional de Cáncer de la Provincia de Biobio; Registro Poblacional de Cáncer Provincia de Concepción); DA Herrmann, S Vargas (Registro Poblacional Region de Los Rios); Goić* (Magallanes, Chile); Colombia : VM Herrera, CJ Uribe (Registro Poblacional de Cáncer Area Metropolitana de Bucaramanga); LE Bravo, LS Garcia (Cali Cancer Registry); NE Arias‐Ortiz, D Morantes (Registro Poblacional de Cáncer de Manizales); DM Jurado, MC Yépez Chamorro (Registro Poblacional de Cáncer del Municipio de Pasto); Costa Rica : S Delgado, M Ramirez (National Registry of Tumors, Costa Rica); Cuba : YH Galán Alvarez, P Torres (Registro Nacional de Cáncer de Cuba); Ecuador : F Martínez‐Reyes (Cuenca Tumor Registry); L Jaramillo, R Quinto (Guayaquil Cancer Registry); J Castillo (Loja Cancer Registry); M Mendoza (Manabí Cancer Registry); P Cueva, JG Yépez (Quito Cancer Registry); France : B Bhakkan, J Deloumeaux (Registre des cancers de la Guadeloupe); C Joachim, J Macni (General Cancer Registry of Martinique); Mexico : R Carrillo, J Shalkow Klincovstein (Centro Nacional para la Salud de la Infancia y la Adolescencia); R Rivera Gomez (Registro Poblacional de Cancer Region Fronteriza Norte de Mexico Zona Tijuana); Peru : P Perez, E Poquioma (Lima Metropolitan Cancer Registry); Puerto Rico : G Tortolero‐Luna, D Zavala (Puerto Rico Central Cancer Registry); Uruguay : R Alonso, E Barrios (Registro Nacional de Cáncer)
America (North)—Canada : A Eckstrand, C Nikiforuk (Alberta Cancer Registry); RR Woods (British Columbia Cancer Registry); G Noonan, D Turner* (Manitoba Cancer Registry); E Kumar, B Zhang (New Brunswick Provincial Cancer Registry); JJ Dowden, GP Doyle (Newfoundland & Labrador Cancer Registry); N Saint‐Jacques, G Walsh (Nova Scotia Cancer Registry); A Anam, P De (Ontario Cancer Registry); CA McClure, KA Vriends (Prince Edward Island Cancer Registry); C Bertrand, AV Ramanakumar (Registre Québécois du Cancer); L Davis, S Kozie (Saskatchewan Cancer Agency); USA : T Freeman, JT George (Alabama Statewide Cancer Registry); RM Avila, DK O'Brien (Alaska Cancer Registry); A Holt (Arkansas Central Cancer Registry); L Almon (Metropolitan Atlanta Registry); S Kwong, C Morris (California State Cancer Registry); R Rycroft (Colorado Central Cancer Registry); L Mueller, CE Phillips (Connecticut Tumor Registry); H Brown, B Cromartie (Delaware Cancer Registry); J Ruterbusch, AG Schwartz (Metropolitan Detroit Cancer Surveillance System); GM Levin, B Wohler (Florida Cancer Data System); R Bayakly (Georgia Cancer Registry); KC Ward (Georgia Cancer Registry; Metropolitan Atlanta Registry); SL Gomez, M McKinley (Greater Bay Area Cancer Registry); R Cress (Cancer Registry of Greater California); J Davis, B Hernandez (Hawaii Tumor Registry); CJ Johnson, BM Morawski (Cancer Data Registry of Idaho); LP Ruppert (Indiana State Cancer Registry); S Bentler, ME Charlton (State Health Registry of Iowa); B Huang, TC Tucker* (Kentucky Cancer Registry); D Deapen, L Liu (Los Angeles Cancer Surveillance Program); MC Hsieh, XC Wu (Louisiana Tumor Registry); M Schwenn (Maine Cancer Registry); K Stern (Maryland Cancer Registry); ST Gershman, RC Knowlton (Massachusetts Cancer Registry); G Alverson, T Weaver (Michigan State Cancer Surveillance Program); J Desai (Minnesota Cancer Reporting System); DB Rogers (Mississippi Cancer Registry); J Jackson‐Thompson (Missouri Cancer Registry and Research Center); D Lemons, HJ Zimmerman (Montana Central Tumor Registry); M Hood, J Roberts‐Johnson (Nebraska Cancer Registry); W Hammond, JR Rees (New Hampshire State Cancer Registry); KS Pawlish, A Stroup (New Jersey State Cancer Registry); C Key, C Wiggins (New Mexico Tumor Registry); AR Kahn, MJ Schymura (New York State Cancer Registry); S Radhakrishnan, C Rao (North Carolina Central Cancer Registry); LK Giljahn, RM Slocumb (Ohio Cancer Incidence Surveillance System); C Dabbs, RE Espinoza (Oklahoma Central Cancer Registry); KG Aird, T Beran (Oregon State Cancer Registry); JJ Rubertone, SJ Slack (Pennsylvania Cancer Registry); J Oh (Rhode Island Cancer Registry); TA Janes, SM Schwartz (Seattle Cancer Surveillance System); SC Chiodini, DM Hurley (South Carolina Central Cancer Registry); MA Whiteside (Tennessee Cancer Registry); KL Musonda, SL Pruitt (Texas Cancer Registry); K Herget, C Sweeney (Utah Cancer Registry); J Kachajian (Vermont Cancer Registry); MB Keitheri Cheteri, P Migliore Santiago (Washington State Cancer Registry); SE Blankenship, JL Conaway (West Virginia Cancer Registry); R Borchers, R Malicki (Wisconsin Department of Health Services); J Espinoza, J Grandpre (Wyoming Cancer Surveillance Program); HK Weir*, R Wilson (Centers for Disease Control and Prevention); BK Edwards*, A Mariotto (National Cancer Institute); C Rodriguez‐Galindo* (St. Jude Children's Research Hospital)
Asia—China : N Wang, L Yang (Beijing Cancer Registry); JS Chen, Y Zhou (Changle City Cancer Registry); YT He, GH Song (Cixian Cancer Registry); XP Gu (Dafeng County Center for Disease Control and Prevention); D Mei, HJ Mu (Dalian Centers for Disease Prevention and Control); HM Ge, TH Wu (Donghai County Center for Disease Prevention and Control); YY Li, DL Zhao (Feicheng County Cancer Registry); F Jin, JH Zhang (Ganyu Center for Disease Prevention and Control); FD Zhu (Guanyun Cancer Registry); Q Junhua, YL Yang (Haimen Cancer Registry); CX Jiang (Haining City Cancer Registry); W Biao, J Wang (Jianhu Cancer Registry); QL Li (Jiashan County Cancer Registry); H Yi, X Zhou (Jintan Cancer Registry); J Dong, W Li (Lianyungang Center for Disease Prevention and Control); FX Fu, SZ Liu (Linzhou Cancer Registry); JG Chen, J Zhu (Qidong County Cancer Registry); YH Li, YQ Lu (Sihui Cancer Registry); M Fan, SQ Huang (Taixing Cancer Registry); GP Guo, H Zhaolai (Cancer Institute of Yangzhong City); K Wei (Zhongshan City Cancer Registry); WQ Chen*, W Wei*, H Zeng (The National Cancer Center); Cyprus : AV Demetriou (Cyprus Cancer Registry); Hong Kong : WK Mang, KC Ngan (Hong Kong Cancer Registry); India : AC Kataki, M Krishnatreya (Guwahati Cancer Registry); PA Jayalekshmi, P Sebastian (Karunagappally Cancer Registry); PS George, A Mathew (Trivandrum Cancer Registry); A Nandakumar* (National Centre for Disease Informatics and Research); Iran : R Malekzadeh, G Roshandel (Golestan Population‐based Cancer Registry); Israel : L Keinan‐Boker, BG Silverman (Israel National Cancer Registry); Japan : H Ito, Y Koyanagi (Aichi Cancer Registry); M Sato, F Tobori (Akita Prefectural Cancer Registry); I Nakata, N Teramoto (Ehime Prefectural Cancer Registry); M Hattori, Y Kaizaki (Fukui Cancer Registry); F Moki (Gunma Prefectural Cancer Registry); H Sugiyama, M Utada (Hiroshima Prefecture Cancer Registry); M Nishimura, K Yoshida (Hyogo Prefectural Cancer Registry); K Kurosawa, Y Nemoto (Ibaraki Prefectural Cancer Registry); H Narimatsu, M Sakaguchi (Kanagawa Cancer Registry); S Kanemura (Miyagi Prefectural Cancer Registry); M Naito, R Narisawa (Niigata Prefecture Cancer Registry); I Miyashiro, K Nakata (Osaka Cancer Registry); D Mori, M Yoshitake (Saga Prefectural Cancer Registry); I Oki (Tochigi Prefectural Cancer Registry); N Fukushima, A Shibata (Yamagata Prefectural Cancer Registry); K Iwasa, C Ono (Yamanashi Cancer Registry); T Matsuda* (National Cancer Center); Jordan : O Nimri (Jordan National Cancer Registry); Korea : KW Jung, YJ Won (Korea Central Cancer Registry); Kuwait : E Alawadhi, A Elbasmi (Kuwait Cancer Registry); Malaysia : A Ab Manan (Malaysia National Cancer Registry); F Adam (Penang Cancer Registry); Mongolia : E Nansalmaa, U Tudev (Cancer Registry of Mongolia); C Ochir (Mongolian National University of Medical Sciences); Qatar : AM Al Khater, MM El Mistiri (Qatar Cancer Registry); Singapore : GH Lim, YY Teo (Singapore Cancer Registry); Taiwan : CJ Chiang, WC Lee (Taiwan Cancer Registry); Thailand : R Buasom, S Sangrajrang (Bangkok Cancer Registry); K Suwanrungruang, P Vatanasapt (Khon Kaen Provincial Cancer Registry); K Daoprasert, D Pongnikorn (Lampang Cancer Registry; Lamphun Cancer Registry); A Leklob, S Sangkitipaiboon (Lopburi Cancer Registry); SL Geater, H Sriplung (Songkhla Cancer Registry); Turkey : O Ceylan, I Kög (Ankara Cancer Registry); O Dirican (Antalya Cancer Registry); T Köse (Bursa Cancer Registry); T Gurbuz (Edirne Cancer Registry); FE Karaşahin, D Turhan (Erzurum Cancer Registry Center); U Aktaş, Y Halat (Eskişehir Cancer Registry); S Eser, CI Yakut (Izmir Cancer Registry); M Altinisik, Y Cavusoglu (Samsun Cancer Registry); A Türkköylü, N Üçüncü (Trabzon Cancer Registry)
Europe—Austria : M Hackl (Austrian National Cancer Registry); Belarus : AA Zborovskaya (Belarus Childhood Cancer Subregistry); OV Aleinikova (Belarusian Research Center for Pediatric Oncology, Hematology and Immunology); Belgium : K Henau, L Van Eycken (Belgian Cancer Registry); Bulgaria : TY Atanasov, Z Valerianova (Bulgarian National Cancer Registry); Croatia : M Šekerija (Croatian National Cancer Registry); Czech Republic : L Dušek, M Zvolský (Czech National Cancer Registry); Denmark : L Steinrud Mørch, H Storm*, C Wessel Skovlund (Danish Cancer Society); Estonia : K Innos, M Mägi (Estonian Cancer Registry); Finland : N Malila, K Seppä (Cancer Society of Finland); France : J Jégu, M Velten (Bas‐Rhin General Cancer Registry); E Cornet, X Troussard (Registre Régional des Hémopathies Malignes de Basse Normandie); AM Bouvier (Registre Bourguignon des Cancers Digestifs); AV Guizard (Registre Général des Tumeurs du Calvados); V Bouvier, G Launoy (Registre des Tumeurs Digestives du Calvados); S Dabakuyo Yonli, ML Poillot (Breast and Gynecologic Cancer Registry of Côte d'Or France); M Maynadié, M Mounier (Hémopathies Malignes de Côte d'Or); L Vaconnet, AS Woronoff (Doubs General Cancer Registry); M Daoulas, M Robaszkiewicz (Finistère Cancer Registry); J Clavel, C Poulalhon (French National Registry of Childhood Hematopoietic Malignancies); E Desandes, B Lacour (National Registry of Childhood Solid Tumors); I Baldi (Gironde Registry of Primary Central Nervous System Tumors); B Amadeo, G Coureau (General Cancer Registry of Gironde Department); A Monnereau, S Orazio (Registre des Hémopathies Malignes de la Gironde); M Audoin, TC D'Almeida (Registre Général des Cancers de Haute‐Vienne); S Boyer, K Hammas (Haut‐Rhin Cancer Registry); B Trétarre (Registre des Tumeurs de l'Hérault); M Colonna, P Delafosse (Registre du Cancer du Département de l'Isère); S Plouvier (Registre Général des Cancers de Lille et de sa Region); A Cowppli‐Bony (Loire‐Atlantique‐Vendée Cancer Registry); F Molinié (Loire‐Atlantique‐Vendée Cancer Registry; French Network of Cancer Registries (FRANCIM)); S Bara (Manche Cancer Registry); O Ganry, B Lapôtre‐Ledoux (Registre du Cancer de la Somme); L Daubisse‐Marliac (Tarn Cancer Registry); N Bossard, Z Uhry (Hospices Civils de Lyon); J Estève (Université Claude Bernard, Lyon); Germany : R Stabenow, H Wilsdorf‐Köhler (Common Cancer Registry of the Federal States); A Eberle, S Luttmann (Bremen Cancer Registry); I Löhden, AL Nennecke (Hamburg Cancer Registry); J Kieschke, E Sirri (Epidemiological Cancer Registry of Lower Saxony); C Behr, C Justenhoven (Rhineland Palatinate Cancer Registry); B Holleczek (Saarland Cancer Registry); N Eisemann, A Katalinic (Schleswig‐Holstein Cancer Registry); Gibraltar : RA Asquez, V Kumar (Gibraltar Cancer Registry); Greece : E Petridou (Nationwide Registry for Childhood Haematological Malignancies and Solid Tumors); Iceland : EJ Ólafsdóttir, L Tryggvadóttir (Icelandic Cancer Registry, Icelandic Cancer Society); Ireland : DE Murray, PM Walsh (National Cancer Registry Ireland); H Sundseth* (European Institute of Women's Health); M Harney* (University of Limerick); Italy : G Mazzoleni, F Vittadello (Registro Tumori Alto Adige); E Coviello, F Cuccaro (Registro Tumori Puglia – Sezione ASL BT); R Galasso (Registro Tumori di Basilicata); G Sampietro (Registro Tumori di Bergamo); A Giacomin† (Piedmont Cancer Registry Provinces of Biella and Vercelli); M Magoni (Registro Tumori Dell'ASL Di Brescia); A Ardizzone (Registro Tumori Brindisi); A D'Argenzio (Caserta Cancer Registry); AA Di Prima, A Ippolito (Integrated Cancer Registry of Catania‐Messina‐Siracusa‐Enna); AM Lavecchia, A Sutera Sardo (Registro Tumori Catanzaro); G Gola (Registro Tumori della Provincia di Como); P Ballotari, E Giacomazzi (Registro Tumori Cremona; Registro Tumori Mantova); S Ferretti (Registro Tumori della Provincia di Ferrara); L Dal Maso, D Serraino (Registro Tumori del Friuli Venezia Giulia); MV Celesia, RA Filiberti (Registro Tumori Regione Liguria); F Pannozzo (Registro Tumori della Provincia di Latina); A Melcarne, F Quarta (Registro Tumori Della Provincia Di Lecce Sezione RTP); A Andreano, AG Russo (Registro Tumori Milano); G Carrozzi, C Cirilli (Registro Tumori della Provincia di Modena); L Cavalieri d'Oro, M Rognoni (Registro Tumori di Monza e Brianza); M Fusco, MF Vitale (Registro Tumori della ASL Napoli 3 Sud); M Usala (Nuoro Cancer Registry); R Cusimano, W Mazzucco (Registro Tumori di Palermo e Provincia); M Michiara, P Sgargi (Registro Tumori della Provincia di Parma); L Boschetti, S Marguati (Cancer Registry of the province of Pavia); G Chiaranda, P Seghini (Registro Tumori Piacenza); MM Maule, F Merletti (Piedmont Childhood Cancer Registry); E Spata, R Tumino (Registro Tumori della Provincia di Ragusa); P Mancuso (Registro Tumori Reggio Emilia); T Cassetti, R Sassatelli (Pancreas Tumor Registry of Reggio Emilia Province); F Falcini (Registro Tumori della Romagna); AL Caiazzo, R Cavallo (Registro Tumori Salerno); D Piras (Registro Tumori Nord Sardegna); F Bella, A Madeddu (Registro Tumori Siracusa); AC Fanetti, S Maspero (Registro Tumori della Provincia di Sondrio); S Carone, A Mincuzzi (Registro Tumori Taranto); G Candela, T Scuderi (Registro Tumori Trapani); MA Gentilini, R Rizzello (Registro Tumori Trento); S Rosso (Piedmont Cancer Registry); A Caldarella, T Intrieri (Registro Tumori della Regione Toscana); F Bianconi (Registro Tumori Umbro di Popolazione); P Contiero, G Tagliabue (Registro Tumori Lombardia, Provincia di Varese); M Rugge, M Zorzi (Registro Tumori Veneto); S Beggiato, A Brustolin (Registro Tumori Della Provincia Di Viterbo); G Gatta (Fondazione IRCCS Istituto Nazionale dei Tumori); R De Angelis (National Centre for Epidemiology); M Vicentini (Italian Association of Cancer Registries (AIRTUM); Registro Tumori Reggio Emilia); R Zanetti* (International Association of Cancer Registries; Piedmont Cancer Registry); F Stracci (Italian Association of Cancer Registries (AIRTUM); Registro Tumori Umbro di Popolazione); Latvia : A Maurina, M Oniščuka (Latvian Cancer Registry); Liechtenstein : M Blum, M Mousavi (Liechtenstein); Lithuania : L Steponaviciene, I Vincerževskienė (Lithuanian Cancer Registry); Malta : MJ Azzopardi, N Calleja (Malta National Cancer Registry); Netherlands : S Siesling, O Visser (Netherlands Cancer Registry, IKNL); Norway : TB Johannesen, S Larønningen (The Cancer Registry of Norway); Poland : M Trojanowski (Wielkopolski Rejestr Nowotworów); P Macek (Świętokrzyski Rejestr Nowotworów); T Mierzwa (Kujawsko‐Pomorski Rejestr Nowotworów); J Rachtan (Małopolski Rejestr Nowotworów); A Rosińska (Łódzki Rejestr Nowotworów); K Kępska (Dolnośląski Rejestr Nowotworów); B Kościańska (Lubelski Rejestr Nowotworów); K Barna (Lubuski Rejestr Nowotworów); U Sulkowska (Mazowiecki Rejestr Nowotworów); T Gebauer (Opolski Rejestr Nowotworów); JB Łapińska (Podlaski Rejestr Nowotworów); J Wójcik‐Tomaszewska (Pomorski Rejestr Nowotworów); M Motnyk (Śląski Rejestr Nowotworów); A Patro (Podkarparcki Rejestr Nowotworów); A Gos (Warmińsko‐Mazurski Rejestr Nowotworów); K Sikorska (Zachodniopomorski Rejestr Nowotworów); M Bielska‐Lasota (National Institute of Public Health, NIH); JA Didkowska, U Wojciechowska (Polish National Cancer Registry); Portugal : G Forjaz de Lacerda, RA Rego (Registo Oncológico Regional dos Açores); B Carrito, A Pais (Registo Oncológico Regional do Centro); MJ Bento, J Rodrigues (Registo Oncológico Regional do Norte); A Lourenço, A Mayer‐da‐Silva (Registo Oncólogico Regional do Sul); Romania : D Coza, AI Todescu (Cancer Institute I. Chiricuta); Russia : MY Valkov (Arkhangelsk Regional Cancer Registry); L Gusenkova, O Lazarevich (Population Cancer Registry of the Republic of Karelia); O Prudnikova, DM Vjushkov (Omsk Regional Cancer Registry); A Egorova, A Orlov (Samara Cancer Regional Registry); LV Pikalova, LD Zhuikova (Population‐Based Cancer Registry of Tomsk); Slovakia : J Adamcik, C Safaei Diba (National Cancer Registry of Slovakia); Slovenia : V Zadnik, T Žagar (Cancer Registry of Republic of Slovenia); A Peterle* (Ljubljana, Slovenia); Spain : M De‐La‐Cruz, A Lopez‐de‐Munain (Basque Country Cancer Registry); A Aleman, D Rojas (Registro Poblacional de Cáncer de la Comunidad Autónoma de Canarias); RJ Chillarón, AIM Navarro (Registro de Cáncer de Cuenca); R Marcos‐Gragera, M Puigdemont (Girona Cancer Registry); M Rodríguez‐Barranco, MJ Sánchez Perez (Granada Cancer Registry); P Franch Sureda, M Ramos Montserrat (Mallorca Cancer Registry); MD Chirlaque López, A Sánchez Gil (Murcia Cancer Registry); E Ardanaz, M Guevara (Registro de Cáncer de Navarra, CIBERESP); A Cañete‐Nieto, R Peris‐Bonet (RETI‐SEHOP, Universidad de Valencia); M Carulla, J Galceran (Tarragona Cancer Registry); F Almela, C Sabater (Comunitat Valenciana Childhood Cancer Registry); Sweden : S Khan, D Pettersson (Swedish Cancer Registry); P Dickman* (Karolinska Institutet, Stockholm); Switzerland : K Staehelin, B Struchen (Basel Cancer Registry); M Blum (East Switzerland Cancer Registry); E Rapiti, R Schaffar (Geneva Cancer Registry); P Went (Cancer Registry Graubünden‐Glarus); SM Mousavi (Cancer Registry Graubünden‐Glarus; East Switzerland Cancer Registry); JL Bulliard, M Maspoli‐Conconi (Registre Neuchâtelois et Jurassien des Tumeurs); BW A van der Linden (Registre Fribourgeois des Tumeurs); CE Kuehni, SM Redmond (Childhood Cancer Registry); A Bordoni, L Ortelli (Registro Tumori Canton Ticino); A Chiolero, I Konzelmann (Registre Valaisan des Tumeurs); S Rohrmann, M Wanner (Cancer Registry Zürich and Zug); United Kingdom : J Broggio, J Rashbass, C Stiller* (National Cancer Registration and Analysis Service England); D Fitzpatrick, A Gavin (Northern Ireland Cancer Registry); DS Morrison, CS Thomson (Scottish Cancer Registry); G Greene, DW Huws (Welsh Cancer Intelligence & Surveillance Unit); M Grayson* (Belfast, UK); H Rawcliffe* (Lancashire, UK); C Allemani*, MP Coleman*, V Di Carlo, F Girardi, M Matz, P Minicozzi, N Sanz, N Ssenyonga (London School of Hygiene & Tropical Medicine); D James* (London, UK); R Stephens* (Patient Advocate, Stevenage)
Oceania—Australia : E Chalker, M Smith (Australian Capital Territory Cancer Registry); J Gugusheff, H You (NSW Cancer Registry); S Qin Li, S Dugdale (Northern Territory of Australia Cancer Registry); J Moore, S Philpot (Queensland Cancer Registry); R Pfeiffer, H Thomas (South Australian Cancer Registry); B Silva Ragaini, AJ Venn (Tasmanian Cancer Registry); SM Evans, L Te Marvelde (Victorian Cancer Registry); V Savietto, R Trevithick (Western Australian Cancer Registry); J Aitken* (Cancer Council Queensland); D Currow* (Cancer Institute NSW); New Zealand : C Fowler, C Lewis (New Zealand Cancer Registry)
* CONCORD Steering Committee
Supporting information
Supporting information
ACKNOWLEDGMENT
The authors have nothing to report.
Matz M, Valkov M, Šekerija M, Luttman S, Caldarella A, Coleman MP, et al. Worldwide trends in esophageal cancer survival, by sub‐site, morphology, and sex: an analysis of 696,974 adults diagnosed in 60 countries during 2000‐2014 (CONCORD‐3). Cancer Commun. 2023;43:963–980. 10.1002/cac2.12457
DATA AVAILABILITY STATEMENT
Research data are not shared due to the ethical and legal constraints that apply to sensitive personal data.
REFERENCES
- 1. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000‐14 (CONCORD‐3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. The Lancet. 2018;391(10125):1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X‐S, et al. Global surveillance of cancer survival 1995‐2009: analysis of individual data for 25,676,887 patients from 279 population‐based registries in 67 countries (CONCORD‐2). The Lancet. 2015;385(9972):977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. The Lancet. 2017;390(10110):2383–96. [DOI] [PubMed] [Google Scholar]
- 4. Schottenfeld D, Fraumeni JF. Cancer epidemiology and prevention. 3rd ed. Oxford; New York: Oxford University Press; 2006. xviii, 1392 p. p. [Google Scholar]
- 5. Crane LM, Schaapveld M, Visser O, Louwman MW, Plukker JT, van Dam GM. Oesophageal cancer in The Netherlands: increasing incidence and mortality but improving survival. European Journal of Cancer. 2007;43(9):1445–51. [DOI] [PubMed] [Google Scholar]
- 6. Dikken JL, Lemmens VE, Wouters MW, Wijnhoven BP, Siersema PD, Nieuwenhuijzen GA, et al. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. European Journal of Cancer. 2012;48(11):1624–32. [DOI] [PubMed] [Google Scholar]
- 7. Gavin AT, Francisci S, Foschi R, Donnelly DW, Lemmens V, Brenner H, et al. Oesophageal cancer survival in Europe: a EUROCARE‐4 study. Cancer Epidemiol. 2012;36(6):505–12. [DOI] [PubMed] [Google Scholar]
- 8. Morgan E, Soerjomataram I, Gavin AT, Rutherford MJ, Gatenby P, Bardot A, et al. International trends in oesophageal cancer survival by histological subtype between 1995 and 2014. Gut. 2021;70(2):234–42 . [DOI] [PubMed] [Google Scholar]
- 9. Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105(1):98–100. [DOI] [PubMed] [Google Scholar]
- 10. Shin A, Won YJ, Jung HK, Kong HJ, Jung KW, Oh CM, et al. Trends in incidence and survival of esophageal cancer in Korea: Analysis of the Korea Central Cancer Registry Database. J Gastroenterol Hepatol. 2018;33(12):1961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sundelof M, Ye W, Dickman PW, Lagergren J. Improved survival in both histologic types of oesophageal cancer in Sweden. Int J Cancer. 2002;99(5):751–4. [DOI] [PubMed] [Google Scholar]
- 12. Fritz AG, Percy C, Jack A, Shanmugaratnam K, Sobin LH, Parkin DM, et al., editors. International Classification of Diseases for Oncology (ICD‐O). First revision of 3rd ed. Geneva: World Health Organisation; 2013. [Google Scholar]
- 13. Cutler SJ, Ederer F. Maximum utilization of the life table method in analyzing survival. J Chronic Dis. 1958;8(6):699–712. [DOI] [PubMed] [Google Scholar]
- 14. Esteve J, Benhamou E, Raymond L. Statistical methods in cancer research. Volume IV. Descriptive epidemiology. IARC Sci Publ. 1994(128):1–302. [PubMed] [Google Scholar]
- 15. Brenner H, Gefeller O. An alternative approach to monitoring cancer patient survival. Cancer. 1996;78:2004–10. [PubMed] [Google Scholar]
- 16. Pohar Perme M, Stare J, Estève J. On estimation in relative survival. Biometrics. 2012;68:113–20. [DOI] [PubMed] [Google Scholar]
- 17. Clerc‐Urmès I, Grzebyk M, Hédelin G. Net survival estimation with stns. Stata Journal. 2014;14:87–102. [Google Scholar]
- 18. Corazziari I, Quinn MJ, Capocaccia R. Standard cancer patient population for age standardising survival ratios. European Journal of Cancer. 2004;40:2307–16. [DOI] [PubMed] [Google Scholar]
- 19. Bytzer P, Christensen PB, Damkier P, Vinding K, Seersholm N. Adenocarcinoma of the esophagus and ’Barrett's esophagus: a population‐based study. Am J Gastroenterol. 1999;94(1):86–91. [DOI] [PubMed] [Google Scholar]
- 20. Lindblad M, Ye W, Lindgren A, Lagergren J. Disparities in the classification of esophageal and cardia adenocarcinomas and their influence on reported incidence rates. Ann Surg. 2006;243(4):479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McColl KE, Going JJ. Aetiology and classification of adenocarcinoma of the gastro‐oesophageal junction/cardia. Gut. 2010;59(3):282–4. [DOI] [PubMed] [Google Scholar]
- 22. Arnold M, Morgan E, Bardot A, Rutherford MJ, Ferlay J, Little A, et al. International variation in oesophageal and gastric cancer survival 2012‐2014: differences by histological subtype and stage at diagnosis (an ICBP SURVMARK‐2 population‐based study). Gut. 2022;71(8):1532–43. [DOI] [PubMed] [Google Scholar]
- 23. Bird‐Lieberman EL, Fitzgerald RC. Early diagnosis of oesophageal cancer. Br J Cancer. 2009;101(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lao‐Sirieix P, Fitzgerald RC. Screening for oesophageal cancer. Nat Rev Clin Oncol. 2012;9(5):278–87. [DOI] [PubMed] [Google Scholar]
- 26. Yang S, Wu S, Huang Y, Shao Y, Chen XY, Xian L, et al. Screening for oesophageal cancer. Cochrane Database Syst Rev. 2012;12:CD007883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamashima C. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol. 2018;48(7):673–83. [DOI] [PubMed] [Google Scholar]
- 28. Hamashima C SD, Yamazaki H, Inoue K, Fukao A, Saito H, Sobue T. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38(4):259–67. [DOI] [PubMed] [Google Scholar]
- 29. Zheng X, Mao X, Xu K, Lu L, Peng X, Wang M, et al. Massive Endoscopic Screening for Esophageal and Gastric Cancers in a High‐Risk Area of China. PLoS One. 2015;10(12):e0145097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi KS, Jun JK, Suh M, Park B, Noh DK, Song SH, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the National Cancer Screening Programme in Korea. Br J Cancer. 2015;112(3):608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
Research data are not shared due to the ethical and legal constraints that apply to sensitive personal data.


