Abstract
BACKGROUND:
With the advent of the molecular era, the diagnosis and treatment systems of glioma have also changed. A single histological type cannot be used for prognosis grade. Only by combining molecular diagnosis can precision medicine be realized.
OBJECTIVE:
To develop an automatic integrated gene detection system (AIGS) for intraoperative detection in glioma and to explore its positive role in intraoperative diagnosis and treatment.
METHODS:
We analyzed the isocitrate dehydrogenase 1 (IDH1) mutation status of 105 glioma samples and evaluated the product's potential value for diagnosis; 37 glioma samples were detected intraoperatively to evaluate the feasibility of using the product in an actual situation. A blinding method was used to evaluate the effect of the detection technology on the accuracy of intraoperative histopathological diagnosis by pathologists. We also reviewed the current research status in the field of intraoperative molecular diagnosis.
RESULTS:
Compared with next-generation sequencing, the accuracy of AIGS in detecting IDH1 was 100% for 105 samples and 37 intraoperative samples. The blind diagnostic results were compared between the 2 groups, and the molecular information provided by AIGS increased the intraoperative diagnostic accuracy of glioma by 16.2%. Using the technical advantages of multipoint synchronous detection, we determined the tumor molecular margins for 5 IDH-positive patients and achieved accurate resection at the molecular level.
CONCLUSION:
AIGS can quickly and accurately provide molecular information during surgery. This methodology not only improves the accuracy of intraoperative pathological diagnosis but also provides an important molecular basis for determining tumor margins to facilitate precision surgery.
KEY WORDS: IDH1 mutations, Intraoperative molecular diagnosis, Glioma, Molecular margin
ABBREVIATIONS:
- AA
anaplastic astrocytoma
- AO
anaplastic oligodendroglioma
- AIGS
automatic integrated gene detection system
- CNS
central nervous system
- DA
diffuse astrocytoma
- FAM
Carboxyfluorescein
- GBM
glioblastoma
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HGG
higher-grade glioma
- IHC
immunohistochemistry
- IDH
isocitrate dehydrogenase
- LGG
lower-grade glioma
- MS
mass spectrum
- NPV
negative predictive value.
- NGS
next-generation sequencing
- OD
oligodendroglioma
- PPV
positive predictive value
- ROX
Rhodamine X
- WHO
World Health Organization.
The accurate diagnosis of glioma increasingly depends on molecular characteristics.1 In 2021, the World Health Organization (WHO) released the fifth edition of the classification standard of central nervous system (CNS) tumors. The molecular markers in the new classification standard have become indispensable in the diagnosis of almost all tumors.2 Among many molecular markers that play a key role in the diagnosis and prognosis of gliomas, the mutation state of isocitrate dehydrogenase (IDH) is critical.3 Many studies have shown that patients with glioma with IDH1/2 mutations have a better prognosis and response to chemotherapy and radiotherapy than other patients.4-6 In glioma, the mutation frequency (>90%) of IDH1 is much higher than that of IDH2, whereas 80%-90% of IDH1 mutations are R132H. The other mutation types are rare and include R132C/G/S/L.7-9
Traditionally, the detection methods based on immunohistochemistry (IHC) and next-generation sequencing (NGS) take a long time and are mostly used for postoperative diagnosis, which cannot meet the needs of intraoperative detection. Studies have shown that quickly understanding IDH mutation information in patients during surgery can enable surgeons to perform more accurate tumor resection procedures in patients with these mutations, which has a positive impact on the long-term survival rate of these patients.10-13 In this article, we developed an IDH mutation detection instrument: AIGS real-time fluorescence PCR. Based on the automatic integrated gene detection system, the instrument integrates nucleic acid extraction and real-time gene amplification detection and achieves fully closed automatic output from samples to genes in the form of a microfluidic card slot. In this study, we evaluated the accuracy of AIGS in intraoperative molecular diagnosis and further studied its practical value in glioma surgery.
METHODS
Tumor Sample Selection
This study was a mixed cohort study. For retrospective samples, tumor samples and basic information of 105 patients were obtained from the “Institute of Brain and Brain-Inspired Science” laboratory sample bank. This batch of samples is from patients who underwent surgery in the Department of Neurosurgery of Qilu Hospital from 2016 to 2018. Their histopathological classification was based on the 2021 CNS tumor classification standard. For prospective samples, after the start of the study, we performed intraoperative detection on 37 patients with gliomas diagnosed preoperatively. The 37 patients were hospitalized in the Department of Neurosurgery of Qilu Hospital from July 2021 to February 2022. All patients underwent rapid histopathological detection and AIGS. All tumors were confirmed as gliomas by postoperative histopathology, and the samples were retained for IHC and NGS. This study was registered in the National Clinical Research Center and approved by the ethics committee of Qilu Hospital of Shandong University. All patients participating in the prospective study provided written informed consent. We used the Standards for Reporting of Diagnostic Accuracy checklist when writing the section of diagnostic studies.
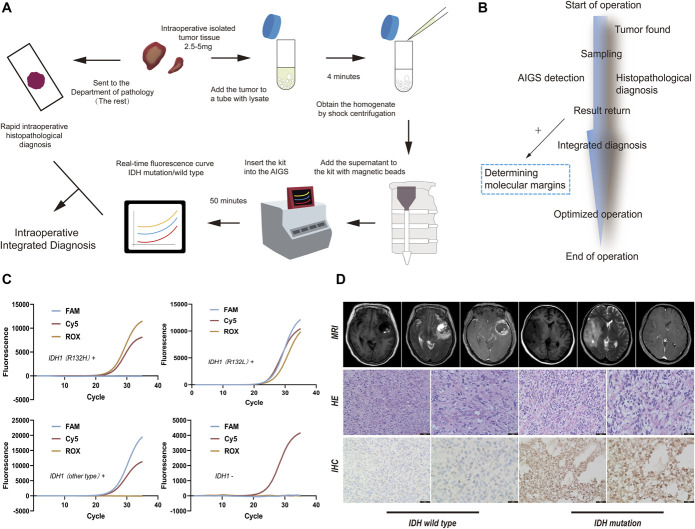
Workflow and Interpretation of the Results from AIGS
We used 2.5–5 mg of tumor samples (as big as a grain of rice), placed them into centrifuge tubes containing lysate (The Solarbio Company), and subjected the samples to shaking and centrifugation (The Beijing Synapse Biotechnology Company). The samples were shaken at 4 °C for 30 seconds and rotated at 6000 rpm for 2.5 minutes. Then, 200 μL of homogenate (Supplemental Digital Content, Table 1, http://links.lww.com/NEU/D526, Method 1, http://links.lww.com/NEU/D527) after centrifugation and 10μL of magnetic beads were added to the integrated nucleic acid detection card box (Supplemental Digital Content, Figure 1, http://links.lww.com/NEU/D528) and fully mixed. The card box was inserted into the card slot of the AIGS, and automatic analysis was performed. The analysis results were obtained within 50 minutes (Figure 1A). After samples are obtained during the operation, AIGS detection and rapid histopathological diagnosis are performed simultaneously and integrated diagnosis is obtained according to the results of the 2 samples. If the molecular diagnosis is positive, multipoint sampling can be performed to determine the molecular margin to optimize the operation (Figure 1B). The results showed 3 fluorescence curves: IDH1 (R132H) mutation Rhodamine X, IDH1 (non-R132H) mutation Carboxyfluorescein, and GAPDH as an internal reference (Cy5, Figure 1C). The sequences of probes and primers are determined by multiple control experiments (Supplemental Digital Content, Table 2, http://links.lww.com/NEU/D529, Method 2, http://links.lww.com/NEU/D530). The interpretation of NGS is represented by a visual Integrated Genomics Viewer (IGV, The GenomiCare Company) diagram (Supplemental Digital Content, Figure 2, http://links.lww.com/NEU/D531). All glioma cases were confirmed by MRI (The Siemens Company), H&E, and IHC (The OriGene Company, Figure 1D).
FIGURE 1.
A, AIGS real-time fluorescence PCR detection process and intraoperative integrated diagnostic standard flowchart. B, The time axis of the application of AIGS in surgery. C, The results of various types of AIGS real-time fluorescence PCR detection images. If the FAM curve and ROX curve show S-type amplification (including the S-curve before the platform stage), it indicates that the sample to be tested harbors the IDH1 (R132L) mutation. If only the FAM curve shows S-type amplification, it indicates that there are other types of mutations, such as IDH1 R132C, in the sample to be tested. If only ROX shows an S-type amplification curve, it indicates that the IDH1 (R132H) mutation is present in the tested sample. If only Cy5 shows an S-type amplification curve, it indicates that an IDH1 mutation does not exist in the detected sample. D, MRI images, H&E staining images (The OriGene Company), and IHC images (anti-IDH1, The OriGene Company) of IDH mutation and IDH wild-type gliomas. AIGS, automatic integrated gene detection system; FAM, Carboxyfluorescein; IDH, isocitrate dehydrogenase; IHC, immunohistochemistry; ROX, Rhodamine X.
Blind Evaluation of the Effect of AIGS on the Accuracy of Intraoperative Rapid Histopathological Diagnosis
The experiment was performed using 37 intraoperative samples of patients with gliomas diagnosed by preoperative imaging (Supplemental Digital Content, Table 3, http://links.lww.com/NEU/D532). After performing surgical resection, the neurosurgeons immediately divided the tumor samples into 2 copies. One copy was tested by AIGS. The inspectors recorded the IDH test results without knowing the tumor histomorphology. The other copy was sent to the pathology department. The pathologists in group A performed rapid morphological diagnosis without knowing the IDH mutation state and then provided the same batch of sections to the pathologists in group B. The pathologists in this group performed rapid histological diagnosis again when the patient’s IDH state was obtained. The final diagnosis results of the pathologists in the 2 groups were submitted to the third pathologist for review and compared with postoperative histopathological results to compare the diagnostic results of the pathologists in the 2 groups.
Statistical Analysis
In this study, the NGS results were used as the gold standard to compare AIGS and IHC. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of AIGS were evaluated using a four-grid table. The statistical significance of AIGS in improving the diagnostic accuracy of glioma was analyzed using the chi-square test. The survival of 105 patients was analyzed and expressed by Kaplan–Meier survival curves. All the above statistical analyses were performed using SPSS 22.0 (International Business Machines Corporation, IBM), and statistical significance was set to P < .05. The figures presented in this article were drawn using GraphPad Prism 9 (The GraphPad Software Company) and Adobe Illustrator (The Adobe Company).
RESULTS
Description of Clinical Characteristics
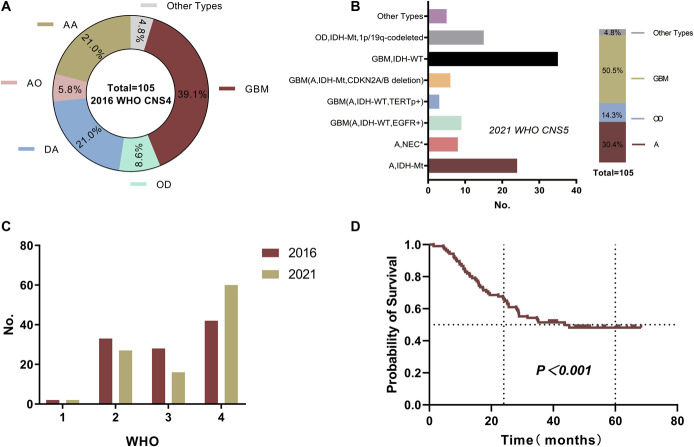
A total of 142 patients were included in this study. Table 1 provides the clinicopathological information of 105 retrospective samples. Glioblastoma accounted for the highest proportion (39.1%), followed by diffuse astrocytoma (21.0%) and AA (21.0%, Figure 2A). We rediagnosed them according to the 2021 classification standard (Figure 2B). The new diagnostic standard was based on molecular information and tissue diagnosis. The results showed that patients with astrocytoma (A), IDH mutation, and homozygous deletion of CDKN2A/B were classified as having glioblastoma in biological behavior. Similarly, patients with astrocytoma, IDH wild-type, and EGFR or TERT promoter (TERTp) mutations were also classified as having glioblastoma in biological behavior. Therefore, the proportion of glioblastoma in the new classification criteria increased significantly (50.5%, Figure 2B). We compared the grading of 2016 with that of 2021 and found that the proportion of grade 4 was significantly increased (Figure 2C), which illustrated the importance of molecular diagnosis for the classification and grading of glioma.
TABLE 1.
Clinicopathological Characteristics of the 105 Patients Who Met Inclusion Criteria
| Variable | Final pathologya | P | ||||
|---|---|---|---|---|---|---|
| Total (n = 105) | Astrocytoma | Oligodendroglioma | Glioblastoma | Other types | ||
| No (%) | No (%) | No (%) | No (%) | No (%) | ||
| Age (mean ± SD, y) | 50.6 ± 14.5 | 46.9 ± 13.3 | 53.0 ± 9.3 | 57.0 ± 13.5 | 28 ± 12.1 | >.05 |
| Sex | ||||||
| Male | 57 (54.3%) | 28 (63.6%) | 8 (53.3%) | 18 (43.9%) | 3 (60.0%) | >.05 |
| Female | 48 (45.7%) | 16 (36.4%) | 7 (46.7%) | 23 (56.1%) | 2 (40.0%) | |
| Location | ||||||
| F | 62 (47.0%) | 25 (44.6%) | 9 (39.1%) | 24 (51.1%) | 4 (66.7%) | >.05 |
| T | 30 (22.7%) | 14 (25.0%) | 6 (26.1%) | 8 (17.0%) | 2 (33.3%) | |
| P | 15 (11.4%) | 7 (12.5%) | 2 (8.7%) | 6 (12.8%) | 0 (0%) | |
| O | 10 (7.6%) | 4 (7.1%) | 1 (4.3%) | 5 (10.6%) | 0 (0%) | |
| I | 9 (6.8%) | 3 (5.4%) | 4 (17.4%) | 2 (4.3%) | 0 (0%) | |
| Others | 6 (4.5%) | 3 (5.4%) | 1 (4.3%) | 2 (4.3%) | 0 (0%) | |
| IDH (NGS) | ||||||
| Mutation | 46 (43.8%) | 24 (54.5%) | 15 (100.0%) | 6 (14.6%) | 1 (20.0%) | <.05 |
| Integrated diagnosis | Astrocytoma, IDH-Mt | Oligodendroglioma, IDH-Mt/1p19q-codeleted | Astrocytoma, IDH-Mt | |||
| Wild type | 59 (56.2%) | 20 (45.5%) | 0 (0%) | 35 (85.4%) | 4 (80.0%) | |
| Integrated diagnosis | Astrocytoma, NEC b | Glioblastoma, IDH-WT | ||||
| IDH (AIGS) | ||||||
| Mutation | 44 (41.9%) | 23 (52.3%) | 13 (86.7%) | 7 (17.1%) | 1 (20.0%) | <.05 |
| Wild type | 61 (58.1%) | 21 (47.7%) | 2 (13.3%) | 34 (82.9%) | 4 (80.0%) | |
| Survival state | ||||||
| Death | 53 (50.5%) | 18 (40.9%) | 1 (6.7%) | 33 (80.5%) | 1 (20.0%) | <.05 |
| Survival | 52 (49.5%) | 26 (59.1%) | 14 (93.3%) | 8 (19.5%) | 4 (80.0%) | |
AIGS, automatic integrated gene detection system; F, Frontal lobe; T, Temporal lobe; I, Insula; IDH, isocitrate dehydrogenase; P, Parietal lobe; O, Occipital lobe; WHO, World Health Organization.
2021 WHO classification standard of central nervous system tumor.
The necessary diagnostic tests had been successfully carried out, but in view of the incompatibility of clinical, histological, immunohistochemical and/or genetic characteristics, WHO integrated diagnosis could not be made.
Bold text indicates that the introduction of new diagnostic standards has changed the original diagnosis of this part. Italic text indicates the 2021 version of WHO integrated diagnostic standards, which is different from the 2016.
FIGURE 2.
A, Proportion distribution of various types for 105 glioma samples according to the 2016 WHO. B, A total of 105 gliomas were reclassified according to the 2021 WHO CNS5 (*histological characteristics and molecular typing do not match). C, Comparison of 2016 and 2021 WHO standard grades. D, Kaplan–Meier survival curve of 105 patients with glioma. AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; GBM, glioblastoma; DA, diffuse astrocytoma; OD, oligodendroglioma, WHO, World Health Organization.
According to the follow-up results, approximately half patients (50.05%) had end point events by the last follow-up, and the median survival time of these patients was 38.9 months. The 5-year OS was 48.2% (Figure 2D).
Evaluation of the Diagnostic Effectiveness of AIGS
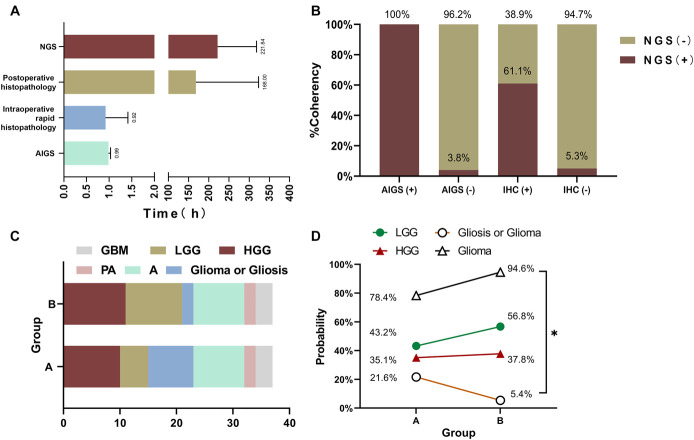
In 105 patients with glioma, 46 patients had IDH mutations based on NGS. The IDH1 (R132H) mutation was detected in 44 patients by AIGS. In Supplemental Digital Content, Table 3, http://links.lww.com/NEU/D532, the results of 3 detection techniques were compared and analyzed in 37 patients. AIGS detected IDH1 (R132H) mutation in 11 patients. Postoperative NGS detected IDH mutations in 12 patients, and IHC detected the IDH1 R132H mutation in 18 patients. We compared the results reporting the time of the 3 detection techniques (Figure 3A). The results show that AIGS has an obvious time advantage and can be used for intraoperative diagnosis.
FIGURE 3.
A, Time comparison. The average time of AIGS real-time fluorescence PCR detection was 59.2 minutes. The average time of intraoperative rapid histopathological detection was approximately 55.4 minutes. Routine pathological results were available 7 days after the operation, and the average time for NGS detection was 9.2 days. B, Taking the NGS results as the gold standard, the results of AIGS real-time fluorescence PCR were compared with IHC (total = 37). C, Comparison of the diagnosis results of the pathologists in group A and group B in intraoperative histopathological diagnosis. D, Double-blind comparison of AIGS in improving the accuracy of pathologists' intraoperative diagnoses. Compared with group A, group B improved the diagnostic accuracy of glioma by 16.2%, and the diagnostic rate of uncertainty decreased. AIGS, automatic integrated gene detection system; NGS, next-generation sequencing.
We evaluated the actual diagnostic efficacy of AIGS in 105 patients with glioma (Table 2). The overall accuracy of AIGS in detecting IDH mutations was 96.2%, and Youden's index was 91.8%. Ideally, IDH2 mutation cases would be excluded, rendering 100% accuracy of IDH1 detection by AIGS. IHC and AIGS were compared based on the sequencing of 37 patients. The results showed that the diagnostic accuracy of IHC was 78.4%, and Youden’s index was 71.4%. By contrast, the diagnostic accuracy of AIGS was 97.3%, and Youden’s index was 91.7% (Figure 3B). If IDH2 mutation cases were removed, AIGS also achieved 100% diagnostic accuracy in IDH1 detection.
TABLE 2.
Comparison of Diagnostic Efficacy of AIGS in 2 Cohorts
| Performance factors | Total = 105 | P b | Total = 37 | P b | |||
|---|---|---|---|---|---|---|---|
| AIGS | AIGSa | IHC | AIGS | AIGSa | |||
| Accuracy | 96.2% | 100% | <.05 | 78.4% | 97.3% | 100% | <0.05 |
| Sensitivity | 93.5% | 100% | 91.7% | 91.7% | 100% | ||
| Specificity | 98.3% | 100% | 72% | 100% | 100% | ||
| Youden's index | 91.8% | 100% | 63.7% | 91.7% | 100% | ||
| PPV | 97.7% | 100% | 61.1% | 100% | 100% | ||
| NPV | 95.1% | 100% | 94.7% | 96.2% | 100% | ||
AIGS, automatic integrated gene detection system; IHC, immunohistochemistry; PPV, positive predictive value; NPV, negative predictive value.
Compared with NGS detection results, IDH2 mutation was excluded.
χ2 test of diagnostic consistency between AIGS and NGS.
AIGS can Assist Pathologists in Intraoperative Diagnosis
A double-blind experiment showed that 8 patients in group A had an uncertain diagnosis of “gliosis, not excepted gliomas.” The pathologists in group B made a rapid morphological diagnosis after obtaining the IDH mutation information of all patients. Without considering the difference in the diagnostic level of the pathologists in the 2 groups, only 2 cases in group B had an uncertain diagnosis (Figure 3C). After analysis, we found that among the 8 patients for whom an accurate diagnosis of glioma could not be made, 6 cases were clearly diagnosed because they provided IDH mutation information. Therefore, through the molecular information provided by AIGS, the intraoperative diagnostic accuracy of glioma was improved by 16.2% (Figure 3D) and almost all cases with uncertain diagnoses were lower-grade gliomas (LGGs), which is also consistent with the fact that some LGGs are indeed difficult to distinguish from gliosis in histology and morphology.
To observe the time matching between AIGS and intraoperative rapid histopathological detection under actual conditions, we recorded the time spent by 37 patients in AIGS and intraoperative rapid histopathological detection and performed statistical analysis. Under normal conditions, certain procedures, such as obtaining materials, registering, checking, sending for examination, and uploading test reports, must be performed. Therefore, the actual average time for pathologists to perform intraoperative rapid histopathological detection was 55.3 minutes, whereas the average time for AIGS in the operating room was 59.2 minutes (Figure 3A).
Determining Tumor Molecular Margins
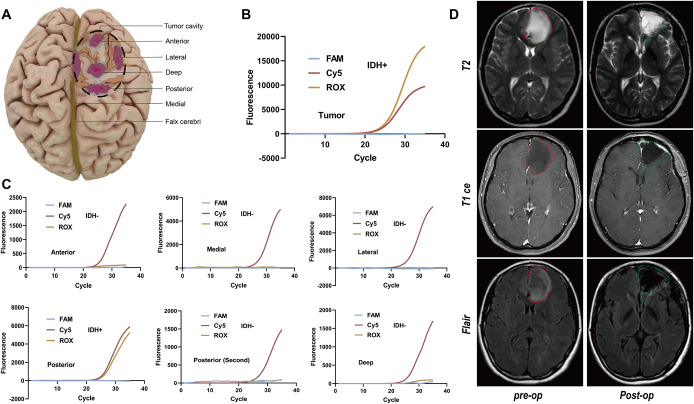
IDH mutations are not present in normal brain tissue. Thus, in patients with glioma with IDH mutations, if the IDH mutation is not present at the margin of the tumor cavity, it is hypothesized that the surgical resection has reached normal brain tissue. According to this hypothesis, we assessed the molecular margins in 5 of the 37 patients who were diagnosed as IDH-positive during surgery. In this article, we show the actual operation of patient 7 (Supplemental Digital Content, Table 3, http://links.lww.com/NEU/D532). The surgical tumor cavity was similar to that of a hemisphere with a downward arc. We determined the suspicious margins in the anterior, posterior, lateral, deep, and medial directions according to the operation conditions and obtained materials from the suspicious parts in each direction (Figure 4A and Video). The results showed that in the other 4 directions, the results were negative except for the area behind the tumor cavity, demonstrating that the operation had reached normal brain tissue. We cleaned and removed the posterior area again and took samples for testing. The second test result showed that IDH was negative, demonstrating that the normal margin was reached after reresection (Figure 4B and 4C). Postoperative MRI showed that the resection was clean, and there was no obvious residue at the edge (Figure 4D).
FIGURE 4.
A, Multipoint sampling of the tumor cavity was performed based on the results of AIGS real-time fluorescence PCR. B, Tumor site (IDH-positive). C, After tumor resection, multipoint samples were obtained from the suspicious boundaries in 5 directions, and IDH status detection was performed. D, Comparison of MRI images preoperation and postoperation. AIGS, automatic integrated gene detection system; IDH, isocitrate dehydrogenase.
VIDEO.
In the form of surgical video, the key steps in the operation are intercepted to demonstrate the process of multipoint material extraction and determination of the tumor molecular boundary.
DISCUSSION
In the field of neuro-oncology, it has been confirmed that IDH mutation status is significant in the prognosis of patients with glioma. Although relevant studies believe that compared with HGG IDH wild type, the scope of surgical resection can affect the recurrence and OS of patients with IDH-mutant glioma ,11-13 it is undeniable that according to the current treatment standards for glioma, regardless of its gene mutation, the principle of surgical resection should be followed to the maximum extent of safety. Nevertheless, it is still of great significance to determine the tumor boundary and nature of surgery for selecting surgical strategies.14
In the past decade, researchers have tried detecting gene mutations quickly through different detection technologies (Table 3).10,14-23 There are 2 main technologies: mass spectrum (MS) and PCR. Between these methods, MS has the advantage of fast detection, but because its detection principle is based on the metabolite 2-hydroxyglutaric acid (2-HG) as an indirect reflection of the mutation status of IDH, the application of MS has certain limitations, namely, it cannot be used for IDH typing or for detecting other molecular mutations. Compared with MS, PCR technology after transformation and upgrading still has no advantage in detection time, but it has high detection sensitivity and specificity. Moreover, different probes can be designed to distinguish IDH subtypes. Similarly, different primers can also be designed to detect various molecular mutations (such as TERTp). Although molecular detection technology has existed for more than 40 years, very few of its applications can be used in surgery and even fewer can achieve clinical transformation or commercialization. Our research is also based on PCR technology, but in contrast to our predecessors, we use highly automated integration of sample detection and result output. Simultaneously, the specific primer and probe design further compresses the time and can support the simultaneous detection of multiple samples, which greatly improves the surgical efficiency and increases the accuracy of the diagnosis and treatment. For intraoperative histopathological diagnosis, the pathologist should assess the nature of the tumor in a short time, so the accuracy of actual diagnosis is affected, especially for LGG. It is occasionally difficult to distinguish gliomas from gliosis based on morphology, prompting neurosurgeons to choose a more conservative surgical scheme and leave residual tumor. Therefore, if the diagnostic information of specific molecules can be obtained during the operation, the diagnostic accuracy of pathologists during the operation will be greatly improved. Our research found that this is indeed the case. According to the IDH mutation information provided by the AIGS, the accuracy of pathologists in intraoperative diagnosis was improved by 16.2%, and a more accurate integrated diagnosis could be obtained during the operation. This information plays an important role in the selection of a surgical strategy and the prediction of prognosis.
TABLE 3.
Summary of published series of intraoperative rapid molecular detection
| Correlational research | Detection method | Detection indexa | Number of cases | Detection duration | Lower limit of detectionb | Sample | Measurement | Clinical transformation/commercialization |
|---|---|---|---|---|---|---|---|---|
| Kanamori, et al (2014)14 | FMCA-Cold PCR | IDH1/2 | 28 (intraoperative) | 60–65 min | – | Tumor | Accuracy: 100% | N |
| Santagata, et al (2014)15 | DESI-MS | 2-HG | 35 (intraoperative) | >10 min | 3 μmol/g | Tumor | Accuracy: 100% | Y |
| Shankar, et al (2015)16 | PNA/LNA-PCR (OperaGen) | IDH1 R132H/L/C/G/S TERTp |
190 (frozen + archived fixed) | <60 min | Allele content>0.1% | Tumor | Sensitivity: 96% Specificity: 100% |
Y |
| Ohka, et al (2017)17 | I-Densy-Regular PCR | IDH1 R132H | 11 (intraoperative) | 90–100 min | 3.25 ng/μL | Tumor/CSF | Accuracy: 100% | Y |
| Xu, et al (2019)18 | GC-MS | 2-HG | 220 (frozen) + 87 (intraoperative) | 40 min | 5.4 μM/g−2 mM/g | Tumor | Accuracy: 100% | N |
| Brown, et al (2019)19 | Mini-MS | 2-HG | 25 (biopsy) | 3 min | - | Tumor | Accuracy: 100% | Y |
| Alfaro,et al (2019)10 | DESI-MS | 2-HG | 37 (frozen) + 25 (biopsy) | 5 min | >100 ng/mg | Tumor | Sensitivity: 100% Specificity: 100% |
Y |
| Sim, et al (2019)20 | HPLC-MS/MS | 2-HG/R-2-HG/S-2-HG | 87 (intraoperative) | 60 min | 10 ng/mL | Tumor | Sensitivity: 97% Specificity: 100% |
N |
| Diplas, et al (2019)21 | LNA-PCR (GliomaDx) | IDH1/2 TERTp |
39 (frozen) | <60 min | 10 ng/mL | Tumor | Sensitivity: 99%–100% | Y |
| Avsar, et al (2020)22 | 3m-ARMS | IDH1/2 | 236 (frozen) | 60 min | <100 fg | Tumor/CSF/blood | Accuracy: 98.3% NPV: 100% |
N |
| Fujioka, (2021)23 | Digital PCR | IDH/TERTp/H3 | 34 (intraoperative) + 11 (preoperative CSF) | 110–147 min | 20 ng/mL | CSF | FP = 0 | Y |
| Our research | AIGS-Realtime PCR | IDH1 R132H/L/C/G/S TERTp |
105 (frozen) + 37 (intraoperative) | 59.2 min | 500 copies/mL | Tumor | Accuracy: 100% | Y |
AIGS, automatic integrated gene detection system; IDH, isocitrate dehydrogenase.
The detection index is only based on the test results of the number of cases included in the author's article.
The difference in the measurement unit of the lower limit of detection is caused by the difference in detection technology and the detection method.
The invasive growth of glioma poses great difficulties in surgical resection. Therefore, achieving maximum safe resection is a common problem faced by all neurosurgeons.24,25 Determination of the tumor margin is critical. Intraoperative fluorescence imaging-assisted technology, intraoperative magnetic resonance technology, neuronavigation technology, and intraoperative wake-up technology represent useful methods. These technologies can be used for maximum removal of the solid margin or functional margin of the tumor.26-29 As early as 2015, Shankar et al16 suggested the application of a molecular detection system to analyze tumor margins in their research. However, because of the lagging development of intraoperative molecular diagnostic technology, this concept cannot be further confirmed. Recently, we have conducted continuous research in molecular diagnostic technology, which makes accurate and rapid molecular detection a reality. Therefore, we proposed the concept of tumor molecular margins. Given the absence of IDH and TERTp mutations in normal brain tissue, we can take multipoint samples from suspicious lesions at the junction between the tumor and normal brain tissue, and these samples can be assessed by AIGS. In this article, the presence of an IDH (or TERTp) mutation indicates the presence of tumor tissue. If the test result of suspicious orientation is negative, it indicates that the operation has reached normal brain tissue. If there is a positive result, the tumor can be resected again in the corresponding orientation. The margin position after resection can be tested again until the result is negative to obtain total resection at the molecular level.
Limitations
At present, AIGS only targets the most common IDH1 mutation in gliomas, achieving a balance between economic interests and actual conditions. The “molecular margin” proposed by us only proves that the site has reached the boundary within a certain sensitivity range through our detection technology, but this does not mean that there are no tumor cells. Because the lower limit of sensitivity of our detection technology is 500 copies/mL (Supplemental Digital Content, Table 4, http://links.lww.com/NEU/D533), the number of cells below this value cannot be detected within the specified time. In our study, we only assessed the molecular margins of 5 samples. Although the results are satisfactory, we still should expand the sample size to verify more scientific conclusions, which is part of our ongoing work. AIGS detection technology theoretically takes 54 min. Although this method takes the shortest time among the methods researched, it may affect the operation that takes a shorter time. Moreover, the time cost of determining the molecular margin by iteration may be further increased. However, we are constantly optimizing our products and can reduce the time to approximately 35 min.
CONCLUSION
AIGS can assist pathologists in making more accurate histological diagnoses by providing molecular information, which is also important for selecting surgical strategies and intratumoral targeted drugs. The multichannel synchronous detection of the AIGS instrument can be used to achieve multipoint and multiple sampling of the suspicious margin of the tumor to clarify the molecular margin in patients with IDH-mutant glioma, thus providing a theoretical basis for and demonstrating the practical feasibility of total resection of glioma at the molecular level.
Acknowledgments
We thank the research and development (R&D) technicians of Beijing Synapse Biotechnology Co., Ltd. for their technical support. We thank the nurses in the neurosurgery operating room of Qilu Hospital of Shandong University for their help and support in intraoperative testing. H. Xue and Z. Han initiated and designed the study and were involved in data collection, data interpretation, and writing the manuscript. G. Li was involved in study design and interpretation. B. Han, M. Qi, and H. Zhang participated in the design and research of pathological experiment. H. Li, X. Li, D. Jia, K. Zhang, J. Gong, H. Wang, Z. Feng, and S. Ni participated in the collection of patient information and the provision of tumor specimens.
Footnotes
Supplemental digital content is available for this article at neurosurgery-online.com.
Hao Xue and Zhe Han contributed equally to this work.
Contributor Information
Hao Xue, Email: xuehao@sdu.edu.cn.
Zhe Han, Email: hzaptx4869@126.com.
Haiyan Li, Email: 13793182112@163.com.
Xueen Li, Email: qlneurolab@163.com.
Deze Jia, Email: jiadeze@163.com.
Mei Qi, Email: qimeiqm@163.com.
Hui Zhang, Email: zhanghui4720@163.com.
Kailiang Zhang, Email: kailiang-zhang@email.sdu.edu.cn.
Jie Gong, Email: smilegongjie@163.com.
Hongwei Wang, Email: tigerwang8000@hotmail.com.
Zichao Feng, Email: zichaof@163.com.
Shilei Ni, Email: nishilei@sdu.edu.cn.
Bo Han, Email: boh@sdu.edu.cn.
Funding
Research on the future application prospect of classical gene marker kit (contract No. 6010122006), Key Clinical Research Project of Clinical Research Center of Shandong University (2020SDUCRCA011), and Taishan Pandeng Scholar Program of Shandong Province (No. tspd20210322).
Disclosures
The relevant authors of this article have no conflicts of interest.
Supplemental Digital Content
Supplementary Method 1. To determine the optimal sampling volume and homogenate volume of AIGS.
Supplementary Method 2. To determine the primers and probes.
Supplementary Figure 1. IDH1 gene mutation detection integrated nucleic acid detection cassette (The Beijing Synapse Biotechnology Company). A, Box body; B, box cover; C, cracking chamber with cracking liquid; D, a first cleaning chamber with a first cleaning solution; E, a second cleaning chamber with a second cleaning solution; F, reaction chamber (including the primer probe mixture of IDH1 gene); G, plunger; H, a magnetic bead that can pass through the plug hole.
Supplementary Figure 2. Results of tumor samples detected by NGS. Integrated Genomics Viewer (IGV, The GenomiCare Company) result diagram. A, IDH1 R132H mutant; B, IDH wild type.
Supplementary Table 1. To determine the optimal sampling volume and homogenate volume of rapid molecular pathology during operation.
Supplementary Table 2. The best primers and probes were selected according to the CT value.
Supplementary Table 3. Clinicopathological characteristics of 37 patients who were detected by the AIGS technique during operation.
Supplementary Table 4. Determining the lower limit of AIGS detection.
REFERENCES
- 1.Kristensen BW, Priesterbach-Ackley LP, Petersen JK, et al. Molecular pathology of tumors of the central nervous system. Ann Oncol. 2019;30(8):1265-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komori T. Grading of adult diffuse gliomas according to the 2021 WHO classification of tumors of the central nervous system. Lab Invest. 2022;102(2):126-133. [DOI] [PubMed] [Google Scholar]
- 4.Aldape K, Zadeh G, Mansouri S, et al. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848. [DOI] [PubMed] [Google Scholar]
- 5.Aoki K, Nakamura H, Suzuki H, et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018;20(1):66-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller JJ, Shih HA, Andronesi OC, et al. Isocitrate dehydrogenase-mutant glioma: evolving clinical and therapeutic implications. Cancer. 2017;123(23):4535-4546. [DOI] [PubMed] [Google Scholar]
- 7.Mu L, Xu W, Li Q, et al. IDH1 R132H mutation is accompanied with malignant progression of paired primary-recurrent astrocytic tumours. J Cancer. 2017;8(14):2704-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes J, Yu Y, Jalbert LE, et al. Genomic analysis of the origins and evolution of multicentric diffuse lower-grade gliomas. Neuro Oncol. 2018;20(5):632-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfaro CM, Pirro V, Keating MF, et al. Intraoperative assessment of isocitrate dehydrogenase mutation status in human gliomas using desorption electrospray ionization-mass spectrometry. J Neurosurg. 2020;132(1):180-187. [DOI] [PubMed] [Google Scholar]
- 11.Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molinaro AM, Hervey-Jumper S, Morshed RA, et al. Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020;6(4):495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SH, Bansal AG, Young EB, et al. Extent of surgical resection in lower-grade gliomas: differential impact based on molecular subtype. AJNR Am J Neuroradiol. 2019;40(7):1149-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanamori M, Kikuchi A, Watanabe M, et al. Rapid and sensitive intraoperative detection of mutations in the isocitrate dehydrogenase 1 and 2 genes during surgery for glioma. J Neurosurg. 2014;120(6):1288-1297. [DOI] [PubMed] [Google Scholar]
- 15.Santagata S, Eberlin LS, Norton I, et al. Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc Natl Acad Sci U S A. 2014;111(30):11121-11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar GM, Francis JM, Rinne ML, et al. Rapid intraoperative molecular characterization of glioma. JAMA Oncol. 2015;1(5):662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohka F, Yamamichi A, Kurimoto M, et al. A novel all-in-one intraoperative genotyping system for IDH1-mutant glioma. Brain Tumor Pathol. 2017;34(2):91-97. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Xia YK, Li CJ, et al. Rapid diagnosis of IDH1-mutated gliomas by 2-HG detection with gas chromatography mass spectrometry. Lab Invest. 2019;99(4):588-598. [DOI] [PubMed] [Google Scholar]
- 19.Brown HM, Pu F, Dey M, et al. Intraoperative detection of isocitrate dehydrogenase mutations in human gliomas using a miniature mass spectrometer. Anal Bioanal Chem. 2019;411(30):7929-7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sim HW, Nejad R, Zhang W, et al. Tissue 2-hydroxyglutarate as a biomarker for isocitrate dehydrogenase mutations in gliomas. Clin Cancer Res. 2019;25(11):3366-3373. [DOI] [PubMed] [Google Scholar]
- 21.Diplas BH, Liu H, Yang R, et al. Sensitive and rapid detection of TERT promoter and IDH mutations in diffuse gliomas. Neuro Oncol. 2019;21(4):440-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avsar T, Sursal A, Turan G, et al. Development of a rapid and sensitive IDH1/2 mutation detection method for glial tumors and a comparative mutation analysis of 236 glial tumor samples. Mol Diagn Ther. 2020;24(3):327-338. [DOI] [PubMed] [Google Scholar]
- 23.Fujioka Y, Hata N, Akagi Y, et al. Molecular diagnosis of diffuse glioma using a chip-based digital PCR system to analyze IDH, TERT, and H3 mutations in the cerebrospinal fluid. J Neurooncol. 2021;152(1):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marko NF, Weil RJ, Schroeder JL, et al. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014;32(8):774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pessina F, Navarria P, Cozzi L, et al. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J Neurooncol. 2017;135(1):129-139. [DOI] [PubMed] [Google Scholar]
- 26.Acerbi F, Broggi M, Schebesch KM, et al. Fluorescein-guided surgery for resection of high-grade gliomas: a multicentric prospective phase II study (FLUOGLIO). Clin Cancer Res. 2018;24(1):52-61. [DOI] [PubMed] [Google Scholar]
- 27.Orillac C, Stummer W, Orringer DA. Fluorescence guidance and intraoperative adjuvants to maximize extent of resection. Neurosurgery. 2021;89(5):727-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkinson MD, Barone DG, Bryant A, et al. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst Rev. 2018;1(1):Cd012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fountain DM, Bryant A, Barone DG, et al. Intraoperative imaging technology to maximise extent of resection for glioma: a network meta-analysis. Cochrane Database Syst Rev. 2021;1(1):Cd013630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Method 1. To determine the optimal sampling volume and homogenate volume of AIGS.
Supplementary Method 2. To determine the primers and probes.
Supplementary Figure 1. IDH1 gene mutation detection integrated nucleic acid detection cassette (The Beijing Synapse Biotechnology Company). A, Box body; B, box cover; C, cracking chamber with cracking liquid; D, a first cleaning chamber with a first cleaning solution; E, a second cleaning chamber with a second cleaning solution; F, reaction chamber (including the primer probe mixture of IDH1 gene); G, plunger; H, a magnetic bead that can pass through the plug hole.
Supplementary Figure 2. Results of tumor samples detected by NGS. Integrated Genomics Viewer (IGV, The GenomiCare Company) result diagram. A, IDH1 R132H mutant; B, IDH wild type.
Supplementary Table 1. To determine the optimal sampling volume and homogenate volume of rapid molecular pathology during operation.
Supplementary Table 2. The best primers and probes were selected according to the CT value.
Supplementary Table 3. Clinicopathological characteristics of 37 patients who were detected by the AIGS technique during operation.
Supplementary Table 4. Determining the lower limit of AIGS detection.