Abstract
Aim:
To evaluate the clinical and economic impact of antiarrhythmic drugs (AADs) compared with ablation both as individual treatments and as combination therapy without/with considering the order of treatment among patients with atrial fibrillation (AFib).
Materials & methods:
A budget impact model over a one-year time horizon was developed to assess the economic impact of AADs (amiodarone, dofetilide, dronedarone, flecainide, propafenone, sotalol, and as a group) versus ablation across three scenarios: direct comparisons of individual treatments, non-temporal combinations, and temporal combinations. The economic analysis was conducted in accordance with CHEERS guidance as per current model objectives. Results are reported as costs per patient per year (PPPY). The impact of individual parameters was evaluated using one-way sensitivity analysis (OWSA).
Results:
In direct comparisons, ablation had the highest annual medication/procedure cost ($29,432), followed by dofetilide ($7661), dronedarone ($6451), sotalol ($4552), propafenone ($3044), flecainide ($2563), and amiodarone ($2538). Flecainide had the highest costs for long-term clinical outcomes ($22,964), followed by dofetilide ($17,462), sotalol ($15,030), amiodarone ($12,450), dronedarone ($10,424), propafenone ($7678) and ablation ($9948). In the non-temporal scenario, total costs incurred for AADs (group) + ablation ($17,278) were lower compared with ablation alone ($39,380). In the temporal scenario, AADs (group) before ablation resulted in PPPY cost savings of ($22,858) compared with AADs (group) after ablation ($19,958). Key factors in OWSA were ablation costs, the proportion of patients having reablation, and withdrawal due to adverse events.
Conclusion:
Utilization of AADs as individual treatment or in combination with ablation demonstrated comparable clinical benefits along with costs savings in patients with AFib.
Keywords: ablation, antiarrhythmic drugs, atrial fibrillation, economic model
Plain language summary
What is this article about?
Atrial fibrillation (AFib) is the most common type of heart rhythm disorder in which the heart beats too slowly, too fast, or in an irregular way. During AFib, the upper and lower chambers of the heart beat out of sync, causing blood flow to be ineffective. Symptoms of AFib include palpitations, lightheadedness, shortness of breath, and fatigue. Guidelines recommend rate control and rhythm control as important treatments for the management of AFib. Rhythm control strategies including antiarrhythmic drugs (AADs) and ablation are promising interventions for maintaining sinus rhythm.
This study assessed the economic impact of AAD use versus ablation in patients with AFib from a US payer's perspective. Three different scenarios were compared: direct comparisons of individual treatments, non-temporal combinations (scenarios where order of treatments was not considered), and temporal combinations (scenarios where order of treatments was considered).
What were the results?
In the direct comparison of AADs versus ablation, per patient per year (PPPY) costs across AADs were 35%–73% lower compared with ablation, mainly due to higher procedural costs of ablation. In the non-temporal scenario, PPPY costs for AADs used in combination with ablation were 44% lower compared with ablation alone. In the temporal scenario, AADs placed before ablation yielded 87% cost savings compared with AADs placed post-ablation, largely due to improved clinical outcomes associated with AAD use and high costs of ablation.
What do the results mean?
Overall, use of AADs alone or in combination with ablation resulted in comparable clinical benefits with lower costs. Decision-makers can use these findings to inform decisions about economical treatment alternatives available for patients with AFib.
Atrial fibrillation (AFib) is the most common sustained cardiac arrhythmia, imposing a substantial impact on mortality and morbidity [1–3]. As per 2019 estimates, AFib was mentioned on 183,321 death certificates in the USA and was the underlying cause of death in 26,535 of those deaths [4]. More than 454,000 hospitalizations have been attributed to AFib as a primary diagnosis each year in the US [5], where prevalence is expected to reach 12.1 million by 2030 [6].
People affected with AFib experience an interruption in their normal sinus rhythm in which the upper chambers of the heart (atria) beat irregularly and out of coordination with the lower chambers of the heart (ventricles) [7]. Asymptomatic episodes of AFib have been reported in approximately 15% to 30% of patients [8]. For symptomatic AFib, patients may experience a range of mild to severe symptoms including heart palpitations, lightheadedness, shortness of breath, and chest pain [7]. AFib is associated with increased risk of thromboembolism, cognitive dysfunction, heart failure (HF), stroke, and premature death, which can adversely affect quality of life (QoL) and lead to enormous economic burden [9]. In the US, management of AFib has been estimated to range in cost from $6–$26 billion annually and reportedly increased hospitalization costs by 11% between 2010 and 2017 [10,11].
A comprehensive therapy approach centered on the Atrial Fibrillation Better Care (ABC) pathway has been suggested to reduce the risk of adverse outcomes for people with AFib [12]. The ABC pathway is guided by three main aspects: “A” Avoid stroke with anticoagulants; “B” Better symptom management, with patient-centered decisions on rate or rhythm control; and “C” Cardiovascular and Comorbidity risk optimization [13]. Increasing evidence supports early rhythm control for most patients recently diagnosed with AFib as well as for those who are symptomatic [14,15]. Treatment options such as antiarrhythmic drugs (AADs), catheter ablation, or a combination of the two are recommended for maintaining sinus rhythm, and the decision to pursue either AADs or catheter ablation is based on a patient's clinical profile, patient decision, and physician discretion [15–17].
For treatment with AADs, amiodarone, dofetilide, dronedarone, flecainide, propafenone, and sotalol are commonly used drugs available in the US for patients with paroxysmal and persistent AFib [18,19]. The clinical benefits of dronedarone were evaluated in the ATHENA trial, demonstrating significantly better cardiovascular (CV) outcomes and fewer hospitalizations in patients with paroxysmal and persistent AFib [14]. Recently, the EAST AFNET4 trial showed that patients undergoing rhythm control via AADs or ablation had a significantly lower risk of CV events over a follow-up period of 5 years compared with patients who received usual care [15]. Despite observed clinical benefits, there is evidence showing that some AADs can be associated with adverse events (AE) [18], hence limiting patient adherence and subsequently their efficacy in rhythm control.
However, some recent studies have demonstrated potentially better CV outcomes with catheter ablation in AFib patients compared with AADs [20]. For example, the CABANA trial showed that patients receiving ablation exhibited significant improvements in survival, risk of AFib recurrence, and QoL compared with patients treated with AADs only [21]. Furthermore, the STOP AF [22] and the EARLY AF [16] trials demonstrated a lower rate of AFib recurrence in patients treated with cryoablation compared with those who received AADs. Results of other RCTs have suggested that ablation as first-line therapy followed by AADs is more effective in maintaining sinus rhythm than AADs as first-line [23]. Although ablation is associated with meaningful clinical benefits and success compared with AADs, there is also major risk for procedural complications [24] and potential high economic burden.
In the present study, we aimed to evaluate the economic impact of AADs compared with ablation, both as individual treatments and as combination therapy, from the US payer perspective. We investigated long-term clinical outcomes (LTCO) associated with treatments as well as procedural complications of ablation and adverse treatment effects of AADs, along with scenarios either taking the order of treatments in combination therapy into account or not.
Methods
Overview
The economic impact of rhythm control treatments was calculated using a one-year model horizon developed in Microsoft Excel 2010 (Microsoft Corp, Redmond, WA). Different treatment scenarios (Figure 1) were compared to assess the economic benefits of AADs (amiodarone, dofetilide, dronedarone, flecainide, propafenone, sotalol, and AADs as a group) versus ablation among US adults with AFib. The economic impact of AADs was calculated as the difference in total annual costs of AADs (individually, or in combination with ablation) with the cost of ablation alone (accounting for a 30% rate of reablation [25]) among the different scenarios. The economic evaluations reported in this study followed CHEERS guidance [26] as per current model requirements.
Figure 1. . Framework of the rhythm control economic model.

AAD: Antiarrhythmic drug; AE: Adverse event; AFib: Atrial fibrillation; LTCO: Long-term clinical outcomes; PPPY: Per patient per year.
Study population & data source
In a retrospective database analysis, a reference AFib population of patients from US Merative MarketScan (formerly Truven MarketScan) meeting the following inclusion criteria was identified:
- Had ≥1 inpatient or ≥2 outpatient claims with a primary or secondary diagnosis of paroxysmal (International Classification of Diseases, 10th Revision [ICD-10] I48.0) or persistent (ICD-10 I48.1) AFib occurring on different days during the identification period (January 2016 to December 2019).
- The index date is the date of the first claim for AFib.
Patients ≥18 years old at 12 months pre-index.
Continuous health plan eligibility during the 12 months pre-index.
No previous diagnosis of AFib during the 12 months pre-index.
No previous diagnosis of atrial flutter during the 12 months pre-index.
No previous hospitalization for HF during the 12 months pre-index.
Treatment scenarios were classified into three categories. The first scenario was individual direct comparisons, including all AADs individually and as a group versus ablation alone. The second scenario was a non-temporal combination of comparisons in which the order of treatment was not taken into account, including all AADs individually and as a group used with ablation versus ablation alone. The third scenario was temporal combination comparisons in which the order of treatment was taken into account, including: AADs as a group given before ablation versus AADs as a group given after ablation; AADs as a group given before ablation versus ablation only; AADs as a group given after ablation versus ablation only. Due to limited availability of data, results for individual AADs were not reported in the temporal scenarios.
The target population included US adult AFib patients. Patients included in the study had not previously used AADs or undergone ablation, i.e., incident patients. The analysis was conducted from a US payer perspective over one year. Direct medical costs to payers included those associated with treatment, outpatient administration, LTCO (i.e., events most often happening a year or more following treatment initiation, as opposed to short-term AE occurring at or around time of initiation), and ablation procedural complications, reported in 2021 USD. Results were reported as cost savings per patient in the target population per year (PPPY).
Model inputs
Data available from the literature, Sanofi internal analyses, and results generated from the retrospective database analysis were included as model inputs. The target population included all treated patients with AFib (paroxysmal and persistent) in a hypothetical health plan of 1,000,000 members.
LTCOs included risks from withdrawal due to AE, proarrhythmia, stroke, and AFib recurrence, based on the most common outcomes reported across clinical trials comparing patients receiving AADs versus placebo (Table 1) [18]. For the scenario of direct comparison of individual therapies, treated risks were calculated by multiplying risk ratios for AADs (except for stroke in dofetilide and propafenone) obtained from a meta-analysis of clinical trials reporting LTCOs associated with use of AADs [18] times the risk of LTCOs observed in the reference AFib population (from the database analyses). Due to a lack of literature evidence, the treated risk for stroke in dofetilide was calculated as the average treated risk for stroke in Vaughan Williams class 3 AADs [27]. The treated risk for stroke in propafenone was taken directly from literature [28]. Treated risks for proarrhythmia in dofetilide and withdrawal due to AEs in flecainide were calculated as being greater than 1.0, suggesting placebo as having lower rates of LTCOs than flecainide and dofetilide, an unlikely conclusion based on weak evidence from published literature (Supplementary Table 1) [18]. Thus, calculated treated risks for LTCOs which were greater than 1.0 were capped at 0.99. For all other scenarios, the risks of LTCOs were include directly from the database analysis, and not calculated by multiplying risk ratios in the literature times risks in the reference AFib population. Costs for LTCO were obtained from the literature and adjusted to 2021 costs utilizing medical inflation rates [29]: withdrawal due to AEs ($6496) [30], AFib recurrence ($10,288) [31], proarrhythmia ($10,952) [32], and stroke ($28,008) [33]. Total costs for LTCOs were calculated as the sum of each LTCO's treated risk multiplied by its cost (Table 2). In the scenarios for combination treatments, PPPY costs for ablation were obtained from the retrospective database analysis and distributed among all users of each AAD and AADs as a group.
Table 1. . Risk of long-term clinical outcomes and ablation procedural complications.
| LTCO/ablation procedural complication | Ablation | Reablation [34] | Ref. |
|---|---|---|---|
| Withdrawal due to AE† | NA | NA | |
| Proarrhythmia | 0.079 | 0.079 | |
| Stroke | 0.020 | 0.005 [35] | |
| AFib recurrence | 0.391 | 0.062 | |
| Pericardial effusion | 0.022 | 0.022 | [31] |
| Cardiac tamponade | 0.013 | 0.013 | [31] |
| Intra-/Post-operative hemorrhage/hematoma requiring transfusion (excluding ESRD/chronic anemia) | 0.019 | 0.019 | [31] |
| Vascular injury/aneurysm/AV fistula (excluding ESRD patient) | 0.011 | 0.011 | [31] |
| Intubation - 96 h | 0.015 | 0.015 | [31] |
| Vascular injury requiring surgical intervention | 0.007 | 0.007 | [31] |
Withdrawal due to AE is only applicable for the AAD therapy and not applicable for ablation and reablation. Based on the literature [34], the risk of complications of reablations (except stroke) is considered the same as the risk of index ablation.
AE: Adverse event; AFib: Atrial fibrillation; AV: Arteriovenous; ESRD: End-stage renal disease; LTCO: Long-term clinical outcome; NA: not applicable.AE: Adverse event; AFib: Atrial fibrillation; AV: Arteriovenous; ESRD: End-stage renal disease; LTCO: Long-term clinical outcome; NA: Not applicable.
Table 2. . Cost of long-term clinical outcomes and ablation procedural complications.
| LTCO/ablation procedural complication | Cost of LTCOs/ablation procedural costs, $ | Ref. |
|---|---|---|
| Withdrawal due to AE | 6496 | [30] |
| Proarrhythmia | 10,952 | [32] |
| Stroke | 28,008 | [33] |
| AFib recurrence | 10,288 | [31] |
| Pericardial effusion | 30,793 | [36,37] |
| Cardiac tamponade | 30,793 | [36,37] |
| Intra-/post-operative hemorrhage/hematoma requiring transfusion (excluding ESRD/chronic anemia) | 27,239 | [36,37] |
| Vascular injury/aneurysm/AV fistula (excluding ESRD patient) | 24,179 | [36,37] |
| Intubation - 96 h | 24,886 | [36,37] |
| Vascular injury requiring surgical intervention | 62,662 | [36,37] |
LTCO costs are inflated to September 2021 prices using the CPI inflation calculator [29].
AE: Adverse event; AFib: Atrial fibrillation; AV: Arteriovenous; ESRD: End-stage renal disease; LTCO: Long-term clinical outcome.
Procedural complications of ablation included pericardial effusion, cardiac tamponade, intra-/post-operative hemorrhage/hematoma requiring transfusion (excluding end-stage renal disease [ESRD]/chronic anemia), vascular injury/aneurysm/arteriovenous [AV] fistula (excluding ESRD patient), intubation (96 h in duration), and vascular injury requiring surgical intervention. These complications were selected because they had an incidence of ≥0.5% in the literature [24]. Sultan et al. reported that the occurrence of procedural complications did not differ between initial and repeat ablations [34], thus the current model assumed the same. Furthermore, Numminen et al. reported that rates of major procedural complications did not differ by sex, body mass index, or age, with no other factors reported on [38]. Thus, the current model assumed that procedural complications of ablation were due to the procedure itself and not impacted by any other factor. Procedure costs of index ablation and reablation were assumed to be the same at $22,640 [33], adjusted to 2021 USD. Costs of ablation procedural complications were based on Diagnosis Related Group (DRG) codes retrieved from Find-A-Code [36] and converted to commercial costs using a factor of 2.24, as suggested by a 2021 report by the RAND Corporation [37].
Costs of AADs were wholesale acquisition costs (WAC; Table 3). The cost of outpatient administration for AADs was assumed to be $131.20, based on an office visit of an established patient (Current Procedural Terminology [CPT] 99214) [39], while inpatient costs for sotalol and dofetilide were considered as $4442 and $4892, respectively based on the literature [40]. In the absence of literature evidence, the inpatient costs for the rest of the AADs were calculated as the average inpatient costs of sotalol and dofetilide. Dosing and administration guidelines in product package inserts informed outpatient and inpatient administration proportions [36,41–45]; i.e., 0% and $0 in sotalol and dofetilide in the outpatient setting which are administered 100% in the inpatient setting at $4442 and $4892, respectively [40], 50% and $65.60 in the outpatient setting and 50% and $2333.50 in the inpatient setting for amiodarone, propafenone, and flecainide, and 100% and $131.20 for dronedarone which is administered entirely in the outpatient setting [40].
Table 3. . Wholesale acquisition costs of antiarrhythmic drugs.
| Treatment | Unit cost, $ | Frequency per day | Average daily cost, $ |
|---|---|---|---|
| Dronedarone | 12.19† | 2‡ | 24.38 |
| Amiodarone | 0.31§ | 2¶ | 0.62 |
| Sotalol | 0.26# | 2†† | 0.52 |
| Flecainide | 0.57‡‡ | 1§§ | 0.57 |
| Propafenone | 0.77¶¶ | 3## | 2.31 |
| Dofetilide | 3.99††† | 2‡‡‡ | 7.98 |
Average daily cost of treatment arms is considered by multiplying unit costs with daily drug intake frequency.
www.drugs.com/price-guide/multaq. Accessed on October 19, 2021.
www.drugs.com/dosage/dronedarone.html. Accessed on October 19, 2021.
www.drugs.com/price-guide/amiodarone. Accessed on October 19, 2021.
www.drugs.com/dosage/amiodarone.html. Accessed on October 19, 2021.
www.drugs.com/price-guide/sotalol. Accessed on October 19, 2021.
www.drugs.com/dosage/sotalol.html. Accessed on October 19, 2021.
www.drugs.com/price-guide/flecainide. Accessed on October 19, 2021.
www.drugs.com/dosage/flecainide.html. Accessed on October 19, 2021.
www.drugs.com/price-guide/propafenone. Accessed on October 19, 2021.
www.drugs.com/dosage/propafenone.html. Accessed on October 19, 2021.
www.drugs.com/price-guide/dofetilide. Accessed on October 19, 2021.
www.drugs.com/dosage/dofetilide.html. Accessed on October 19, 2021.
Patient cost-sharing (copay/coinsurance) was considered to be paid once per refill frequency. A discount rate of 20% was included for dronedarone [46]. Monitoring costs for administration and usage of AADs and for the ablation post-procedural period were not included in analysis.
Assumptions
The model did not differentiate between techniques used for catheter ablation, such as cryoballoon and radio-frequency ablation. Patients could undergo at most two ablation procedures (i.e., one index ablation and one reablation) within a year. An index ablation with a 30% incidence rate of reablation was assumed based on the literature [25]. Analysis did not account for drug adherence and persistence. LTCO costs for treatments were reported annually and not by event.
Sensitivity analysis
A one-way sensitivity analysis (OWSA) was conducted to evaluate the impact of individual parameters on model results. Variables included in the OWSA were epidemiology inputs (plan population, proportion of treated patients, and proportion of patients requiring a reablation) and cost inputs (annual costs of AADs, ablation procedural costs, annual costs of AADs in combination with ablation, copayment and coinsurance for each tier, LTCO costs, ablation procedural complication costs, and discounts on branded drugs). The range used in OWSA for population and cost parameters was set to ±30% from baseline.
Results
Direct comparison of individual therapies
Total costs PPPY for individual therapies are shown in Table 4. In this scenario, comparison of AADs with ablation resulted in PPPY cost savings of $28,658 for propafenone, $24,392 for amiodarone, $22,505 for dronedarone, $19,799 for sotalol, $13,853 for flecainide, and $16,581 for AADs as a group. Thus, PPPY cost for all AADs were 35%–73% lower compared with ablation. Greater cost savings of AADs were mainly driven by higher procedural costs of ablation. LTCO costs (including procedural complication costs for ablation) were comparable between ablation ($9948) and AADs ($7678–$22,964) (Table 4). LTCO accounted for 90% of PPPY costs for flecainide, 83% for amiodarone, 77% for sotalol, 72% for propafenone, 70% for dofetilide, 62% for dronedarone, 25% for ablation, and 80% for AADs as a group.
Table 4. . Cost savings of antiarrhythmic drugs when compared with ablation (direct comparison scenario).
| Treatment | Annual medication costs/ablation procedure costs, $ | LTCO costs (including procedural complications for ablation), $ | Total costs PPPY, $† | Cost savings‡ PPPY, $ |
|---|---|---|---|---|
| Dronedarone (400 mg) | 6451 | 10,424 | 16,875 | 22,505 |
| Amiodarone (200 mg) | 2538 | 12,450 | 14,988 | 24,392 |
| Sotalol (120 mg) | 4552 | 15,030 | 19,581 | 19,799 |
| Flecainide (100 mg) | 2563 | 22,964 | 25,527 | 13,853 |
| Propafenone (225 mg) | 3044 | 7678 | 10,722 | 28,658 |
| Dofetilide (125 mg) | 7661 | 17,462 | 25,122 | 14,258 |
| AADs (group) | 4468 | 18,331 | 22,799 | 16,581 |
| Ablation | 29,432 | 9948 | 39,380 | - |
Total cost is the sum of annual medication costs or ablation procedure costs and LTCO costs.
Cost savings is calculated from the difference between ablation cost and each drug cost.
AAD: Antiarrhythmic drug; LTCO: Long-term clinical outcomes; PPPY: Per patient per year.
Non-temporal comparison of combination therapies
Total costs PPPY for combination therapies not taking order of treatments into account are shown in Table 5. PPPY costs for combination therapies were lower than ablation alone by $24,466 for flecainide + ablation, $24,402 for amiodarone + ablation, $23,995 for propafenone + ablation, $21,772 for sotalol + ablation, $19,213 for dronedarone + ablation, $17,190 for dofetilide + ablation, and $22,102 for AADs as a group + ablation. The combination of AADs with ablation resulted in PPPY cost savings when compared with ablation due to higher procedural costs associated with ablation ($29,432). LTCO costs (including procedural complications for ablation) were comparable between ablation ($9948) and the combination therapies ($10,079–$11,780) (Table 5). LTCO accounted for 73% of PPPY costs for amiodarone + ablation, 72% for propafenone + ablation, 68% for flecainide + ablation, 65% for sotalol + ablation, 63% for dofetilide + ablation, 53% for dofetilide + ablation, and 53% of for dronedarone + ablation.
Table 5. . Cost savings of antiarrhythmic drugs when compared with ablation (non-temporal scenarios).
| Treatment | Annual medication costs/ablation procedure costs, $ | LTCO costs (including procedural complications for ablation), $ | Total costs† PPPY, $ | Cost savings‡ PPPY, $ |
|---|---|---|---|---|
| Dronedarone + ablation | 8894 | 11,273 | 20,166.89 | 19,213 |
| Amiodarone + ablation | 3986 | 10,992 | 14,977.86 | 24,402 |
| Sotalol + ablation | 6217 | 11,391 | 17,608.27 | 21,772 |
| Flecainide + ablation | 4835 | 10,079 | 14,913.71 | 24,466 |
| Propafenone + ablation | 4305 | 11,080 | 15,385.28 | 23,995 |
| Dofetilide + ablation | 10,410 | 11,780 | 22,190.19 | 17,190 |
| AADs (group) + ablation | 6441 | 10,837 | 17,277.89 | 22,102 |
| Ablation | 29,432 | 9948 | 39,380.04 |
Total cost is the sum of annual medication costs or ablation procedure costs and LTCO costs.
Cost savings is calculated as the difference between ablation cost and the combination of each drug with ablation cost.
AAD: Antiarrhythmic drug; LTCO: Long-term clinical outcome; PPPY: Per patient per year.
Temporal comparison of combination therapies
Total costs PPPY for combination therapies taking order of treatments into account are shown in Table 6. AADs given before ablation resulted in PPPY cost savings of $2900 compared with ablation coming before AADs. LTCO costs were the driving factor for cost savings ($10,080 for AADs before ablation and $12,981 for ablation before AADs), accounting for 61% of PPPY costs for AADs before ablation and 67% for ablation before AADs. Compared with ablation alone, PPPY cost savings were $22,858 for AADs before ablation and $19,958 for ablation before AADs (Table 6). Thus, PPPY costs for AADs before ablation were 15% lower compared with ablation before AADs and 58% lower when compared with ablation alone.
Table 6. . Cost savings of antiarrhythmic drugs when compared with ablation (temporal scenarios).
| Treatment | Annual medication costs/ablation procedure costs, $ | LTCO costs (including procedural complications for ablation), $ | Total costs† PPPY, $ | Cost savings, PPPY $ |
|---|---|---|---|---|
| AADs before ablation | $6441 | $10,080 | $16,522 | $22,858‡ |
| Ablation before AADs | $6441 | $12,981 | $19,422 | $19,958§ |
Total cost is the sum of annual medication costs or ablation procedure costs and LTCO costs.
Cost savings is calculated as the difference between ablation cost and cost of AADs before ablation.
Cost savings is calculated as the difference between ablation cost and cost of ablation before AADs.
AAD: Antiarrhythmic drug; LTCO: Long-term clinical outcome; PPPY: Per patient per year.
Sensitivity analysis: direct comparison scenario
In OWSA of the direct comparison scenario, index ablation costs, the proportion of patients undergoing reablation, the cost of proarrhythmia, and the annual cost of AADs as a group were the most impactful variables in the model (Figure 2). A 30% increase in index ablation costs increased PPPY savings for AADs by $8830 from its base value ($16,581). Other key variables influencing OWSA results are depicted in Figure 2.
Figure 2. . Tornado diagram – sensitivity analysis results (direct comparison scenarios).
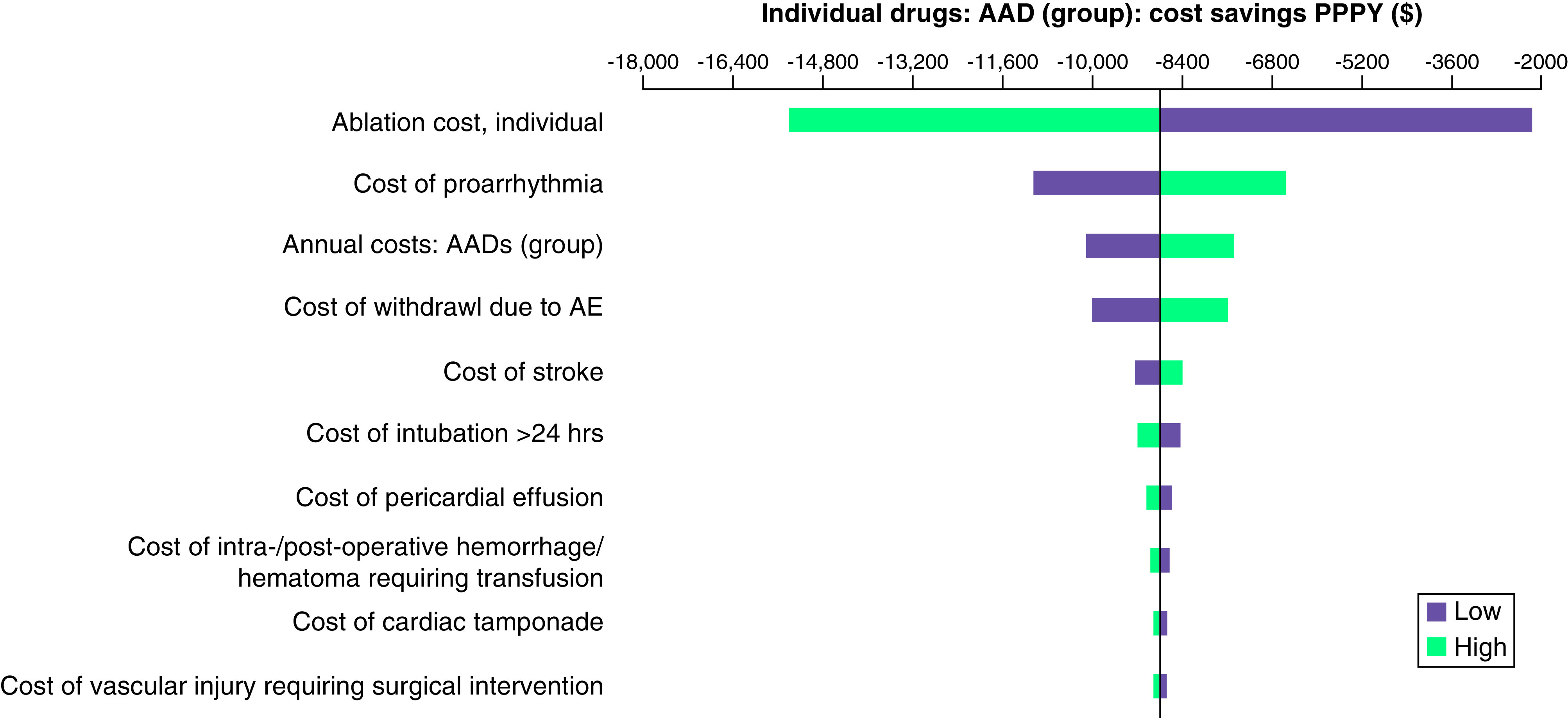
The sensitivity analysis for the direct comparison scenario shows the cost savings PPPY ($) of AADs as a group versus ablation alone.
AAD: Antiarrhythmic drug; AE: Adverse event; PPPY: Per patient per year.
Sensitivity analysis: non-temporal comparison of combination therapies
In OWSA of non-temporal comparisons of combination therapies, index ablation costs, the proportion of patients undergoing reablation, the annual cost of AADs as a group, and the ablation cost associated with AADs as a group had the greatest influence on results (Figure 3). A 30% increase in index ablation costs increased PPPY savings for AADs as a group in combination with ablation by $8830 from its base value ($22,102). Similarly, a 30% change in the proportion of patients undergoing reablation, the annual cost of AADs as a group, and ablation cost associated with AADs as a group resulted in PPPY savings of $2048, $1340, and $592, from the base value, respectively.
Figure 3. . Tornado diagram – sensitivity analysis results (non-temporal combination comparison scenarios).
The non-temporal comparison scenario shows the cost savings PPPY ($) of AADs as a group in combination with ablation compared to ablation wherein treatment order was not taken into consideration.
AADs: Antiarrhythmic drugs; PPPY: Per patients per year.
Sensitivity analysis: temporal comparisons of combination therapies
In OWSA of temporal comparisons of combination therapies, results were predominantly driven by costs of LTCO (Figure 4). A 30% increase in proarrhythmia costs increased PPPY savings for AADs as a group before ablation by $1026 from its base value ($22,859). Similarly, a 30% change in cost of AFib recurrence, stroke, and withdrawal due to AE resulted in PPPY savings of $4280, $1713, and $697, from the base value, respectively.
Figure 4. . Tornado diagram – sensitivity analysis results (temporal comparison scenarios).

The temporal comparison scenario shows the cost savings PPPY ($) of AADs as a group given before ablation compared with ablation given before AADs as a group.
AADs: Antiarrhythmic drugs; AE: Adverse events; AFib: Atrial fibrillation; PPPY: Per patients per year.
Discussion
In this study, we investigated the clinical and economic outcomes of rhythm control treatment among AFib patients via an economic model from a US payer perspective to assess the cost savings of AADs compared with ablation, both as an individual treatment option and as combination therapy. The analysis presented herein also addressed the LTCO associated with treatments as well as procedural complications of ablation, along with non-temporal and temporal scenarios for combination therapy.
Long-term use of AADs is recommended as the first-line strategy for maintaining sinus rhythm, but utilization of these drugs is limited by risk of LTCOs (e.g., proarrhythmic events, stroke, all-cause mortality) and risks of AFib recurrence [18]. In addition to LTCOs, use of amiodarone has been found to be associated with severe adverse outcomes such as pulmonary toxic effects, diffuse alveolar hemorrhage, acute respiratory distress syndrome, and eosinophilic pneumonia [47,48]. Given the lower incidences of AFib recurrence and hospitalization rates, ablation represents an attractive therapeutic option in maintaining sinus rhythm. However, the clinical benefits of ablation are usually offset by its high upfront costs and risk of procedural complications. Thus, there exists a trade-off between high costs and better clinical outcomes between ablation versus AADs.
The current analysis demonstrated a reduction in incremental costs PPPY when AADs were used in combination with ablation therapy compared with ablation alone, a finding which can be mainly attributed to distribution of ablation costs among patients taking AADs (with or without ablation) versus concentration of ablation costs among patients having ablation (with or without AADs). Previous research has also documented better clinical outcomes associated with the use of AADs as an adjuvant therapy [21]. In the EAST-AFNET 4 study, patients who received rhythm control therapy primarily with AADs had a 21% reduced composite outcome of death from CV causes, stroke, or hospitalization with worsening HF or acute coronary syndrome compared with those who received usual care (hazards ratio [HR] 0.79; 96% confidence interval [CI] 0.66 - 0.94; p = 0.005) [15]. Furthermore, in a systematic review and meta-analysis comparing the relative safety and efficacy of ablation to AADs, Calkins et al. reported a higher success rate (77%) in the cohort undergoing multiple ablations when using AADs as an adjuvant therapy versus the cohort undergoing ablation without AADs (71%) [49]. Similar findings were reported by Gunawardena et al. in a UK-based study, where the majority of AFib patients were routinely taking AADs alone or as combination therapy prior to ablation treatment [50]. Likewise, in the CABANA trial, patients with HF who received an ablation procedure were taking AADs during the post-blanking follow-up period [21].
Furthermore, we observed higher economic benefits when AADs were used as a first-line therapy before ablation compared with the scenario when AADs were used post-ablation. This result is attributable to improved clinical outcomes associated with AAD use (i.e., lower frequency of proarrhythmia, AFib recurrence, and stroke) and the high upfront cost of ablation and reablation. Similarly, a post-hoc analysis of data from the ATHENA trial found that the risk of AFib recurrence was significantly lower in AFib patients who received dronedarone (57%) compared with placebo (71%) during the post-ablation period [51].
In OWSA, we observed cost savings from AADs placed before ablation were predominantly driven by lower risks and associated costs of LTCO. In addition, the most influential parameters impacting results in the scenario for direct comparison of individual therapies were index ablation costs, the proportion of patients undergoing reablation, and the cost of proarrhythmia, which are in accordance with previous findings [52].
While our study findings suggest comparable clinical outcomes and lower costs associated with AAD use either as individual treatment or in combination with ablation, the overall benefits of ablation have been well reported. For example, the 2020 ESC guidelines recommend catheter ablation as a safe and effective treatment option for maintaining sinus rhythm and improving QoL [23]. Furthermore, in a systematic literature review and meta-analysis, Asad et al. reported significant benefits of catheter ablation in men and younger patients compared with women and older patients (≥65 years) [53]. In addition, AFib patients with HF with reduced ejection fraction demonstrated lower rates of all-cause mortality with ablation compared with medical therapy [53]. However, in the present study we have observed that combination therapy, specifically, placing AADs ahead of ablation, resulted in cost–effectiveness and better LTCO. Hence, further investigation of combinational therapy as a treatment option in a wide spectrum of AFib patients is warranted.
Finally, ablation has been shown to have a positive impact on patients' QoL compared with treatment with AADs [54–56]. Though not included in our analysis, validated patient-reported outcome measures such as the Short-Form 36 version 2 (SF-36v2) [57] and EQ-5D-5L (Euroqol) [58] questionnaires, along with associated calculations for quality adjusted life years, would be an important addition to better understand all aspects of patients' health as well as inform discussions around value-based contracting and pricing decisions.
Overall, the use of AADs either as individual treatment or in combination with ablation resulted in comparable clinical benefits but with lower costs. Notably, AADs placed as first-line therapy ahead of ablation resulted in costs savings compared with AADs placed as second-line therapy. From the perspective of providers, payers, and patients, the lower overall costs of AADs either as individual treatment or as combination therapy, along with comparable clinical outcomes, support the use of AADs as first-line therapy followed by ablation in the management of AFib.
Limitations
This model prioritized evidence from the literature over results generated from analysis or real-world data (RWD). Where there were gaps in the literature, inputs from RWD were utilized. There are several limitations common to RWD, including incompleteness or inaccuracies in patients' medical records in claims data. Furthermore, patients' entire medical history is seldom captured within a single medical claims database, thus, there may be uncertainty around longitudinal measures and potential misclassification bias. The model did not take drug adherence and persistence or polypharmacy of AADs into account. Likewise, monitoring costs were not considered, which may have resulted in total costs across treatments being lower than what would occur in clinical and real-world settings [59]. Furthermore, cost associated with monitoring (e.g., adverse reactions at baseline and over time for patients taking amiodarone) were not taken into consideration in this study. Due to limited availability of data, the model did not differentiate between different AADs in temporal scenarios. Thus, comparisons of individual AADs in combination with ablation as first- versus second-line therapy were not able to be ascertained. Another limitation of the model was its short time horizon of one year. Within this one year, it was assumed that patients could receive at most two ablations. However, patients may receive more than two ablations in the real-world over an extended period of time. This cohort of patients experiencing multiple ablation failures represents a population in need of great medical intervention and monitoring for which limited data on clinical and economic outcomes exists. Furthermore, the results presented herein focused only on a general cohort of patients with AFib and did not investigate sub-cohorts of patients with structural heart disease, HF with reduced ejection fraction, or other cardiac implications. Finally, though inclusion of LTCO and ablation procedural complications is a strength of this model, the list of conditions was not exhaustive. Thus, there are additional risks and costs associated with rhythm control that were not accounted for.
Conclusion
An economic model was developed to understand the value of rhythm control with AADs versus and in combination with ablation from a US payer perspective over a one-year time horizon. This was achieved by performing a) direct comparison analysis of individual AADs versus ablation, b) non-temporal analysis of individual AADs in combination with ablation in which the order of treatments was not considered and c) temporal analysis of AADs as a group in combination with ablation where the order of treatments was considered. Use of AADs, individual or in combination with ablation, resulted in comparable clinical outcomes and overall cost savings due to high procedural costs of ablation. Specifically, AADs placed before ablation resulted in $2900 cost savings compared with ablation before AADs. Overall, results of this economic model suggest that AADs as first-line therapy for patients with AFib is a cost-effective treatment option, with potentially greater savings compared with ablation alone or placed ahead of AADs in combination therapy. Study findings can be used by decision-makers to inform formulary placement and utilization controls.
Summary points
Atrial fibrillation (AFib) is the most common type of heart rhythm disorder in which the heart beats too slowly, too fast, or in an irregular way.
Rhythm control strategies including antiarrhythmic drugs (AADs) and ablation are promising interventions for maintaining sinus rhythm.
This study aimed to evaluate the clinical and economic impact of AADs compared with ablation both as individual treatments and as combination therapy without/with considering the order of treatment among patients with AFib using a budget impact model over a one-year time horizon among a hypothetical plan population of 1 million patients.
In direct comparisons, ablation had the highest annual medication/procedure cost ($29,432), followed by dofetilide ($7661), dronedarone ($6451), sotalol ($4552), propafenone ($3044), flecainide ($2563), and amiodarone ($2538).
Costs for long-term clinical outcomes were highest for flecainide ($22,964), followed by dofetilide ($17,462), sotalol ($15,030), amiodarone ($12,450), dronedarone ($10,424), propafenone ($7678), and ablation ($9948).
In the non-temporal scenario, total costs for AADs + ablation ($17,278) were lower compared with ablation alone ($39,380).
In the temporal scenario, AADs before ablation resulted in per-patient-per-year cost savings of ($22,858) compared with AADs after ablation ($19,958).
Utilization of AADs as individual treatment or in combination with ablation demonstrated comparable clinical benefits along with cost savings in patients with AFib.
Supplementary Material
Acknowledgments
This study was presented in part at ISPOR Europe Conference, November 6–9, 2022. The authors thank Amrita Dubey (PhD) and Ajit Kumar Jaiswal (MPhil) of Axtria Pvt. Ltd., India, for providing medical writing/editorial support.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2023-0065
Author contributions
All authors contributed to the study design, interpretation of findings, and critical revision of the manuscript. All authors approved the submitted version of the manuscript and agree to be accountable for all aspects of the work.
Financial & competing interests disclosure
This study was funded by Sanofi U.S. Inc. (Bridgewater, NJ, USA). J Ken-Opurum and SSS Srinivas, are employees of Axtria, which received funding from Sanofi for this analysis. S Park, S Charland, A Revel, and R Preblick are employees and stockholders of Sanofi stock. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support was provided by Amrita Dubey and Ajit Kumar Jaiswal of Axtria Pvt. Ltd., India and was funded by Sanofi U.S. Inc.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Chugh SS, Havmoeller R, Narayanan K et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129(8), 837–847 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98(10), 946–952 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Kalantarian S, Ay H, Gollub RL et al. Association between atrial fibrillation and silent cerebral infarctions: a systematic review and meta-analysis. Ann. Intern. Med. 161(9), 650–658 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Atrial Fibrillation. www.cdc.gov/heartdisease/atrial_fibrillation.htm. (2023).
- 5.Benjamin EJ, Muntner P, Alonso A et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139(10), e56–e528 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Tsao CW, Aday AW, Almarzooq ZI et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation 145(8), e153–e639 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Mayo Clinic. Atrial fibrillation. www.mayoclinic.org/diseases-conditions/atrial-fibrillation/symptoms-causes/syc-20350624. (2023).
- 8.Rienstra M, Lubitz SA, Mahida S et al. Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation 125(23), 2933–2943 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health 9(5), 348–356 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Abugroun A, Taha A, Abdel-Rahman M, Volgman AS. Economic impact of atrial fibrillation on hospitalization outcomes of acute heart failure in the United States. Am. J. Cardiol. 138, 124–127 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ. Cardiovasc. Qual. Outcomes 4(3), 313–320 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Comprehensive management with the ABC (Atrial Fibrillation Better Care) Pathway in clinically complex patients with atrial fibrillation: a post hoc ancillary analysis from the AFFIRM Trial. J. Am. Heart Assoc. 9(10), e014932 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat. Rev. Cardiol. 14(11), 627–628 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Hohnloser SH, Crijns HJ, van Eickels M et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N. Engl. J. Med. 360(7), 668–678 (2009). [DOI] [PubMed] [Google Scholar]; •• This study helped in understanding safety of dronedarone.
- 15.Kirchhof P, Camm AJ, Goette A et al. Early rhythm-control therapy in patients with atrial fibrillation. N. Engl. J. Med. 383(14), 1305–1316 (2020). [DOI] [PubMed] [Google Scholar]; •• This study provided insight to understand the decision-making behavior for rhythm-control therapy in patients with early AFib.
- 16.Andrade JG, Wells GA, Deyell MW et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N. Engl. J. Med. 384(4), 305–315 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Kuck KH, Lebedev DS, Mikhaylov EN et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace 23(3), 362–369 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valembois L, Audureau E, Takeda A, Jarzebowski W, Belmin J, Lafuente-Lafuente C. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 9(9), (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study helped in calculating the risks of long-term clinical outcomes (LTCOs) for antiarrhythmic drugs (AADs), which provided a base to compare dronedarone with other AADs in scenario analysis.
- 19.January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 64(21), e1–e76 (2014). [DOI] [PubMed] [Google Scholar]; • This study provided recommendations for the management of patients with atrial fibrillation (AFib).
- 20.Marrouche NF, Brachmann J, Andresen D et al. Catheter ablation for atrial fibrillation with heart failure. N. Engl. J. Med. 378(5), 417–427 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Packer DL, Piccini JP, Monahan KH et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA Trial. Circulation 143(14), 1377–1390 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study provided insights on the benefits of catheter ablation compared to drug therapies in patients with symptomatic AFib.
- 22.Wazni OM, Dandamudi G, Sood N et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N. Engl. J. Med. 384(4), 316–324 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Hindricks G, Potpara T, Dagres N et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42(5), 373–498 (2021). [DOI] [PubMed] [Google Scholar]; •• This guideline provided insights on the recommendations to treat AFib.
- 24.Wu L, Narasimhan B, Ho KS, Zheng Y, Shah AN, Kantharia BK. Safety and complications of catheter ablation for atrial fibrillation: predictors of complications from an updated analysis the National Inpatient Database. J. Cardiovasc. Electrophysiol. 32(4), 1024–1034 (2021). [DOI] [PubMed] [Google Scholar]; •• This study provided insights on the safety and predictors of ablation procedural complications.
- 25.Nyong J, Amit G, Adler AJ et al. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst. Rev. 11(11), (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Husereau D, Drummond M, Augustovski F et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations.. CHEERS 2022 ISPOR Good Research Practices Task Force. [DOI] [PubMed] [Google Scholar]
- 27.Lei M, Wu L, Terrar DA, Huang CL. Modernized classification of cardiac antiarrhythmic drugs. Circulation 138(17), 1879–1896 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Freemantle N, Lafuente-Lafuente C, Mitchell S, Eckert L, Reynolds M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace 13(3), 329–345 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Consumer Price Index Inflation Calculator. (bls.gov), (2021). www.bls.gov/data/inflation_calculator.htm
- 30.Reynolds MR, Zimetbaum P, Josephson ME, Ellis E, Danilov T, Cohen DJ. Cost-effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ. Arrhythm Electrophysiol. 2(4), 362–369 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruggenjurgen B, Kohler S, Ezzat N, Reinhold T, Willich SN. Cost effectiveness of antiarrhythmic medications in patients suffering from atrial fibrillation. Pharmacoeconomics 31(3), 195–213 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Podrid PJ, Kowey PR, Frishman WH et al. Comparative cost-effectiveness analysis of quinidine, procainamide and mexiletine. Am. J. Cardiol. 68(17), 1662–1667 (1991). [DOI] [PubMed] [Google Scholar]
- 33.Anderson LH, Black EJ, Civello KC, Martinson MS, Kress DC. Cost-effectiveness of the convergent procedure and catheter ablation for non-paroxysmal atrial fibrillation. J. Med. Econ. 17(7), 481–491 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Sultan A, Luker J, Andresen D et al. Predictors of atrial fibrillation recurrence after catheter ablation: data from the German Ablation Registry. Sci. Rep. 7(1), (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szegedi N, Széplaki G, Herczeg S et al. Repeat procedure is a new independent predictor of complications of atrial fibrillation ablation. Europace. 21(5), 732–737 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Findacode.com. www.findacode.com/index.html. (2022).
- 37.Whaley CM, Briscombe B, Kerber R, O'Neill B, Kofner A. Prices Paid to Hospitals by Private Health Plans: Findings from Round 4 of an Employer-Led Transparency Initiative. Rand Health Q. 10(1), 5 (2022). [PMC free article] [PubMed] [Google Scholar]
- 38.Numminen A, Penttila T, Arola O et al. Treatment success and its predictors as well as the complications of catheter ablation for atrial fibrillation in a high-volume centre. J. Interv. Card. Electrophysiol. 63(2), 357–367 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Center for Medicare & Medicaid Services. Physician Fee Schedule Look-Up Tool (HCPCS code 99214). www.cms.gov/medicare/physician-fee-schedule/search?Y=0&T=4&HT=0&CT=3&H1=99214&M=5. (2021).
- 40.Kim MH, Klingman D, Lin J, Pathak P, Battleman DS. Cost of hospital admission for antiarrhythmic drug initiation in atrial fibrillation. Ann. Pharmacother. 43(5), 840–848 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Dronedarone label (fda.gov). MULTAQ® (dronedarone) tablets, for oral use Prescribing Information. www.accessdata.fda.gov/drugsatfda_docs/label/2011/022425s007lbl.pdf (2021).
- 42.Amiodarone label (fda.gov). www.accessdata.fda.gov/drugsatfda_docs/label/2018/018972s054lbl.pdf (2021).
- 43.Sotalol label (fda.gov). Sotalol IV (sotalol hydrochloride injection 10mL vial [15mg/mL]). www.accessdata.fda.gov/drugsatfda_docs/label/2020/022306s005lblrpl.pdf (2021).
- 44.Flecainide: Package Insert (Drugs.com). Flecainide: Package Insert / Prescribing Information. www.drugs.com/pro/flecainide.html (2021).
- 45.Propafenone label (fda.gov). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/019151s015lbl.pdf (2021).
- 46.MULTAQ Savings Card Program. MULTAQ® (dronedarone) Tablet 400 mg. www.multaq.com/savings-and-support/multaq-savings-card-program. (2023).
- 47.Ruzieh M, Moroi MK, Aboujamous NM et al. Meta-analysis comparing the relative risk of adverse events for amiodarone versus placebo. Am. J. Cardiol. 124(12), 1889–1893 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Mason JW. Amiodarone. N. Engl. J. Med. 316(8), 455–466 (1987). [DOI] [PubMed] [Google Scholar]
- 49.Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 20(1), e1–e160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunawardena RF, Furniss SS, Shepherd E, Santarpia G, Lord SW, Bourke JP. Outcomes following catheter ablation of atrial fibrillation in the UK: a single-centre cohort analysis. Brit. J. Cardiol. 17, 271–276 (2010). [Google Scholar]
- 51.Vamos M, Calkins H, Kowey PR et al. Efficacy and safety of dronedarone in patients with a prior ablation for atrial fibrillation/flutter: insights from the ATHENA study. Clin. Cardiol. 43(3), 291–297 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ken-Opurum J, Srinivas SS, Vadagam P et al. A value-based budget impact model for dronedarone compared with other rhythm control strategies. J. Comp. Eff. Res. 12(4), e220196 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Results from this study helped in informing the pricing and formulary decisions related to dronedarone.
- 53.Asad ZUA, Yousif A, Khan MS, Al-Khatib SM, Stavrakis S. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Circ. Arrhythm Electrophysiol. 12(9), e007414 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Blomstrom-Lundqvist C, Gizurarson S, Schwieler J et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 321(11), 1059–1068 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Packer DL, Mark DB, Robb RA et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 321(13), 1261–1274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mark DB, Anstrom KJ, Sheng S et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 321(13), 1275–1285 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The SF-36v2® Health Survey. Measure functional health and well-being from the patient's point of view with the SF-36v2® Health Survey. www.qualitymetric.com/health-surveys/the-sf-36v2-health-survey/. (2023).
- 58.EQ-5D. EUROQOL INSTRUMENTS. https://euroqol.org/ (2023).
- 59.Kibert JL, Franck JB, Maltese Dietrich N, Quffa LH, Franck AJ. Impact of a pharmacy-cardiology collaborative management program during initiation of antiarrhythmic drugs. Clin. Pharm. Res. Rep. 3(1), 30–35 (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



