Abstract
Nonfastidious aerobic gram-negative bacilli (GNB) are commonly isolated from blood cultures. The feasibility of using an electrochemical method for direct antimicrobial susceptibility testing of GNB in positive blood cultures was evaluated. An aliquot (10 μl) of 1:10-diluted positive blood cultures containing GNB was inoculated into the Bactometer module well (bioMérieux Vitek, Hazelwood, Mo.) containing 1 ml of Mueller-Hinton broth supplemented with an antibiotic. Susceptibility tests were performed in a breakpoint broth dilution format, with the results being categorized as resistant, intermediate, or susceptible. Seven antibiotics (ampicillin, cephalothin, gentamicin, amikacin, cefamandole, cefotaxime, and ciprofloxacin) were used in this study, with each agent being tested at the two interpretive breakpoint concentrations. The inoculated modules were incubated at 35°C, and the change in impedance in each well was continuously monitored for 24 h by the Bactometer. The MICs of the seven antibiotics for each blood isolate were also determined by the standardized broth microdilution method. Of 146 positive blood cultures (1,022 microorganism-antibiotic combinations) containing GNB tested by the direct method, the rates of very major, major, and minor errors were 0, 1.1, and 2.5%, respectively. The impedance method was simple; no centrifugation, preincubation, or standardization of the inocula was required, and the susceptibility results were normally available within 3 to 6 h after inoculation. The rapid method may allow proper antimicrobial treatment almost 30 to 40 h before the results of the standard methods are available.
The isolation of any significant microorganism from a blood culture is an occurrence that requires careful evaluation by the clinician, and prompt action is usually necessary. If the results of clinical microbiological analyses are to contribute in a meaningful way to the diagnosis and management of patients with bacteremia, they must be made available to the clinician in a relevant time frame (1, 3, 15).
Most clinical laboratories use liquid media for the detection of microorganisms in blood, and the antimicrobial susceptibility tests are performed with colonies obtained on subculture plates. After a positive blood culture is detected, the standard procedures may take as long as 2 days to provide the susceptibility results. Recognizing this, efforts have been made to devise analytical procedures which can provide results more quickly.
Rapid techniques for testing the susceptibilities of organisms in blood cultures include the direct disk diffusion test (3, 4, 9, 11, 16) and automated or semiautomated instrument systems. Direct disk diffusion susceptibility testing of the organisms in positive blood cultures has been shown to be reliable for most microorganisms and antimicrobial agents (4, 6, 16, 24); this technique can save 18 to 24 h compared to the times required for the standardized protocols. Additional time savings can be obtained by early reading (6 to 10 h) of the plates after direct incubation (1, 12, 13); however, the test accuracy is sacrificed and some plates may not be readable due to limited bacterial growth.
Several automated systems for antimicrobial susceptibility testing have been described. These systems include the Vitek system (bioMérieux Vitek, Hazelwood, Mo.) (17, 20, 22), MicroScan (Baxter MicroScan, West Sacramento, Calif.) (14, 22), and the MS-2 system (Abbott Laboratories, Irving, Tex.) (2, 19). Although direct inoculation of positive blood culture broths into these systems has been suggested, serial steps of blood cell lysis, differential centrifugation, or preincubation in a broth followed by adjustment of the inoculum are recommended before inoculation. These additional procedures are subject to contamination and are impractical for routine analyses.
A novel method that uses electrochemical measurement was recently proposed for the direct detection of oxacillin-resistant Staphylococcus aureus in blood culture bottles (25). The method is based on the phenomenon that electrical changes (e.g., impedance, conductance, or capacitance) will occur in the media, provided that the test microorganism can grow to a population of approximately 106 to 107 CFU/ml (7). The method is simple and rapid and has a high degree of accuracy.
The purpose of this study was to evaluate the feasibility of direct antimicrobial susceptibility testing of nonfastidious aerobic gram-negative bacilli (GNB) in positive blood cultures by the electrochemical method.
MATERIALS AND METHODS
Selection of electrical signal for susceptibility testing.
The measurement of electrical changes in the culture broth was conducted with the Bactometer M-128 (bioMérieux Vitek) instrument. Three electrical signals (impedance, conductance, and capacitance) were available from the instrument. To determine which signal was best for monitoring the bacterial growth, the three signals were obtained for two clinical isolates (Escherichia coli 1966 and Klebsiella pneumoniae 1374). Each module (bioMérieux Vitek) well contained 1 ml of Mueller-Hinton broth and was inoculated with 50 μl of a 1:10-diluted bacterial suspension with a turbidity equivalent to that of a 0.5 McFarland standard. The inoculated modules (each module contained 16 wells) were inserted into the Bactometer incubator set at 35°C. The change in the electrical signals in the module wells was continuously monitored by the instrument at 6-min intervals for 24 h, and the results were graphically displayed as the percent changes in the three signals.
The detection time (DT; in hours) for each module well was automatically determined by the instrument software when three consecutive readings of the signal change exceeded the default value in the instrument or was manually determined by locating the inflection point (where an accelerating change in the signal was evident) on the growth curve.
Validation of the electrochemical method for susceptibility testing.
To verify the electrochemical technique for susceptibility testing, the MICs for 5 strains of GNB were determined with the Bactometer, with each strain being tested against two randomly selected antimicrobial agents. The strains (antibiotics) tested were E. coli ATCC 23501 (cephalothin and gentamicin), Pseudomonas aeruginosa ATCC 27853 (gentamicin and amikacin), K. pneumoniae 9367 (gentamicin and ciprofloxacin), Enterobacter cloacae 9950 (cephalothin and amikacin), and Citrobacter freundii 8311 (amikacin and ciprofloxacin). The procedures were the same as those described above, except that the culture broth was supplemented with various concentrations of an antimicrobial agent and impedance was used to monitor the growth of the bacteria. The MIC was defined as the lowest concentration of an antibiotic that completely abolished the change in the impedance during an incubation period of 20 h at 35°C.
Direct susceptibility testing of positive blood cultures.
Blood specimens were collected at the National Cheng Kung University Hospital during a 6-month period in 1997. The BACTEC Aerobic and Aerobic Plus bottles (Becton Dickinson Microbiology Systems, Sparks, Md.) were normally inoculated with 5 to 10 ml of blood from the patients, inserted into BACTEC NR-9240 instruments (Becton Dickinson Microbiology Systems), and incubated at 37°C. Samples from positive bottles showing growth of GNB, as determined by Gram staining, were used for direct inoculation into the Bactometer. Smears showing mixed cultures were excluded from the study.
Seven antimicrobial agents were used for susceptibility testing, with each agent being tested at the two interpretive breakpoint concentrations (ampicillin, cephalothin, and cefamandole, 8 and 32 μg/ml; gentamicin, 4 and 16 μg/ml; amikacin, 16 and 64 μg/ml; cefotaxime, 8 and 64 μg/ml; ciprofloxacin, 1 and 4 μg/ml) as defined by the National Committee for Clinical Laboratory Standards (18). The positive culture broths containing GNB were diluted 1:10 with sterile water, and 10 μl of the diluted samples was inoculated into each module well. The inoculated modules were incubated at 35°C. A positive control (no antibiotic in the inoculated well) and a negative control (culture broth only) were included in tests with each blood specimen. Interpretive categorization of the blood isolate by the direct method was based on the inhibition of the microorganism at the two breakpoint concentrations (18).
All blood isolates obtained on subculture plates were identified by conventional microbiological procedures. The MICs of the seven antimicrobial agents for each isolate were determined by the standardized broth microdilution method (18). The MIC data for each isolate were used for categorization of the interpretive susceptibility (18).
Analysis of discrepancy.
The results from the direct impedance tests were compared with those from the microdilution method, and discrepancies were classified as very major, major, or minor errors (4). A very major error was a susceptible result by the direct method and a resistant result by the standard method. A major error was a resistant result by the direct method and a susceptible result by the standard method. A minor error was any change involving an intermediate result.
RESULTS
Selection of electrical signal.
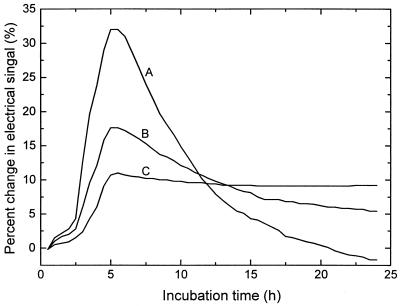
The growth curves for E. coli 1966, as monitored with the three electrical signals, are shown in Fig. 1. Usually, the capacitance change during the growth of bacteria was most prominent, followed by changes in the impedance and conductance. However, the DTs (2.5 h) were not influenced by the use of any of these signals. Similar results were obtained for K. pneumoniae 1374 (data not shown). For some GNB isolates, the change in the capacitance signal was so large that an overscale response was encountered, whereas the change in the conductance signal for some strains (e.g., Acinetobacter and Stenotrophomonas) was too small to reveal active growth. Therefore, the impedance signal was used in the following susceptibility experiments.
FIG. 1.
Growth curves for E. coli 1966 (a clinical isolate) as measured by the changes in capacitance (curve A), impedance (curve B), and conductance (curve C). The change in the capacitance signal was most prominent, followed by changes in the impedance and conductance signals.
Validation of the impedance method for susceptibility testing.
At the beginning of the study, it was necessary to prove that the MICs determined by the electrochemical method were comparable to those obtained by standardized procedures. The comparison was conducted with five strains of GNB (E. coli, P. aeruginosa, K. pneumoniae, E. cloacae, and C. freundii), with each strain being tested with two randomly selected antibiotics. It appeared that the MICs determined by the impedance method were comparable or equivalent to those obtained by the microdilution method (Table 1).
TABLE 1.
Comparison of the MICs for five strains of GNB determined by the impedance method and the broth microdilution method
| Microorganism | MICa (μg/ml)
|
|
|---|---|---|
| First antibiotic | Second antibiotic | |
| E. coli ATCC 23501 | 0.5, 0.25 (gentamicin) | 8, 8 (cephalothin) |
| P. aeruginosa ATCC 27853 | 0.25, 0.25 (gentamicin) | 0.5, 1 (amikacin) |
| K. pneumoniae 9367 | 0.5, 0.5 (gentamicin) | 0.032, 0.032 (ciprofloxacin) |
| E. cloacae 9950 | >512, >512 (cephalothin) | 0.5, 1 (amikacin) |
| C. freundii 8311 | 1, 2 (amikacin) | 1, 1 (ciprofloxacin) |
Each strain was tested against two randomly selected antimicrobial agents. The first MIC in each pair was determined by the broth microdilution method, while the second MIC was determined by the impedance method.
Direct antimicrobial susceptibility testing by the impedance method.
A total of 150 positive blood cultures containing GNB were analyzed by the impedance method, with each culture being tested with seven antibiotics. Among the 150 blood cultures, 4 samples contained mixed cultures and were excluded from the data analysis. The distribution of microorganisms in the 146 blood cultures is shown in Table 2, with E. coli (51 strains; 35%) being the most frequently occurring isolate, followed by K. pneumoniae (29 strains; 19.8%) P. aeruginosa (14 strains; 9.6%), E. cloacae (10 strains; 6.8%), and other minor species.
TABLE 2.
Numbers of different isolates recovered from the 146 positive blood cultures containing GNB
| Microorganism | No. of strains |
|---|---|
| Escherichia coli | 51 |
| Klebsiella pneumoniae | 29 |
| Pseudomonas aeruginosa | 14 |
| Enterobacter cloacae | 10 |
| Stenotrophomonas maltophilia | 6 |
| Proteus mirabilis | 5 |
| Aeromonas hydrophila | 4 |
| Acinetobacter calcoaceticus | 4 |
| Vibrio vulnificus | 3 |
| Enterobacter aerogenes | 3 |
| Pseudomonas spp. | 3 |
| Acinetobacter spp. | 3 |
| Aeromonas spp. | 3 |
| Citrobacter freundii | 2 |
| Salmonella enteritidis | 2 |
| Unidentified gram-negative bacilli | 4 |
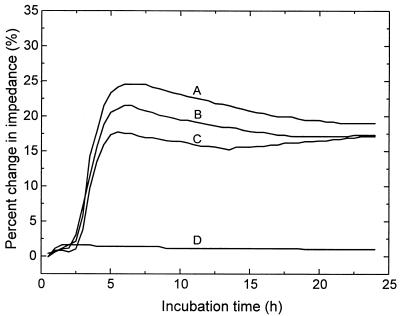
Figure 2 shows typical growth curves obtained with the impedance signal and generated by direct inoculation of a positive blood culture into the Bactometer. The impedance measurement was obtained by a real-time, on-line process. It was evident that the strain in the culture bottle (E. coli 8892) was resistant to ampicillin (32 μg/ml; curve B) and ciprofloxacin (4 μg/ml; curve C) but was susceptible to gentamicin (16 μg/ml; curve D). The DT for the positive control (no antibiotic in the inoculated well; curve A) was only 2.3 h, and at about this time the susceptibility of the organism to other antibiotics was readily discernible. Since most aerobic GNB from blood cultures were fast-growing organisms, the antimicrobial susceptibility patterns were normally available within 3 to 6 h after direct inoculation into the Bactometer.
FIG. 2.
Antimicrobial susceptibility patterns generated by direct inoculation of a positive blood culture into the Bactometer. The growth curves were monitored by detecting changes in impedance during incubation. It was evident that the microorganism (E. coli 8892) in the blood culture was resistant to ampicillin (curve B) and ciprofloxacin (curve C) but susceptible to gentamicin (curve D). It is noteworthy that the DT for the positive control (no antibiotic in the inoculated well; curve A) was only 2.3 h.
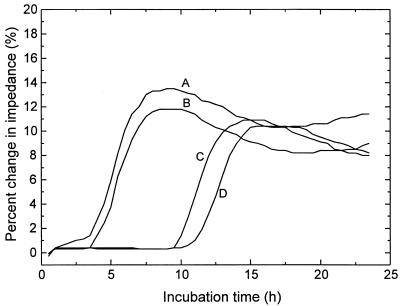
Occasionally, a long delay in DT compared with that for the positive control was found for some blood samples (Fig. 3); this indicated the presence of a heterogeneous resistant subpopulation in the blood cultures. For example, the isolate for which data are presented in Fig. 3 (E. cloacae 0333) was resistant to gentamicin (16 μg/ml; curve B), cefamandole (32 μg/ml; curve C), and cefotaxime (64 μg/ml; curve D); however curves C and D had DTs of 9.0 and 10.3 h, respectively. The lag in DTs was about 6 h compared to that for the positive control (DT, 3.5 h). The microorganisms in the module wells containing cefamandole and cefotaxime were subcultured, and the MICs were determined to be 128 and 512 μg/ml, respectively, by the microdilution method. In contrast, the MICs of cefamandole and cefotaxime were only 16 and 1.0 μg/ml, respectively, for colonies obtained after subculture of the positive blood culture on an agar plate. The discrepancies caused by the direct inoculation method and the conventional method would be that the resistant subpopulation, which represented only a minor population in the original bottle, grew to a majority in the presence of an antibiotic. However, the minor resistant bacteria had little chance of being sampled for MIC determination after subculture on an agar plate.
FIG. 3.
Detection of minor resistant subpopulation by direct inoculation of a positive blood culture into the Bactometer. The blood isolate (E. cloacae 0333) was resistant to gentamicin (curve B), cefamandole (curve C), and cefotaxime (curve D). However, the DTs in the presence of cefamandole (9 h) and cefotaxime (10.3 h) were much longer than that (3.5 h) for the positive control. The MICs (cefamandole, 128 μg/ml; cefotaxime, 512 μg/ml) determined for subcultures obtained from the module wells were much higher than those (cefamandole, 16 μg/ml; cefotaxime, 1 μg/ml) for organisms subcultured from the original blood bottle. The detection time of positive control (curve A) was 3.5 h.
When 146 blood cultures containing aerobic GNB were tested against the seven antimicrobial agents (a total of 1,022 microorganism-antibiotic combinations) by the impedance method and the microdilution technique, the overall agreement between the two methods in terms of the interpretive categories (susceptible, intermediate, and resistant) was 96.4%. There were 11 (1.1%) major errors and 26 (2.5%) minor errors caused by the direct method, but no very major error was found. The major discrepancies were observed for strains of E. coli, E. cloacae, Acinetobacter spp., and Stenotrophomonas maltophilia when testing cefamandole, cefotaxime, or aminoglycosides (gentamicin and amikacin) (Table 3).
TABLE 3.
Results of major errors caused by the impedance method after testing 146 positive cultures containing nonfastidious aerobic GNB with seven antibiotics
| Specimen no. | Microorganism | Antibiotic | Susceptibility test resulta
|
|
|---|---|---|---|---|
| Direct method | Standard method | |||
| 0109 | Enterobacter cloacae | Cefotaxime | R | S |
| 0333 | Enterobacter cloacae | Cefotaxime | R | S |
| 1771 | Escherichia coli | Cefamandole | R | S |
| 1788 | Escherichia coli | Cefamandole | R | S |
| 8782 | Escherichia coli | Gentamicin | R | S |
| 3399 | Acinetobacter baumannii | Gentamicin | R | S |
| 3399 | Acinetobacter baumannii | Amikacin | R | S |
| 8203 | Klebsiella pneumoniae | Ciprofloxacin | R | S |
| 9548 | Acinetobacter calcoaceticus | Gentamicin | R | S |
| 9582 | Stenotrophomonas maltophilia | Gentamicin | R | S |
| 9582 | Stenotrophomonas maltophilia | Amikacin | R | S |
R, resistant; S, susceptible.
Among the 26 minor errors produced by the direct method, 19 were false resistance, with the remaining 7 being false susceptibility. Of these minor errors, 21 were observed when testing β-lactam antibiotics, with the frequencies of occurrence being as follows: ampicillin, three samples; cephalothin, six samples; cefamandole, eight samples; cefotaxime, five samples; and other antibiotics, four samples. No specific microorganism-antibiotic combination was responsible for these minor discrepancies. The major and minor errors caused by the direct method were reconfirmed by testing the colonies grown on subculture plates by the E test (AB Biodisk, Solna, Sweden).
Susceptibility tests with mixed cultures.
Although smears apparently containing mixed cultures were not used in the direct susceptibility test, four blood samples appeared to contain multiple species, as revealed on subculture plates and identified by conventional procedures. Three of the four specimens contained two different species of GNB, with the remaining specimen containing two different strains of E. coli. Table 4 demonstrates the susceptibility test results for two of the four mixed cultures. When a polymicrobial infection was encountered, the impedance method detected the more resistant side of the mixed flora. For example, a mixed blood culture (specimen 9758) contained E. coli (cephalothin resistant) and K. pneumoniae (cephalothin susceptible), and the impedance method detected resistance when cephalothin was tested.
TABLE 4.
Results of susceptibility tests for mixed cultures
| Specimen no. | Microorganism | Results of susceptibility testa
|
||||||
|---|---|---|---|---|---|---|---|---|
| Ampicillin | Cephalothin | Gentamicin | Amikacin | Cefamandole | Cefotaxime | Ciprofloxacin | ||
| 7748 | E. coli/E. colib | R/R (R) | I/R (R) | S/R (R) | S/S (S) | S/R (R) | S/S (S) | S/S (S) |
| 9758 | K. pneumoniae/E. coli | R/R (R) | S/R (R) | R/I (R) | S/S (S) | R/R (R) | S/S (S) | S/R (R) |
Antimicrobial susceptibility results were obtained by the standardized microdilution method; the results in parentheses were obtained by the impedance method with the Bactometer. R, resistant; I, intermediate; S, susceptible.
Two different strains of E. coli.
DISCUSSION
A direct antimicrobial susceptibility test based on the measurement of changes in impedance was developed for blood cultures containing aerobic GNB. The method was performed in a breakpoint broth dilution format with results expressed in the form of susceptibility categories (resistant, intermediate, and susceptible). Under most conditions, the susceptibility results were available within 3 to 6 h after inoculation by the direct method, whereas by routine procedures results are available in an average of 40 to 48 h. This allowed the susceptibility patterns to be available on the same day that the positive blood culture bottles were detected in the clinical laboratories. The direct method had a level of agreement of 96.4% with the standardized microdilution technique performed with pure cultures grown on subculture plates. The frequencies of major errors (1.1%) and minor errors (2.5%) by the impedance method were low.
The impedance technique described here was simple; only a single step of inoculating 10 μl of a 1:10-diluted positive culture broth into the module wells was required. Among the seven antimicrobial agents tested, ampicillin, cephalothin, and gentamicin represented group A antibiotics, with the remaining four (amikacin, cefamandole, cefotaxime, and ciprofloxacin) being group B antibiotics; both groups are recommended by the National Committee for Clinical Laboratory Standards for routine testing and reporting (18).
Since trimethoprim-sulfamethoxazole is also commonly used for the treatment of bacteremia caused by GNB, at the end of this study 28 blood cultures containing GNB were tested with this antimicrobial agent combination. The results demonstrated that the direct method had only one minor error and a 96.3% agreement with the reference microdilution method (data not shown). It seems that the impedance method can be applied to a broad spectrum of microorganisms and antimicrobial agents.
The impact of rapid antimicrobial susceptibility testing on infectious disease outcome has been systematically assessed by Doern et al. (5). The benefits include significant reductions in the numbers of microbiology tests, subsequent positive blood cultures, serum antibiotic assays, some imaging procedures, and days of intubation and reductions in the length of time spent in an intensive care area. It was important to find that the mortality rate was much lower (8.8%) for the rapid test group than for the control group (15.3%) for which conventional overnight techniques were used for susceptibility testing (5). Trenholme et al. (21) also demonstrated that rapid susceptibility testing of blood isolates could result in an earlier initiation of an appropriate therapy or a change to the use of more effective and less expensive antibiotics. In addition, the rapid availability of susceptibility information was more likely to be followed by the treatment of patients by clinicians.
Direct disk diffusion susceptibility testing of the organisms found in blood cultures has been shown to be reliable for most microorganism-antimicrobial agent combinations (6, 16, 24). Before direct inoculation, some investigators proposed that positive blood samples should be subcultured in a liquid broth followed by adjustment of the inoculum density. However, several studies with inocula taken directly from positive blood bottles also obtained good results (3, 4), but an incubation period of 16 to 20 h is normally required for the direct disk diffusion test.
Several instrument-assisted susceptibility test systems have been developed, and these systems were claimed to provide results in a matter of hours rather than days. These instruments include MicroScan, the Vitek Automicrobic system, and the Cobasbact system (10, 14, 21–23). However, several steps including sample centrifugation, blood cell lysis, and standardization of the inoculum are recommended before direct inoculation into these systems (2, 19, 20), or a preincubation step followed by adjustment of the inoculum density is required (11). The detection principles for these systems are usually based on the measurement of changes in optical properties (turbidity or fluorescence) and are more sensitive to interferences from the blood specimens.
The present impedance method has two advantages. The first is that the measurement of an electrical property was basically not influenced by the color or turbidity of the blood samples. The second was that signal detection in the Bactometer was a continuous, real-time process, and susceptibility patterns could be obtained by a real-time comparison with the growth curve for the positive control.
Direct susceptibility testing has an additional advantage for the testing of a broader representation of the bacterial population present in blood cultures (8) and is more likely to detect the heterogeneous resistant bacteria which represent only a minor subpopulation in positive blood culture bottles. Theoretically, about 105 cells were inoculated into each module well of the Bactometer, whereas only three to five colonies on subculture plates were sampled for inoculum preparation by the conventional microdilution protocol (18). This might explain the observation that on most occasions in which discrepant results occurred the direct method detected the more-resistant organism of the mixed cultures and very major errors were not found.
Although polymicrobial infections were excluded from the data analysis in this study, it was interesting that the impedance method detected the more resistant side of the mixed flora (Table 4). This result would be desirable if the direct method were used to guide a clinician in starting antimicrobial therapy for patients.
In view of the high rate of isolation of aerobic GNB from patients with bacteremia and the high mortality rates from bacteremia caused by aerobic GNB, a rapid method for the antimicrobial susceptibility testing of GNB may be beneficial for patients with bacteremia. The impedance method is proposed as a test that can be used as a supplement to the standardized procedures for the earlier determination of the susceptibility patterns of aerobic GNB from blood cultures.
ACKNOWLEDGMENTS
This project was supported by a grant (grant NSC 88-2314-B-006-078) from the National Science Council, Taipei, Taiwan, and by a grant (NCKUH 87-065) from National Cheng Kung University Hospital, Tainan, Taiwan, Republic of China.
REFERENCES
- 1.Coyle M B, McGonagle L A, Plorde J J, Clausen C R, Schoenknecht F D. Rapid antimicrobial susceptibility testing of isolates from blood cultures by direct inoculation and early reading of disk diffusion tests. J Clin Microbiol. 1984;20:473–477. doi: 10.1128/jcm.20.3.473-477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dipersio J R, Ficorilli S M, Varga F J. Direct identification and susceptibility testing of gram-negative bacilli from BACTEC bottles by use of the MS-2 system with updated bacterial identification software. J Clin Microbiol. 1984;20:1202–1204. doi: 10.1128/jcm.20.6.1202-1204.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doern G V, Scott D R, Rashad A L. Clinical impact of rapid antimicrobial susceptibility testing of blood culture isolates. Antimicrob Agents Chemother. 1982;21:1023–1024. doi: 10.1128/aac.21.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doern G V, Scott D R, Rashad A L, Kim K S. Evaluation of a direct blood culture disk diffusion antimicrobial susceptibility test. Antimicrob Agents Chemother. 1981;20:696–698. doi: 10.1128/aac.20.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doern G V, Vautour R, Gaudet M, Levy B. Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol. 1994;32:1757–1762. doi: 10.1128/jcm.32.7.1757-1762.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fay D, Oldfather J E. Standardization of direct susceptibility test for blood cultures. J Clin Microbiol. 1979;9:347–350. doi: 10.1128/jcm.9.3.347-350.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Firstenberg-Eden R, Eden G. Impedance microbiology. New York, N.Y: John Wiley & Sons, Inc.; 1984. pp. 7–90. [Google Scholar]
- 8.Hong T, Ndamukong J. Direct application of Etest to gram-positive cocci from blood cultures: quick and reliable minimum inhibitory concentration data. Diagn Microbiol Infect Dis. 1996;25:21–25. doi: 10.1016/0732-8893(96)00062-4. [DOI] [PubMed] [Google Scholar]
- 9.Johnson J E, Washington J A., III Comparison of direct and standardized antimicrobial susceptibility testing of positive blood cultures. Antimicrob Agents Chemother. 1976;10:211–214. doi: 10.1128/aac.10.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamm W, Bille J. Evaluation of the Cobasbact system for rapid antimicrobial susceptibility testing of positive blood culture broths. Eur J Clin Microbiol. 1985;4:579–582. doi: 10.1007/BF02013399. [DOI] [PubMed] [Google Scholar]
- 11.Kiehn T E, Capitolo C, Armstrong D. Comparison of direct and standard microtiter broth dilution susceptibility testing of blood culture isolates. J Clin Microbiol. 1982;16:96–98. doi: 10.1128/jcm.16.1.96-98.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluge R M. Accuracy of Kirby-Bauer susceptibility tests read at 4, 8, and 12 hours of incubation: comparison with readings at 18 to 20 hours. Antimicrob Agents Chemother. 1975;8:139–145. doi: 10.1128/aac.8.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberman D F, Robertson R G. Evaluation of a rapid Bauer-Kirby antibiotic susceptibility testing for bacteria isolated from blood. Antimicrob Agents Chemother. 1975;7:250–255. doi: 10.1128/aac.7.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGregor A, Schio F, Beaton S, Boulton V, Perman M, Gilbert G. The MicroScan WalkAway diagnostic microbiology system—an evaluation. Pathology. 1995;27:172–176. doi: 10.1080/00313029500169822. [DOI] [PubMed] [Google Scholar]
- 15.Mirrett S. Antimicrobial susceptibility testing and blood cultures. Clin Lab Med. 1994;14:171–179. [PubMed] [Google Scholar]
- 16.Mirrett S, Reller L B. Comparison of direct and standard antimicrobial disk susceptibility testing for bacteria isolated from blood. J Clin Microbiol. 1979;10:482–487. doi: 10.1128/jcm.10.4.482-487.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore D F, Hamada S S, Marso E, Martin W J. Rapid identification and antimicrobial susceptibility testing of gram-negative bacilli from blood cultures by the AutoMicrobic system. J Clin Microbiol. 1981;13:934–939. doi: 10.1128/jcm.13.5.934-939.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 19.Nolte F S, Contestable P B, Lincalis D, Punsalang A., Jr Rapid, direct antibiotic susceptibility testing of blood culture isolates using the Abbott Advantage System®. Am J Clin Pathol. 1986;86:665–669. doi: 10.1093/ajcp/86.5.665. [DOI] [PubMed] [Google Scholar]
- 20.Sahm D L, Boonlayangoor S, Morello J A. Direct susceptibility testing of blood culture isolates with the AutoMicrobic system (AMS) Diagn Microbiol Infect. 1987;8:1–11. doi: 10.1016/0732-8893(87)90040-x. [DOI] [PubMed] [Google Scholar]
- 21.Trenholme G M, Kaplan R L, Karakusis P H, Stine T, Fuhrer J, Landan W, Levin S. Clinical impact of rapid identification and susceptibility testing of bacterial blood culture isolates. J Clin Microbiol. 1989;27:1342–1345. doi: 10.1128/jcm.27.6.1342-1345.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visser M R, Bogaards L, Rozenberg-Arska M, Verhoef J. Comparison of the autoSCAN-W/A and Vitek Automicrobic systems for identification and susceptibility testing of bacteria. Eur J Clin Microbiol Infect Dis. 1992;11:979–984. doi: 10.1007/BF01967786. [DOI] [PubMed] [Google Scholar]
- 23.Waites K B, Brookings E S, Moser S A, McKinnon M L, Van Pelt L. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Direct susceptibility testing from positive BacT/Alert® blood culture specimens using MicroScan® overnight and rapid panels, abstr. C-313; p. 56. [Google Scholar]
- 24.Wegner D A, Mathis C R, Neblett T R. Direct method to determine the antibiotic susceptibility of rapidly growing blood pathogens. Antimicrob Agents Chemother. 1976;9:861–862. doi: 10.1128/aac.9.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J J, Huang A H, Dai J H, Chang T C. Rapid detection of oxacillin-resistant Staphylococcus aureus in blood cultures by an impedance method. J Clin Microbiol. 1997;35:1460–1464. doi: 10.1128/jcm.35.6.1460-1464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]