Abstract
Chronic kidney disease is a worldwide public health issue with rising incidence, morbidity/mortality, and cost. Depression and chronic renal disease often coexist, and psychological illnesses are associated with poor results. Early identification of depression reduces morbidity and death. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) are reported as practical biomarkers of inflammation and immune system activation. In this study, we aimed to determine the association of NLR and PLR with depression in dialysis patients. This study included 71 adults over 18 without known hematologic or oncologic disease, drug use, or chronic inflammatory diseases. Comorbid chronic diseases, laboratory data, and Beck depression inventory scores were prospectively recorded. A comparison of 2 groups according to the existence of depression was made, and a binomial logistic regression test was used to determine the association between the variables and the presence of depression after adjusting for confounding factors. A receiver operating curve analysis was used to differentiate groups with and without severe depression. Seventy-one patients met the study criteria, with 46 hemodialysis and 25 peritoneal dialysis patients. The majority had hypertension and diabetes mellitus, with 47.89% having minimal-minor depression and 52.11% having moderate-major depression. The 2 groups were similar regarding chronic diseases, with no significant differences in serum creatinine levels, glucose, lipid profiles, or electrolytes. However, when the NLR of the 2 groups was compared, the median was higher in patients with moderate or major depression. Multivariate analysis showed no significant differences between the groups in PLR, triglyceride to glucose ratio, and C-reactive peptide to albumin ratio. The best NLR cutoff value was 3.26, with 48.6% sensitivity, 88.2% specificity, 81.8% positive predictive value, 61.2% negative predictive value, and 67.6% test accuracy. Depression is one of the most common psychiatric conditions in dialysis patients and is linked to increased morbidity, mortality, treatment failure, expense, and hospitalization. NLR helped predict moderate-to-major depression in dialysis patients, even after controlling for confounding factors in multivariate analysis. This study indicated that an NLR successfully identified depressive groups, and patients with an NLR value >3.26 were 6.1 times more likely to have moderate or major depression.
Keywords: chronic kidney disease, depression, dialysis, neutrophil-lymphocyte ratio
1. Introduction
Chronic kidney disease (CKD) is a global public health problem with increasing incidence, high morbidity/mortality rates, and financial burden on countries, affecting approximately 10% of the worldwide population.[1–3] CKD has emerged as one of the leading causes of death worldwide and is one of the few non-communicable medical causes that has seen an increase in disease-related deaths in the last 20 years. Still, the presence of other comorbidities makes the condition even more dangerous.[1] Psychiatric disorders in this patient population increase morbidity and mortality, leading to increased health expenditures and increased incidence of hospitalization.[4] Depression is one of the most common psychiatric disorders in individuals with CKD, with a prevalence of 23% to 29%, and rates as high as 47% have been reported.[5–8] The emergence of psychiatric symptoms in many individuals with CKD who need dialysis treatment worsens the quality of life of patients and their caregivers.[9] Studies on end-stage renal disease patients have shown the association of depressive symptoms with adverse health outcomes. A meta-analysis by Fabrazzo et al[10] showed that patients with depressive symptoms had a higher risk of death.
Early recognition of depression in CKD patients is essential to reduce morbidity and mortality and improve quality of life. There are many studies suggesting that depression and CKD are associated with underlying inflammatory triggers,[11–14] and inflammation is known to be effective both in the occurrence and progression of CKD[15] and in the severity of depression.[16]
The neutrophil to lymphocyte ratio (NLR) is the ratio of the absolute neutrophil count to the total lymphocyte count in the blood. The NLR has been found to be a valuable marker of inflammation and immune system activation, with higher ratios indicating a greater degree of inflammation and immune system activation. In the literature, there are studies related to the use of the Neutrophil/Lymphocyte ratio, an inflammatory marker, as an indicator of inflammation levels in depressive patients.[17,18] NLR, an easily accessible and low-cost marker of inflammation, has been shown to have predictive value in several different medical conditions, including cardiovascular disease, malignancies, and infectious diseases.[19] For example, a higher NLR has been associated with poorer outcomes in patients with heart disease or cancer and increased mortality in patients with sepsis.[19]
Similar to NLR, platelet-to-lymphocyte ratio (PLR) has been studied in various medical conditions and has been found to be a useful prognostic marker in some cases. For example, a meta-analysis of studies on colorectal cancer patients found that elevated preoperative or pretreatment PLR was associated with poor prognosis.[20] PLR has been correlated with poor survival in many malignancies and has been verified as predictive of intraductal papillary mucinous neoplasms.[20,21] PLR has also been studied as a predictor of treatment response to neoadjuvant therapy in esophageal cancer.[22]
Using a biological parameter associated with depression in dialysis patients can provide clinicians with an objective tool to predict the need for psychiatric evaluation and treatment in the follow-up of these patients. In this study, we aim to investigate the association between the neutrophil/lymphocyte ratio and the platelet/lymphocyte ratio, which are noninvasive, inexpensive, easily accessible tests, and can be determined only from a blood count, with depression in dialysis patients.
2. Methods
Between January 15, 2023 and March 1, 2023, patients receiving hemodialysis and peritoneal dialysis treatment at Hitit University Faculty of Medicine Department of Nephrology Hemodialysis Unit were selected as the subjects of the study. Seventy-one patients over 18 years of age without known hematologic or oncologic disease, without drug use that would affect laboratory values, and without chronic inflammatory diseases were included in the study. Patients age, gender, type of dialysis, and the existence of chronic diseases such as diabetes mellitus, hypertension, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and peripheric artery disease were recorded. After overnight fasting serum glucose, triglyceride, total cholesterol, low density lipoprotein, high density lipoprotein, sodium, potassium, phosphorus levels, white blood cell count (WBC), neutrophil, lymphocyte, monocyte, platelet counts, hemoglobin, platelet distribution width (PDW), C-reactive peptide (CRP), parathormone, ferritin, vitamin B12, folate, thyroid stimulant hormone (TSH), T4, blood urea nitrogen, creatinine, uric acid, calcium, albumin levels, and kt/V values were also recorded prospectively. NLR, PLR, triglyceride to glucose ratio (TyG), and C-reactive peptide to albumin ratio (CAR) values were calculated using the formulas NLR = neutrophil count/lymphocyte count, PLR = platelet count/lymphocyte count, TyG = serum triglyceride/serum glucose, and CAR = serum CRP/serum albumin and added to the study. Hemodialysis patients had blood drawn just before the middle of the week dialysis sessions, whereas peritoneal dialysis patients had their blood drawn during their outpatient clinical controls. The chronic kidney disease epidemiology collaboration equation was used to generate glomerular filtration rate estimates. Kt/Vurea was used to determine peritoneal dialysis and hemodialysis qualification. Beck depression inventory was utilized to determine the severity of depression. No patients were lost to follow-up, and no missing data was found at the end for the patient selection and inclusion stage. This study was approved by the Hitit University Faculty of Medicine Clinical Research Ethics Committee, and consent forms were obtained from all of the participants (Decision No: 2023-02/Date:12/01/2023).
This study is planned prospectively. In the a priori power analysis performed by examining the related studies in the literature, the sample size required to obtain a significant result was calculated as 71 patients (G-Power v3.1.9.7).[23] All statistical analysis was performed using IBM SPSS Statistics for Windows software (version 26; IBM Corp., Armonk, NY). Descriptive statistics were reported as counts and percentages for categorical variables, the mean ± standard deviation for normally distributed numeric variables, and the median value followed by the minimum and maximum values in parentheses for non-normally distributed numeric variables. The Shapiro–Wilks test was used to assess data distribution. In accordance with the data distribution, the Pearson or Spearman correlation coefficients were used to analyze the associations between the variables. A comparison of numerical measurements for 2 independent research groups for age, blood urea nitrogen, creatinine, uric acid, calcium, hemoglobin, T4, WBC, and PDW was done with a Student t test, and for serum glucose, triglyceride, total cholesterol, low-density lipoprotein, high-density lipoprotein, sodium, potassium, phosphorus levels, neutrophil, lymphocyte, monocyte, platelet counts, CRP, parathormone, ferritin, vitamin B12, folate, TSH, albumin levels, kt/V, NLR, TyG, CAR values, and Beck depression inventory scores were evaluated by a Mann–Whitney U test in accordance with the distribution of the data. The ratio comparisons of the type of dialysis, gender, diabetes mellitus, hypertension, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and peripheric artery disease distributions between research groups were evaluated using a Chi-square test. A binomial logistic regression test was used for the multivariate analysis to determine the association between the variables and the existence of depression after adjusting for confounding factors. A receiver operating curve (ROC) analysis was done to differentiate groups with and without severe depression, and the optimal NLR cutoff values were found using the area under the curve and the Youden index. For these cutoff values, sensitivity, specificity, positive predictive value, negative predictive value, test accuracy, and odds ratio values were calculated. For statistical significance, P < .05 was accepted.
3. Results
Seventy-one patients who met the study criteria were included in the study. There were 46 (64.79%) hemodialysis patients and 25 (35.21%) peritoneal dialysis patients. The mean age was 54.58 ± 12.51 years. 45 (63.38%) of the patients were male, and 26 (36.62%) were female. The majority (74.65%) of the patients had hypertension, and diabetes mellitus (25.35%) was the second most common disease (Table 1). Total 34 (47.89%) patients had minimal or minor depression, and 37 (52.11%) had moderate or major depression.
Table 1.
Baseline characteristics, univariate and multivariate analysis between depression groups.
| Variables | All patients (n = 71) | Univariate analysis | Multivariate logistic regression analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Minimal and minor depression (n = 34) | Moderate and major depression (n = 37) | Statistical significance | Wald | Exp (B) (CI %95) | Statistical significance | |||
| Dialysis type | Hemodialysis | 46 (64.79%) | 22 (64.71%) | 24 (64.86%) | 0.989 | 0.03 | 1.158 (0.218–6.165) | 0.863 |
| Peritoneal dialysis | 25 (35.21%) | 12 (35.29%) | 13 (35.14%) | |||||
| Age | 54.58 ± 12.51 | 52.15 ± 13.18 | 56.81 ± 11.59 | 0.117 | 2.951 | 1.052 (0.993–1.116) | 0.086 | |
| Gender | Male | 45 (63.38%) | 23 (67.65%) | 22 (59.46%) | 0.474 | 0.925 | 0.528 (0.144–1.941) | 0.336 |
| Female | 26 (36.62%) | 11 (32.35%) | 15 (40.54%) | |||||
| DM | 18 (25.35%) | 8 (23.53%) | 10 (27.03%) | 0.735 | 0.026 | 1.158 (0.192–6.977) | 0.873 | |
| HT | 53 (74.65%) | 24 (70.59%) | 29 (78.38%) | 0.451 | 0.020 | 0.895 (0.192–4.173) | 0.887 | |
| CAD | 11 (15.49%) | 4 (11.76%) | 7 (18.92%) | 0.518 | 0.205 | 0.675 (0.124–3.69) | 0.651 | |
| CHF | 11 (15.49%) | 5 (14.71%) | 6 (16.22%) | 0.861 | 0.243 | 1.809 (0.171–19.105) | 0.622 | |
| COPD | 6 (8.45%) | 4 (11.76%) | 2 (5.41%) | 0.417 | 0.509 | 2.468 (0.207–29.494) | 0.475 | |
| PAD | 2 (2.82%) | 0 (0%) | 2 (5.41%) | 0.494 | ||||
| BUN | 58.03 ± 18.21 | 56.82 ± 14.6 | 59.14 ± 21.13 | 0.591 | ||||
| Creatinine | 8.27 ± 2.49 | 8.48 ± 2.09 | 8.07 ± 2.82 | 0.492 | ||||
| Glucose | 98 (60–360) | 99 (67–360) | 93 (60–200) | 0.538 | ||||
| Triglyceride | 149 (52–698) | 150 (64–550) | 140 (52–698) | 0.447 | ||||
| Total cholesterol | 159 (91–331) | 162 (101–331) | 157 (91–287) | 0.434 | ||||
| LDL | 87 (38–253) | 89 (38–253) | 80 (38–204) | 0.53 | ||||
| HDL | 40 (21–80) | 40.5 (21–80) | 40 (28–60) | 0.504 | ||||
| Uric Acid | 6.15 ± 1.34 | 6.2 ± 1.46 | 6.11 ± 1.24 | 0.769 | ||||
| Sodium | 138 (129–142) | 138 (129–142) | 138 (130–142) | 0.736 | ||||
| Potassium | 5.3 (3.4–8) | 5.35 (3.4–7) | 5.2 (3.4–8) | 0.596 | ||||
| Calcium | 8.58 ± 0.72 | 8.66 ± 0.76 | 8.51 ± 0.68 | 0.399 | ||||
| Phosphorus | 4.6 (2.5–8.3) | 4.65 (2.7–6.3) | 4.6 (2.5–8.3) | 0.986 | ||||
| Hemoglobin | 10.69 ± 1.36 | 10.96 ± 1.16 | 10.45 ± 1.5 | 0.114 | ||||
| Neutrophil | 3.96 (1.68–7.92) | 3.28 (1.85–5.64) | 4.63 (1.68–7.92) | <0.001 | ||||
| Lymphocyte | 1.5 (0.52–3.8) | 1.5 (0.52–3.19) | 1.47 (0.72–3.8) | 0.858 | ||||
| WBC | 7.02 ± 1.94 | 6.72 ± 2.02 | 7.31 ± 1.86 | 0.205 | ||||
| Platelet | 213 (42–520) | 201.5 (42–480) | 215 (126–520) | 0.486 | ||||
| PDW | 11.9 ± 1.86 | 11.7 ± 1.74 | 12.08 ± 1.98 | 0.404 | ||||
| CRP | 8 (0.9–31) | 3.51 (0.9–26) | 8 (3–31) | 0.083 | ||||
| Parathormone | 365 (1–1660) | 395.5 (1–1660) | 365 (1–1494) | 0.95 | 0.732 | 1.001 (0.999–1.003) | 0.392 | |
| Ferritin | 463 (57–2000) | 471.5 (57–2000) | 452 (94–2000) | 0.831 | 1.765 | 0.999 (0.998–1) | 0.184 | |
| kt/V | 1.7 (0.9–4.93) | 1.92 (0.9–3.45) | 1.67 (1.22–4.93) | 0.245 | 0.211 | 1.338 (0.386–4.635) | 0.646 | |
| Vitamin B12 | 480 (150–2000) | 404 (150–2000) | 530 (225–1613) | 0.165 | 0.654 | 1.001 (0.999–1.003) | 0.419 | |
| Folate | 12.3 (2–20) | 8.1 (2.2–20) | 17.6 (2–20) | 0.211 | 0.484 | 1.038 (0.934–1.154) | 0.487 | |
| TSH | 1.6 (0.33–8.7) | 1.42 (0.33–5.7) | 2 (0.4–8.7) | 0.437 | 1.760 | 1.315 (0.877–1.972) | 0.185 | |
| T4 | 1.15 ± 0.21 | 1.13 ± 0.19 | 1.17 ± 0.23 | 0.43 | ||||
| Monocyte | 0.56 (0.25–1.3) | 0.55 (0.26–1.3) | 0.58 (0.25–1.29) | 0.904 | ||||
| Albumin | 38 (20–47) | 37 (22–47) | 38 (20–44) | 0.768 | ||||
| NLR | 2.44 (0.84–8.27) | 2.23 (0.84–8.27) | 3.21 (0.84–7.45) | 0.004 | 6.477 | 2.554 (1.241–5.258) | 0.011 | |
| PLR | 134.76 (42–553.19) | 140.75 (42–312.06) | 131.79 (55.26–553.19) | 0.849 | 1.891 | 0.992 (0.98–1.004) | 0.169 | |
| TyG | 1.33 (0.32–9.97) | 1.35 (0.32–5.25) | 1.33 (0.34–9.97) | 0.809 | 0.483 | 1.209 (0.707–2.068) | 0.487 | |
| CAR | 0.2 (0.02–1.18) | 0.11 (0.02–1.18) | 0.22 (0.08–0.89) | 0.049 | 0.321 | 2.151 (0.152–30.423) | 0.571 | |
| BDI score | 17 (0–52) | 12 (0–16) | 27 (17–52) | <0.001 | ||||
| Depression | Minimal and minor | 34 (47.89%) | ||||||
| Moderate and major | 37 (52.11%) | |||||||
BDI = Beck depression inventory, BUN = blood urea nitrogen, CAD = coronary artery disease, CAR = C-reactive peptide albumin ratio, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, CRP = C-reactive peptide, DM = diabetes mellitus, HDL = high-density lipoprotein, HT = hypertension, LDL = low-density lipoprotein, NLR = neutrophil-lymphocyte ratio, PAD = peripheral artery disease, PDW = platelet distribution width, PLR = platelet-lymphocyte ratio, TSH = thyroid stimulating hormone, TyG = triglyceride-lymphocyte ratio, WBC = white blood cell count.
Patients were divided into 2 groups according to the severity of their depression. Patients who had minimal or minor depression were designated as Group 1, and those who had moderate or major depression were designated as Group 2.
When the 2 groups were univariately compared, no statistically significant difference was observed between groups in terms of dialysis type, age, or gender (P = .989, P = .117, P = .474, respectively).
The 2 groups were similar in terms of chronic diseases, and there were no significant differences in the prevalence of comorbidities such as hypertension, diabetes, and cardiovascular disease (P = .451, P = .735, P = .518, respectively) (Table 1). The mean serum creatinine level of Group 1 was 8.48 ± 2.09, and the mean serum creatinine level of Group 2 was 8.07 ± 2.82, indicating no statistically significant difference between the 2 groups (P = .492). There were no significant differences found in serum glucose levels, blood lipid profiles, or electrolytes between the 2 groups (Table 1). The difference in mean hemoglobin levels between the 2 groups was found to be not statistically significant (P = .23); the mean hemoglobin level of Group 1 was 10.96 ± 1.16 and was 10.45 ± 1.5 for Group 2.
The median neutrophil count was higher in Group 2 compared to Group 1 (P < .001), with a median count of 4,63 (1.68–7.92) for Group 2 and 3.28 (1.85–5.64) for Group 1, respectively. But there was no significant difference in the lymphocyte count between the 2 groups (P = .858), with a median count of 1.5 (0.52–3.19) for Group 2 and 1.47 (0.72–3.8) for Group 1, respectively. There was no difference in terms of WBC, platelet count, PDW, CRP, parathormone, ferritin, vitamin B12, folate, kt/V, TSH, T4, monocyte count, and albumin levels (Table 1).
When the NLR of the 2 groups was compared, the median was found to be higher in patients with moderate or major depression (P = .004), 3.21 (0.84–7.45) for Group 2, and 2.23 (0.84–8.27) for Group 1. Similarly, patients with moderate to major depression have had higher CRP levels than patients with minimal or minor depression (0.11 (0.02–1.18) vs. 0.22 (0.08–0.89), P = .049). The PLR and TyG ratios did not differ between groups (P = .849, P = .809).
A multivariate analysis including age, gender, dialysis type, comorbidities, parathormone, ferritin, kt/V, vitamin B12, folate, and TSH as covariates also showed no significant differences in PLR, TyG, and CRP-albumin ratios between the groups (P = .169, P = .487 and P = .571, respectively). The binomial logistic regression model correctly classified 77.5% of the cases (R2 = 0.343, P < .05), and NLR remained an independent predictor even after adjusting for potential confounders in the multivariate analysis (Exp [B] with 95% confidence interval [CI] = 2.554 [1.241–5.258], P = .011).
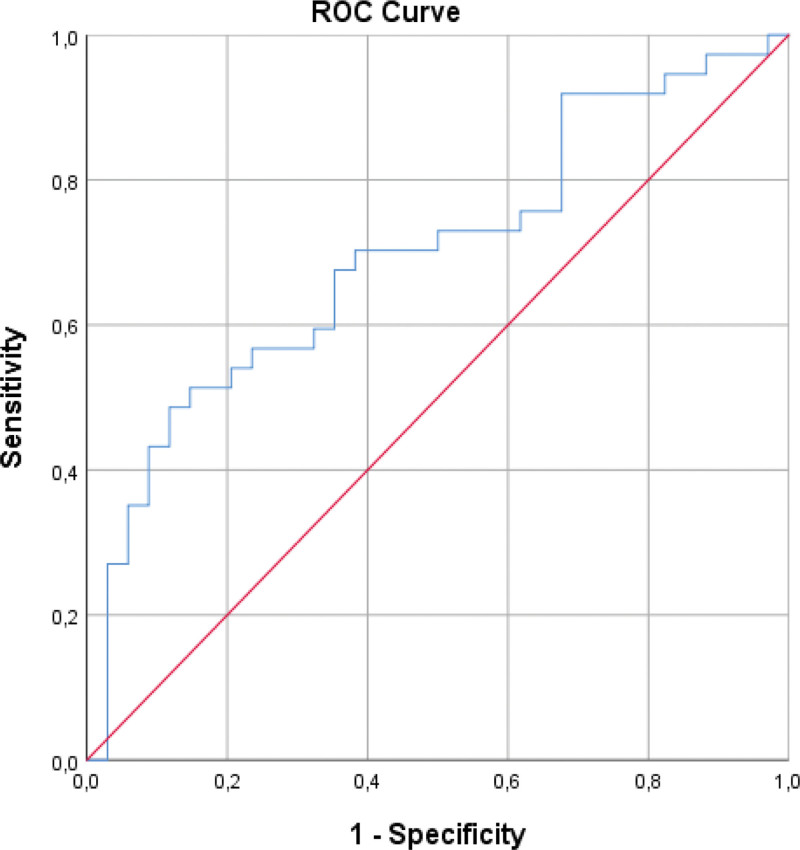
To determine the optimal NLR cutoff value for the distinction of depression groups, a ROC analysis was used (area under curve [standard error] 0.698 [0.063], CI%95 [0.575–0.821], P = .004) (Fig. 1). The best NLR cutoff was found to be 3.26 with 48.6% sensitivity, 88.2% specificity, 81.8% positive predictive value, 61.2% negative predictive value, and 67.6% test accuracy (odds ratio 7.105, 95% CI 2.084–24.221, P = .001) (Table 2). A patient whose NLR is higher than 3.26 is approximately 6.1 times more likely to belong to the moderate or major depression group than those with a lower NLR.
Figure 1.
Receiver operating curve of NLR in classification of depression in dialysis patients.
Table 2.
Diagnostic values of variables and results of receiver operating curve analysis.
| Variables | Cut-off | Diagnostic values | ROC analysis | Odds ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Accuracy | Area (SE) | %95 CI | P | Odds ratio | %95 CI | P | ||
| NLR | 3.26 | 48.6% | 88.2% | 81.8% | 61.2% | 67.6% | 0.698 (0.063) | 0.575–0.821 | .004 | 7105 | 2084–24.221 | .001 |
CI = confidence interval, NPV = negative predictive value, P = statistical significance, PPV = positive predictive value, ROC = receiver operating curve, SE = standard error.
4. Discussion
Depression is the most common psychiatric disorder in dialysis patients, affecting 22% to 39% of patients.[24] In our study, the rate of depression was found to be 47.89%, which was higher than in the literature. The presence of comorbid depression in dialysis patients has been associated with increased morbidity, mortality, inadequate response to treatment, and increased treatment cost and duration of hospitalization.[25–27] Depression can impair the dialysis process, treatment adherence, immune system function, and nutritional status.[24,28] Depressed dialysis patients have elevated levels of proinflammatory cytokines, and depression is associated with poor outcomes.[29] Chronic inflammation has been implicated in atherosclerosis, osteoporosis, diabetes, cancer, depression, and CKD.[30]
Inflammation, an immune response that safeguards the body against harmful agents, can exert intricate effects on the central nervous system, potentially influencing the development and progression of depression.[31,32] A growing body of scientific evidence supports a strong association between inflammation and depression, indicating that chronic or excessive inflammation may significantly impact mood regulation and brain function.[31–34]
Prior research has shown that inflammation is associated with higher odds of developing depression.[33–35] Depressed patients are associated with elevated markers of inflammation, such as inflammatory cytokines and acute-phase proteins. Additionally, the administration of inflammatory stimuli has been linked to the onset of depressive symptoms.[35] Proinflammatory agents have been found to contribute to the development of depressive symptoms, and patients with depression have increased levels of both central and peripheral proinflammatory cytokines.[36] Significantly, the pathophysiology of depression may involve oxidative stress due to the release of reactive oxygen radicals from activated neutrophils.[37,38]
One of the suggested mechanisms through which inflammation affects depression involves the activation of the immune system. Following exposure to stressors, the immune system releases pro-inflammatory cytokines, including interleukin-1, interleukin-6, and tumor necrosis factor-alpha.[32] These cytokines can affect the brain composure through various pathways, such as crossing the blood-brain barrier or activating the vagus nerve, thereby triggering alterations in neurotransmitters activity such as serotonin, dopamine, and norepinephrine, which play pivotal roles in regulating mood.[31] Imbalances in these neurotransmitters have been linked to the development of depressive symptoms.[31]
Chronic inflammation may also negatively impact neuroplasticity, the brain ability to adapt and form new connections.[39] Reduced neuroplasticity, particularly in brain regions like the hippocampus and prefrontal cortex, has been associated with depression.[39] The activation of the hypothalamic-pituitary-adrenal axis, a critical system involved in the body stress response, is another suggested mechanism by which inflammation affects depression.[40] Prolonged activation of the hypothalamic-pituitary-adrenal axis can lead to increased cortisol levels, which may contribute to the development of depression.[40,41] Moreover, inflammation can disrupt the gut-brain axis, a bidirectional communication system between the gut microbiota and the brain and can contribute to depressive symptoms.[42]
NLR is a measure that combines 2 WBC subsets that reflect 2 inversely linked immune pathways. It is a more consistent measurement than individual WBC counts and is simply determined using differential WBC counts.[43] NLR is frequently used in many fields because it is a low-cost, easily-accessible biomarker and is effective at indicating the degree of inflammation. Studies have found a relationship between NLR and the progression of end-stage renal failure.[23,44] Another study found that NLR is a biomarker for predicting systemic involvement in adult IgA vasculitis patients.[45]
Several studies have suggested that a higher NLR may be associated with an increased risk of developing depression. Wang et al[46] found an association between NLR and clinically relevant depressive symptoms in people with diabetes. In a cross-sectional study, Feng et al[47] found high NLR is a significant predictor of depressive symptoms in patients. There are also works contradicting the relationship between NLR and depression. Zhu et al[48] explored the relationship between inflammation and depression using NLR, MLR, and PLR as inflammatory markers and found NLR to be not correlated with depression.
Several mechanisms could account for the connection between NLR and depressive symptoms, and both neutrophilia and lymphocytopenia are well-known inflammatory reactions to diverse stressors.[49–52] NLR predictive ability in dialysis patients is thought to be reliant on the link between inflammation and nutritional state.[52] Also, in hemodialysis patients, lymphocytopenia has been identified as a sign of malnutrition.[51] Hence, recent evidence linking inflammation, malnourishment, and depression in dialysis patients might explain NLR predictive capacity to anticipate depressive symptoms in this community.[49,50]
This study found that NLR is an important predictor for moderate-to-major depression in patients undergoing dialysis, even after adjusting for potential confounders. This finding is consistent with previous studies that have shown an association between inflammation and depression in the general population.[46,47] ROC analysis determined that an NLR cutoff of 3.26 had 88.2% specificity and 48.6% sensitivity in distinguishing between depression groups. Patients with an NLR higher than 3.26 were approximately 6.1 times more likely to belong to the moderate or major depression group than those with a lower NLR.
Notably, the PLR, TyG, and CAR values did not differ between the depression groups, indicating that these biomarkers may not be as useful for identifying depression in this population. The multivariate analysis also failed to show significant differences in PLR, TyG, and CRP-albumin ratios between the groups after adjusting for potential confounders.
Our research has a number of limitations. Due to the nature of an observational study, we can only discuss the association between inflammation, inflammatory markers, and depression; this work cannot establish a causal relationship. The findings may not be representative of the overall population because of the relatively small sample size and reliance on a single hospital. Still, it can guide clinicians about depression in maintenance hemodialysis patients.
5. Conclusion
In conclusion, we found a significant frequency of moderate to major depression among dialysis patients. These findings offer valuable clinical implications, wherein NLR monitoring could aid in early depression detection and intervention, ultimately enhancing patient outcomes and quality of life. Accessible and affordable, NLR might serve as a useful novel indicator for predicting the prevalence of major depressive symptoms in patients undergoing hemodialysis and peritoneal dialysis treatments. However, it is important to note that NLR is not a definitive diagnostic tool and should be used in conjunction with other clinical and laboratory parameters to make treatment decisions.
Author contributions
Conceptualization: Duygu Tutan, Ayşe Erdoğan Kaya.
Data curation: Duygu Tutan, Bariş Eser.
Formal analysis: Duygu Tutan, Ayşe Erdoğan Kaya.
Investigation: Duygu Tutan, Ayşe Erdoğan Kaya, Bariş Eser.
Methodology: Duygu Tutan, Ayşe Erdoğan Kaya.
Project administration: Duygu Tutan.
Resources: Duygu Tutan, Ayşe Erdoğan Kaya.
Software: Duygu Tutan, Ayşe Erdoğan Kaya, Bariş Eser.
Supervision: Duygu Tutan, Bariş Eser.
Validation: Duygu Tutan, Bariş Eser.
Visualization: Duygu Tutan.
Writing – original draft: Duygu Tutan, Ayşe Erdoğan Kaya, Bariş Eser.
Writing – review & editing: Duygu Tutan, Ayşe Erdoğan Kaya.
Abbreviations:
- CAR
- C-reactive peptide to albumin ratio
- CI
- confidence interval
- CKD
- chronic kidney disease
- CRP
- C-reactive peptide
- NLR
- neutrophil to lymphocyte ratio
- PDW
- platelet distribution width
- PLR
- platelet to lymphocyte ratio
- ROC
- receiver operating curve
- TSH
- thyroid stimulant hormone
- TyG
- triglyceride to glucose ratio
- WBC
- white blood cell count
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Tutan D, Erdoğan Kaya A, Eser B. The relationship between neutrophil lymphocyte ratio, platelet lymphocyte ratio, and depression in dialysis patients. Medicine 2023;102:37(e35197).
Contributor Information
Ayşe Erdoğan Kaya, Email: dr.ayserdogan@gmail.com.
Bariş Eser, Email: baris.eser@saglik.gov.tr.
References
- [1].Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022;12:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66:1310–4. [DOI] [PubMed] [Google Scholar]
- [3].Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kimmel PL. Psychosocial factors in adult end-stage renal disease patients treated with hemodialysis: correlates and outcomes. Am J Kidney Dis. 2000;35:S132–40. [DOI] [PubMed] [Google Scholar]
- [5].Palmer S, Vecchio M, Craig JC, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84:179–91. [DOI] [PubMed] [Google Scholar]
- [6].Foster GF, Cohn GL, McKegney PF. Psychobiologic factors and individual survival on chronic renal hemodialysis. A two year follow-up: I. Psychosom Med. 1973;35:64–82. [DOI] [PubMed] [Google Scholar]
- [7].Liu M, Zhang Y, Yang S, et al. Bidirectional relations between depression symptoms and chronic kidney disease. J Affect Disord. 2022;311:224–30. [DOI] [PubMed] [Google Scholar]
- [8].Bautovich A, Katz I, Smith M, et al. Depression and chronic kidney disease: a review for clinicians. Aust N Z J Psychiatry. 2014;48:530–41. [DOI] [PubMed] [Google Scholar]
- [9].Zalai D, Szeifert L, Novak M. Psychological distress and depression in patients with chronic kidney disease. Semin Dial. 2012;25:428–38. [DOI] [PubMed] [Google Scholar]
- [10].Fabrazzo M, De Santo RM. Depression in chronic kidney disease. Semin Nephrol. 2006;26:56–60. [DOI] [PubMed] [Google Scholar]
- [11].Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135–51. [DOI] [PubMed] [Google Scholar]
- [12].Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang T, Fu X, Chen Q, et al. Arachidonic acid metabolism and kidney inflammation. Int J Mol Sci. 2019;20:3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30:234–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fu Y, Xiang Y, Li H, et al. Inflammation in kidney repair: mechanism and therapeutic potential. Pharmacol Ther. 2022;237:108240. [DOI] [PubMed] [Google Scholar]
- [16].Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107:234–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Su M, Ouyang X, Song Y. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and monocyte to lymphocyte ratio in depression: a meta-analysis. J Affect Disord. 2022;308:375–83. [DOI] [PubMed] [Google Scholar]
- [18].Kinoshita H, Takekawa D, Kudo T, et al. Higher neutrophil-lymphocyte ratio is associated with depressive symptoms in Japanese general male population. Sci Rep. 2022;12:9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ni J, Wang H, Li Y, et al. Neutrophil to lymphocyte ratio (NLR) as a prognostic marker for in-hospital mortality of patients with sepsis: a secondary analysis based on a single-center, retrospective, cohort study. Medicine (Baltim). 2019;98:e18029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang J, Zhang HY, Li J, et al. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:68837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Luvira V, Kamsa-Ard S, Pugkhem A, et al. Predictive utility of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in intraductal papillary neoplasm of the bile duct. Clin Exp Hepatol. 2019;5:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McLaren PJ, Bronson NW, Hart KD, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios can predict treatment response to neoadjuvant therapy in esophageal cancer. J Gastrointest Surg. 2017;21:607–13. [DOI] [PubMed] [Google Scholar]
- [23].Altunoren O, Akkus G, Sezal DT, et al. Does neutrophyl to lymphocyte ratio really predict chronic kidney disease progression? Int Urol Nephrol. 2019;51:129–37. [DOI] [PubMed] [Google Scholar]
- [24].King-Wing Ma T, Kam-Tao Li P. Depression in dialysis patients. Nephrology (Carlton). 2016;21:639–46. [DOI] [PubMed] [Google Scholar]
- [25].Farrokhi F, Abedi N, Beyene J, et al. Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63:623–35. [DOI] [PubMed] [Google Scholar]
- [26].Soucie JM, McClellan WM. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. J Am Soc Nephrol. 1996;7:2169–75. [DOI] [PubMed] [Google Scholar]
- [27].Drayer RA, Piraino B, Reynolds CF, et al. Characteristics of depression in hemodialysis patients: symptoms, quality of life and mortality risk. Gen Hosp Psychiatry. 2006;28:306–12. [DOI] [PubMed] [Google Scholar]
- [28].Kimmel PL, Phillips TM, Simmens SJ, et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Taraz M, Taraz S, Dashti-Khavidaki S. Association between depression and inflammatory/anti-inflammatory cytokines in chronic kidney disease and end-stage renal disease patients: a review of literature. Hemodial Int. 2015;19:11–22. [DOI] [PubMed] [Google Scholar]
- [30].Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Casaril AM, Dantzer R, Bas-Orth C. Neuronal mitochondrial dysfunction and bioenergetic failure in inflammation-associated depression. Front Neurosci. 2021;15:725547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Uzzan S, Azab AN. Anti-tnf-α compounds as a treatment for depression. Molecules. 2021;26:2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jaremka LM, Lindgren ME, Kiecolt-Glaser JK. Synergistic relationships among stress, depression, and troubled relationships: insights from psychoneuroimmunology. Depress Anxiety. 2013;30:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172:1075–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kohler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–87. [DOI] [PubMed] [Google Scholar]
- [37].Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim YK, Na KS, Myint AM, et al. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:277–84. [DOI] [PubMed] [Google Scholar]
- [39].Lecca D, Jung YJ, Scerba MT, et al. Role of chronic neuroinflammation in neuroplasticity and cognitive function: a hypothesis. Alzheimers Dement. 2022;18:2327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dziurkowska E, Wesolowski M. Cortisol as a biomarker of mental disorder severity. J Clin Med. 2021;10:5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhu F, Tu H, Chen T. The microbiota-gut-brain axis in depression: the potential pathophysiological mechanisms and microbiota combined antidepression effect. Nutrients. 2022;14:2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kounis NG, Soufras GD, Tsigkas G, et al. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin Appl Thromb Hemost. 2015;21:139–43. [DOI] [PubMed] [Google Scholar]
- [44].Yuan Q, Wang J, Peng Z, et al. Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: results from the Chinese cohort study of chronic kidney disease (c-stride). J Transl Med. 2019;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nagy GR, Kemeny L, Bata-Csorgo Z. Neutrophil-to-lymphocyte ratio: a biomarker for predicting systemic involvement in adult IgA vasculitis patients. J Eur Acad Dermatol Venereol. 2017;31:1033–7. [DOI] [PubMed] [Google Scholar]
- [46].Wang J, Zhou D, Li X. The association between neutrophil-to-lymphocyte ratio and diabetic depression in U.S. Adults with diabetes: findings from the 2009–2016 national health and nutrition examination survey (NHANES). Biomed Res Int. 2020;2020:8297628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Feng J, Lu X, Li H, et al. High neutrophil-to-lymphocyte ratio is a significant predictor of depressive symptoms in maintenance hemodialysis patients: a cross-sectional study. BMC Psychiatry. 2022;22:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhu X, Li R, Zhu Y, et al. Neutrophil/lymphocyte, platelet/lymphocyte, monocyte/lymphocyte ratios and systemic immune-inflammation index in patients with depression. Bratisl Lek Listy. 2023;124:471–4. [DOI] [PubMed] [Google Scholar]
- [49].Guenzani D, Buoli M, Caldiroli L, et al. Malnutrition and inflammation are associated with severity of depressive and cognitive symptoms of old patients affected by chronic kidney disease. J Psychosom Res. 2019;124:109783. [DOI] [PubMed] [Google Scholar]
- [50].Brys ADH, Di Stasio E, Lenaert B, et al. Serum interleukin-6 and endotoxin levels and their relationship with fatigue and depressive symptoms in patients on chronic haemodialysis. Cytokine. 2020;125:154823. [DOI] [PubMed] [Google Scholar]
- [51].Jung YS, You G, Shin HS, et al. Relationship between geriatric nutritional risk index and total lymphocyte count and mortality of hemodialysis patients. Hemodial Int. 2014;18:104–12. [DOI] [PubMed] [Google Scholar]
- [52].Balboul Y, Gurshumov A, Azar A, et al. Biological basis of lymphocyte ratios for survival prediction in hemodialysis patients: a longitudinal study. Int Urol Nephrol. 2020;52:1345–56. [DOI] [PubMed] [Google Scholar]