Abstract
Objective
This article is a preliminary study to compare the ability of 0.05% chlorhexidine diacetate (CD) and 1% povidone‐iodine (PI) solutions to reduce bacterial contamination on the canine external ear canal during initial patient preparation and comparison of the incidence of immediate tissue reactions.
Study design
The study is a multi‐institutional, randomised, clinical prospective study.
Animals or sample population
Dogs (n = 19) undergoing total ear canal ablation with bulla osteotomy (TECABO).
Methods
The external ear of each dog was cleaned with the assigned antiseptic solution. Culture of the ear was performed by standard techniques to semi‐quantitatively evaluate bacterial growth and to identify bacterial organisms pre‐ and post‐antiseptic use.
Results
Both antiseptic groups showed a significant reduction in bacterial growth score (BGS) between pre‐ and post‐antiseptic use (CD p = 0.009, PI p = 0.005). There was no difference in the reduction of BGS between CD and PI solutions (p = 0.53). Minor adverse skin reactions occurred in 25% of cases. There was no significant difference in the occurrence of adverse skin reactions between antiseptics (p = 0.63).
Conclusion
CD and PI were similarly able to decrease the number of bacteria on the external ear following initial preparation. No difference in the incidence of adverse tissue reactions was found.
Clinical significance
Properly diluted aqueous formulations of either antiseptic may be used for safe preparation limited to the external ear canal of dogs. Additional studies evaluating outcomes such as duration of bacterial inhibition and incidence of surgical site infections are needed to fully elucidate differences between CD and PI antiseptics prior to TECABO.
Keywords: antimicrobials, bacterial, soft tissue, surgery
The effectiveness of dilute chlorhexidine diacetate and povidone‐iodine in preparation of the canine ear prior to total ear canal ablation and bulla osteotomy was compared. There was no difference in bacterial load reduction or incidence of adverse tissue reactions. The authors conclude that properly diluted aqueous formulations of either antiseptic may be used for safe preparation limited to the external ear canal of dogs.

1. INTRODUCTION
The total ear canal ablation with bulla osteotomy (TECABO) procedure is indicated for the removal of neoplasms, extensive benign disease and, most commonly, chronic otitis affecting the external and/or middle ear cavity (White & Pomeroy, 1990). TECABO is often classified as a dirty procedure due to the presence of active bacterial infection encountered at the time of surgery. Infections are typically of mixed organisms and, potentially, multi‐drug resistant bacteria, likely due to prior treatment with antimicrobials (Guedeja‐Marrón et al., 1998; Penna et al., 2010).
Surgical site infections (SSIs) remain a major concern in human and veterinary medicine, and their incidence is related to the surgical wound class and contamination of the surgical site (Eugster et al., 2004; Garibaldi et al., 1991; Vasseur et al., 1988). Not surprisingly, most studies have identified incisional infections occurring in up to 10.7% of TECABO cases in the short‐ and long‐term postoperative period (Beckman et al., 1990; Guillamumot et al., 2011; Mason et al., 1988; Matthiesen & Scavelli, 1990; Sharp, 1990; Smeak & Dehoff, 1986). A more recent report followed dogs at least 6 months postoperatively and found an incidence of 24.9%; 46.2% of which required revision surgery (Folk et al., 2022).
As one aspect of SSI prevention, aseptic preparation of surgical patients aims to prevent wound contamination by the removal, inhibition or destruction of transient organisms and reduction of resident flora on the proposed operative site (H. W. Boothe, 1998). This is commonly achieved through the use of topical antiseptics such as chlorhexidine and iodophors. Currently, a dilute aqueous solution of povidone‐iodine (PI) is recommended for preoperative preparation of the external ear canal of patients prior to TECABO procedures due to the often‐irritated epithelium in patients with chronic otitis (Bacon, 2018).
Previous veterinary studies evaluating dilute PI have found decreased efficacy in the presence of organic matter, such as purulent material (Zamora et al., 1985). Additionally, investigations of antiseptic effectiveness comparing dilute preparations of PI and chlorhexidine have demonstrated chlorhexidine's superior ability in preventing wound infections (Amber et al., 1983; Sanchez, Swaim, et al., 1988). Most recently, Neihaus et al. found that in preparation of the canine prepuce, PI resulted in more positive bacterial cultures than chlorhexidine diacetate (CD) and a higher, though not statistically significant, incidence of adverse tissue reactions (Neihaus et al., 2011).
Given these previous findings, the objective of this study was to compare the ability of 0.05% CD and 1.0% PI solutions to reduce bacterial contamination on the canine external ear canal during initial patient preparation and to compare the incidence of immediate tissue reactions. The authors hypothesised that CD would display a significantly greater reduction in bacterial load when compared to PI and result in fewer adverse tissue reactions.
2. MATERIALS AND METHODS
2.1. Patient selection
Twenty dogs presenting to Texas A&M Veterinary Medical Teaching Hospital or Dallas Veterinary Surgical Center for a TECABO were sampled. This study was approved by the primary institution's animal care and use committee (TAMU IACUC 2019‐0231 CA), and informed consent was obtained from all owners prior to the sampling procedure. If dogs were undergoing a bilateral TECABO procedure, only one side was sampled. Dogs requiring a TECABO for any indication were eligible for inclusion in the study. Dogs were assigned to either CD or PI groups randomly. A random number generator was used to assign treatment groups, and the groups were evenly distributed within each institution.
2.2. Patient sampling
Antiseptic solutions were prepared prior to each procedure. The 0.05% CD solution was prepared by adding 12.5 mL of 2% CD solution (Nolvasan 2% solution; Fort Dodge Animal Health) to 237.5 mL of 0.9% saline. The 1% PI solution was prepared by adding 25 mL of 10% povidone‐iodine solution (ReadyPrep PVP 10% solution; Medline) to 225 mL of 0.9% saline.
After induction of anaesthesia, all dogs were placed in lateral recumbency, and a standard clip with a #40 clipper blade was performed on the pinna and surrounding head and neck. A commercial swab (BD BBL CultureSwab Collection & Transport System, Becton Dickinson) containing transport media was used for sampling before and after the initial prep. An initial swab was performed circumferentially around the external ear canal opening without entering the vertical portion of the ear canal (Figure 1). An initial preparation using the assigned solution was performed with cotton‐tipped applicators and cotton balls until gross debris was removed from the external ear canal and the area adjacent to the ear canal opening, ensuring at least a 5‐min contact time with the solution. After the preparation was completed, the area was flushed with 40 mL of 0.9% sterile saline. A second swab was performed tracing the same path as the initial swab, again not entering the vertical portion of the ear canal. Photos of the prepared area were taken prior to each sampling. The surrounding area to be included in the surgical field was then prepared as per the standard of the institution. Patient preparation and sample collection were performed by veterinary technicians who had undergone training on the procedures for this study.
FIGURE 1.
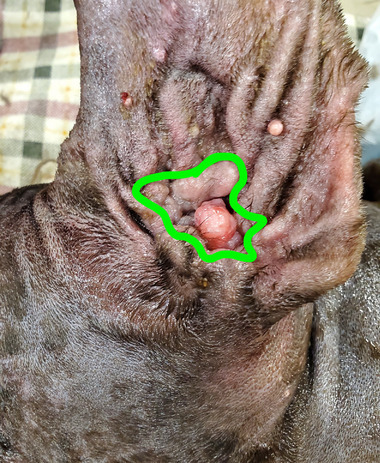
Example outline, in green, of the swabbing pattern used around the external ear canals of client‐owned dogs undergoing initial surgical preparation for total ear canal ablation with bulla osteotomy (TECABO).
At the end of study enrollment, all photos were scrutinised by a single observer (Alicia K. Nye) for the presence of any adverse reactions such as erythema, wheals or weeping of serum. This observer was blinded to the subject's group.
2.3. Sample handling and preparation
All swabs were plated within 24–72 h of collection at the same reference diagnostic laboratory (Clinical Microbiology, Texas A&M Veterinary Medical Teaching Hospital, College Station, TX) by the principal investigator (AN); swabs collected at Dallas Veterinary Surgical Center required transport to the laboratory for processing. Swabs were plated on 5% bovine blood agar, MacConkey agar and Columbia NaladixicAcid (CNA) Agar with Sheep Blood sequentially and isolated using a quadrant streak method. Swabs were also inoculated into enriched Tryptose Broth to confirm any potential cases of negative growth on plates. Blood and Columbia CNA plates were incubated at 37°C in 5% carbon dioxide. MacConkey agar plates were incubated at 37°C in ambient air. Cultures were examined after 24, 48 and 72 h of incubation. All culture examinations were performed by technicians and microbiologists blinded to the antiseptic used for preparation. Semi‐quantitative scoring of total bacterial growth was based on the highest level of growth on any of the three plates. A standard bacterial growth score (BGS) was assigned as follows: growth was recorded as 0 for none, 1 for 10 or fewer colonies in the first quadrant only, 2 for more than 10 colonies in the first quadrant only, 3 for growth spreading into the second quadrant, 4 for growth spreading into the third quadrant and 5 for growth spreading into the fourth quadrant. Bacterial species and their prevalence were noted prior to subculturing for identification purposes. Organisms were identified at the species level using conventional biochemical testing.
2.4. Statistical analysis
BGSs at both sampling times (pre‐ and post‐preparation) and reduction in BGS between sampling times were compared between antiseptic groups and institutions using the Wilcoxon rank‐sum test. Samples with high bacterial growth (defined as BGS of 4 or 5) at each sampling time and association with a high bacterial growth and reduction in BGS were compared between antiseptic groups and institutions using the Fisher's exact test. The level of significance was set at p < 0.05. Samples with negative pre‐preparation cultures (BGS = 0) were excluded from the analysis. Each antiseptic group's post‐preparation BGS scores were assigned either a high BGS (4–5) or low BGS (0–3) for post hoc sample size calculation. A sample size calculation was performed and 942 dogs total, 471 per group, would be needed in order to achieve a power of 80%.
3. RESULTS
Twenty dogs were enrolled in the study. One dog was excluded from the analysis due to negative bacterial growth at the pre‐preparation sampling time. Therefore, 19 dogs were included in the study; 8/19 (42%) were performed at Institution 1 and 11/19 (58%) were performed at Institution 2. The median age of all dogs was 10 years (range 3–14). The breeds included were Shih Tzu (n = 5), Cocker Spaniel (n = 5) and one each of West Highland Terrier, Blue Tick Hound, Labrador Retriever, Chihuahua, Maltese, Yorkshire Terrier, Cairn Terrier, English Bulldog, Basset Hound and Boxer. The most common indication for the TECABO procedure was chronic otitis in 17/20 (85%) of dogs. Six of the 20 (30%) dogs had a presenting complaint of a mass or proliferative tissue within the ear canal with or without the presence of otitis. Fifteen of 20 (75%) dogs were being administered topical or systemic antimicrobials at the time of presentation to the hospital.
Figure 2 illustrates the BGS scores for each antiseptic group pre‐ and post‐preparation. No difference in the reduction of BGS was detected between antiseptic groups (CD vs. PI; p = 0.53; Table 1). Both antiseptic groups showed a significant reduction in BGS between pre‐ and post‐ antiseptic use (n = 19; CD p = 0.009, PI p = 0.005). The median reduction in BGS following antiseptic preparation for all cases was 2 (n = 19; interquartile range (IQR) 1–3). Overall, high bacterial growth at the pre‐preparation sampling time was not associated with the degree of reduction in BGS post‐preparation (p = 0.50).
FIGURE 2.
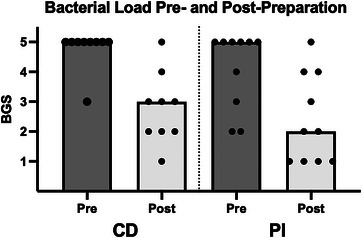
Semi‐quantitative bacterial load detected by culture around the external ear canal of client‐owned dogs (n = 19) undergoing initial surgical preparation for TECABO, pre‐ and post‐preparation with chlorhexidine diacetate (CD) or povidone‐iodine (PI). Bars indicate the median BGS for each antiseptic group. Dots indicate individual values. p < 0.05. BGS = bacterial growth score.
TABLE 1.
Summary of the median scores, percentage of high acterial growth score (BGS), percentage of adverse skin reactions encountered on client‐owned dogs (n = 19) undergoing initial surgical preparation for total ear canal ablation with bulla osteotomy (TECABO), pre‐ and post‐preparation (prep) with chlorhexidine diacetate (CD) or povidone‐iodine (PI).
| Antiseptic group | |||
|---|---|---|---|
| CD | PI | p‐value | |
| Median BGS Pre‐Prep | 5 (5–5) | 5 (2.8–5) | 0.15 |
| High BGS Pre‐prep (%) | 89 | 70 | 0.58 |
| Median BGS Post‐Prep | 3 (2–3.5) | 2 (1–4) | 0.45 |
| High BGS Post‐prep (%) | 22 | 30 | 1.00 |
| Median BGS Reduction | 2 (1–3) | 1 (1–3) | 0.53 |
| Adverse skin reactions (%) | 20 | 30 | 0.63 |
Note: Interquartile range is noted within parentheses.
The median BGS of all samples pre‐preparation was 5 (n = 19; IQR 4–5), and there was no difference between antiseptic groups (p = 0.15). A high level of bacterial growth (BGS 4 or 5) was present in 89% and 70% of the CD and PI antiseptic group samples, respectively. This difference was not significant (p = 0.58).
No difference in median BGS was found between antiseptic groups post‐preparation (p = 0.45); the overall median BGS was 2 (n = 19; IQR 1–4). Twenty‐two percent of the CD group had a high bacterial growth post‐preparation, compared to 30% of the PI group; this finding was not significant between antiseptic groups (p = 1.0).
No difference was found between institutions regarding median BGS post‐preparation or the degree of BGS reduction (p = 0.063; 0.76). A significant difference was found between institutions when assessing the median BGS pre‐preparation (p = 0.033) and the proportion of samples with a high BGS both pre‐ and post‐preparation (p = 0.018 and 0.045, respectively).
Bacterial growth was identified by culture on all but one pre‐preparation sample and all post‐preparation samples; 65% of cultures grew three or more organisms. The identified organisms matched pre‐and post‐preparation (determined by no newly identified organisms in the post‐preparation sample) in 60% of cases. Commonly cultured organisms at each sampling time are listed in Table 2.
TABLE 2.
Summary of the bacterial organisms cultured from the area surrounding the external ear canal of client‐owned dogs (n = 20) undergoing initial surgical preparation for TECABO, pre‐ and post‐preparation (prep) with CD or PI.
|
Pre‐/post‐prep frequency (n = 20) |
CD pre‐/ post‐prep frequency (n = 10) | PI pre‐/ post‐prep frequency (n = 10) | |
|---|---|---|---|
| Staphylococcus | |||
| S. pseudintermedius | 12 (60%)/10 (50%) | 6 (60%)/6 (60%) | 6 (60%)/4 (40%) |
| S. schleiferi | 5 (25%)/5 (25%) | 2 (20%)/2 (20%) | 3 (30%)/3 (30%) |
| Enterococcus faecalis | 5 (25%)/5 (25%) | 1 (10%)/0 (0%) | 4 (40%)/5 (50%) |
| Escherichia coli | 2 (10%) / 3 (15%) | 0 (0%) / 1 (10%) | 2 (20%) / 2 (20%) |
| Corynebacterium | |||
| C. auriscanis | 9 (45%)/5 (25%) | 4 (40%)/2 (20%) | 5 (50%)/3 (30%) |
| C. amycolatum | 2 (10%)/1 (5%) | 1 (10%)/0 (0%) | 1 (10%)/1 (10%) |
| Pseudomonas aeruginosa | 4 (20%)/3 (15%) | 1 (10%)/0 (0%) | 3 (30%)/3 (30%) |
| Proteus mirabilis | 3 (15%)/4 (20%) | 1 (10%)/2 (20%) | 2 (20%)/2 (20%) |
| Streptococcus | |||
| S. canis | 5 (25%)/5 (25%) | 1 (10%)/3 (30%) | 4 (40%)/2 (20%) |
| S. halichoeri | 4 (20%)/5 (25%) | 2 (20%)/3 (30%) | 2 (20%)/2 (20%) |
| Bacillus spp. | 2 (10%)/0 (0%) | 0 (0%)/0 (0%) | 2 (20%)/0 (0%) |
| Other | 6 (30%)/4 (20%) | 4 (40%)/2 (20%) | 2 (20%)/2 (20%) |
Note: Relative frequency is indicated in parentheses.
Subjective examination of pre‐ and post‐preparation photos found only minor adverse skin reactions consisting of localised erythema in 5/20 (25%) cases. Of these, three were in the PI group, and two were in the CD group. There was no significant difference in the occurrence of adverse skin reactions between groups (p = 0.63) or study institutions (p = 1.0).
4. DISCUSSION
The results of this study found no difference in the reduction of bacterial load or incidence of adverse skin reactions between the evaluated antiseptics; thus, the hypothesis was rejected. Both CD and PI resulted in a significant reduction in BGS following preparation.
Neither antiseptic achieved negative post‐preparation growth. This finding was expected as growth was evaluated only after an initial preparation, which involves removing gross contamination prior to the final surgical preparation in the operating room. While the assumption can be made that the number of bacteria present would be further decreased after a second preparation, the surgical preparation used between institutions was not controlled and additional load reduction could not be evaluated. Further, the authors wished to obtain data regarding the safety and efficacy of the two antiseptic solutions prior to changing the entire preparation process. The authors chose to evaluate antiseptic effectiveness by comparing the bacterial load reduction instead of the incidence of SSIs. This method of comparison was chosen because complications, specifically SSIs, may occur after the TECABO procedure for several reasons other than the effectiveness of surgical preparation, including contamination of the site with contents of the middle ear during surgery (Cole et al., 1998; Hettlich et al., 2005; Vogel et al., 1999), incomplete removal of the middle ear epithelium (Smeak, 2016; Smeak et al., 1996) and lack of a normal skin barrier present in dogs prone to infections at this site. However, the incidence of SSIs may be the more clinically applicable method for determining antiseptic effectiveness, and additional studies should be considered to determine if any difference in bacterial load or incidence of SSIs exists between antiseptics following the final surgical preparation.
When evaluated in experimentally contaminated wounds, potential differences in the effectiveness of CD and PI have been noted at dilute concentrations. Amber et al. and Sanchez et al. both found CD was more effective than PI at reducing the incidence of infection in wounds contaminated with Staphylococcus aureus, and dilute PI failed to show any significant bactericidal activity (Amber et al., 1983; Sanchez, Swaim, et al., 1988). A potential explanation for the difference between the antiseptic effectiveness in vivo is PI's interaction with organic material. Iodine may be neutralised when forming complexes and undergoing chemical reactions with organic matter such as blood and purulent discharge. Neutralisation of PI is even more apparent in dilute solutions that have a decreased reservoir of iodine (Ghogawala & Furtado, 1990; Zamora et al., 1985). However, the present study did not find a significant difference between CD and PI effectiveness, and a high bacterial load pre‐preparation had no effect on the BGS reduction. While ears with chronic otitis often have a significant amount of debris, the amount of organic matter present may not correlate with the overall bacterial load. In the current study, the amount of debris was not evaluated, and it is therefore possible that a difference in PI effectiveness may be seen with large amounts of organic matter, and a higher concentration of PI may be indicated (Ghogawala & Furtado, 1990).
Concentrations of the antiseptics evaluated in this study were based on the concentrations that are recommended and used clinically on open wounds, have been shown to exhibit bactericidal activity and have minimal effects on wound healing (Amber et al., 1983; Sanchez, Nusbaum, et al., 1988; Sanchez, Swaim, et al., 1988). Additionally, the 0.05% concentration of chlorhexidine does not cause ototoxic effects, which is a concern with higher concentrations (Harvey, 2006; Merchant et al., 2015). Cats, however, may display transient ototoxicity and middle ear mucosal injury even at this low concentration (Igarashi & Oka, 1988), and therefore use of dilute chlorhexidine in preparation for this species should be avoided.
The present study found only minor adverse tissue reactions with both antiseptics resulting in mild erythema of the external ear and concave pinna. This differs from Neihaus et al. in which more adverse reactions were found to dilute PI solution than to dilute CD on preputial and penile membranes; however, the difference was not statistically significant (Neihaus et al., 2011). In the present study, fewer adverse reactions may have been detected due to a difference in the anatomic location, as the contact times and concentrations were similar, and the preparation of the ear is likely more abrasive than flushing of the prepuce. As in the Neihaus et al. study, it is possible that lavage of the site after preparation could have masked the incidence or severity of adverse tissue reactions that would occur with a contact time of longer than 5 min. Additionally, the use of photos to evaluate the preparation site retrospectively may have altered the appearance of the skin or missed reactions that may have occurred at a later time. While the present study did not evaluate for reactions to the preparation used on surrounding areas, one case that had chlorhexidine scrub (4%) applied to the head and neck had an adverse reaction consisting of erythema and wheals. Importantly, this patient was in the CD group, and no skin reaction was observed on the external ear or concave pinna with the dilute solution. In humans, hypersensitivity reactions to chlorhexidine formulations range from urticaria to life‐threatening anaphylaxis (Heinemann et al., 2002). Reactions to chlorhexidine in veterinary species are reportedly low (D. M. Boothe & Boothe, 2015); however, cases of hypersensitivity are likely underestimated due to poor recognition or recording of this adverse reaction (Heinemann et al., 2002). In contrast, when prepared with PI scrub, skin reactions may occur in nearly half of dogs, regardless of any history of skin disease (Osuna et al., 1990).
The TECABO procedure has a high incidence of long‐term complications, including SSIs. Preparation of the ear is particularly challenging as antiseptics are unable to reach the entirety of the gross contamination within the external and middle ear prior to surgery. The present study chose to evaluate the effectiveness of antiseptics on the external ear as opposed to the ear canal. This area was chosen as it is the location of the incision, is accessible to antiseptic preparation in all patients regardless of the degree of canal stenosis and is representative of organisms that will be left superficially at the surgical site as well as provide a source of contamination throughout the entirety of the surgical procedure. Identification of organisms present in the dog ear can be quite difficult as cultures often display variation in isolates and susceptibility, even when performed at the same location within the ear canal (Graham‐Mize & Rosser, 2004). This may explain why bacterial species cultured pre‐ and post‐preparation matched exactly in only 60% of our cases. As variation can also be seen between laboratories, all samples in the current study were performed by a single laboratory. Only one case had a negative pre‐preparation culture confirmed by lack of growth in enrichment broth, and surprisingly Escherichia coli was isolated on the post‐preparation culture. While this may represent an example of the aforementioned variation between samples, it is likely that this was a contaminant as E. coli is not a commonly cultured organism in healthy or affected dog ears (Tang et al., 2020).
The culturing technique used in the present study is limited in its ability to quantify bacteria. A semi‐quantitative method was chosen as the bacterial load can be underestimated by culture due to the assumption that a single organism is responsible for each colony‐forming unit (AnKh et al., 2020). Due to the presence of active infection, the growth on the majority of the pre‐preparation samples (14/20) had the highest score possible (BGS 5) when using the chosen semi‐quantitative method. This makes it difficult to say if pre‐preparation samples between groups were truly similar in their bacterial load. Serial dilutions of the samples followed by colony counts would have potentially allowed for better comparison between groups as well as evaluation of the bacterial load reduction. An additional limitation of culture is that results may be affected by storage or transport, which may falsely decrease or increase the bacterial quantity. In the present study, 12 of 20 culture swabs required transport to the microbiology laboratory, which may account for the differences found between institutions. Transported samples were more likely to have a higher median pre‐preparation BGS as well as a high (grade 4 or 5) BGS pre‐ and post‐preparation. This may have been due to exposure to temperatures above those recommended by the swab manufacturer during transport, allowing for the propagation of bacterial organisms. Regardless, swabs from each patient (pre‐ and post‐preparation) were transported together and subjected to the same conditions to allow for pairwise comparison.
Veterinary studies have shown chlorhexidine's persistence of action at a dilution of 0.05% (Amber et al., 1983; Sanchez, Swaim, et al., 1988) and on the skin, and this is due to protein binding within the stratum corneum (Sebben, 1983). This feature poses a theoretical advantage to the TECABO incision site in the immediate postoperative period, as the procedure does not completely remove skin that is prone to dermatitis in these patients. However, this characteristic, while beneficial to surgical wounds, can lead to potential overestimation of antiseptic effectiveness by continued bactericidal activity within samples if methods of neutralisation are not utilised (Kampf, 2019; Reichel et al., 2008; Sutton et al., 2002). Neutralisation of antiseptics may be accomplished by dilution or chemical neutralisation. For the current investigation, dilution of the residual antiseptic was performed with lavage of the area with sterile saline. Assuming that 10 mL of antiseptic is an overestimation of the maximum residual volume present after preparation, the quantity of 40 mL for post‐preparation lavage was chosen in order to reach a maximum concentration of 0.01% and 0.02% for CD and PI, respectively. This residual concentration is well below the minimally effective concentration for each antiseptic (Sanchez, Swaim, et al., 1988). Clinically, lavage of the area is not typically performed after preparation, and it is possible that this step may have further reduced the bacterial load on the area and overestimated the effectiveness of each antiseptic.
An important limitation of the current study is the small sample size, based on power calculation, leading to the possibility of a Type II error. However, given the lack of an obvious disadvantage or incidence of adverse effects, the authors conclude that either antiseptic could be used for safe preparation limited to the external ear canal of dogs.
5. CONCLUSION
CD and PI were similarly able to decrease the number of bacteria found on the ear by culture following initial preparation. No significant difference in the ability of either antiseptic to reduce bacterial load was found and no difference in the incidence of adverse tissue reactions was found. Properly diluted aqueous formulations of either antiseptic may be used for safe preparation limited to the external ear canal of dogs.
AUTHOR CONTRIBUTIONS
Study design, acquisition of data and drafting of work: Alicia K. Nye. Acquisition of data and review of manuscript: Matthew Lazarus and Amanda Amore. Conception of study, study design, revision of content and final approval of the version to be published: Artem Rogovskyy and Kelley Thieman Mankin.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest related to this report.
FUNDING INFORMATION
The authors received no specific funding for this work.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. This study was approved by the primary institution's animal care and use committee (TAMU IACUC 2019‐0231 CA), and informed consent was obtained from all owners prior to the sampling procedure.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1200.
ACKNOWLEDGEMENTS
Joe Hauptman for statistical analysis. Heather Noecker, Mallory Worley, Marina Harrison and Dana Whitaker for sample collection.
Nye, A. K. , Rogovskyy, A. , Lazarus, M. A. , Amore, R. , & Mankin, K. M. T. (2023). Effectiveness of chlorhexidine diacetate and povidone‐iodine in antiseptic preparation of the canine external ear canal prior to total ear canal ablation with bulla osteotomy procedure: A preliminary study. Veterinary Medicine and Science, 9, 1998–2005. 10.1002/vms3.1200
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Amber, E. I. , Henderson, R. A. , Swaim, S. F. , & Gray, B. W. (1983). A comparison of antimicrobial efficacy and tissue reaction of four antiseptics on canine wounds. Veterinary Surgery, 12(2), 63–68. 10.1111/j.1532-950x.1983.tb00708.x [DOI] [Google Scholar]
- AnKh, B. , AlKh, B. , Kuluev, B. R. , KYu, S. , Yamidanov, R. S. , Matniyazov, R. T. , Chemeris, D. A. , Zubov, V. V. , YaI, A. , Mavzyutov, A. R. , YaA, I. , & Chemeris, A. V. (2020). Modern approaches to differentiation of live and dead bacteria using selective amplification of nucleic acids. Microbiology, 89(1), 13–27. 10.1134/s0026261720010038 [DOI] [Google Scholar]
- Bacon, N. J. (2018). Pinna and external ear canal. In K. M. Tobias & S. A. Johnston (Eds.), Veterinary surgery: Small animal ( 2nd ed., Vol 2 , pp. 2310–2327). Elsevier. [Google Scholar]
- Beckman, S. L. , Henry, W. B. , & Cechner, P. (1990). Total ear canal ablation combining bulla osteotomy and curettage in dogs with chronic otitis externa and media. Journal of the American Veterinary Medical Association, 196(1), 84–90. [PubMed] [Google Scholar]
- Boothe, D. M. , & Boothe, H. W. (2015). Antimicrobial considerations in the perioperative patient. Veterinary Clinics of North America: Small Animal Practice, 45(3), 585–608. 10.1016/j.cvsm.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Boothe, H. W. (1998). Antiseptics and disinfectants. Veterinary Clinics of North America: Small Animal Practice, 28(2), 233–248. 10.1016/s0195-5616(98)82003-2 [DOI] [PubMed] [Google Scholar]
- Cole, L. K. , Kwochka, K. W. , Kowalski, J. J. , & Hillier, A. (1998). Microbial flora and antimicrobial susceptibility patterns of isolated pathogens from the horizontal ear canal and middle ear in dogs with otitis media. Journal of the American Veterinary Medical Association, 212(4), 534–538. [PubMed] [Google Scholar]
- Eugster, S. , Schawalder, P. , Gaschen, F. , & Boerlin, P. (2004). A prospective study of postoperative surgical site infections in dogs and cats. Veterinary Surgery, 33(5), 542–550. 10.1111/j.1532-950x.2004.04076.x [DOI] [PubMed] [Google Scholar]
- Folk, C. A. , Lux, C. N. , Sun, X. , & Fryer, K. J. (2022). Effect of empirical versus definitive antimicrobial selection on postoperative complications in dogs and cats undergoing total ear canal ablation with lateral bulla osteotomy: 120 cases (2009–2019). Journal of the American Veterinary Medical Association, 260(8), 899–910. 10.2460/javma.21.10.0462 [DOI] [PubMed] [Google Scholar]
- Garibaldi, R. A. , Cushing, D. , & Lerer, T. (1991). Risk factors for postoperative infection. The American Journal of Medicine, 91(3), S158–S163. 10.1016/0002-9343(91)90362-2 [DOI] [PubMed] [Google Scholar]
- Ghogawala, Z. , & Furtado, D. (1990). In vitro and in vivo bactericidal activities of 10%, 2.5%, and 1% povidone‐iodine solution. American Journal of Hospital Pharmacy, 47, 1562–1566. [PubMed] [Google Scholar]
- Graham‐Mize, C. A. , & Rosser, E. J. (2004). Comparison of microbial isolates and susceptibility patterns from the external ear canal of dogs with otitis externa. Journal of the American Animal Hospital Association, 40(2), 102–108. 10.5326/0400102 [DOI] [PubMed] [Google Scholar]
- Guedeja‐Marrón, J. , Blanco, J. L. , Ruperez, C. , & Garcia, M. ‐E. (1998). Susceptibility of bacterial isolates from chronic canine otitis externa to twenty antibiotics. Journal of Veterinary Medicine, Series B, 45(1‐10), 507–512. 10.1111/j.1439-0450.1998.tb00821.x [DOI] [PubMed] [Google Scholar]
- Guillamumot, P. , Poncet, C. , & Bouvy, B. (2011). Outcome after total ear canal ablation and subtotal bulla osteotomy (TECASBO) in 23 dogs. Wiener Tierärztliche Monatsschrift, 98(5/6), 106–113. [Google Scholar]
- Harvey, R. (2006). Use of topical ear cleaners in small animals. In Practice, 28(3), 131–135. 10.1136/inpract.28.3.131 [DOI] [Google Scholar]
- Heinemann, C. , Sinaiko, R. , & Maibach, H. I. (2002). Immunological contact urticaria and anaphylaxis to chlorhexidine: Overview. Current Problems in Dermatology, 1(4), 186–194. 10.1159/000066145 [DOI] [Google Scholar]
- Hettlich, B. F. , Boothe, H. W. , Simpson, R. B. , DuBose, K. A. , Boothe, D. M. , & Carpenter, M. (2005). Effect of tympanic cavity evacuation and flushing on microbial isolates during total ear canal ablation with lateral bulla osteotomy in dogs. Journal of the American Veterinary Medical Association, 227(5), 748–755. 10.2460/javma.2005.227.748 [DOI] [PubMed] [Google Scholar]
- Igarashi, Y. , & Oka, Y. (1988). Mucosal injuries following intratympanic applications of chlorhexidine gluconate in the cat. Archives of Oto‐Rhino‐Laryngology, 245(5), 273–278. 10.1007/bf00464629 [DOI] [PubMed] [Google Scholar]
- Kampf, G. (2019). Antibiotic resistance can be enhanced in gram‐positive species by some biocidal agents used for disinfection. Antibiotics, 8(1), 13. 10.3390/antibiotics8010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, L. K. , Harvey, C. E. , & Orsher, R. J. (1988). Total ear canal ablation combined with lateral bulla osteotomy for end‐stage otitis in dogs results in thirty dogs. Veterinary Surgery, 17(5), 263–268. 10.1111/j.1532-950x.1988.tb01012.x [DOI] [PubMed] [Google Scholar]
- Matthiesen, D. T. , & Scavelli, T. (1990). Total ear canal ablation and lateral bulla osteotomy in 38 dogs. Journal of the American Animal Hospital Association, 26, 257–267. [Google Scholar]
- Merchant, S. R. , Neer, T. M. , Tedford, B. L. , Twedt, A. C. , Cheramie, P. M. , & Strain, G. M. (2015). Ototoxicity assessment of a chlorhexidine otic preparation in dogs. Progress in Veterinary Neurology, 4(3), 72–75. [Google Scholar]
- Neihaus, S. A. , Hathcock, T. L. , Boothe, D. M. , & Goring, R. L. (2011). Presurgical antiseptic efficacy of chlorhexidine diacetate and providone‐iodine in the canine preputial cavity. Journal of the American Animal Hospital Association, 47(6), 406–412. 10.5326/jaaha-ms-5681 [DOI] [PubMed] [Google Scholar]
- Osuna, D. J. , DeYoung, D. J. , & Walker, R. L. (1990). Comparison of three skin preparation techniques Part 2: Clinical trial in 100 dogs. Veterinary Surgery, 19(1), 20–23. 10.1111/j.1532-950x.1990.tb01137.x [DOI] [PubMed] [Google Scholar]
- Penna, B. , Varges, R. , Medeiros, L. , Martins, G. M. , Martins, R. R. , & Lilenbaum, W. (2010). Species distribution and antimicrobial susceptibility of staphylococci isolated from canine otitis externa. Veterinary Dermatology, 21(3), 292–296. 10.1111/j.1365-3164.2009.00842.x [DOI] [PubMed] [Google Scholar]
- Reichel, M. , Heisig, P. , & Kampf, G. (2008). Pitfalls in efficacy testing—How important is the validation of neutralization of chlorhexidine digluconate? Annals of Clinical Microbiology and Antimicrobials, 7(1), 20. 10.1186/1476-0711-7-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, I. R. , Nusbaum, K. E. , Swaim, S. F. , Hale, A. S. , Henderson, R. A. , & McGuire, J. A. (1988). Chlorhexidine diacetate and povidone‐iodine cytotoxicity to canine embryonic fibroblasts and Staphylococcus aureus . Veterinary Surgery, 17(4), 182–185. 10.1111/j.1532-950x.1988.tb00995.x [DOI] [PubMed] [Google Scholar]
- Sanchez, I. R. , Swaim, S. F. , Nusbaum, K. E. , Hale, A. S. , Henderson, R. A. , & McGuire, J. A. (1988). Effects of chlorhexidine diacetate and povidone‐lodine on wound healing in dogs. Veterinary Surgery, 17(6), 291–295. 10.1111/j.1532-950x.1988.tb01019.x [DOI] [PubMed] [Google Scholar]
- Sebben, J. E. (1983). Surgical antiseptics. Journal of the American Academy of Dermatology, 9, 759–765. [DOI] [PubMed] [Google Scholar]
- Sharp, N. J. H. (1990). Chronic otitis externa and otitis media treated by total ear canal ablation and ventral bulla osteotomy in thirteen dogs. Veterinary Surgery, 19(2), 162–166. 10.1111/j.1532-950x.1990.tb01159.x [DOI] [PubMed] [Google Scholar]
- Smeak, D. D. (2016). Treatment of persistent deep infection after total ear canal ablation and lateral bulla osteotomy. Veterinary Clinics of North America: Small Animal Practice, 46(4), 609–621. 10.1016/j.cvsm.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Smeak, D. D. , Crocker, C. B. , & Birchard, S. J. (1996). Treatment of recurrent otitis media that developed after total ear canal ablation and lateral bulla osteotomy in dogs: Nine cases (1986‐1994). Journal of the American Veterinary Medical Association, 209(5), 937–942. [PubMed] [Google Scholar]
- Smeak, D. D. , & Dehoff, W. D. (1986). Total ear canal ablation clinical results in the dog and cat. Veterinary Surgery, 15(2), 161–170. 10.1111/j.1532-950x.1986.tb00197.x [DOI] [Google Scholar]
- Sutton, S. V. W. , Proud, D. W. , Rachui, S. , & Brannan, D. K. (2002). Validation of microbial recovery from disinfectants. Journal of Pharmaceutical Science and Technology, 56(5), 255–266. [PubMed] [Google Scholar]
- Tang, S. , Prem, A. , Tjokrosurjo, J. , Sary, M. , Bel, M. A. V. , Rodrigues‐Hoffmann, A. , Kavanagh, M. , Wu, G. , Eden, M. E. V. , & Krumbeck, J. A. (2020). The canine skin and ear microbiome: A comprehensive survey of pathogens implicated in canine skin and ear infections using a novel next‐generation‐sequencing‐based assay. Veterinary Microbiology, 247, 108764. 10.1016/j.vetmic.2020.108764 [DOI] [PubMed] [Google Scholar]
- Vasseur, P. B. , Levy, J. , Dowd, E. , & Eliot, J. (1988). Surgical wound infection rates in dogs and cats data from a teaching hospital. Veterinary Surgery, 17(2), 60–64. 10.1111/j.1532-950x.1988.tb00278.x [DOI] [PubMed] [Google Scholar]
- Vogel, P. L. , Komtebedde, J. , Hirsh, D. C. , & Kass, P. H. (1999). Wound contamination and antimicrobial susceptibility of bacteria cultured during total ear canal ablation and lateral bulla osteotomy in dogs. Journal of the American Veterinary Medical Association, 214(11), 1641–1643. [PubMed] [Google Scholar]
- White, R. A. S. , & Pomeroy, C. J. (1990). Total ear canal ablation and lateral bulla osteotomy in the dog. Journal of Small Animal Practice, 31(11), 547–553. 10.1111/j.1748-5827.1990.tb00683.x [DOI] [Google Scholar]
- Zamora, J. L. , Price, M. F. , Chuang, P. , & Gentry, L. O. (1985). Inhibition of povidone‐iodine's bactericidal activity by common organic substances: An experimental study. Surgery, 98(1), 25–29. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


