Abstract
Background:
Normobaric hyperoxia (NBH) and hyperbaric oxygen therapy (HBOT) are effective treatment plan for traumatic brain injury (TBI). The aim of this study was to compare cognitive outcome after mild TBI between NBH and HBOT so as to provide a more suitable treatment strategy for patients with mild TBI.
Methods:
A prospective research was conducted between October 2017 and March 2023, enrolling patients with mild TBI (Glasgow coma scale score: 13–15 points) within 24 hours of injury in Cangzhou Central Hospital. Patients were randomized into 3 groups: group control (C), group NBH and group HBOT. The patients in HBOT group received hyperbaric oxygen therapy in high pressure oxygen chamber and patients in NBH group received hyperbaric oxygen therapy. at 0 minute before NBH or HBOT (T1), 0 minute after NBH or HBOT (T2) and 30 days after NBH or HBOT (T3), level of S100β, NSE, GFAP, HIF-1α, and MDA were determined by ELISA. At the same time, the detection was performed for MoCA and MMSE scores, along with rSO2.
Results:
The results showed both NBH and HBOT could improve the score of MoCA and MMSE, as well as the decrease the level of S100β, NSE, GFAP, HIF-1α, MDA, and rSO2 compared with group C. Furthermore, the patients in group HBOT have higher score of MoCA and MMSE and lower level of S100β, NSE, GFAP, HIF-1α, MDA, and rSO2.
Conclusion:
Both NBH and HBOT can effectively improve cognitive outcome for patients with mild TBI by improving cerebral hypoxia and alleviating brain injury, while HBOT exert better effect than NBH.
Keywords: cerebral oxygen metabolism, cognitive outcome, hyperbaric oxygen, normobaric hyperoxia, traumatic brain injury
1. Introduction
In children and young adults, traumatic brain injury (TBI) is among the leading causes of morbidity and mortality.[1] The mortality rate associated with TBI in China ranged from 2.7% to 21.8%.[2] In the recent period of time, people under 45 years of age die and suffer disabilities more frequently from TBI than ever before. Those who suffer from TBI may suffer cognitive impairments that affect their quality of life, psychosocial outcomes, family functioning, and employment prospects.[3] In the aftermath of a TBI, a longer recovery time from cognitive dysfunction is predictive of a worse prognosis, so shortening the duration of cognitive impairment may improve outcomes.[4] There is a significant increase in the number of people reporting cognitive problems several years after a major head injury, and even patients who suffered a mild TBI may continue to suffer long-term cognitive dysfunction. Therefore, the cognitive impairment induced by TBI needs the attention of medical personnel, but there are currently no effective prevention and treatment measures.
Although the mechanism underlying the pathogenesis of TBI-induced cognitive impairment is still poorly understood, studies have revealed that cerebral oxygen metabolism disorder, hippocampal neuronal apoptosis, and neuroinflammation are closely related to the pathogenesis of it, and thus several measures have been investigated in the prevention of TBI-induced cognitive impairment in animal models and clinical patients.[5,6] Normobaric hyperoxia (NBH)[7] and hyperbaric oxygen therapy (HBOT)[8] are effective treatment plan for TBI. Numerous studies have confirmed that both NBH and HBOT can increase blood oxygen content and blood oxygen diffusion, improve brain tissue oxygen metabolism ability, reduce brain damage levels caused by hypoxia, and improve cognitive impairment in TBI. However, no study has been conducted to compare the cerebroprotection of NBH and HBOT.
Thus, the aim of this study was to compare effect of improvement on cognitive function after mild TBI between NBH and HBOT, which will provide theoretical basis and practical reference for clinical intervention and prevention and treatment strategies of complications in TBI.
2. Methods
This prospective, randomized, controlled trial has been approved by the Ethics Committee of Cangzhou Central Hospital (2017-025-01) and complies with the Helsinki Declaration. The subjects’ enrollment started in October 2017 and the study intervention completed in March 2023. Based on the previous study,[9–11] participants suffered from mild TBI [Glasgow coma scale score: 13–15 point] were recruited from the third department of Neurosurgery, Cangzhou Central Hospital and the nearby universities in the area after having signed an informed consent for participation in the trial.
2.1. Study population
The inclusion criteria included:
-
(1)
MRI and CT scans confirmed the diagnosis of TBI,
-
(2)
Regardless of gender, between the ages of 18 and 65,
-
(3)
Admission within 72 hours of injury, the condition remained stable and they were not affected by any significant language impairments in the hospital,
-
(4)
Be willing to sign an informed consent form and undergo a 1-month follow-up,
-
(5)
Neither primary consciousness disorder nor limb functional activity disorder was present,
The exclusion criteria included:
-
(1)
Accompanied by severe visual or hearing impairment, having difficulty communicating,
-
(2)
The patient has severe liver dysfunction, severe kidney dysfunction, severe infection, severe diabetes, inner ear disease and claustrophobia,
-
(3)
Contraindications to hyperbaric oxygen therapy such as untreated pneumothorax, multiple sternal fractures, open chest wall trauma, cavernous pulmonary tuberculosis, and retinal detachment,
-
(4)
Drugs that treat epilepsy or depression currently being taken,
-
(5)
Patients cannot complete all HBOT or NBH courses on time,
-
(6)
During the study period, secondary brain injuries, strokes, epilepsy, and hydrocephalus were observed, or need for surgery. The trail will be stopped if serious complications of the central nervous system occur during the experiment, putting the patient in a critical condition. Study participants were not allowed to smoke.
All enrolled participants were requested to maintain their daily routines/activities, lifestyles, and medications throughout the study.
2.2. Sample size estimation, randomization and blinding
In the current study, a sample size calculation was carried out using the G*Power program (V.3.1.9). The aim of this study was to evaluate the cognitive difference among 3 groups. It is thus suggested that the sample size should be 32 subjects per group with 80% power and a two-tailed error of 5%, based on preliminary experimental results.[12] The number of patients in each group had to be at least 35 because of the high dropout rate.
Based on the above results, one hundred and 10 individuals completed a baseline assessment and were randomly divided into 3 groups using the random number table method: control group (group C, n = 36), NBH group (n = 37) and HBOT group (n = 37). There was blinding of all patients to the group allocations. Physicians who carried out NBH or HBOT intervention, assessed, and analyzed the patients did not know anything about the groupings.
2.3. Study design and intervention
All patients were routinely treated for dehydration, prevention of infection, support, neuronutrition, and prevention of complications. On this basis, the patients in HBOT group received hyperbaric oxygen therapy in High pressure oxygen chamber based on the previous study.[13] Each hyperbaric oxygen therapy lasts for 135 minutes at 2.0 atmospheres absolute, including air pressurization 20 minutes, oxygen inhalation under stable pressure 40 minutes, 10-minutes rest, oxygen inhalation 40minutes, pressure reduction 25 minutes. For a total of 8 weeks, HBOT is administered once a day, 5 times a week. The patients in NBH group received hyperbaric oxygen therapy as previous described.[14] Briefly, patients receive 100% FiO2 oxygen for 3 hours at 1.0 atmospheres absolute, once a day for 8 weeks.
2.4. Sample collection and detection
Each group was given a 4ml blood sample at 0 min prior to hyperbaric oxygenation or hyperbaric oxygenation (T1), 0 min after hyperbaric oxygen therapy or hyperbaric oxygen therapy(T2) and 30 days after hyperbaric oxygen therapy or hyperbaric oxygen therapy (T3). Having been centrifuged at 3000 g for 15 minutes, the serum collected was stored at −80°C. In accordance with the manufacturer’s instructions, serum concentrations of S100β, neuron-specific enolase (NSE), glial fibrillary acidic protein (GFAP), hypoxiainducible factor-1α (HIF-1α), and malondialdehyde (MDA) were determined by ELISA.
At T1-3, rSO2 saturation was measured. A Cerebral oxygen saturation detector (Covidine II, USA) was used to measure cerebral oxygen saturation (rSO2). An electrode was placed on each side of the forehead, 4 cm away from the eyebrow arch. The mean of left and right rSO2 was recorded as the patients’ rSO2.
2.5. Cognitive function evaluation
An assessment of cognitive function was conducted using the Montreal Cognitive Function Assessment (MoCA)[15] and the mini-mental state examination (MMSE)[16] scale at T1-3. MoCA includes testing items in 8 cognitive domains: visual space and executive ability, naming, memory, attention, language, abstraction, delayed recall, and orientation. MoCA includes testing items in 8 cognitive domains: visual space and executive ability, naming, memory, attention, language, abstraction, delayed recall, and orientation. MoCA has a total score of 30 points, with a score of generally greater than or equal to 26 being normal, a score of 18 to 26 being mild cognitive impairment, a score of 10 to 17 being moderate cognitive impairment, and a score of less than 10 being severe cognitive impairment. MMSE includes 7 aspects and 30 questions: time orientation, location orientation, immediate memory, attention and computational power, delayed memory, language, and visual space. Generally, a score greater than or equal to 26 is considered normal, a score of 21 to 26 is considered mild cognitive impairment, a score of 10 to 20 is considered moderate cognitive impairment, and a score less than 10 is considered severe cognitive impairment.
2.6. Statistical analyses
Data present as group means ± standard deviation of the means. Baseline characteristics of the participants among the different groups were compared using one-factor analysis of variance (ANOVA) for continuous variables and Chi-square test for categorical variables. Paired t test or Wilcoxon signed-rank test (if the data failed a normality test) was conducted to determine a significance within-group changes before (i.e., baseline) vs after intervention. In addition, the intervention-induced relative change (%) from the baseline was analyzed using one-factor ANOVA to determine the difference in the changes among the groups. P < .05 was regarded as statistically significant. Statistical analysis of all experimental data were performed using SPSS 21.0 software (SPSS, Inc., Chicago, IL).
3. Results
3.1. Demographic characters of patients
Flow diagram of study participants illustrates the enrollment of study participants. One hundred and forty-nine individuals were evaluated for eligibility and signed informed consent form. Thirty-nine of them were excluded because of not meeting inclusion criteria and declining to participate. Total 3 participants missed the therapy during intervention in group NBH and group HBOT. During the follow-up process, 2 patients in Group C were forced to terminate the study due to early discharge. During the analysis process, one patient’s blood sample was contaminated and could not be analyzed in group NBH. So a final population sample of 104 participants was completed the study.
Table 1 summarizes the participants’ age, gender, body mass index, education years, Glasgow coma scale score, time from injury to admission, as well as the cause of injury. None of these characteristics was statistically different among the groups.
Table 1.
Characteristics of the study participants.
| Characteristics | Group C (n = 36) | Group NBH (n = 37) | Group HBOT (n = 37) | P value |
|---|---|---|---|---|
| Age (yr) | 53.6 ± 5.7 | 51.6 ± 5.9 | 50.9 ± 5.3 | .389 |
| Gender | ||||
| Male [(n) %] | 17 (47.22%) | 16 (43.24%) | 18 (48.65%) | .763 |
| Female [(n) %] | 19 (52.78%) | 21 (56.76%) | 19 (51.35%) | |
| BMI (kg/m2) | 24.7 ± 2.4 | 23.9 ± 2.2 | 24.2 ± 2.5 | .445 |
| Years of education (yr) | 7.7 ± 2.3 | 7.9 ± 2.5 | 8.2 ± 2.6 | .531 |
| GCS score | 14.2 ± 1.2 | 14.4 ± 1.4 | 13.9 ± 1.3 | .506 |
| Time from injury to admission (days) | 7.4 ± 2.03 | 8.1 ± 2.3 | 8.2 ± 2.4 | .372 |
| Cause of injury | ||||
| Car accident injury [(n) %] | 17 (47.2%) | 15 (40.5%) | 16 (43.2%) | .501 |
| Falling injury [(n) %] | 10 (27.8%) | 12 (32.4%) | 11 (29.7%) | .263 |
| Hit injury [(n) %] | 5 (13.9%) | 6 (16.2%) | 5 (13.5%) | .408 |
| Other reason [(n) %] | 4 (11.1%) | 4 (10.8%) | 5 (13.5%) | .534 |
BMI = body mass index, GCS = Glasgow Coma Scale, TBI = traumatic brain injury.
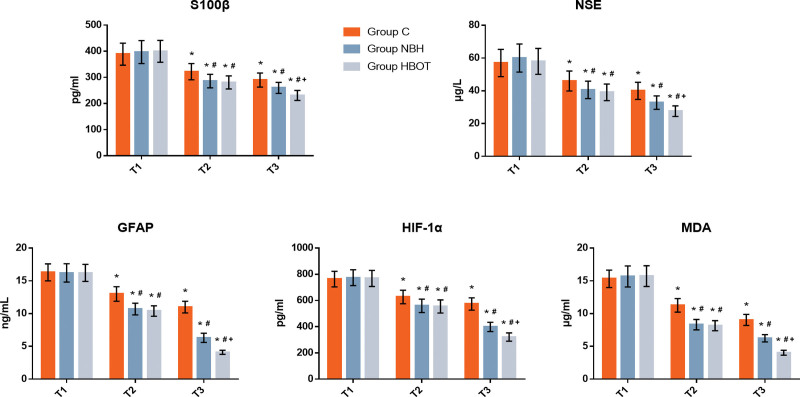
3.2. S100β, NSE, GFAP, HIF-1α, and MDA levels
Figure 1 illustrates NSE, GFAP, HIF-1α, and MDA levels before and after the intervention., there was no significant difference in baseline level of NSE, GFAP, HIF-1α and MDA among the 3 groups at T1 suggested by two-factor ANOVA (P > .05). Compared with the baseline at T1, the level of S100β, NSE, GFAP, HIF-1α, and MDA in 3 groups were significantly decreased at T2-3 (P < .05). Compared with group C, the level of S100β, NSE, GFAP, HIF-1α, and MDA in group NBH and HBOT at T2-3 were significantly decreased (P < .05). Compared with group NBH, the level of S100β, NSE, GFAP, HIF-1α, and MDA in group HBOT were significantly decreased at T3 (P < .05), while there was no statistical differences between 2 groups at T2 (P > .05).
Figure 1.
Concentrations of serum NSE (A), GFAP (B), HIF-1α (C), and MDA (D). Compared with T1, *P < .05; Compared with Group C, #P < .05; Compared with Group NBH, +P < .05. GFAP = glial fibrillary acidic protein, HIF-1α = Hypoxiainducible factor-1α, MDA = malondialdehyde, NBH = normobaric hyperoxia, NSE = neuron-specific enolase.
3.3. Cerebral oxygen saturation
Table 2 presents the cerebral oxygen saturation level between groups before and after the intervention. In all 3 groups, rSO2 fluctuated within normal limits throughout the observation. Compared with the baseline at T1, the values of rSO2 on two sides in 3 groups were increased at T2-3 (P < .05). Compared with group C, the values of rSO2 on two sides in group NBH and HBOT at T2-3 were significantly increased (P < .05). Compared with group NBH, the values of rSO2 on two sides in group HBOT were significantly increased at T3 (P < .05), while there was no statistical differences between 2 groups at T2 (P > .05).
Table 2.
Comparison of cerebral oxygen saturation (χ ± s).
| Time | Group C (n = 36) | Group NBH (n = 37) | Group HBOT (n = 37) | P value |
|---|---|---|---|---|
| T1 | 58.3 ± 5.9 | 59.4 ± 6.2 | 58.8 ± 6.1 | .524 |
| T2 | 64.5 ± 6.1* | 73.9 ± 7*,† | 75.2 ± 6.9*,† | .024 |
| T3 | 74.1 ± 7.1* | 80.1 ± 7.6*,† | 85.6 ± 7.9*,†,‡ | <.001 |
T1: 0 min prior to hyperbaric oxygenation or hyperbaric oxygenation, T2: 0 min after hyperbaric oxygen therapy or hyperbaric oxygen therapy, T3: 30 days after hyperbaric oxygen therapy or hyperbaric oxygen therapy.
HBOT = hyperbaric oxygen therapy, NBH = normobaric hyperoxia.
Compared with T1, P < .05.
Compared with Group C, P < .05.
Compared with Group NBH, P < .05.
3.4. Cognitive function
As shown in Table 3, the score of MoCA and MMSE increased at T2 and T3 in 3 groups compared with the baseline at T1 (P < .05). Compared with, group C, the score of MoCA and MMSE in group NBH and HBOT at T2-3 were significantly increased (P < .05). Compared with group NBH, the score of MoCA and MMSE in group HBOT were significantly increased at T3 (P < .05), while there was no statistical differences between 2 groups at T2 (P > .05).
Table 3.
Comparison of cognitive function score (χ ± s).
| Time | Group C (n = 36) | Group NBH (n = 37) | Group HBOT (n = 37) | P value | |
|---|---|---|---|---|---|
| T1 | MoCA | 15.1 ± 1.8 | 15.4 ± 2.0 | 15.5 ± 2.1 | .361 |
| MMSE | 16.7 ± 2.4 | 16.9 ± 2.6 | 16.7 ± 2.5 | .544 | |
| T2 | MoCA | 18.4 ± 2.0* | 21.3 ± 2.3*,† | 21.7 ± 2.4*,† | .012 |
| MMSE | 18.9 ± 2.4* | 21.5 ± 2.8*,† | 21.9 ± 2.8*,† | .008 | |
| T3 | MoCA | 19.9 ± 2.6* | 21.9 ± 2.3*,† | 24.9 ± 3.0*,†‡ | <.001 |
| MMSE | 20.4 ± 2.7* | 22.3 ± 2.4*,† | 25.3 ± 3.1*,†,‡ | <.001 |
T1: 0 min prior to hyperbaric oxygenation or hyperbaric oxygenation, T2: 0 min after hyperbaric oxygen therapy or hyperbaric oxygen therapy, T3: 30 days after hyperbaric oxygen therapy or hyperbaric oxygen therapy.
HBOT = hyperbaric oxygen therapy, MoCA = Montreal Cognitive Function Assessment; MMSE = mini-mental state examination, NBH = normobaric hyperoxia.
Compared with T1, P < .05.
Compared with Group C, P < .05.
Compared with Group NBH, P < .05.
4. Discussion
TBI has a high incidence rate, especially in contemporary societies with highly developed industries and economies. TBI has many sequelae and a high disability and mortality rate, which not only brings great pain to patients and their families, but also imposes a heavy burden on society. Cognitive impairment is one of the most common and persistent complications of TBI. In this study, we compare effect of improvement on cognitive function after mild TBI between NBH and HBOT so as to provide theoretical basis and practical reference for clinical intervention and prevention and treatment strategies of complications in TBI. The results of the current showed that NBH and HBOT can improve the cognitive outcome for patients with mild TBI by improving cerebral hypoxia and alleviating brain injury which is consistent with previous research,[12,14] while, HBOT exert better effect than NBH.
Structural damage to brain tissue[17] and increased levels of oxidative stress[18] are key factors in TBI induced cognitive impairment. For the higher cognitive function of the brain, cognitive impairment will occur in multiple parts of either side, especially in functional areas such as frontal lobe and hippocampus.[19] Studies have shown that the cognitive impairment is more severe, especially the damage in the frontal lobe. Executive functions in the frontal lobe include abstract ability, concept formation, selective memory, and cognitive transfer processes. Patients with frontal lobe brain damage, especially the left dorsal frontal lobe damage, have significantly impaired cognitive function, difficult to organize and implement planning, difficult to handle and solve problems, and difficult to correct errors.[20] The frontal lobe has a corresponding and responsible area for purposeful active memory (including short and long-term memory, as well as memory of words, objects and spatial relationships). Some scholars believe that the left paraventricular frontal lobe is closely related to thinking and emotion, while the right paraventricular frontal lobe is closely related to language and spatial memory functions.[21,22] Therefore, after frontal lobe damage caused by trauma or cerebrovascular disease, patients mainly exhibit language dysfunction, mental disorders, and ataxia.[23–25] Oxidative stress damage may also be an important pathogenesis in the pathological process of TBI. TBI-induced cognitive impairment is associated with oxidative stress, which produces reactive oxygen species and reactive nitrogen species. As a result of disequilibrium between the biochemical processes that produce reactive oxygen species, and the enzymatic processes that remove them, oxidative stress may occur.[26] As a result of mitochondrial dysfunction, breakdown of the blood-brain barrier, sensory-motor dysfunction, and secondary neuronal dysfunction, oxidative stress can lead to brain edema.[27] Animal experimental studies have found that oxidative stress damage to hippocampal neurons in rats is an important cause of cognitive dysfunction, and hippocampal CA1 functional damage is the most typical and severe site of cognitive decline in mice.[28] Preclinical and clinical studies have shown that mitochondrial targeted antioxidants can reduce oxidative stress and alleviate permanent cognitive impairment.[29]
Researchers have explored many related preventive measures to address the important role of increased levels of oxidative stress in TBI induced cognitive impairment. Oxygen therapy refers to the use of various methods to transport oxygen-containing substances to the human body, increase dissolved oxygen in plasma, improve tissue oxygen supply, and prevent or correct hypoxemia. NBH and HBOT are the 2 most commonly used oxygen therapy methods in clinical practice. In China, HBOT is widely used for traumatic brain injury. HBOTt has neuroprotective effects on TBI patients, improving consciousness, cognitive impairment, prognosis, and reducing mortality. This conclusion has also been included in the “Consensus of Chinese Experts on Neurocritical Rehabilitation.” HBOT involves placing TBI patients in an environment that significantly increases oxygen uptake, resulting in an increase in blood oxygen concentration and thus increased O2 diffusion.[30] HBOH treatment has been shown to improve neurological function in TBI patients.[31] In TBI patients, it promotes angiogenesis, improves brain metabolism, and preserves the function of newly born and regenerated neurons, especially memory impairment, attention impairment, and executive dysfunction.[32]
NBH therapy is applied earlier than HBOT, has lower environmental requirements for implementation, and is more easily accepted by patients.[33] It has high application value in the early prehospital emergency treatment of TBI and some primary hospitals with limited medical conditions. Compared with hyperbaric oxygen therapy, it has the advantages of simple equipment requirements, noninvasive, and can be used for early treatment. Especially in the battlefield treatment, disaster area rescue, field rescue and other aspects have a great application prospects. After TBI, brain tissues are damaged, internal environments are disturbed, and inflammation occurs, which requires more energy supply than is normal to repair them. Under normal conditions, brain tissue absorbs a large amount of oxygen per unit of blood. Oxygen uptake rate per unit volume of blood remains low after injury and hypoxia. As a result of injury, blood vessels rupture and contract, which reduces cerebral blood flow and affects oxygen utilization. The above factors cause a serious imbalance in the supply and demand of brain oxygen. NBO treatment has been shown to significantly increase local oxygen partial pressure and enhance brain tissue oxygen supply.[34,35] A prospective clinical study by Rockswold et al demonstrated that the NBO group increased oxygen partial pressure by (86 ± 12) mm Hg compared to the normal control group, significantly improving the patient’s aerobic metabolism level and prognosis.[36] In addition, NBH treatment can also improve mitochondrial function,[34] reduce intracranial pressure,[37] and improve the internal environment of damaged brain tissue in TBI patients. Although numerous studies have confirmed that both HBOT and NBH can effectively improve cognitive impairment caused by TBI, there are currently no scholars who have compared the effects of the two.
The S100β protein and GFAP are highly specific relative to the brain injury. S100β protein is a class of acidic calcium binding proteins with small molecular weight (21 kD) widely distributed in different tissues. S100β protein is elevated in serum and cerebrospinal fluid during brain injury and ischemia. It may be related to the destruction of glial cells and the blood-brain barrier. According to Woertgen et al, the early plasma S100B protein peak exceeding 2.0 μg/L or the secondary increase exceeding 2.0 μg/L, often indicating severe craniocerebral injury. Often accompanied by higher case fatality rates and disability rates.[38] In the central nervous system, GFAP is mainly found in astrocytes and participates in cytoske formation. In TBI, blood GFAP levels are correlated with clinical severity and extent of intracranial pathology.[39] NSE is a glycolytic enzyme mainly expressed in the cytoplasm of neurons, and serum NSE levels still have high clinical value in evaluating the therapeutic effect and prognosis of TBI.[40] HIF-1α is a transcription factor closely associated with hypoxia, and MDA is the end product of free radical and lipid peroxidation. They are all representative indicators of TBI oxidative stress levels. In the current study, we found NBH and HBOT could improve brain oxygen metabolism rate, reduce oxidative stress levels, improve TBI induced brain structural damage and cognitive function. The MMSE was developed by Folsteill et al in 1975,[41] and it has become the world’s most influential, popular, and commonly used cognitive screening tool. Due to its simple operation, short time consumption, and highest clinical acceptance, MMSE is often used as a tool for evaluating and screening cognitive function. MoCA is an assessment tool used for rapid screening of cognitive dysfunction, which includes 11 examination items in 8 cognitive domain. Previous study have confirmed that MOCA has good sensitivity and specificity for TBI patients with Mild cognitive impairment.[42] After 8 weeks of intervention treatment, there was no significant difference in MMSE scores between the two. However, during the follow-up process after 30 days, patients in the HBOT group showed better cognitive and cerebral oxygen metabolism levels, indicating that HBOT is more beneficial for cognitive recovery in mild TBI patients compared to NBH.
A main limitation of the study is the follow-up period for this study is only 30 days, and longer follow-up periods are necessary to evaluate the effectiveness of NBH or HBOT on cognitive recovery for patients with mild TBI. In addition, the results of the current study would be tested and verified by a multicenter randomized controlled clinical trials in the future to make the results more clinically instructive.
5. Conclusion
Taken together, both NBH and HBOT can effectively improve cognitive outcome for patients with mild TBI by improving cerebral hypoxia and alleviating brain injury, while HBOT exert better effect than NBH.
Acknowledgments
We thank Professor Li Zhang from School of Mathematics and Statistics, Cangzhou Normal University for his guidance on data statistics processing and “Home for Researchers” for its great help on manuscript polishing and grammar error checking.
Author contributions
Conceptualization: Zhiguo Liu.
Data curation: Xirui Wang.
Formal analysis: Xirui Wang, Zhiyou Wu, Haibin Chu.
Investigation: Zhiguo Liu, Zhiyou Wu.
Methodology: Zhiguo Liu, Zhiyou Wu.
Resources: Gangfeng Yin.
Software: Haibin Chu, Pengyue Zhao.
Validation: Pengyue Zhao.
Writing – review & editing: Gangfeng Yin.
Abbreviations:
- ANOVA
- one-factor analysis of variance
- GFAP
- glial fibrillary acidic protein
- HBOT
- hyperbaric oxygen therapy
- HIF-1α
- hypoxiainducible factor-1α
- MDA
- malondialdehyde
- MMSE
- mini-mental state examination
- MoCA
- Montreal Cognitive Function Assessment
- NBH
- normobaric hyperoxia
- NSE
- neuron-specific enolase
- rSO2
- cerebral oxygen saturation
- TBI
- traumatic brain injury
This study was approved by the institutional review board of the Cangzhou Central Hospital (2017-025-01) in compliance with the declaration of Helsinki.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Liu Z, Wang X, Wu Z, Yin G, Chu H, Zhao P. HBOT has a better cognitive outcome than NBH for patients with mild traumatic brain injury: A randomized controlled clinical trial. Medicine 2023;102:37(e35215).
Contributor Information
Xirui Wang, Email: 4682036970@qq.com.
Zhiyou Wu, Email: wzy13303063269@126.com.
Gangfeng Yin, Email: yingf1968@163.com.
Haibin Chu, Email: dcochb@163.com.
Pengyue Zhao, Email: 365113207@qq.com.
References
- [1].Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–41. [DOI] [PubMed] [Google Scholar]
- [2].Li Y, Gu J, Zhou J, et al. The epidemiology of traumatic brain injury in civilian inpatients of Chinese Military Hospitals, 2001-2007. Brain Inj. 2015;29:981–8. [DOI] [PubMed] [Google Scholar]
- [3].Jiang JY, Gao GY, Feng JF, et al. Traumatic brain injury in China. Lancet Neurol. 2019;18:286–95. [DOI] [PubMed] [Google Scholar]
- [4].Liu J, Xue X, Wu Y, et al. Efficacy and safety of electro-acupuncture treatment in improving the consciousness of patients with traumatic brain injury: study protocol for a randomized controlled trial. Trials. 2018;19:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Khellaf A, Khan DZ, Helmy A. Recent advances in traumatic brain injury. J Neurol. 2019;266:2878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alouani AT, Elfouly T. Traumatic Brain Injury (TBI) detection: past, present, and future. Biomedicines. 2022;10:2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wijayatilake DS, Jigajinni SV, Sherren PB. Traumatic brain injury: physiological targets for clinical practice in the prehospital setting and on the Neuro-ICU. Curr Opin Anaesthesiol. 2015;28:517–24. [DOI] [PubMed] [Google Scholar]
- [8].Chen Y, Wang L, You W, et al. Hyperbaric oxygen therapy promotes consciousness, cognitive function, and prognosis recovery in patients following traumatic brain injury through various pathways. Front Neurol. 2022;13:929386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yuh EL, Jain S, Sun X, et al.; TRACK-TBI Investigators for the CENTER-TBI Investigators. Pathological computed tomography features associated with adverse outcomes after mild traumatic brain injury: a track-TBI study with external validation in center-TBI. JAMA Neurol. 2021;78:1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roberts I, Shakur-Still H, Aeron-Thomas A, et al. Tranexamic acid to reduce head injury death in people with traumatic brain injury: the CRASH-3 international RCT. Health Technol Assess. 2021;25:1–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jia H, Chen Y, Wang Y, et al. The neuroprotective effect of electro-acupuncture on cognitive recovery for patients with mild traumatic brain injury: a randomized controlled clinical trial. Medicine (Baltimore). 2023;102:e32885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ablin JN, Lang E, Catalogna M, et al. Hyperbaric oxygen therapy compared to pharmacological intervention in fibromyalgia patients following traumatic brain injury: a randomized, controlled trial. PLoS One. 2023;18:e0282406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harch PG, Andrews SR, Fogarty EF, et al. A phase I study of low-pressure hyperbaric oxygen therapy for blast-induced post-concussion syndrome and post-traumatic stress disorder. J Neurotrauma. 2012;29:168–85. [DOI] [PubMed] [Google Scholar]
- [14].Rockswold SB, Rockswold GL, Zaun DA, et al. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J Neurosurg. 2010;112:1080–94. [DOI] [PubMed] [Google Scholar]
- [15].Hobson J. The montreal cognitive assessment (MoCA). Occup Med (Lond). 2015;65:764–5. [DOI] [PubMed] [Google Scholar]
- [16].Li H, Jia J, Yang Z. Mini-Mental state examination in elderly chinese: a population-based normative study. J Alzheimers Dis. 2016;53:487–96. [DOI] [PubMed] [Google Scholar]
- [17].Thapa K, Khan H, Singh TG, et al. Traumatic brain injury: mechanistic insight on pathophysiology and potential therapeutic targets. J Mol Neurosci. 2021;71:1725–42. [DOI] [PubMed] [Google Scholar]
- [18].Fesharaki-Zadeh A. Oxidative stress in traumatic brain injury. Int J Mol Sci. 2022;23:13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Komoltsev I, Shalneva D, Kostyunina O, et al. Delayed TBI-induced neuronal death in the ipsilateral hippocampus and behavioral deficits in rats: influence of corticosterone-dependent survivorship bias? Int J Mol Sci. 2023;24:4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Howlett JR, Nelson LD, Stein MB. Mental health consequences of traumatic brain injury. Biol Psychiatry. 2022;91:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. 2013;28:1483–91. [DOI] [PubMed] [Google Scholar]
- [22].Friederici AD. The brain basis of language processing: from structure to function. Physiol Rev. 2011;91:1357–92. [DOI] [PubMed] [Google Scholar]
- [23].Nolan AL, Sohal VS, Rosi S. Selective inhibitory circuit dysfunction after chronic frontal lobe contusion. J Neurosci. 2022;42:5361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bernier RA, Hillary FG. Traumatic brain injury and frontal lobe plasticity. Handb Clin Neurol. 2019;163:411–31. [DOI] [PubMed] [Google Scholar]
- [25].Weber E, Spirou A, Chiaravalloti N, et al. Impact of frontal neurobehavioral symptoms on employment in individuals with TBI. Rehabil Psychol. 2018;63:383–91. [DOI] [PubMed] [Google Scholar]
- [26].Frati A, Cerretani D, Fiaschi AI, et al. Diffuse axonal injury and oxidative stress: a comprehensive review. Int J Mol Sci. 2017;18:2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lu XY, Wang HD, Xu JG, et al. NADPH oxidase inhibition improves neurological outcome in experimental traumatic brain injury. Neurochem Int. 2014;69:14–9. [DOI] [PubMed] [Google Scholar]
- [28].Rehman SU, Ikram M, Ullah N, et al. Neurological enhancement effects of melatonin against brain Injury-induced oxidative stress, neuroinflammation, and neurodegeneration via AMPK/CREB signaling. Cells. 2019;8:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hakiminia B, Alikiaii B, Khorvash F, et al. Oxidative stress and mitochondrial dysfunction following traumatic brain injury: From mechanistic view to targeted therapeutic opportunities. Fundam Clin Pharmacol. 2022;36:612–62. [DOI] [PubMed] [Google Scholar]
- [30].Rockswold SB, Rockswold GL, Defillo A. Hyperbaric oxygen in traumatic brain injury. Neurol Res. 2007;29:162–72. [DOI] [PubMed] [Google Scholar]
- [31].Lu Y, Zhou X, Cheng J, et al. Early intensified rehabilitation training with hyperbaric oxygen therapy improves functional disorders and prognosis of patients with traumatic brain injury. Adv Wound Care (New Rochelle). 2021;10:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hadanny A, Efrati S. Editorial: Hyperbaric oxygen and the brain. Front Neurol. 2022;13:1078544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Daly S, Thorpe M, Rockswold S, et al. Hyperbaric oxygen therapy in the treatment of acute severe traumatic brain injury: a systematic review. J Neurotrauma. 2018;35:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sahoo S, Sheshadri V, Sriganesh K, et al. Effect of hyperoxia on cerebral blood flow velocity and regional oxygen saturation in patients operated on for severe traumatic brain injury-the influence of cerebral blood flow autoregulation. World Neurosurg. 2017;98:211–6. [DOI] [PubMed] [Google Scholar]
- [35].Puccio AM, Hoffman LA, Bayir H, et al. Effect of short periods of normobaric hyperoxia on local brain tissue oxygenation and cerebrospinal fluid oxidative stress markers in severe traumatic brain injury. J Neurotrauma. 2009;26:1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rockswold SB, Rockswold GL, Zaun DA, et al. A prospective, randomized phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injury. J Neurosurg. 2013;118:1317–28. [DOI] [PubMed] [Google Scholar]
- [37].Vilalta A, Sahuquillo J, Merino MA, et al. Normobaric hyperoxia in traumatic brain injury: does brain metabolic state influence the response to hyperoxic challenge? J Neurotrauma. 2011;28:1139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Agoston DV, Shutes-David A, Peskind ER. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017;31:1195–203. [DOI] [PubMed] [Google Scholar]
- [39].Abdelhak A, Foschi M, Abu-Rumeileh S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022;18:158–72. [DOI] [PubMed] [Google Scholar]
- [40].Mercier E, Tardif PA, Cameron PA, et al. Prognostic value of neuron-specific enolase (NSE) for prediction of post-concussion symptoms following a mild traumatic brain injury: a systematic review. Brain Inj. 2018;32:29–40. [DOI] [PubMed] [Google Scholar]
- [41].Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–35. [DOI] [PubMed] [Google Scholar]
- [42].Smith T, Gildeh N, Holmes C. The montreal cognitive assessment: validity and utility in a memory clinic setting. Can J Psychiatry. 2007;52:329–32. [DOI] [PubMed] [Google Scholar]