Abstract
Background:
Long-term data on cardiovascular disease (CVD) and mortality in female carriers of the transthyretin (TTR) V122I (pV142I) mutation, one of the most common mutations of hereditary transthyretin cardiac amyloidosis, are sparse and the effects of blood pressure, heart rate, body mass index and physical activity on CVD outcomes remain largely unknown.
Objective:
The aim was to first examine the relationship of TTR V122I (pV142I) carrier status with CVD and mortality and second to investigate the effects of blood pressure, heart rate, body mass index, and physical activity in a large cohort of postmenopausal women.
Methods:
Our study population consisted of 9,862 non-Hispanic Black/African American women, 9,529 non-carriers and 333 TTR V122I carriers, enrolled in the Women’s Health Initiative at 40 US centers. Women were generally healthy and postmenopausal at the time of enrollment (1993–1998). CVD was defined as a composite endpoint consisting of coronary heart disease (CHD), stroke, acute heart failure or CVD death, and all-cause mortality. CVD cases were based on self-reported annual mailed health updates. All information was centrally adjudicated by trained physicians. Hazard ratios (HR) and 95% confidence intervals (95% CI) were obtained from adjusted Cox proportional hazards models.
Results:
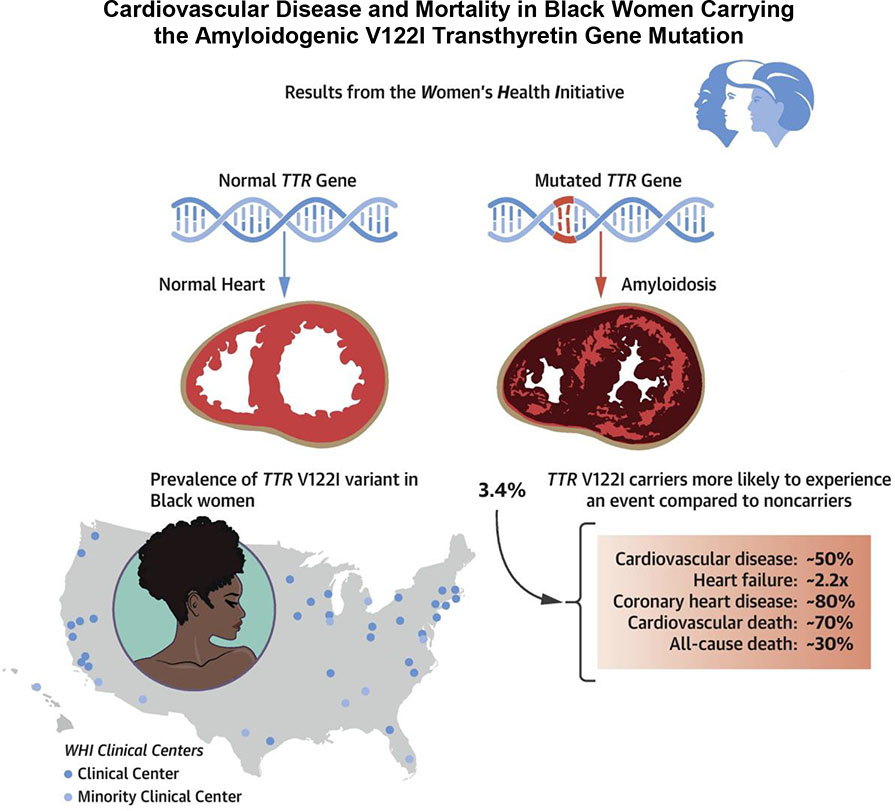
Among 9,862 Black female participants (mean age, 62 years [interquartile range, 56–67 years]), the population frequency of the TTR V122I variant was 3.4% (333 variant carriers and 9,529 non-carriers). During a mean follow-up of 16.1 years (interquartile range, 9.7–22.2 years), incident CVD occurred in 2,229 non-carriers and 96 carriers, whereas 2,689 non-carriers and 108 carriers died. In adjusted models including demographic, lifestyle and medical history covariates, TTR V122I carriers were at higher risk of the composite endpoint CVD [HR (95% CI), 1.52 (1.22, 1.88)], acute heart failure [2.21 (1.53, 3.18)], CHD [1.80 (1.30, 2.47)], CVD death [1.70 (1.26, 2.30)] and all-cause mortality [1.28 (1.04, 1.56)]. We found a significant interaction by age but not by blood pressure, heart rate, body mass index or physical activity.
Conclusions:
Black female TTR V122I (pV142I) carriers have a higher CVD and all-cause mortality risk compared to noncarriers. In case of clinical suspicion of amyloidosis, they should be screened for TTR V122I (pV142I) carrier status to ensure early treatment onset.
Keywords: Transthyretin Cardiac Amyloidosis, V122I, pV142I, Cardiovascular Disease, Mortality, Older Women
Keywords: Transthyretin Amyloidosis, Postmenopausal Women
Condensed Abstract
Long-term data on cardiovascular disease and mortality in Black women carrying the amyloidogenic transthyretin (TTR) V122I (pV142I) variant are sparse. Our study consisted of 9,862 non-Hispanic Black/African American postmenopausal women, including 9,529 non-carriers and 333 TTR V122I carriers. CVD and all-cause mortality risk was significantly higher in TTR V122I carriers compared to non-carriers. This increased risk was largely driven by heart failure, coronary heart disease and cardiovascular death. A significant interaction by age but not by blood pressure, heart rate, body mass index or physical activity was detected. African-American women with signs of cardiomyopathy, extracardiac amyloidosis red flags or relevant family history should be screened for TTR V122I (pV142I) variant carrier status to ensure early treatment onset.
Introduction
Cardiac amyloidosis is a progressive infiltrative cardiomyopathy that leads to an increase in ventricular wall thickness and stiffness of the heart. It is characterized by the deposition of misfolded transthyretin (TTR) protein in the myocardium due to mutations that lead to misfolding of a precursor protein and multisystem disease.(1–3) According to the THAOS registry, a global observational database, one of the most common disease-associated TTR variant in the USA is the valine-to-isoleucine substitution (TTR V122I; pV142I).(4,5)
Approximately 3% to 4% of the Black population carry the TTR V122I mutation in the United States.(6) Although the mutation has been reported to be generally absent outside of African ancestry, recent reports of higher-than-expected prevalence in European-ancestry populations question the African specificity of this allele.(7–9) Results from the Atherosclerosis Risk in Communities Study (ARIC) including individuals with a mean age of 50 to 52 years with long-time follow-up found an increased risk for of heart failure in TTR V122I carriers but did not observe any differences according to sex or detect a significant difference in mortality between carriers and non-carriers.(10) A likely explanation is that ARIC participants were mostly too young for the TTR V122I allele to cause clinically significant disease. A recent analysis from the REGARDS (Geographic and Racial Differences in Stroke) study with a mean age of 62 years extended these findings by additionally reporting an association between carrier status and mortality.(11)
Transthyretin amyloid cardiomyopathy has been predominantly diagnosed in men.(12,13) Earlier studies reported that affected women have a more favorable cardiac phenotype, less aggressive disease trajectory, and did not observe mortality differences compared to non-carriers.(14,15) Emerging evidence, however, suggests that women diagnosed with transthyretin cardiomyopathy are more symptomatic than men, potentially suggesting a delayed diagnosis.(16,17) Unfortunately, outcome studies with long-time follow-up focusing on female TTR V122I carriers are sparse.(18)
Due to accumulating protein deposition in amyloid cardiomyopathy, the myocardium stiffens over time. Consequently, TTR V122I carriers suffer from a decline in blood pressure (BP) and increases in heart rate due to progressive diastolic dysfunction and declining stroke volumes. The ventricle becomes more sensitive to preload and afterload changes.(2,19) This implies a pivotal role of age, BP, heart rate on cardiovascular risk in variant carriers, yet such data are largely not available. Moreover, some reports suggest that affected individuals have lower body mass index (BMI) and increased antecedent physical activity but their effect on CVD risk is largely unclear.(20,21)
The aim was to first examine the relationship of TTR V122I (pV142I) carrier status with CVD and mortality and second to investigate the effects of blood pressure, heart rate, body mass index, and physical activity in a large cohort of postmenopausal women.
Methods
Study Population
The study population consisted of women enrolled in the Women’s Health Initiative (WHI) who were part of the Population Architecture using Genomics and Epidemiology (PAGE II) and SNP Health Association Resource (SHARe) genetic epidemiology studies and who participated in the WHI observational study or randomized controlled trials.(22,23) Detailed information about the WHI has been previously described.(24–26) In brief, women were eligible to participate in WHI if they were 50 to 79 years old, generally healthy and postmenopausal at the time of enrollment. PAGE II was designed to characterize the genetic architecture of complex traits through large-scale genetics and epidemiological research.(23) SHARe was an NHLBI sponsored effort to genotype the African American and Hispanic sub-cohort of WHI. All participants provided written informed consent, and institutional review board approval was received by all participating institutions.
Women participating in the WHI PAGE II (n=12,439) and SHARe (n=11,992) underwent genotyping which included the hereditary TTR V122I variant (pV142I; rs76992529).(4,23,27). After combining WHI PAGE II and SHARe datasets and removing overlap as well as 11 participants with no follow up, our study population consisted of 9,862 non-Hispanic Black / African American Women with quality controlled genotyped data available including 9,529 non-carriers and 333 TTR V122I carriers (332 heterozygous; 1 homozygous). In the multivariable models, data on a total of 9,160 participants, including 303 carriers and 8,857 non-carriers, were used due to missing covariate data.
Genotyping
The WHI samples were genotyped as part of the larger PAGE II study on the Multi-Ethnic Genotyping Array by the Center for Inherited Disease Research. Quality control was carried out by the University of Washington Genetic Analysis center and included technical filters, removing variant with discordant calls in duplicates or Mendelian errors, and Hardy Weinberg outliers.(23) The WHI SHARe samples were genotyped at Affymtrix Inc on the Genome-wide Human SNP Array 6.0. Quality control was carried out at the Fred Hutchinson and included removal of relatives, SNPs with low call rates or concordance rates, and Hardy Weinberg outliers.(28)
Assessment of Blood Pressure, Heart Rate, Body Mass Index and Physical Activity
Blood pressure and heart rate were measured by WHI staff following a standardized measurement protocol.(24,26) Two BP measurements, taken 30 seconds apart, were recorded and averaged. Heart rate was measured by palpating the radial pulse for 30 seconds. In women participating in the WHI clinical trials BP and heart rate were determined annually, while it was measured at baseline and at year 3 visit in women participating in the WHI Observational Study. The mean number of measurements for BP, heart rate, BMI and physical activity was 5 (interquartile range, 2 – 8) and 4 (interquartile range, 3 – 6). Trained clinic staff measured height and weight at baseline using a calibrated clinical scale and stadiometer. Body mass index was calculated as weight in kilograms divided by height in meters squared. Recreational physical activity was assessed using a questionnaire on frequency and duration of several activity types, which were summarized as metabolic equivalent-hours per week (MET-hrs/wk).
Cardiovascular Disease and All-cause Mortality
CVD was a composite endpoint including incident CHD (including fatal or non-fatal myocardial infarction (MI), coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI)), fatal or non-fatal ischemic or hemorrhagic stroke, acute heart failure (HF), or CVD death events. CVD cases were based on self-reported mailed health updates annually. All information was centrally adjudicated by trained physicians. In the main analysis, acute HF was defined as a definite or possible acute hospitalized HF event. In sensitivity analyses, the definition of HF was expanded and included incident definite or possible acute hospitalized HF and chronic stable HF events. All-cause mortality was ascertained by extracting health information from hospital records or the National Death Index.(24–26,29)
Covariates
Information was collected via self-reported surveys or by physical measurements at WHI baseline.(24,26) Hypertension was defined as self-reported current anti-hypertensive use or values of systolic blood pressure (SBP) ≥ 140mmHg or diastolic blood pressure (DBP) ≥ 90mmHg at baseline visit in accordance with clinical guidelines used at the time of WHI enrollment.(30) Antihypertensive medication information was collected by inventory and inspection of all prescription medications brought to an in-person clinic visit. Women were classified as having diabetes based on self-report of diabetes or self-report of diabetes treatment. Smoking status was categorized as a current, former, or never smoker based on self-report.
Statistical Analysis
Descriptive statistics by TTR V122I carrier status were created for baseline demographic variables.
In order to adjust for ancestral differences in the genotype data that remain after selecting self-identified Black/African American participants, principal components (PCs) were calculated separately for the PAGE II and SHARe studies.(31) For PAGE II, PCs were calculated among unrelated individuals using SNPs that passed variant QC for the study and then estimated by projection for those who were related to other study participants. Relationships were determined from the genotype data by estimating kinship coefficients with GENESIS R package, and PCs were calculated using the SNPRelate R package. For SHARe, PCs were calculated with R using a subset of 5,665 SNPs and all non-duplicate samples across WHI GWAS studies. All analyses were adjusted for the main effect of PAGE II vs. SHARe and interactions with the first 10 principal components. Hazard ratios (HRs) and 95% confidence intervals (CI) for risk of incident CVD and all-cause mortality were estimated from Cox proportional hazards regression models, which controlled for age, BMI (kg/m2), smoking status, history of CVD (MI/CABG/PCI/CHF/stroke), history of hypertension, history of diabetes, physical activity (MET hrs/wk), Clinical Trial vs. Observational Study participation and Hormone Therapy and Diet Modification Trial randomization arms. Time to CVD or death was calculated from WHI enrollment to date of death or CVD event and time to event was censored at last known follow-up or death. For all models utilizing outcomes other than all-cause mortality, competing events were censored in the Cox regression models, and risk factors that are common to the events of interest and competing events were included. Sensitivity analyses further controlled for antihypertensive medication use (never used, used at all visits, new start during follow-up) in separate models. Kaplan-Meier curves for CVD and total mortality are presented by carrier status with age as the underlying time variable. Interactions of age, SBP, DBP, heart rate, BMI and physical activity with carrier status were assessed in corresponding multivariable adjusted Cox models additionally including the main effect and interaction term; the p-value for interaction was based on incorporating time-varying data for each subgroup except age at screening. Finally, we examined trajectories of mean SBP, DBP and heart rate measurements of carriers compared to non-carriers by age and significant differences in trajectories were assessed using general linear models with repeated measures.
Analyses were performed using SAS software (v 9.4, SAS Inc, Cary, NC) and the R statistical computing environment (v 4.2.0, R Foundation for Statistical Computing, Vienna, Austria). A 2-sided p < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics of study participants by carrier status are presented in Table 1. The mean age of our study population was approximately 61 years. Only slight differences across characteristics between TTR V122I carriers and non-carriers were detected. Carriers of the TTR V122I variant were more likely to have a BMI < 25kg/m2, to be physically active, to be current smokers and less likely to suffer from diabetes or from CVD. Other CVD risk factors such as age, heart rate, systolic and diastolic BP and history of hypertension, were similar across groups.
Table 1.
Baseline characteristics
| Non-Carrier TTR V122I (N=9,529) | Carrier TTR V122I (N=333) | |||
|---|---|---|---|---|
|
| ||||
| N | % or Mean (SD) | N | % or Mean (SD) | |
|
| ||||
| Age at screening, years | 9529 | 61.5 (7.0) | 333 | 61.2 (7.2) |
| 50 – 59 | 3982 | 41.8 | 154 | 46.2 |
| 60 – 69 | 4110 | 43.1 | 129 | 38.7 |
| 70 – 79 | 1437 | 15.1 | 50 | 15.0 |
|
| ||||
| Systolic blood pressure, mmHg | 9528 | 132.1 (17.8) | 333 | 131.5 (18.7) |
| Diastolic blood pressure, mmHg | 9523 | 78.1 (9.4) | 333 | 78.0 (10.2) |
| Heart rate, bpm | 9516 | 70.8 (13.3) | 331 | 70.6 (14.0) |
|
| ||||
| Body mass index, kg/m2 | ||||
| <25 | 1497 | 15.8 | 68 | 20.7 |
| 25 – 30 | 3121 | 33.0 | 115 | 35.0 |
| ≥30 | 4830 | 51.1 | 146 | 44.4 |
|
| ||||
| Smoking status | ||||
| Never | 4541 | 48.6 | 153 | 47.5 |
| Past | 3730 | 39.9 | 126 | 39.1 |
| Current | 1067 | 11.4 | 43 | 13.4 |
| History of hypertension | 5821 | 61.1 | 203 | 61.0 |
| History of CVD (MI/CABG/PCI/CHF/stroke) | 806 | 8.6 | 16 | 5.0 |
| History of diabetes | 1405 | 14.8 | 44 | 13.2 |
|
| ||||
| Physical activity, MET hrs/wk | ||||
| None | 2150 | 23.2 | 78 | 23.6 |
| >0 – 5.0 | 2522 | 27.2 | 93 | 28.2 |
| >5.0 – 14.25 | 2408 | 26.0 | 75 | 22.7 |
| >14.25 | 2197 | 23.7 | 84 | 25.5 |
The mean duration of follow-up for the overall study sample was 16.1 years (standard deviation (SD), 6.8 years), with non-carriers having an average (SD) of 16.1 (6.8) years follow-up and carriers having an average (SD) 15.7 (6.6) years. The average age for carriers vs. non-carriers at time of CVD was 74.8 (8.8) vs 74.3 (8.9) years and for death it was 78.8 (8.5) vs 77.7 (9.2) years. The annualized risk among participants with complete case data for a CVD event was 2.08% for TTR V122I carriers and 1.55% for non-carriers whereas the annualized all-cause mortality risk was 2.08% for carriers and 1.73% for non-carriers. Kaplan-Meier Curves for incident CVD and all-cause mortality events by carrier status with age as the underlying time variable are shown (Figure 1 & 2).
Figure 1.
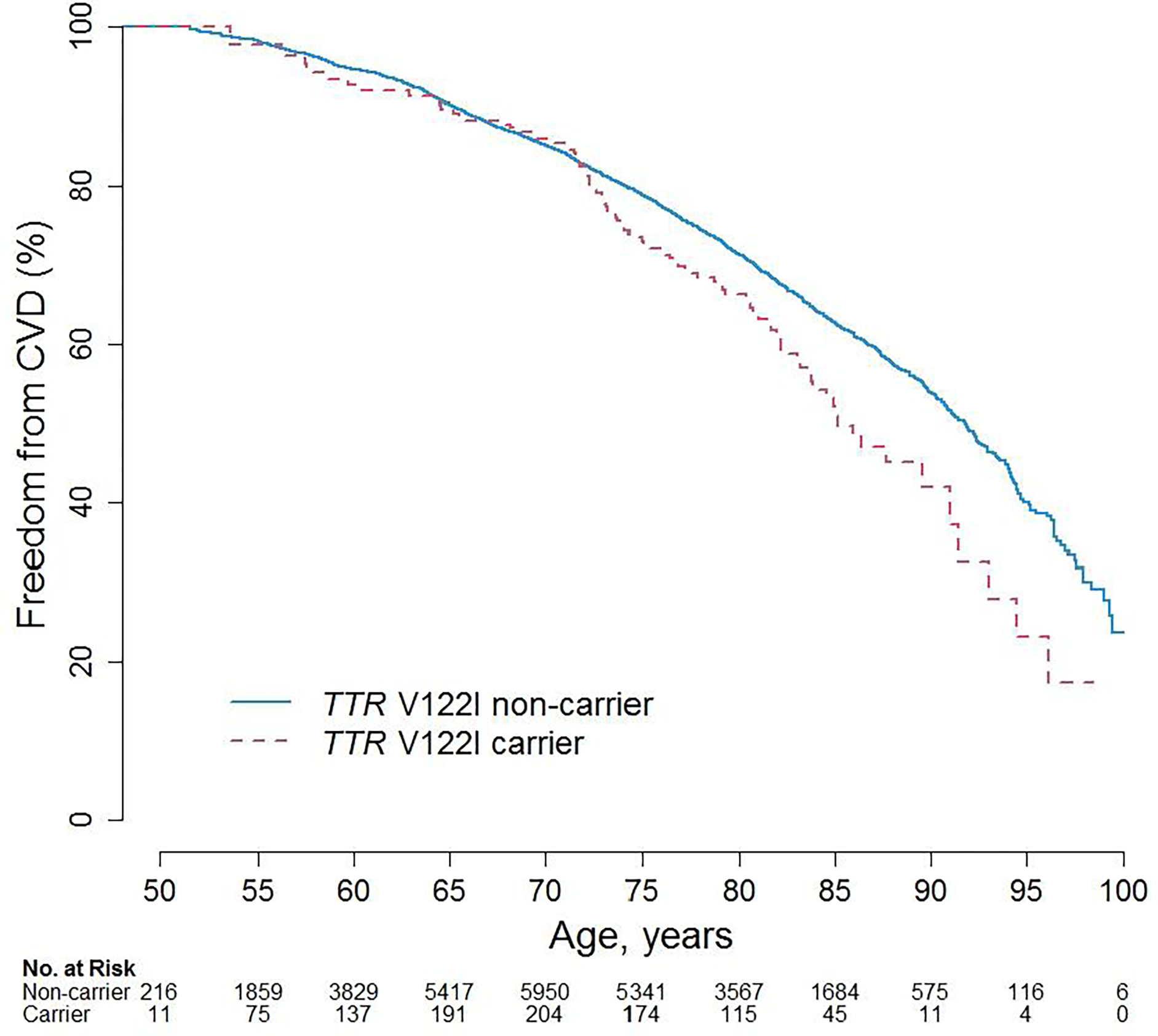
Kaplan-Meier Curves for CVD by TTR V122I carrier status
Figure 2.
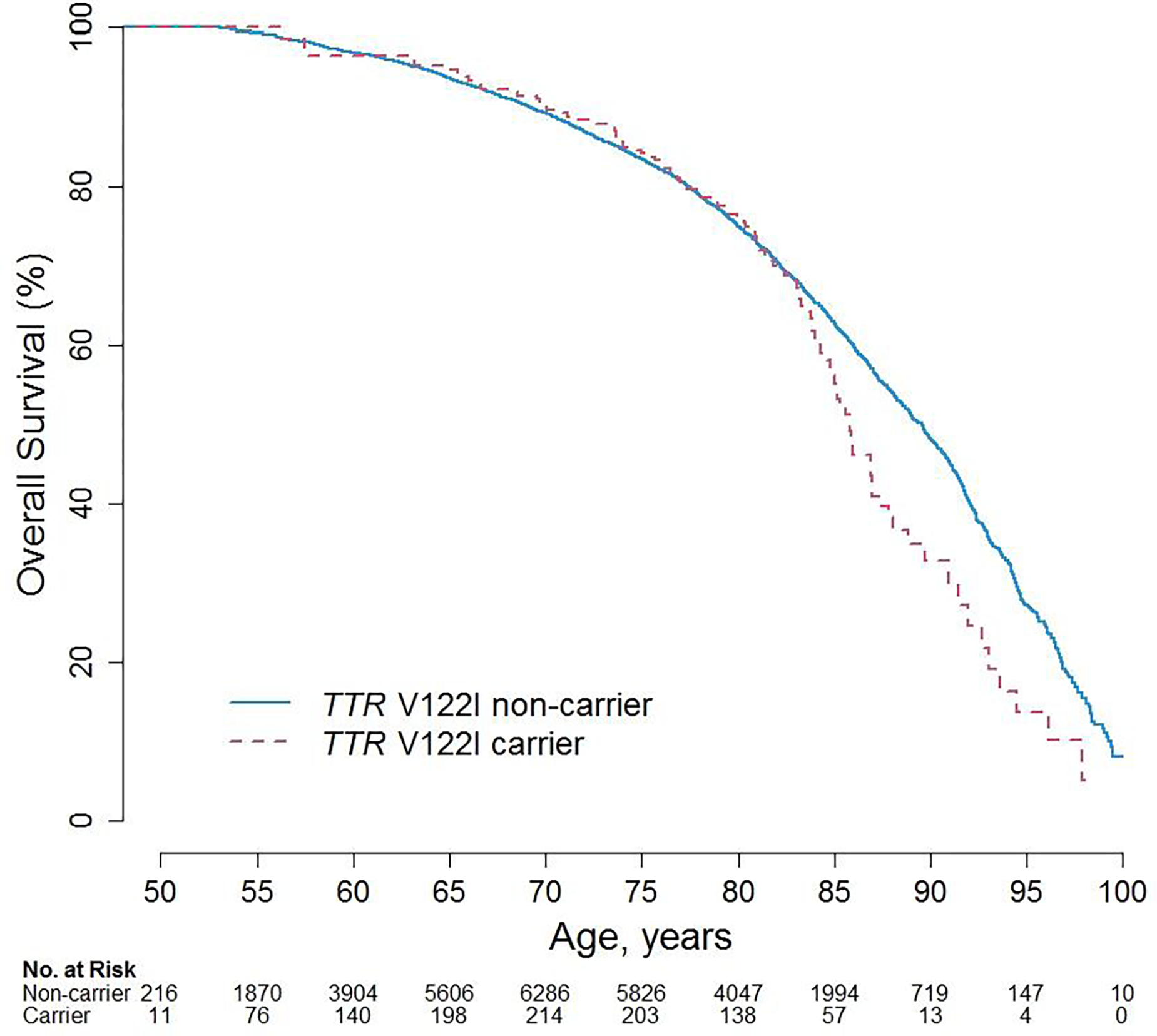
Kaplan-Meier Curves for Survival by TTR V122I carrier status
The results of Cox proportional hazards analysis for CVD and all-cause mortality are given in Table 2. Compared to non-carriers, and after fully adjusting for demographic, lifestyle and medical history covariates, TTR V122I carriers were at higher risk for overall CVD (HR 1.52; 95% CI 1.22, 1.88; p-value=0.0001), acute HF (HR 2.21; 95% CI 1.53, 3.18; p-value<0.0001), CHD (HR 1.80; 95% CI 1.30, 2.47; p-value=0.0003), CVD death (HR 1.70; 95% CI 1.26, 2.30; p-value=0.001) and all-cause mortality (HR 1.28; 95% CI 1.04, 1.56; p-value=0.02). No significant risk for stroke (HR 1.03; 95% CI 0.68, 1.58, p-value=0.87) was detected. In sensitivity analysis, we combined acute and chronic HF events. This increased the number of HF cases and the effect sizes. When we additionally controlled our analysis for antihypertensive medication use, our results were not changed substantially (Supplemental Table 1).
Table 2.
Hazard Ratios (95% CIs) and annualized rates for CVD and all-cause mortality risk for TTR V122I carriers versus non-carriers
| Non-Carrier TTR V122I | Carrier TTR V122I | HR | (95% CI) | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Total N | Cases | (Ann %) | Total N | Cases | (Ann %) | ||||
|
| |||||||||
| CVD (CHD/acute HF/stroke/CVD death) | |||||||||
| Age adjusted1 | 9529 | 2229 | (1.56%) | 333 | 96 | (2.02%) | 1.33 | (1.09, 1.64) | 0.01 |
| Multivariate adjusted2 | 8857 | 2045 | (1.55%) | 303 | 89 | (2.08%) | 1.52 | (1.22, 1.88) | 0.0001 |
| Heart failure, acute | |||||||||
| Age adjusted1 | 9529 | 582 | (0.39%) | 333 | 32 | (0.63%) | 1.73 | (1.21, 2.48) | 0.003 |
| Multivariate adjusted2 | 8857 | 531 | (0.38%) | 303 | 31 | (0.68%) | 2.21 | (1.53, 3.18) | <0.0001 |
| Stroke | |||||||||
| Age adjusted1 | 9529 | 718 | (0.48%) | 333 | 24 | (0.47%) | 1.02 | (0.68, 1.53) | 0.94 |
| Multivariate adjusted2 | 8857 | 680 | (0.49%) | 303 | 22 | (0.47%) | 1.03 | (0.68, 1.58) | 0.87 |
| CHD (MI/CABG/PTCA) | |||||||||
| Age adjusted1 | 9529 | 863 | (0.59%) | 333 | 44 | (0.90%) | 1.58 | (1.17, 2.14) | 0.003 |
| Multivariate adjusted2 | 8857 | 786 | (0.58%) | 303 | 40 | (0.91%) | 1.80 | (1.30, 2.47) | 0.0003 |
| CVD death | |||||||||
| Age adjusted1 | 9529 | 984 | (0.64%) | 333 | 51 | (0.98%) | 1.57 | (1.19, 2.09) | 0.002 |
| Multivariate adjusted2 | 8857 | 895 | (0.63%) | 303 | 46 | (0.98%) | 1.70 | (1.26, 2.30) | 0.001 |
| All-cause mortality | |||||||||
| Age adjusted1 | 9529 | 2689 | (1.75%) | 333 | 108 | (2.07%) | 1.25 | (1.03, 1.52) | 0.02 |
| Multivariate adjusted2 | 8857 | 2469 | (1.73%) | 303 | 98 | (2.08%) | 1.28 | (1.04, 1.56) | 0.02 |
| Sensitivity analyses: | |||||||||
| CVD (CHD/acute+chronic HF/ stroke/CVD death) | |||||||||
| Age adjusted1 | 9529 | 2292 | (1.61%) | 333 | 101 | (2.13%) | 1.37 | (1.12, 1.67) | 0.002 |
| Multivariate adjusted2 | 8857 | 2106 | (1.59%) | 303 | 94 | (2.20%) | 1.56 | (1.27, 1.92) | <0.0001 |
| Heart failure, acute+chronic | |||||||||
| Age adjusted1 | 9529 | 745 | (0.50%) | 333 | 45 | (0.89%) | 1.91 | (1.41, 2.59) | <0.0001 |
| Multivariate adjusted2 | 8857 | 685 | (0.49%) | 303 | 43 | (0.94%) | 2.39 | (1.75, 3.27) | <0.0001 |
HR, Hazard Ratio; CI, confidence interval; Ann %. Annualized Rate (%)
Hazard ratios, 95% confidence intervals and p-values are adjusted for age, PAGE II vs. SHARe study and the first 10 principal components (PCs) plus interaction terms of the PCs with PAGE II vs. SHARe study.
Hazard ratios, 95% confidence intervals and p-values are adjusted for age, body mass index (kg/m2), smoking status, history of CVD (MI/CABG/PCI/CHF/stroke), history of hypertension, history of diabetes, physical activity (MET hrs/wk), Clinical Trial vs. Observational Study participation, Hormone Therapy and Diet Modification Trial randomization arms, PAGE II vs. SHARe study and the first 10 principal components (PCs) plus interaction terms of the PCs with PAGE II vs. SHARe study.
When we examined the interactions between age, BP, heart rate, BMI, physical activity, TTR V122I carrier status and CVD, we did not observe significant findings except for age (P=0.03) (Figure 3). In particular, the risk of CVD associated with the mutation among participants ages 60 and older was significantly higher compared to non-carriers of the same age group (ages 60–64: HR, 2.03; 95% CI 1.39, 2.95; P=0.0002; ages 65 and older: HR, 1.66; 95% CI 1.21, 2.30; P=0.002). Similarly, the risk of all-cause mortality associated with the mutation among women 65 years and older was significantly higher compared to non-carriers (HR, 1.54; 95% CI 1.17, 2.04; P=0.002) (Supplemental Table 2 & 3). Finally, we present SBP, DBP and heart rate trajectories by age in carriers vs. non-carriers and did not detect significant differences (Supplemental Figure 1).
Figure 3.
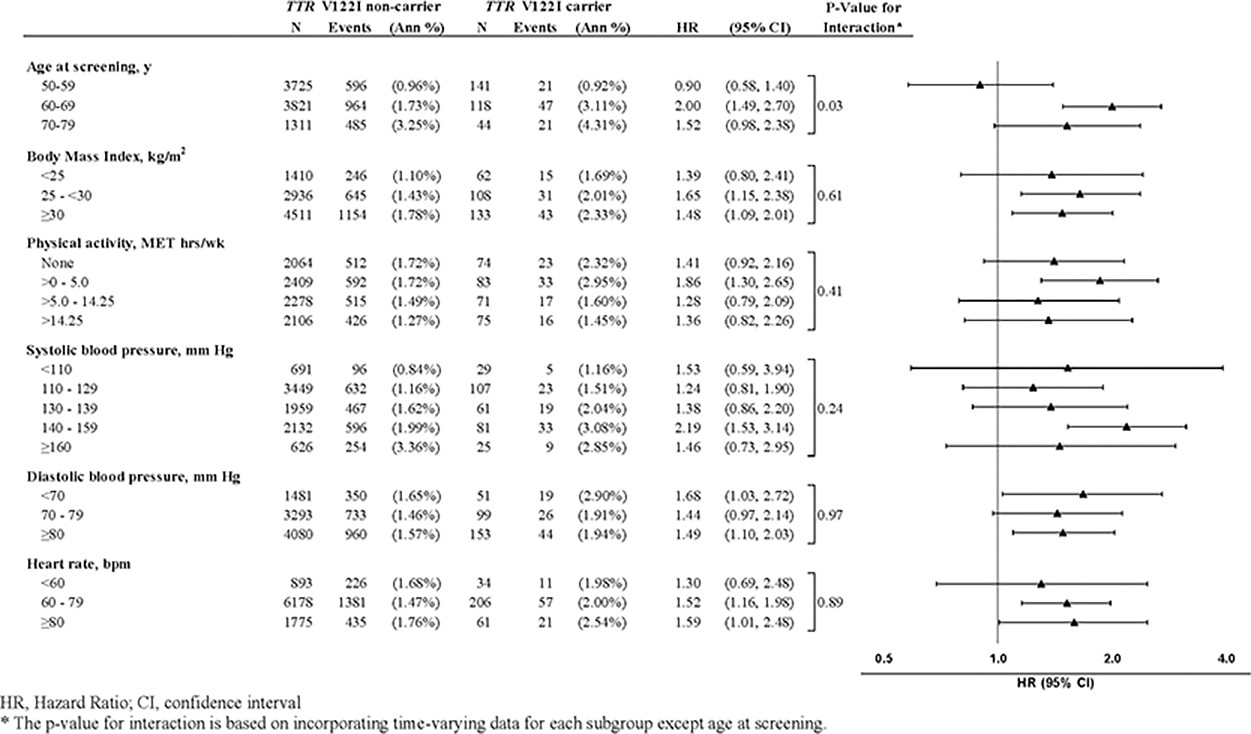
CVD Rates and Hazard Ratios (95% CIs) for TTR V122I carriers versus non-carriers
Discussion
Age and gender have been previously reported to play a role in TTR V122I penetrance with male carriers to be affected more severely.(2,18) In a large nationwide cohort, we found older Black women carrying the TTR V122I variant to have a substantially elevated risk for CVD and mortality compared to non-carriers. In comparison, results from the ARIC study which comprised 124 individuals with a mean age of around 50 to 53 (approximately 64% women) only showed a moderately elevated risk for heart failure (HR 1.47; 95% CI, 1.03 to 2.10; P=0.04). Recent data from the REGARDS study based on 232 carriers (approximately 61% women) at a similar age as our study but with shorter follow-up time point to a strong association with heart failure (HR 2.43; 95% CI 95%, 1.71 to 3.46; P < 0.001) and mortality (HR, 1.46; 95% CI, 1.19 to 1.78; P < 0.001) in line with our observations.(10,11) Moreover, we found the time to incident CVD and mortality to be considerably shorter in carriers older than 65 compared to non-carriers. Our results thus support the notion that TTR V122I carrier status imparts a significant risk increase which will inevitably become more important with age. In fact, an earlier autopsy study reported that, after age 65, all TTR V122I carriers exhibit some degree of cardiac amyloid deposition.(1)
It is known that affected individuals are more likely to be diagnosed with other diagnoses at first symptom such as carpal tunnel syndrome before receiving the diagnosis of transthyretin amyloidosis.(2,32) Our results of a higher CHD but not stroke risk in carriers point to a similar direction. Given that carriers typically present with left ventricular wall thickening, often have elevated troponin and Nt-proBNP levels, it appears plausible that these individuals are more likely to receive invasive coronary diagnostic work up for coronary artery disease before other differential diagnoses come into consideration. From a clinical standpoint, this suggests that cardiac amyloidosis may be substantially misinterpreted as long-standing hypertension, or other forms of cardiomyopathy. This argument is supported by the fact that Black individuals suffer from a higher burden of CVD risk factors and other disadvantages and inequalities and are more likely to access healthcare through the emergency department.(33–35) Recognizing cardiac amyloidosis as the responsible pathophysiological mechanism on the one hand and hypertension, coronary artery disease, etc. as watching bystanders and concomitant disease processes on the other hand may be challenging in daily practice. Interestingly, individuals exhibiting early transthyretin amyloidosis symptoms have been reported to have a lower BMI and higher levels of prior physical activity levels.(20,21) Our long-term data indicate that neither BMI or physical activity significantly interacts with later CVD or all-cause mortality risk.
Cardiac amyloidosis is characterized by restrictive left ventricular filling and reduced cardiac output due to increased ventricular wall thickness and myocardial stiffening over time.(2,3,36) As clinicians are thus regularly faced with the question which BP and heart rate levels to target in TTR V122I carriers knowing that the myocardium is stiffening, we proceeded to examine various BP and heart rate levels by carrier status but found no interaction or differences in BP or heart rate trajectory patterns over time.
The TTR V122I mutation has until recently not been included in the list of clinically actionable deleterious variants compiled by the American College of Medical Genetics and Genomics.(37,38) Given the prevalence of the mutation, the clinical consequences of the TTR V122I mutation currently remain underappreciated and cardiac amyloidosis is most likely under-diagnosed in clinical practice.(2) Our data in conjunction with results from other population-based cohort studies and biobanks, emphasize the risk associated TTR V122I variant carrier status and provide an outlook on the importance of genetic screening for carrier status and close follow-up of affected individuals.(10,11,39) This risk becomes strikingly apparent after age 65 with subclinical cardiac changes to be already detected around midlife (at 50 years). In light of approved therapeutics targeting transthyretin amyloid cardiomyopathy, recognizing, diagnosing, and treating affected individuals should happen early in the course of disease process as therapy may be more efficacious at this point.(36,40–42) The European Society of Cardiology currently recommends for asymptomatic genetic carriers to start clinical follow-up 10 years before the age of disease onset of affected relatives or predicted usual onset age for the specific mutation.(41) Following our data and recent results from the REGARDS study, TTR V122I variant carriers should undergo a cardiac assessment at the age of 50 to 55 years with follow-up and potential evaluation for start of early treatment.(11,40,42) Further data on clinical penetrance, and disease progression risk as well as clinical trials testing the utility of screening are warranted.
A strength of this analysis is the use of a nationwide population-based cohort of older women and the results are based on a large number of TTR V122I variant carriers. A standardized characterization of participants, adjudication of outcomes and long-time follow-up are other important points. Some limitations, however, should be noted. As we did not have echocardiographic data, endomyocardial biopsy specimens or autopsy specimens available, we could not evaluate the degree of cardiac involvement in carriers. We also could not report on homozygous women due to insufficient number. These limitations are similar to other analyses.(10,11) Finally, due to the observational character of our study, residual and unmeasured confounding could be partly responsible for the results.
In summary, the TTR V122I variant carries a significant increased risk of CVD and all-cause mortality. Our findings suggest that TTR V122I variant carrier status may not be as benign as previously thought among Black women. Women between age 50 to 59 years with increased left ventricular wall-thickness or signs of cardiomyopathy, amyloidosis red flags or relevant family history should be considered as screening candidates for TTR V122I variant carrier status to ensure early treatment onset.
Supplementary Material
Perspectives.
Competency in Medical Knowledge Competency in Patient Care
The TTR V122I variant carries a significant increased risk of CVD and all-cause mortality for Black women which will become more important with increasing age. The CVD risk is largely driven by heart failure, CHD and CVD death events, whereas no association with stroke was detected.
Black women showing red flags of amyloidosis should be screened for TTR V122I carrier status to ensure early treatment onset as the availability of newer therapeutics brings the promise of improving clinical outcomes.
Acknowledgments
The authors thank the WHI participants, clinical sites, investigators, and staff for their dedicated efforts. A list of current WHI investigators is available online at: https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/WHI-Investigator-Short-List.pdf
We thank Armin Schweitzer, Saarland University Hospital, for his help in preparing the central illustration of this manuscript.
Central Illustration.
Cardiovascular disease and mortality in Black women carrying the amyloidogenic V122I transthyretin gene mutation
Sources of Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. The opinions expressed in this manuscript are those of the authors and do not necessarily reflect the views of the Department of Health and Human Services/National Institutes of Health. The WHI PAGE 2 study was supported by grant U01HG007376.
Abbreviations:
- BMI
body-mass index
- BP
blood pressure
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HF
heart failure
- TTR
transthyretin
- WHI
Women’s Health Initiative
Footnotes
Disclosures
None
References
- 1.Jacobson DR, Pastore RD, Yaghoubian R et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med 1997;336:466–73. [DOI] [PubMed] [Google Scholar]
- 2.Buxbaum JN, Ruberg FL. Transthyretin V122I (pV142I)* cardiac amyloidosis: an age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med 2017;19:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012;126:1286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buxbaum JN, Dispenzieri A, Eisenberg DS et al. Amyloid nomenclature 2022: update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2022;29:213–219. [DOI] [PubMed] [Google Scholar]
- 5.Maurer MS, Hanna M, Grogan M et al. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol 2016;68:161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid 2015;22:171–4. [DOI] [PubMed] [Google Scholar]
- 7.Shah KB, Mankad AK, Castano A et al. Transthyretin Cardiac Amyloidosis in Black Americans. Circ Heart Fail 2016;9:e002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dungu JN, Papadopoulou SA, Wykes K et al. Afro-Caribbean Heart Failure in the United Kingdom: Cause, Outcomes, and ATTR V122I Cardiac Amyloidosis. Circ Heart Fail 2016;9. [DOI] [PubMed] [Google Scholar]
- 9.Mazzarotto F, Argirò A, Zampieri M et al. Investigation on the high recurrence of the ATTRv-causing transthyretin variant Val142Ile in central Italy. Eur J Hum Genet 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quarta CC, Buxbaum JN, Shah AM et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med 2015;372:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parcha V, Malla G, Irvin MR et al. Association of Transthyretin Val122Ile Variant With Incident Heart Failure Among Black Individuals. Jama 2022;327:1368–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin 2013;29:63–76. [DOI] [PubMed] [Google Scholar]
- 13.Dispenzieri A, Coelho T, Conceição I et al. Clinical and genetic profile of patients enrolled in the Transthyretin Amyloidosis Outcomes Survey (THAOS): 14-year update. Orphanet J Rare Dis 2022;17:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buxbaum J, Alexander A, Koziol J, Tagoe C, Fox E, Kitzman D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J 2010;159:864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caponetti AG, Rapezzi C, Gagliardi C et al. Sex-Related Risk of Cardiac Involvement in Hereditary Transthyretin Amyloidosis: Insights From THAOS. JACC Heart Fail 2021;9:736–746. [DOI] [PubMed] [Google Scholar]
- 16.Rapezzi C, Emdin M, Aimo A. Unravelling the role of sex in the pathophysiology, phenotypic expression and diagnosis of cardiac amyloidosis. Eur J Heart Fail 2022;24:2364–2366. [DOI] [PubMed] [Google Scholar]
- 17.Patel RK, Ioannou A, Razvi Y et al. Sex differences among patients with transthyretin amyloid cardiomyopathy - from diagnosis to prognosis. Eur J Heart Fail 2022;24:2355–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandrashekar P, Alhuneafat L, Mannello M et al. Prevalence and Outcomes of p.Val142Ile TTR Amyloidosis Cardiomyopathy: A Systematic Review. Circ Genom Precis Med 2021;14:e003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gertz MA, Benson MD, Dyck PJ et al. Diagnosis, Prognosis, and Therapy of Transthyretin Amyloidosis. J Am Coll Cardiol 2015;66:2451–2466. [DOI] [PubMed] [Google Scholar]
- 20.Poterucha TJ, Kurian D, Raiszadeh F et al. Relation of Body Mass Index to Transthyretin Cardiac Amyloidosis Particularly in Black and Hispanic Patients (from the SCAN-MP Study). Am J Cardiol 2022;177:116–120. [DOI] [PubMed] [Google Scholar]
- 21.Lee YZJ, Fajardo J, Brown E, D’Adamo CR, Judge DP. Wild-Type Transthyretin Cardiac Amyloidosis Is Associated with Increased Antecedent Physical Activity. J Cardiovasc Transl Res 2022;15:689–691. [DOI] [PubMed] [Google Scholar]
- 22.NCBI Database of Genotypes and Phenotypes (dbGaP). https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000386.v8.p3. Last accessed 02-17-23.
- 23.Wojcik GL, Graff M, Nishimura KK et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature 2019;570:514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 25.Rossouw JE, Anderson GL, Prentice RL et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 26.Anderson GL, Manson J, Wallace R et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol 2003;13:S5–17. [DOI] [PubMed] [Google Scholar]
- 27.Wojcik GL, Fuchsberger C, Taliun D et al. Imputation-Aware Tag SNP Selection To Improve Power for Large-Scale, Multi-ethnic Association Studies. G3 (Bethesda) 2018;8:3255–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutter CM, Young AM, Ochs-Balcom HM et al. Replication of breast cancer GWAS susceptibility loci in the Women’s Health Initiative African American SHARe Study. Cancer Epidemiol Biomarkers Prev 2011;20:1950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curb JD, McTiernan A, Heckbert SR et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol 2003;13:S122–8. [DOI] [PubMed] [Google Scholar]
- 30.Chobanian AV, Bakris GL, Black HR et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 31.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006;38:904–9. [DOI] [PubMed] [Google Scholar]
- 32.Bishop E, Brown EE, Fajardo J, Barouch LA, Judge DP, Halushka MK. Seven factors predict a delayed diagnosis of cardiac amyloidosis. Amyloid 2018;25:174–179. [DOI] [PubMed] [Google Scholar]
- 33.Brown LE, Burton R, Hixon B et al. Factors influencing emergency department preference for access to healthcare. West J Emerg Med 2012;13:410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carnethon MR, Pu J, Howard G et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 35.Bell CN, Thorpe RJ Jr. Bowie JV, LaVeist TA Race disparities in cardiovascular disease risk factors within socioeconomic status strata. Ann Epidemiol 2018;28:147–152. [DOI] [PubMed] [Google Scholar]
- 36.Kittleson MM, Maurer MS, Ambardekar AV et al. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020;142:e7–e22. [DOI] [PubMed] [Google Scholar]
- 37.Miller DT, Lee K, Gordon AS et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2021 update: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2021;23:1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller DT, Lee K, Abul-Husn NS et al. ACMG SF v3.1 list for reporting of secondary findings in clinical exome and genome sequencing: A policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2022;24:1407–1414. [DOI] [PubMed] [Google Scholar]
- 39.Damrauer SM, Chaudhary K, Cho JH et al. Association of the V122I Hereditary Transthyretin Amyloidosis Genetic Variant With Heart Failure Among Individuals of African or Hispanic/Latino Ancestry. Jama 2019;322:2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maurer MS, Schwartz JH, Gundapaneni B et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Pavia P, Rapezzi C, Adler Y et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2021;42:1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rapezzi C, Elliott P, Damy T et al. Efficacy of Tafamidis in Patients With Hereditary and Wild-Type Transthyretin Amyloid Cardiomyopathy: Further Analyses From ATTR-ACT. JACC Heart Fail 2021;9:115–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.