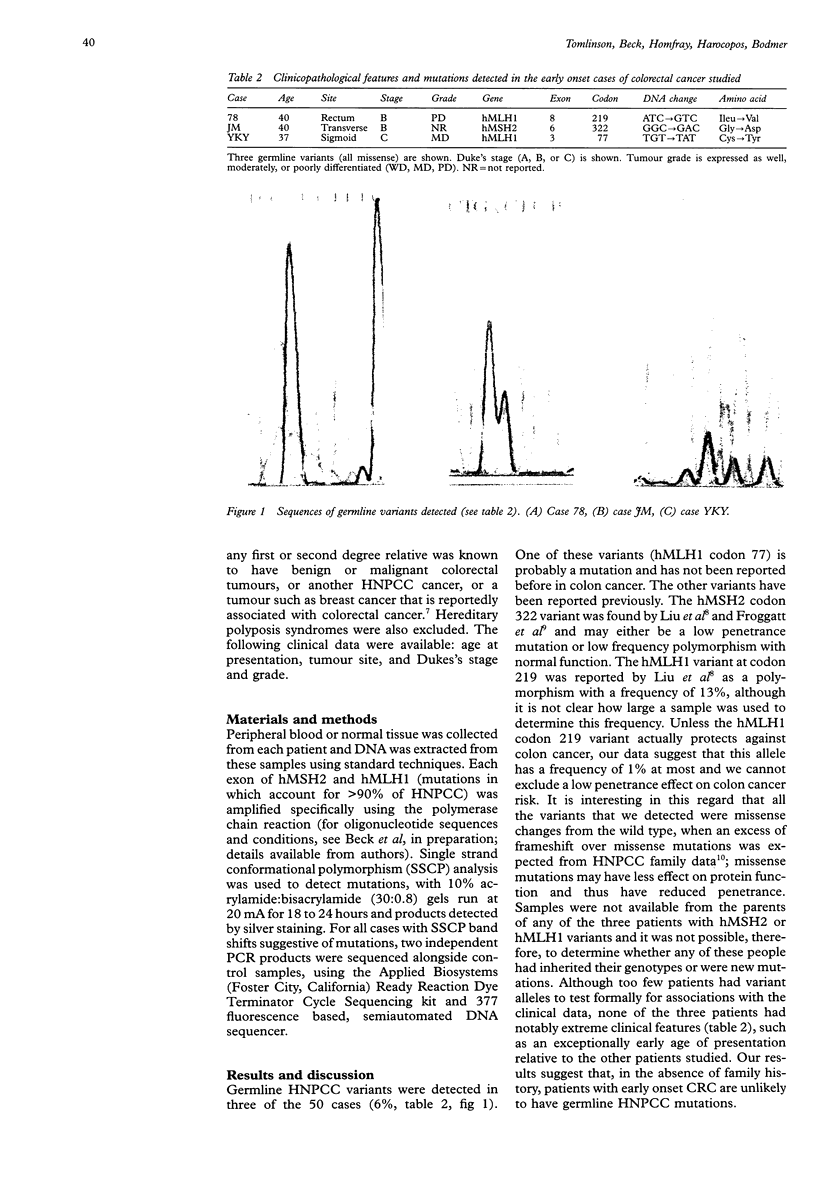
Abstract
Hereditary non-polyposis colorectal cancer (HNPCC) is a syndrome of inherited bowel and other cancers that has been said to account for up to 15% of all colorectal carcinomas (CRCs). HNPCC can now be diagnosed at the molecular level by detecting germline mutations in genes involved in mismatch repair. A current problem is to determine the prevalence of HNPCC mutations in colon cancer patients with limited or no family history, especially in cases of early onset. We have identified 50 cases of non-polyposis colorectal cancer without a family history of CRC or any other HNPCC cancer, who presented under the age of 45 years. Germline HNPCC variants (at the hMSH2 or hMLH1 loci) were detected in a small minority of cases (6%). The variants that we have found may be new or low penetrance mutations, or even polymorphisms. It remains possible that some of our sample have an inherited predisposition to CRC that is not caused by HNPCC mutations or by known polyposis syndromes. Our data suggest that most HNPCC mutations occur in families and have high or moderate penetrance. New or low penetrance HNPCC mutations probably do not contribute significantly to the risk of colorectal cancer in the general population and probably account for much fewer than 15% of all CRCs. Our results question whether mass population genetic screening programmes are worthwhile for diseases such as HNPCC using current technology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Ford D., Easton D. F., Bishop D. T., Narod S. A., Goldgar D. E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994 Mar 19;343(8899):692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Friedl W., Meuschel S., Caspari R., Lamberti C., Krieger S., Sengteller M., Propping P. Attenuated familial adenomatous polyposis due to a mutation in the 3' part of the APC gene. A clue for understanding the function of the APC protein. Hum Genet. 1996 May;97(5):579–584. doi: 10.1007/BF02281864. [DOI] [PubMed] [Google Scholar]
- Froggatt N. J., Joyce J. A., Evans D. G., Lunt P. W., Koch D. J., Ponder B. J., Maher E. R. MSH2 sequence variations and inherited colorectal cancer susceptibility. Eur J Cancer. 1996 Jan;32A(1):178–178. doi: 10.1016/0959-8049(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Hodgson S. V., Bishop D. T., Dunlop M. G., Evans D. G., Northover J. M. Suggested screening guidelines for familial colorectal cancer. J Med Screen. 1995;2(1):45–51. doi: 10.1177/096914139500200112. [DOI] [PubMed] [Google Scholar]
- Houlston R. S., Murday V., Harocopos C., Williams C. B., Slack J. Screening and genetic counselling for relatives of patients with colorectal cancer in a family cancer clinic. BMJ. 1990 Aug 18;301(6748):366–368. doi: 10.1136/bmj.301.6748.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Liu B., Farrington S. M., Petersen G. M., Hamilton S. R., Parsons R., Papadopoulos N., Fujiwara T., Jen J., Kinzler K. W., Wyllie A. H. Genetic instability occurs in the majority of young patients with colorectal cancer. Nat Med. 1995 Apr;1(4):348–352. doi: 10.1038/nm0495-348. [DOI] [PubMed] [Google Scholar]
- Liu B., Nicolaides N. C., Markowitz S., Willson J. K., Parsons R. E., Jen J., Papadopolous N., Peltomäki P., de la Chapelle A., Hamilton S. R. Mismatch repair gene defects in sporadic colorectal cancers with microsatellite instability. Nat Genet. 1995 Jan;9(1):48–55. doi: 10.1038/ng0195-48. [DOI] [PubMed] [Google Scholar]
- Liu B., Parsons R., Papadopoulos N., Nicolaides N. C., Lynch H. T., Watson P., Jass J. R., Dunlop M., Wyllie A., Peltomäki P. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996 Feb;2(2):169–174. doi: 10.1038/nm0296-169. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T., Lynch J. F. Overview of natural history, pathology, molecular genetics and management of HNPCC (Lynch Syndrome). Int J Cancer. 1996 Feb 20;69(1):38–43. doi: 10.1002/(SICI)1097-0215(19960220)69:1<38::AID-IJC9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Nicolaides N. C., Papadopoulos N., Liu B., Wei Y. F., Carter K. C., Ruben S. M., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994 Sep 1;371(6492):75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994 Mar 18;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- Plummer S. J., Casey G. Are we any closer to genetic testing for common malignancies? Nat Med. 1996 Feb;2(2):156–158. doi: 10.1038/nm0296-156. [DOI] [PubMed] [Google Scholar]
- Ravnik-Glavac M., Glavac D., Dean M. Sensitivity of single-strand conformation polymorphism and heteroduplex method for mutation detection in the cystic fibrosis gene. Hum Mol Genet. 1994 May;3(5):801–807. doi: 10.1093/hmg/3.5.801. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Beck J. S., Kwitek A. E., Sandstrom D. W., Stone E. M. The sensitivity of single-strand conformation polymorphism analysis for the detection of single base substitutions. Genomics. 1993 May;16(2):325–332. doi: 10.1006/geno.1993.1193. [DOI] [PubMed] [Google Scholar]
- Spirio L., Olschwang S., Groden J., Robertson M., Samowitz W., Joslyn G., Gelbert L., Thliveris A., Carlson M., Otterud B. Alleles of the APC gene: an attenuated form of familial polyposis. Cell. 1993 Dec 3;75(5):951–957. doi: 10.1016/0092-8674(93)90538-2. [DOI] [PubMed] [Google Scholar]
- Ushijima T., Hosoya Y., Suzuki T., Sofuni T., Sugimura T., Nagao M. A rapid method for detection of mutations in the lacI gene using PCR-single strand conformation polymorphism analysis: demonstration of its high sensitivity. Mutat Res. 1995 Jun;334(3):283–292. doi: 10.1016/0165-1161(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig A., Moller D. E. Comparative sensitivity of alternative single-strand conformation polymorphism (SSCP) methods. Biotechniques. 1994 Sep;17(3):490-2, 494, 496. [PubMed] [Google Scholar]