Abstract
We conduct a comparative evaluation of the visual systems from the retina to the muscles of the mouse and the macaque monkey noting the differences and similarities between these two species. The topics covered include (1) visual-field overlap, (2) visual spatial resolution, (3) V1 cortical point-image [i.e., V1 tissue dedicated to analyzing a unit receptive field], (4) object versus motion encoding (5) oculomotor range, (6) eye, head, and body movement coordination, and (7) neocortical and cerebellar function. We also discuss blindsight in rodents and primates which provides insights on how the neocortex mediates conscious vision in these species. This review is timely because the field of visuomotor neurophysiology is expanding beyond the macaque monkey to include the mouse; there is therefore a need for a comparative analysis between these two species on how the brain generates visuomotor responses.
Keywords: cortical point-image, objects, motion, blindsight, oculomotor range, neocortex, cerebellum, mouse, macaque monkey
1. Introduction
There are many parallels between the visual systems of the mouse and the macaque monkey, the latter of which has been studied to finest detail over the last half century (Schiller and Tehovnik 2015). With the advent of optogenetics and two-photon imaging, two methods that are heavily focused on the mouse, there has been renewed interest in studying the visual system of the mouse (Froudarakis et al. 2019; Koch and Reid 2012). Presently, research efforts have concentrated on deducing the genetic, anatomic, and electrophysiologic characteristics of the mouse brain and on the computational analysis towards an algorithmic understanding of visual processing. Much less effort has gone toward performing a direct comparison of the visual systems of the mouse and the macaque monkey while keeping in mind the vast differences in their behavioral capacities. Here we discuss the following: visual-field overlap which has implications for stereovision, visual spatial resolution which limits how well visual scenes can be resolved, and the V1 cortical point-image which assesses the amount of tissue devoted to analyzing the visual attributes of an image based on the cortical magnification factor. Moreover, the substrates for both object vision and motion perception are considered for both cortical and subcortical brain regions, including the cerebellum. Additionally, the motor characteristics of visual processing are compared which includes the oculomotor range and how the eyes, head, and body of an animal are made to move with respect to a visual image. Finally, we revisit how the brain mediates conscious vision in rodents (i.e., the mouse, hamster, and gerbil) and primates (i.e., the macaque monkey and human) vis-à-vis blindsight.
2. Field of View, Spatial Resolution, and Retino-V1 Connectivity
Striking differences exist between the mouse and the macaque monkey with respect to their visual field of view and visual spatial resolution via the primary visual cortex (V1). These differences impact the way the visual systems of these two animals are innervated at the level of V1 by way of the lateral geniculate nucleus (LGN) which receives projection from the retina. The mouse unlike the macaque monkey has laterally displaced eyes such that the eyes project outward at approximately 50 degrees with respect to the longitudinal axis of the head (Heesy 2004; Samonds et al. 2019) thereby restricting the aligned binocular overlap to about 40 degrees of visual angle for straight-ahead viewing (Fig. 1, Rodent). Furthermore, the eyes of the mouse are oriented slightly upwards which provides an animal with an expansive view of the world such that objects approaching from the front, the side, overhead, or behind can be viewed (Van Alphen et al. 2010). In the case of the macaque monkey, the eyes are oriented forward and roughly parallel with respect to the longitudinal axis of the head when the eyes are centered in the orbit (Fig. 1, Primate). This permits for a higher degree of binocular overlap (about 130 degrees) which can be utilized for stereovision. The shortcoming here is that macaque monkeys cannot see objects approaching from above and behind.
Figure 1.
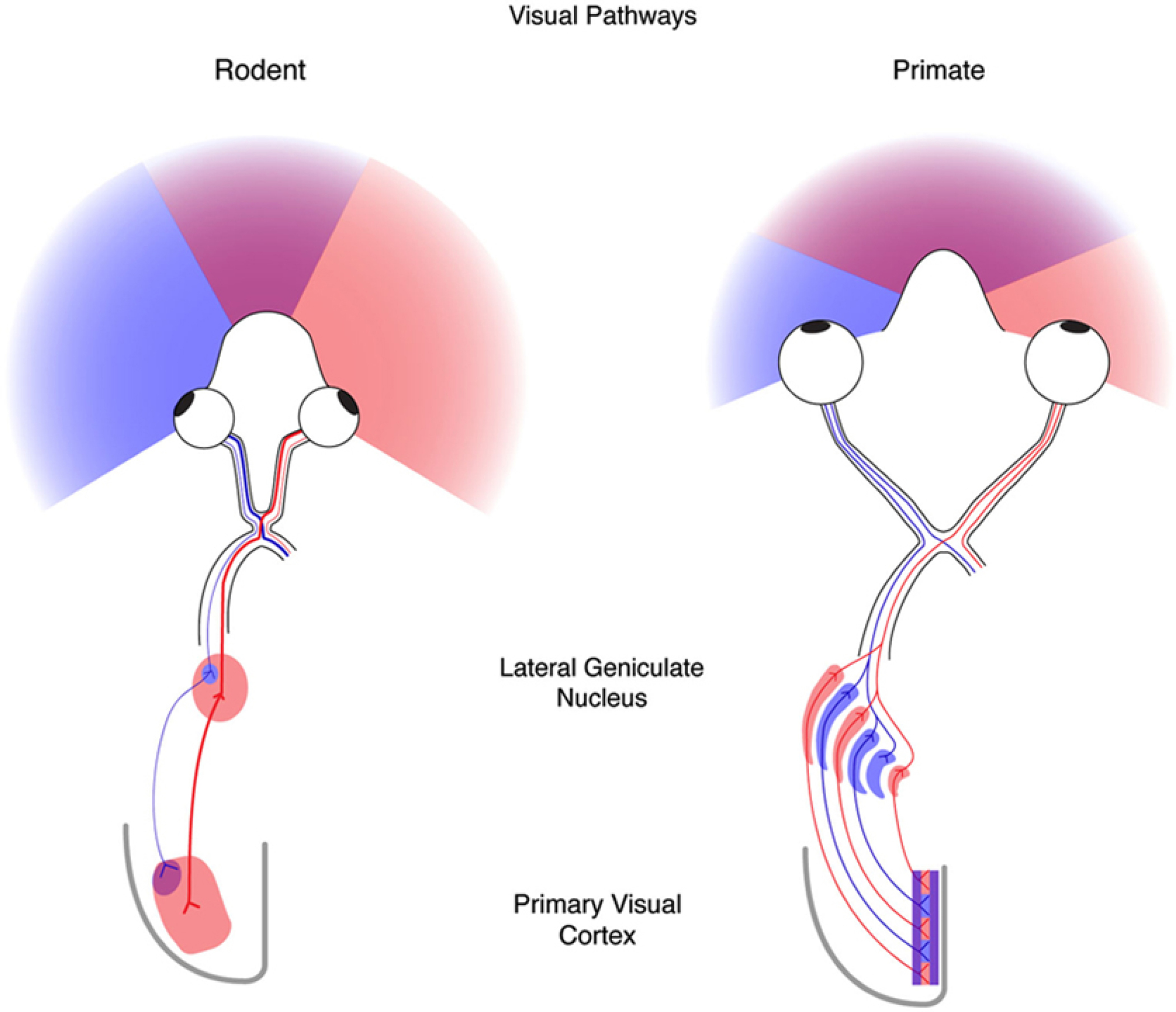
On the left is shown the field of view of the rodent (e.g. the mouse) and on the right is shown the field of view of the primate (e.g., the macaque monkey/human). Shown is the innervation scheme between the retina and V1, which is the first major station of the neocortex that receives visual information in these animals (from Priebe and McGee 2014).
This difference in viewing experience translates to a varied wiring between the retina and area V1, the first station in the neocortex to receive a substantial input from the visual thalamus, the lateral geniculate nucleus (Fig. 1). Unlike the macaque monkey, the visual projections in the mouse are largely crossed with only around 10% of the projections passing ipsilaterally to the lateral geniculate nucleus (Guido 2018). The macaque monkey has a far more developed lateral geniculate nucleus that is composed of six layers, three dedicated to projections from one eye and the remaining dedicated to projections from the other eye (Hubel and Wiesel 1977). A consequence of this segregation is that area V1 is organized into ocular dominance columns such that at the level of lamina IV (the input layer) the cells receive input from one eye only. As one advances an electrode parallel to the V1 surface the ocular dominance shifts every 0.5 mm or so from being left eye dominant to right eye dominant and so on. It is the segregation of ocular dominance columns that sets up the cortical wiring for stereovision (Poggio and Fischer 1977). In the case of the mouse, which has no ocular dominance columns (Dräger 1975), it is believed that depth perception is mediated mainly by monocular cues such as motion parallax (Ellard et al. 1986; Legg and Lambert 1990), which maps the differential movements of objects across the retina to different depth planes. Cells in mouse V1 respond to a wide range of velocities (from 5 to 200 degrees/sec) that can support parallax evaluation (Dräger 1975; Ellard et al. 1986; Legg and Lambert 1990).
In addition, mouse V1 contains neurons that respond to inputs from the two eyes encoding visual field regions of binocular overlap (Dräger 1975; Garrett et al. 2014; Scholl et al. 2013). It has been estimated that the disparity tuning of mouse V1 binocular cells is about 2 to 10 degrees of visual angle (Samonds et al. 2019; Scholl et al. 2013) which is far less sensitive for stereopsis than the tuning of V1 binocular cells in the macaque monkey (i.e., 0.2 to 0.5 degrees, Poggio and Fischer 1977). Macaque monkeys have no difficulty detecting stereo-depth cues presented at a disparity of 0.1 degrees of visual angle (Schiller and Tehovnik 2015), whereas mice can discriminate disparities from 2 to 5 degrees of visual angle (Samonds et al. 2019). Finally, monocular depth cues, such as interposition, perspective, and shading, could be used by both the mouse and the macaque monkey, although these cues have yet to be studied systematically in these species.
Another difference between the mouse and the macaque monkey is in how the visual field is represented in area V1. In the case of the mouse, the entire visual field is represented on the surface of the neocortex with the center of gaze (i.e., the zero coordinates of the azimuth and elevation visual axes) represented centrally and with the nasal field represented antero-laterally and the temporal field represented postero-medially in the neocortex (Fig. 2, derived from Garrett et al. 2014). In the case of the macaque monkey, half the visual field out to 7 degrees is represented on the surface of the neocortex (also called the operculum) with the remainder folded in a sulcus (Fig. 2, Schiller and Tehovnik 2015). Furthermore, the foveal representation (which is highly magnified) in the macaque monkey is situated laterally in the neocortex and the temporal representation of the visual field is located medially. The mouse does not have a fovea, but the region of the retina subserving the primary optical axis into the nasal representation is minimally magnified (Fig. 7A,D of Garrett et al. 2014).
Figure 2.
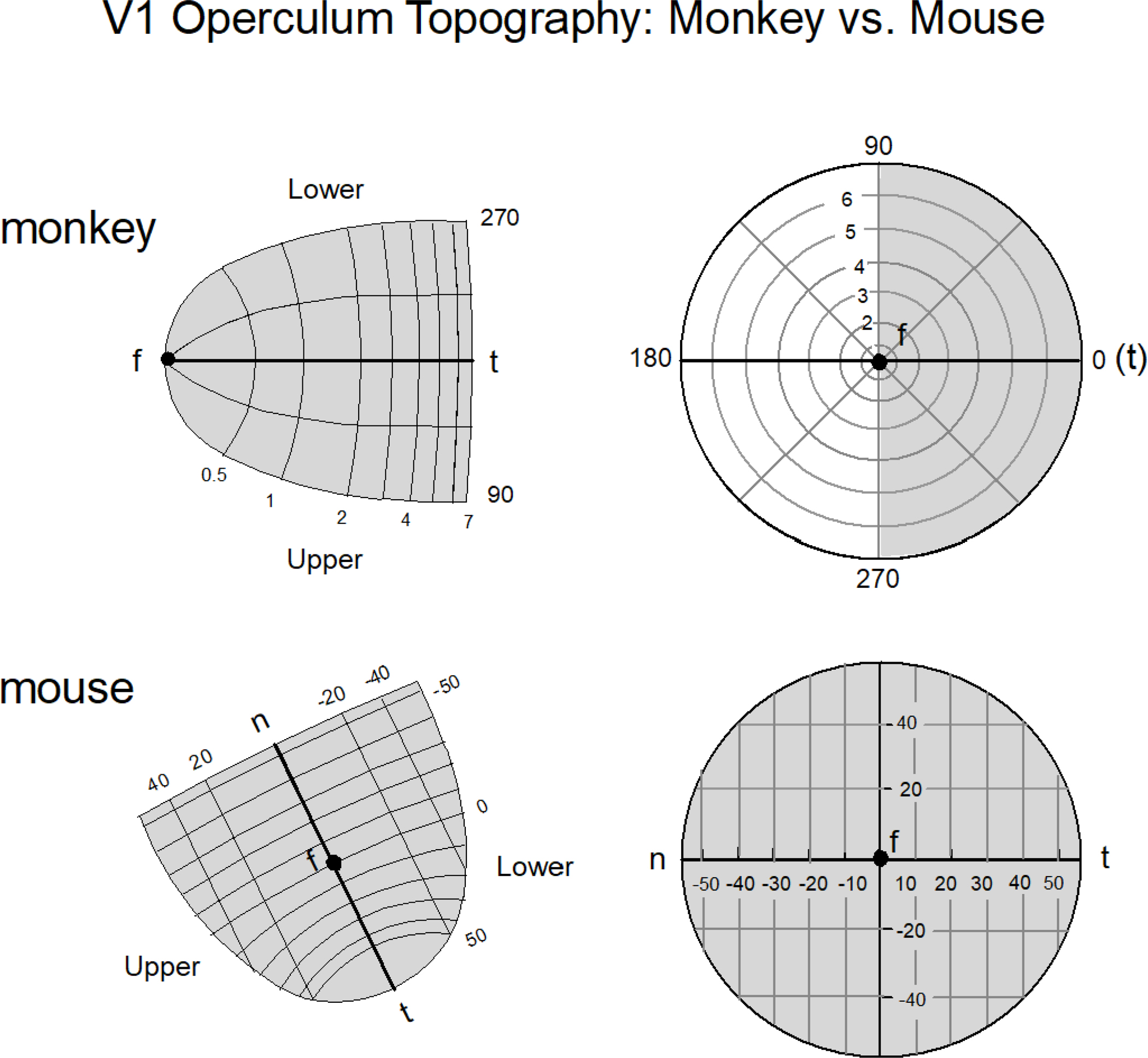
The layout of area V1 of the macaque monkey and the mouse as it pertains to the operculum (i.e., the exposed area of neocortex) is shown. In the case of the macaque monkey only 7 degrees of the visual field of one hemifield is represented in the operculum (top panel)(derived from Schiller and Tehovnik 2008); in the case of the mouse the arrangement is different (bottom panel): the entire visual field is encoded by the operculum and the center of gaze marked by ‘f’ is situated in the center of the map with ‘n’ representing the nasal field and ‘t’ representing temporal field (derived from Fig. 1G,H & Fig. 7A,D of Garret et al. 2014). One operculum in the mouse encodes the entire visual field from a viewing eye as illustrated. Notice the slight magnification of the visual representation beyond the center of gaze ‘f’ for the nasal representation of the mouse; for the macaque monkey the magnification is more extreme which accounts for its superior visual spatial resolution. The magnification is a rough approximation. For precise depictions see Schiller and Tehovnik (2008) and Garret et al. (2014).
Figure 7.
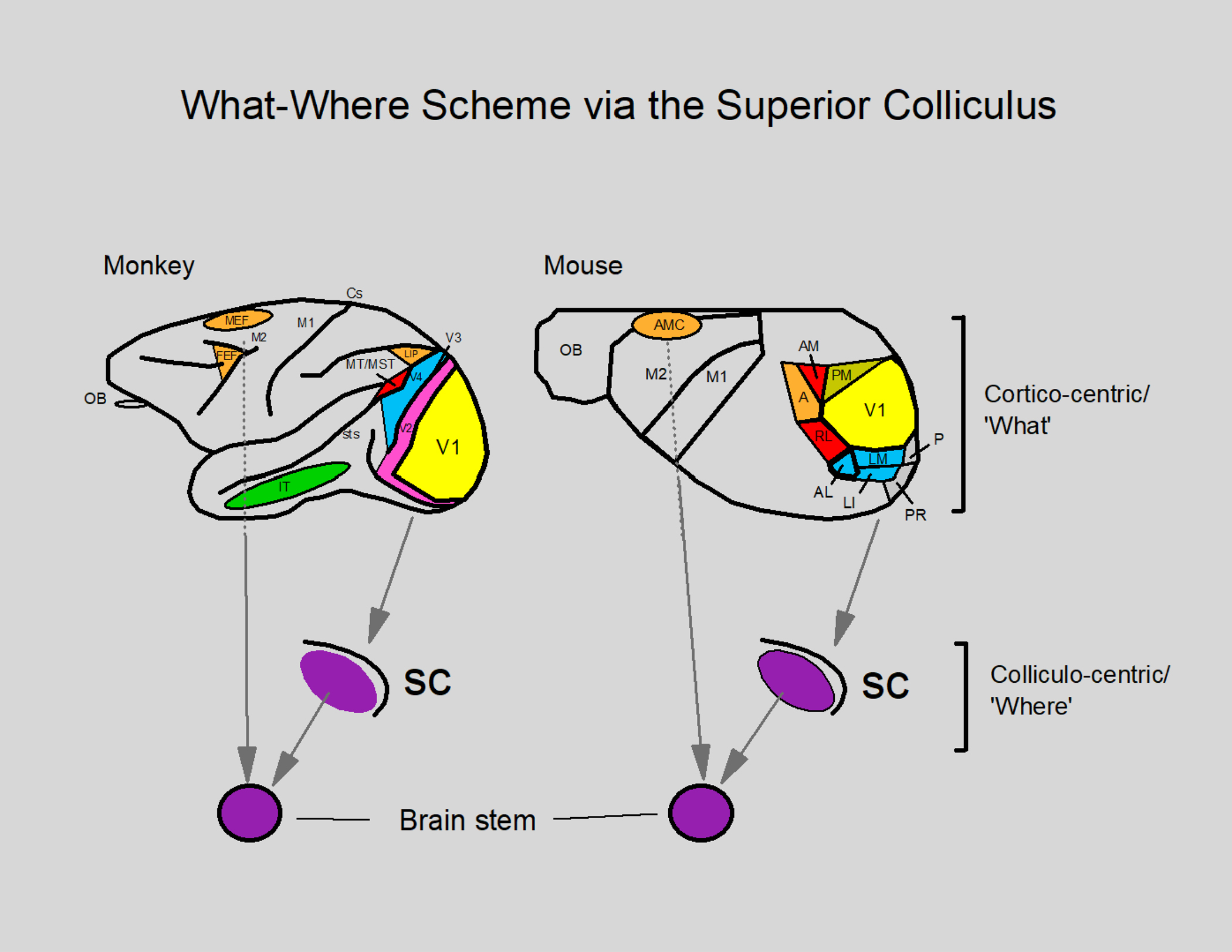
The what-where scheme as originally proposed by Ingle and colleagues in 1967 with respect to the superior colliculus. In this scheme the neocortex is designated as a feature detector (cortico-centric ‘what’) which then sends information to the superior colliculus (SC) for orienting the eyes and head toward peripherally located objects using a retinotopic map in both the mouse and the macaque monkey. In order to move the eyes and head, the brain stem (which is innervated by the superior colliculus and the eye fields in the frontal cortex—FEF and MEF/AMC) contains neurons whose firing rate increases to bring about a precise orientation of the eyes and head to position a visual target in the center of gaze by contracting the muscles (Ingle 1973; Ingle et al. 1967; Schiller and Tehovnik 2015). For other details see the caption of Figure 5.
The visual spatial resolution of rodents including mice is a couple orders of magnitude below that of the primate (Fig. 3). Rodents have a visual spatial resolution of 0.5 to 2.0 cycles per degree with mice exhibiting acuity at the lower end of this range at 0.5 cycles per degree (Ingle 1981; Prusky et al. 2000). The visual spatial resolution of macaque monkeys as well as humans extends to about 60 cycles per degree (Schiller and Tehovnik 2015; Souza et al. 2011). In macaque monkeys the acuity is maximal at the fovea which is restricted to a 1-degree regions of the central visual field and which contains a high concentration of cone receptors (Schiller and Tehovnik 2015); for the mouse, the acuity is more uniform throughout the visual field at 0.5 cycles per degree (Prusky et al. 2000). Also, the mouse (unlike the macaque monkey) is sensitive to ultraviolet light originating from the sky above which is an adaptation for the detection of over-head flying predators (Szatko et al. 2019; Tan et al. 2015).
Figure 3.
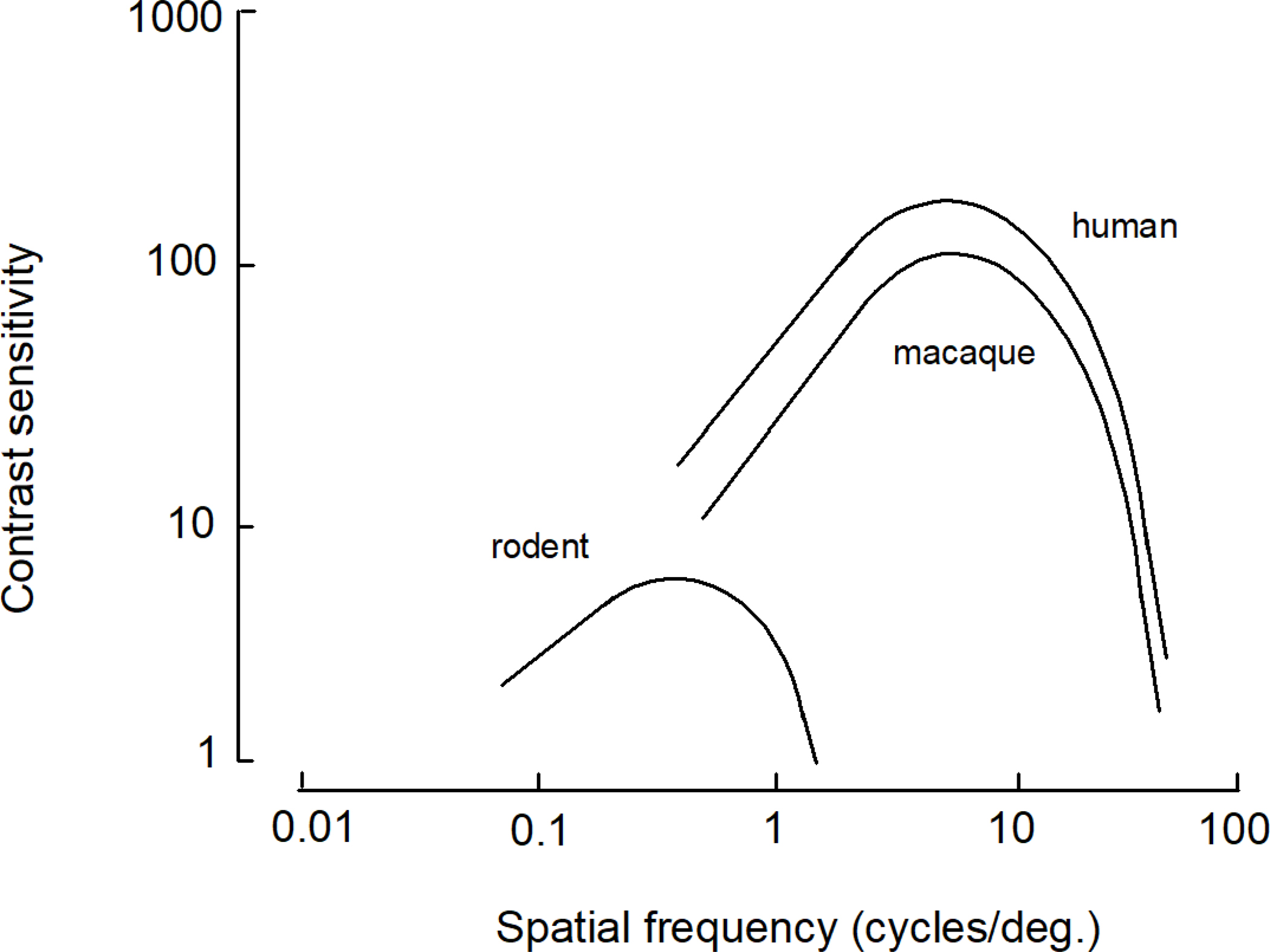
Contrast sensitivity functions plotted as a function of spatial frequency in cycles per degree for primates (e.g., the macaque monkey and human) and rodents (e.g., rats, mice, gerbils, etc.; derived from Souza et al. 2011).
This difference in visual spatial resolution between the mouse and the macaque monkey is further highlighted by the following. If we consider 30 μm of V1 tissue in both the mouse and the macaque monkey, which is the amount of tissue believed to encode a single feature (Ji et al. 2015; Ohki and Reid 2007; Peters 1994), this amount of tissue in the mouse represents about 1 degree of visual angle (i.e., 3,600 seconds), while in the macaque monkey it represents about 1 second of visual angle at the fovea. These estimates are based on the magnification factor of mouse and macaque monkey V1 (Garrett et al. 2014; Tehovnik and Slocum 2007). That these values are reasonable is supported by the observation that the visual spatial resolution of the mouse does not surpass 1 cycle per degree (Prusky et al. 2000) and that the hyperacuity of primates (i.e., of humans) amounts to several seconds of visual angle at the fovea (Westheimer and McKee 1977).
In the mouse, like all mammals including the macaque monkey, the lateral geniculate nucleus has a standard retinal topography: anterior represents the central to the nasal visual field, posterior represents the peripheral visual field, medial represents the lower visual field, and lateral represents the upper visual field (Kerschensteiner and Guido 2017; Reese and Jeffery 1983; Schiller and Tehovnik 2015). The retinal projections to the lateral geniculate are far less numerous in the mouse than they are in the macaque monkey (cf., 45,000 vs. 1.6 million, Koch and Reid 2012; Perry and Cowey 1985). Like macaque retinal ganglion neurons, the retinal ganglion neurons of the mouse that innervate the lateral geniculate nucleus have center-surround properties with either an ON-center field or an OFF-center field that responds to a spot of light (Kerschensteiner and Guido 2017; Stone and Pinto 1993; Schiller and Tehovnik 2015; Tang et al. 2016). In addition, the retinal ganglion cells of the mouse can detect the direction of motion, information that is conveyed to V1 (Borst and Euler 2011; Kerschensteiner and Guido 2017; Sanes and Masland 2015; Tang et al. 2016) and neurons in the lateral geniculate nucleus are orientation tuned (Kondo and Ohki 2016; Roth et al. 2016). In the macaque monkey, all orientation and direction tuning starts at the level of V1 (Schiller and Tehovnik 2015). Unlike the macaque monkey that has three cone types each for resolving blue, green, or red for trichromatic visual processing (Schiller and Tehovnik 2015), the mouse does not have trichromatic vision. Yet it has ‘cone’ retinal receptors that are tuned to either green (i.e., 500 nm) or ultraviolet light (i.e., 350 nm, Szatko et al. 2019); the green-sensitive receptors are concentrated in the dorsal retina, whereas the ultraviolet-sensitive receptors are concentrated in the ventral retina. This functional bifurcation allows for the viewing of a green terrain and an ultraviolet emitting sky (Szatko et al. 2019).
In both the mouse and the macaque monkey, the strongest projections from the lateral geniculate nucleus (dorsalis) terminate in area V1 (Frost and Caviness 1980; Schiller and Tehovnik 2015; Simmons et al. 1982). In the case of the mouse there are weaker projections to extrastriate areas (e.g., the lateromedial area, the lateral intermediate area, the posterior area, and the postrhinal area) including the retrosplenial cortex (Ji et al. 2015). The macaque monkey, however, has few such projections terminating in extrastriate cortex albeit there are konicellular projections that directly innervate the middle temporal cortex, area MT (Warner et al. 2010). The terminations of the lateral geniculate nucleus in V1 of both the mouse and the macaque monkey are ordered topographically such that they terminate in regions represented by patches as defined by acetylcholine staining (i.e., muscarinic acetylcholine receptor staining or acetylcholine esterase staining) in the mouse and by acetylcholine or cytochrome oxidase staining in the macaque monkey (Horton 1984; Ji et al. 2015). These patches are repeated systematically through the retinotopic map of V1. The patches have been used to determine the amount of V1 tissue dedicated to representing features falling within the receptive field of neurons at a V1 map location. The features include orientation, spatial frequency, direction of motion, binocular disparity, and color. The extent of this tissue is called a V1 cortical point-image. For both the mouse and the macaque monkey it has been estimated that seven patches (each arranged in a hexagonal configuration to optimize spacing) are sufficient to represent the V1 cortical point-image (Ji et al. 2015). For the mouse this represents roughly 240 μm by 240 μm of tissue running parallel to the V1 surface, whereas for the macaque monkey this represents 1,000 μm by 1,000 μm of tissue (Blasdel 1992; Fahey et al. 2019; Ji et al. 2015; Marshel et al. 2011; Nauhaus et al. 2016; Ohki and Reid 2007). What this means is that this tissue in the mouse and the macaque monkey is sufficient to encode orientation, spatial frequency, the direction of motion, binocular disparity, and color for a given region of visual space as defined by the receptive field of the neurons at a V1 map location (Ji et al. 2015; Schiller and Tehovnik 2015). In terms of visual-field coverage, the V1 cortical point-image of the mouse represents a region of 13 degrees by 13 degrees throughout most of the visual field, which is the approximate size of the receptive field (Ji et al. 2015); the receptive field size is largely uniform throughout the map of V1 of the mouse, since the cortical magnification is minimal. The V1 cortical point-image of the macaque monkey represents a region as small as 0.2 by 0.2 degree2 of visual field at the fovea (Tehovnik and Slocum 2007), which is related to the cortical magnification factor and which attests to the superior visual spatial resolution of the macaque monkey at the center of gaze.
Accordingly, V1 of the mouse is much less sensitive for the processing of visual information per unit receptive field than is V1 of the macaque monkey, and as such these two species employ different behavioral strategies when using their visual systems. As well, the anatomical differences of the visual systems of these two species reflect different behavioral adaptations: mice are ground dwellers and as such are prey to many species, whereas macaque monkeys can live in the trees and they are well-known for their predation of many species.
3. Object versus Motion Vision in Extrastriate Cortex
Area V1 of the mouse innervates nine distinct extrastriate areas that have been defined using the nomenclature of the Allen Mouse Brain Connectivity Atlas (Fig. 4A, Froudarakis et al. 2019; Wang and Burkhalter 2007; Wang et al. 2011, 2012). Extrastriate areas proximate to V1 tend to receive stronger innervations than areas distal to V1, which is in keeping with the projection scheme found in macaque monkeys (Felleman and Van Essen 1991). Recently, Froudarakis et al. (2020) trained mice to discriminate between objects in their home cage with the purpose of identifying regions in the visual cortex that are responsive to objects. Once the mice were trained, the entire extrastriate cortex and area V1 were studied using two-photon calcium imaging so that the responses of individual neurons could be recorded, as the trained mice were presented with objects whose size, orientation, shading, and background clutter varied from the training set. It was found that three areas in particular were selective for objects: the anterolateral area, the lateromedial area, and the lateral intermediate area (Fig. 4B). It is noteworthy that these areas are all located lateral to the areas that have been identified for motion processing including the rostrolateral area and the anteromedial area (Garrett et al. 2014; Marshel et al. 2011; Rasmussen et al. 2020). Also, the anterolateral area contains neurons that encode motion (Marshel et al. 2011); the anterolateral area may be a transition point between the object encoding areas and motion encoding areas. Significantly, the extrastriate cortex of the mouse is organized roughly similar to that of the macaque monkey whereby objects are represented in lateral regions of the neocortex and motion is represented in medial regions (Fig. 5; Felleman and Van Essen 1991; Schiller and Tehovnik 2015).
Figure 4.
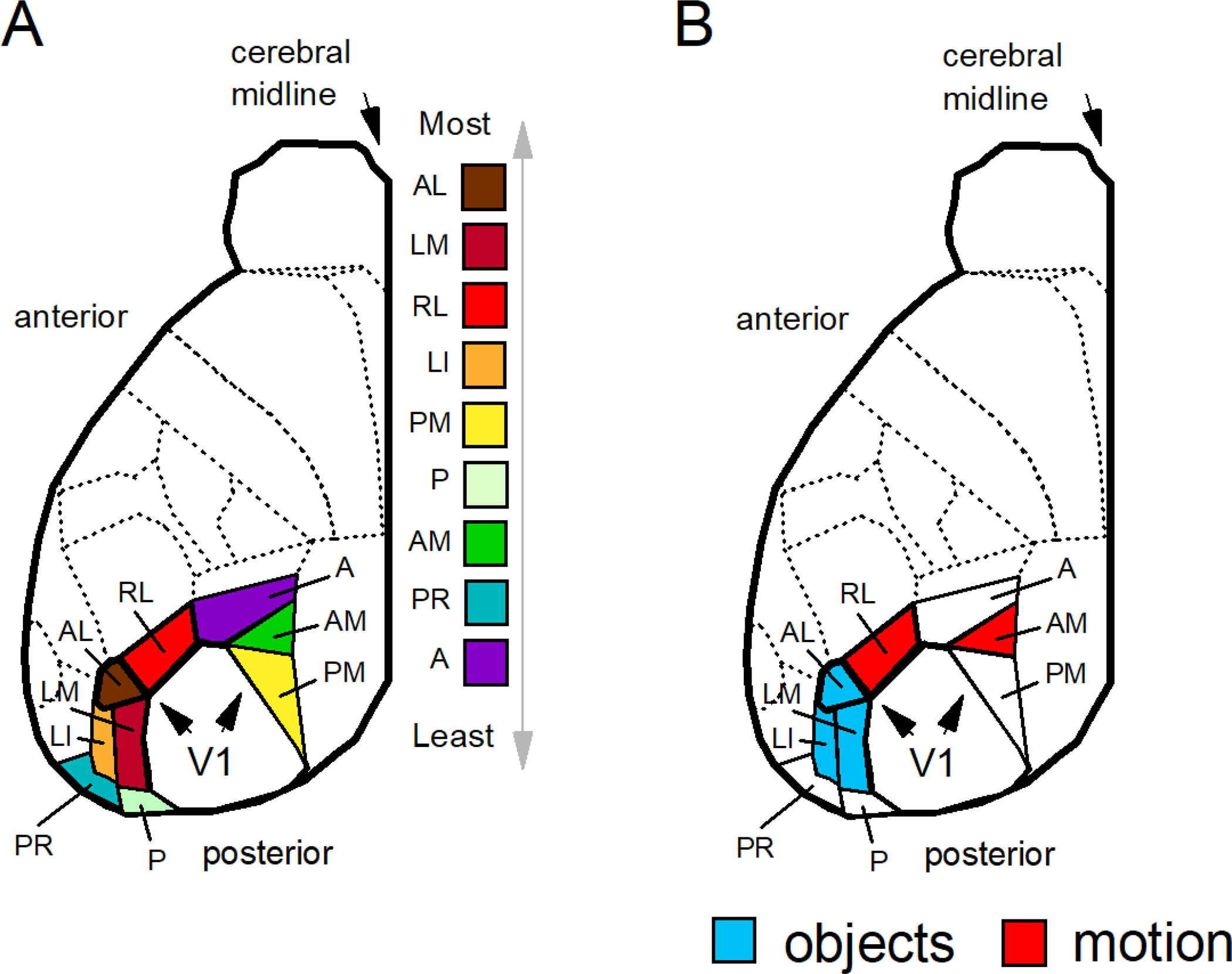
(A) Extrastriate areas of the mouse are listed from top to bottom according to the density of innervation from V1 from maximal to minimal as derived from Froudarakis et al. (2019): the anterolateral area (AL), the lateromedial area (LM), the rostrolateral area (RL), the lateral intermediate area (LI), the posteromedial area (PM), the posterior area (P), the anteromedial area (AM), the postrhinal area (PR), and the anterior area (A). (B) Neurons that are modulated by objects have been identified in the anterolateral (AL), lateromedial (LM), and lateral intermediate (LI) areas (defined in blue); neurons modulated by complex motion stimuli (e.g. flow fields) have be identified in the rostrolateral (RL) and anteromedial (AM) areas (defined in red). Regions of the extrastriate cortex that encode objects are located lateral to the regions that encode motion. Complete details of the mouse visual cortex can be found in Froudarakis et al. (2019, 2020), Garrett et al. (2014), Marshel et al. (2011), Rasmussen et al. (2020), and Wang et al. (2012).
Figure 5.
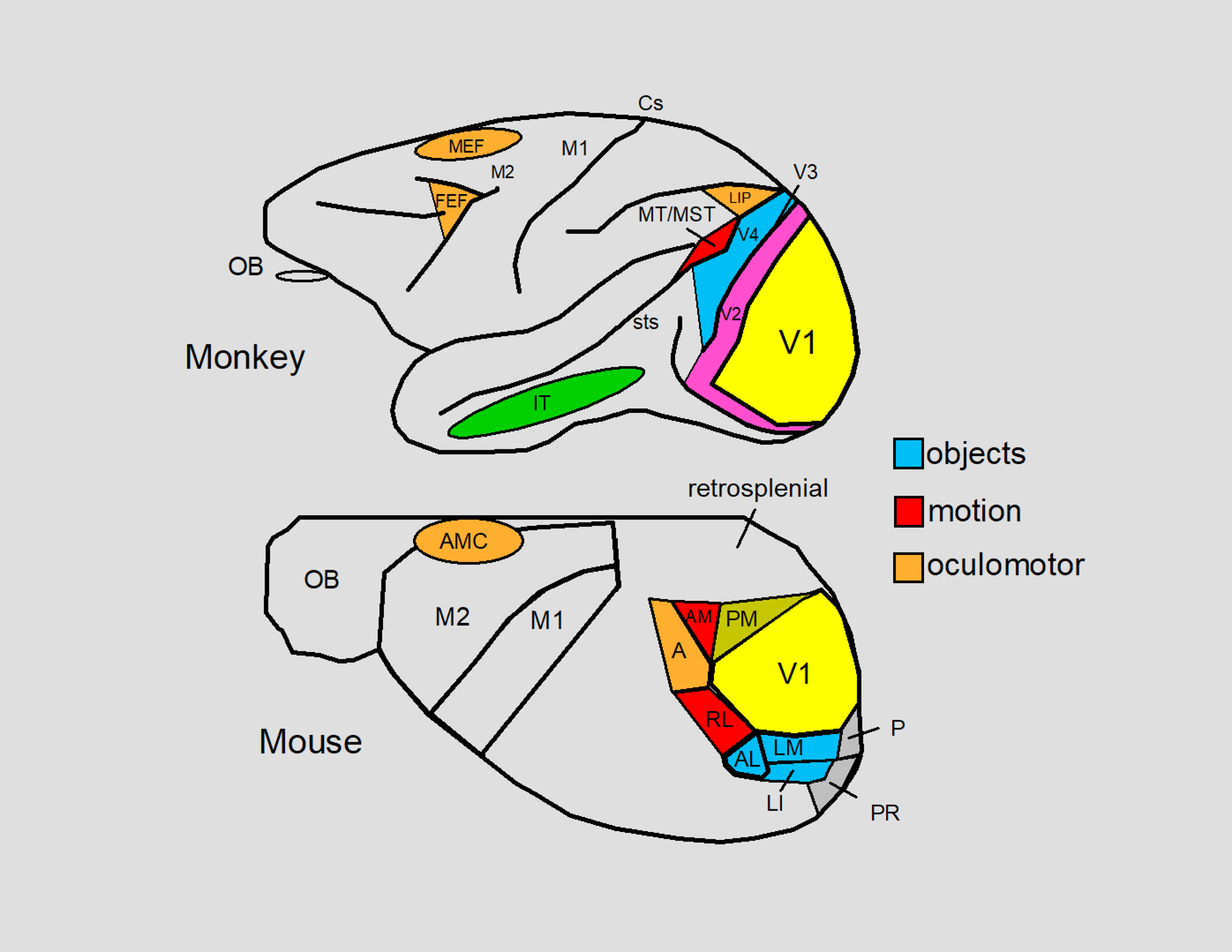
The visual areas of the neocortex of the macaque monkey and the mouse are summarized. Both species have homologous areas for processing visual information starting at V1 which process stationary and moving oriented lines. Object encoding has been described for V4 in the macaque monkey and for areas AL (anterolateral), LM (lateromedial) and LI (lateral intermediate) in the mouse. V4 of the macaque monkey ultimately innervates the IT (inferior temporal) cortex which contains cells that respond to faces and other complex objects. The object encoding areas of both the mouse and the macaque monkey contain a central-field representation. Motion encoding has been described for MT/MST (middle temporal cortex/middle superior temporal cortex) in the macaque monkey and for areas AM (anteromedial) and RL (rostrolateral) in the mouse. MT and MST ultimately innervate LIP (the lateral interparietal area) which is an oculomotor area that mediates eye movements and active fixation in macaque monkeys (Andersen and Mountcastle 1983; Mountcastle et al. 1975). Area A (anterior) of the mouse may be a homologue of LIP for eye movements can be evoked from this region (Itokazu et al. 2018) and this area has been implicated in spatial vision in rats (Kolb and Walkey 1987). In the macaque monkey, the visual signals of the posterior cortex eventually arrive in the frontal lobes at one of the two major oculomotor areas: the FEF (the frontal eye fields) and the MEF (the medial eye fields). The FEF is a central controller of eye movements (saccadic, smooth pursuit, and vergence) and the MEF is involved in eye, head, and body part coordination. Activation of the AMC (anteromedial cortex) in the mouse evokes eye movements (Itokazu et al. 2018) as well as head movements in rodents such as rats (Tehovnik and Yeomans 1987). Whether the AMC contains FEF and MEF homologues is not known. V2, V3, sts (superior temporal sulcus), and Cs (central sulcus) are indicated for the macaque monkey and areas PM (posteromedial), P (posterior), and PR (postrhinal) are indicated for the mouse. The remaining labels include M1, M2, the retrosplenial cortex, and the olfactory bulb (OB). The inset to the right color codes some of the areas according to function: objects (blue), motion (red), and oculomotor (orange). For further details see: Froudarakis et al. (2019), Garrett et al. (2014), Marshel et al. (2011), Rasmussen et al. (2020), and Schiller and Tehovnik (2015).
Whether the mouse has a definitive area V2 is unclear. After V1, areas posteromedial and lateromedial have the largest coverage of the visual field after V1 at 56% and 44%, respectively, as compared to the coverage of V1 set to 100% (Fig. 6). Also, these two areas are the largest immediately after V1 (cf., Felleman and Van Essen 1991; Froudarakis et al. 2019). There is no clear agreement on what structure is homologous with primate V2, but some have suggested that area lateromedial satisfies this distinction (Wang and Burkhalter 2007). In the macaque monkey, area V2 is distinct from V1 for having neurons that respond to illusory contours (Von der Heydt and Peterhans 1989).
Figure 6.
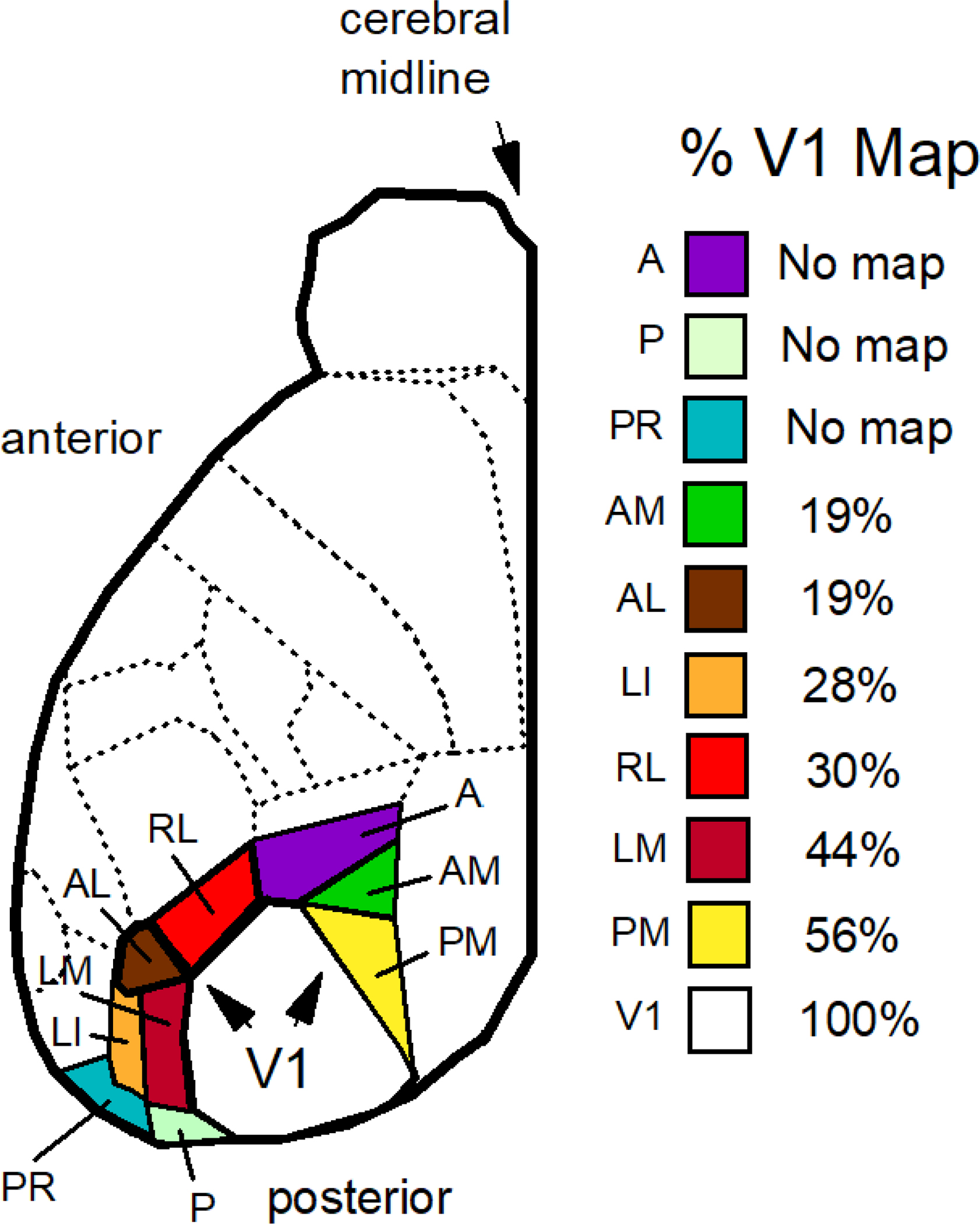
Extrastriate areas of the mouse are listed from top to bottom according to the percent of the visual field represented as a fraction of that represented by the V1 map using the data of figures 5B,C of Garrett et al. (2014). All maps contained a central visual field encoding the primary optical axis. Regions are list from no topographic coverage (no map) to maximal coverage: the anterior area (A), the posterior area (P), postrhinal area (PR), the anteromedial area (AM), the anterolateral area (AL), the lateral intermediate area (LI), the rostrolateral area (RL), the lateromedial area (LM), the posteromedial area (PM), and area V1 (V1).
Finally, in the mouse it has been shown that areas anterior, rostrolateral, and anterolateral send and receive axonal projections by way of the anteromedial cortex of the frontal neocortex (Fig. 5, areas A, RL, AL, and AMC). When areas anterior, rostrolateral, or anterolateral are stimulated electrically with pulses of 200 μA (at 0.3-ms pulse duration) saccadic eye movements are induced (Itokazu et al. 2018). Such current activates axons directly within 1 mm from the electrode tip (Tehovnik 1996) which means the induced saccades cannot be attributed to passive current spreading from areas anterior, rostrolateral, or anterolateral to the anteromedial cortex, which is located 4 mm anterior to these areas (Kirkcaldie et al. 2012) and which contains neurons mediating eye movements as demonstrated by neural stimulation (Fig. 5, AMC; Itokazu et al. 2018; Zhang et al. 2014). Of course, this does not rule out antidromic activation which can only be tested by disabling the anteromedial cortex and stimulating areas anterior, rostrolateral, or anterolateral of the mouse. Most relevant here for the mouse, however, is that area anterior (i.e., a lateral intraparietal area homologue; Kolb and Walkey 1987), area rostrolateral (i.e., a motion encoding area), and area anterolateral (i.e., an object encoding area) have direct access to frontal regions that control eye movements, which concurs with what is found in the macaque monkey for functionally comparable areas, namely, the lateral intraparietal area, areas MT/MST (middle temporal cortex/middle superior temporal cortex), and area V4 (Felleman and Van Essen 1991; Rao et al. 2016; Schiller and Tehovnik 2015).
In conclusion, our homology scheme for the mouse coincides with that proposed by others (e.g., Laramée and Boire 2015; Murakami et al. 2017). To strengthen this scheme, additional behavioral experiments will be required as have been done on macaque monkeys (e.g., described in chapters 6 to 13 of Schiller and Tehovnik 2015).
4. Eye Movements in the Mouse and the Macaque Monkey
Eye movements are central to object vision both for object identification as well as a means by which to minimize retinal adaptation (Schiller and Tehovnik 2015; Yarbus 1967). Unlike a mouse and other rodents whose eyes remain relatively immobile as well as centered in orbit and conjugate during head-fixed conditions (Payne and Raymond 2017; Van Alphen et al. 2010; Wallace et al. 2013), the eyes of a macaque monkey are in constant motion being displaced within ±30 degrees of the primary optical axis roughly three times per second and achieving velocities as high as 800 degrees per second (Schiller and Tehovnik 2015). Such displacement continues for the duration of a monkey’s life and most eye movements generated in macaque monkeys are yoked such that the eyes move together (even when disjunctive through vergence) so that objects situated in depth can be identified effectively by projecting the image precisely onto the fovea while adjusting the lens for accommodation. The mouse, however, during head-fixed conditions does exhibit low-amplitude (< 5 degrees) and low velocity (< 10 degrees/sec) spontaneously-generated eye movements (Payne and Raymond 2017) which refreshes the image on the retina. And with training, a mouse can be made to evoke 5 to 15 degree conjugate saccadic eye movements toward visual targets while the head is fixed (Itokazu et al. 2018). It is unclear whether rodents including mice have a well-developed system of vergence and accommodation by which to adaptively focus visual images (Hughes 1977). In this regard, rodents (including mice) are known for exhibiting non-yoked, independent eye movements during which there can be no vergence (Meister and Cox 2013; Wallace et al. 2013; Samonds et al. 2019).
When the head is free to move in both the mouse and the macaque monkey (as well as in all other vertebrates), the visual image is stabilized on the retina by way of the vestibulo-ocular reflex (Payne and Raymond 2017; Schiller and Tehovnik 2015). After an abrupt head displacement, the eyes remain fixated on a visual target (as the head moves) but thereafter the eyes are re-centered in the orbit with an eye displacement so that visual analysis can commence from an optimal eye-head orientation for the acquisition of new targets. In the mouse, this optimal orientation is centered at roughly 50 degrees with respect to the longitudinal axis of the head, and in the macaque monkey, it is centered at roughly zero degrees with respect to the longitudinal axis (Heesy 2004; Samonds et al. 2019; Schiller and Tehovnik 2015). This interplay between the head and eyes to maintain a stable visual image on the retina also extends the oculomotor range (with respect to the body axis) well beyond the head-fixed condition (oculomotor range will be discussed later in detail). The mouse is more dependent on head movements than is the macaque monkey to extend this range but both animals rely on head movements for their survival in their natural habitats (Froudarakis et al. 2019; Schiller and Tehovnik 2015).
5. What-Where Scheme and the Superior Colliculus
The developers of the ‘What and Where Hypothesis’, David Ingle and his associates, asserted that the mammalian neocortex identifies objects whereas the superior colliculus orients an animal to locate them irrespective of what the object is (Ingle et al. 1967). Future generations intent on moving ‘what’ and ‘where’ into the neocortex posited that the ventral stream of primates passing through area V4 mediates ‘what’ and the dorsal stream passing through area MT mediates ‘where’ (Mishkin et al. 1983). This cortico-centric hypothesis has had two shortcomings. First, it produced an evolutionary partition between primates and other mammals whose visual systems depend more (but only superficially so) on the superior colliculus for locating objects. Second, there are several unexplained observations: Damage of areas MT (a putative ‘where’ module) and V4 (a putative ‘what’ module) in macaque monkeys spares visual functions such as stereopsis and color vision (Schiller and Tehovnik 2015). Motion (a MT ‘where’ function) can be used to provide information about object shape as in structure-from-motion, and eye movements (a putative ‘where’ attribute) are used to identify objects such that damage to the inferior temporal cortex can disable movements that scan the outline of objects (Ingle 1973; Schiller and Tehovnik 2015; Yarbus 1967). As well, ‘what’ and ‘where’ are coupled in many visual areas that have retinotopic maps (Schiller and Tehovnik 2015). Therefore, the scheme adopted in this report is to have the superior colliculus assume the function of locating objects in visual space, which concurs with the views of many (e.g., Goldberg and Wurtz 1972; Mlinar and Goodale 1984; Robinson 1972; Schiller and Stryker 1972; Schneider 1969; Sparks 1986; Tehovnik 1989; Tehovnik and Yeomans 1986). Indeed, as early as 1946 the mammalian superior colliculus was implicated in the ‘visual grasp reflex’ by Hess and colleagues based on the electrical stimulation of the colliculus in alert, behaving cats (Hess et al. 1946).
In both the rodent and the monkey including the macaque monkey, the posterior neocortex gains access to the oculomotor generator in the brain stem via the superior colliculus, whereas the frontal cortex gains access to this region by way of direct projections, albeit the frontal cortex also innervates the superior colliculus directly (Fig. 7; note that the fronto-collicular projections are not illustrated; Barret et al. 2020; Benavidez et al. 2020; Comoli et al. 2012; Fries 1984; Froudarakis et al. 2019; Ingle 1973; Kunzle and Akert 1977; Kuypers and Lawrence 1967; Leichnetz 1981; Lund 1966; Sherman et al. 1979; Shook et al. 1990; Spatz et al. 1970; Stanton et al.1988; Tehovnik et al. 1989). The superior colliculus of mammals is a seven-layered structure such that superficial layers (layers I to III) receive direct visual input whereas the intermediate and deep layers (layers IV to VII) receive strong input from the neocortex and they send motor projections into the brain stem (Ingle 1973; Schiller and Tehovnik 2015). If the superior colliculus is lesioned in the macaque monkey, all ocular responses evoked electrically from the posterior neocortex (i.e., from V1 to the lateral intraparietal area) are abolished while sparing such responses elicited from the frontal and medial eye fields (Keating and Gooley 1988; Keating et al. 1983; Schiller 1977; Tehovnik et al. 1994). Furthermore, employing suprathreshold currents in the macaque monkey, the shortest-to-longest latency for evoking saccadic eye movements occurs in the frontal eye fields [15 ms], the medial eye fields [23 ms], the lateral intraparietal area [25 ms], and finally in area V1 [50 ms; Robinson and Fuchs 1969; Shibutani et al. 1984; Tehovnik and Lee 1993; Tehovnik et al. 1994; Tehovnik et al. 2003]. The latency value for the superior colliculus is 20 ms (Robinson 1972). This systematic increase in latency moving from anterior to posterior regions of the neocortex of the macaque monkey is indicative of a diminished directness and robustness of connectivity between the neocortex and the brain stem for evoking ocular responses.
Finally, and most importantly, if both the frontal eye fields and superior colliculus are removed bilaterally in macaque monkeys, the animals lose all ability to generate visually guide eye movements even though skeletomotor orienting responses remain intact, i.e., the animals can still turn their heads toward and reach to visual targets (Schiller et al. 1980). Also, the vestibulo-ocular reflex and optokinetic nystagmus remain intact even though the gains are reduced following the paired ablations (Schiller et al. 1980). These findings reinforce the notion that neocortical oculomotor-control is made up of a posterior system going through the colliculus on the way to the brain stem and an anterior system having direct access to the brain stem (Schiller and Tehovnik 2001), as illustrated in figure 7. The foregoing has yet to be established in the mouse, but similar results would be expected based on the known anatomy of the rodent (including the mouse), which is similar to that of the macaque monkey, as referenced above.
As one moves closer to the oculomotor controller of the brain stem of rodents and macaque monkeys (as depicted in Fig. 7), there is a gradual transition from sensory encoding to motor encoding such that in the neocortex a full range of sensory computations are performed (e.g., orientation, spatial frequency, direction of motion, binocular disparity, and color) but by the time the signal reaches the superior colliculus, the sensory-encoding capacity of the cells is reduced to specifying the retinotopic location of a target even if the cells contain visual receptive fields (Dräger and Hubel 1975; Ingle 1973; Schiller and Tehovnik 2015). In the hamster, it is known that if the occipital cortex is removed the motion encoding capability of cells in the superficial and intermediate layers of the superior colliculus is lost (Rhoades and Chalupa 1978) and gerbils with lesions of the visual cortex can no longer anticipate the trajectory of moving targets (Ingle 1981; Ingle et al.1979). Such lesions in gerbils abolish motion parallax used to compute the distance of edges (Ellard et al. 1986). Finally, by the time the signal reaches the brain stem, which houses the oculomotor controller, the location signal that is encoded according to a retinotopic map is converted into a code that is devoid of visuo-sensory attributes such that the greater the discharge of the neurons the greater the magnitude of the response as initiated by the contraction of the ocular and skeletal muscles, a process realized by a firing-rate code to contract the muscles (Schiller and Tehovnik 2015).
6. Blind-Sight, Superior Colliculus and Tegmentum
Blindsight is believed to be mediated by subcortical mechanisms. This phenomenon was first discovered in human subjects who had their visual cortex, specifically area V1, damaged bilaterally (Pöppel et al. 1973); however, evidence for this idea first came from work done on macaque monkeys showing that all pattern, color, and motion vision is abolished following damage to the visual cortex (Humphrey and Weiskrantz 1963; Weiskrantz 1963). As well, stereopsis is eliminated (Schiller and Tehovnik 2015). In primates, if a high contrast spot of light (e.g., > 95% contrast) is presented to V1-damaged subjects they can discern its location even though there is no awareness of having done so in humans (Cowey and Stoering 1995; Ingle 1981; Moore et al. 1995; Pöppel et al. 1973; Segraves et al. 1987). It is believed that the superior colliculus mediates this residual vision although projections from the lateral geniculate nucleus to the extrastriate cortex may be sufficient for the mediation (Schmid et al. 2010).
In rodents (e.g., hamsters and gerbils) when the visual cortex including the putative object and motion encoding areas (see Figs. 5 and 7) is lesioned, the animals can still orient to visual targets (Mlinar and Goodale 1984; Schneider 1969), but they lose the capacity to perform feature vision such as discriminating between horizontal versus vertical black and white stripes or between speckled patterns versus diagonal stripes (Schneider 1969). Moreover, orientation discrimination is abolished in such animals (i.e., in mice, Schnabel et al. 2018) and the tracking of component motion as assessed using plaid stimuli is compromised (i.e., in mice, Palagina et al. 2017). As already mentioned, animals (i.e., gerbils) with lesions of the visual cortex fail to anticipate the trajectory of moving stimuli and to perform motion parallax (Ellard et al. 1986; Ingle 1981; Ingle et al.1979). Animals (i.e., hamsters and gerbils) that receive only collicular lesions can still discriminate between patterned stimuli and demonstrate motion parallax (Schneider 1969; Ellard et al. 1986), but they fail to orient to punctate targets (at 98% contrast) beyond 40 degrees of eccentricity (Mlinar and Goodale 1984) and they fail to respond to looming visual stimuli (> 20 degrees in size) throughout their ‘panoramic’ visual field [Schneider 1969; also see Shang et al. 2018 for pulvinar participation in response to looming stimuli], a function that moreover depends on an intact retrosplenial cortex (Ellard and Chapman 1991). Note that the superior colliculus of rodents (i.e., mice) contains neurons that respond to expanding flow fields presented from overhead (Dräger and Hubel 1975; also see Li et al. 2020). When both the visual cortex and the superior colliculus are lesioned, gerbils are no longer able to orient to visual stimuli anywhere in the visual field including to high contrast targets of 98% (Mlinar and Goodale 1984). Hence, rodents with lesions of the visual cortex and colliculus are rendered totally blind, failing to exhibit blindsight.
A structure not discussed much in the literature is the pretectum, which is composed of several nuclei situated immediately anterior to the superior colliculus and which is best known for mediating the pupillary reflex in both the rodent and primate (Clarke and Gamlin 1995; Clarke and Ikeda 1985). A pupillary enlargement, as part of the reflex, is triggered when animals entering a dark tunnel. Ingle (1981) found that gerbils with lesions of the superior colliculus (much like normal animals) had no difficulty acquiring a low-contrast aperture (a brown tunnel) located on a striped background such that to get a sunflower seed an animal had to enter a chamber by going through the aperture. The lesioned gerbils performed as well as the normal animals for aperture locations anywhere within 90 degrees with respect to the left and right side of the head in the horizontal visual field. This result concurs with the results based on frogs and toads whose pretectal nuclei have been found to mediate aperture detection (Ingle 1973, 1980). If the gerbils (i.e., those with collicular lesions or those with no lesions) were given lesions of V1, they failed to orient to the low-contrast aperture, but if the aperture was of high contrast (a black aperture on a white background) the animals could respond to the aperture, which could be considered an additional type of blindsight (Ingle 1980). Indeed, a human subject with bilateral V1 damage and with no visual awareness was able to walk around and avoid large, salient obstacles as placed within a hallway (De Gelder et al. 2008).
So, how does one secure total blindness, namely, the abolition of blindsight (to punctate targets, for instance) along with pattern vision? There are two ways to accomplish this. If one lesions the superior colliculus and the dorsal tegmentum, which carry neocortical fibres from extrastriate cortex as well as from the retrosplenial, temporal, and parietal cortices (Ingle 1973), an animal becomes totally blind to the presentation of visual stimuli (Casagrande et al. 1972). Another way to achieve total blindness is to lesion area V1 and the extrastriate cortex including the retrosplenial, temporal, and parietal cortices (Ingle 1973; Schmidt et al. 2010). Accordingly, information about object vision is communicated to the brain stem via the superior colliculus and dorsal tegmentum after being processed and finalized in the posterior neocortex.
7. The Oculomotor Range and the Superior Colliculus
If the superior colliculus is an eye movement controller (Stryker and Schiller 1975), which contains a ‘sensory’ retinotopic map that extends well beyond the oculomotor range—i.e., beyond 40 degrees peripherally in all mammals—how are the eyes put on target for objects situated beyond this range? Macaque monkeys, which confine their eye movements to within 30 degrees of central gaze under head-fixed conditions (Schiller and Tehovnik 2015), exhibit a head movement when the head is free to move to extend the oculomotor range (Stryker and Schiller 1975). What this does is orient the head in the direction of a peripherally-located visual object such that as the head moves a series of saccadic eye movements are generated each punctuated by fixations (controlled by the vestibulo-ocular reflex) such that at the end of the gaze shift the eyes are centered in orbit (Land 1999) so that the object can be manipulated efficiently with the forelimbs, for example. Under head-fixed conditions, rodents exhibit few eye movements (i.e., in mice, Froudarakis et al. 2019; Meyer et al. 2018), but once the head is free to move these animals exhibit a combination of eye and head movements to keep the eyes centered in orbit (Meyer et al. 2018; Michaiel et al. 2020; Van Alphen et al. 2001, 2010). It is noteworthy that some have suggested that the superior colliculus controls both eye and head movements to produce gaze shifts to extend the oculomotor range (e.g., Freedman et al. 1996), but there are some concerns about these experiments since there was no explicit control over head position (Chen and Tehovnik 2007).
The superior colliculus in rodents is configured such that anterior regions encode central ‘nasal’ gaze (for grasping prey) and posterior, lateral, and medial regions encode, respectively, peripheral gaze, lower-field lateral gaze, and overhead gaze (all for avoiding predators) (i.e., in mice, Dräger and Hubel 1975). This topographic layout is similar in macaque monkeys (Schiller and Tehovnik 2015) but there is no overhead representation since the eyes project forward (Fig. 1). McHaffie and Stein (1982) electrically stimulated the colliculus of head-fixed rats and hamsters and found that the size of an evoked saccadic eye movement depended on the site of activation (which concurs with what is found in the macaque monkey); if stimulation was continued a series of saccades were induced, each of the same amplitude. Eighteen degrees was the largest saccadic eye movement evoked from the caudal superior colliculus directed temporally. What this means is that the maximal amplitude of the evoked eye movement falls short of the temporal visual field representation of the colliculus, which in rodents surpasses 50 degrees (i.e., in mice, rats, and hamsters, Dräger and Hubel 1975; Siminoff et al. 1966; Tiao and Blakemore 1976). Note that even behaviorally evoked eye movements by head-free rodents fall short of this representation (i.e., all within 35 degrees for mice and rats, Van Alphen et al. 2010; Wallace et al. 2013). The only way the oculomotor range can be extended is through head movements accompanied by multiple discrete eye movements (Michaiel et al. 2020; Van Alphen et al. 2001, 2010) as occurs in the macaque monkey (Stryker and Schiller 1975). Just how the superior colliculus coordinates this process with head movement control systems is unclear.
Most experiments that study the vestibular system of primates confine their investigations to eye-in-orbit deviations of no more than 50 degrees of visual angle (e.g., Lisberger et al. 1984). If the eyes are deviated beyond this limit when the head is allowed to move with respect to the body, a reflex triggered by the neck proprioceptors evokes a blink of the eyes (i.e., in humans, Berkovic et al. 1985; Schaefer et al. 1979; Tinuper 1989) during which time the eyes can be returned to the center of orbit ready to continue their visual analysis upon opening. This process extends the oculomotor range of primates so that there is a correspondence between the visual-field representation of the retinotopic maps [e.g., up to and beyond 70 degrees of visual field as encoded by the primate superior colliculus and area V1, Adams and Horton 2003; Schiller and Tehovnik 2015; Sparks 2002] and the oculomotor output. A similar mechanism may exist in rodents to complement their highly developed vestibular reflexes (Van Alphen et al. 2001, 2010). Major portions of the neocortex (e.g., the frontal and medial eye fields, the motor and premotor cortex, and the parietal cortex including the intraparietal area) and brain stem are devoted to blinking at least in the primate (i.e., in humans and macaque monkeys, Benbadis et al. 1996; Bodis-Wollner et al. 1999; Esteban 1999; Shibutani et al. 1984).
8. The Frontal and Medial Eye Fields
The frontal and medial eye fields of macaque monkeys (as illustrated in Fig. 7) coordinate the eye movements and the gaze by centering the eyes in orbit to expedite an efficient transformation between retinal and skeleto-motor space for optimal object manipulation or avoidance. The frontal eye fields contain a topographic map that encodes the direction and amplitude of saccadic eye movements (Bruce and Goldberg 1985; Bruce et al. 1985). Moreover, neurons have been identified in the frontal eye fields that encode smooth pursuit and vergence eye movements (Gamlin and Yoon 2000; Gottlieb et al. 1993, 1994). The frontal eye fields have properties that are very similar to those of the superior colliculus (Hikosaka and Wurtz 1985; Schiller and Tehovnik 2003, 2015): electrical stimulation evokes fixed-vector saccadic eye movements and multiple saccades of the same size and direction are elicited when using long durations of stimulation (i.e., to evoke staircase saccades); when muscimol, a GABA agonist, is injected into either region, saccadic eye movements made into the receptive field of the affected neurons are disrupted; when bicuculine, a GABA antagonist, is injected into either region, irrepressible saccades are induced whose size and direction are determined by the location of the injection site. These results indicate that the frontal eye fields, much like the superior colliculus, encode eye movements with respect to the fovea. Since ablation of both the frontal eye fields and superior colliculus abolish all visually guided saccadic eye movements while preserving skeleto-motor responses (i.e. in the macaque monkey, Schiller et al. 1980), this reinforces the idea that these regions are dedicated to eye-movement control.
The medial eye fields of the macaque monkey have a totally different organization from that of the frontal eye fields. When electrical stimulation is delivered to this region the size and direction of the eye movements varies are a function of starting eye position such that the eye movements are made to terminate in one location of craniotopic space (i.e., eye in orbit with respect to the head; Schiller and Tehovnik 2015; Tehovnik and Lee 1993; Tehovnik et al. 1998). Furthermore, the termination location varies topographically such that stimulation of anterior sites terminates the eye movement in extreme parts of contralateral craniotopic space, stimulation of posterior sites terminates the eye movement in central craniotopic space, stimulation of medial sites terminates the eye movement in lower craniotopic space, and stimulation of lateral sites terminates the eye movement in upper craniotopic space. If the head of an animal is tilted with respect to the gravitational axis, the termination positions remain fixed in relation to the head. Also, if the stimulation is maintained while the eyes are in a termination position, all visually-evoked saccades are delayed for the duration of stimulation. Neurons have been identified in the medial eye fields that respond maximally when the eyes fixate targets positioned in a termination position as defined by electrical stimulation (Lee and Tehovnik 1995). When the medial eye fields are disabled there are few deficits in eye movement control in head-fixed macaque monkeys (as reviewed in Tehovnik et al. 2000), albeit animals are impaired at generating saccades to multiple remembered target positions (Sommer and Tehovnik 1999). Accordingly, this region maintains a record of the location of the eyes in orbit. It is believed that the medial eye fields are involved in centering the eyes in orbit once a saccadic eye movement has been initiated thereby participating in the vestibulo-ocular reflex in the head-free condition (Chen and Tehovnik 2007; Fukushima et al. 2011).
So, how do the foregoing results coincide with studies done on rodents and especially on the mouse? It is known that stimulation of the anteromedial cortex (AMC) of the mouse (illustrated in Figs. 5 and 7) can evoke saccadic eye movements using both electrical stimulation and optogenetics (Itokazu et al. 2018; Zhang et al. 2014). As well, this region sends projections to area V1 that modulate the gain of orientation-tuned neurons when transitioning between locomotion and immobility (Niell and Stryker 2010; Zhang et al. 2014) and this region projects to parietal and extrastriate cortex as mentioned previously (Itokazu et al. 2018). Neurons in the anteromedial cortex are modulated by visually guided saccadic eye movements and inactivation of this area abolishes saccadic eye movements directed contralateral to the side of inactivation (Itokazu et al. 2018). Additionally, this region innervates V1 topographically conveying information about head and body orientation (Bouvier et al. 2020; Leinweber et al. 2017). Electrical stimulation of the anteromedial cortex in freely moving rats evokes contraversive head and body movements such that the orientation of the head with respect to the axis of the body (as measured from overhead) is about 70 degrees and the animal continues to circle for stimulation train durations up to 40 seconds (Tehovnik and Yeomans 1987; Yeomans and Tehovnik 1988). Moreover, the stimulation evokes vertical head movements as well as vibrissa movements. Since 1909 it has been known that electrical stimulation of the medial eye fields in unrestrained primates (i.e., first in humans and later verified in macaque monkeys) elicits head movements such that the head begins to move before the eyes which differs from the frontal eye fields for which the eyes begin to move before the head when and if a head movement is even evoked (Chen 2006; Chen and Walton 2005; Levinsohn 1909). Furthermore, lesions of the internal capsule at the caudate nucleus abolish all head movements evoked from the frontal lobes (Jansen et al. 1955; also see Yeomans and Buckenham 1992). Whether the anteromedial cortex of the mouse contains representations of the frontal and medial eye fields is currently not known, but parts of the anteromedial cortex of rodents that encode head movements send a robust projection to the superior colliculus, the tegmentum, and the pontine nuclei that innervate the cerebellum (i.e., in the rat, Tehovnik et al. 1989), all regions that have been implicated in oculomotor control.
9. The Brain Stem including the Cerebellum
As indicated in figure 7, once the visual signal propagates into the brain stem it is converted into a firing-rate code which is utilized by the muscles to bring about a muscle contraction [Adrian 1922; Enoka and Duchateau 2017; Gasser 1930; Schiller and Tehovnik 2015; neural recruitment is also involved in this process and some have speculate that the pattern of spike discharge affects the contraction of skeletal muscles, Zhurov and Brezina 2006]. In the macaque monkey, the oculomotor nuclei and many of the neurons in the brain stem composing the eye movement generator operate such that increasing the pulse frequency and/or train duration of electrical stimulation increases the magnitude of an ocular displacement (Cohen and Komatsuzuki 1972; Schiller and Stryker 1972). Furthermore, the greater the displacement the higher the firing frequency of the neurons (Fuchs et al. 1985; Schiller 1970). Finally, head displacement and the rate of lateral locomotion (i.e., circling behavior), as induced from the brain stem via stimulation, increases systematically as a function of pulse frequency and train duration, as illustrated in rats (Tehovnik and Yeomans 1986, 1987; Yeomans and Tehovnik 1988). Whether the patten of neural firing affects ocular responses evoked from the brain stem has yet to be deduced.
In primates (e.g., macaque monkeys), the cerebellum also utilizes a firing-rate code when transmitting signals to the cerebellar nuclei, which are the output neurons that innervate the eye movement generator as well as circuits that regulate head and body movements (Kheradmand and Zee 1991; Manto et al. 2012). By increasing the frequency of pulses or train duration, the size of saccadic eye movements evoked electrically from the cerebellar vermis (lobules VI and VII) increases (Noda and Fujikado 1987b). Moreover, the discharge duration of cerebellar neurons defines the size of a saccadic eye movement such that the longer the duration, the greater the displacement (Fuchs et al. 1993; Ohtsuka and Noda 1991). Furthermore, current pulses as low as 2–3 μA delivered in 20 ms trains are sufficient for evoking saccades from the cerebellum (Noda and Fujikado 1987a). By comparison, current pulses higher than 10 μA and at much longer train durations (i.e., from 50 to 200 ms) are needed to generate ocular responses from the neocortex (Schiller and Tehovnik 2015; Tehovnik and Slocum 2004; Tehovnik and Sommer 1997). A major reason for this difference is that Purkinje cells respond well to high-frequency stimulation (i.e., optimally at 600 Hz, Noda and Fujikado 1987b), which attests to the extreme excitability of their axon initial segments (Foust et al. 2010). Finally, if electrical stimulation is introduced to the cerebellum during an ongoing saccadic eye movement, the movement is interrupted instantly and a new saccade is generated as encoded by the site of stimulation (Noda and Fujikado 1987a; Noda et al. 1991). A similar result occurs for stimulation of the oculomotor generator (Cohen and Komatsuzuki 1972). Accordingly, the cerebellum has priority access to the oculomotor generator in the brain stem for saccade execution. This is not true of the neocortex. Electrically-evoked ocular responses elicited from the neocortex can readily be interrupted by an animal’s ongoing behavior (Chen and Tehovnik 2007; Tehovnik and Slocum 2004). Thus, the cerebellum’s oculomotor control is predicated on having direct access to the movement controllers by way of a firing-rate code. This permits for the execution of effortless body movements as triggered by a specific sensory context once a task has been learned (Swain et al. 2011; Thach et al. 1992).
The neocortex is connected to the cerebellum via the pontine nuclei. These nuclei project to the cerebellar cortex via the middle cerebellar peduncle whose massive size (as compared to the other peduncles) indicates the importance of this pathway for transferring sensory information between the neocortex and cerebellum (Baumann et al. 2015; Kratochwil et al. 2017; Ramnani 2006). Incidentally, the object encoding areas of macaque monkey temporal and extrastriate cortex do not project directly to the pontine nuclei for access to the cerebellum (Baumann et al. 2015). The superior colliculus, which is innervated by the posterior neocortex (Fig. 7), is an alternative path for the transfer of visual information from the inferior temporal cortex and area V4 to the pontine nuclei en route to the cerebellum (Kratochwil et al. 2017; Manni and Petrosini 2004; Matsuzaki and Kyuhou 1997). The oculomotor regions of the frontal cortex in both the rodent and macaque monkey, however, send direct projections to the pontine nuclei (Shook et al. 1990; Stanton et al. 1988; Tehovnik et al. 1989).
The cerebellum has been associated with computing the eye, head, and limb position with respect to the body (Fuchs and Kornhuber 1969; Fukushima et al. 2011; Lisberger and Fuchs 1978; Thach et al. 1992). If macaque monkeys are required to generate saccadic eye movements to a remembered target position in darkness, once their eyes are displaced by electrically stimulating the brain with a brief train of pulses it is typical for the eyes to arrive on target following the displacements via a corrective saccade (Schiller and Sandell 1983; Sparks and Mays 1983; Tehovnik and Sommer 1996). This occurs when stimulating sites at or dorsal to the superior colliculus including the frontal and medial eye fields. If, on the other hand, the eyes are perturbed by stimulating cerebellar sites, the eyes never arrive on target as though to suggest that the visual image has been shifted by the stimulation (Noda et al. 1991). A similar result occurs when stimulating sites within the oculomotor generator including the oculomotor nuclei (Schiller and Sandell 1983; Sparks et al. 1987). If the proprioceptors of the eyes are stimulated, human subjects report that a visual image is made to jump (Roll and Roll 1987; Roll et al. 1991; Valey et al. 1994, 1995, 1997). Indeed, the cerebellum resolves the discrepancy between the proprioceptive signal and the visual signal by shifting the visual percept in favor of proprioception given that proprioceptive signals arrive in the cerebellum at latencies as short as 3 ms, whereas it takes a visual signal over 30 ms to reach the cerebellum (Fuchs and Kornhuber 1969).
That the cerebellum is involved in computing the position of the body is, furthermore, supported by the finding that prism adaptation is abolished in macaque monkeys and humans following cerebellar damage (Braizer and Glickstein 1973; Braizer et al. 1999; Deuschl et al. 1996; Martin et al. 1996; Morton and Bastian 2004; Thach et al. 1992; Weiner et al. 1983). When human subjects are asked to throw a dart once the eyes have been deviated by 15 degrees using a prism, under normal circumstances they adapt to this situation after twenty or so trials (Thach et al. 1992). Following cerebellar damage, there is no adaptation. The foregoing has implications for object vision. It has been known for some time that if human subjects wear a prism that bends a physical straight line, that after wearing the prism for an extended period of time the subjects will perceive the line as straight (Hebb 1969). This adaptation comes about by having the visual system put the curved line, as induced by the prism, in register with the non-visual senses of the body. It is believed that the cerebellum has the last say in this process by integrating all the senses to produce a coherent motor response through learning (Swain et al. 2011; Thach et al. 1992).
The cerebellum of humans is polysynaptically connect to the neocortex, including to regions that process object vision. The cerebellum consists of three lobes: an anterior lobe composed of lobules I to V, a mediolateral lobe composed of lobules VI to VII, and a posterior lobe composed of lobules VIII to IX (Fig. 8, based mainly on Boillat et al. 2020). The somatotopy for all the lobes starts with an eye represented in the mediolateral lobe and terminates with a foot representation in each of the other two lobes (a property of the macaque monkey as well, Adrian 1943; Manni and Petrosini 2004; Noda and Fujikado 1987ab; Thach et al. 1992); the somatotopy for the mediolateral lobe is least well developed. There is also a head representation in lobule VI and a vestibular representation in lobule X for vestibulo-ocular control (Lisberger and Fuchs 1978). Using resting-state functional-connectivity MRI in humans, it has been found that the sensorimotor regions of the neocortex (i.e., M1 and S1) are preferentially linked to the anterior lobe, that the object encoding areas including the extrastriate, temporal, and orbital cortices are linked to the mediolateral lobe, and that the motion encoding areas including MT, MST, STS (superior temporal sulcus), and posterior parietal and supplementary motor cortices are linking to the posterior lobe (Buckner 2013; Buckner et al. 2011; Diedrichsen et al. 2019; Marek et al. 2018). Using both fMRI and brain-damaged patients, the mediolateral lobe has been implicated in language processing and mathematics (Guell et al. 2018; Mariën et al. 2017; Schmahmann and Sherman 1998) both of which rely heavily on object vision. Thus far, the precise topographic order of the mouse cerebellum has yet to be deduced. Given that the mouse has a well-developed object encoding system in neocortex (Figs. 4 and 5) and that its cerebellum includes a mediolateral sector composed of lobules VI to VII (White and Sillitoe 2012) it would be surprising if it did not have real estate dedicated to object vision. On this point, using two-photon calcium imaging, neurons (i.e., granular cells) have been identified in lobule VI of the mouse that respond to visual stimuli and that can be conditioned to evoke an eye-blink response to those stimuli (Figs. 1–3 of Giovannucci et al. 2017). Muscimol inactivation of lobule VI abolished the conditioned response without affecting the unconditioned response. The conditioning of cerebellar neurons has been described as the creation of an efference-copy representation (Giovannucci et al. 2017).
Figure 8.
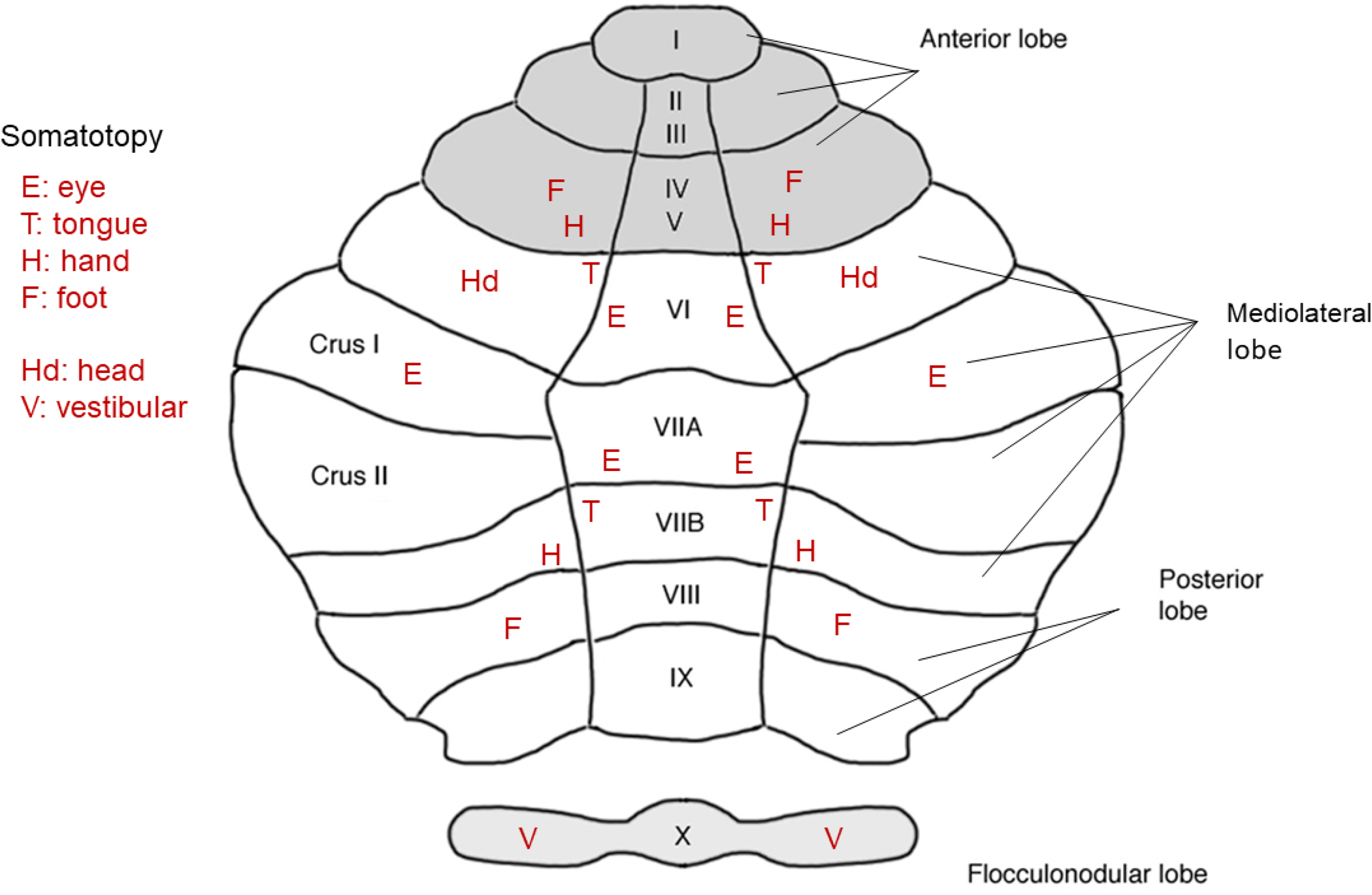
Schematic of a top view of human cerebellum divided into three lobes: anterior, mediolateral, and posterior. According to the fMRI experiments of Boillat et al. (2020) who used a 7-tesla scanner, a somatotopy—for eye, tongue, hand, and foot—is found for anterior and posterior lobes, but an eye representation without a clear somatotopy is found for the mediolateral lobe. A head representation is found in lobule VI (Manni and Petrosini 2004) and vestibular control of the head is found in lobule X (Lisberger and Fuchs 1978). The text inset in the upper left defines the movement of the body part or sense that triggered a maximal response within the cerebellar cortex.
The cerebellum of rodents and primates contains about 80% of all neurons in the brain with the remainder residing mainly in the neocortex (Herculano-Houzel 2009, 2010, 2012). As well, in these animals the number of neurons in the cerebellum varies positively and systematically with the number of neurons in the neocortex (Fig. 4B of Herculano-Houzel 2010). The large number of neurons in the cerebellum that is mainly composed of granular cells is believed to permit the storage of learned motor routines (Giovannucci et al. 2017; Huang 2008). The internal circuitry of the cerebellar cortex is made up of Purkinje neurons whose discharge inhibits the firing of the neurons of the cerebellar nuclei, the exclusive output channel of the cerebellum. Thus, increases in Purkinje discharge decreases the firing of the deep cerebellar nuclei neurons whereas decreases in the discharge increase their firing (Lisberger and Fuchs 1978; Noda et al. 1988; Swain et al. 2011; Thach et al. 1992). Purkinje neurons receive two inputs, one from the mossy fibres and a second from the climbing fibres. The mossy fibres carry sensory input from the neocortex, brain stem, and spinal cord; the climbing fibres, which originate from the inferior olive, are able to change the gain of firing of Purkinje neurons when new routines are being learned by eliciting complex spikes in the Purkinje neurons. The process, which is documented by a large amount of experimental data (e.g., Boyden et al. 2004; Gilbert and Thach 1977; Kitazawa et al.1998; Medina and Lisberger 2008; Miles and Lisberger 1981; Soetedjo and Fuchs 2006; Soetedjo et al. 2008; Swain et al. 2011; Thach et al. 1992; Yang and Lisberger 2014), works as follows: when complex spikes are emitted at a low rate (< 2 Hz) the firing frequency of Purkinje spikes (i.e., the simple spikes) is elevated whereas when the complex spikes are emitted at a high rate (> 2 Hz) the firing frequency of these spikes is reduced. This bi-directionally change to the gain of Purkinje neurons has recently been confirmed by others (Loyola et al. 2019; Shadmehr 2020) and has implications for the storage of learned routines (Huang 2008; Swain et al. 2011; Thach et al. 1992; Yang and Lisberger 2014). Indeed, optogenetic perturbation of Purkinje circuits in mice disrupts left versus right, vibrissae tactile-memory (Fig. 1g,h of Gao et al. 2018), suggesting that the recollection of touch and body position, i.e. proprioception, is mediated by the cerebellum.
Using modern methods on the cerebellum of the mouse [e.g., optogenetics, two-photon calcium imaging, and patch-clamp recording, Froudarakis et al. 2020; Gao et al. 2018; Giovannucci et al. 2017; Scala et al. 2020] the following questions can now be answered: (1) Does the pattern of spike discharge at the level of the cerebellum have an effect on the learned behaviors mediated by the cerebellum given that a spike-rate code is believed to be operative here. (2) How are communications established between the object encoding areas vis-à-vis the neocortex and cerebellum as mice learn to discriminate between objects (e.g., using the methods of Froudarakis et al. 2020, Gao et al. 2018, and Giovannucci et al. 2017). (3) Can the bi-direction gain-control mechanism of the Purkinje cells be manipulated to alter systematically the relationship between vision and movement as has been done using the electrical perturbation of cerebellar circuits and the donning of prisms.
10. Outstanding Items
10.1. Conventional versus modern methodologies
Many studies conducted on the mouse visual system have utilized optogenetics and two-photon calcium imaging (Froudarakis et al. 2019). In the experiments conducted on macaque monkeys it has been typical to use older methodologies: single-cell recording, electrical brain stimulation, and lesion methods from ablations to chemical inactivation or excitation (Schiller and Tehovnik 2015). One concern when comparing the mouse to the macaque monkey is that the differences that may arise are based on nothing more than methodological differences. It is known that optogenetics has generally failed to evoke saccadic eye movements from the neocortex of macaque monkeys yet electrical stimulation readily evokes such movements (Schiller and Tehovnik 2015), but if the behavioral conditions for eliciting saccades are optimized (Tehovnik et al. 2003), saccades can be induced from the neocortex using optogenetics (Jazayeri et al. 2012). A major difference between the two techniques is that optogenetic stimulation activates a fraction of the neurons within a tissue volume, whereas electrical stimulation likely drives most neurons and the axonal fibres of passage within the volume. Moreover, the latency to evoke spike discharges using optogenetics can be as long as 8–9 ms (Isa et al. 2020), which would delay the evocation of optogenetically-elicited behavior. When evoking eye movements electrically from the abducens nucleus the latency can be as short as 3 to 4 ms (Miyashita and Hikosaka 1996). Finally, it is known that calcium imaging can be used optimally for those neurons that have moderate firing frequencies thereby failing to capture extremely low and high firing rates (Nauhaus et al. 2012; Tehovnik and Slocum 2013). This is less of a problem for single cell recording (Schiller and Tehovnik 2015).
10.2. The function of the binocular overlap in the mouse
In macaque monkeys, it has been common to attribute the binocular overlap of the visual field of the two eyes for the purpose of conducting stereopsis. The overlap in the mouse is much less. Nevertheless, these animals can perform coarse stereovision both at the level of V1 and extrastriate cortex including area lateromedial, which has been implicated in object encoding (Fig. 4; La Chioma et al. 2020; Samonds et al. 2019; Scholl et al. 2013). Also, it has been suggested that an additional purpose of the overlap in rodents is to enhance an animal’s ability to detect moving stimuli in the field of overlap and to orient the head. Ingle (1981) showed that gerbils can anticipate the trajectory of a moving stimulus (a disc bated with a sunflower seed) such that a 30 to 40 degree per second movement (temporally or nasally) compelled the animal to orient its head and body 5 to 10 degrees in advance of the movement to intercept the stimulus. However, beyond 40 degrees (i.e., outside the region of binocular overlap), the animals treated the disc as stationary. This result will no doubt need to be replicated in the mouse who has a similar visual system to that of the gerbil.
10.3. Object encoding in the mouse
In Froudarakis et al. (2020), three areas in mouse extrastriate cortex were activated by objects based on previous training: the anterolateral area, the lateromedial area, and the lateral intermediate area. Two areas were noticeably non-active: the posterior area and the postrhinal area, which could be homologues of the inferotemporal cortex (Wang et al. 2012), an object encoding area in macaque monkeys. It is noteworthy that bilateral lesions at and anterior to area postrhinal disrupt object recognition memory in rodents (i.e., in rats, Ho et al. 2011). There are four possible reasons for the non-activity of the posterior and postrhinal areas. First, these areas are best observed by viewing the cortex from the side and back rather than from overhead; all experiments conducted by Froudarakis et al. were done by imaging from overhead. Second, passive viewing was used to identify objects even though the animals learned the object sets in their home cage. It is well known that neurons in the visual cortex are best activated if animals (i.e., macaque monkeys) are required to use visual information to perform a behavioral task for reward especially if studying regions beyond V1 (e.g., Haenny and Schiller 1988). Third, in the experiments of Froudarakis et al. the mice were trained for only three weeks on the various objects such that the overall identification of objects did not surpass 70% correctness (with chance at 50%). Some believe that in order for objects to be archived in the temporal lobes an extensive period of training is required (Hikosaka et al. 2014). Indeed, large medial temporal lobe lesions in elderly adult humans can induce retrograde amnesia of ‘declarative’ objects that spans 40 to 50 years (Squire et al. 2001). Finally, the posterior and postrhinal areas of the mouse may have nothing to do with object vision. Which of the foregoing best accounts for the lack of activity to objects in the posterior and postrhinal areas needs clarification.
10.4. Expanding the oculomotor range in the mouse
Head movements extends the oculomotor range in mice and primates including macaque monkeys. In these animals, input from vestibular sensors, which coordinate eye and head movements, are highly distributed. These inputs reach vast sections of the neocortex including the supplementary motor area (which includes the medial eye field), the neck and forelimb representation of M1 and S1, the orbital cortex (area 13), the temporal lobe (area 14), and areas LIP (lateral intraparietal), MST, and STS, and the retrosplenial cortical area (Chen et al. 2011; Fukushima et al. 2011; Guldin and Grüsser 1998; Rancz et al. 2015). Also, the vestibular system transmits to the thalamus, the striatum, and the superior and inferior colliculi. In order to deduce how the mouse extends its oculomotor range, eye and head movements will need to be recorded simultaneously in freely-moving mice orient toward and away from visual stimuli (e.g., Meyer et al. 2018) as neurons are examined at various sites within the eye and head movement control system.
10.5. Efference-copy representation and the cerebellum
The consensus is that the cerebellum is responsible for producing the efference-copy representation for all movements that arises once animals, such as mice and macaque monkeys, become highly trained on a task (Ebner and Pasalar 2008; Giovannucci et al. 2017; Huang et al. 2013; Ito 2008; Miall et al. 1993; Robinson 1981; Wolpert et al. 1998). This has been studied best for the vestibulo-ocular reflex such that the movement of the head triggers an eye movement that counter-rotates with respect to the head at a latency as short as 12 ms following the head movement (Miles and Lisberger 1981), a process which stabilizes the visual image on the retina. This short latency cannot be attributed to vision given that it takes over 30 ms for a visual signal to arrive in the brain stem (Fuchs and Kornhuber 1969; Miles and Lisberger 1981) Cerebellar circuits are involved in tuning the short latency (Miles et al. 1980; Miles and Lisberger 1981). Furthermore, adapting the vestibulo-ocular reflex to prisms is abolished following cerebellar lesions (Lisberger et al. 1984). When studying the grey matter volume of the cerebellum in athletes (all under the age of thirty) it was found that ballet performers had an enhancement of the posterior lobe including the vestibular circuitry (Dordevic et al. 2018), sprinters had an augmentation of the anterior lobe which subserve lower limb locomotion (Wenzel et al. 2014), and basketball players had a potentiated mediolateral lobe which subserves eye-hand, object coordination (Park et al. 2009). The latter, the mediolateral lobe, mediates object vision as well as language (Buckner et al. 2011; Diedrichsen et al. 2019; Guell et al. 2018; Mariën et al. 2017; Park et al. 2009; Schmahmann and Sherman 1998; Sendhilnathan et al. 2020; see Fig. 8 for details of lobe location). In the case of language, it can take as much as a decade to acquire through reading (which depends on object vision), writing, and speaking but once acquired the anticipatory interchange between two individuals in conversation occurs at a fraction of a second (Levinson and Torreira 2015), which could not be realized without an efference-copy representation of speech anticipation. Incidentally, patient HM who had bilateral removal of his hippocampus—but an intact cerebellum—could still engage in conversation even though minutes later he would not remember having had the conversation (Annese et al. 2014; Corkin 2002).
10.6. Information transfer vis-à-vis objects
Information transfer expressed in bits (as derived from the number of possibilities) per unit time has been used to quantify the behaviors produced by rodents, monkeys, and humans (Tehovnik and Chen 2015; Tehovnik and Teixeira-e-Silva 2014; Tehovnik et al. 2013). In humans, learning compresses information flows (Miller 1956) by adding more sensory items into memory and by having each item evoke a precise motor output at the shortest latency (e.g., via the cerebellum: Huang 2008; Sultan and Heck 2003; Tehovnik and Chen 2015). Once versed in a language, one can transfer over 40 bits per second as delivered through speaking (Reed and Durlach 1998). By comparison, the ant pheromone system transfers 1.4 bits per second based on its 20 pheromone-alphabet (Hölldobler and Wilson 1990; McIndoo 1914). The late physicist, Stephen Hawking who suffered from ALS (amyotrophic lateral sclerosis) was able to transfer 0.4 bits of information per second without the assistance of his computer (De Lange 2011), a computer that acted like a cerebellum to increase his rate of communication. Every animal must store in its memory a collection of objects (expressed as the number of possibilities) so that upon encountering those objects a behavioral response is evoked at the shortest latency. In the case of the mouse, the collection of objects necessary for survival is found in the animal’s natural habitat (Gibson 1972). The upper limit to the storage of objects for a mouse is not known, but the carrying capacity of the mouse brain is 0.08% of the human brain based on the total number of neurons, with the cerebellum possessing most of this capacity [i.e., {(71 × 106) / (86 × 109)} × 100, Herculano-Houzel 2009]. An outstanding question is how the neocortex in combination with the cerebellum establishes the high information transfer rates needed to survive in an ever changing environment. In the case of the human cerebellum, it has been estimated to have an information storage capacity of 1014 bits based on the large number of granular cells and their connectivity (Huang 2008). Only now are investigators beginning to study the relationship between the neocortex and cerebellum in behaving animals (e.g., in the mouse, Gao et al. 2018), which has the potential to address the issue of object-based information flow so that the storage and transfer of information can be estimated for different species.
11. Conclusions
The visual spatial resolution of a mouse is many orders of magnitude less than the visual spatial resolution of a macaque monkey; a mouse does not have a fovea but instead has a visual system designed for panoramic and overhead viewing.
The extrastriate cortex of the mouse and macaque monkey are similarly organized such that lateral regions encode objects and medial regions encode motion. In both species these regions mediates complex perception that depends on learning.
Areas in the frontal cortex along with the superior colliculus coordinate the eye movements and gaze shifts of the mouse and macaque monkey by centering the eyes in orbit to expedite an efficient transformation between retinal and skeleton-motor space for optimal object manipulation or avoidance.
The cerebellum much like the visual cortex contains a separate representation for objects and motion processing as demonstrated in humans. It is believed that this structure in mice and macaque monkeys stores efference-copy routines for the immediate execution of well-learned behaviors.
The brain stem of the mouse and macaque monkey utilizes a firing-rate code independent of sensory information to contract the eye and head muscles to orient the body toward or away from visual objects, a coding scheme that is also utilized by the cerebellum.
Acknowledgements
We would like to dedicate this work to Professor Peter H. Schiller whose empirical work is largely responsible for allowing us to make explicit comparisons between the mouse and the macaque monkey because of his fifty years of study on how information travels between the retina and the muscles in behaving rhesus monkeys. Special thanks to Professors Fabrizio Gabbiani and Jacob Reimer for the many thoughtful comments made toward improving this work. Research reported in this publication was supported by the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke under Award Number U19MH114830. Also, supported by the Intelligence Advanced Research Projects Activity (IARPA) via Department of Interior/Interior Business Center (DoI/IBC) contract number D16PC00003. R01 EY109272 and R01 NS113890 was awarded to S. Smirnakis. The U.S. Government is authorized to reproduce and distribute reprints for Governmental purposes notwithstanding copyright. Disclaimer: The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements (either expressed or implied) of IARPA, DoI/IBC, or the U.S. Government including the National Institutes of Health. Additional funding includes: R01 EY026927, DARPA #5N66001, R01 MH109556, HR0011-18-2-0025, NeuroNex NSF-1707400, NeuroNex NSF-1707359 and Big Data NSF IIS-1546273.
Text Abbreviations
- ALS
amyotrophic lateral sclerosis
- AMC
anteromedial cortex
- LGN
lateral geniculate nucleus
- LIP
lateral intraparietal area
- MT
middle temporal cortex
- MST
middle superior temporal cortex
- STS
superior temporal sulcus
- V1
primary visual cortex
References
- Adams DL, Horton JC, 2003. A precise retinotopic map of primate striate cortex generated from representation of angioscotomas. J. Neurosci. 23, 3771–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED, 1922. The relation between the stimulus and the electrical response in a single muscle fiber. Arch. Neérl. Physiol. 7, 330–332. [Google Scholar]
- Adrian ED, 1943. Afferent areas in the cerebellum connected with the limbs. Brain 66, 289–315. [Google Scholar]
- Andersen RA, Mountcastle VB, 1983. The influence of the angle of gaze upon the excitability of the light-sensitive neurons of the posterior parietal cortex. J. Neurosci. 3, 532–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annese J, Schenker-Ahmed NM, Bartsch H, Maechler P, Sheh C, Thomas N, Kayano J, Ghatan A, Bresler N, Frosch MP, Klaming R, Corkin S, 2014. Postmortem examination of patients H.M.’s brain based on histological sectioning and digital 3D reconstruction. Nature Comm. doi: 10.1038/ncomms4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barret RLC, Dawson M, Dyrby TB, Krug K, Pitito M,…Catani M, 2020. Differences in frontal network anatomy across primate species. J. Neurosci. 40, 2094–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C,…Sokolov AA, 2015. Consensus paper: The role of the cerebellum in perceptual processing. Cerebellum 14, 197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavidez NL, Bienkowksi MS, Khanjani N, Bowmajn I, Fayzullina M,…Dong H-W, 2020. The mouse cortico-tectal projectome. bioRxiv doi: 10.1101/2020.03.24.006775. [DOI] [Google Scholar]
- Benbadis SR, Kotagal P, Klem GH, 1996. Unilateral blinking: a sign in partial seizures. Neurol. 46, 45–48. [DOI] [PubMed] [Google Scholar]
- Berkovic SF, Rubin M, Andermann F, Gauthier S, 1985. Secondary paroxysmal kinesigenic choreoatitosis misdiagnosed as epilepsy: a study of 3 cases (Abstract). 16th Epilepsy International Congress, Hamburg. [Google Scholar]
- Blasdel GG, 1992. Orientation selectivity, preference, and continuity in monkey striate cortex. J. Neurosci. 12, 3139–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodis-Wollner I, Bucher SF, Seelos KC, 1999. Cortical activation patterns during voluntary blinks and voluntary saccades. Neurology 53, 1800–1805. [DOI] [PubMed] [Google Scholar]
- Boillat Y, Bazin P-L, Van der Zwaag W, 2020. Whole-body somatotopic maps in the cerebellum revealed with 7T fMRI. NeuroImage doi: 10.1016/j.neuroimage.2020.116624. [DOI] [PubMed] [Google Scholar]
- Borst A, Euler T, 2011. Seeing things in motion: Models, circuits, and mechanisms. Neuron 71, 974–994. [DOI] [PubMed] [Google Scholar]
- Bouvier G, Senzai Y, Scanziani M, 2020. Head movements control the activity of primary visual cortex in a luminance dependent manner. bioRxiv doi: 10.1101/2020.01.913160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Katoh A, Raymond JL 2004. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu. Rev. Neurosci. 27, 581–609. [DOI] [PubMed] [Google Scholar]
- Braizer J, Glickstein M, 1973. Role of cerebellum in prism adaptation. J. Physiol. Lond. 236, 34–35. [PubMed] [Google Scholar]
- Braizer J, Kralj-Hans I, Glickstein M, 1999. Cerebellar lesions and prism adaptation in macaque monkeys. J. Neurophysiol. 81, 1960–1965. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, 1985. Primate frontal eye fields. I. Single neurons discharge before saccades. J. Neurophysiol. 53, 603–635. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GA, 1985. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J. Neurophysiol. 54, 714–734. [DOI] [PubMed] [Google Scholar]
- Buckner RL, 2013. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807–815. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo TT, 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande VA, Harting JK, Hall WC, Diamond IT, Martin GF, 1972. Superior colliculus of the tree shrew: a structural and functional subdivision into superficial and deep layers. Science 177, 444–447. [DOI] [PubMed] [Google Scholar]
- Chen A, DeAngelis GC, Angelaki DE, 2011. A comparison of vestibular spatiotemporal tuning in macaque parietoinsular vestibular cortex, ventral intraparietal area, and medial superior temporal area. J. Neurosci. 31, 3082–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, 2006. Head movements evoked by electrical stimulation in the frontal eye fields of the monkey: evidence for independent eye and head control. J. Neurophysiol. 95, 3528–3542. [DOI] [PubMed] [Google Scholar]
- Chen LL, Tehovnik EJ, 2007. Cortical control of eye and head movements: integration of movements and percepts. Eur. J. Neurosci. 25, 1253–1264. [DOI] [PubMed] [Google Scholar]
- Chen LL, Walton MM, 2005. Head movements evoked by electrical stimulation in the supplementary eye field of the macaque monkey. J. Neurophysiol. 94, 4502–2519. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Gamlin PDR, 1995. The role of the pretectum in the pupillary light reflex. In: Robbins JR et al. , eds., Basic and Clinical Perspective in Clinical Research. Springer, New York, pp. 149–159. [Google Scholar]
- Clarke RJ, Ikeda H, 1985. Luminance and darkness detectors in the olivary and posterior pretectal nuclei and their relationship to the pupillary light reflex in the rat. I Studies with steady luminance levels. Exp. Brain. Res. 57, 224–232. [DOI] [PubMed] [Google Scholar]
- Cohen B, Komatsuzuki A, 1972. Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp. Neurol. 36, 101–117. [DOI] [PubMed] [Google Scholar]
- Comoli E, Das Neves Favero P, Vaudrellie N, Leriche M, Overton PG, Redgrave P, 2012. Segregated anatomical input to subregions of the rodent superior colliculus associated with approach and defense. Front. Neuroanat. doi: 10.3389/fnana.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S, 2002. What’s new with the amnesic patient H.M.? Nature Rev. Neurosci. 3, 153–160. [DOI] [PubMed] [Google Scholar]
- Cowey A, Stoering P, 1995. Blindsight in monkeys. Nature 373, 247–249. [DOI] [PubMed] [Google Scholar]
- De Gelder B, Tamietto M, Van Boxtel G, Goebel R, Sahraie A, Van der Stock J, Stienen BMC, Weiskrantz L, Pegna A, 2008. Intact navigation skills after bilateral loss of striate cortex. Curr. Biol. 18, PR1128–PR1129. [DOI] [PubMed] [Google Scholar]
- De Lange C, 2011. The man who saved Stephen Hawking’s voice. New Scientist, December issue. [Google Scholar]
- Deuschl G, Toro C, Zeffiro T, Massaquoi S, Hallett M, 1996. Adaptation motor learning of arm movements in patients with cerebellar disease. J. Neurol. Neurosurg. Psychiatr. 60, 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, King M, Hernandez-Castillo C, Sereno M, Ivry RB, 2019. Universal transform or multiple functionality? Understanding the contribution of human cerebellum across task domains. Neuron doi: 10.1016/j.neuron.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordevic M, Schrader R, Taubert M, Muller P, Hokelmann A, Muller NG, 2018. Vestibular-hippocampal function is enhanced and brain structure altered in professional ballet dancers. Front. Integr. Neurosci. doi: 10.3389/fnint.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dräger UC, 1975. Receptive fields of single cells and topography in the mouse visual cortex. J. Comp. Neurol. 160, 269–290. [DOI] [PubMed] [Google Scholar]
- Dräger UC, Hubel DH, 1975. Response to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J. Neurophysiol. 38, 690–713. [DOI] [PubMed] [Google Scholar]
- Ebner TJ, Pasalar S, 2008. Cerebellum predicts the future motor state. Cerebellum 7, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard CG, Chapman DG, 1991. The effects of posterior cortical lesions on responses to visual threats in the Mongolian gerbil (Meriones unguiculatus). Behav. Brain Res. 44, 163–167. [DOI] [PubMed] [Google Scholar]
- Ellard CG, Goodale D, Scorfield DM, Lawrence C, 1986. Visual cortical lesions abolish the use of motion parallax in the Mongolian gerbil. Exp. Brain Res. 64, 599–602. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J, 2017. Rate coding and the control of muscle force. Cold Spring Harbor Perspect. Med doi:10:1101/cshperspect.a029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban A, 1999. A neurophysiological approach to the brainstem reflex. Blink reflex. Neurophysiol. Clin. 29, 7–38, doi: 10.1016/S0987-7053(99)80039-2. [DOI] [PubMed] [Google Scholar]
- Fahey PG, Muhammad T, Smith C, Froudarakis E, Cobos E, Fu J, Walker EY, Yatsenko D, Sinz FH, Reimer J, Tolias AS, 2019. A global map of orientation tuning in mouse visual cortex. bioRxiv doi: 10.1101/745323. [DOI] [Google Scholar]
- Felleman DJ, Van Essen DC, 1991. Distributed hierarchical processing in the primate cerebellar cortex. Cereb. Cortex 1, 1–47. [DOI] [PubMed] [Google Scholar]
- Foust A, Popovic M, Zecevic D, McCormick DA, 2010. Action potentials initiate in axon initial segment and propagation through axon collaterals reliably in cerebellar Purkinje neurons. J. Neurosci 30, 6891–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL, 1996. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus of macaque monkeys. J. Neurophysiol. 76, 927–952. [DOI] [PubMed] [Google Scholar]
- Fries W (1984) Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol 230:55–76. [DOI] [PubMed] [Google Scholar]
- Frost DO, Caviness VS Jr., 1980. Radial organization of thalamic projections to the neocortex in the mouse. J. Comp Neurol. 194, 369–393. [DOI] [PubMed] [Google Scholar]
- Froudarakis E, Cohen U, Diamantaki M, Walker EY, Reimer J, Berens P, Sompolinsky H, Tolias AS, 2020. Object manifold geometry across the mouse cortical visual hierarchy. bioRxiv doi: 10.1101/2020.08.20.258798. [DOI] [Google Scholar]
- Froudarakis E, Fahey PG, Reimer J, Smirnakis SM, Tehovnik EJ, Tolias AT, 2019. The visual system in context. Annu. Rev. Vis. Sci. doi: 10.1146/annurev-vision-091517-034407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Kaneko CR, Scudder CA, 1985. Brainstem control of saccadic eye movements. Annu. Rev. Neurosci. 8, 307–337. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Kornhuber HH, 1969. Extraocular muscle afferents to the cerebellum of the cat. J. Physiol. Lond. 200, 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Robinson FR, Straube A, 1993. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge patterns. J. Neurophysiol. 70, 1723–1740. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Fukushima J, Warabi T, 2011. Vestibular-related frontal cortical areas and their role in smooth-pursuit eye movements: representation of neck velocity, neck-vestibular interactions, and memory-based smooth pursuit. Front. Neurol. doi: 10.3389/fneur.2011.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, Yoon K, 2000. An area for vergence eye movement in primate frontal cortex. Nature 407, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Gao Z, Davis C, Thomas AM, Economo MN, Abrego AM, Svoboda K, De Zeeuw CI, Li N, 2018. A cortico-cerebellar loop for motor planning. Nature doi: 10.1038/s41586-018-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett ME, Nauhaus I, Marshel JH, Callaway EM, 2014. Topography and areal organization of mouse visual cortex. J. Neurosci. 34, 12587–12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser HS, 1930. Contraction of skeletal muscle. Physiol. Rev. doi: 10.1152/physrev.1930.10.135. [DOI] [Google Scholar]
- Gibson JJ, 1972. A Theory of Direct Visual Perception. In: Royce J, Rozenboom W, eds., The Psychology of Knowing. Gordon and Breach, New York. [Google Scholar]
- Gilbert PF, Thach WT, 1977. Purkinje cell activity during motor learning. Brain Res. 128, 309–328. [DOI] [PubMed] [Google Scholar]
- Giovannucci A, Badura A, Deverett B, Najafi F, Pereira TD, Gao Z, Ozden I, Kloth AD, Pnevmatkakis E, Paninski L, De Zeeuw CI, Medina JF, Wang SS-H, 2017. Cerebellar granular cells acquire a widespread predictive feedback signal during motor learning. Nat. Neurosci. 20, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Wurtz RE, 1972. Activity of superior colliculus in behaving monkey. I. Visual receptive fields of single neurons. J. Neurophysiol. 35, 542–559. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Bruce CJ, MacAvoy MG, 1993. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J. Neurophysiol. 69, 786–799. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, MacAvoy MG, Bruce CJ, 1994. Neural responses related to smooth pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J. Neurophysiol. 72, 1634–1653. [DOI] [PubMed] [Google Scholar]
- Guell X, Gabrieli JDE, Schmahmann JD, 2018. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analysis in a single large cohort. NeuroImage 172, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W, 2018. Development, form, and function of the mouse thalamus. J. Neurophysiol. 120, 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldin WO, Grüsser OJ, 1998. Is there a vestibular cortex? Trends Neurosci. 21, 254–259. [DOI] [PubMed] [Google Scholar]
- Haenny PE, Schiller PH, 1988. State dependent activity in monkey visual cortex. I. Single unit activity in V4 and V1 on visual tasks. Exp. Brain Res. 69, 225–244. [DOI] [PubMed] [Google Scholar]
- Hebb DO, 1969. The mechanism of perception. In: The Conceptual Nervous System, Buchtel HA, ed., Pergamon Press, Oxford, UK, 1982. [Google Scholar]
- Heesy CP, 2004. On the relationship between orbit orientation and binocular visual field overlap in mammals. Anat. Rec. Part A 281a, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, 2009. The human brain in numbers: a linearly scaled-up primate brain. Front. Neurosci. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, 2010. Coordinated scaling of cortical and cerebellar numbers of neurons. Front. Neuroanat. doi: 10.3389/fnama.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, 2012. The remarkable, yet not extraordinary, human brain as scaled-up primate brain and its associated cost. Proc. Natl. Acad. Sci. USA 109, 10661–10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess WR, Burgi S, Bücher V, 1946. Motorische funktionen des tektal- und tegmentalgebietes. Monatsschr. Psychiatr. Neurol. 112, 1–52. [PubMed] [Google Scholar]
- Hikosaka O, Kim HF, Yasuda M, Yamamoto S, 2014. Basal ganglia circuits for reward value-guided behavior. Annu. Rev. Neurosci. 37, 289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH, 1985. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J. Neurophysiol. 53, 266–291. [DOI] [PubMed] [Google Scholar]
- Ho JW, Narduzzo KE, Outram A, Tinsley CJ, Henley JM, Warbuton EC, Brown MW, 2011. Contribution of area Te2 to rat recognition memory. Lean. Mem. 18, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO, 1990. The Ants. Harvard University Press, Cambridge MA. [Google Scholar]
- Horton JC, 1984. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Phil. Trans. R. Soc. Lond. B Biol Sci 304:199–253. [DOI] [PubMed] [Google Scholar]
- Huang C, 2008. Implications on cerebellar function from information coding. Cerebellum doi: 10.1007/s12311-008-0032-1. [DOI] [PubMed] [Google Scholar]
- Huang C-C, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, Nelson SB, Adam W, Hantman AW 2013. Convergence of pontine and proprioceptive streams onto multimodal cerebellar granular cells. eLife 2, e00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, 1977. Functional architecture of macaque monkey visual cortex. Proc. R. Soc. Lond. B 198, 1–59. [DOI] [PubMed] [Google Scholar]
- Hughes A, 1977. The refractive state of the rat eye. Vision Res. 17, 927–939. [DOI] [PubMed] [Google Scholar]
- Humphrey NK, Weiskrantz L 1963. Vision in monkey after removal of the striate cortex. Nature 215, 595–597. [DOI] [PubMed] [Google Scholar]
- Ingle D, 1973. Evolutionary perspectives on the function of the optic tectum. Brain Behav. Evol. 8, 211–237. [DOI] [PubMed] [Google Scholar]
- Ingle DJ, 1980. Some effects of pretectum lesions on the frog’s detection of stationary objects. Behav. Brain Res. 1, 139–163. [DOI] [PubMed] [Google Scholar]
- Ingle DJ, 1981. New methods for analysis of vision in the gerbil. Behav. Brain Res. 3, 151–173. [DOI] [PubMed] [Google Scholar]
- Ingle DJ, Cheal M, Dizio P, 1979. Cine analysis of visual orientation and pursuit by the Mongolian gerbil. J. Comp. Physiol. Psych. 93, 919–928. [Google Scholar]
- Ingle D, Schneider G, Trevarthen C, Held R, 1967. Locating and identifying: two modes of visual processing. Psych. Forschung. 31, 42–43. [Google Scholar]
- Isa K, Sooksawate T, Kobayashi K, Kobayashi K, Redgrave P, Isa T, 2020. Dissecting the tectal output channels for orienting and defense response. eNeuro doi: 10.1523/ENEURO.0271-20-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, 2008. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 9, 304–313. [DOI] [PubMed] [Google Scholar]
- Itokazu T, Hasegawa M, Kimura R, Osaki H, Albrecht U-R,…Sato TR, 2018. Streamlined sensory motor communication through cortical reciprocal connectivity in a visually guided eye movement task. Nat. Comm. doi: 10.1038/s41467-017-02501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J Jr., Andersen P, Kaada BR, 1955. Subcortical mechanisms in the ‘searching’ or ‘attention’ response elicited by prefrontal cortical stimulation in unanesthetized cats. Yale J. Biol. Med. 28, 331–341. [PMC free article] [PubMed] [Google Scholar]
- Jazayeri PR, Lindbloom-Brown Z, Horwitz GD, 2012. Saccadic eye movements evoked by optogenetic activation of primate V1. Nat. Neurosci. 15, 1368–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Gamanut R, Bista P, D’Souza RD, Wang Q, Burkhalter A, 2015. Modularity in the organization of mouse primary visual cortex. Neuron doi: 10.1016/j.neuron.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating EG, Gooley SG, 1988. Disconnection of parietal and occipital access to the saccadic oculomotor system. Exp. Brain Res. 70, 385–398. [DOI] [PubMed] [Google Scholar]
- Keating EG, Gooley SG, Pratt SE, Kelsey JE, 1983. Removing the superior colliculus silences eye movements normally evoked from stimulation of the parietal and occipital eye fields. Brain Res. 269,145–148. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D, Guido W, 2017. Organization of the dorsal lateral geniculate nucleus in the mouse. Vision Neurosci. doi: 10.1017/S0952523817000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand A, Zee DS, 2011. Cerebellum and ocular motor control. Front. Neurol. doi: 10.3389/fneur.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkcaldie M, Watson CR, Paxinos G, Franklin KBJ, 2012. Straightening out mouse neocortex. Australian National Society. January Issue. [Google Scholar]
- Kitazawa S, Kimura T, Yin P-B., 1998. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature 392, 494–497. [DOI] [PubMed] [Google Scholar]
- Koch C, Reid RC, 2012. Observations of the mind. Nature 483, 397–398. [DOI] [PubMed] [Google Scholar]
- Kolb B, Walkey J, 1987. Behavioral and anatomical studies of the posterior parietal cortex in the rat. Behav. Brain Res. 23, 125–145. [DOI] [PubMed] [Google Scholar]
- Kondo S, Ohki K, 2016. Laminar differences in the orientation selectivity of geniculate afferents in mouse primary visual cortex. Nature Neurosci. 19, 316–319. [DOI] [PubMed] [Google Scholar]
- Kratochwil CF, Maheshwari U, Rijli FM, 2017. The long journey of pontine nuclei neurons: from rhombic lip to cortico-ponto-cerebellar circuitry. Front. Neural Circuits doi: 10.3389/fncir.2017.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzle H, Akert K, 1977. Efferent connections of cortical area 8 (frontal eye field) in the Macaca fascicularis. An reinvestigation using autoradiographic technique. J. Comp. Neurol. 173, 147–164. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM, Lawrence DG, 1967. Cortical projections to the red nucleus and the brainstem in the macaque monkey. Brain Res. 4, 151–188. [DOI] [PubMed] [Google Scholar]
- La Chioma A, Bonhoeffer T, Hübener M, 2020. Disparity sensitivity and binocular integration in mouse visual cortex areas. J. Neurosci. doi: 10.1523/JNEUROSCI.1060-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land MF, 1999. Motion and vision: why animals move their eyes. J. Comp. Physiol. A 185:341–352. [DOI] [PubMed] [Google Scholar]
- Laramée M-E, Boire D, 2015. Visual cortical areas of the mouse: comparison of parcellation and network structure with primates. Front. Neural Circuits doi: 10.3389/fncir.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K-M, Tehovnik EJ, 1995. Topographic distribution of fixation-related units in the dorsomedial frontal cortex of the macaque monkey. Eur. J. Neurosci. 7, 1005–1011. [DOI] [PubMed] [Google Scholar]
- Legg CR, Lambert S, 1990. Distance estimation in the hooded rat: experimental evidence for the role of motion cues. Behav. Brain Res. 41, 11–20. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, 1981. The prefrontal cortico-oculomotor trajectories in the monkey. J. Neurol. Sci. 49, 387–392. [DOI] [PubMed] [Google Scholar]
- Leinweber M, Ward DR, Sobczak JM, Attinger A, Keller GB, 2017. A sensorimotor circuit in mouse cortex for visual flow predictions. Neuron 95, 1420–1432. [DOI] [PubMed] [Google Scholar]
- Levinsohn G, 1909. Uber die Beziehungen der Grosshirnrinde beim Affen zu den Bewegungen des Auges. Graefe Arch 71, 313–378. [Google Scholar]
- Levinson SC, Torreira F, 2015. Timing in turn-taking and its implications for processing models of language. Front. Cog. Psych. doi: 10.3389/fpsyg.2015.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Turan Z, Meister M, 2020. Functional architecture of motion direction in mouse superior colliculus. Curr. Biol. doi: 10.1016/j.cub.2020.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Fuchs AL, 1978. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J. Neurophysiol. 41:733–763. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Miles FA, Zee DS, 1984. Signals used to compute errors in monkey vestibuloocular reflex: possible role of flocculus. J. Neurophysiol. 52,1140–1153. [DOI] [PubMed] [Google Scholar]
- Loyola S, Bosman LWJ, De Gruijl JR, De Jeu MTG, Negrello M, Hoogland TM, De Zeeuw CI, 2019. Inferior olive: all ins and outs. In: Manto M, et al. , eds., Handbook of the Cerebellum and Cerebellar Disorders. Springer Nature, Switzerland, doi: 10.1007/978-3-319-97911-3_43-2. [DOI] [Google Scholar]
- Lund RD, 1966. The occipitotectal pathway of the rat. J. Anat. 100, 51–62. [PMC free article] [PubMed] [Google Scholar]
- Manni E, Petrosini L, 2004. A century of cerebellar somatotopy: a debated representation. Nature Rev. Neurosci. 5, 241–249. [DOI] [PubMed] [Google Scholar]
- Manto M, Conforto JM, Deglado-Garcia JM, Farias da Guarda SM, Gerwig M, Habas C,…Timmann D, 2012. Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum 11, 457–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Siegal JS, Gorden EM, Raut RV, Gratton C,…Dosenbach NUF, (2018) Spatial and temporal organization of the human cerebellum. Neuron 100, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P, van Dun K, Van Dormael J, Vandernborre D, Keulen S, Manto M, Verhoeven J, Abutalebi J, 2017. Cerebellar induced differential polyglot aphasia: a neurolinguistic and fMRI study. Brain Language 175, 18–28. [DOI] [PubMed] [Google Scholar]
- Marshel JH, Garrett ME, Nauhaus I, Callaway EM, 2011. Functional specialization of seven mouse visual cortical areas. Neuron 72, 1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT, 1996. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119, 1183–1198. [DOI] [PubMed] [Google Scholar]
- Matsuzaki R, Kyuhou S, 1997. Pontine neurons which relay projections from the superior colliculus to the posterior vermis of the cerebellum in the cat: distribution and visual properties. Neurosci. Lett. 236, 99–102. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stein BE, 1982. Eye movements evoked by electrical stimulation in the superior colliculus of rats and hamsters. Brain Res. 247, 243–253. [DOI] [PubMed] [Google Scholar]
- McIndoo NE, 1914. The olfactory sense of hymenoptera. Proc. Acad. Nat. Sci. Phil. 66, 294–341. [Google Scholar]
- Medina JF, Lisberger SG, 2008. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nature Neurosci. 11, 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Cox D, 1913. Rats maintain a binocular field centered on the horizon. F1000Research 2:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AF, Poort J, O’Keefe J, Sahani M, Linden JF, 2018. A head-mounted camera system integrates detailed behavioral monitoring with multichannel electrophysiology in freely moving mice. Neuron 100, 46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Wolpert DM, Stein JF, 1993. Is the cerebellum a smith predictor? J. Motor. Behav. 25, 203–216. [DOI] [PubMed] [Google Scholar]
- Michaiel AM, Abe ETT, Niell CM, 2020. Dynamics of gaze control during prey capture in freely moving mice. eLife doi: 10.7554/eLife.57458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FA, Braitman DJ, Dow BM, 1980. Long-term adaptive changes in primate vestibuloocular reflex. IV. Electrophysiological observations in flocculus of adapted monkeys. J. Neurophysiol. 43, 1477–1493. [DOI] [PubMed] [Google Scholar]
- Miles RA, Lisberger SG, 1981. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu. Rev. Neurosci. 4, 273–299. [DOI] [PubMed] [Google Scholar]
- Miller GA, 1956. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psych. Rev. 63, 81–97. [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA, 1983. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 6, 414–417. [Google Scholar]
- Miyashita N, Hikosaka O, 1996. Minimal synaptic delay in the saccadic output pathway of the superior colliculus studied in awake monkey. Exp. Brain Res. 112, 187–196. [DOI] [PubMed] [Google Scholar]
- Mlinar EJ, Goodale MA, 1984. Cortical and tectal cortical control of visual orientation in the gerbil: evidence for parallel channels. Exp. Brain Res. 55, 33–48. [DOI] [PubMed] [Google Scholar]
- Moore T, Rodman HR, Rep AB, Gross CG, 1995. Localization of visual stimuli after striate cortex damage in monkeys: parallels with human blindsight. Proc. Natl. Acad. Sci. USA 92, 8215–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ, 2004. Cerebellar control of balance and locomotion. Neuroscientist 10, 247–259. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos H, Sakata H, Acuna C, 1975. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J. Neurophysiol. 38, 871–908. [DOI] [PubMed] [Google Scholar]
- Murakami T, Matsui T, Ohki K, 2017. Functional segregation and development of mouse higher visual areas. J. Neurosci. 37, 9424–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauhaus I, Nielson KJ, Callaway EM, 2012. Nonlinearity of two-photon Ca2+ imaging yields distorted measurements of tuning for V1 neuronal populations. J. Neurophysiol. doi: 10.1152/jn.00725.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauhaus I, Nielson KJ, Callaway EM, 2016. Efficient receptive field tiling in primate V1. Neuron doi: 10.1016/j.neuron.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP, 2010. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Fujikado T, 1987a. Involvement of Purkinje cells in evoking saccadic eye movements by microstimulation of the posterior cerebellar vermis of monkeys. J. Neurophysiol. 57, 1247–1261. [DOI] [PubMed] [Google Scholar]
- Noda H, Fujikado T, 1987b. Topography of the oculomotor area of the cerebellar vermis in macaques as determined by microstimulation. J. Neurophysiol. 58, 359–378. [DOI] [PubMed] [Google Scholar]
- Noda H, Murakami S, Yamada J, Tamaki Y, Aso T, 1988. Saccadic eye movements evoked by microstimulation of the fastigial nucleus of macaque monkeys. J Neurophysiol. 60, 1036–1052. [DOI] [PubMed] [Google Scholar]
- Noda H, Shinji M, Warabi T, 1991. Effects of fastigial stimulation upon visually-directed saccades in macaque monkeys. Neurosci. Res. 10, 188–199. [DOI] [PubMed] [Google Scholar]
- Ohki K, Reid RC, 2007. Specificity and randomness in the visual cortex. Curr. Opin. Neurobiol. 17, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K, Noda H, 1991. Saccadic burst neurons in the oculomotor region of the fastigial nucleus of macaque monkeys. J. Neurophysiol. 65, 1422–1434. [DOI] [PubMed] [Google Scholar]
- Palagina G, Meyer JF, Smirnakis SM, 2017. Complex visual motion representation in mouse area V1. J. Neurosci. 37, 164–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I, Lee K, Han J, Lee N, Lee W, Park K, et al. , 2009. Experience-dependent plasticity of cerebellar vermis in basketball players. Cerebellum 8, 334–339. [DOI] [PubMed] [Google Scholar]
- Payne HL, Raymond JR, 2017. Magnetic eye tracking in mice. eLife doi: 10.7554/eLife.29222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Cowey A, 1985. The ganglion cell and cone distributions in the monkey’s retina: Implications for central magnification factors. Vision Res. 25, 1795–1810. [DOI] [PubMed] [Google Scholar]
- Peters A, 1994. The organization of the primate visual cortex in the macaque. In: Peters A, Jones EG, eds., Cerebral Cortex, Plenum Press, New York, pp. 1–35. [Google Scholar]
- Poggio GF, Fischer B, 1977. Binocular interaction and depth sensitivity in striate and prestriate cortex of behaving macaque monkey. J. Neurophysiol. 40, 1392–1405. [DOI] [PubMed] [Google Scholar]
- Pöppel E, Held RM, Frost DO, 1973. Residual visual function after brain wounds involving the central visual pathways in man. Nature 243, 295–296. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, McGee AW, 2014. Mouse vision as a gateway for understanding how experience shapes neural circuits. Front. Neural Circuits doi:3389/fncir.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, West WR, Douglas RM, 2000. Behavioral assessment of visual acuity in mice and rats. Vision Res. 40, 2201–2209. [DOI] [PubMed] [Google Scholar]
- Ramnani N, 2006. The primate cortico-cerebellar system: anatomy and function. Nature Rev. Neurosci. 7, 511–522. [DOI] [PubMed] [Google Scholar]
- Rancz EA, Moya J, Drawitsch F, Brichta AM, Canals S, Margrie TW, 2015. Widespread vestibular activation of the rodent cortex. J. Neurosci. 35, 5926–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao HM, Mayo JP, Sommer MA, 2016. Circuits for presaccadic visual remapping. J. Neurophysiol. 116, 2624–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen RN, Matsumoto A, Arvin S, Yonehara K, 2020. Binocular integration of retinal motion information underlies optic flow processing by the cortex. bioRxiv doi: 10.1101/2020.10.16.342402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CM, Durlach NI, 1998. Note on information transfer rates in human communication. Presence: Teleoperators and Virtual Environments 7, 509–518. [Google Scholar]
- Reese BE, Jeffery G, 1983. Crossed and uncrossed visual topography in dorsal lateral geniculate nucleus of the pigmented rat. J. Neurophysiol. 49, 877–885. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Chalupa LM, 1978. Functional properties of the corticotectal projection in the golden hamster. J. Comp. Neurol. 180, 617–634. [DOI] [PubMed] [Google Scholar]
- Robinson DA, 1972. Eye movements evoke by collicular stimulation in the alert monkey. Vison Res. 12, 1795–1808. [DOI] [PubMed] [Google Scholar]
- Robinson DA, 1981. Models of the mechanics of eye movements. In: Zuber BL (ed.), Models of Oculomotor Behavior and Control, CRC Press, Boca Raton, Florida, pp. 21–41. [Google Scholar]
- Robinson DA, Fuchs AF, 1969. Eye movements evoked by stimulation of frontal eye fields. J. Neurophysiol. 32, 637–648. [DOI] [PubMed] [Google Scholar]
- Roll JP, Roll R, 1987. Kinaesthetic and motor effects of extraocular muscle vibration in man. In: O’Regan JK, A Lévy-Schoen A, eds., Eye Movements: from Physiology to Cognition, Elsevier, Amsterdam, pp. 57–68. [Google Scholar]
- Roll R, Velay JL, Roll JP, 1991. Eye and neck proprioceptive messages contribute to the spatial coding of retinal input in visually oriented activities. Exp. Brain Res 85, 423–431. [DOI] [PubMed] [Google Scholar]
- Roth MM, Dehmen JC, Muir DR, Imhof F, Martini FJ, Hofer SA, 2016. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nature Neurosci. 19, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonds JM, Chei C, Priebe NJ, 2019. Mice discriminate stereoscopic surfaces without fixating in depth. J. Neurosci. doi: 10.1523/JNEUROSCI.0895-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Masland RH, 2015. The types of retinal ganglion cells: Current status and implications for neural classification. Annu. Rev. Neurosci. 38, 221–246. [DOI] [PubMed] [Google Scholar]
- Scala F, Kobak D, Bernabucci M, Bernaerts Y, Cadwell CR … Tolias, A.S., 2020. Phenotypic variation of transcriptomic cell types in mouse motor cortex. Nature 10.1038/s41586-020-2907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer KP, Meyer DL, Zierav H, 1979. Neural activity pattern in different brain stem structures during eye-head movements. Prog. Brain Res 50, 805–812. [DOI] [PubMed] [Google Scholar]
- Schiller PH, 1970. The discharge characteristics of single units in the oculomotor and abducens nuclei of the unanesthetized monkey. Exp. Brain Res. 10, 247–362. [DOI] [PubMed] [Google Scholar]
- Schiller PH, 1977. The effect of superior colliculus ablation on saccades elicited by cortical stimulation. Brain Res. 122, 154–156. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, 1983. Interaction between visually and electrically elicited saccades before and after superior colliculus and frontal eye field ablations in the macaque monkey. Exp. Brain Res 49, 381–392. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Stryker M, 1972. Single-unit recording and stimulation in the superior colliculus of the alert macaque monkey. J. Neurophysiol. 35, 915–924. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ, 2001. Look and see: how the brain moves the eyes about. Prog. Brain Res. 134, 127–142. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ, 2003. Cortical inhibitory circuits in eye movement generation. Eur. J. Neurosci. 18, 3127–3133. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ, 2008. Visual prosthesis. Perception 37, 1529–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ, 2015. Vision and the Visual System. Oxford University Press, New York. [Google Scholar]
- Schiller PH, True SD, Conway JL, 1980. Deficits in eye movements following frontal eye field and superior colliculus ablations. J. Neurophysiol. 44, 1175–1189. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC, 1998. The cerebellar cognitive affective syndrome. Brain 121, 561–579. [DOI] [PubMed] [Google Scholar]
- Schmid MC, Mrowka SW, Turchi J, Saunders RC, Wilke M, Peters AJ, Ye FQ, Leopold DA, 2010. Blindsight depends on the lateral geniculate nucleus. Nature 466, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel UH, Kirchberger L, van Beest EH, Mukherjee S, Barsegyan A, Lorteije JAM, van der Togt C, Self MW, Roelfsema PR, 2018. Feedforward and feedback processing during figure-ground perception in mice. bioRxiv doi: 10.1101/456459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider GE, 1969. Two visual systems. Science 163, 895–902. [DOI] [PubMed] [Google Scholar]
- Scholl B, Burge J, Priebe NJ, 2013. Binocular integration and disparity selectivity in mouse primary visual cortex. J. Neurophysiol. 109, 3013–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves MA, Goldberg MA, Deng S-Y, Bruce CJ, Ungerleider LG, Mishkin M, 1987. The role of striate cortex in the guidance of eye movements in the monkey. J. Neurosci. 7, 3040–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendhilnathan N, Ipata AE, Goldberg ME, 2020. Neural correlates of reinforcement learning in mid-lateral cerebellum. Neuron 106, 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, 2020. Population coding in the cerebellum and its implications for learning from error. bioRxiv doi: 10.1101/2020.05.18.102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C, Chen Z, Liu A, Li Y, Zhang J,…Cao P, 2018. Divergent midbrain circuits orchestrate escape and freezing responses to looming stimuli in mice. Nature Comm. doi: 10.1038/s41467-018-03580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman HB, Caviness VS Jr., Ingle DJ, 1979. Corticotectal connections in the gerbil. Neurosci. Abstr. 4, 120. [Google Scholar]
- Shibutani H, Sakata H, Hyvärinen J, 1984. Saccade and blinking evoked by microstimulation of the posterior parietal association cortex of the monkey. Exp. Brain Res. 55, 1–8. [DOI] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, Schlag MJ, 1990. Primate supplementary eye field: I. Comparative aspects of mesencephalic and pontine connections. J. Comp. Neurol. 301, 618–642. [DOI] [PubMed] [Google Scholar]
- Siminoff R, Schwassmann HO, Kruger L, 1966. An electrophysiological study of the visual projection to the superior colliculus of the rat. J. Comp. Neurol. 127, 435–444. [DOI] [PubMed] [Google Scholar]
- Simmons PA, Lemmon V, Pearlman AL, 1982. Afferent and efferent connections of the striate and extrastriate visual cortex of the normal and reeler mouse. J. Comp. Neurol. 211, 295–308. [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Fuchs AF 2006. Complex spike activity of Purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J. Neurosci. 26, 7741–7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetedjo R, Kojima Y, Fuchs AF 2008. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning. J. Neurophysiol. 100, 1949–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Tehovnik EJ, 1999. Reversible inactivation of macaque dorsomedial frontal cortex. Exp. Brain Res. 124, 429–446. [DOI] [PubMed] [Google Scholar]
- Souza G-S, Gomes BD, Silveira LCL, 2011. Comparative neurophysiology of spatial luminance contrast sensitivity. Psych. Neurosci. doi: 10.3922/j.psns.2011.1.005. [DOI] [Google Scholar]
- Sparks DL, 1986. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol. Rev. 66, 118–171. [DOI] [PubMed] [Google Scholar]
- Sparks DL, 2002. The brainstem control of saccadic eye movements. Nature Rev. Neurosci. 3, 952–964. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE, 1983. Spatial localization of saccade targets. I. Compensation of stimulation-induced perturbation in eye position. J. Neurophysiol. 49, 45–63. [DOI] [PubMed] [Google Scholar]
- Sparks DL,. Mays LE, Porter JD, 1987. Eye movements induced by pontine stimulation: interaction with visually triggered saccades. J. Neurophysiol. 58, 300–318. [DOI] [PubMed] [Google Scholar]
- Spatz WB, Tigges J, Tigges M, 1970. Subcortical projections, cortical associations, and some intrinsic interlaminar connections of the striate cortex in the squirrel monkey (Saimiri). J. Comp. Neurol. 140, 155–173. [DOI] [PubMed] [Google Scholar]
- Squire LR, Clark RE, Knowlton BJ, 2001. Retrograde amnesia. Hippocampus 11, 50–55. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ, 1988. Frontal eye field efferents in the macaque monkey: I. Subcortical pathways and topography of striatal and thalamic terminal fields. J. Comp. Neurol. 271, 473–492. [DOI] [PubMed] [Google Scholar]
- Stone C, Pinto LH, 1993. Response properties of ganglion cells in the isolated mouse retina. Vision Neurosci. 10, 31–39. [DOI] [PubMed] [Google Scholar]
- Stryker MP, Schiller PH, 1975. Eye and head movements evoked by electrical stimulation of monkey superior colliculus. Exp. Brain Res. 23, 103–112. [DOI] [PubMed] [Google Scholar]
- Sultan F, Heck D, 2003. Detection of sequences in the cerebellar cortex: numerical estimate of the possible number of tidal-wave inducing sequences represented. J. Physiol. Paris 97, 591–600. [DOI] [PubMed] [Google Scholar]
- Swain RA, Kerr AL, Thompson RF, 2011. The cerebellum: a neural system for the study of reinforcement learning. Front. Behav. Neurosci. doi: 10.3389/fnbeh.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatko KP, Korympidou MM, Ran Y, Berens P, Dalkara D, Schubert T, Euler T, Franke K, 2019. Neural circuits in the mouse retina support color vision in the upper visual field. bioRxiv doi: 10.1101/745539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z, Sun W, Chen T-W, Kim D, Ji N, 2015. Neural representation of ultraviolet visual stimuli in mouse primary visual cortex. Sci. Report doi: 10.1038/srep12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Ardila Jimenez SC, Chakraborty S,, Schultz SR, 2016. Visual receptive field properties of neurons in the mouse lateral geniculate nucleus. PLoS ONE doi: 10.1371/journal.pone.0146017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, 1989. Head and body movements evoked electrically form the caudal superior colliculus of rats: pulse frequency effects. Behav. Brain Res. 34, 71–78. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, 1996. Electrical stimulation of neural tissue to evoke behavioral responses. J. Neurosci. Meth 65, 1–17. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Chen LL, 2015. Brain control and information transfer. Exp. Brain Res. 233, 3335–3347. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Lee K-M, 1993. The dorsomedial frontal cortex of the macaque monkey: topographic representation of saccades evoked by electrical stimulation. Exp. Brain Res. 96, 430–442. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Lee K-M, Schiller PH, 1994. Stimulation evoke saccades from the dorsomedial frontal cortex of the macaque monkey following lesions of the frontal eye fields and superior colliculus. Exp. Brain Res. 98, 179–190. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, 2004. Behavioural state affects saccades elicited electrically from neocortex. Neurosci. Biobehav. Rev. 28, 13–25. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, 2007. Phosphene induction by microstimulation of macaque V1. Brain Res. Rev. 53, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, 2013. Two-photon imaging and the activation of cortical neurons. Neurosci. 245, 12–25. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Schiller PH, 2003. Saccadic eye movements evoked by microstimulation of striate cortex. Eur. J. Neurosci. 17, 870–878. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Tolias AS, Schiller PH, 1998. Saccades induced electrically from the dorsomedial frontal cortex: evidence for a head-centered representation. Brain Res. 795, 287–291. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, 1996. Compensatory saccades made to remembered targets following orbital displacement by electrical stimulation of the dorsomedial frontal cortex or frontal eye fields of primates. Brain Res. 727, 221–224. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, 1997. Electrically evoked saccades from dorsomedial frontal cortex and frontal eye fields: a parametric evaluation reveals differences between areas. Exp. Brain Res. 117, 369–378. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH, 2000. Eye fields in the frontal lobes of primates. Brain Res. Rev. 32, 413–448. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Spence SJ, Saint-Cyr JA, 1989. Efferent projections of the anteromedial cortex of the rat as described by Phaseolus vulgaris leucoagglutinin immunohistochemistry. Behav. Brain Res. 35, 153–162. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Teixeira-e-Silva Z, 2014. Brain-to-brain interface for real-time sharing of sensorimotor information: a commentary. OA Neurosciences Jan 01, 2(1), 2. [Google Scholar]
- Tehovnik EJ, Woods LC, Slocum WM, 2013. Transfer of information by BMI. Neuroscience, 255, 134–146. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Yeomans JS, 1986. Two converging brainstem pathways mediating circling behavior. Brain Res. 385, 329–342. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Yeomans JS, 1987. Circling elicited from the anteromedial cortex and medial pons: refractory periods and summation. Brain Res. 407, 240–252. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG, 1992. The cerebellum and the adaptive coordination of movement. Annu. Rev. Neurosci. 15, 403–442. [DOI] [PubMed] [Google Scholar]
- Tiao T-C, Blakemore C, 1976. Functional organization in the superior colliculus of the golden hamster. J. Comp. Neurol. 168, 483–504. [DOI] [PubMed] [Google Scholar]
- Tinuper P, Montagna P, Laudadio S, Ripamoni L, Lugaresis E, 1989. Paroxysmol blinking provoked by head movements. Eur. Neurol. 29, 298–300. [DOI] [PubMed] [Google Scholar]
- Valey JL, Allin F, Bouquerel A, 1997. Motor and perceptual responses to horizontal and vertical eye vibration in humans. Vision Res. 37, 2631–2638. [DOI] [PubMed] [Google Scholar]
- Valey JL, Roll R, Demaria JL, Bouguerel A, Roll JP, 1995. Human eye muscle proprioceptive feedback is involved in target velocity perception during smooth pursuit. In: Findlay JM, Walker R, Kentridge RW, eds., Eye Movement Research: Mechanisms, Processes and Applications, Amsterdam: Elsevier, pp. 79–85. [Google Scholar]
- Valey JL, Roll R, Lennerstrand G, Roll JP, 1994. Eye proprioception and visual localization in humans: influence of ocular dominance and visual context. Vision Res. 34, 2169–2176. [DOI] [PubMed] [Google Scholar]
- Van Alphen AM, Stahl JS, De Zeeuw CI, 2001. The dynamic characteristics of the mouse horizontal vestibulo-ocular and optokinetic response. Brain Res. 890, 296–395. [DOI] [PubMed] [Google Scholar]
- Van Alphen B, Winkelman BHJ, Frens MA, 2010. Three-dimensional optokinetic eye movements in C57BL/6J mouse. Investiga. Ophthalmol. Vis. Sci. 51, 623–630. [DOI] [PubMed] [Google Scholar]
- Von der Heydt R, Peterhans E, 1989. Mechanisms of contour perception in monkey visual cortex. I. Lines of pattern discontinuity. J. Neurosci. 9, 1731–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DJ, Greenberg DS, Sawinski J, Rulla S, Notaro F, Kerr JND, 2013. Rats maintain an overhead binocular field at the expense of constant fusion. Nature 498, 65–69. [DOI] [PubMed] [Google Scholar]
- Wang Q, Burkhalter A, 2007. Area map of mouse visual cortex. J. Comp. Neurol. 502, 339–357. [DOI] [PubMed] [Google Scholar]
- Wang Q, Gao E, Burkhalter A, 2011. Gateways of ventral and dorsal streams in mouse visual cortex. J. Neurosci. 31, 1905–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sporns O, Burkhalter A, 2012. Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. J. Neurosci. 32, 4386–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner CE, Goldshmit Y, Brourne JA, 2010. Retinal afferents synapse with relay cells targeting the middle temporal area in pulvinar and lateral geniculate nuclei. Front. Neuroanat. doi: 10.3389/neuro.05.008.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH, 1983. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurol. 33, 766–772. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, 1963. Contour discrimination in a young monkey with striate cortex ablation. Neuropsychologia 1, 145. [Google Scholar]
- Wenzel U, Taubert M, Ragert P, Krug J, Villringer A, 2014. Functional and structural correlations of motor speed in cerebellar anterior lobe. PLoS ONE doi: 10.1371/journal.pone.0096871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G, McKee SP, 1977. Spatial configuration for hyperacuity. Vision Res. 17, 941–947. [DOI] [PubMed] [Google Scholar]
- White JJ, Sillitoe RV, 2012. Development of the cerebellum: from gene expression patterns to circuit maps. WIREs Dev. Biol. doi: 10.1002/wdev.65. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M, 1998. Internal models in the cerebellum. Trends Cogn. Sci. 2, 338–347. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lisberger SG, 2014. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature doi: 10.1038/nature13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbus AL, 1967. Eye Movement and Vision. Plenum Original, New York. [Google Scholar]
- Yeomans JS, Buckenham K, 1992. Electrically evoked turning: asymmetric and symmetric collision between anteromedial cortex and striatum. Brain Res. 570, 279–2923. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Tehovnik EJ, 1988. Turning responses evoked by stimulation of visuomotor pathways. Brain Res. Rev. 13, 235–259. [DOI] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang W-C, Jenvay S, Miyamichi K, Luo L, Dan Y, 2014. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurov Y, Brezina V, 2006. Variability of motor neuron spike timing maintains and shapes contractions of the accessory radula closure muscle of Aplysia. J. Neurosci. 26, 7056–7070. [DOI] [PMC free article] [PubMed] [Google Scholar]


