Abstract
Background:
Hemorrhoidectomy is a common surgical procedure associated with significant postoperative pain. The conventional analgesic methods used for hemorrhoidectomy often have adverse effects and may not provide adequate pain relief. The sacral erector spinae plane block (ESPB) is a newly introduced technique that has shown promise in various surgical procedures. This prospective, randomized, controlled trial aimed to evaluate the analgesic effects of sacral ESPB following hemorrhoidectomy.
Methods:
Seventy patients undergoing hemorrhoidectomy were divided into 2 groups: the control group and the sacral ESPB group. Bilateral sacral ESPB was performed in the sacral ESPB group, whereas no intervention was performed in the control group. The numeric rating scale at rest and during the active period (mobilizing) was used as the primary outcome measure. Secondary outcome measures were the cumulative doses of tramadol, the number of patients who required rescue analgesia postoperatively, and quality of recovery-15 Turkish version patient recovery quality.
Results:
The sacral ESPB group had significantly low numeric rating scale scores at various time points (P < .05). More patients in the control group needed rescue analgesia during the postoperative period (P < .001). The dosages of tramadol consumption after the first 24 hours postoperatively were significantly lower in the sacral ESPB group compared with the control group (P < .001). Furthermore, quality of recovery-15 Turkish version scores were high in the sacral ESPB group (P < .001).
Conclusion:
The results suggest that sacral ESPB is an effective method for post-hemorrhoidectomy pain management, reducing the need for additional analgesics and improving patient recovery.
Keywords: hemorrhoidectomy, numeric rating scale, QoR-15T patient recovery quality, sacral erector spinae plane block
1. Introduction
Hemorrhoids are common surgical conditions that affect the anorectal area. These conditions are characterized by symptoms such as pain, bleeding, and the presence of a protruding mass from the anal opening. Fear of postoperative pain is one of the main reasons patients avoid surgical interventions.[1] Postoperative pain is a significant concern, with over 80% of patients experiencing moderate to severe pain. This heightened pain level increases the risk of complications, including atelectasis, thromboembolism, myocardial ischemia, cardiac arrhythmia, electrolyte imbalance, urinary retention, and ileus.[2] The 2 main unresolved issues after surgery are postoperative pain and urinary retention. In addition to improving patient satisfaction, pain management decreases urinary retention and constipation, especially in the first 24 hours following surgery.[3] Previous research has shown that, even with analgesic therapy, 20% to 40% of patients who underwent hemorrhoidectomy experience severe postoperative pain.[4,5] Commonly used pain relievers, such as nonsteroidal anti-inflammatory drugs (NSAIDs), paracetamol, and opioids, often produce adverse effects, such as dizziness, nausea, vomiting, constipation, and the potential for tolerance. These side effects can prevent full recovery and lead to a poor prognosis.[6–8] While bilateral pudendal nerve blocks are known to significantly reduce postoperative pain, they are technically challenging and require specific positioning.[9] Furthermore, the administration of pudendal nerve block carries the risk of complications, such as hematoma formation, sciatic nerve injury, and accidental rectal puncture.[10] Therefore, an alternative analgesic method with minimal adverse effects would be beneficial.
The erector spinae plane block (ESPB) is initially introduced as an interfascial plane block performed at the upper thoracic levels to alleviate neuropathic pain.[11] Subsequently, its application expanded to include a range of thoracic interventions, including mastectomy, video-assisted thoracoscopy, and cardiac surgery. It has also been utilized at lumbar levels for procedures such as abdominal surgery, prostatectomy, lumbar spine surgery, total hip arthroplasty, and proximal femur surgery.[12] A recently documented method called the sacral ESPB has demonstrated its effectiveness in various surgical procedures through case studies. Specifically, it has shown promise in managing radicular pain at the L5-S1 level after sex reassignment surgery and hypospadias surgery and has provided analgesia for the posterior branches of the sacral nerves during pilonidal sinus surgery.[13–17]
Our hypothesis was that performing sacral ESPB would result in effective analgesia following hemorrhoidectomy. We also hypothesized that sacral ESPB would reduce the need for additional analgesics after hemorrhoidectomy and improve the quality of patient recovery. In this prospective, randomized, controlled trial, our main objective was to examine the postoperative analgesic effects of sacral ESPB following hemorrhoidectomy.
2. Materials and methods
2.1. Trial design
This prospective and randomized study was conducted at the Department of Anesthesiology and Reanimation, Health Sciences University Konya City Hospital in Turkey. The study received approval from the Clinical Research Ethics Committee at Health Sciences University Ankara City Hospital (Decision Number: E1-23-3742, Date: 21.06.2023). This investigation was registered with ClinacalTrials.gov (NCT05965674). Written informed consent was obtained from all participants. All techniques performed on human volunteers in studies conformed to the ethical norms of the Institutional Research Committee and the 1964 Helsinki Statement and its later revisions or other comparable ethical standards.
2.2. Participants and eligibility criteria
Seventy patients who had undergone hemorrhoidectomy were enrolled in the study. The inclusion criteria for patient selection were as follows: aged between 18 and 65 years and American Society of Anesthesiologists (ASA) status of 1 to 2. Patients under the age of 18 years, pregnant individuals, those with significant hematopoietic, cardiovascular, liver, or kidney disorders, patients unable to comply with medical instructions, individuals on anticoagulant therapy, and those with contraindications to regional anesthetic agents or a history of previous hemorrhoidectomy were excluded from the study.
Participants were randomly assigned into the control group (Group N) and the sacral ESPB group (Group S), using sequentially numbered opaque envelopes. The allocation was sealed and created by an anesthesiologist who was not involved in the investigation. At the end of the surgery, all patients in Group S received bilateral sacral ESPB, whereas patients in Group N received no intervention.
2.3. Anesthesia application and analgesic protocol
The patients underwent standard monitoring methods, including electrocardiography, noninvasive blood pressure monitoring, and peripheral oxygen saturation measurements. Intravenous access was established using a 22-gauge intravenous needle, and isotonic fluid was infused at a rate of 15 mL/kg/h. Spinal anesthesia was uniformly administered to all surgical patients, either in the sitting or lateral decubitus positions. A 25-gauge Quincke spinal needle (B. Braun Melsungen, Germany) was used for spinal anesthesia at the L3–L4 or L4–L5 interspace. Following observation of spinal fluid, 2 to 2.5 mL of 0.5% bupivacaine hydrochloride was injected into the intrathecal space, and the patients were subsequently placed in the supine position. After waiting for 5 to 10 minutes to confirm the spinal block level reaching the T10 dermatome, the patients were repositioned into the prone position. Oxygen was administered via the nasal cannula at a rate of 2 L/min. If a decrease in blood pressure of more than 20% from the baseline value was observed, ephedrine (0.1 mg/kg) was administered. If the heart rate drops below 50 bpm, atropine (0.5 mg/kg) is administered.
At the end of the operation, each patient received a single intravenous dose of paracetamol at a rate of 15 mg/kg, along with intravenous diclofenac at a rate of 1.5 mg/kg (with a maximum dose of 75 mg). During the postoperative period, all patients received intravenous paracetamol every 8 hours at a dosage of 15 mg/kg, while intravenous diclofenac was administered every 12 hours at a dosage of 1.5 mg/kg (with a maximum dose of 75 mg). In cases where the numeric rating scale (NRS) score exceeded 3, intravenous tramadol at a dosage of 1.5 mg/kg was provided as rescue analgesia.
2.4. Ultrasound-guided sacral ESPB
Following asepsis and antisepsis protocols, patients in Group S underwent a procedure in which a high-frequency linear ultrasound probe (Clarius, 205-2980 Virtual Way, Vancouver, BC, Canada) was positioned on the transverse plane, specifically on the fifth spinous process. The probe was then moved downward to visualize the first and second median sacral crests. Subsequently, the transducer was placed 3 to 4 cm laterally to the second medial sacral crest to visualize the intermediate sacral crest. In the interfascial plane, 20 mL of local anesthetic solution (consisting of 10 mL of 0.5% bupivacaine, 5 mL of 2% lidocaine, and 5 mL of normal saline) was injected between the erector spinae muscles and the intermediate sacral crest. The same procedure was performed on the contralateral side.
2.5. Outcome measures
The primary outcome was to assess pain intensity at rest and during active (mobilizing) periods using an NRS ranging from 0 (no pain) to 10 (worst pain) at 0, 2, 4, 6, 12, and 24 hours after the procedure. The secondary outcome measures included the cumulative doses of tramadol, the number of patients requiring rescue medication in the postoperative period, and the 15-item quality of recovery (QoR-15T).
Furthermore, the preoperative age, sex, weight, height, ASA, body mass index, and operative times of the patients in both groups were recorded.
2.6. Sample size calculation
For the sample size analysis, it was determined that each group should have 33 patients, with a power ratio of 95% and an alpha margin of error of 0.05. The effect used for this calculation was 0.826, derived from studies with similar sample sizes, and the actual power was calculated as 0.953. As a result of the analysis, considering the dropout rate, it was planned to include 35 patients in each group.
2.7. Statistical analysis
Statistical analyses were performed using the SPSS 21.0 program (IBM Inc., Chicago, IL). Descriptive statistics of the numerical and categorical data obtained in the study were analyzed. Numerical parameters were expressed as mean ± SD or median (min–max) (interquartile range [IQR]), whereas categorical variables were expressed as frequency and percentage. The conformance of numerical variables to the normal distribution was assessed using the Kolmogorov–Smirnov test, histogram graphs, and Q–Q plot graphs. Levene’s test was performed to analyze the homogeneity properties of numerical parameters. Correlation relationships between numerical data were examined using 2-way Pearson’s or Spearman’s correlation analyses. The Wilcoxon test was employed to compare the values of the 2 dependent groups. An independent t test or Mann–Whitney U test was used for pairwise comparisons of independent groups. Relationships between categorical variables were examined using Pearson’s chi-square analysis. The relationships between categorical groups and numerical parameters were summarized using boxplot graphics. In this study, the type-I error rate was set at 5%, and a P value of <.05 was considered significant.
3. Results
Seventy-two patients were initially enrolled in the study. However, 2 participants were subsequently excluded from the investigation due to a change in the surgical procedure. Among the remaining 70 patients, 35 were allocated to the sacral ESPB group, while the remaining 35 were assigned to the control group.
Demographic characteristics of the patients in both cohorts were subjected to a comparative analysis. The results revealed no statistically significant differences between the 2 groups in terms of age, height, weight, body mass index, duration of the surgical procedure, gender distribution, and ASA physical status classification (Table 1).
Table 1.
Analysis of demographic and clinical variables between sample groups.
| Parameter | Group N (control) (n = 35) | Group S (ESPB applied) (n = 35) | P |
|---|---|---|---|
| Mean ± SD | |||
| Age (yr) | 38.91 ± 12.45 | 41.03 ± 12.11 | .47 |
| Height (cm) | 171.43 ± 9.19 | 169.71 ± 7.31 | .39 |
| Weight (kg) | 73.8 ± 13.85 | 73.49 ± 9.37 | .91 |
| BMI (kg/m2) | 25.1 ± 4.43 | 25.52 ± 3.14 | .64 |
| Operation duration (min) | 15 (15–25) | 15 (15–25) | .99 |
| Frequency (%) | |||
| Gender | |||
| Female | 18 (52.9%) | 16 (47.1%) | .81 |
| Male | 17 (47.2%) | 19 (52.8%) | |
| ASA Group | |||
| ASA 1 | 22 (47.5%) | 24 (52.2%) | .80 |
| ASA 2 | 13 (54.2%) | 11 (45.8%) | |
ESPB = erector spinae plane block.
When assessing the NRS values, significantly higher scores were observed in Group N compared to Group S at the 6th, 12th, and 24th hours during rest, as well as at the 4th, 6th, 12th, and 24th hours during active periods (mobilizing) (P < .05 in each) (Table 2).
Table 2.
Analysis of NRS scores.
| Parameter | Group N (control) (n = 35) | Group S (ESPB applied) (n = 35) | P |
|---|---|---|---|
| Median (min–max) | |||
| NRS-R, 0 h | 0 (0–0) | 0 (0–0) | 1 |
| NRS-R, 2 h | 0 (0–0) | 0 (0–0) | 1 |
| NRS-R, 4 h | 0 (0–3) | 0 (0–0) | .99 |
| NRS-R, 6 h | 4 (0–7) | 0 (0–5) | <.001 * |
| NRS-R, 12 h | 1 (0–3) | 0 (0–5) | .02 ** |
| NRS-R, 24 h | 1 (0–2) | 0 (0–2) | .008 ** |
| NRS-A, 0 h | 0 (0–0) | 0 (0–0) | 1 |
| NRS-A, 2 h | 0 (0–2) | 0 (0–0) | .31 |
| NRS-A, 4 h | 0 (0–3) | 0 (0–0) | .01 ** |
| NRS-A, 6 h | 5 (0–8) | 0 (0–5) | <.001 * |
| NRS-A, 12 h | 2 (0–3) | 0 (0–6) | <.001 * |
| NRS-A, 24 h | 1 (0–2) | 0 (0–2) | .008 ** |
A = active (mobilizing), ESPB = erector spinae plane block, NRS = numeric rating scale, R = resting.
P < .001.
P < .05.
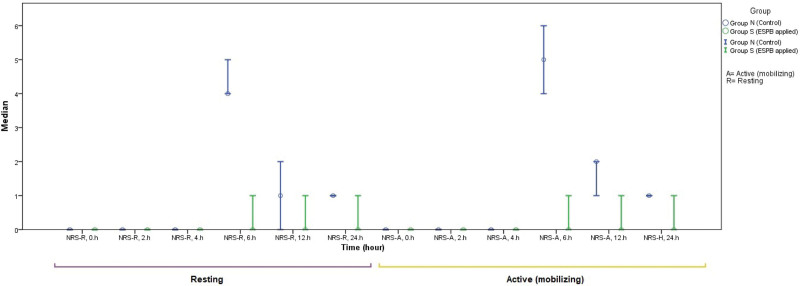
In comparison to the resting period, the NRS value at the 6th hour was found to be higher during active movement (IQR: 2 [0–8] vs 2 [0–7], and mean ranks: 16.53 vs 15.50, P < .001). Similarly, the NRS value at the 12th hour was also observed to be higher during active movement compared to the resting period (IQR: 2 [1, 0–6] vs 0 [0–5], P = .007) (Fig. 1).
Figure 1.
Summary of the distribution of NRS at different time intervals in both the resting and active periods (mobilizing). NRS = numeric rating scale.
In Group N, a notably higher proportion of patients needed postoperative rescue analgesia (90.3% vs 9.7%, P < .001) (Table 3). Furthermore, when the total amount of rescue tramadol consumption was compared during the initial 24 hours after surgery was significantly lower in Group S in comparison to Group N, 101.67 ± 2.89 mg and 109.5 ± 20.98 mg, respectively (P < .001) (Table 3).
Table 3.
Comparison of tramadol consumption as rescue analgesia.
| Parameter | Group N (control) (n = 35) | Group S (ESPB applied) (n = 35) | P |
|---|---|---|---|
| Mean ± SD (mg) | |||
| Total tramadol (dose) (mg) | 109.5 ± 20.98 | 101.67 ± 2.89 | <.001 * |
| Frequency (%) | |||
| Rescue analgesic required | |||
| Yes | 28 (90.3%) | 3 (9.7%) | <.001 * |
| No | 7 (17.9%) | 32 (82.1%) | |
ESPB = erector spinae plane block.
P < .001.
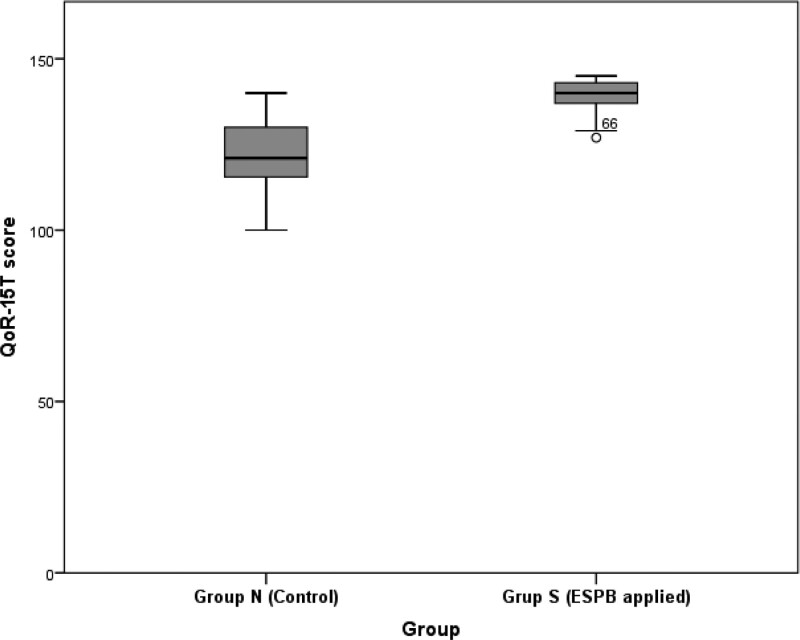
A significant difference between Groups N and S was observed in the QoR-15T scores; higher QoR-15T scores were recorded in Group S compared to Group N (IQR: 140 [127–145] vs 121 [100–140], P < .001) (Fig. 2).
Figure 2.
Comparison of QoR-15T scores by group and summary of distribution (P < .001). QoR-15T = quality of recovery-15 Turkish version.
4. Discussion
This randomized controlled study aimed to assess the impact of sacral ESPB on postoperative pain, analgesic consumption, and the quality of patient recovery following hemorrhoidectomy. The findings unequivocally reveal that implementing sacral ESPB after hemorrhoidectomy leads to a statistically significant reduction in postoperative pain among patients. Moreover, the sacral ESPB group exhibited a reduced demand for analgesics within 24 hours following the surgery.
Given the frequently encountered significant postoperative pain associated with this common surgical procedure, ensuring effective pain management remains paramount.[5,18] Pain following hemorrhoidectomy is typically most severe in the first 24 hours after surgery and then tends to decline gradually, starting from the second postoperative day onwards. Various techniques, including conventional analgesics, local perianal anesthesia, and pudendal nerve block, have been successfully used to control pain caused by hemorrhoidectomy.[19,20] Even though these techniques can effectively provide analgesia during hemorrhoidectomy, they may have potential adverse effects.[21] Despite being a recently introduced technique, the utilization of ESPB has gained widespread recognition and has been applied for postoperative pain management across various surgical procedures.[11] Sacral ESPB is a recently introduced modification of the technique originally described by Tulgar et al in 2019 for pilonidal cyst surgery targeting the posterior branches of the sacral nerves. Tulgar et al emphasized the incorporation of bilateral sacral ESPB as an integral component of a multimodal approach to postoperative pain control. In their study, the researchers administered a bilateral approach with an overall amount of 40 mL of local anesthetic in the interfascial space located between the erector spinae muscles and the intermediate sacral crest. They reported that patients who underwent sacral ESPB had an NRS score of less than 3 out of 10 for pain in the first 13 hours after surgery.[13] Roy et al investigated the use of bilateral ultrasound-guided sacral ESPB as a method of postoperative analgesia in 10 cases of perianal surgeries, including hemorrhoidectomy, minimally invasive procedure for hemorrhoid (MIPH), fistulectomy, anal fissure, etc. They reported that sacral ESPB provides adequate analgesia during the postoperative period as part of a multimodal analgesia regimen.[9] The existing literature on the effectiveness of sacral ESPB for perineal and anorectal surgeries primarily consists of case reports and small observational studies. This study represents the initial randomized controlled trial within the existing literature to apply sacral ESPB for pain management after hemorrhoidectomy. In our randomized controlled study, we applied 20 ml of local anesthetic mixture bilaterally and 40 ml of local anesthetic between the erector spinae muscles and the intermediate sacral crest. A significant difference in NRS scores was observed between the sacral ESPB and the control group, both at rest and during active periods (mobilizing). The NRS score was considerably lower than that of the control group. Compared with the sacral ESPB group, the NRS scores were significantly higher in the control group, particularly following the decline of the spinal anesthesia effect at the 6th hour after the surgery. The findings of this research underscore the efficacy of sacral ESPB in alleviating postoperative pain among patients undergoing hemorrhoidectomy. The low NRS scores in the sacral ESPB group demonstrate the potential benefits of incorporating this technique into a multimodal analgesic approach. Previous studies have shown positive results with the use of sacral ESPB for its bi-level use in various surgical procedures. Kaya et al applied bilateral sacral ESPB at the S2 and S4 levels for surgical anesthesia in ambulatory anorectal surgeries, and Kukreja et al[15] applied bilateral sacral ESPB at the S2 and S4 levels for a major gender reassignment surgery. In our study, we applied sacral ESPB from the bilateral S2 for postoperative pain management. This approach is similar to the research carried out by Roy et al[9] and Tulgar et al[13] who also performed sacral ESPB bilaterally at the S2 level for postoperative pain control. As a result of the application of sacral ESPB at the bilateral S2 level for hemorrhoidectomy, we achieved adequate postoperative pain control, and no complications related to sacral ESPB were observed or reported. Sacral ESPB demonstrates the potential for blockage as it can spread across multiple levels, enabling the blockage of the sacral nerves and the pudendal nerve (S2–S4), as well as the lumbar plexus through cephalad spread.[9]
Tramadol and NSAIDs are commonly used to treat hemorrhoidectomy pain. However, their application is often limited to a short period due to concerns about potential side effects. Tramadol exhibits adverse effects, such as drowsiness, nausea, constipation, and potential dependency or addiction.[22,23] Therefore, reducing the use of drugs, such as tramadol and NSAIDs, during the postoperative period can aid in minimizing their associated side effects. In the present study, patients who received sacral ESPB for postoperative analgesia required a lower dose of tramadol during the first 24 hours postoperatively. In addition, rescue analgesics were required in only 3 patients during the first 24 hours postoperatively from patients who had undergone sacral ESPB. However, among the total number of patients in the control group, 27 patients required rescue analgesics during the first 24 hours after surgery. It is important to reduce the need for rescue analgesics in the postoperative period, as it has several potential benefits, including reduced risk of side effects associated with these drugs and improved patient comfort.
Hemorrhoidectomy surgery necessitates profound anesthesia due to the multiple nerves’ complex innervation of this region. Local anesthesia and sedation techniques are more cost-effective and result in shorter recovery periods.[24] However, when a patient undergoes local anesthesia and sedation, there is an elevated risk of severe pain, often accompanied by symptoms such as tachypnea and laryngospasm.[25] General anesthesia techniques are linked to a greater likelihood of postoperative nausea and vomiting.[26] Efficient pain management within 24 hours post-surgery mitigates complications and enhances patient comfort. Although intrathecal morphine is frequently employed for postoperative analgesia following surgery, its usage is associated with potential risks and side effects, including nausea, vomiting, pruritus, urinary retention, and the possibility of respiratory depression.[27] As a consequence, we prioritize spinal anesthesia and have chosen not to incorporate opioids into the clinical practice of spinal anesthesia.
Quality of life is a measure of a patient’s health status following surgery. It encompasses 5 dimensions of health: physical comfort, physical independence, psychological support, emotional well-being, and pain management. The QoR-15T scale is commonly employed to assess the QoR of patients who underwent diverse surgical and anesthetic interventions. This scale is a shortened version of the 40-item QoR scale and assigns a score ranging from 0 to 150, with 150 corresponding to the best possible outcome.[28–30] Kara et al[31] developed the Turkish version of QoR-15T through a translation and cultural adaptation process to evaluate the validity, reliability, and responsiveness of the QoR-15T for Turkish patients. In this study, we employed the QoR-15T score to assess the quality of recovery after surgery. The outcomes of this study exhibited a notably higher QoR-15T score within the first 24 hours postoperatively in the sacral ESPB group compared with the control group. This finding indicates that patients undergoing sacral ESPB after hemorrhoidectomy experience better recovery quality, including improved physical comfort, reduced pain levels, and overall higher satisfaction with postoperative outcomes.
The present study exhibits some limitations. First, the study had a small sample size, which can affect the generalizability of the findings and limit the statistical power to detect significant differences. Second, the research was conducted in a single center, which may restrict the diversity and ability to generalize the results to different healthcare settings or populations. Finally, the short-term follow-up period in this study may not capture the long-term effects or sustainability of the analgesic effects of sacral ESPB.
5. Conclusion
This study investigated the analgesic efficacy of sacral ESPB compared to a control group in patients undergoing hemorrhoidectomy. The results demonstrated that the sacral ESPB was more effective in providing pain relief within the first 24 hours postoperatively. The sacral ESPB group had lower pain scores, lower analgesic consumption, and higher patient recovery quality compared with the control group.
Acknowledgments
The authors gratefully acknowledge all volunteers who participated in this study.
Author contributions
Conceptualization: Aydin Mermer, Yasin Tire.
Data curation: Aydin Mermer, Yasin Tire.
Formal analysis: Aydin Mermer.
Methodology: Aydin Mermer, Hasan Alp Mermer.
Supervision: Gurcan Simsek, Betül Kozanhan.
Writing – original draft: Aydin Mermer, Yasin Tire.
Writing – review & editing: Aydin Mermer.
Abbreviations:
- ASA
- American Society of Anesthesiologists
- ESPB
- erector spinae plane block
- IQR
- interquartile range
- NRS
- numeric rating scale
- NSAIDs
- nonsteroidal anti-inflammatory drugs
- QoR-15T
- quality of recovery-15 Turkish version
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Mermer A, Simsek G, Mermer HA, Tire Y, Kozanhan B. Effect of sacral erector spinae plane block on post-hemorrhoidectomy pain: A randomized controlled trial. Medicine 2023;102:37(e35168).
Contributor Information
Hasan Alp Mermer, Email: akifsenemli@gmail.com.
Yasin Tire, Email: dryasintire@hotmail.com.
Betül Kozanhan, Email: betulkozanhan@gmail.com.
References
- [1].Borges LA, da Cunha Leal P, Rey Moura EC, et al. Randomized clinical study on the analgesic effect of local infiltration versus spinal block for hemorrhoidectomy. Sao Paulo Med J. 2017;135:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nygren J, Thacker J, Carli F, et al.; Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin Nutr. 2012;31:801–16. [DOI] [PubMed] [Google Scholar]
- [3].Ferrandis C, De Faucal D, Fabreguette J-M, et al. Efficacy of Doppler-guided hemorrhoidal artery ligation with mucopexy, in the short and long terms for patients with hemorrhoidal disease. Tech Coloproctol. 2020;24:165–71. [DOI] [PubMed] [Google Scholar]
- [4].Gerbershagen HJ, Aduckathil S, van Wijck AJ, et al. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118:934–44. [DOI] [PubMed] [Google Scholar]
- [5].Medina-Gallardo A, Curbelo-Peña Y, De Castro X, et al. Is the severe pain after Milligan-Morgan hemorrhoidectomy still currently remaining a major postoperative problem despite being one of the oldest surgical techniques described? A case series of 117 consecutive patients. Int J Surg Case Rep. 2017;30:73–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Culpepper-Morgan JA, Inturrisi CE, Portenoy RK, et al. Treatment of opioid-induced constipation with oral naloxone: a pilot study. Clin Pharmacol. 1992;52:90–5. [DOI] [PubMed] [Google Scholar]
- [7].Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105. [PubMed] [Google Scholar]
- [8].Fowler P. Aspirin, paracetamol and non-steroidal anti-inflammatory drugs. Med Toxicol Adverse Drug Exp. 1987;2:338–66. [DOI] [PubMed] [Google Scholar]
- [9].Roy R, Agarwal G, Patel A, et al. Sacral multifidus plane block for post-operative analgesia in perianal procedures. J Clin Anesth. 2020;68:110060. [DOI] [PubMed] [Google Scholar]
- [10].Rajabi M, Hosseinpour M, Jalalvand F, et al. Ischiorectal block with bupivacaine for post hemorrhoidectomy pain. Korean J Pain. 2012;25:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth. 2016;41:621–27. [DOI] [PubMed] [Google Scholar]
- [12].De Cassai A, Bonvicini D, Correale C, et al. Erector spinae plane block: a systematic qualitative review. Minerva Anestesiol. 2019;85:308–19. [DOI] [PubMed] [Google Scholar]
- [13].Tulgar S, Senturk O, Thomas DT, et al. A new technique for sensory blockage of posterior branches of sacral nerves: ultrasound guided sacral erector spinae plane block. J Clin Anesth. 2019;57:129–30. [DOI] [PubMed] [Google Scholar]
- [14].Piraccini E, Antioco M, Maitan S. Ultrasound guided sacral erector spinae plane block: a useful tool for radicular pain treatment. J Clin Anesth. 2020;59:11–2. [DOI] [PubMed] [Google Scholar]
- [15].Kukreja P, Deichmann P, Selph JP, et al. Sacral erector spinae plane block for gender reassignment surgery. Cureus. 2020;12:7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kilicaslan A, Uyel Y. Novel lumbosacral approach for erector spinae plane block (LS-ESPB) in hip surgery. J Clin Anesth. 2019;60:83–4. [DOI] [PubMed] [Google Scholar]
- [17].Aksu C, Gürkan Y. Sacral erector spinae plane block with longitudinal midline approach: could it be the new era for pediatric postoperative analgesia? J Clin Anesth. 2019;59:38–9. [DOI] [PubMed] [Google Scholar]
- [18].Simillis C, Thoukididou S, Slesser A, et al. Systematic review and network meta-analysis comparing clinical outcomes and effectiveness of surgical treatments for haemorrhoids. J Br Surg. 2015;102:1603–18. [DOI] [PubMed] [Google Scholar]
- [19].Anannamcharoen S, Cheeranont P, Boonya-usadon C. Local perianal nerve block versus spinal block for closed hemorrhoidectomy: a ramdomized controlled trial. Med J Med Assoc Thai. 2008;91:1862. [PubMed] [Google Scholar]
- [20].Jinjil K, Dwivedi D, Bhatnagar V, et al. Perianal block: is it as good as spinal anesthesia for closed hemorrhoidectomies? Anesth Essays Res. 2018;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Morgan GE, Jr, Mikhail MS, Murray MJ. Pain management-pudendal nerve block. Lange Clin Anesthesiol. 2002;3:309–43. [Google Scholar]
- [22].Liu J-W, Lin C-C, Kiu K-T, et al. Effect of glyceryl trinitrate ointment on pain control after hemorrhoidectomy: a meta-analysis of randomized controlled trials. World J Surg. 2016;40:215–24. [DOI] [PubMed] [Google Scholar]
- [23].Ruiz-Castro M, San José Santos M, Rodríguez-Miguel A, et al. Intraspinal administration of morphine hydrochloride combined with low doses of bupivacaine in hemorrhoidectomy: a clinical randomized trial. Minerva Anestesiol. 2017;83:930–8. [DOI] [PubMed] [Google Scholar]
- [24].Kushwaha R, Hutchings W, Davies C, et al. Randomized clinical trial comparing day-care open haemorrhoidectomy under local versus general anaesthesia. J Br Surg. 2008;95:555–63. [DOI] [PubMed] [Google Scholar]
- [25].Proudfoot J. Analgesia, anesthesia, and conscious sedation. Emerg Med Clin North Am. 1995;13:357–79. [PubMed] [Google Scholar]
- [26].Apfel C, Kranke P, Eberhart L, et al. Comparison of predictive models for postoperative nausea and vomiting. Br J Anaesth. 2002;88:234–40. [DOI] [PubMed] [Google Scholar]
- [27].Gwirtz KH, Young JV, Byers RS, et al. The safety and efficacy of intrathecal opioid analgesia for acute postoperative pain: seven years’ experience with 5969 surgical patients at Indiana University Hospital. Anesth Analg. 1999;88:599–604. [DOI] [PubMed] [Google Scholar]
- [28].Chazapis M, Walker E, Rooms M, et al. Measuring quality of recovery-15 after day case surgery. Br J Anaesth. 2016;116:241–8. [DOI] [PubMed] [Google Scholar]
- [29].Finnerty DT, McMahon A, McNamara JR, et al. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery: a randomised clinical trial. Br J Anaesth. 2020;125:802–10. [DOI] [PubMed] [Google Scholar]
- [30].Lu J, Wang J-F, Guo C-L, et al. Intravenously injected lidocaine or magnesium improves the quality of early recovery after laparoscopic cholecystectomy: a randomised controlled trial. Eur J Anaesthesiol. 2021;38:S1–8. [DOI] [PubMed] [Google Scholar]
- [31].Kara U, Şimşek F, Kamburoğlu H, et al. Linguistic validation of a widely used recovery score: quality of recovery-15 (QoR-15). Turk J Med Sci. 2022;52:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]