Abbreviations
- HCC
hepatocellular carcinoma
- IVCTT
inferior vena cava tumor thrombus
- CAR
Chimeric antigen receptor
- GPC3
glypican‐3
- CNLC
china liver cancer staging
- TACE
transcatheter arterial chemoembolization
- MWA
microwave ablation
- GKRS
gamma knife radiosurgery
- AFP
alpha‐fetoprotein
- COVID
coronavirus disease
- MRI
magnetic resonance imaging
- LM
lymphatic metastasis
- RECIST
response evaluation criteria in solid tumors
- CTCs
circulating tumor cells
Dear Editor,
Available evidence regarding the most suitable treatment strategies for hepatocellular carcinoma (HCC) with inferior vena cava tumor thrombus (IVCTT) is extremely limited, and the median overall survival time for these patients after liver resection is only 17.76 months [1]. Other local or systemic treatments for HCC with IVCTT result in a median overall survival time ranging from 5.88 to 15.36 months [1, 2, 3]. Thus, new therapeutic strategies are urgently needed to improve the survival of HCC patients with IVCTT. Chimeric antigen receptor (CAR) T‐cell therapy has seen success in treating B‐cell neoplasms with impressive outcomes [4]. However, this therapy alone has shown limited efficacy on solid tumors, such as HCC [5]. In this study, we put forward a proof‐of‐concept treatment strategy that local therapy plus CAR‐glypican‐3 (CAR‐GPC3) T‐cell therapy might be effective for advanced HCC patients and reported the application of this combination in two GPC3‐positive HCC patients with rapidly progressing IVCTT. The study protocol can be found in the Supplementary Materials. In brief, both patients received local therapy to treat liver lesions and IVCTT, followed by sequential infusions of CAR‐GPC3 T‐cells, and achieved more than 5‐year disease‐free survival and more than 8‐year overall survival.
For case 1, a 50‐year‐old male patient (Patient A) with hepatitis B cirrhosis was diagnosed with Ib‐stage HCC according to China Liver Cancer Staging (CNLC) in December 2014. The patient's liver function was graded as Child‐Pugh A at diagnosis. After two sessions of transcatheter arterial chemoembolization (TACE) resulting in partial response (PR), the patient underwent microwave ablation (MWA) as a radical treatment in March 2015. However, his disease rapidly progressed, and he developed IVCTT within 6 weeks after the MWA treatment (Figure 1A‐B). Considering the financial reason, the patient refused systemic therapy and was voluntarily enrolled in our clinical trial (NCT02395250) and received rescue therapy with MWA for liver lesion and gamma knife radiosurgery (GKRS) for IVCTT right after that (Figure 1A, C). CAR‐GPC3 T‐cells were successfully manufactured as previously described [5] and were administrated from a starting split dose of 0.04 × 108 cells and gradually increased until the final total dose of the split infusion reached 2.32 × 108 cells. A total of 7.10 × 108 CAR‐GPC3 T‐cells were administered (Figure 1D). Promisingly, alpha‐fetoprotein (AFP) rapidly reduced from 1,210 ng/mL to 121 ng/mL at day 14 and gradually returned to the normal range within the next two months (Figure 1D). No tumor recurrence was observed after administration of CAR‐GPC3 T‐cells (Supplementary Figure S1‐S2), and the patient has been free of detectable cancer for more than 5 years without any further treatment, with overall survival time more than 8 years. No CAR‐GPC3 T‐cell‐related toxicities in major organs were observed. Due to the coronavirus disease (COVID)‐19 pandemic, the patient underwent the latest examinations, including a liver ultrasound and AFP test at a local hospital in November 2022, which showed no tumor recurrence and a normal AFP level.
FIGURE 1.
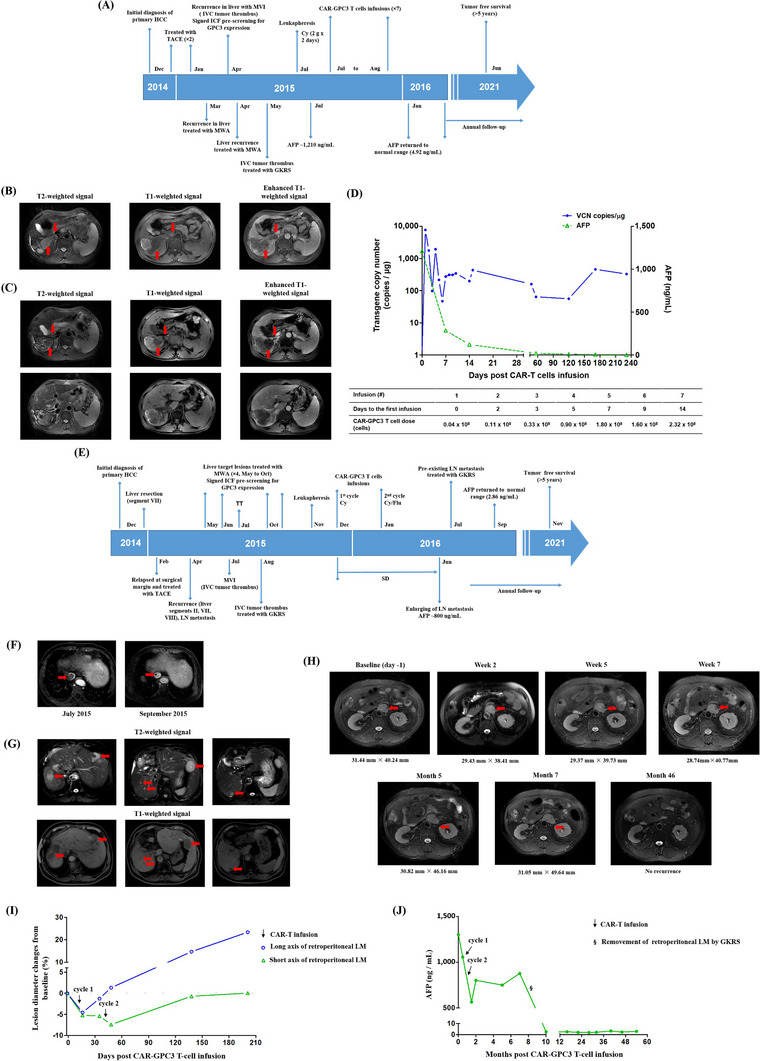
Combined local therapy and CAR‐GPC3 T‐cell therapy in two advanced HCC patients with IVCTT. (A) Overview of disease course and treatment timeline of patient A. (B) MRI images showed intrahepatic tumor complication of inferior vena cava tumor thrombus (April 2015; red arrows). (C) MRI images before administration of CAR‐GPC3 T‐cells. The patient was treated with MWA and GKRS for these lesions (red arrows). No active lesions were shown on T2, T1, and T1 contrast‐enhanced images at the baseline before the patient received CAR‐GPC3 T‐cell infusion (July 2015). (D) DNA was extracted from peripheral blood, and the CAR‐GPC3 transgene copies per microgram of DNA were evaluated by quantitative PCR assay (left y‐axis). AFP level was also monitored (right y‐axis). Dose‐schedule of each CAR‐GPC3 T‐cell infusion is listed below. (E) Overview of disease course and treatment timeline of patient B. (F) MRI scan of vascular invasion before enrollment. Inferior vena cava filling defect was shown on T1 contrast‐enhanced image, indicating tumor thrombus (July 2015; red arrows) and non‐active scar tissue after GKRS treatment (September 2015; red arrows). (G) Multiple liver lesions (red arrows) were shown on T1 and T2 weighted MRI six weeks before the patient received CAR‐GPC3 T‐cell infusion (October 2015). The patient was treated with MWA for these lesions. (H) MRI imaging on retroperitoneal lymphatic metastasis before and after CAR‐GPC3 T‐cell infusions (red arrows). (I) Dynamic changes in the retroperitoneal lymphatic metastasis after CAR‐GPC3 T‐cell infusions. The lesion changes are presented as a percentage based on the baseline value. The green line with triangles represents the changes in the short axis, and the blue line with circles represents the changes in the long axis. The patient remained in SD per RECIST criteria. (J) Reduction of tumor biomarker AFP after CAR‐GPC3 T‐cell infusions. Cycle 1 included six split infusions with a total dose of 40.7 × 108 CAR‐GPC3 T‐cells; Cycle 2 included one infusion with 11.1 × 108 CAR‐GPC3 T‐cells. Abbreviations: CAR‐GPC3, chimeric antigen receptor–glypican‐3; HCC, hepatocellular carcinoma; IVCTT, inferior vena cava tumor thrombus; MRI, magnetic resonance imaging; MWA, microwave ablation; GKRS, gamma knife radiosurgery; DNA, deoxyribonucleic acid; AFP,alpha‐fetoprotein; SD, standard deviation; RECIST, response evaluation criteria in solid tumors; Cy, cyclophosphamide; Flu, fludarabine; MVI, macrovascular invasion; LN, lymph nodes; VCN, vector copy number; TT, tumor thrombus.
For case 2, a 54‐year‐old male patient (Patient B) with hepatitis B cirrhosis was diagnosed with Ib‐stage HCC according to CNLC and underwent surgical resection of the tumor in December 2014. At diagnosis, his liver function was graded as Child‐Pugh A. The tumor recurred at surgical resection margins 6 weeks after surgery in February 2015. One TACE session and two MWA sessions were performed to treat the recurrences between February and June 2015. In July 2015, magnetic resonance imaging (MRI) indicated the disease had rapidly progressed with multifocal lesions in the liver, IVCTT (Figure 1E‐F), and retroperitoneal lymphatic metastasis (LM). The patient received GKRS to treat the IVCTT in August 2015 and two additional MWA sessions as salvage therapy in October 2015 for multiple liver lesions (Figure 1E, G). The patient did not receive systemic therapy for the disease progression because of financial reasons and was voluntarily enrolled in our clinical trial. Two cycles of infusions with a total dose of 51.80 × 108 CAR‐GPC3 T‐cells were administrated between December 2015 and January 2016. The size of the retroperitoneal LM began to decrease 2 weeks after the initial infusion; the diameters of the short axis and the long axis of the lesion were reduced by 5.2% and 4.5%, respectively. The short axis of the target lesion further decreased following the final administration of CAR‐GPC3 T‐cells (by 7.4% at day 7 post‐seventh infusion) (Figure 1H‐I). Simultaneously, the AFP level reduced by 56.6% (from 1,301 ng/mL to 565 ng/mL) when CAR‐GPC3 T‐cells reached peak expansion after the last infusion (Figure 1J). The patient remained at stable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1) for 7 months. However, considering the elevated AFP and enlargement of the retroperitoneal LM shown by MRI in June 2016 (Figure 1H), the patient underwent GKRS to remove the target lesion in July 2016. The AFP level gradually decreased to the normal range post‐procedure, and the patient has experienced cancer‐free status since then (Supplementary Figure S3‐S4). Interestingly, the patient remained disease‐free for more than 5 years without any further anticancer treatment and has an overall survival time of over 8 years. Pyrexia, fatigue, transient leukopenia, thrombocytopenia, and grade 1 cytokine release syndrome were reported during CAR‐GPC3 T‐cell infusions. Due to the COVID‐19 pandemic, the last follow‐up date of patient B was November 2021. At the last telephone follow‐up in March 2023, patient B was reported to be in good physical condition.
To date, CAR T‐cell therapy has given rise to breakthroughs in treating hematological malignancies. However, despite extensive research, their success in treating solid tumors remains limited, probably due to the immune‐suppressive tumor microenvironment and low infiltration of T cells in solid tumors [6]. Therefore, many effects have been made to focus on developing strategies to mitigate tumor antigen heterogeneity and escape immune suppression [7, 8, 9]. In our previous phase I study, we reported the initial safety profile of CAR‐GPC3 T‐cell therapy in patients with advanced HCC [5]. Among them, two patients with IVCTT underwent local therapy to reduce the tumor load prior to CAR T‐cell infusion. Therefore, we report the 5‐year follow‐up data of the two specific patients in our current manuscript. The results support our hypothesis that CAR T‐cells can be used to eliminate the potential microlesion and circulating tumor cells (CTCs), thus avoiding the limitations of the tumor microenvironment in solid tumors. In the first case, despite the absence of MRI‐visible tumors before CAR T‐cell infusion, the high level of AFP suggested potential MRI‐invisible tumors or CTCs. AFP remarkably reduced within 2 weeks and returned to normal within 3 months after CAR T‐cell infusion. In the second case, the MRI‐visible metastatic lesion transiently decreased after CAR‐GPC3 T‐cell therapy and no new lesions occurred. More importantly, both patients had disease‐free survival for more than 5 years. Taken together, the results of the two patients suggest that although CAR T‐cells cannot eliminate the large solid tumor so far, they may have greater potential in treating tumor recurrence and metastasis by killing CTCs. Unfortunately, we did not monitor the changes in CTCs before and after CAR T‐cell therapy which is considered a limitation of this study. Another limitation of our findings is that the sample size is relatively small. The study treatment should be tested in a larger number of patients in the future before it could be considered a therapeutic option for patients with HCC and IVCTT.
In summary, our study demonstrated that local therapy is responsible for removing image‐visible tumors, while the following CAR T‐cell therapy may eliminate image‐invisible lesions and CTCs. This concept should be further confirmed in a larger study.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Bo Zhai, Haojie Jin, Zonghai Li and Yaoping Shi: Conceptualization, Writing‐Original Draft, Supervision, or Funding acquisition. Yaoping Shi, Donghua Shi, Jiachang Chi, Dan Cui, Xiaoyin Tang, Yan Lin, Siying Wang and Bo Zhai: Investigation and Formal analysis. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
Zonghai Li reports a patent on the antibody against glypican‐3 (CN201610626384.7) issued to CARsgen Therapeutics. No potential conflicts of interest were disclosed by the other authors.
FUNDING
This clinical study (NCT02395250) was supported by the Research Fund of the State Key Laboratory of Oncogenes and Related Genes (91‐15‐04), the Program of Shanghai Subject Chief Scientist (No. 16XD1402600), the National Natural Science Foundation (Nos. 81502672, 82222047, 82073039, and 82070619), the Program of Shanghai Academic/Technology Research Leader (No. 22XD1423100), the Shanghai Science and Technology Innovation Action Plan (No. 16DZ1910700), and Shanghai Science and Technology Commission Grant (No. 23ZR1439000).
CONSENT FOR PUBLICATION
Written informed consent for publication was obtained from all participants.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
A phase I clinical study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board of Renji Hospital, affiliated with Shanghai Jiao Tong University. All participants signed the informed consent form. This trial is registered at www.clinicaltrials.gov as NCT02395250.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank the patients and families for their corporation. They also thank the study staff of Dr. Jun Xiao, Ms. Jie Zhang, Ms. Chunyan He, and Ms. Huaying Ruan, Dr. Zhen Liu, Dr. Xiaoou Zhou (CARsgen Therapeutics).
Contributor Information
Zonghai Li, Email: zonghaili@163.com.
Haojie Jin, Email: hjjin1986@126.com.
Bo Zhai, Email: zhaiboshi@sina.com.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article and its supplementary information files or from the corresponding author upon reasonable request.
REFERENCES
- 1. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: a Japanese nationwide survey. Hepatology. 2017;66(2):510–7. [DOI] [PubMed] [Google Scholar]
- 2. Lou J, Li Y, Liang K, Guo Y, Song C, Chen L, et al. Hypofractionated radiotherapy as a salvage treatment for recurrent hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: a multi‐center analysis. BMC Cancer. 2019;19(1):668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rim CH, Jeong BK, Kim TH, Hee Kim J, Kang HC, Seong J. Effectiveness and feasibility of external beam radiotherapy for hepatocellular carcinoma with inferior vena cava and/or right atrium involvement: a multicenter trial in Korea (KROG 17‐10). Int J Radiat Biol. 2020;96(6):759–66. [DOI] [PubMed] [Google Scholar]
- 4. June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MCJS. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–5. [DOI] [PubMed] [Google Scholar]
- 5. Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, et al. Chimeric Antigen Receptor‐Glypican‐3 T‐Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I TrialsPhase I Trials of CAR‐GPC3 T Cells for Advanced HCC. Clin Cancer Res. 2020;26(15):3979–89. [DOI] [PubMed] [Google Scholar]
- 6. Hou AJ, Chen LC, Chen YYJNRDD. Navigating CAR‐T cells through the solid‐tumour microenvironment. Nat Rev Drug Discov. 2021;20(7):531–50. [DOI] [PubMed] [Google Scholar]
- 7. Larson RC, Kann MC, Bailey SR, Haradhvala NJ, Llopis PM, Bouffard AA, et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature. 2022;604(7906):563–70. [DOI] [PubMed] [Google Scholar]
- 8. Martinez M, EKJFii Moon. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. An Z, Hu Y, Bai Y, Zhang C, Xu C, Kang X, et al. Antitumor activity of the third generation EphA2 CAR‐T cells against glioblastoma is associated with interferon gamma induced PD‐L1. Oncoimmunology. 2021;10(1):1960728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files or from the corresponding author upon reasonable request.


