ABSTRACT
This study compared the efficacy of flomoxef with other β-lactam antibiotics against extended-spectrum β-lactamases (ESBL)-producing bacteria of clinical relevance. First, the prevalence and β-lactamase genotypes of ESBL-producing strains among Escherichia coli and Klebsiella pneumoniae isolates collected in Japan from 2004 to 2018 were investigated. High MIC90 values (>64 µg/mL) of ceftriaxone, cefepime, and ceftazidime and low MIC90 values (≤0.06–2 µg/mL) of flomoxef, cefmetazole, and meropenem against both species were observed. Second, a chemostat model was used to analyze the efficacy of humanized regimens of three oxacephem/cephamycin antibiotics (flomoxef, cefmetazole, cefoxitin) and two other antibiotics (meropenem and piperacillin/tazobactam) in suppressing the growth of five ESBL-producing E. coli and two K. pneumoniae strains. Flomoxef, piperacillin/tazobactam, and meropenem showed good bactericidal effects with >4 log10 CFU/mL reduction without bacterial regrowth at 24 h even when the MIC of test isolates was >MIC90. Cefmetazole and cefoxitin resulted in regrowth of test isolates with MIC ≥MIC90 at 24 h. Cefmetazole, cefoxitin, flomoxef, and meropenem showed increased MICs for regrown samples. A clear relationship between the proportion of time that the free drug concentration exceeded the MIC (%fT>MIC) and antibiotic efficacy was found for flomoxef, cefoxitin, and cefmetazole, and flomoxef had the highest %fT>MIC, whereas discrepancies between Clinical and Laboratory Standards Institute breakpoint and bactericidal activity were observed for cefmetazole. Flomoxef was effective in preventing the growth of all ESBL-producing strains, even those with an MIC eight times the MIC90. Thus, flomoxef may be a good alternative to meropenem in context of carbapenems sparing stewardship.
KEYWORDS: extended-spectrum beta-lactamases, antimicrobial, in vitro chemostat model, flomoxef, carbapenem sparing
INTRODUCTION
The emergence and spread of extended-spectrum β-lactamases (ESBL) among human pathogens have become a serious public health concern worldwide, narrowing the therapeutic options for the treatment of hospital and community-acquired infections (1). ESBL hydrolyze some commonly used β-lactam antibiotics, including penicillin and cephalosporins, and make these drugs ineffective for treating infections. ESBL-producing Enterobacterales have become major multidrug-resistant pathogens in the last two decades, and the multifactorial nature of its expansion poses a major challenge in the efforts to control them (1). Mobile genetic elements are thought to be responsible for ESBL spread (2), and ESBL-producing bacteria are associated with increased mortality rates, longer hospital stays, and increased costs for healthcare systems (3, 4). Therefore, finding adequate therapies to eradicate these infectious agents has become critical.
Previously, TEM- and SHV-type ESBL were the predominant families of ESBL. Currently, cefotaxime (CTX)-M type enzymes are the most commonly found ESBL type with the CTX-M-15 variant being the most prevalent worldwide (5). The increasing prevalence of CTX-M-type ESBL-producing bacteria has become a serious problem. Additionally, acquired AmpC-type β-lactamases are clinically important cephalosporinases for some species of Enterobacterales, as they mediate resistance to cephalothin, cefazolin, cefoxitin, penicillin, and β-lactam/β-lactamase inhibitor (such as amoxicillin/clavulanic acid, ampicillin/sulbactam, and piperacillin/tazobactam) combinations. The treatment options for infections caused by ESBL-producing pathogens are limited because they are resistant to many commonly prescribed antibiotics. Therefore, the antimicrobials effective against ESBL-producing microorganisms need to be developed and tested. The urgency of the development of these antimicrobials was indicated in a pathogens list previously published by the World Health Organization, wherein they have been included in the “critical” category (https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12) and in an antibiotic resistance threats list published by the Centers for Disease Control and Prevention as one of the serious threats (https://www.cdc.gov/drugresistance/biggest-threats.html).
Typically, the first-line choice against ESBL-producing bacteria is a carbapenem (6). However, carbapenem-sparing approaches are gaining acceptance in the context of antimicrobial stewardship programs, as the overuse of these agents has been linked to the development of resistance against carbapenems (7). It has been reported that cephamycins and oxacephems are not degraded by ESBLs (8); therefore, they may be valid options for treating infections caused by ESBL-producing microbes.
The present study aimed to compare, using an in vitro chemostat model, the efficacy of flomoxef (FMOX), an oxacephem antibiotic, and cephamycins against ESBL-producing bacteria of clinical relevance to assess appropriate treatment options other than carbapenems. FMOX was first synthesized in Japan in 1980s and is currently marketed only in East Asia such as Japan, China, South Korea, and Taiwan (9, 10). FMOX is widely active against Gram-positive, Gram-negative, and anaerobic bacteria, and its activity against ESBL-producing bacteria has recently attracted attention (8, 10 - 13). The clinical isolates used in this research were collected during a multicenter study conducted in Japan from 2004 to 2018.
RESULTS
Prevalence of ESBL-producing bacteria and major betaβ-lactamase families
Among Escherichia coli clinical isolates, the prevalence of ESBL-producing strains was revealing a strong increase from 2008 to 2012 (Fig. 1A). The rate of Klebsiella pneumoniae isolates producing ESBL also increased during the period analyzed, but much more slowly, and decreased again in 2018 to reach a value similar to that before 2010. Considering the whole 14-y period analyzed, the frequency of ESBL-producing strains was 14.1% (180/1274) for E. coli and 4.6% (33/720) for K. pneumoniae.
Fig 1.
(A) Prevalence of extended-spectrum β-lactamases (ESBL)-producing Escherichia coli and Klebsiella pneumoniae in Japan. (B) Types of enzymes in ESBL-producing E. coli. (C) Types of enzymes in ESBL-producing K. pneumoniae.
Both ESBL-producing isolates of E. coli and K. pneumoniae mostly belonged to the CTX-M-9 group, followed by the CTX-M-1 group (Fig. 1B and C).
Table 1 shows the susceptibility profile of these ESBL-producing isolates for several antibiotics, and includes the minimum inhibitory concentration (MIC)50 and MIC90 values calculated for each drug. While the high MIC90 values (>64 µg/mL) of ceftriaxone (CTRX), cefepime (CFPM), and ceftazidime (CAZ) against both species were observed, FMOX, cefmetazole (CMZ), cefoxitin (CFX), and meropenem (MEPM) showed lower MIC90 values against both species (0.25–0.5 µg/mL, 4 µg/mL, 16–32 µg/mL, and ≤0.06 µg/mL, respectively). Of note, among 180 ESBL-producing E. coli isolates, only two were AmpC co-producers, and no K. pneumoniae isolate displayed this feature. Though rare, these strains were resistant to cephems and cephamycins, showing relatively high MICs for these antimicrobials. One of these strains simultaneously produced CTX-M-1 and DHA, and the other one produced CTX-M-9 and CMY; both have been included as “CTX-M +AmpC” in Fig. 1B. “Others” in Fig. 1B include other ESBL (SHV) producers, ESBL co-producers (CTX-M-1 group and CTX-M-9 group, CTX-M-9 group and TEM-ESBL, and CTX-M-1 group and SHV-ESBL), and co-producers of CTX-M-1 group and OXA-1. Regarding FMOX, two isolates with high MIC, namely, CMY-type AmpC-producing E. coli (MIC >64 µg/mL) and DHA-type AmpC-producing E. coli (MIC 8 µg/mL), were AmpC producers.
TABLE 1.
MIC distribution against extended-spectrum β-lactamases-producing Escherichia coli and Klebsiella pneumoniae isolated in Japan between 2004 and 2018b
| MIC (μg/mL) | MIC50 | MIC90 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | >64 | (μg/ mL) | (μg/ mL) | %Sa | %Ra |
| ESBL-producing E. coli isolated between 2004 and 2018 (180)b | ||||||||||||||||
| Ceftazidime | 0 | 0 | 0 | 4 | 15 | 26 | 27 | 45 | 19 | 13 | 10 | 21 | 8 | >64 | 40.0 | 35.0 |
| Ceftriaxone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 175 | >64 | >64 | 0 | 100 |
| Cefepime | 0 | 0 | 1 | 1 | 1 | 4 | 15 | 34 | 26 | 24 | 11 | 63 | 32 | >64 | 3.9 | 68.9 |
| Flomoxef | 49 | 76 | 30 | 14 | 5 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 0.12 | 0.5 | NA | NA |
| Cefmetazole | 0 | 0 | 0 | 8 | 72 | 53 | 29 | 13 | 2 | 1 | 2 | 0 | 2 | 4 | 98.3 | 1.1 |
| Cefoxitin | 0 | 0 | 0 | 0 | 1 | 12 | 73 | 55 | 24 | 8 | 5 | 2 | 8 | 16 | 78.3 | 8.3 |
| Piperacillin/tazobactam | 0 | 0 | 0 | 0 | 8 | 86 | 47 | 12 | 14 | 3 | 6 | 4 | 2/4 | 16/4 | 85.0 | 7.2 |
| Meropenem | 179 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ≤0.06 | ≤0.06 | 100 | 0 |
| ESBL-producing K. pneumoniae isolated between 2004 and 2018 (33)b | ||||||||||||||||
| Ceftazidime | 1 | 0 | 0 | 4 | 5 | 5 | 3 | 2 | 3 | 3 | 3 | 4 | 4 | >64 | 54.5 | 39.4 |
| Ceftriaxone | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | 4 | 24 | >64 | >64 | 3.0 | 93.9 |
| Cefepime | 1 | 0 | 0 | 2 | 3 | 4 | 3 | 5 | 3 | 0 | 3 | 9 | 8 | >64 | 30.3 | 45.5 |
| Flomoxef | 16 | 10 | 5 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0.12 | 0.25 | NA | NA |
| Cefmetazole | 0 | 0 | 0 | 7 | 15 | 4 | 5 | 1 | 0 | 1 | 0 | 0 | 1 | 4 | 97.0 | 0 |
| Cefoxitin | 0 | 0 | 0 | 0 | 0 | 14 | 7 | 4 | 4 | 3 | 1 | 0 | 4 | 32 | 75.8 | 12.1 |
| Piperacillin/tazobactam | 0 | 0 | 0 | 0 | 2 | 9 | 6 | 5 | 6 | 2 | 0 | 3 | 4/4 | 32/4 | 66.7 | 15.2 |
| Meropenem | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ≤0.06 | ≤0.06 | 100 | 0 | |
%S and %R were defined based on CLSI guideline M100-Ed32 (15).
ESBL, extended-spectrum beta-lactamases; CLSI, Clinical and Laboratory Standards Institute; S, susceptible; R, resistance; NA, not available; underlined numbers, MIC50; bold numbers, MIC90.
Antimicrobial effects on selected ESBL-producing isolates assessed by a chemostat assay
We used five E. coli and two K. pneumoniae isolates that produced CTX-M-type ESBL to carry out the chemostat assays; Table S2 shows the main characteristics of these seven strains. FMOX and two cephamycin antibiotics, CMZ and CFX, were evaluated because oxacephems and cephamycins are considered to be possible option as carbapenem-sparing therapy due to their activity against ESBL producers (6, 15). MEPM and piperacillin/tazobactam (PIPC/TAZ) were used as a control to compare the potential as carbapenem-sparing therapy. It may be observed that isolates had MIC values similar to those representing MIC90 values for each antibiotic during the entire period of analyses (2004–2018). However, to observe the efficacy of FMOX with MIC above MIC90, we also included one strain of E. coli, SR43056, with MIC value of 4 µg/mL, which is eight-fold higher than the MIC90 for FMOX. Additionally, a strain of K. pneumoniae, SR34688, was used as a positive control to confirm the efficacy of each antibiotic against susceptible isolates.
The daily time course of antibiotic concentrations (free form) in human plasma when administered at their standard regimes and the pharmacokinetic parameters used to predict them is shown in Fig. S1. All drugs peaked at about 1 h after each dose due to the 1 h infusion regimen, and then the concentration diminished slowly up to the next dose. The difference in the free concentration of antibiotics may be partly due to the differences in their protein-binding properties. FMOX, CMZ, and CFX showed similar concentration–time curves; however, FMOX reached higher free concentration levels (μg/mL) than did the other cephamycins.
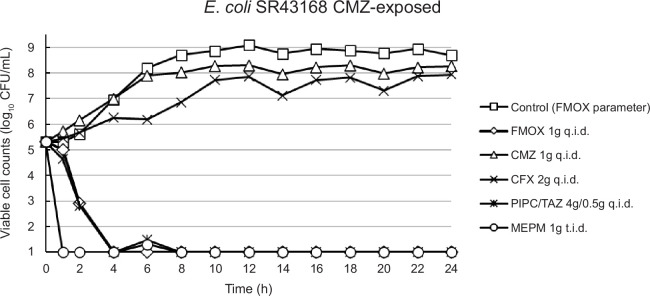
Fig. 2 summarizes the time–kill curves under humanized pharmacokinetic regimens in the chemostat assay. Under human pharmacokinetic reproducible conditions, FMOX 1 g q.i.d. showed good bactericidal effects with >4 log10 CFU/mL reduction and no regrowth at 24 h for all isolates with MIC ≤4 µg/mL. For CFX 2 g q.i.d. and CMZ 1 g q.i.d., bactericidal activity at 24 h was achieved only for one isolate with lower MIC that was used as a positive control and was not achieved for all other test isolates with MIC close to MIC90 (16–32 µg/mL for CFX and 4–16 µg/mL for CMZ). It should be noted that these isolates are defined as intermediate or resistant to CFX, but susceptible to CMZ based on Clinical and Laboratory Standards Institute (CLSI) interpretation based on CLSI guideline M100-Ed32 (14).
Fig 2.
Time course of viable cell counts for seven extended-spectrum β-lactamase-producing clinical isolates in the presence of different antibiotics
From the regrown samples, some colonies were picked up and the MICs of several antibiotic classes were determined through standard broth microdilution method (Table S3). Both CMZ and CFX-exposed E. coli strains showed elevations not only in the MIC values of CMZ and CFX, but also for other β-lactam drugs to which the parent strains had not been exposed, including FMOX and MEPM. Although MIC values of FMOX and MEPM for the CMZ-exposed strain were as high as 4 µg/mL and 0.5 µg/mL, respectively, the maximum clinical dose of FMOX recreated in this model was shown to have a strong antibacterial effect against this strain (Fig. 3).
Fig 3.
Time course of viable cell counts for cefmetazole-exposed strain in the presence of different antibiotics. FMOX, flomoxef; CMZ, cefmetazole; CFX, cefoxitin; PIPC/TAZ, piperacillin/tazobactam; MEPM, meropenem.
Bactericidal effect and %fT>MIC
The overall results from our chemostat assay are summarized in Table 2. A clear relationship between the proportion of time that the free (unbound) drug concentration exceeded the MIC (%fT>MIC) and the efficacy was found through chemostat analysis. FMOX at a dose of 1 g q.i.d. was effective in preventing the growth of all ESBL-producing strains tested, even those showing high MIC against this antimicrobial (8 × MIC90). Based on our chemostat model, our results suggest that for FMOX, %fT>MIC of at least 40% is sufficient to reduce >4 log10 CFU/mL of these bacterial populations. However, CMZ applied at its standard dose (1 g q.i.d.) against E. coli strains considered susceptible according to CLSI breakpoints in CLSI guideline (14) (MICs of 4–16 µg/mL) resulted in regrowth, in line with %fT>MIC of <30%. These concentrations were insufficient to suppress bacterial growth and resulted in the emergence of resistance in only 24 h. Furthermore, CFX at 2 g q.i.d. could not suppress the growth of E. coli strains classified as resistant or intermediate and resulted in regrowth, with only a slight bactericidal activity against one susceptible strain of K. pneumoniae with <2 log10 CFU/mL reduction, where this antimicrobial achieved around 30% of %fT>MIC. PIPC/TAZ at 4 g/0.5 g q.i.d. showed bactericidal activity in all tested strains, including one resistant strain.
TABLE 2.
Percentage of time the free form of flomoxef, cefmetazole, cefoxitin, and piperacillin/tazobactam above MIC and change of log10 CFU/mL from initial at 24 h against each strain of Escherichia coli and Klebsiella pneumonia
| Change of | ||||||
|---|---|---|---|---|---|---|
| MIC | S/I/Ra | log10 CFU/mL | ||||
| Agent | Strain | ESBLa genotype | (μg/mL) | (CLSI)a | %fT>MICb | from initial at 24 h |
| Flomoxef | K. pneumoniae SR34688 | CTX-M-15 | 0.12 | NAa | 100 | −4.58 |
| 1 g | E. coli SR43132 | CTX-M-28 | 0.25 | NAa | 100 | −4.66 |
| q.i.d. | E. coli SR43168 | CTX-M-14 | 0.25 | NAa | 100 | −4.56 |
| E. coli SR43170 | CTX-M-14 | 0.25 | NAa | 100 | −4.79 | |
| E. coli SR53100 | CTX-M-28 | 0.5 | NAa | 91.6 | −4.70 | |
| K. pneumoniae SR34844 | CTX-M-14 | 0.5 | NAa | 91.6 | −4.80 | |
| E. coli SR43056 | CTX-M-28 | 4 | NAa | 46.6 | −4.66 | |
| E. coli SR43168-CMZ exposed | CTX-M-14 | 4 | NAa | 46.6 | −4.31 | |
| Cefmetazole | K. pneumoniae SR34688 | CTX-M-15 | 1 | Sa | 59.1 | −4.58 |
| 1 g | E. coli SR43132 | CTX-M-28 | 4 | Sa | 28.7 | 3.19 |
| q.i.d. | E. coli SR43170 | CTX-M-14 | 4 | Sa | 28.7 | 2.92 |
| E. coli SR53100 | CTX-M-28 | 4 | Sa | 28.7 | 2.64 | |
| E. coli SR43168 | CTX-M-14 | 8 | Sa | 8.0 | 3.20 | |
| K. pneumoniae SR34844 | CTX-M-14 | 8 | Sa | 8.0 | 2.27 | |
| E. coli SR43056 | CTX-M-28 | 16 | Sa | 0 | 2.87 | |
| E. coli SR43168-CMZ exposed | CTX-M-14 | 32 | Ra | 0 | 2.96 | |
| Cefoxitin | K. pneumoniae SR34688 | CTX-M-15 | 4 | Sa | 32.2 | −1.94 |
| 2 g | E. coli SR43132 | CTX-M-28 | 16 | Ia | 8.8 | 2.38 |
| q.i.d. | E. coli SR43168 | CTX-M-14 | 16 | Ia | 8.8 | 1.86 |
| E. coli SR43170 | CTX-M-14 | 16 | Ia | 8.8 | 0.69 | |
| E. coli SR53100 | CTX-M-28 | 16 | Ia | 8.8 | 2.32 | |
| E. coli SR43056 | CTX-M-28 | 32 | Ra | 0 | 2.17 | |
| K. pneumoniae SR34844 | CTX-M-14 | 32 | Ra | 0 | 2.60 | |
| E. coli SR43168-CMZ exposed | CTX-M-14 | 64 | Ra | 0 | 2.64 | |
| Piperacillin/tazobactam | E. coli SR43056 | CTX-M-28 | 4/4 | Sa | 100 | −4.66 |
| 4 g/0.5 g | E. coli SR43132 | CTX-M-28 | 4/4 | Sa | 100 | −4.66 |
| q.i.d. | E. coli SR43168 | CTX-M-14 | 8/4 | Sa | 92.4 | −4.56 |
| K. pneumoniae SR34688 | CTX-M-15 | 8/4 | Sa | 92.4 | −4.58 | |
| E. coli SR43170 | CTX-M-14 | 16/4 | SDDa | 74.3 | −4.79 | |
| E. coli SR53100 | CTX-M-28 | 16/4 | SDDa | 74.3 | −4.70 | |
| K. pneumoniae SR34844 | CTX-M-14 | 16/4 | SDDa | 74.3 | −4.80 | |
| E. coli SR43168-CMZ exposed | CTX-M-14 | >64/4 | Ra | <37.2 | −4.31 |
S/I/R were defined based on CLSI guideline M100-Ed32 (15). ESBL, extended-spectrum β-lactamases; CLSI, Clinical and Laboratory Standards Institute; S, susceptible; I, intermediate; R, resistance; SDD, susceptible dose-dependent; NA, not available.
%fT>MIC, % time above MIC of free drug.
Summing up, some discrepancies between the susceptible/intermediate/resistant profiles based on CLSI breakpoints in CLSI guideline (14) and the bactericidal activity of CMZ and PIPC/TAZ were revealed through this chemostat assay. The emergence of resistant isolates seems to be associated with insufficient bactericidal activity, but FMOX showed good activity as resistant isolates did not appear. This could be the consequence of a high %fT>MIC for FMOX, which allowed the control of resistant isolates that could have appeared during the therapy.
DISCUSSION
It is well-known that carbapenems have excellent efficacy against ESBL-producing microorganisms, but the excessive use of these agents may result in the emergence of resistance against carbapenems. This study showed that CTX-M-9 and CTX-M-1 were the most prevalent ESBL genotypes among the clinical isolates collected in Japan during a 14 y period, which is consistent with the findings of other reports (16 - 18). The AmpC type β-lactamase genotype was observed at a very low frequency, and FMOX, CMZ, and CFX were not active against these isolates. In our chemostat model, FMOX displayed potent antimicrobial activity against these ESBL-producing isolates. Our findings regarding ESBL producers and MIC of antibiotics are consistent with several other reports on strains isolated from Japan and other countries (11, 16 - 22).
FMOX and CMZ showed a potent antimicrobial activity against most ESBL-producing E. coli and K. pneumoniae strains isolated in Japan, and the MIC90 values of FMOX were the second lowest, following that of MEPM. Based on the MIC distribution profile of CAZ, CFX, CMZ, and FMOX (Table 1), the percentage of susceptible isolates to CAZ was around 50%. The susceptibility percentage for CMZ (MIC ≤16 µg/mL) was around 90%, but based on this result, CMZ seemed to be effective against the isolates with MIC ≤2 µg/mL, suggesting that the susceptibility percentage could be around 50%. FMOX has been shown to be effective against isolates with MIC of up to 4 µg/mL, suggesting that the susceptibility rate was nearly 100%. This sufficient bactericidal effect of >4 log10 CFU/mL reduction against a resistant strain needs further investigations.
Furthermore, positive clinical outcome of MEPM and PIPC/TAZ against infections caused by ESBL-producing Enterobacterales has been reported frequently (6 - 30 - 6). The findings of the MERINO trial (31) have revealed that among patients with E. coli or K. pneumoniae bloodstream infection and CTRX resistance, definitive treatment with PIPC/TAZ compared with MEPM did not result in a noninferior 30 d mortality. Though MEPM showed good efficacy against the strains isolated in our study, carbapenem-sparing therapies are needed to reduce the risk of carbapenem resistance emergence. The combination PIPC/TAZ has been proposed in the context of carbapenem-sparing approaches, but its efficacy has been insufficient in some cases, and MEPM has been prescribed more frequently.
It has been indicated that cephamycins can be used as carbapenem-sparing therapy, but only a little preclinical and clinical information is available on their efficacy against ESBL producers (15, 32). Therefore, we evaluated the efficacy of cephamycins and oxacephem with MICs around MIC90 values against ESBL producers to predict their efficacy against most pathogens in clinical environments by deriving their plasma concentration curves using in vitro culture media. The in vitro pharmacodynamic trial carried out in this study recreated the variations in human blood plasma concentrations when used at the maximum clinical doses. Using multiple ESBL-producing E. coli and K. pneumoniae clinical isolates, our findings revealed that the bactericidal effects of FMOX and PIPC/TAZ were superior to those of CMZ and CFX.
We also found that it is important to ensure that appropriate dosing interval is maintained such that the drug concentration remains above its MIC (%fT>MIC) well-known pharmacokinetics/pharmacodynamics parameter for β-lactam antibiotics to be effective against the infecting pathogen (33, 34). This would ensure appropriate bactericidal effect and prevent the eventual regrowth of more resistant clones, leading to satisfactory patient outcomes.
The CLSI breakpoint for CMZ has not been updated for several years. The findings of our study reveal that the breakpoint of CMZ may not be appropriate because this compound showed no efficacy even against CMZ susceptible isolates and its %fT>MIC did not reach 30% in most cases. The CLSI breakpoint for FMOX has not been defined yet; the breakpoint for latamoxef (4 µg/mL) is generally used as a provisional breakpoint for FMOX in Japan. Our findings reveal that FMOX needs 40% fT>MIC to show bactericidal effect, and at this concentration, its efficacy even on bacteria against which MIC was 4 µg/mL was recorded. Considering the MIC distribution of FMOX against ESBL-producing E. coli and K. pneumoniae obtained in our study (Table 1), FMOX is expected to be effective against 97.8% (176/180) of ESBL-producing E. coli and 100% (33/33) of ESBL-producing K. pneumoniae in our collected isolates. The ratios of strains with FMOX MIC of 4 µg/mL or less among ESBL-producing bacteria in other surveys in Japan and other countries are as follows: 93.5% (29/31) of ESBL-producing E. coli and K. pneumoniae isolated from postoperative intra-abdominal infections in Japan, 92.5% (37/40) of ESBL-producing Enterobacteriaceae isolated from surgical site infections in Japan, 96.6% (112/116) of ESBL-producing E. coli, K. pneumoniae, and Proteus mirabilis isolated from various clinical specimens in China, and 88.6% (156/176) of ESBL-producing E. coli and K. pneumoniae from clinical isolates in Korea (12, 20, 22, 35). Therefore, FMOX 1 g q.i.d. would be effective on most ESBL-producing isolates of clinical significance obtained in Japan and other East Asian countries. The findings of our study should be further verified in in vivo model systems and clinical trials.
Conclusion
There is an unmet medical need for controlling ESBL-producing bacteria effectively. Carbapenem-sparing is recommended to prevent the emergence of carbapenem resistance, and new non-carbapenem drugs are needed for controlling ESBL-producing bacteria. The results of our study suggest that maximum clinical doses of FMOX and PIPC/TAZ are expected to be effective on more than 90% of the ESBL-producing strains responsible for causing infections in Japan. FMOX showed a strong bactericidal activity against ESBL-producing bacteria, for which it is expected to become a promising treatment option, allowing the reduction in the use of carbapenems.
MATERIALS AND METHODS
Test strains used
During a surveillance study conducted by Shionogi & Co., Ltd., clinical isolates were collected from 15 to 17 medical centers in Japan eight times every 2 y between 2004 and 2018, and 1,274 isolates of E. coli and 720 isolates of K. pneumoniae were collected. The medical facilities here are tertiary medical institutions, and one to three facilities were selected from all eight regions in Japan. For each collection, 10 strains/facility/time for E. coli and six strains/facility/time for K. pneumoniae were collected randomly regardless of drug susceptibility and without duplication.
Susceptibility testing and molecular characterization
The MICs of FMOX, CMZ, CFX, PIPC/TAZ, MEPM, CTRX, CFPM, and CAZ were determined using the broth microdilution method, according to the CLSI guideline M07-Ed11 (36). FMOX, CMZ, CFX, PIPC/TAZ, and MEPM, which are known to be active against ESBL-producing isolate, were selected from the viewpoint of carbapenem-sparing strategy (15). On the other hand, CTRX, CFPM, and CAZ, which are substrates of ESBL and can be degraded, were selected in order to investigate the trend of ESBL-producing pathogens in Japan and their degree of resistance to these antibiotics. CLSI breakpoints described in CLSI guideline (14) were used for the interpretation of susceptibility (susceptible/intermediate/resistant) to each isolate except for FMOX, for which CLSI breakpoints are unavailable.
Possible ESBL-producing isolates of E. coli and K. pneumoniae were selected based on the MIC of CTX or CAZ with or without clavulanic acid (in line with the CLSI guidelines) and screened for the presence of the following ESBL genes. The presence of genes encoding Ambler class A ESBL (TEM-type, CTX-M-1 group, CTX-M-2 group, CTX-M-8 group, CTX-M-9 group, and SHV-type) was assessed by polymerase chain reaction (PCR) using the commercial Cica Geneus ESBL Genotype Detection KIT2 (Kanto Chemical Co., Inc., Tokyo, Japan), followed by DNA sequencing. When the addition of 3-aminophenylboronic acid led to shifts in CAZ or CTX MIC values for ESBL-producing isolates, those strains were considered AmpC producers. The presence of genes encoding Ambler class C β-lactamases (CIT family, DHA family, FOX family, ACT family, ACC family, and MOX family) was examined by PCR using the commercial Cica Geneus AmpC Genotype Detection KIT (Kanto Chemical Co., Inc., Tokyo, Japan), followed by DNA sequencing.
Antimicrobial activity assessed using an in vitro chemostat model
Five antibiotic regimes based on the maximum dosages indicated in the package insert of each commercial product (Japan) were selected (FMOX, 1 g q.i.d., 1 h infusion; CMZ, 1 g q.i.d., 1 h infusion; CFX, 2 g q.i.d., 1 h infusion; PIPC/TAZ, 4 g/0.5 g q.i.d., 0.5 h infusion; and MEPM, 1 g t.i.d., 0.5 h infusion), and the plasma variations of the corresponding active ingredients (free forms) over 24 h based on the parameters determined previously (Table S1) in Phase 1 human pharmacokinetic studies (37 - 47) and protein-binding rate of CMZ described in a package insert were recreated to compare their efficacy on five ESBL-producing E. coli (SR43168, SR43170, SR43132, SR53100, and SR43056) and two K. pneumoniae (SR34844 and SR34688) isolates. These isolates were obtained in the surveillance study described previously.
All these strains had the CTX-M-type ESBL. E. coli strains SR43168 and SR43170 had CTX-M-14 (CTX-M-9 group) ESBL and strains SR43132, SR53100, and SR43056 had CTX-M-28 (CTX-M-1 group) ESBL. K. pneumoniae strain SR34844 had CTX-M-14 ESBL and strain SR34688 had CTX-M-15 (CTX-M-1 group) ESBL.
A computer-controlled system (48) was used to recreate the expected time–concentration curves of these antibiotics (as free form) in human plasma and evaluate their effect on bacterial growth following a published protocol (49). Briefly, the test strains at 5 × 105 CFU/mL were inoculated into the bacterial culture bottle containing cation-adjusted Mueller-Hinton broth (CAMHB) and continuously stirred at 37℃. The time–concentration curve of each antibiotic (as free form) was achieved by adding the antibiotics solutions and medium and collecting a portion of bacterial cultures. A control sample without any antibiotic was set with the same parameters as FMOX 1 g q.i.d., draining the bacterial culture and adding fresh medium. Sampling was performed every 2 h. The collected samples were kept at 4°C, diluted, and spread on agar plates. The number of colonies was counted the following day. The lower limit of quantitation was one log10 CFU/mL.
Determination of antibiotics concentrations in the medium
Determination of FMOX, CMZ, CFX, MEPM, PIPC, and TAZ in CAMHB was performed using liquid chromatography-tandem mass spectrometry (LC/MS/MS). The supernatants obtained by protein precipitation of medium sample (20 µL) with methanol/formic acid (1,000:1, by vol., 300 µL) were analyzed by LC/MS/MS. Operating condition details were described in Table S4.
Correlation between bactericidal effect and %fT>MIC
The percentage of time the unbound (free) form of the antibiotics tested exceeded MIC (%fT>MIC) was determined, and the correlation between this parameter and the changes in viable cell numbers after 24 h of antibiotic exposure was evaluated.
ACKNOWLEDGMENTS
Editorial support, in the form of medical writing, assembling tables, and creating high-resolution images based on authors' detailed directions, collating author comments, copyediting, fact checking, and referencing was provided by Editage, Cactus Communications, and funded by Shionogi & Co., Ltd.
Hidenori Yamashiro, Naomi Anan, Miki Takemura, and Yoshinori Yamano are employees of Shionogi & Co., Ltd. Yu Kasamatsu received speaker honoraria from Shionogi, Gilead, Viiv, Janssen, Astellas, and Hitachi High-Tech.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Hidenori Yamashiro, Email: hidenori.yamashiro@shionogi.co.jp.
Ryan K. Shields, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
DATA AVAILABILITY
The datasets generated and/or analyzed in this study are not publicly available, but are available from the corresponding author on a reasonable request.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.00258-23.
Supplemental figures and tables.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Doi Y, Iovleva A, Bonomo RA. 2017. The ecology of extended-spectrum β-lactamases (ESBLs) in the developed world. J Travel Med 24:S44–S51. doi: 10.1093/jtm/taw102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stadler T, Meinel D, Aguilar-Bultet L, Huisman JS, Schindler R, Egli A, Seth-Smith HMB, Eichenberger L, Brodmann P, Hübner P, Bagutti C, Tschudin-Sutter S. 2018. Transmission of ESBL-producing Enterobacteriaceae and their mobile genetic elements—identification of sources by whole genome sequencing: study protocol for an observational study in Switzerland. BMJ Open 8:e021823. doi: 10.1136/bmjopen-2018-021823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pitout JDD. 2010. Infections with extended-spectrum β-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment. Drugs 70:313–333. doi: 10.2165/11533040-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 4. Nelson RE, Hyun D, Jezek A, Samore MH. 2022. Mortality, length of stay, and healthcare costs associated with multidrug-resistant bacterial infections among elderly hospitalized patients in the United States. Clin Infect Dis 74:1070–1080. doi: 10.1093/cid/ciab696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castanheira M, Simner PJ, Bradford PA. 2021. Extended-spectrum β-lactamases: an update on their characteristics, epidemiology and detection. JAC Antimicrob Resist 3:dlab092. doi: 10.1093/jacamr/dlab092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2022. Infectious diseases society of america 2022 guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis 75:187–212. doi: 10.1093/cid/ciac268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corcione S, Lupia T, Maraolo AE, Mornese Pinna S, Gentile I, De Rosa FG. 2019. Carbapenem-sparing strategy: carbapenemase, treatment, and stewardship. Curr Opin Infect Dis 32:663–673. doi: 10.1097/QCO.0000000000000598 [DOI] [PubMed] [Google Scholar]
- 8. Jacoby GA, Carreras I. 1990. Activities of β-lactam antibiotics against Escherichia coli strains producing extended-spectrum β-lactamases. Antimicrob Agents Chemother 34:858–862. doi: 10.1128/AAC.34.5.858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsuji T, Satoh H, Narisada M, Hamashima Y, Yoshida T. 1985. Synthesis and antibacterial activity of 6315-S, a new member of the oxacephem antibiotic. J Antibiot (Tokyo) 38:466–476. doi: 10.7164/antibiotics.38.466 [DOI] [PubMed] [Google Scholar]
- 10. Darlow CA, Hope W. 2022. Flomoxef for neonates: extending options for treatment of neonatal sepsis caused by ESBL-producing Enterobacterales. J Antimicrob Chemother 77:711–718. doi: 10.1093/jac/dkab468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Q, Zhang H, Cheng J, Xu Z, Xu Y, Cao B, Kong H, Ni Y, Yu Y, Sun Z, Hu B, Huang W, Wang Y, Wu A, Feng X, Liao K, Shen D, Hu Z, Chu Y, Lu J, Su J, Gui B, Duan Q, Zhang S, Shao H. 2015. In vitro activity of flomoxef and comparators against Escherichia coli, Klebsiella pneumoniae and proteus mirabilis producing extended-spectrum β-lactamases in China. Int J Antimicrob Agents 45:485–490. doi: 10.1016/j.ijantimicag.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 12. Yang Q, Zhang H, Cheng J, Xu Z, Hou X, Xu Y. 2015. Flomoxef showed excellent in vitro activity against clinically important gram-positive and gram-negative pathogens causing community- and hospital-associated infections. Diagn Microbiol Infect Dis 81:269–274. doi: 10.1016/j.diagmicrobio.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 13. Matsumura Y, Yamamoto M, Nagao M, Komori T, Fujita N, Hayashi A, Shimizu T, Watanabe H, Doi S, Tanaka M, Takakura S, Ichiyama S. 2015. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-β-lactamase-producing Escherichia coli bacteremia. Antimicrob Agents Chemother 59:5107–5113. doi: 10.1128/AAC.00701-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. CLSI . 2023. CLSI supplement M100. In performance standards for antimicrobial susceptibility testing. 32nd ed. Clinical and Laboratory Standards Institute. [Google Scholar]
- 15. Karaiskos I, Giamarellou H. 2020. Carbapenem-sparing strategies for ESBL producers: when and how. Antibiotics (Basel) 9:61. doi: 10.3390/antibiotics9020061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shibasaki M, Komatsu M, Sueyoshi N, Maeda M, Uchida T, Yonezawa H, Inagaki K, Omi A, Matsumoto H, Murotani M, Iwamoto T, Kodaka Y, Kieda H, Tokiwa M, Masuwa B, Kinoshita M, Saito K, Katou M. 2016. Community spread of extended-spectrum β-lactamase-producing bacteria detected in social insurance hospitals throughout Japan. J Infect Chemother 22:395–399. doi: 10.1016/j.jiac.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 17. Hara T, Sato T, Horiyama T, Kanazawa S, Yamaguchi T, Maki H. 2015. Prevalence and molecular characterization of CTX-M extended-spectrum β-lactamase-producing Escherichia coli from 2000 to 2010 in Japan. Jpn J Antibiot 68:75–84. [PubMed] [Google Scholar]
- 18. Sato T, Hara T, Horiyama T, Kanazawa S, Yamaguchi T, Maki H. 2015. Mechanism of resistance and antibacterial susceptibility in extended-spectrum β-lactamase phenotype Klebsiella pneumoniae and Klebsiella oxytoca isolated between 2000 and 2010 in Japan. J Med Microbiol 64:538–543. doi: 10.1099/jmm.0.000057 [DOI] [PubMed] [Google Scholar]
- 19. Komatsu Y, Kasahara K, Inoue T, Lee S-T, Muratani T, Yano H, Kirita T, Mikasa K. 2018. Molecular epidemiology and clinical features of extended-spectrum β-lactamase-or carbapenemase-producing Escherichia coli bacteremia in Japan. PLoS One 13:e0202276. doi: 10.1371/journal.pone.0202276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jung Y, Lee SS, Song W, Kim H-S, Uh Y. 2019. In vitro activity of flomoxef against extended-spectrum β-lactamase–producing Escherichia coli and Klebsiella pneumoniae in Korea. Diagn Microbiol Infect Dis 94:88–92. doi: 10.1016/j.diagmicrobio.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 21. Ngoi ST, Teh CSJ, Chong CW, Abdul Jabar K, Tan SC, Yu LH, Leong KC, Tee LH, AbuBakar S. 2021. In vitro efficacy of flomoxef against extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae associated with urinary tract infections in Malaysia. Antibiotics (Basel) 10:181. doi: 10.3390/antibiotics10020181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takesue Y, Kusachi S, Mikamo H, Sato J, Watanabe A, Kiyota H, Iwata S, Kaku M, Hanaki H, Sumiyama Y, Kitagawa Y, Mizuguchi T, Ambo Y, Konosu M, Ishibashi K, Matsuda A, Hase K, Harihara Y, Okabayashi K, Seki S, Hara T, Matsui K, Matsuo Y, Kobayashi M, Kubo S, Uchiyama K, Shimizu J, Kawabata R, Ohge H, Akagi S, Oka M, Wakatsuki T, Suzuki K, Okamoto K, Yanagihara K. 2018. Antimicrobial susceptibility of pathogens isolated from surgical site infections in Japan: comparison of data from nationwide surveillance studies conducted in 2010 and 2014–2015. J Infect Chemother 24:156–157. doi: 10.1016/j.jiac.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 23. Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. 2013. Community-associated extended-spectrum β-lactamase–producing Escherichia coli infection in the United States. Clin Infect Dis 56:641–648. doi: 10.1093/cid/cis942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE, Antibacterial Resistance Leadership Group . 2015. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis 60:1319–1325. doi: 10.1093/cid/civ003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burgess DS, Hall RG, Lewis JS, Jorgensen JH, Patterson JE. 2003. Clinical and microbiologic analysis of a hospital's extended-spectrum β-lactamase-producing isolates over a 2-year period. Pharmacotherapy 23:1232–1237. doi: 10.1592/phco.23.12.1232.32706 [DOI] [PubMed] [Google Scholar]
- 26. Du B, Long Y, Liu H, Chen D, Liu D, Xu Y, Xie X. 2002. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae bloodstream infection: risk factors and clinical outcome. Intensive Care Med 28:1718–1723. doi: 10.1007/s00134-002-1521-1 [DOI] [PubMed] [Google Scholar]
- 27. Kang C-I, Kim S-H, Park WB, Lee K-D, Kim H-B, Kim E-C, Oh M-D, Choe K-W. 2004. Bloodstream infections due to extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob Agents Chemother 48:4574–4581. doi: 10.1128/AAC.48.12.4574-4581.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paterson DL, Ko W-C, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum β-lactamase production in nosocomial infections. Ann Intern Med 140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008 [DOI] [PubMed] [Google Scholar]
- 29. Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, de Cueto M, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, Martínez-Martínez L, Pitout J, Akova M, Peña C, Molina J, Hernández A, Venditti M, Prim N, Origüen J, Bou G, Tacconelli E, Tumbarello M, Hamprecht A, Giamarellou H, Almela M, Pérez F, Schwaber MJ, Bermejo J, Lowman W, Hsueh P-R, Mora-Rillo M, Natera C, Souli M, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J. 2016. A multinational, preregistered cohort study of β-lactam/β-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:4159–4169. doi: 10.1128/AAC.00365-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Namikawa H, Yamada K, Yamairi K, Shibata W, Fujimoto H, Takizawa E, Niki M, Nakaie K, Oinuma K-I, Niki M, Takemoto Y, Kaneko Y, Shuto T, Kakeya H. 2019. Mortality caused by extended-spectrum β-lactamase–producing Enterobacteriaceae bacteremia; a case control study: alert to Enterobacteriaceae strains with high minimum inhibitory concentrations of piperacillin/tazobactam. Diagn Microbiol Infect Dis 94:287–292. doi: 10.1016/j.diagmicrobio.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 31. Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL, MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN) . 2018. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 320:984–994. doi: 10.1001/jama.2018.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tamma PD, Rodriguez-Bano J. 2017. The use of noncarbapenem β-lactams for the treatment of extended-spectrum β-lactamase infections. Clin Infect Dis 64:972–980. doi: 10.1093/cid/cix034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 34. Craig WA. 2001. Dose the dose matter? Clin Infect Dis 33 Suppl 3:S233–7. doi: 10.1086/321854 [DOI] [PubMed] [Google Scholar]
- 35. Takesue Y, Kusachi S, Mikamo H, Sato J, Watanabe A, Kiyota H, Iwata S, Kaku M, Hanaki H, Sumiyama Y, Kitagawa Y, Nakajima K, Ueda T, Uchino M, Mizuguchi T, Ambo Y, Konosu M, Ishibashi K, Matsuda A, Hase K, Harihara Y, Okabayashi K, Seki S, Hara T, Matsui K, Matsuo Y, Kobayashi M, Kubo S, Uchiyama K, Shimizu J, Kawabata R, Ohge H, Akagi S, Oka M, Wakatsuki T, Suzuki K, Okamoto K, Yanagihara K. 2018. Antimicrobial susceptibility of common pathogens isolated from postoperative intra-abdominal infections in Japan. J Infect Chemother 24:330–340. doi: 10.1016/j.jiac.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 36. CLSI . 2018. CLSI standard M07. In methods for dilution antimicrobial susceptibility tests for bacteria that grow Aerobically. 11th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 37. Maeda T, Kumuro M, Matsushita H. 1994. Pharmacokinetic study of Tazobactam/piperacillin in experimental animals. Chemotherapy 42 (S-2):206–216. doi: 10.11250/chemotherapy1953.42.Supplement2_206 [DOI] [Google Scholar]
- 38. Isla A, Trocóniz IF, de Tejada IL, Vázquez S, Canut A, López JM, Solinís MÁ, Rodríguez Gascón A. 2012. Population pharmacokinetics of prophylactic cefoxitin in patients undergoing colorectal surgery. Eur J Clin Pharmacol 68:735–745. doi: 10.1007/s00228-011-1206-1 [DOI] [PubMed] [Google Scholar]
- 39. Yamasaku F, Suzuki Y. 1981. Pharmacokinetics of Cefazolin and Cefamycin derivatives (Cefmetazole and Cefoxitin) in healthy volunteers after I.V. constant infusion. Chemotherapy (Tokyo) 29:857–864. doi: 10.11250/chemotherapy1953.29.857 [DOI] [Google Scholar]
- 40. Fujii A, Nakanishi T, Matsumoto O, Kataoka N, Sia IC, Kamidono S, Kawabata G, Arakawa S, Ishigami J, Umezu K, Hara S, Izumi T. 1987. 6315-S (Flomoxef) in urinary tract infection. Chemotherapy (Tokyo) 35 (S-1):1059–1069. doi: 10.11250/chemotherapy1953.35.Supplement1_1059 [DOI] [Google Scholar]
- 41. Kimura Y, Nakashimizu H, Nakano M, Otsubo R, Matsubara H, Yoshida T. 1987. Pharmacokinetic characterization of 6315-S (Flomoxef) in experimental animals. Chemotherapy (Tokyo) 35 (S-1):161–175. doi: 10.11250/chemotherapy1953.35.Supplement1_161 [DOI] [Google Scholar]
- 42. Ohkawa MI, Orito MA, Sugata TO, Shimamura MA, Sawaki M, Nakashita E, Kuroda K, Sasahara K. 1980. Pharmacokinetics of cefmetazole in normal subjects and in patients with impaired renal function. Antimicrob Agents Chemother 18:386–389. doi: 10.1128/AAC.18.3.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shiba K. 2010. Phase I study of tazobactam/piperacillin in healthy volunteers. Jpn J Chemother 58 (S-1):1–10. [Google Scholar]
- 44. Nakashima M, Uematsu T, Kanamaru M, Ueno K. 1992. Clinical phase I study of Meropenem. Chemotherapy (Tokyo) 40 (S-1):258–275. doi: 10.11250/chemotherapy1953.40.Supplement1_258 [DOI] [Google Scholar]
- 45. Iba K, Yoshitake A, Harrison H, Hutchison M. 1992. Pharmacokinetics of [14C] meropenem in dogs and monkeys. Chemotherapy (Tokyo) 40:145–153. doi: 10.11250/chemotherapy1953.40.Supplement1_145 [DOI] [Google Scholar]
- 46. Yasunaga K, Okamoto Y, Maehara K, Mase K, Iida Y, Yoshioka S, Yamada H, Yoshida T, Oguma T, Kimura Y, Hirauchi M. 1987. Clinical study on 6315-S (Flomoxef). Chemotherapy (Tokyo) 35 (S-1):494–517. doi: 10.11250/chemotherapy1953.35.Supplement1_494 [DOI] [Google Scholar]
- 47. Saito R, Kato Y, Ishikawa K, Odagaki E, Shinohara M, Fukuhara I, Tomizawa M, Nakayama I, Sato K, Yoshida R. 1987. Studies on 6315-S (Flomoxef). Chemotherapy (Tokyo) 35 (S-1):523–540. doi: 10.11250/chemotherapy1953.35.Supplement1_523 [DOI] [Google Scholar]
- 48. Matsumoto S, Kanazawa S, Sato T, Yamano Y. 2020. Activities of cefiderocol with simulated human plasma concentrations against carbapenem-resistant Gram-negative bacilli in an in vitro chemostat model. Antimicrob Agents Chemother 64:e01128-20. doi: 10.1128/AAC.01128-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Homma T, Hori T, Ohshiro M, Maki H, Yamano Y, Shimada J, Kuwahara S. 2010. In vitro pharmacokinetic and pharmacodynamic evaluation of S-013420 against haemophilus influenzae and Streptococcus pneumoniae. Antimicrob Agents Chemother 54:4300–4305. doi: 10.1128/AAC.00214-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures and tables.
Data Availability Statement
The datasets generated and/or analyzed in this study are not publicly available, but are available from the corresponding author on a reasonable request.